Open Access Article
Open Access ArticleA review on assessment of ionic liquids in extraction of lithium, nickel, and cobalt vis-à-vis conventional methods
Pratima Meshram ab,
Nikita Agarwala and
Abhilash
ab,
Nikita Agarwala and
Abhilash *ab
*ab
aCSIR-National Metallurgical Laboratory, Jamshedpur, 831007, India. E-mail: abhilash@nml.res.in
bAcademy of Scientific and Innovative Research (AcSIR-NML), Jamshedpur, India
First published on 18th March 2025
Abstract
This review discusses the extraction of critical metals (Li, Co, and Ni) using ionic liquids. Here, ionic liquids act as solvents for the separation and extraction of metals. In addition to extraction, they can be used as a lixiviant to leach out metals from spent lithium-ion batteries. Leaching and extraction of metals from the leachate can be performed using a single ionic liquid solvent. Lithium, cobalt, and nickel have been discussed in detail as per their reactivity towards an ionic liquid based on the extraction efficiency and reusability of the ionic liquid. Recycling and reusability of ionic liquids are crucial parameters to be considered while using them as solvents for extracting metals. Moreover, all the other methods such as solvent extraction, ion exchange, ionic liquids, and DES-based separation of metals are compared with respect to their extraction efficiency, cost-effectiveness, and reusability.
Introduction
Ionic liquids are popularly known as “designer solvents” owing to their proficiency in tuning and modifying thermo-physical properties.1 In recent times, ionic liquids are regarded as a new category of solvents having the ability to flow, consisting of an organic cation and inorganic or organic anions. Moreover, they have remarkable physicochemical properties, including inflammability, thermal stability, increased conductivity, non-volatility, and recyclability; hence, they are also referred to as “green solvents”. The term “green solvents” also refer to their characteristics of decreasing the possibility of polluting the atmosphere and minimizing the quantity of catalysts used for a reaction.2,3 Ionic liquids can be used in several fields such as electrolytes in batteries, metal plating, solar panels: solvents in reactions, extraction procedures in hydrometallurgy, coating materials and lubricants. This review will focus on ionic liquids and their applications in hydrometallurgy.In recent years, metals have become an integral part of our daily lives; they have shaped the course of history through various applications. Consequently, the demand for ferrous and non-ferrous metals, rare earth metals, critical metals, and precious metals is increasing day by day.4,5 Critical metals play an essential role in energy industries, especially in battery storage, but are scarce in recent times. An economically significant metal at high supply risk is considered a critical metal. Natural resources of essential metals cannot be renewed and are exhausting owing to their high demand.6,7 Lithium, cobalt, and nickel, among other metals, are distinguished as critical metals, and since the world is going further with substantial and material-based systems rather than fuel-based systems, the demand for these metals is rising.8 For the extraction of critical metals, the feasible alternative to hard rocks is saline water mining as saline water is loaded with dissolved metals.9
Critical metals such as Li, Co, Ni, and Mn are also used for the production of lithium-ion batteries and can thus be extracted from spent Li-ion batteries. The anode and cathode are the two main components of Li-ion batteries; the anode comprises graphite, whereas the cathode is layered with the lithium metal oxides LiCoO2 (LCO), LiMn2O4 (LMO), LiFePO4 (LFP) and LiNiMnCo2 (NMC).10–13 Extracting metals from spent LIBs is a recycling process that is necessary not only for extracting critical metals but also for environmental reasons, as some metals and electrolytes present in the battery can harm the environment if released without any recycling treatment. Recycling can be done using pyrometallurgical or hydrometallurgical routes. Hydrometallurgy includes discharging, the dismantling of LIBs, and the separation of different components of the battery. After separating all the components, dissolution or leaching is done to recover critical metals. Hydrometallurgy includes less thermal treatment when compared to pyrometallurgy and helps to obtain pure metals.14 The dissolution of metals is followed by solvent extraction, precipitation, and other methods to get pure metal salts. Solvent extraction has been used recently to recover valuable metals using organic solvents, and metal recovery usually depends on the loading capacity of the organic solvent.15
However, metal separation can be performed using ionic liquids instead of organic solvents. This review discusses ionic liquids, their properties, synthesis, and advantages over organic solvents for extracting and separating Li, Ni and Co from the processed and synthetic solutions, primarly on batteries.
Ionic liquids
Structure and examples
An ionic liquid is a class of salt that behaves as a liquid at a low temperature (room temperature). NaCl, a salt, is made up of Na+ and Cl− ions that neutralize each other to form a stable compound. Similarly, ionic liquids are also made up of cations and anions.Traditionally, at room temperature, salts do not exist in liquid state and are termed molten salts above 800 °C.
Table salts such as NaCl have small-sized cations and anions that are arranged in an orderly manner and packed closely, resulting in a crystalline structure. These ions have strong interactions with each other, forming a well-defined lattice structure. Displacing ions from their position requires high energy due to the tightly packed arrangement of ions in these salt crystals.
However, in ionic liquids, the cation size is more significant, and the anion size has variability. These irregularities in the sizes of cations and anions make it challenging to create a lattice structure, leading to their liquid state. The structures of molten salt and ionic liquid are shown in Fig. 1.
Fig. 2 illustrates that the cations used to synthesize ionic liquids are mostly heterocyclic rings with one or more N-atoms and can be paired up with the anions shown in the same figure.
History of ionic liquids
In 1914, Paul Walden pioneered the idea of ionic liquids by reporting the physical properties of ethyl ammonium nitrate ([EtNH3][NO3]) at a melting point of 13–14 °C.16 Ethylamine was made to react with concentrated nitric acid to give the neutralized product, ethyl ammonium nitrate, using distillation to remove water, as shown in Fig. 3.17–19 In the 1950s, a concept of halide salt ionic liquid with a mixture of aluminum chloride and ethyl pyridinium bromide (EtPyBr) was found in a mole ratio of 2/1 to form a low-temperature molten salt.20 These molten salts were of high interest because of their negligible vapor pressure, high ionic conductivities, and wide temperature ranges. In some cases, these salts also behave as Lewis acid catalysts. Earlier, the findings focused on the imidazolium or pyridinium cations and their mixture with chloroaluminate anions.21 Chloroaluminate ions control the chemistry of these ionic liquids with the change in their proportions. Changing the proportions can change the melt composition. These ionic liquids have been used for the deposition of metals and aluminum batteries, and they have helped to conduct several reactions as a medium.22,23 One example of chloroaluminate ionic liquid is given in Fig. 4, where N-ethyl pyridinium bromide is also a constituent of the ionic liquid with aluminum chloride.However, the two ionic liquids discussed above demand complete, sophisticated handling as they are not water-stable and need an entire water-free state. Therefore, a new generation of ionic liquids came into the picture in 1992, as reported by Wilkes and Zaworotko, to overcome the handling issues. These ILs have air- and water-stable imidazolium cations with alkyl substituents and are inadequately coordinated with anions such as tetrafluoroborate [BF4] and hexafluorophosphate [PF6]. These ions have been changed recently because of their restricted stability by a new set of stable anions such as tris(pentafluoroethyl)trifluorophosphate, triflate [CF3SO3] or [OTf], and particularly bis(trifluoromethanesulfonyl)amide [(CF3SO2)2N] or [NTf2], along with the halide-free anions methylsulfate [CSO4], acetate [C1COO], thiocyanate [SCN], and others.24,25
Properties of ionic liquids
Ionic liquids comprise an asymmetric organic cation and an anion, which can be organic or inorganic.26 Various types of bonds link together these cations-anions. They relate to many properties including all the physical and chemical properties, making the vapor pressure almost negligible. These salts possess large ions due to the delocalization of charges, lowering the force of attraction between the ions and decreasing the melting temperature of ionic liquids.27,28 Its low vapor pressure does not allow it to evaporate at room temperature.23 This is one of the properties of ionic liquids, which decreases the exposure risk factor compared to other volatile solvents. Moreover, this non-volatile property makes it less flammable under normal conditions.29,30 Nowadays, ionic liquids are also used in industries not just for their low vapor pressure but also for their air and water stability.31ILs are environmentally friendly for many reasons. It is non-volatile, inflammable, mixes with other organic solvents or water, and has better solubility of organic and inorganic materials. Conventionally used volatile and toxic solvents can be replaced by ionic liquids. Moreover, ILs have high ionic conductivity, potential windows, and thermal stability.23,32
All these properties of ionic liquids can be tuned or changed by a proper choice of anions and cations, which can also give us an enormous number of combinations. Thus, the properties of ILs are tunable and primarily dependent on the structure of cations and anions and can be called ‘designer solvents’. Properties such as hydrophilicity-lipophilicity, viscosity, surface tension, and density depend on the length of the alkyl chain attached to the cation and its symmetry, including the type of anion.27
These properties of ionic liquids can be studied with the help of a cation that has been used most recently. Methylimidazolium-based ionic liquids are considered the most favorable cationic species for this discussion. They are stable in air and water, hold a wide range of liquids, obviously stay liquid at room temperature, and have more significant viscosity and density. Moreover, N-methylimidazole is commercially available making its alkylation easier than any other cations and becomes one of the most popular cations used in the synthesis of ionic liquids.33 It is also seen that adding alkyl groups or increasing the number of carbon atoms in the alkyl chain can change the properties of ionic liquids.34,35 The substituents linked to the imidazole molecule and the type of counter anion present decide the solubility of ionic liquids in water.24
Synthesis of ionic liquids
Ionic liquids can be synthesized in two steps.(1) Formation of the desired action
(2) Anion exchange
Initially, the desired cation can be synthesized by the protonation of amine, alkylation, or quaternization of amines. In addition, the anions were exchanged by changing the halide through anion metathesis or using a Lewis acid to form a Lewis-acid-based ionic liquid.16,23
A general representation of the synthesis of ionic liquids including both the steps is shown in Fig. 5.23 Alkyl halide was used to form the desired cation from a N-based compound and anion was exchanged as the second step using any Lewis/Brønsted acids or via ion exchange resins.23,29
One of the elementary ways to prepare imidazolium-based ionic liquids is the reaction of methylated imidazole molecules with alkyl halide. Eventually, desired room-temperature ionic liquids were achieved after a metathesis of halide salt.24
In Fig. 6, several ionic liquids were synthesized, following the mentioned steps. To synthesize the cation, methylimidazole was taken with an alkyl halide and kept in reflux to get 1-alkyl-3-methylimidazolium chloride. The halide ion product was treated with several other acids and salt to change the anion.31,36
Usually, alkylation, anion metathesis, acid–base reactions, and heterocyclic compound reactions were used to synthesize ionic liquids.
The amination of ionic liquids could be reached by the quaternization of ammonia, its derivatives and phosphine derivatives. Hydrophilic and hydrophobic products were formed during metathesis of an anion through an intermediate. The intermediate anion was then substituted by any other anion, giving the hydrophobic or hydrophilic ionic liquid products.
Application of ionic liquids in metal recovery via hydrometallurgy
Leaching
Depleted Li-ion batteries can be recycled using hydrometallurgy, including leaching, solvent extraction, and precipitation. Traditionally, inorganic acids, organic acids, or oxidants were used to leach metals from depleted lithium-ion batteries (LCO, LMO, NMC, LFP, etc.), producing a large quantity of acidic waste. The separation of metals from the leachate was done using the solvent extraction method. Solvent extraction includes the use of organic solvents such as Cyanex, D2EHPA, and TBP, which again creates a problem as these solvents are highly volatile, and toxic for the environment, and multiple extractants are required to recover metals selectively.37,38 Therefore, to overcome these disadvantages, ionometallurgy is introduced, where ionic liquids play a major role as solvents, and their ability to coordinate strongly is highly important to dissociate the reactive metals. Leaching via ionic liquids may show a higher metal selectivity than that of inorganic or organic acids. Generally, Brønsted acidic ionic liquids are used for leaching, as this class of ionic liquids can donate H+ protons to the solution. The donated H+ in the solution reacts with metal oxides and can lead to the formation of water, showing the dissolution capability of metal oxides.39,40Lithium can be recovered from brines via solvent extraction, but to recover lithium as well as other metals such as Co and Ni from depleted lithium-ion batteries, leaching is a necessary step for the dissolution of metals. The dissolution of metals can be explained with the help of an example of extraction of cobalt from LCO batteries using ionic liquids. Approximately, 25–30 wt% of a portable lithium-ion battery consists of cobalt as a part of the cathode material in the LiCoO2 (LCO) battery.41–43
Ionic liquid [Hbet][NTf2] was applied for the leaching of LCO but cobalt oxides do not have high solubility in pure [Hbet][NTf2]. The addition of chloride increased the solubilities of cobalt oxides and improved the rate of dissolution. IL cation [Hbet]+ donated acidic protons to hydrate the oxygen atoms at the oxide surface, as shown in eqn (1) and the unused betaine coordinated the cobalt ions. This resulted in the diffusion of dissolved species into the solution; also, betaine got displaced by chloride as Co showed great affinity towards chloride in ligand exchange reactions. Cobalt(III) got reduced, leading to the formation of Cl3− enabling the oxidative leaching of Co parallelly.44,45
| Co(II) + [Hbet][NTf2] + [Hbet]Cl (4LiCoO2 + 40[Hbet]+ + 16Cl−) = 4[Li(bet)3]+ + 4[CoCl4]2− + 28[Hbet]+ + 6H2O + O2↑ | (1) |
It took 2–3 hours to complete the dissolution of LCO, at 150 °C in a closed flask. The molar ratio of leaching reactants LCO/[Hbet][NTf2]/[Hbet]Cl was 1/10/2. After the dissolution, [CoCl4]2− was formed as one of the products. Direct electrodeposition occurred to change [CoCl4]2− into [Co(H2O)6]2+ in the presence of water, as represented in eqn (2). [Co(H2O)6]2+ migrates to the cathode, displacing the chloride ions.44,45 The temperature of leaching does not exceed 150 °C, and this low temperature is advantageous to the IL reusability.46 The reaction environment created by ionic liquids comprising only ions establishes exceptional dissolution properties for metals.44,45,47,48
| [CoCl4]2− + 6H2O = [Co(H2O)6]2+ + 4Cl− | (2) |
One more example of an ionic liquid as a lixiviant for the leaching of depleted lithium-ion batteries is an imidazolium-based ionic liquid, imidazolium glycol [1-(2,3-dihydroxypropyl)-3-methylimidazolium chloride]. To recover metals from spent LCO batteries, imidazolium glycol behaves as a non-decomposable leaching agent. Under heating conditions, imidazolium glycol can generate the oxygen anion redox of transition metal-ions as illustrated in eqn (3) and shows a high leaching efficiency of up to 100% Li and >99% Co. Imidazolium glycol can be easily recycled and reused as it does not go through redox decomposition. This procedure for the extraction of metals via imidazolium glycol is environmentally suitable and achievable, demonstrating significant and capable applications in LIB recycling.49
| 10[DHPMIm]+ + 10Cl− + 2LiCoO2 = [DHPMIm]4[Co2Cl8] + 6[DHPMIm]+ + 2Li+ + 2Cl− + 2O2− | (3) |
Imidazolium glycol was recovered and reused after every cycle of leaching, as it does not decompose during the process without producing any toxic gases.37
Solvent extraction
Solvent extraction refers to the diffusion of a solute between two non-miscible liquids, when in contact, distributing the solute into two phases. It is the most favored technique used for metal recovery due to its simplicity, speed, and wide range.27 As metal ions have a wide range of applications in the industrial area of metallurgy, electronics, catalysis, synthetic chemicals, etc., metal ion extraction has always driven ample recognition from researchers.50 It has stimulated the rapid growth of industries and delivered a significant economic profit, yet also raised environmental problems. Metal extraction is applied for three primary business cases: (a) extraction of metal ions from natural resources, (b) recycling the materials from secondary resources, and (c) wastewater treatment.51Solvent extraction employs water-immiscible organic solvents that can be toxic, flammable, or volatile. Given the rising costs for their eventual disposal and the growing awareness of the environmental impact associated with their use, replacing these solvents with less harmful alternatives is desirable.
Ionic liquid used as a solvent
Ionic liquids show various properties that make them sound like solvents including a wide liquid range, thermal stability, ability to dissolve an extensive range of solutes, absence of vapor pressure, and many more factors.27 Moreover, it can be referred to as an environment-friendly solvent and less toxic than traditionally used organic solvents.28 Nowadays, an essential aspect of research is its effect on the environment and whether it is green or not. Keeping these conditions in mind, the ionic liquid was designed to be safe, and its eco-friendly separation procedures have created a vital role in clean manufacturing processes and the cleanup of areas affected by manufacturing technology from an earlier generation.Lately, significant attraction has been paid to the room-temperature ionic liquids as solvents for several reactions such as alkylation, acylation, and polymerization. This perspective allows the controlled production of wanted products from reactants with the least amount of waste generation via side reactions, as ionic liquids tend to repress the solvation.52
The hydrophobic characteristics of some ILs allow the extraction of several heavy metals such as zinc, mercury, lead, cadmium, iron, chromium, copper, and nickel, from their aqueous solutions. Despite their hydrophobic character, ILs offer high solubility for salts and can be used to extract salts. The solubility of ILs in water strongly depends on the type of cations and anions. ILs possessing acid anions can also be used for the leaching of metals from ores.27
The extraction of metals from brine solutions (lithium) or from spent lithium-ion batteries (lithium, cobalt, nickel, etc.) can be achieved using a pathway shown in Fig. 7. Leachates of lithium-ion batteries (cathode material) or brine solutions are taken as aqueous phases where the addition of ionic liquids is carried out to separate metals. Metals usually get transferred to an organic phase reacting with the anionic part of the ionic liquid and the ionic liquid cation gets transferred to the aqueous raffinate phase. After separation, the organic phase is stripped using an acid/base/salt to obtain an ionic liquid-free aqueous metal solution. Ionic liquids can be regenerated again for further separation and extraction of metals.
Extraction of metals using ionic liquids
Lithium exists in two states: mineral and liquid. The absence of adequate availability of lithium as a mineral has directed the industries towards its other forms of resources. The other form is liquid, which contains 85% more retrievable lithium in the world.56,57
One of the primary lithium resources is enclosed in the brines of lake sediments. Primarily, 60–65% of the lithium in total is stored in brines.53,55,58 Apart from brines, the use of lithium in lithium-ion batteries increased from 23% to 74% in the last few years, which exceeded the consumption of glass and ceramics.53
Thus, the necessity of lithium is increasing for several applications, and there is a call for its treatment/extraction from all practical resources. The extraction procedure of lithium from minerals/ores consists of roasting and leaching, while its recovery from brines follows evaporation, precipitation, adsorption, and ion exchange. However, lithium extraction can be done from Li-ion batteries using hydrometallurgical methods, including leaching, precipitation, and ion exchange or solvent extraction.54 Nowadays, solvent extraction is thought to be the most effective method for extracting lithium from its aqueous solutions (brine solution or Li-ion batteries) as it is eco-friendly, highly selective, easily accessible, and efficient.57–60
Solvent extraction of lithium from its brine or aqueous solution was usually performed using the extraction system of tributyl phosphate (TBP)/FeCl3 and kerosene. TBP is an organophosphorus extractant, highly popular for the traditional extraction procedure where kerosene is used as a diluent. Ferric chloride (FeCl3) solution works as a co-extracting agent.57–59 Various analyses from the literature show that the TBP–Kerosene–FeCl3 system satisfies the separation of lithium from its solution as explained in eqn (4).57
Acidic conditions were needed for this system to work and to prevent the hydrolysis of the remaining Fe+3 ions in the aqueous solution, producing vast quantities of acidic wastage at the time of extraction. Moreover, the stripping process after solvent extraction required a high concentration of acids, which can harm the environment. Moreover, these highly acidic parameters can damage the equipment by corrosion.53 Therefore, improvement in the extraction system is necessary with an alternative of green extraction methods, which can address and control the negative impact of traditionally used solvents on the environment.61
 | (4) |
A better approach to decrease the usage of organic solvents is needed, and this can be done by researching a different system of extraction that can remove these unwanted characteristics. The ionic liquid is chosen as an alternative to organic solvents. ILs fulfill the desired requirements of the solvents for lithium extraction, and researchers nowadays are interested in ionic liquids because of their tunable properties such as hydrophilicity/hydrophobicity, low-flammability, non-volatility, high selectivity, high thermal stability, and greater efficiency towards solvent extraction.61,62
Ionic liquids used in lithium extraction with their concentrations, extraction efficiency, conditions, stripping parameters, and reusability are shown in Table 1.
| Li (conc.) | Extraction (IL) | Stripping of loaded IL layer | Regeneration of ILs (cycles) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| ILs (conc.) | Conditions (T, t, pH) | IL/aqueous | Extraction% | Stripping agent | Stripping conditions | Stripping recovery% | |||
| (0.5 g L−1) | [P4444][BTMPP] (0.85 mol L−1) in 5 mL toluene | 30 min, 293 K, pH = 6.28 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | HCl (0.5 mol L−1) | 293 K | 94.07 | 3 | 58 |
| (0.291 mol L−1) | [OHEmim][NTf2] (0.383 mol L−1) in TBP (2.197 mol L−1) | 30 min, 278.15 K, pH < 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95.01 | HCl (1.0 mol L−1) | 278.15 K | 92.86 | 5 | 63 |
200 mg L−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Omim-TTA (0.1 M) in Omim-TFSI (0.1 M) | pH ≈ 4.3, 120 min, EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co (1.1 Co (1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
82.4 | HCl | — | >90 | 3 | 62 |
| 0.5 g L−1 | [N4444][DEHP] (1.06 mol L−1) in methylbenzene | 293 K, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 | HCl (0.5 mol L−1) | 293 K | >90 | Possible | 61 |
| 0.10 mol L−1 LiCl | [N1888][P507] 0.025 mol L−1 + TBP (60%) + FeCl3 | 298 K, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63.84 | HCl (1.5 mol L−1) | 298 K, 20 min | 90 | 6 | 64 |
| 0.5 g L−1 | [N4444][EHPMEH] 0.6 mol L−1 In 10 mL methylbenzene | 298 K, 2 h | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>90 | HCl (0.5 mol L−1) | 298 K | 94.2 | — | 65 |
| 2.088 g L−1 | [C4mim][PF6] (TBP/IL of 9/1) (v/v) | 10 min, pH = 5.58 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90.93 | HCl (0.1 M) | 80 °C | 96.28 | — | 66 |
| 3.85 g L−1 | (Carboxymethyl trimethyl bis(trifluoromethyl)sulfonamide) [(CH3)3 NCH2COOH] + TBP | 20 min, 298.15 K, pH = 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96.80 (after 5 stages) | H2SO4 (1 M) | 298.15 K | 99.6 | 5 | 67 |
| LiNTf2 (1 M) | [BMIm][NTf2] (0.5 M), 0.2 M crown ether, DCM. | 1 h, 293.15 K | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
26.3 | — | — | — | — | 68 |
| LiCl (0.33 M) | [C4mim][PF6] + TIBP + kerosene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8:1) v/v 8:1) v/v |
298.15 K, pH = 5, 10 min | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74.14 | HCl (1 M) | 298.15 K | 86.36 | 4 | 69 |
| LiCl (0.3 M) | [C4MIM]Cl (0.8 M) + NaOH (1.6 M) | 298 K, 20 min | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52 | NaCl (5 M) | — | 100 (6 times) | Possible | 70 |
| LiCl (1 M) 6Li | ([EMIm][NTf2]) + DB15C5 (0.2 M) + anisole | 60 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.022 (20 stages) | HCl (1 M) | 1 h | 100 (3 times) | Possible | 71 |
| 0.766 g L−1 (brine sol) | [OHEMIM][Tf2N] (0.09 mol L−1) + TOP | 30 min, pH = 7 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83.16 in 1st stage (99.09% in 5 stages) | NaCl (0.5 M), LiCl (0.1 M) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.90 | 6 | 72 |
| LiCl (250 mg L−1) | [Aliquat][DEHPA] (1 M) diluted in dodecane | pH = 5, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 | HCl (1 M) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.1 ± 2.2 | Possible | 73 |
| 1.5 g L−1 Li | 15% [OHEMIM][NTf2] + 85% Cyanex923 | pH = 10.68, temp. = 298 K, 20 min | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2 stages) | HCl (0.5 M) | 80 | 74 | ||
| 0.16 mol L−1 | [Hmim][BTA] (0.5 M) in 2-octane | pH = 8.87, 6 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | LiCl (0.25 M), HCl (1 M) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95.43 | >10 | 75 |
| 0.35 g L−1 | [Bmim]Bph4 + TBP + CH2BrCl | 10 min, temp. = 298 K | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.47 (4 stages) | NaCl (2.56 M), LiCl (0.10 M), Na2CO3 (2 M) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | >10 | 76 |
| 0.52 g L−1 Li in HCl | Cyphos IL-101 | Temp. – 60 °C, pH = 0–0.3, 10 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86.2 | HCl (2 M) | 74.2 | 10 | 77 | |
| [Li2CO3 + NaCl + H2O] Li (0.5 g L−1) | [A336]TTA (0.5 M) + TRPO | pH = 13.11, temp. = 298 K | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
86.64 | HCl (0.5 M) | 97.16 | 78 | ||
| 0.29 M | [OHEMIM][NTf2] (15% vol) in TBP | pH = 6, temp. = 303 K, 15 min | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94.2 | LiCl (0.6 M) + NaCl (1.8 M), HCl (1 M) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 | 7 | 79 |
An example of lithium extraction with the chemical equation and structure is shown in eqn (5). Ionic liquid, [OHEmim][NTf2] (1-hydroxyethyl-3-methyl imidazolium bis(trifluoromethylsulfonyl)imide) (Fig. 8) and TBP as diluents were employed to extract lithium from its aqueous brine solution containing Mg, Na, etc. Simply IL or TBP couldn't extract lithium effectively, but IL and TBP together as an organic media created a synergistic effect and extracted lithium efficiently.65
| Li+(aq) + TBP(org) + [OHEmim][NTf2](org) = Li·TBP·[NTf2](org) + [OHEmim]+(aq) | (5) |
The anion of IL (NTf2) moved closer to the hydrated lithium in the aqueous solution from its organic phase due to the coulombic force of attraction and hydrogen bonding interaction initiating the extraction.
Since the structure of lithium in the aqueous solution got deformed, it facilitated the interaction of TBP with the lithium ion in the aqueous solution. Because of this interaction of TBP with lithium, the aqueous phase of lithium changed to the organic phase, and the IL cation [OHEMim] was left in the aqueous solution with other metals as the raffinate.65
From Table 1, various similarities between extraction systems can be drawn, as the temperature used for extracting lithium was around 298 K in most of the extraction systems. The pH range is usually between 2 and 6, and the stirring time is 20–60 min. In addition to extraction, HCl was often used for the stripping process, and that too in a shallow concentration range of 0.5–1 M with satisfactory extraction results. In ionic liquids, when compared to organic solvent extraction systems, TBP was used in both approaches. Still, when TBP was used with an ionic liquid, its presence in an ionic liquid supported the extraction of lithium ions more than usual. Moreover, ionic liquids with nitrogen and phosphorus atoms attached to them gave better extraction results than ionic liquids containing only carbon due to the electronegative properties of N and P, which help in the comfortable distribution of electrons. The positive charge will mostly disperse on carbon atoms, and the positive charge dispersion increases the ionic liquid's extraction ability. Moreover, increasing or decreasing the hydrophobicity of ionic liquids can change the extraction efficiency. Hydrophobicity can be improved by increasing the alkyl chain length of the cation. However, further increasing the alkyl chain can also create steric hindrance and hamper the extraction.68
For instance, 1-butyl-3-methyl imidazolium [BMIM]+, as shown in Fig. 9, is an imidazole-based cation containing two N-atoms and two alkyl groups bonded to the N-atoms. Here, the N-atoms can increase the dispersion of positive charge on the imidazolium cation and weaken the interaction between the cation and anion, allowing for an easy extraction route for lithium. Moreover, the butyl and methyl chains in the cation increase the hydrophobic properties, which is beneficial for lithium extraction. However, if these chains are increased in length to improve hydrophobicity, it can obstruct the extraction procedure by multiplying the steric crowd and creating hindrance.68
Extraction from secondary sources can be possible using solvent extraction as a method, as it is a successful way of recovering Co, Ni, and many other metals.83 Previously, CYANEX 301, CYANEX 302, CYANEX 921 and CYANEX 923 have been extensively used as a solvent for Co extraction from its acetate and sulphate solutions.84–86 Cyanex 272 and D2HEPA are dissolved in cobalt from kerosene extract in its aqueous solution and separate from nickel.87 Since Co(II) oxidizes to Co(III) in the presence of air, it becomes inert, and its stripping becomes difficult with the organic solvent taken for solvent extraction. Moreover, the extraction efficiency could be more satisfying when using these organic solvents.88
Therefore, ionic liquids can be used as alternatives to organic solvents to extract cobalt from their aqueous solutions. Ionic liquids used in solvent extraction for Co extraction, along with their concentrations, extraction efficiency, conditions, stripping parameters, and reusability, are shown in Table 2.
| Co (conc.) | Extraction (IL) | Stripping of loaded IL layer | Regeneration of ILs (cycles) | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|
| ILs (conc.) | Conditions (T, t, pH) | IL/A | Extraction% | Stripping agent | Stripping conditions | Stripping recovery% | |||
| 75 mg L−1 | [A336][CA-12] | 298 K, 1 h, 4.3 pH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | H2SO4 (8 × 10−4 mol L−1) | 298 K | 99 | — | 89 |
| 0.43 g (2 M H2SO4) | R4N–SCN (0.36 mol L−1 in kerosene) | pH – 5.6, 20 min, 308 K | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91.6 | Water (70 °C) | 99.9 | Possible | 90 | |
| H2SO4 (2 M, 25 °C) | 99.9 | ||||||||
| HCl (3 M, 25 °C) | 99.9 | ||||||||
| 100 mg L−1 | 20 mL [P66614][SCN] (0.1 M) in toluene | 2 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio (2.6 1 mole ratio (2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
95 | EDTA (0.2 M) | (2–3) h | 100 | Possible | 91 |
| 0.05 mol L−1 | [THN][Dca] | 298 K, 12 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.3 | Na2SO4 (1 mol L−1) | — | 93 | 3 | 92 |
| 1 g L−1 (1 M H2SO4, 5 M HCl) | [P8888][Cl] | 298 K, 10 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
98.3 | H2O | 298 K, 20 min | 100 | — | 93 |
| HCl (0.1 M) | 99.4 | ||||||||
| 0.5 g L−1 H2SO4 (4 M) (NH4)2SO4 (3 M) | [C101][Cl] | 75 °C, 45 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>85 | H2O | 30 min, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99 | 3 | 94 |
| 5.12 g L−1 in HCl | Cyphos IL-101 | 10 min, 60 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90.5 | HCl (2 M) | 87.4 | 10 | 77 | |
| 100 mg L−1 in 5 M HCl | P88812R2POO (0.01–0.1)M in toluene | 3 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
99 | H2O | 99.8 | Possible | 95 | |
| 5 mg L−1 (CoCl2∙6H2O) | [P6,6,6,14][BTMPP] (5 mmol) or [P6,6,6,14][SCN] (5 mmol) | Temp. – 303 K, 6 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | H2O | 96 | |||
| 100 mg L−1 (1 mL) | [HMIM][BF4] (0.7 g) and NaCl (0.5 g) | 5 min | ∼100 | H2O or NaPF6 (15 mL of 0.02 M) | 20 °C | 90 | Possible | 97 | |
| Co(SCN)3− (25 mg L−1) and NH4SCN (0.1 M) | [BBIM]Br (0.1 M) in DCM | Temp. – 25 °C, pH – 4.5, 3 min at 1200 rpm | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.20 | NH3 (2 M) | 93.4 | 98 | ||
| 1 g L−1 Co2+, (CoCl2 + NaCl) | [P8888+][oleate−] | 2 h, 2500 rpm | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | Na2CO3 (1 M) | 98 | Possible | 99 | |
| 5 g L−1 Co2+ (CoCl2) in 7.6 M HCl | Cyphos IL 101 (14 wt%) | 50 °C, 16 h, 750 rpm | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | H2O | >99.8 (3 stages) | Possible | 100 | |
| 1.5 g kg−1 | [P44414][Cl] (40 wt%)+ NaCl (11 wt%) | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
101 | |||||
| 10 ng mL−1 | [Hmim][BF4] (25 mg) NaPF6 (0.5 mL, 80 mg mL−1), ethanol (diluent), Schiff base (0.02 M) | pH = 7, 5 min | 98–101 | 102 | |||||
| 100 ng mL−1 Co2+, in Co(NO3)2·6H2O | [Hmim][PF6] (0.8 mg μL−1, 64 mg), and [Hmim][Tf2N] (0.5 mg μL−1), ethanol (diluent), 5-Br-PADAP ligand (1.5 × 10−5 M), NaNO3 (8%) | 35 °C, 0 °C, 8 min, 2500 rpm, pH = 7.5 | ∼100 | 103 | |||||
Table 2 shows the application of different ionic liquids for solvent extraction of cobalt from its aqueous solution. These ionic liquids can be grouped into several categories according to the elements used in the cation formation. For example, ionic liquids [P8888][Cl], [P66614][SCN], and [P44414][Cl], all contain phosphorus in their cationic fractions along with long alkyl chains such as butyl, hexyl, and octyl. Therefore, these three can be categorized as a group of ionic liquids and can be compared with the other groups. Similarly, Cyphos 101 and [C101][Cl] (trihexyltetradecyl phosphonium chloride) can also be grouped in the category of ionic liquids containing phosphorus. For cobalt extraction, many ionic liquids containing phosphorus are assumed to give 95–10% extraction recovery of cobalt. All these ionic liquids work under different conditions with different temperatures, stirring times, and pH. The ratio of ionic liquids to the aqueous solution of cobalt was 1/1 in most cases and 2/1 in very few. They are now talking about the N-containing ionic liquids, which are also used very frequently for cobalt extraction, like [A336][CA-12], R4N–SCN, and [THN][Dca] with an extraction efficiency of 90–100%. The temperature range for most of the extraction systems was 298–308 K, and the pH varied from 4 to 8. Lastly, the imidazolium-based ionic liquids used for cobalt extraction were [Hmim][PF6], [Hmim][BF4], and [BBIM]Br, showing an extraction efficiency from 98% to 100%. In most extraction systems, water is used for stripping, providing 100% stripping efficiency. Most importantly, these ionic liquids can be regenerated and reused after the extraction for different cycles.
Table 3 discusses nickel extraction using ionic liquids such as Alamine 336 with Cyanex 272 and D2EHPA, imidazole-based ionic liquid [Bmim] [PF6] and amine-based ionic liquids. The conditions for nickel extractions are room temperature, a low pH range (1–6), and varying the stirring time of extraction from 5 min to 180 min to obtain >99.9% extraction efficiency. Stripping with acids (HCl and H2SO4) can regenerate the ionic liquid and separate the nickel metal from the organic solution. The regenerated solvent can be used again in further extraction cycles.
| Ni (conc.) | Solvent extraction (IL) | Stripping of loaded IL layer | Regeneration of ILs (cycles) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| ILs (conc.) | Conditions (T, t, pH) | IL/A | Extraction% | Stripping agent | Stripping conditions | Stripping recovery% | |||
| 100 mg L−1 in 3 M HCl + 2 M NaCl (Ni, Li sol) | (0.1 M) Alamine 336 and Cyanex 272/(D2EHPA) | pH 3.15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99.9 | 0.1 M H2SO4 | A/O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99.9 | 108 | |
| 5.28 g in 2 M H2SO4 | (0.36 M) R4N–Cl in kerosene | pH 6.4, 110 min, 298 K | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.2 | Scrubbing (0.1 M) HCl, 0.4 M HNO3 | O/A = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
>99.6 (5.2 g) | Possible | 90 |
| Stripping (0.02 M) H2SO4 | |||||||||
| 0.110 g Ni | D2EHPA + Aliquat 336 + [Benzet][TCM] (6.7 g of IL in 15 cm3 of toluene) | pH 2.5, 318 K, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84.5 | 0.5 M H2SO4 | 20 min, 323 K | 109 wt% | 109 | |
| 1.40 g L−1 (chloride salt) | [P8888][oleate] + water (10 wt%) | 5 min, pH 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99 | 0.1 M HCl | ∼100 | Possible | 110 | |
| 1 mM | [Bmim][PF6] in 2-amino thiophenol | 180 min, 298 K, pH 4–6 | 57 | 3% H2O2 in 0.1 HNO3 | 20 min | >98 | 111 | ||
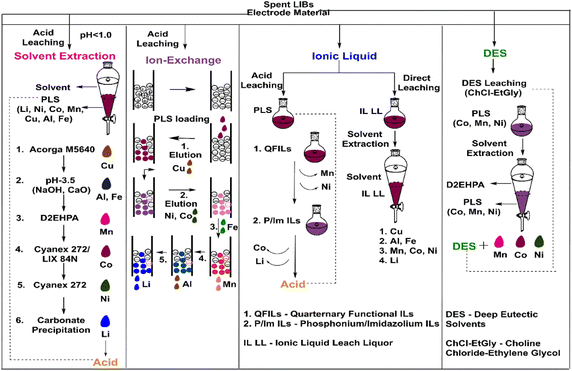
Comparative assessment among the various prevalent methods with IL-based separation
The high cost of ILs (exceeding up to $800/kg) is the main concern for their low adaptation and industrial scaling, making several industrial utilizations unjustified and unsustainable. Currently, ILs are produced in small scales, which is one of the main reasons for their high price.112,113 In this section, our effort is to provide a lay-man's comparative outlook among the various processes employed in the separation of metals during spent LIB recycling. The process considered here takes into account an inorganic acid-based leaching process of 100 kg spent NMC-611 battery scrap from its cathode material as the starting feed, unless specified otherwise. The two majorly discussed and practised processes in critical metal extraction/separation from spent LIBs are solvent extraction and ion exchange, as depicted in Fig. 10. Solvent extraction (SX) is one of the most robust methods in metal separation, and thus, it is applied on an industrial scale. The major demerit is shown in Fig. 10, which is the number of sub-unit operations desired to first separate impurities, followed by Mn, Co, Ni and Li; however, there is an important merit of highly pure salts as well as high regeneration efficiency. Another widely used method is ion-exchange wherein functionalised metal-specific resin depending upon their efficacy adsorbs Cu, Ni, Co, Fe, Mn, Al and Li. The ion-exchange process still governs by the use of multiple functionalised resins in columns for adsorption followed by their preferential elution to recover the metal ion. The other two methods, namely ILs and DESs, discussed are relatively new, and thus, their implication or assessment among the existing ones is deeply desired. The merit of ILs is the use of QFLs and PIL or IMILs, which makes the separation among Mn–NI and Co–Li possible. Another inherent method is the use of ILs in the leaching stage, which substantially reduces the acid consumption. Although it has been widely covered, the need for SX will be invariably still needed to separate these metals. DESs also function similar to ILs acting as leaching and separation agents. It has been effective for all battery metals and has shown non-reactivity towards Li. DESs containing only non-ionic species can be prepared easily, which exhibit more benefits than the polar or hydrophilic DESs as they are inexpensive, non-toxic in nature, and can be regenerated by evaporation only. The extraction of alkali or transition metals or polycyclic aromatic hydrocarbons from water can be done using non-ionic DESs. Metal ions or metal salts can be removed from water using non-ionic DESs containing decanoic acid and lidocaine in a molar ratio of 2/1. Positively charged metal ions probably exchanged with the moderately charged lidocaine ion executing an ion exchange process removing all the transition metals. Moreover, the non-ionic DES can be regenerated using Na2C2O4 and reused by increasing the decanoic-to-lidocaine ratio.114Since ILs and DESs are more expensive than organic solvents and have complexities in synthesis, their reusability and recyclability can only promise their economic feasibility for large-scale applications. The cost-competitiveness of ILs can be achieved by the regeneration of ILs and their reuse for different applications without creating any additional changes in the functionality of ILs. ILs are likely to be judged. They have the potential to lower the environmental impact of solvents in metal extraction when compared to organic solvents and DES reagents.112
Conclusions
In this review, the importance and usefulness of ionic liquids have been discussed, as well as their usage in the extraction of Li, Co, and Ni from their different sources. The ionic liquid is a solvent that is liquid at room temperature, as its volatility is very low or negligible. This is one of the main reasons for considering ionic liquids more than conventional solvents for metal extraction. Ionic liquids can be synthesized according to one's own choice and can be tuned by changing the anions or adding substituents to the cation molecule. For metal extraction, the choice of solvent and the extraction parameters depend on the behavior of the metal ion in the aqueous solution, whether it is acidic or alkaline. The structure of an ionic liquid determines its properties, such as hydrophobicity/hydrophilicity and weak interactions between cations and anions. According to the previously published works, extraction parameters are mild when compared to the traditionally used solvents. For instance, temperature and stirring time used for extraction vary between 293 and 298 K and 20 and 60 min with a pH range of 2–6. In addition to parameters, the extraction efficiency shown by the ionic liquid extractants was highly satisfying. For stripping, HCl was frequently used to strip >90% of lithium ions from the organic phase. The critical part is the regeneration of ionic liquids after the extraction procedure. All the ionic liquid extractants can be recycled and reused for further extraction cycles for Li, Co, and Ni.Future directions of research
Solvents such as ionic liquids contain only ions rather than any other neutral molecules, and have a low vapor pressure, and are almost non-volatile. This absence of volatility shows that ILs are safer to use and are environment-friendlier than any other traditionally used organic solvents, which are usually volatile organic compounds.115 However, as we compare other aspects of organic solvents and ionic liquids, viscosity is one of the factors that increases in ionic liquids. The viscosity of a solvent can disturb the transfer of ions and decrease the rate of chemical reactions. Similarly, high production costs are also a factor that can be seen as a disadvantage for ILs.116,117 ILs can be used as a solvent in metal extraction, not only for separation purposes, but also for leaching. They can also be used in the electro-winning and electro-refining of metals acting as electrolytes.117Abbreviations
| IL | Ionic liquid |
| LIB | Lithium-ion battery |
| D2EHPA | Di(2-ethylhexyl)phosphoric acid |
| TBP | Tributyl phosphate |
| Cyphos 101/[C101][Cl] | Trihexyl(tetradecyl)phosphonium chloride |
| [P8888][Cl] | Tetraoctylphosphonium chloride |
| [P66614][SCN] | trihexyl(tetradecyl)phosphonium thiocyanate |
| [P44414][Cl] | Tributyl(tetradecyl)phosphonium chloride |
| [A336][CA-12] | Tricaprylmethylammonium sec-octylphenoxy acetic acid |
| R4N–SCN | Tetraalkylammonium thiocyanate |
| [THN][Dca] | Tetrahexylammonium dicyanamide |
| [Hmim][PF6] | 1-Hexyl-3-methylimidazolium hexafluorophosphate |
| [Hmim][BF4] | 1-Hexyl-3-methylimidazolium tetrafluoroborate |
| [Bmim]Br | 1-Butyl-3-methylimidazoloium bromide |
| [P8888][oleate] | Tetraoctylphosphonium oleate |
| LCA | Life cycle assessment |
| LCO | Lithium cobalt oxide |
| NMC | Lithium nickel manganese cobalt Oxide |
| LFP | Lithium iron phosphate |
| LMO | Lithium manganese oxide |
| [Hmim][NTf2] | 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| CoCl4 | Cobalt tetrachloride |
| [OHEmim][NTf2] | 1-Hydroxyethyl-3-methyl imidazolium bis(trifluoromethylsulfonyl)imide |
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Author contributions
Pratima: supervision, software, project administration, funding acquisition, and formal analysis. Nikita: writing – original draft and investigation. Abhilash: writing – review & editing, validation, supervision, resources, methodology, data curation, and conceptualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding support from the Waste Management and Technology Board of the Department of Science and Technology, Government of India, and CSIR Mission Project for carrying out this study under DST/TDT/WMT/Power Ind Waste/2021/01(G) and MMP-085201, respectively.References
- G. Kaur, H. Kumar and M. Singla, J. Mol. Liq., 2022, 351, 118556 CrossRef CAS
.
- S. Mahajan, R. Sharma and R. K. Mahajan, Langmuir, 2012, 28, 17238–17246 CrossRef CAS PubMed
.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 Search PubMed
.
- S. Zhang, Y. Ding, B. Liu and C. C. Chang, Waste Manage., 2017, 65, 113–127 CrossRef PubMed
.
- M. Sethurajan and S. Gaydardzhiev, Resour., Conserv. Recycl., 2021, 165, 105225 CrossRef CAS
.
- S. Y. Jiang, W. Wang and H. M. Su, J. Earth Sci., 2023, 34, 1295–1298 CrossRef CAS
.
- S. E. Can Sener, V. M. Thomas, D. E. Hogan, R. M. Maier, M. Carbajales-Dale, M. D. Barton and G. L. Amy, ACS Sustainable Chem. Eng., 2021, 9, 11616–11634 CrossRef CAS
.
- X. Sun, Front. Energy Res., 2022, 10, 957884 CrossRef
.
- G. Inman, I. C. Nlebedim and D. Prodius, Energies, 2022, 15, 628 CrossRef CAS
.
- A. Crawford, J. L. Seefeldt, R. Kent, M. Helbert, G. P. Guzman, A. González, Z. Chen and A. Abbott, One Earth, 2021, 4, 323–326 CrossRef
.
- W. Li, S. Lee and A. Manthiram, Adv. Mater., 2020, 32, 2002718 CrossRef CAS PubMed
.
- P. Meshram, R. V. Jaiswal, C. Baiju and R. L. Gardas, J. Mol. Liq., 2024, 400, 124594 CrossRef CAS
.
- N. Peeters, K. Binnemans and S. Riaño, Green Chem., 2020, 22, 4210–4221 RSC
.
- U. Kesieme, A. Chrysanthou, M. Catulli and C. Y. Cheng, J. Chem. Technol. Biotechnol., 2018, 93, 3374–3385 CrossRef CAS
.
- R. Ratti, Adv. Chem., 2014, 1, 729842 Search PubMed
.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC
.
- G. Boeck, ChemTexts, 2019, 5, 6 CrossRef
.
- A. J. McIntosh, J. Griffith and J. Gräsvik, Appl., Purif., Recovery Ionic Liq., 2016, 59–99 CAS
.
- R. A. Osteryoung, Molten Salt Chem., 1987, 329–364 CAS
.
- R. Holze, Molten Salts and Ionic Liquids, 2016, p. 738 Search PubMed
.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 1, 70–71 RSC
.
- A. P. Abbott, G. Capper, D. L. Davies and R. Rasheed, Inorg. Chem., 2004, 43, 3447–3452 CrossRef CAS PubMed
.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 1120388 CrossRef
.
- A. Ghanem, R. D. Alharthy, S. M. Desouky and R. A. El-Nagar, Materials, 2022, 15, 1600 CrossRef CAS PubMed
.
- T. J. Schubert, Commercial Applications of Ionic Liquids, 2020, 191–208 Search PubMed
.
- A. Ray and B. Saruhan, Materials, 2021, 14, 2942 CrossRef CAS PubMed
.
- Y. A. El-Nadi, Sep. Purif. Rev., 2017, 46, 195–215 CrossRef CAS
.
- B. Pospiech and W. Kujawski, Rev. Chem. Eng., 2015, 31, 179–191 CAS
.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 112038 CrossRef CAS
.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS
.
- A. A. Shamsuria and D. K. Abdullah, Makara J. Sci., 2011, 14, 19 Search PubMed
.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC
.
- A. J. Greer, J. Jacquemin and C. Hardacre, Molecules, 2020, 25, 5207 CrossRef CAS
.
- D. Depuydt, A. Van den Bossche, W. Dehaen and K. Binnemans, Chem. Commun., 2017, 53, 5271–5274 RSC
.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 16, 1765–1766 RSC
.
- A. I. Siriwardana, I. R. Crossley, A. A. Torriero, I. M. Burgar, N. F. Dunlop, A. M. Bond, G. B. Deacon and D. R. MacFarlane, J. Org. Chem., 2008, 73, 4676–4679 CrossRef CAS
.
- A. Chagnes and B. Pospiech, J. Chem. Technol. Biotechnol., 2013, 88, 1191–1199 CrossRef CAS
.
- W. S. Chen and H. J. Ho, Metals, 2018, 8, 321 CrossRef
.
- R. Chen, J. Ionic Liq., 2023, 3, 100070 CrossRef
.
- E. Quijada-Maldonado, F. Olea, R. Sepúlveda, J. Castillo, R. Cabezas, G. Merlet and J. Romero, Sep. Purif. Technol., 2020, 251, 117289 CrossRef CAS
.
- A. Boyden, V. K. Soo and M. Doolan, Procedia CIRP, 2016, 48, 188–193 CrossRef
.
- J. Xu, H. R. Thomas, R. W. Francis, K. R. Lum, J. Wang and B. Liang, J. Power Sources, 2008, 177, 512–527 CrossRef CAS
.
- T. Georgi-Maschler, B. Friedrich, R. Weyhe, H. Heegn and M. Rutz, J. Power Sources, 2012, 207, 173–182 CrossRef CAS
.
- P. G. Schiavi, P. Altimari, E. Sturabotti, A. Giacomo Marrani, G. Simonetti and F. Pagnanelli, ChemSusChem, 2022, 15, e202200966 CrossRef
.
- I. M. Pateli, D. Thompson, S. S. Alabdullah, A. P. Abbott, G. R. Jenkin and J. M. Hartley, Green Chem., 2020, 22, 5476–5486 RSC
.
- J. Richter, T. Pietsch and M. Ruck, ChemSusChem, 2023, 16, e202300090 CrossRef CAS
.
- K. Larsson and K. Binnemans, Green Chem., 2014, 16, 4595–4603 RSC
.
- G. Inman, I. C. Nlebedim and D. Prodius, Energies, 2022, 15, 628 CrossRef CAS
.
- Y. Hu, M. Yang, Q. Dong, X. Zou, J. Yu, S. Guo and F. Yan, Energy Environ. Sci., 2024, 17, 4238–4247 RSC
.
- J. A. Whitehead, G. A. Lawrance and A. McCluskey, Aust. J. Chem., 2004, 57, 151–155 CrossRef CAS
.
- J. Narbutt, Liq.-Phase Extr., 2020, 121–155 CAS
.
- B. Clare, A. Sirwardana and D. R. MacFarlane, Top. Curr. Chem., 2010, 290, 1–40 Search PubMed
.
- Y. Liu, B. Ma, Y. Lü and C. Wang, Chen, A review of lithium extraction from natural resources, Int. J. Miner., Metall. Mater., 2023, 30, 209–224 CrossRef CAS
.
- Z. Zhang, Y. Jia, B. Liu, H. Sun, Y. Jing, Q. Zhang, F. Shao and Y. Yao, J. Mol. Liq., 2021, 324, 114709 CrossRef CAS
.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Hydrometallurgy, 2014, 150, 192–208 CrossRef CAS
.
- L. Talens Peiró, G. Villalba Méndez and R. U. Ayres, JOM, 2013, 65, 986–996 CrossRef
.
- P. W. Harben and G. H. Edwards, JOM, 1997, 49, 21 CrossRef
.
- C. Shi, Y. Jing, J. Xiao, X. Wang and Y. Jia, Hydrometallurgy, 2017, 169, 314–320 CrossRef CAS
.
- Z. Zhou, W. Qin, Y. Liu and W. Fei, J. Chem. Eng. Data, 2012, 57, 82–86 CrossRef CAS
.
- C. Cai, T. Hanada, A. T. Fajar and M. Goto, Desalination, 2021, 509, 115073 CrossRef CAS
.
- C. Shi, Y. Jing, J. Xiao, X. Wang, Y. Yao and Y. Jia, Sep. Purif. Technol., 2017, 172, 473–479 CrossRef CAS
.
- R. Morina, D. Merli, P. Mustarelli and C. Ferrara, ChemElectroChem, 2023, 10, e202201059 CrossRef CAS
.
- R. Bai, J. Wang, L. Cui, S. Yang, W. Qian, P. Cui and Y. Zhang, Chin. J. Chem., 2020, 38, 1743–1751 CrossRef CAS
.
- R. Bai, J. Wang, D. Wang, Y. Zhang and J. Cui, Sep. Purif. Technol., 2021, 274, 119051 CrossRef CAS
.
- X. Zhao, H. Wu, M. Duan, X. Hao, Q. Yang, Q. Zhang and X. Huang, Fluid Phase Equilib., 2018, 459, 129–137 CrossRef CAS
.
- C. Shi, D. Duan, Y. Jia and Y. Jing, J. Mol. Liq., 2014, 200, 191–195 CrossRef CAS
.
- H. Zheng, T. Dong, Y. Sha, D. Jiang, H. Zhang and S. Zhang, ACS Sustainable Chem. Eng., 2021, 9, 7022–7029 CrossRef CAS
.
- W. Zhu, Y. Jia, Q. Zhang, J. Sun, Y. Jing and J. Li, J. Mol. Liq., 2019, 285, 75–83 CrossRef CAS
.
- D. Gao, Y. Guo, X. Yu, S. Wang and T. Deng, J. Chem. Eng. Jpn., 2018, 49, 104–110 CrossRef
.
- X. Jingjing, L. Zaijun, G. Zhiguo, W. Guangli and L. Junkang, J. Radioanal. Nucl. Chem., 2013, 295, 2103–2110 CrossRef
.
- H. Sun, Y. Jia, B. Liu, Y. Jing, Q. Zhang, F. Shao and Y. Yao, Fusion Eng. Des., 2019, 149, 111338 CrossRef CAS
.
- G. Yu, X. Zhang, T. Hubach, B. Chen and C. Held, Chem. Eng. Sci., 2024, 286, 119682 CrossRef CAS
.
- G. Zante, D. Trébouet and M. Boltoeva, Appl. Geochem., 2020, 123, 104783 CrossRef CAS
.
- S. Yang, G. Liu, J. Wang, L. Cui and Y. Chen, Fluid Phase Equilib., 2019, 493, 129–136 CrossRef CAS
.
- R. Li, Y. Wang, L. Chen, W. Duan, Z. Ren and Z. Zhou, Desalination, 2024, 574, 117274 CrossRef CAS
.
- R. Li, W. Wang, Y. Wang, X. Wei, Z. Cai and Z. Zhou, Sep. Purif. Technol., 2021, 277, 119471 CrossRef CAS
.
- L. Xu, C. Chen and M. L. Fu, Hydrometallurgy, 2020, 197, 105439 CrossRef CAS
.
- J. Wang, S. Yang, X. Zhang, Y. Wang, D. Wang, W. Li, M. A. Ashraf, A. H. A. Park and X. Li, Energy Fuels, 2020, 34, 11581–11589 CrossRef CAS
.
- W. Zhou, S. Xu and Z. Li, J. Sustain. Metall., 2021, 7, 256–265 CrossRef
.
- Y. Zhou, X. Wei, L. Huang and H. Wang, Environ. Sci. Pollut. Res., 2023, 30, 16930–16946 CrossRef PubMed
.
- S. H. Farjana, N. Huda and M. P. Mahmud, J. Sustain. Min., 2019, 18, 150–161 CrossRef
.
- S. Banerjee and S. Basu, J. Anal. Environ. Cult. Herit. Chem., 2004, 94, 581–590 CAS
.
- G. Alvial-Hein, H. Mahandra and A. Ghahreman, J. Cleaner Prod., 2021, 297, 126592 CrossRef CAS
.
- N. A. Grigorieva and I. Y. Fleitlikh, Solvent Extr. Ion Exch., 2015, 33, 278–294 CrossRef CAS
.
- P. E. Tsakiridis and S. L. Agatzini, Hydrometallurgy, 2004, 72, 269–278 CrossRef CAS
.
- B. Menoyo and M. P. Elizalde, Solvent Extr. Ion Exch., 1997, 15, 97–113 CrossRef CAS
.
- G. Granata, E. Moscardini, F. Pagnanelli, F. Trabucco and L. Toro, J. Power Sources, 2012, 206, 393–401 CrossRef CAS
.
- V. N. H. Nguyen, T. H. Nguyen and M. S. Lee, Metals, 2020, 10, 1105 CrossRef CAS
.
- X. Sun, Y. Ji, L. Zhang, J. Chen and D. Li, J. Hazard. Mater., 2010, 182, 447–452 CrossRef CAS PubMed
.
- A. A. Nayl, J. Hazard. Mater., 2010, 173, 223–230 CrossRef CAS PubMed
.
- S. A. Satyawirawan, R. W. Cattrall, S. D. Kolev and M. I. G. Almeida, J. Mol. Liq., 2023, 380, 121764 CrossRef CAS
.
- S. Boudesocque, L. Viau and L. Dupont, J. Environ. Chem. Eng., 2020, 8, 104319 CrossRef CAS
.
- D. E. Chaverra, O. J. Restrepo-Baena and M. C. Ruiz, ACS Omega, 2020, 5, 5643–5650 CrossRef CAS PubMed
.
- B. Onghena, S. Valgaeren, T. Vander Hoogerstraete and K. Binnemans, RSC Adv., 2017, 7, 35992–35999 RSC
.
- M. L. Firmansyah, A. T. Fajar, R. R. Mukti, T. Ilmi, G. T. Kadja and M. Goto, Solvent Extr. Res. Dev., Jpn., 2021, 28, 79–93 CrossRef CAS
.
- A. Łukomska, A. Wiśniewska, Z. Dąbrowski and U. Domańska, J. Mol. Liq., 2020, 307, 112955 CrossRef
.
- J. Flieger, M. Tatarczak-Michalewska, E. Blicharska, A. Madejska, W. Flieger and A. Adamczuk, Sep. Purif. Technol., 2019, 209, 984–989 CrossRef CAS
.
- V. Eyupoglu, E. Polat, A. Kunduracioglu and H. I. Turgut, J. Dispersion
Sci. Technol., 2015, 36, 1704–1720 CrossRef CAS
.
- E. A. Othman, A. Ham, H. Miedema and S. R. A. Kersten, J. Chem. Eng. Process Technol., 2019, 10(2), 397 Search PubMed
.
- S. Wellens, R. Goovaerts, C. Möller, J. Luyten, B. Thijs and K. Binnemans, Green Chem., 2013, 15, 3160–3164 RSC
.
- B. Onghena, T. Opsomer and K. Binnemans, Chem. Commun., 2015, 51, 15932–15935 RSC
.
- M. Hosseini, N. Dalali and S. Moghaddasifar, J. Anal. Chem., 2014, 69, 1141–1146 CrossRef CAS
.
- M. Mirzaei and N. Amirtaimoury, J. Anal. Chem., 2014, 69, 503–508 CrossRef CAS
.
- B. I. Whittington and D. Muir, Miner. Process. Extr. Metall. Rev., 2000, 21, 527–599 CrossRef CAS
.
- B. R. Reddy and K. H. Park, Sep. Sci. Technol., 2007, 42, 2067–2080 CrossRef CAS
.
- D. S. Flett, J. Organomet. Chem., 2005, 690, 2426–2438 CrossRef CAS
.
- S. Chauhan and T. Patel, Int. J. Eng. Res. Technol., 2014, 3, 1321–1326 Search PubMed
.
- V. N. H. Nguyen and M. S. Lee, Physicochem. Probl. Miner. Process., 2021, 57, 1–17 CrossRef CAS
.
- A. Łukomska, A. Wiśniewska, Z. Dąbrowski, D. Kolasa, S. Luchcińska and U. Domańska, J. Mol. Liq., 2021, 343, 117694 CrossRef
.
- E. A. Othman, A. G. van der Ham, H. Miedema and S. R. Kersten, Sep. Purif. Technol., 2020, 252, 117435 CrossRef CAS
.
- R. Lertlapwasin, N. Bhawawet, A. Imyim and S. Fuangswasdi, Sep. Purif. Technol., 2010, 72, 70–76 CrossRef CAS
.
- A. H. Tullo, Chem. Eng. News, 2020, 98, 5 Search PubMed
.
- G. Choudhary, J. Dhariwal, M. Saha, S. Trivedi, M. K. Banjare, R. Kanaoujiya and K. Behera, Environ. Sci. Pollut. Res., 2024, 31, 10296–10316 CrossRef CAS PubMed
.
- D. J. G. P. van Osch, D. Parmentier, C. H. J. T. Dietz, A. van den Bruinhorst, R. Tuinier and M. C. Kroon, Chem. Commun., 2016, 52, 11987–11990 RSC
.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2023, 9, 423–438 CrossRef
.
- M. R. Asrami, N. N. Tran, K. D. P. Nigam and V. Hessel, Sep. Purif. Technol., 2021, 262, 118289 CrossRef CAS
.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2017, 3, 570–600 CrossRef
.
| This journal is © The Royal Society of Chemistry 2025 |