Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in isoxazole chemistry and their role in drug discovery
Glanish Jude Martis
and
Santosh L. Gaonkar
 *
*
Department of Chemistry, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, 576104, Karnataka, India. E-mail: sl.gaonkar@manipal.edu
First published on 17th March 2025
Abstract
Isoxazoles are a class of five-membered heterocyclic compounds that have gained significant attention in medicinal chemistry due to their diverse biological activities and therapeutic potential. Recent advances in isoxazole chemistry have led to the development of novel synthetic strategies, enabling the creation of a wide array of isoxazole derivatives with enhanced bioactivity and selectivity. This review explores the latest progress in isoxazole synthesis, highlighting key methodologies such as transition metal-catalyzed cycloadditions, green chemistry approaches, and regioselective functionalization techniques. These advances have not only improved the efficiency of isoxazole synthesis but have also facilitated the design of more complex and bioactive derivatives. In addition to their synthetic advances, isoxazoles have demonstrated a broad spectrum of biological activities, including antimicrobial, anticancer, anti-inflammatory, and neuroprotective effects, making them attractive candidates in drug discovery. This review discusses the structural modifications that enhance their pharmacological properties and their potential for developing therapies for diseases such as cancer, neurodegenerative disorders, and infections. Moreover, we examine the emerging trends in isoxazole-based drug discovery, such as the development of multi-targeted therapies and personalized medicine approaches. The evolving role of isoxazoles in drug discovery underscores their continued importance in modern pharmaceutical research and their potential to address unmet medical needs.
1 Introduction
Isoxazole is a five-membered heterocycle with one nitrogen and oxygen connected adjacent to each other. Analogous to this, there is another structure that is partially saturated, isoxazoline. Several biologically and pharmacologically active compounds have these moieties, which make them the best candidates for medicinal diversity.1–4 In recent years, several strategies and methods have been developed to synthesize isoxazole/isoxazoline derivatives. The formation of various fused heterocycles from isoxazoles is noteworthy and substantial.5 The reason for this approach is that these derivatives possess various biological properties such as antioxidant,6,7 antibacterial,8,9 antifungal,10,11 anticancer,12,13 insecticidal,14 anti-inflammatory,15,16 antidiabetic,17,18 and analgesic19 properties. These derivatives are also effective against Alzheimer's disease.20,21 In particular, isoxazole has gained much attention and importance because of its electron-rich aromatic structure. Moreover, the weak nitrogen and oxygen bond is attributed to ring cleavage reactions.22,23 Currently, isoxazole is present not only in pharmaceuticals, but also in natural products and agrochemicals.24 Direct extraction of chemical constituents from plant sources results in low pharmacological effects. Thus, it is necessary for inducing structural modifications to improve their biological activity, pharmacological efficiency and drug selectivity. By doing so, it is also possible to have different activities from the parent source.25 Isoxazoles are found in various pharmaceutical drugs such as danazol,26 muscimol,27,28 zonisamide,29 isoxaben,30 isoxaflutole,31 sulfisoxazole,32,33 valdecoxib,34,35 fluxametamide,36,37 risperidone,38,39 leflunomide,40 pleconaril,41,42 broxaterol,43 isocarboxazid,44 sulfamethoxazole,45 ibotenic acid,46 and parecoxib.47 The substituents present on the isoxazole moiety play a vital role in complex formation, especially, when functional groups are present.48,49 Thus, the structure of isoxazoles has undergone several modifications and alterations to increase their biological activity.50 However, by including isoxazole in medicinal targets, there can be improvement in pharmacokinetic profiles, increased efficacy and decrease in toxicity.51,52 However, the scope of functionalized isoxazoles is wide and has delivered promising results.53 (Fig. 1).2 Synthetic strategies for isoxazoles and their derivatives
2.1 Cycloaddition reactions
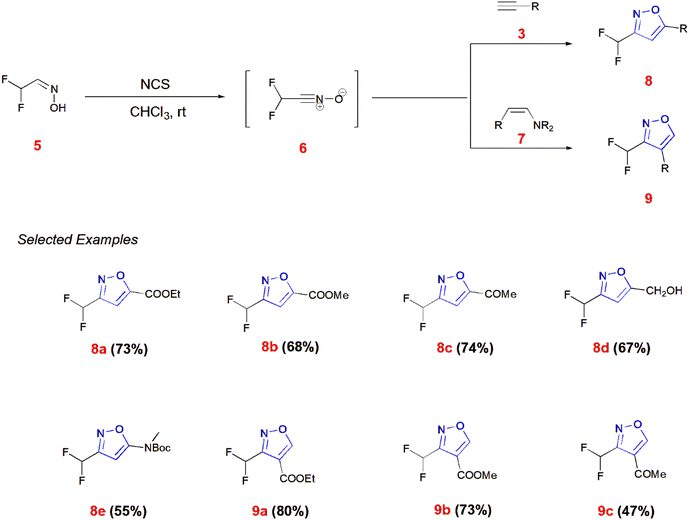
Mykhailiuk and co-workers generated difluoromethyl isoxazoles 8 and 9 from difluoro oxime 5 reacting with N-chloro-succinimide (NCS) in the presence of chloroform as solvent to give the dipole difluoro nitrile oxide 6. This dipole 6 reacts with terminal alkynes 3 and enamines 7 to give 3,5-disubstituted 8 and 3,4-disubstituted isoxazoles 9, respectively.55 (Scheme 2).
Wu et al., synthesized 3-trifluoromethyl-4-iodoisoxazoles 11 from trifluoroacetohydroximoyl chloride 10, terminal alkynes 3 and N-iodosuccinimide in a one-pot reaction containing sodium bicarbonate as the base and dichloromethane as the solvent at room temperature. This reaction afforded up to 81% yield.56 (Scheme 3).
Shibata et al., reported the synthesis of 4-difluoromethyl isoxazoles 14 from difluoromethyl alkynes 12 and imidoyl chloride 13 using triethylamine as the base with dichloromethane. Difluoromethyl alkynes 12 were obtained from terminal alkynes 3 via reaction with fluoroform (source of difluorocarbene) in the presence of potassium tert-butoxide and n-decane heated at 80–100 °C for 3 h.57 (Scheme 4).
Gopi and co-workers illustrated an orthogonal cycloaddition reaction leading to the formation of doubly conjugated peptide 17 bearing isoxazole and triazole moieties. First, nitro-alkane tethered peptide 15 was treated with phenyl isocyanate and N-Cbz-propargylamine 16 in THF at room temperature to yield 81% of the peptide containing isoxazole 17. This isoxazole scaffold 17 was made to undergo alkyne–azide cycloaddition with phenylacetylene 18 in the presence of sodium ascorbate, CuSO4·5H2O in equimolar THF/water to yield 82% of triazole–isoxazole peptide 19.58 (Scheme 5).
Nitrile oxides obtained from imidoyl chloride 13 undergo 1,3-dipolar cycloaddition of mono- or di-fluorinated propargyl thioethers 21 catalyzed by a copper sulfate/sodium ascorbate line to give isoxazoles 22. First, propargyl thioethers 20 are electrochemically reacted with HF salt to give partially fluorinated terminal alkynes 21.59 (Scheme 6).
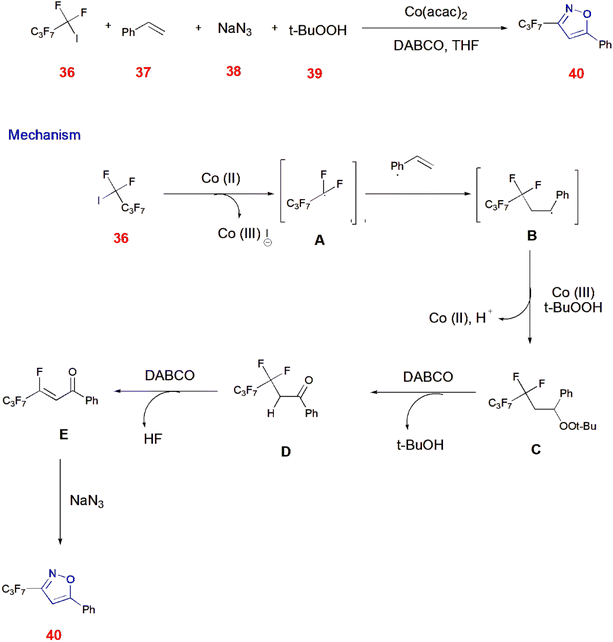
Yang et al., demonstrated the generation of nitrile oxide from 2-ethylazaarene 23, KNO3 and K2SO8 by selective oxidation. Then it underwent copper-catalyzed 1,3-dipolar cycloaddition reaction with terminal alkynes 3 to give quinoline–isoxazole derivatives 24. The plausible mechanism shows the role of KNO3 and K2SO8 in giving products along with Cu catalyst.60 (Scheme 7).
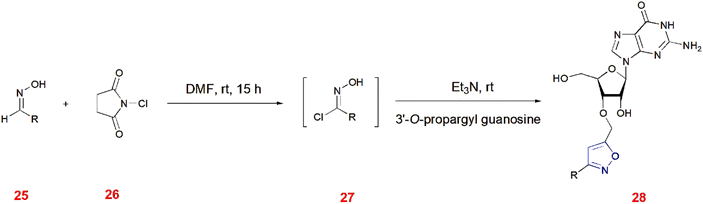
Kore and co-workers illustrated the 1,3-dipolar cycloaddition of 3′-O-propargyl guanosine with chloroximes 27 to give isoxazoles 28 in the presence of triethylamine at room temperature. Prior to this, different aldoximes 25 were chlorinated with N-chlorosuccinimide 26 in DMF at room temperature to generate chloroximes 27. The different derivatives and their yields are given in Table 1.61 (Scheme 8).
| R | Yield (%) |
|---|---|
| Phenyl | 84 |
| 4-Methoxy-phenyl | 74 |
| 9-Anthranyl | 72 |
| 3-Pyridyl | 77 |
| 3-Indolyl | 79 |
| 2-Chlorophenyl | 70 |
| 4-Nitrophenyl | 77 |
| 4-Bromo-2-thiophenyl | 70 |
Isoxazoles were generated from potassium poly(heptazine imide) (K-PHI) after artificial photosynthesis to O2 by maintaining a pressure of 1 bar at 461 nm. Next, the aldoximes 25 were quenched with 1O2 to form nitrile oxides, which react with alkyl nitriles 31 in a [3 + 2] cycloaddition fashion to give isoxazoles 32.63 (Scheme 10).
Zhao et al., proposed another method involving copper catalyzed [3 + 2] cycloaddition of phenylacetylene 18 with nitrile oxides derived from nitroso radical and copper carbene. A three component reaction of 18 with tert-butyl nitrite 33 and ethyl diazoacetate 34 in the presence of Cu(OAc)2·H2O as catalyst and 1,4-diazabicyclo[2.2.2]octane (DABCO) as base in toluene at 130 °C gave 3,5-disubstituted isoxazole 35.64 (Scheme 11).
Tang et al., annulated sulfoxonium ylides 41 with tert-butyl nitrite 42 catalyzed by Cu(TFA)2 with sodium acetate as the base and dioxane as the solvent heated at 80 °C for 12 h to give isoxazoles 43. This was one of the novel preparations of isoxazole cores involving [2 + 1 + 1 + 1] cycloaddition.66 (Scheme 13).
2.2 Condensation reactions
Langer and co-workers synthesized 5-trifluoromethyl isoxazoles 48 from hydrazone dianions 45, which were prepared from the reaction of n-BuLi with oximes 44. These dianions were then treated with trifluoroacetate 46 to give 5-trifluoromethyl isoxazoles 48 via 47 through reflux.67 (Scheme 14).Several 3-methylthio-5aryl-isoxazoles 50 were synthesized from β-oxodithioesters 49 reacting with hydroxylamine hydrochloride via sodium acetate and acetic acid under acidic conditions at 90 °C for 2–10 h. One key point here is that the use of acetic acid is necessary for the formation of the isoxazole ring with possible mechanistic approach.68 (Scheme 15).
Reddy et al., proposed a synthetic strategy for 3,5-disubstituted isoxazoles 32. In this method, ynones 51 react with trimethylsilylazide 52 through syn-Michael addition via trichloroethylene in open air at room temperature to yield 3,5-disubstituted isoxazoles 32.69 (Scheme 16).
Similarly, ynones react with azide ion to form TMS-ynamides 53 and then react to give 5-aminoisoxazoles 54 using potassium carbonate in aqueous media at room temperature followed by the addition of sodium azide 38 in the presence of ammonium chloride. In this method, the TMS group eliminated first under basic conditions, followed by the formation of corresponding 5-aminoisoxazoles 54 from syn-Michael adducts.70 (Scheme 17).
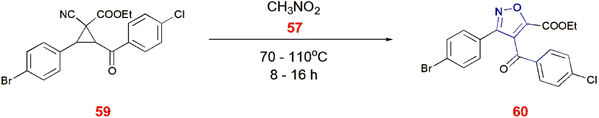
Bondarenko and co-workers reported a method of producing 5-bromoisoxazoles 58. Here, 2 aryl-1,1-dibromocyclopropanes 55 undergo nitrosation with nitrosyl chloride 56 in the presence of nitromethane 57 to give 5-bromoisoxazoles 58 at room temperature.71 (Scheme 18) Similarly, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)-facilitated ring opening of the aryl-cyclopropane 59 generated isoxazole-5-carboxylate 60 in the presence of nitromethane 57 as a driving force for ring cleavage maintained at 70–110 °C for 8–16 h. DBU resulted 90% of the yield with DMF as the solvent whereas acetonitrile and THF produced no yield.72 (Scheme 19).
2.3 Microwave-induced synthesis
Kulkarni developed a solvent free method for synthesizing 3,4-disubstituted isoxazole-5(4H)-ones 63 via microwave-induced organic synthesis. In this method, substituted aldehydes 61 were treated with hydroxylamine hydrochloride and ethyl acetoacetate 62 in the presence of catalysts such as KBr, KCl, NaOAc, MgCl2 etc and irradiated at 200 W to obtain 3,4-disubstituted isoxazole-5(4H)-ones 63.73 (Scheme 20).Sebbar et al., illustrated microwave synthesis for 3,5-disubstituted isoxazole 66 from alkyne of benzimidazole-2-one 64 and hydroxy-4-methoxy-benzene-carboximidoyl chloride 65 in the presence of triethylamine, DMF and catalyst Cu.74 (Scheme 21).
Qiang Gu and others gave an efficient method for synthesizing 3-substituted bis isoxazole ether 69 from chloro derived pyridyl oxime 68 and 3-substituted phenyl-5-((prop-2-yn-1-yloxy)methyl) isoxazoles 67 in the presence of sodium bicarbonate as a base and acid-binding agent in THF and aqueous media followed by microwave irradiation of the reaction mixture.75 (Scheme 22).
Microwave synthesis was also found to be helpful in isomerization reactions. Furfuryl ketone 70 was reacted with hydroxylamine hydrochloride to form furfuryl oxime 71 which was then treated with m-chloroperbenzoic acid (m-CPBA) at 0 °C for 1 h. The addition of trifluoroacetic acid at 0 °C and then bringing to room temperature gave 4-(3-phenylisoxazol-5-yl)but-3-en-2-one 72 as a mixture of E and Z isomers at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. The mixture 72 was made to undergo iodine-mediated isomerization at 140 °C in the presence of toluene in microwave synthesizer to obtain the E form of isomer 73.76 (Scheme 23).
30. The mixture 72 was made to undergo iodine-mediated isomerization at 140 °C in the presence of toluene in microwave synthesizer to obtain the E form of isomer 73.76 (Scheme 23).
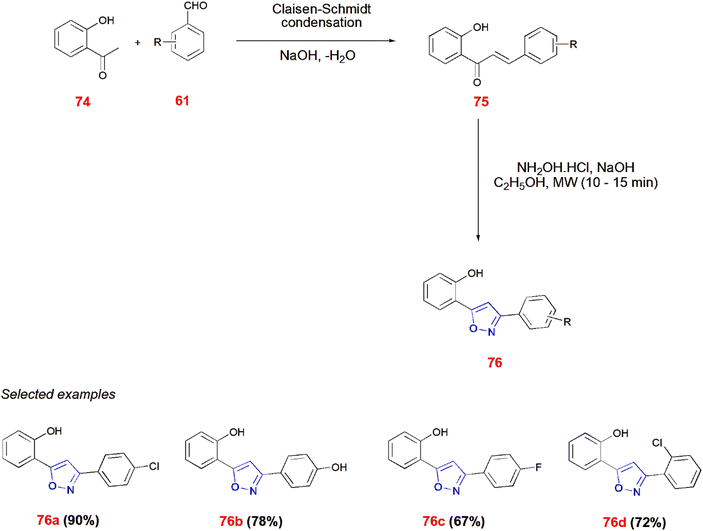
O-Hydroxyacetophenone 74 undergoes Claisen–Schmidt condensation with substituted aldehydes 61 in the presence of NaOH to give chalcones 75. When irradiated in Microwave with hydroxylamine hydrochloride in ethanol for 10–15 min, these chalcones 75 afforded isoxazole 76.77 (Scheme 24).
Trifluoromethylated flavonol 77 on treatment with aromatic oxime 78 in the presence of triethylamine and CuI in DMF generated flavonoids with isoxazole ring 79. These reactions were carried out via microwave irradiation (250 W) for 5 min.78 (Scheme 25).
2.4 Cycloisomerization
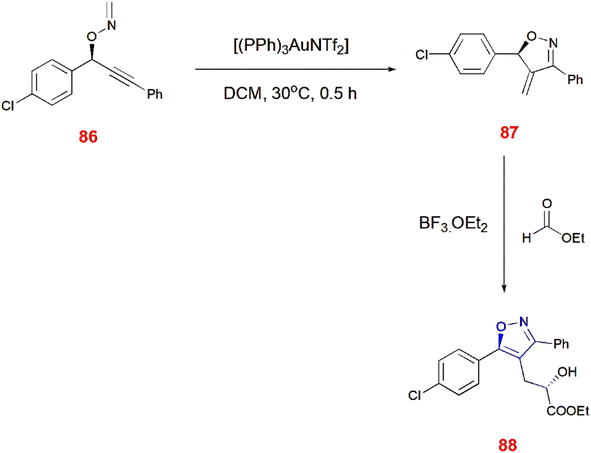
Nakamura and co-workers illustrated the preparation of 4-methylated isoxazoline analogues 81 by the rearrangement of O-propargylic formaldoxime 80 via intermolecular methylene group transfer reaction using gold catalyst. Later, ene or isomerization reaction of isoxazolines 81 takes place with maleimide, glyoxalate, potassium tert-butoxide and azodicarboxylate to yield corresponding isoxazole derivatives 82–85.79 (Scheme 26) Similarly, chiral isoxazoles 88 can be made from O-propargylic oxime 86 facilitated through chirality transfer. Furthermore, the chirality of O-propargylic oxime was retained without any changes leading to the formation of isooxazoline derivatives 87 which undergoes treatment with glyoxalate in the presence of boron trifluoride etherate to give expected chiral isoxazole 88.80 (Scheme 27).4-[Alkoxy(aryl)methyl]-substituted isoxazoles 91 were synthesized from aryl acetals 90 and alkynyl-O-methyl oximes 89. This is promoted by the oxocarbenium cations, which cyclize alkynyl-O-methyl oximes 89 intramolecularly. For this occurrence, triple bond of alkynyl-O-methyl oxime 89 must be activated by oxocarbenium cations generated from aryl acetals 90 in the presence of boron trifluoride etherate, acetonitrile at room temperature for 10–15 min which led to the formation of isoxazoles 91 via intramolecular 5-endo cyclization.81 (Scheme 28).
Chlorinative cyclization of NCS and trimethylsilyl chloride led to the formation of 4-chloroisoxazoles 93. (E/Z)-alkynyl-O-methyl oximes 92 were isomerized to its Z form to cyclize isoxazole 93 in the presence of nitromethane 57 at room temperature for 1 h.82 (Scheme 29).
2.5 Direct functionalization
The direct functionalization of the isoxazole ring at C-3, C-4 and C-5 is important because it is labile under basic conditions. This is accomplished either by C–H activation or transition metal cross coupling reactions. Propargyl phenyl ethers 94 were made to react with hydroxymoyl chloride 95 in the presence of CuI and potassium carbonate in diethyl ether to give isoxazole 96. This intermediate was then cyclized using Pd(II) complex generating tricyclic fused isoxazoles 97.83 (Scheme 30).Negishi coupling of isoxazole zinc pivalates 98 with bromopyridine derivative 99 which is highly functionalized in the presence of the XPhos Pd G3 catalyst, produced the drug-like scaffold 100. The reactivities of organozinc pivalates can be determined from several experimental methods.84 (Scheme 31).
3,4-Disubstituted isoxazoles 101 underwent C–H arylation via 1,2-bis(diphenylphosphino)benzene (dppBz), which facilitated the coupling at C-5 of the isoxazole ring with aryl iodides 102 to yield coupled product 103.85 (Scheme 32).
Tang et al., demonstrated various methods of fluorinating isoxazole acids 104 directly by decarboxylation in the presence of KF in 1,2-dichloroethane/water at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 70 °C for 15 h using Selectfluor™ 105 to yield fluorinated isoxazole 106
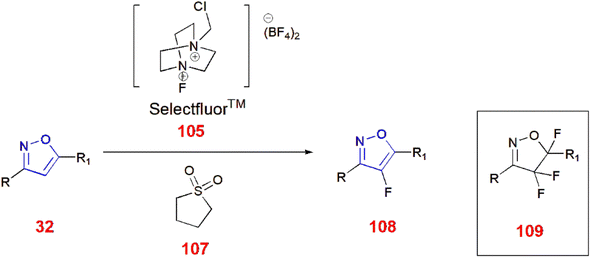
1 at 70 °C for 15 h using Selectfluor™ 105 to yield fluorinated isoxazole 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 (Scheme 33). Moreover, Selectfluor™ 105 was also used to fluorinate 3,5-disubstituted isoxazoles 32 at the C-4 position to give 108 in modest yields. Excess use of Selectfluor™ gave isoxazolines 109 with difluoro and monofluoro substituents at C-4 and C-5, respectively.87 (Scheme 34).
86 (Scheme 33). Moreover, Selectfluor™ 105 was also used to fluorinate 3,5-disubstituted isoxazoles 32 at the C-4 position to give 108 in modest yields. Excess use of Selectfluor™ gave isoxazolines 109 with difluoro and monofluoro substituents at C-4 and C-5, respectively.87 (Scheme 34).
5-Aminoisoxazole 112 underwent difluoromethylthiolation using N-difluoromethylthiophthalimide 111 to give 113. N-difluoromethylthiophthalimide 111 was prepared from reacting benzyl difluoromethylthioether 110 with chlorine in chloroform to generate dichlorocarbene at −30 °C to room temperature for 2 h. Furthermore, potassium phthalimide was added to give the desired reagent 111.88,89 (Scheme 35).
3 Biology of isoxazoles
3.1 Antibacterial and antifungal activity
Antibacterial agents fall under the class of antimicrobials that can be bacteriostatic90 or bactericidal91 in nature. Thus, several commercially available drug molecules containing isoxazoles have been approved for the treatment of acute bronchitis92 and infections in the urinary tract.93 However, isoxazole-containing drugs such as oxacillin,94 flucloxacillin,95 dicloxacillin,96 cloxacillin,97 sulfamethoxazole45 and sulfisoxazole,98,99 which are commercially available, are listed in Table 2.Raju et al., synthesized novel urea and thiourea derivatives of 6-fluoro-3-(piperidin-4-yl)benzo[d] isoxazole and evaluated their antimicrobial activity keeping tetracycline as the standard reference drug. Among the six derivatives, compounds 111–113 showed their inhibition against Bacillus subtilis and Staphylococcus aureus. Furthermore, 111 and 113 showed additional inhibition against Escherichia coli and Pseudomonas aeruginosa. Structure–activity relationship (SAR) studies revealed that the presence of electron withdrawing groups like trifluoro and chloro increased the activity to a greater extent.100
Khazi et al., synthesized new sulfones and sulfides of methylene-bridged benzisoxazolylimidazo[2,1-b][1,3,4]thiadiazoles, tested them against E. coli ATCC 35218 and B. subtilis ATCC 6633 and concluded that most had significant to modest activity when compared with the standard drug ampicillin. Compounds 114–117 showed good activity against these strains. Additionally, compounds 115–117 showed activity against fungal species such as Candida albicans and the activity of 115 was almost equivalent to the standard drug Clotrimazole. Furthermore, compounds 114 and 118 showed good inhibitory effect against Aspergillus fumigatus. Coumarin and methoxy substituents made these compounds more active against fungal and bacterial strains. Especially, the delocalization of π-electrons in 114 and 115 resulted in penetration into lipid membranes as they are lipophilic in nature.101
Anjani et al., prepared novel derivatives of 1,3,5-triazines containing aminopyridines, isoxazoles and acetyl pyrazoline moieties, checked for their antimicrobial proficiency and determined their minimum inhibitory concentration (MIC). Compound 119 was effective against S. aureus bearing MIC value of 100 μg ml−1, quite good when compared with ampicillin with 250 μg ml−1 but not better than chloramphenicol and ciprofloxacin, both with MIC of 50 μg ml−1. Compounds 121 and 123 both showed MIC values of 100 and 125 μg ml−1 against Streptococcus pyogenes, which is equivalent to ampicillin with MIC 100 μg ml−1 but moderate towards chloramphenicol and ciprofloxacin with 50 μg ml−1. When Gram-negative bacteria such as E. coli is taken, compound 122 showed MIC value of 100 μg ml−1 which was again equivalent to ampicillin but was quite higher than chloramphenicol and ciprofloxacin having MIC of 50 and 25 μg ml−1, respectively. Compounds 121 and 122 showed MIC values of 200 μg ml−1 same as that of ampicillin against S. aureus. Notably, compound 122 showed great antifungal activity against C. albicans with MIC value of 250 μg ml−1. Compounds 119–121 showed same activity as that of standard drug griseofulvin showing MIC value of 500 μg ml−1. However, the SAR studies revealed that the presence of CF3 in the compounds 119–123 made them active as antimicrobial scaffolds. Among them, 119 and 123 showed highest antimicrobial activity. Such electronic changes or modifications enable molecules to increase the binding energy with the microbial target site, thereby enhancing potency.102 (Fig. 2).
3.2 Anticancer activity
Isoxazoles have good anticancer activities and can be used to treat various carcinomic conditions. Several commercially available isoxazole-containing drug molecules such as acivicin,103 XN05,104,105 PNZ5,106 NVP-AUY922![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107–109 shown in Table 3.
107–109 shown in Table 3.
Eid et al., synthesized and evaluated the biological performance of new isoxazole–amide analogues. As a result of the anticancer evaluation, these derivatives were tested against HeLa, Hep3B, and MCF-7 cell lines, their IC50 (half-maximal inhibitory concentration) values were compared with that of doxorubicin. It was found that, compound 124 was most active against HeLa cell line with IC50 value of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 ± 0
48 ± 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89 μg ml−1. However, compound 125 was considerably active against HeLa showing IC50 value of 18
89 μg ml−1. However, compound 125 was considerably active against HeLa showing IC50 value of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 ± 0
62 ± 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79 μg ml−1. Compounds 124 and 126 showed anticancer activity against Hep3B cell line with IC50 23
79 μg ml−1. Compounds 124 and 126 showed anticancer activity against Hep3B cell line with IC50 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 ± 1
98 ± 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 μg ml−1 and 23
83 μg ml−1 and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 ± 1
44 ± 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 μg ml−1, respectively.110
99 μg ml−1, respectively.110
Hawash et al., synthesized and studied the biological activity of phenyl-isoxazole–carboxamide analogues. These derivatives were tested for their anticancer profiles against HeLa, MCF-7, Hep3B, HepG2, and Hek293T cell lines comparing with those of doxorubicin. Compounds 127–129 showed quite good anticancer activity against Hep3B cell lines showing the IC50 values (μM) of 5.96 ± 0.87, 6.93 ± 1.88 and 8.02 ± 1.33, respectively. Compound 130 was active against the MCF-7 cell line with IC50 value (μM) of 4.56 ± 2.32. Compound 129 showed excellent anticancer activity against HeLa cell line with IC50 value (μM) of 0.91 ± 1.03.111
Ketan and co-workers produced some isoxazole derivatives with phenyl rings linked with a diazo group and evaluated their anticancer activity against PC3 and HEK normal cell lines. Compounds 131–134 showed anticancer activity against PC3 among which 134 was highly potent. However, these compounds showed anticancer activity only with high doses (640 μM) in normal cell lines. The SAR studies revealed the significance of the electron-withdrawing groups such as –F, –Cl, and –Br. The ortho-substituted bromo compound 134 demonstrated valuable cytotoxic effects than the rest of the halogen substituted analogues.112
Jarina et al., generated novel isoxazole derivatives, tested their anticancer activity against MCF-7 cell lines and reported their IC50 values (μg ml−1). Among the six derivatives, compounds 135 and 136 showed good anticancer activity reflecting IC50 values (μg ml−1) of −26.32 and −29.57 comparing with that of standard drug Adriamycin. Moreover, molecular docking studies demonstrated the binding affinity of −9, favouring hydrophobic binding interactions with topoisomerase II.113
Panathur et al., produced isoxazoles linked with indole and studied their antiproliferation activity against MCF-7 and HT-29 cell lines comparing with Gemcitabine. Compounds 137 and 138 were effective against MCF-7 cell lines showing IC50 values (μM) 7.72 and 5.51. Compounds 139–142 were tested against HT-29 cell lines and showed interesting IC50 values (μM) −4.82, −2.59, −4.80 and −4.83, respectively. The docking analysis proved that compound 140 containing a trifluoromethyl benzyl ether linkage was responsible for the increased cytotoxicity and is one of the lead compounds that could furnish significant anti-proliferative activity along with SIRT1 enzyme.114 (Fig. 3).
3.3 Anti-inflammatory activity
Isoxazoles are reported to have good anti-inflammatory activities and they control inflammation as there is a great need to reduce or treat edema. Normally, they follow two pathways namely, the cyclooxygenase (COX) and lipoxygenase (LOX) pathways.115–117 Some of the isoxazole-containing drugs such as Parecoxib,118 Valdecoxib,119,120 Mofezolac,121,122 Leflunomide123 are shown in Table 4.Abdellal worked on synthesizing novel isoxazoles derivatives and checked for their anti-inflammatory activity using rat paw edema model induced by carrageenan124 as a suitable domain to perform experiments. Among various isoxazoles, compound 143 showed the ED50 (mg kg−1) value 45 when compared to standard drug bearing 40. While, other compounds 144 and 145 showed ED50 (mg kg−1) value in the range of 48–50. Thus, compound 143 was closer and more effective in reducing inflammation in rat paws. The molecular docking scores with the range −14.77 to −15.63 kcal mol−1 demonstrated the high selectivity of compounds 143–145 towards COX-2 receptor site and revealed well-established bonding interaction with active pharmacophores such as :O: of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and :N of isoxazole ring.125
O and :N of isoxazole ring.125
Bhupinder Kumar et al., synthesized indole-linked isoxazoles and tested their anti-inflammatory activity using the most accepted paw edema model. After injecting the rats with carrageenan to develop edema, these compounds were tested and the changes in the volume of paw edema at 1 h, 2 h and 4 h intervals, respectively. After thorough examination, compound 146 showed highest anti-inflammatory activity with 77.42% reduction after 4 h. Compounds 147 and 148 also showed good anti-inflammatory by reducing paw edema by 67.74 and 61.29%. Apart from these three compounds, there was an interesting result with respect to compound 149 which showed anti-inflammatory activity at 2 h interval and reduced in later hours which the biologists suggested the methoxy group at meta position had affected for such kind of deviation by inactivating the compound. However, the strong evidence was laid from the SAR studies which demonstrated the presence of 4-methoxy and 3-methoxy in compounds 147 and 148 favoured COX-2 inhibitory, anti-inflammatory and analgesic action. The selectivity index for COX-2 was highest with methoxy group at 4th position and resulted in slight decrease with the shift to 3rd position.126
Khanum et al., produced few novel 3-phenyl-5-furan isoxazole derivatives and evaluated their anti-inflammatory activity. Among the synthesized derivatives, compound 150 exhibited potency in inhibiting COX-2 with IC50 value (μM) 9.16 ± 0.38 in relation with the reference drugs indomethacin and diclofenac sodium. Through molecular docking studies, compounds 151–154 showed their affinity towards COX-1 rather than COX-2. For exhibiting COX-2 affinity, it is very essential for the isoxazole substrate to be hydrophobic and compound 150 had two chloro groups at ortho and para positions which makes them to interact with the active site. Apart from COX-2, it has also shown good 15-LOX inhibition with IC50 value (μM) 8.15 ± 0.16. These inhibitions are the great evidence for them to act as anti-inflammatory agents and lead molecules for the development of newer drug molecules.127
Khan et al., gave some isoxazole derivatives and carried out 5-LOX inhibitory assay for the designed compounds. Compound 155 showed promising 5-LOX inhibition with IC50 value 3.67 μM. Additionally, compounds 156 showed good 5-LOX inhibition next to compound 155. Compound 156 and 157 showed promising results in COX-2 inhibition.128,129 (Fig. 4).
3.4 Antioxidant activity
Hawash and co-workers determined the potential of novel isoxazole derivatives with respect to their antioxidant capacity. The derivatives of fluoro-phenyl-carboxamide were synthesized previously and then checked for their antioxidant properties using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Trolox was used as the control for the experiments. Its IC50 value (μg ml−1) was found to be 3.10 ± 0.92. After evaluation, compounds 158 and 159 showed potent antioxidant activity with IC50 values (μg ml−1) 0.45 ± 0.21 and 0.47 ± 0.33, respectively. Usually, antioxidant compounds exhibit radical scavenging properties wherein the radical will abstract a proton from the antioxidant scaffold. As there are different substituents on the phenyl ring, compound 158 has tert-butyl group, whose absence showed very less antioxidant properties. Thus, it was made clear that the presence of electron donating groups were responsible for exhibiting antioxidant properties.130Chalka et al., investigated the antioxidant activity of novel functionalized isoxazoles using DPPH method of screening, taking BHT as the reference standard having IC50 value (μg ml−1) 28.81 ± 1.84. Among the synthesized isoxazoles, compound 160 showed great antioxidant property with IC50 value (μg ml−1) of 63.51 ± 1.80. Other than this compound, some other compounds 161–163 exhibited free radical scavenging characteristics having IC50 values (μg ml−1) of 79.85 ± 1.90, 83.69 ± 1.92 and 87.76 ± 1.94, respectively. The remaining isoxazoles showed moderate DPPH activity. However, the ‘drug-likeness’ evaluation suggested that compounds 161 and 163 were closely adhering to the Lipinski's ‘Rule of Five’ and compound 163 persisted significant oral bioavailability threshold (>55% F). Erstwhile, all compounds 160–164 validated Egan's rule for balanced consideration of lipophilicity and molecular weight.131
Nagaraju et al., screened quinazolinone-based isoxazole derivatives for antioxidant activity using DPPH assay. Ascorbic acid was used as the control for the experiments. It was found that, compound 164 exhibited best activity with inhibitory concentrations 1.28 ± 0.33 and 1.39 ± 0.38 μM. However, compound 165 showed significant activity succeeding the prior compound with inhibitory concentrations 2.72 ± 0.34 and 2.78 ± 0.41 μM. Furthermore, these compounds 164 and 165 were evaluated against NADP oxidase as it plays a key role in generating reactive oxygen species. As a result of this, compound 164 best fitted into the groove of NADP oxidase with quite acceptable ADME properties without violating Lipinski's rule, exploring the potential in developing into therapeutic agent.132
Victor and co-workers synthesized and studied the antioxidant properties of 4-arylhydrazinylidene-isoxazoles containing polyfluoroalkyl groups. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)133,134 and ferric reducing antioxidant power (FRAP)135,136 free radical scavenging assays were carried out for evaluating antioxidant properties taking Trolox as the reference standard with Trolox equivalent antioxidant capacity (TEAC) 1. Compounds 166–169 were found to be lead compounds in ABTS assay having TEAC 1.50 ± 0.07, 1.80 ± 0.06, 2.00 ± 0.10 and 1.90 ± 0.09, respectively. Compounds 170 and 171 were found to be leads for FRAP assay as they effectively reduced the Fe3+ complex.137 (Fig. 5).
3.5 Antitubercular activity
Mycobacterium tuberculosis causes Tuberculosis, an air-borne lung infection i.e., contagious in nature.138 Mycobacterium tuberculosis falls under the three major classes of the genus Mycobacterium that cause tuberculosis, leprosy and other non-tuberculous mycobacteria.139–141 Thus, many compounds were developed to treat tuberculosis among which isoxazole containing molecules have gained great attention and importance. Quinoline–isoxazole containing compounds have been widely studied and some of these include compounds 172–176.142–146Sharabu and Pappula synthesized and studied the antitubercular activity of isoxazole clubbed pyrimidine derivatives. Mycobacterium tuberculosis H37Rv was used for this study and Pyrazinamide was used as the reference antitubercular drug and was carried out using dilution method at various concentrations ranging from 0.78 to 100 μg ml−1. Compound 177 and 178 showed excellent activity at MIC 0.78 μg ml−1. Compounds 179–183 showed better activity with MIC 1.56 μg ml−1 than the reference drug (MIC 3.125 μg ml−1).147
Marco Pieroni and others evaluated the antitubercular activity of 5-(2-aminothiazol-4-yl)isoxazole-3-carboxamides taking Streptomycin and Isoniazid as reference standards and REMA method for determining MIC toward the tubercular strain. M. tuberculosis H37Rv strain was used for this study and it revealed that compounds 184 and 185 showed maximum antitubercular activity with MIC (μg ml−1) 1.0 and 0.5, respectively.148
Sahoo et al., synthesized several novel chalcone linked 5-phenyl-3-isoxazolecarboxylic acid methyl esters and tested for their antitubercular activity against M. tuberculosis H37Rv strain taking isoniazid, streptomycin, rifampicin and ethambutol as reference drugs. After careful examination, they concluded that compound 186 and 187 were very potent antitubercular agents with MIC (μg ml−1) 0.25 and 0.12, respectively.149 (Fig. 6).
4 Conclusion
Isoxazoles are synthesized via unique reactions such as cycloadditions and the inclusion of click reactions makes them more attractive. The green synthesis of isoxazoles has also attained increased attention, as the environment is a major concern for chemists working on molecules worldwide. The direct functionalization of isoxazoles illustrates the basics of organic reactions and how unique they are upon arylation. In addition to synthetic methods, isoxazoles are also important medicinally. The biological implications of isoxazoles have made them more attributable to clinical dimensions. Most of the isoxazole analogues discussed in this review were successful in inhibiting various bacterial and fungal strains that cause various infections and other health-related diseases. However, such derivatives were also effective with anti-proliferative action when tested against various cancer cell lines such as HeLa, MCF-7, Hep3B, PC3, HEK, HT-29 etc. Such findings accomplish the potential of the isoxazole cores as anticancer agents with the existing standard anticancer drugs. The ability of isoxazoles to inhibit cyclooxygenase (COX) and lipoxygenase (5-LOX) pathways led for the strong anti-inflammatory action. The scope of isoxazoles is further broadened by their remarkable antioxidant and antitubercular actions supported by the ‘drug-likeness’ evaluation. Some isoxazole-containing compounds have shown even better results than the standard reference drugs which are commercially available. Thus, isoxazoles can be fascinating molecules in the world of organic chemistry for chemists to work in academic research and pharmaceutical research and development.Abbreviations
| NCS: | N-Chlorosuccinimide |
| NIS: | N-Iodosuccinimide |
| DCM: | Dichloromethane |
| DCE: | Dichloroethane |
| THF: | Tetrahydrofuran |
| DME: | Dimethyl ether |
| ACN: | Acetonitrile |
| DMF: | Dimethylformamide |
| TBAB: | Tetrabutylammonium bromide |
| DMAC: | Dimethylacetamide |
| K-PHI: | Potassium poly(heptazine imide) |
| DABCO: | 1,4-Diazabicyclo[2.2.2]octane |
| TMSN3: | Trimethylsilyl azide |
| TMSCl: | Trimethylsilyl chloride |
| DBU: | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| MW: | Microwave |
| m-CPBA: | meta-Chloroperbenzoic acid |
| TFA: | Trifluoroacetic acid |
| SAR: | Structure–activity relationship |
| ATCC: | American type culture collection |
| MIC: | Minimum inhibitory concentration |
| HeLa: | Henrietta lacks |
| MCF-7: | Michigan cancer foundation-7 |
| PC3: | Human prostate cancer cell line |
| IC50: | Half-maximal inhibitory concentration |
| ED50: | Median effective dose 50 |
| HEK: | Human embryonic kidney cell line |
| SIRT1: | Silent information regulator sirutin1 |
| COX: | Cyclooxygenase |
| LOX: | Lipooxygenase |
| DNA: | Deoxyribonucleic acid |
| DPPH: | 2,2-Diphenyl-1-picrylhydrazyl |
| NADP: | Nicotinamide adenine dinucleotide phosphate |
| ADME: | Absorption, distribution, metabolism, excretion |
| ABTS: | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP: | Ferric reducing antioxidant power |
| TEAC: | Trolox equivalent antioxidant capacity |
| REMA: | Resazurin microtiter assay plate |
Ethical statement
This study doesn't involve the use of any humans or animals.Data availability
All data has been obtained from peer-reviewed articles cited in the reference list, with no additional datasets utilized.Author contributions
Glanish Jude Martis: writing – original draft, software. Santosh L. Gaonkar: writing – review & editing, supervision.Conflicts of interest
On behalf of the authors, the corresponding author declare no interests.References
- G. Kumar and R. Shankar, ChemMedChem, 2021, 16, 430–447 Search PubMed.
- S. Tilvi and K. S. Singh, Curr. Org. Chem., 2016, 20, 898–929 Search PubMed.
- C. P. Pandhurnekar, H. C. Pandhurnekar, A. J. Mungole, S. S. Butoliya and B. G. Yadao, J. Heterocycl. Chem., 2023, 60, 537–565 Search PubMed.
- J. Zhu, J. Mo, H. Lin, Y. Chen and H. Sun, Bioorg. Med. Chem., 2018, 26, 3065–3075 Search PubMed.
- A. V Galenko, A. F. Khlebnikov, M. S. Novikov, V. V Pakalnis and N. V Rostovskii, Russ. Chem. Rev., 2015, 84, 335–377 Search PubMed.
- M. Gul and S. Eryılmaz, Lett. Org. Chem., 2019, 16, 501–510 CrossRef CAS.
- V. V. Pothuri, P. V. S. Machiraju and V. S. S. Rao, Russ. J. Gen. Chem., 2020, 90, 889–894 CrossRef CAS.
- M. Aarjane, S. Slassi, A. Ghaleb, B. Tazi and A. Amine, Arabian J. Chem., 2021, 14, 103057 CrossRef CAS.
- A. Shaik, R. R. Bhandare, K. Palleapati, S. Nissankararao, V. Kancharlapalli and S. Shaik, Molecules, 2020, 25, 1047 CrossRef CAS PubMed.
- O. S. Trefzger, N. V. Barbosa, R. L. Scapolatempo, A. R. das Neves, M. L. F. S. Ortale, D. B. Carvalho, A. M. Honorato, M. R. Fragoso, C. Y. K. Shuiguemoto, R. T. Perdomo, M. F. C. Matos, M. R. Chang, C. C. P. Arruda and A. C. M. Baroni, Arch. Pharm., 2020, 353, 1900241 CrossRef CAS PubMed.
- T. Zhang, M. Dong, J. Zhao, X. Zhang and X. Mei, J. Pestic. Sci., 2019, 44, 181–185 CrossRef CAS PubMed.
- G. C. Arya, K. Kaur and V. Jaitak, Eur. J. Med. Chem., 2021, 221, 113511 CrossRef CAS PubMed.
- K. Kaur, V. Kumar, A. K. Sharma and G. K. Gupta, Eur. J. Med. Chem., 2014, 77, 121–133 CrossRef CAS PubMed.
- S. Huang, B. Zhu, K. Wang, M. Yu, Z. Wang, Y. Li, Y. Liu, P. Zhang, S. Li, Y. Li, A. Liu and Q. Wang, Pest Manage. Sci., 2022, 78, 2011–2021 CrossRef CAS PubMed.
- A. A. Abu-Hashem and M. El-Shazly, Med Chem, 2018, 14, 356–371 CrossRef CAS PubMed.
- F. V. B. Mota, M. S. de Araújo Neta, E. de Souza Franco, I. V. G. A. Bastos, L. C. C. da Araújo, S. C. da Silva, T. B. de Oliveira, E. K. Souza, V. M. de Almeida, R. M. Ximenes, M. B. de Sousa Maia, F. J. B. M. Junior, P. Marchand, A. R. de Faria and T. G. da Silva, MedChemComm, 2019, 10, 1916–1925 RSC.
- Z. Li, C. Liu, W. Shi, X. Cai, Y. Dai, C. Liao, W. Huang and H. Qian, Bioorg. Med. Chem., 2018, 26, 703–711 CrossRef CAS PubMed.
- S. Fettach, F. Z. Thari, Z. Hafidi, K. Karrouchi, K. Bouathmany, Y. Cherrah, M. El Achouri, L. Benbacer, M. El Mzibri, H. Sefrioui, K. Bougrin and M. E. A. Faouzi, J. Biomol. Struct. Dyn., 2023, 41, 1072–1084 CrossRef CAS PubMed.
- K. V. Chikkula and R. Sundararajan, Med. Chem. Res., 2017, 26, 3026–3037 CrossRef CAS.
- A. Rastegari, M. Safavi, F. Vafadarnejad, Z. Najafi, R. Hariri, S. N. A. Bukhari, A. Iraji, N. Edraki, O. Firuzi, M. Saeedi, M. Mahdavi and T. Akbarzadeh, Mol. Diversity, 2022, 26, 409–428 CrossRef CAS PubMed.
- P. Patil, A. Thakur, A. Sharma and S. J. S. Flora, Drug Dev. Res., 2020, 81, 165–183 CrossRef CAS PubMed.
- A. Sysak and B. Obmińska-Mrukowicz, Eur. J. Med. Chem., 2017, 137, 292–309 CrossRef CAS PubMed.
- M. Tugrak, H. I. Gul, K. Bandow, H. Sakagami, I. Gulcin, Y. Ozkay and C. T. Supuran, Bioorg. Chem., 2019, 90, 103095 CrossRef CAS PubMed.
- A. Sysak and B. Obmińska-Mrukowicz, Eur. J. Med. Chem., 2017, 137, 292–309 CrossRef CAS PubMed.
- H. Yao, J. Liu, S. Xu, Z. Zhu and J. Xu, Expert Opin. Drug Discovery, 2017, 12, 121–140 CrossRef CAS PubMed.
- R. L. Barbieri, S. Evans and R. W. Kistner, Fertil. Steril., 1982, 37, 737–746 CrossRef CAS PubMed.
- H. A. Ramawad, P. Paridari, S. Jabermoradi, P. Gharin, A. Toloui, S. Safari and M. Yousefifard, Korean J. Pain, 2023, 36, 425–440 CrossRef CAS PubMed.
- D. Rivera-Illanes and G. Recabarren-Gajardo, ACS Chem. Neurosci., 2024, 15, 3257–3269 CrossRef CAS PubMed.
- I. E. Leppik, Seizure, 2004, 13, S5–S9 CrossRef PubMed.
- C. Lamberth, J. Heterocycl. Chem., 2018, 55, 2035–2045 CrossRef CAS.
- K. E. Pallett, S. M. Cramp, J. P. Little, P. Veerasekaran, A. J. Crudace and A. E. Slater, Pest Manage. Sci., 2001, 57, 133–142 CrossRef CAS.
- S. Costanzi, T. Santhosh Kumar, R. Balasubramanian, T. Kendall Harden and K. A. Jacobson, Bioorg. Med. Chem., 2012, 20, 5254–5261 CrossRef CAS PubMed.
- Y. Kanda, T. Takahashi, Y. Araki, T. Konoike, S. Mihara and M. Fujimoto, Bioorg. Med. Chem. Lett., 2000, 10, 1875–1878 CrossRef CAS PubMed.
- S. Dadiboyena and A. Nefzi, Eur. J. Med. Chem., 2010, 45, 4697–4707 CrossRef CAS PubMed.
- M. L. Chavez and C. J. DeKorte, Clin. Ther., 2003, 25, 817–851 CrossRef CAS PubMed.
- S. Jeanmart, A. J. F. Edmunds, C. Lamberth, M. Pouliot and J. A. Morris, Bioorg. Med. Chem., 2021, 39, 116162 CrossRef CAS PubMed.
- M. Asahi, M. Kobayashi, T. Kagami, K. Nakahira, Y. Furukawa and Y. Ozoe, Pestic. Biochem. Physiol., 2018, 151, 67–72 CrossRef CAS PubMed.
- D. Kim, M.-S. Kang, J. S. Kim and J.-H. Jeong, Arch. Pharmacal Res., 2005, 28, 1019–1022 CrossRef CAS PubMed.
- M. Agrawal, S. Saraf, S. Saraf, S. G. Antimisiaris, M. B. Chougule, S. A. Shoyele and A. Alexander, J. Controlled Release, 2018, 281, 139–177 CrossRef CAS PubMed.
- R. I. Fox, M. L. Herrmann, C. G. Frangou, G. M. Wahl, R. E. Morris, V. Strand and B. J. Kirschbaum, Clin. Immunol., 1999, 93, 198–208 CrossRef CAS PubMed.
- J. R. Romero, Expert Opin. Invest. Drugs, 2001, 10, 369–379 CrossRef CAS PubMed.
- J. K. Aronson, In Meyler's Side Effects of Drugs, Elsevier, 2016, p. 834 Search PubMed.
- D. Della Bella, Respiration, 1989, 55, 10–14 CrossRef CAS PubMed.
- J. Davidson and C. Turnbull, J. Affective Disord., 1983, 5, 183–189 Search PubMed.
- S. Rostamizadeh, Z. Daneshfar and H. Moghimi, Eur. J. Med. Chem., 2019, 171, 364–371 CrossRef CAS PubMed.
- S. Obermaier and M. Müller, Angew. Chem., 2020, 132, 12532–12535 Search PubMed.
- R. J. Noveck and R. C. Hubbard, J. Clin. Pharmacol., 2004, 44, 474–480 CrossRef CAS PubMed.
- L. Johnson, J. Powers, F. Ma, K. Jendza, B. Wang, E. Meredith and N. Mainolfi, Synthesis, 2012, 45, 171–173 Search PubMed.
- C.-J. Hsieh, K. Xu, I. Lee, T. J. A. Graham, Z. Tu, D. Dhavale, P. Kotzbauer and R. H. Mach, ACS Omega, 2018, 3, 4486–4493 Search PubMed.
- N. Agrawal and P. Mishra, Med. Chem. Res., 2018, 27, 1309–1344 CrossRef CAS PubMed.
- P. Anand and B. Singh, Mini-Rev. Med. Chem., 2014, 14, 623–627 CrossRef CAS PubMed.
- M. A. Barmade, P. R. Murumkar, M. Kumar Sharma and M. Ram Yadav, Curr. Top. Med. Chem., 2016, 16, 2863–2883 Search PubMed.
- T. Morita, S. Yugandar, S. Fuse and H. Nakamura, Tetrahedron Lett., 2018, 59, 1159–1171 CrossRef CAS.
- J.-S. Poh, C. García-Ruiz, A. Zúñiga, F. Meroni, D. C. Blakemore, D. L. Browne and S. V. Ley, Org. Biomol. Chem., 2016, 14, 5983–5991 Search PubMed.
- A. Khutorianskyi, B. Chalyk, P. Borysko, A. Kondratiuk and P. K. Mykhailiuk, Eur. J. Org. Chem., 2017, 2017, 3935–3940 CrossRef CAS.
- Y. Guo, X. Wang, Z. Zhu, J. Zhang and Y. Wu, Synlett, 2016, 27, 2259–2263 Search PubMed.
- S. Okusu, E. Tokunaga and N. Shibata, Org. Lett., 2015, 17, 3802–3805 CrossRef CAS PubMed.
- R. M. Reja, S. Sunny and H. N. Gopi, Org. Lett., 2017, 19, 3572–3575 CrossRef CAS PubMed.
- S. Kuribayashi, N. Shida, S. Inagi and T. Fuchigami, Tetrahedron, 2016, 72, 5343–5349 Search PubMed.
- G.-W. Wang, M.-X. Cheng, R.-S. Ma and S.-D. Yang, Chem. Commun., 2015, 51, 6308–6311 RSC.
- M. Shanmugasundaram, A. Senthilvelan and A. R. Kore, Tetrahedron Lett., 2020, 61, 152464 CrossRef CAS PubMed.
- C. Chen and S. Cui, J. Org. Chem., 2019, 84, 12157–12164 CrossRef CAS PubMed.
- A. Savateev, N. V. Tarakina, V. Strauss, T. Hussain, K. ten Brummelhuis, J. M. Sánchez Vadillo, Y. Markushyna, S. Mazzanti, A. P. Tyutyunnik, R. Walczak, M. Oschatz, D. M. Guldi, A. Karton and M. Antonietti, Angew. Chem., Int. Ed., 2020, 59, 15061–15068 CrossRef CAS PubMed.
- X.-D. Wang, L.-H. Zhu, P. Liu, X.-Y. Wang, H.-Y. Yuan and Y.-L. Zhao, J. Org. Chem., 2019, 84, 16214–16221 CrossRef CAS PubMed.
- Y. Chen, L. Li, X. He and Z. Li, ACS Catal., 2019, 9, 9098–9102 CrossRef CAS.
- Z. Tang, Y. Zhou and Q. Song, Org. Lett., 2019, 21, 5273–5276 CrossRef CAS PubMed.
- T. N. Ngo, S. A. Ejaz, T. Q. Hung, T. T. Dang, J. Iqbal, J. Lecka, J. Sévigny and P. Langer, Org. Biomol. Chem., 2015, 13, 8277–8290 RSC.
- J. Li, W. Ma, W. Ming, C. Xu, N. Wei and M. Wang, J. Org. Chem., 2015, 80, 11138–11142 CrossRef CAS PubMed.
- G. R. Kumar, Y. K. Kumar and M. S. Reddy, Chem. Commun., 2016, 52, 6589–6592 RSC.
- M. V. Andreev, A. S. Medvedeva, L. I. Larina and M. M. Demina, Mendeleev Commun., 2017, 27, 175–177 CrossRef CAS.
- O. B. Bondarenko, A. A. Vinogradov, P. A. Danilov, S. N. Nikolaeva, A. Yu. Gavrilova and N. V. Zyk, Tetrahedron Lett., 2015, 56, 6577–6579 CrossRef CAS.
- S. Xue, J. Liu and C. Wang, Eur. J. Org. Chem., 2016, 2016, 2450–2456 CrossRef CAS.
- P. Kulkarni, J. Indian Chem. Soc., 2021, 98, 100013 CrossRef CAS.
- M. Zouhair, L. El Ghayati, H. El Monfalouti, H. Abchihi, T. Hökelek, M. Ahmed, J. T. Mague and N. K. Sebbar, Acta Crystallogr., Sect. E:Crystallogr. Commun., 2023, 79, 1179–1182 CrossRef CAS PubMed.
- R. Zheng, F. Feng, Z. Zhang, J. Fu, Q. Su, Y. Zhang and Q. Gu, Mol. Diversity, 2020, 24, 423–435 CrossRef CAS PubMed.
- N. Sawengngen, A. A. Kolodina and O. V. Serdyuk, Molbank, 2019, 2019, M1081 CrossRef.
- K. C. Panda, B. V. V. R. Kumar and B. M. Sahoo, Nat. Volatiles Essent. Oils, 2021, 8, 11503–11510 CAS.
- F. K. Algethami, I. Saidi, H. N. Abdelhamid, M. R. Elamin, B. Y. Abdulkhair, A. Chrouda and H. Ben Jannet, Molecules, 2021, 26, 5214 CrossRef CAS PubMed.
- I. Nakamura, S. Gima, Y. Kudo and M. Terada, Angew. Chem., Int. Ed., 2015, 54, 7154–7157 CrossRef CAS PubMed.
- S. Gima, I. Nakamura and M. Terada, Eur. J. Org. Chem., 2017, 2017, 4375–4378 CrossRef CAS.
- M. Jonušis, L. Šteinys, R. Bukšnaitienė and I. Čikotienė, Synthesis, 2016, 49, 1122–1130 CrossRef.
- W. Kaewsri, C. Thongsornkleeb, J. Tummatorn and S. Ruchirawat, RSC Adv., 2016, 6, 48666–48675 RSC.
- D.-C. Guo, C. Zhang, F. Li, F. Zhang, F. Yu and Y.-P. He, Synthesis, 2016, 49, 1356–1370 CrossRef.
- T. J. Greshock, K. P. Moore, R. T. McClain, A. Bellomo, C. K. Chung, S. D. Dreher, P. S. Kutchukian, Z. Peng, I. W. Davies, P. Vachal, M. Ellwart, S. M. Manolikakes, P. Knochel and P. G. Nantermet, Angew. Chem., Int. Ed., 2016, 55, 13714–13718 CrossRef CAS PubMed.
- M. Shigenobu, K. Takenaka and H. Sasai, Angew. Chem., Int. Ed., 2015, 54, 9572–9576 CrossRef CAS PubMed.
- X. Zhang, G. Liu, X. Sun, L.-S. Wan and Y. Zhou, J. Org. Chem., 2024, 89, 14591–14595 CrossRef CAS PubMed.
- K. Sato, G. Sandford, K. Shimizu, S. Akiyama, M. J. Lancashire, D. S. Yufit, A. Tarui, M. Omote, I. Kumadaki, S. Harusawa and A. Ando, Tetrahedron, 2016, 72, 1690–1698 CrossRef CAS.
- D. Zhu, Y. Gu, L. Lu and Q. Shen, J. Am. Chem. Soc., 2015, 137, 10547–10553 CrossRef CAS PubMed.
- D. Zhu, X. Hong, D. Li, L. Lu and Q. Shen, Org. Process Res. Dev., 2017, 21, 1383–1387 CrossRef CAS.
- D. F. Basri, L. W. Xian, N. I. Abdul Shukor and J. Latip, BioMed Res. Int., 2014, 2014, 1–8 CrossRef PubMed.
- F. Baquero and B. R. Levin, Nat. Rev. Microbiol., 2021, 19, 123–132 CrossRef CAS PubMed.
- C. Llor, A. Moragas, C. Bayona, R. Morros, H. Pera, O. Plana-Ripoll, J. M. Cots and M. Miravitlles, BMJ, 2013, 347, f5762 CrossRef PubMed.
- T. Jancel, West. J. Med., 2002, 176, 51–55 CrossRef PubMed.
- M. Pasticci, A. Moretti, G. Stagni, V. Ravasio, L. Soavi, A. Raglio, F. Vailati, A. Cardaccia, A. Santucci, R. Papili, A. Sgrelli, C. Pallotto and F. Baldelli, Ann. Clin. Microbiol. Antimicrob., 2011, 10, 26 CrossRef CAS PubMed.
- M. N. de Menezes, B. A. de Marco, F. A. M. Fiorentino, A. Zimmermann, A. C. Kogawa and H. R. N. Salgado, Crit. Rev. Anal. Chem., 2019, 49, 67–77 CrossRef PubMed.
- S. S. Castle, in xPharm: The Comprehensive Pharmacology Reference, Elsevier, 2007, pp. 1–5 Search PubMed.
- T. Akbarzadeh, A. Fallah Tafti, N. Samadi, A. Foroumadi, M. Amanlou, M. A. Faramarzi and A. Shafiee, Daru, J. Fac. Pharm., Tehran Univ. Med. Sci., 2010, 18, 118–123 CAS.
- E.-J. Im, C.-H. Lee, P.-G. Moon, G. G. Rangaswamy, B. Lee, J. M. Lee, J.-C. Lee, J.-G. Jee, J.-S. Bae, T.-K. Kwon, K.-W. Kang, M.-S. Jeong, J.-E. Lee, H.-S. Jung, H.-J. Ro, S. Jun, W. Kang, S.-Y. Seo, Y.-E. Cho, B.-J. Song and M.-C. Baek, Nat. Commun., 2019, 10, 1387 CrossRef CAS PubMed.
- P. Fonseka, S. V. Chitti, R. Sanwlani and S. Mathivanan, Nat. Commun., 2021, 12, 977 CrossRef CAS PubMed.
- H. Sudhamani, S. T. Basha, N. Venkateswarlu, T. Vijaya and C. N. Raju, J. Chem. Sci., 2015, 127, 1739–1746 CrossRef CAS.
- N. S. Belavagi, M. G. Sunagar, R. S. Lamani, N. Deshapande and I. A. M. Khazi, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 1580–1587 CrossRef CAS.
- A. Solankee and R. Tailor, Elixir Org. Chem., 2015, 87, 35620–35627 Search PubMed.
- A. Galbiati, A. Zana, C. Borsari, M. Persico, S. Bova, O. Tkachuk, A. I. Corfu, L. Tamborini, N. Basilico, C. Fattorusso, S. Bruno, S. Parapini and P. Conti, Molecules, 2023, 28, 3172 CrossRef CAS PubMed.
- J. Zhu, J. Mo, H. Lin, Y. Chen and H. Sun, Bioorg. Med. Chem., 2018, 26, 3065–3075 CrossRef CAS PubMed.
- R. Wu, W. Ding, T. Liu, H. Zhu, Y. Hu, B. Yang and Q. He, Cancer Lett., 2009, 285, 13–22 CrossRef CAS PubMed.
- R. C. Montenegro, P. G. K. Clark, A. Howarth, X. Wan, A. Ceroni, P. Siejka, G. A. Nunez-Alonso, O. Monteiro, C. Rogers, V. Gamble, R. Burbano, P. E. Brennan, C. Tallant, D. Ebner, O. Fedorov, E. O'Neill, S. Knapp, D. Dixon and S. Müller, Oncotarget, 2016, 7, 43997–44012 CrossRef PubMed.
- G. Augello, M. R. Emma, A. Cusimano, A. Azzolina, S. Mongiovì, R. Puleio, G. Cassata, A. Gulino, B. Belmonte, R. Gramignoli, S. C. Strom, J. A. McCubrey, G. Montalto and M. Cervello, Int. J. Cancer, 2019, 144, 2613–2624 CrossRef CAS PubMed.
- M. R. Jensen, J. Schoepfer, T. Radimerski, A. Massey, C. T. Guy, J. Brueggen, C. Quadt, A. Buckler, R. Cozens, M. J. Drysdale, C. Garcia-Echeverria and P. Chène, Breast Cancer Res., 2008, 10, R33 CrossRef PubMed.
- K. Lee, J. Lee, S. Han, S. Im, T. Kim, D. Oh and Y. Bang, Cancer Sci., 2011, 102, 1388–1395 CrossRef CAS PubMed.
- A. M. Eid, M. Hawash, J. Amer, A. Jarrar, S. Qadri, I. Alnimer, A. Sharaf, R. Zalmoot, O. Hammoudie, S. Hameedi and A. Mousa, BioMed Res. Int., 2021, 2021, 1–9 Search PubMed.
- M. Hawash, N. Jaradat, N. Bawwab, K. Salem, H. Arafat, Y. Hajyousef, T. Shtayeh and S. Sobuh, Heterocycl. Commun., 2021, 27, 133–141 CrossRef CAS.
- K. Vashisht, P. Sethi, A. Bansal, T. Singh, R. Kumar, H. S. Tuli and S. Saini, Eur. J. Clin. Exp. Med., 2024, 22, 376–387 CrossRef.
- A. Jarina, S. Kavimani, V. M. Mounnissamy and J. Abdul, J. Pharm. Negat. Results, 2022, 13, 2634–2642 CAS.
- N. Panathur, N. Gokhale, U. Dalimba, P. V. Koushik, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2015, 25, 2768–2772 CrossRef CAS PubMed.
- S. Fiorucci, R. Meli, M. Bucci and G. Cirino, Biochem. Pharmacol., 2001, 62, 1433–1438 CrossRef CAS PubMed.
- C. D. Funk, Science, 2001, 294, 1871–1875 CrossRef CAS PubMed.
- C. Charlier and C. Michaux, Eur. J. Med. Chem., 2003, 38, 645–659 CrossRef CAS PubMed.
- A. Urdaneta, A. Siso, B. Urdaneta, R. Cardenas, L. Quintero, R. Avila and H. Suarez-Roca, Brain Res. Bull., 2009, 80, 56–61 CrossRef CAS PubMed.
- J. J. Talley, D. L. Brown, J. S. Carter, M. J. Graneto, C. M. Koboldt, J. L. Masferrer, W. E. Perkins, R. S. Rogers, A. F. Shaffer, Y. Y. Zhang, B. S. Zweifel and K. Seibert, J. Med. Chem., 2000, 43, 775–777 CrossRef CAS PubMed.
- N. A. Nussmeier, A. A. Whelton, M. T. Brown, R. M. Langford, A. Hoeft, J. L. Parlow, S. W. Boyce and K. M. Verburg, N. Engl. J. Med., 2005, 352, 1081–1091 CrossRef CAS PubMed.
- M. L. Pati, P. Vitale, S. Ferorelli, M. Iaselli, M. Miciaccia, A. Boccarelli, G. D. Di Mauro, C. G. Fortuna, T. F. Souza Domingos, L. C. Rodrigues Pereira da Silva, M. de Pádula, L. M. Cabral, P. C. Sathler, A. Vacca, A. Scilimati and M. G. Perrone, Eur. J. Med. Chem., 2019, 164, 59–76 CrossRef CAS PubMed.
- G. Cingolani, A. Panella, M. G. Perrone, P. Vitale, G. Di Mauro, C. G. Fortuna, R. S. Armen, S. Ferorelli, W. L. Smith and A. Scilimati, Eur. J. Med. Chem., 2017, 138, 661–668 CrossRef CAS PubMed.
- M. Cutolo, S. Capellino, P. Montagna, A. Sulli, B. Seriolo and B. Villaggio, Ann. Rheum. Dis., 2006, 65, 728–735 CrossRef CAS PubMed.
- M. Zimmermann, Pain, 1983, 16, 109–110 CrossRef PubMed.
- E. K. A. Abdelall, Bioorg. Chem., 2020, 94, 103441 CrossRef CAS PubMed.
- R. Bhatia, A. Vyas, S. M. El-Bahy, M. M. Hessien, G. A. M. Mersal, M. M. Ibrahim, R. Dogra and B. Kumar, ChemistrySelect, 2022, 7, e202200800 CrossRef CAS.
- H. M. Pallavi, F. H. Al-Ostoot, H. K. Vivek and S. A. Khanum, J. Mol. Struct., 2022, 1250, 131812 CrossRef.
- W. Alam, H. Khan, M. S. Jan, H. W. Darwish, M. Daglia and A. A. Elhenawy, PLoS One, 2024, 19, e0297398 CrossRef CAS PubMed.
- W. Alam, H. Khan, M. Saeed Jan, U. Rashid, A. Abusharha and M. Daglia, Front. Chem., 2023, 11, 1222047 CrossRef CAS PubMed.
- M. Hawash, N. Jaradat, M. Abualhasan, M. Thaher, R. Sawalhi, N. Younes, A. Shanaa, M. Nuseirat and A. Mousa, Sci. Rep., 2022, 12, 18223 CrossRef CAS PubMed.
- A. Arzine, O. Abchir, M. Chalkha, K. Chebbac, Y. Rhazi, N. Barghady, I. Yamari, A. EL Moussaoui, A. Nakkabi, M. Akhazzane, M. Bakhouch, S. Chtita and M. EL Yazidi, Comput. Biol. Chem., 2024, 108, 107993 CrossRef CAS PubMed.
- N. Myakala, V. Thumma, K. Kandula, N. Rayala, L. S. Boddu and K. D. B. Anagani, Mol. Diversity, 2024 DOI:10.1007/s11030-024-11032-2.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237 CrossRef CAS PubMed.
- G. F. Makhaeva, N. A. Elkina, E. V. Shchegolkov, N. P. Boltneva, S. V. Lushchekina, O. G. Serebryakova, E. V. Rudakova, N. V. Kovaleva, E. V. Radchenko, V. A. Palyulin, Y. V. Burgart, V. I. Saloutin, S. O. Bachurin and R. J. Richardson, Bioorg. Chem., 2019, 91, 103097 CrossRef CAS PubMed.
- I. F. F. Benzie and J. J. Strain, Methods Enzymol., 1999, 299, 15–27 CAS.
- G. F. Makhaeva, N. V. Kovaleva, E. V. Rudakova, N. P. Boltneva, S. V. Lushchekina, I. I. Faingold, D. A. Poletaeva, Y. V. Soldatova, R. A. Kotelnikova, I. V. Serkov, A. K. Ustinov, A. N. Proshin, E. V. Radchenko, V. A. Palyulin and R. J. Richardson, Molecules, 2020, 25, 5891 CrossRef CAS PubMed.
- N. A. Elkina, E. V. Shchegolkov, Y. V. Burgart, N. A. Agafonova, A. N. Perminova, N. A. Gerasimova, G. F. Makhaeva, E. V. Rudakova, N. V. Kovaleva, N. P. Boltneva, O. G. Serebryakova, S. S. Borisevich, N. P. Evstigneeva, N. V. Zilberberg, N. V. Kungurov and V. I. Saloutin, J. Fluorine Chem., 2022, 254, 109935 CrossRef CAS.
- Q. Chai, Y. Zhang and C. H. Liu, Front. Cell. Infect. Microbiol., 2018, 8, 158 CrossRef PubMed.
- M. A. De Groote and G. Huitt, Clin. Infect. Dis., 2006, 42, 1756–1763 CrossRef PubMed.
- M. W. Bratschi, P. Steinmann, A. Wickenden and T. P. Gillis, Lepr. Rev., 2015, 86, 142–155 CrossRef PubMed.
- V. N. Dahl, M. Mølhave, A. Fløe, J. van Ingen, T. Schön, T. Lillebaek, A. B. Andersen and C. Wejse, Int. J. Infect. Dis., 2022, 125, 120–131 CrossRef PubMed.
- N. Scheinfeld, Dermatol. Online J., 2016, 22(6), 1–9 Search PubMed.
- J. Mao, H. Yuan, Y. Wang, B. Wan, M. Pieroni, Q. Huang, R. B. van Breemen, A. P. Kozikowski and S. G. Franzblau, J. Med. Chem., 2009, 52, 6966–6978 CrossRef CAS PubMed.
- A. Lilienkampf, J. Mao, B. Wan, Y. Wang, S. G. Franzblau and A. P. Kozikowski, J. Med. Chem., 2009, 52, 2109–2118 CrossRef CAS PubMed.
- P. P. Jain, M. S. Degani, A. Raju, A. Anantram, M. Seervi, S. Sathaye, M. Ray and M. G. R. Rajan, Bioorg. Med. Chem. Lett., 2016, 26, 645–649 CrossRef CAS PubMed.
- R. S. Keri and S. A. Patil, Biomed. Pharmacother., 2014, 68, 1161–1175 CrossRef CAS PubMed.
- N. Pappula and R. Sharabu, J. Pharm. Res. Int., 2021, 69–79 CrossRef.
- M. Girardini, F. Ferlenghi, G. Annunziato, G. Degiacomi, B. Papotti, C. Marchi, J. C. Sammartino, S. S. Rasheed, A. Contini, M. R. Pasca, F. Vacondio, J. C. Evans, T. Dick, R. Müller, G. Costantino and M. Pieroni, Eur. J. Med. Chem., 2023, 245, 114916 CrossRef CAS PubMed.
- S. K. Sahoo, B. Rani, N. B. Gaikwad, M. N. Ahmad, G. Kaul, M. Shukla, S. Nanduri, A. Dasgupta, S. Chopra and V. M. Yaddanapudi, Eur. J. Med. Chem., 2021, 222, 113580 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |