Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective dual-mode detection of reactive oxygen species and metal ions by chemodosimetric vs. chelation pathways: fluorescence ‘turn-on’ with OCl− and Zn2+/Mn2+, employing theoretical, practical, and bioimaging applications†
Malavika S.
Kumar
a,
Avijit Kumar
Das
 *a,
Yatheesharadhya
Bylappa
b and
Anish
Nag
b
*a,
Yatheesharadhya
Bylappa
b and
Anish
Nag
b
aDepartment of Chemistry, Christ University, Hosur Road, Bangalore, Karnataka 560029, India. E-mail: avijitkumar.das@christuniversity.in
bDepartment of Life Science, Christ University, Hosur Road, Bangalore, Karnataka 560029, India
First published on 28th February 2025
Abstract
An indole-coupled diaminomaleonitrile-based fluorescent chemosensor IMA has been designed and developed for the selective detection of ROS (OCl−) and metal ions Zn2+ and Mn2+ via chemodosimetric and chelation pathways respectively. The selective sensing of OCl− is induced by a method of oxidatively cleaving of the imine bond of IMA, forming free indole aldehyde, which results in a 21-fold enhancement of fluorescence at 521 nm, with a detection limit of 2.8 µM. On the other hand, the selective binding of IMA with Zn2+ and Mn2+ results in chelation-induced enhanced fluorescence (CHEF) and increased intermolecular charge transfer (ICT), leading to a 4-fold and 3-fold fluorescence enhancement at 432 nm and 435 nm, with the detection limits of 12.71 µM and 17.34 µM, respectively. UV-vis spectroscopy, fluorescence, DFT study, mass spectra, 1H-NMR analysis, and Job's plot analysis have been used to validate the sensing mechanism of IMA with OCl−, Zn2+, and Mn2+. For practical applications, the binding of IMA with OCl− has been utilized in the detection of commercial samples like bleaching powder and water analysis. Bio-imaging studies were conducted with IMA in the presence of OCl− and Zn2+ using green gram seeds in a physiological medium.
1. Introduction
The development of chemosensors for the detection of environmentally and physiologically active analytes has been crucial in recent years.1 Chemosensors offer highly selective and sensitive detection of metal ions and anions with great specificity at a very low cost. Many chemosensors have been designed based on specific host–guest interactions, such as hydrogen bonding, electrostatic forces, metal–ligand binding, hydrophobic interactions, and van der Waals forces, enabling the selective qualitative detection of various target molecules. For simplicity, affordability, and ease of use, the majority of these chemosensors have been employed in solution through spectroscopic evaluation.2,3 Thus, considering the fact that reactive oxygen species (ROS) are involved in many physiological and pathological processes, there is growing concern about their detection.4–6 Significant ROS, such as OCl−, have antibacterial and anti-inflammatory characteristics and play a crucial role in the human immune system.7–9 The fact that OCl− is widely utilized in industrial domains, such as a bleaching and disinfection agent, makes its measurement all the more crucial in environmental systems.10,11 Numerous illnesses, including inflammatory and cardiovascular diseases, are caused by abnormally high levels of OCl− in living organisms.12–16 Therefore, the development of practical and selective sensors to measure OCl− in living systems is indispensable.17,18 Numerous techniques, including colorimetric, luminescent, electrochemical, and chromatographic techniques, have been effectively developed in response to this constant demand.19 Strong oxidizing agents like OCl− have been extensively used in the development of extremely efficient fluorescent chemosensors in recent years.20–22 Notable reports of fluorescent probes whose spectral responses have been triggered by selective and sensitive OCl−-mediated oxidation involve the use of organic functionalities such as hydrazone,23 oxime,24,25 thiol,26,27 boronate ester,28 sulfonhydrazone,29 selenide,30 secondary amine, and primary amine.31–34On the other hand, a substantial aspect of many biological, chemical, and environmental processes involves metal ions.35,36 Various metal ions, such as zinc, copper, iron, and manganese, exhibit paradoxical behavior since they can have detrimental impacts on human health whether present in excess or inadequate amounts. Manganese is one of the metal ions that is vital for many biological processes. It is necessary for the normal development of the brain and skeleton in the perinatal and neonatal stages of human life,37 and it functions as a cofactor in many vital enzymes, including glutamine synthetase and superoxide dismutase. However, unchecked manganese exposure to the human body can cause manganism, a disorder that causes psychosis, obsessive behaviors, depression, and mood swings.38 In addition to being an important nutrient, zinc is the second most prevalent transition metal ion in mammals, after iron.39 It is involved in numerous fundamental biological processes, including gene transcription, apoptosis, natural signal transmission or modulation, and serving as structural and catalytic cofactors. While zinc(II) ions are generally non-toxic, excessive consumption suppresses the absorption of copper and iron, leading to deficiencies in both minerals.40,41 Conversely, deficiencies in zinc ions affect the kidney, liver, and brain. Since these metal ions are crucial to human health, it is necessary to address both qualitative and quantitative metal sensing.42,43
Thus, we have created a multifunctional chemosensor [2-(((Z)-(1H-indol-3-yl)methylene)amino)-3-diaminomaleonitrile] (IMA) for selective detection of anion OCl− and cations Zn2+ and Mn2+ by dual pathways via chemodosimetric and chelation respectively. The ligand IMA has been synthesized by one step Schiff base condensation reaction between indole-3-carbaldehyde and 2,3-diaminomaleonitrile in ethanol in presence of catalytic amount of glacial acetic acid with 62% yields. The chemical structure of IMA has been confirmed by NMR and mass spectroscopic technique (Fig. S8–S9, ESI†).
2. Experimental
2.1 Materials and instrumentation
2.2 Synthesis and characterization of IMA
To a solution of indole-3-carbaldehyde (300 mg, 2.06 mmol) in ethanol, 2,3-diaminomaleonitrile (222 mg, 2.05 mmol) was added followed by the addition of catalytic amount of glacial acetic acid. The resultant mixture was heated in oil bath for almost 3 min until all the reactants were dissolved and then the reaction mixture was left on stirring at room temperature for 48 h. A precipitation was separated out, filtered, washed with cold ethanol, and dried under vacuum to get a greenish yellow precipitate of IMA in pure form (Scheme 1).44Yield: 300 mg, 62%. Mp: 230–235 °C. 1H-NMR (DMSO-d6, 400 MHz): 11.93 (s, 1H, –NH), 8.51 (s, 1H), 8.45 (d, 1H, J = 7.6 Hz), 8.15 (s, 1H), 7.46 (d, 1H, J = 8 Hz), 7.20 (m, 4H). Mass (m/z, %): M+ calculated for C13H9N5 is 235.086; found: 236.100 (M + H)+. Elemental analysis: calculated: C, 66.37; H, 3.86; N, 29.77 found: C, 66.35; H, 3.87; N, 29.78.
3. Results and discussion
3.1 Binding study of probe IMA with OCl−, Zn2+ and Mn2+
The binding study of IMA towards OCl−, Zn2+ and Mn2+ were performed by UV-vis and fluorescence experiments in CH3CN/HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH 7.4). The ligand IMA itself exhibited two intense absorption bands at 263 nm and 378 nm respectively. However, increasing concentration of OCl−, the absorption bands of IMA at 263 nm and 378 nm were gradually diminished. Similarly, with incremental concentration of Zn2+ and Mn2+ to the ligand solution, the absorption signals at 263 nm and 378 nm were gradually decreased (Fig. 1).
3, v/v, pH 7.4). The ligand IMA itself exhibited two intense absorption bands at 263 nm and 378 nm respectively. However, increasing concentration of OCl−, the absorption bands of IMA at 263 nm and 378 nm were gradually diminished. Similarly, with incremental concentration of Zn2+ and Mn2+ to the ligand solution, the absorption signals at 263 nm and 378 nm were gradually decreased (Fig. 1).
Fluorescence studies of IMA towards different analytes were conducted in CH3CN/HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
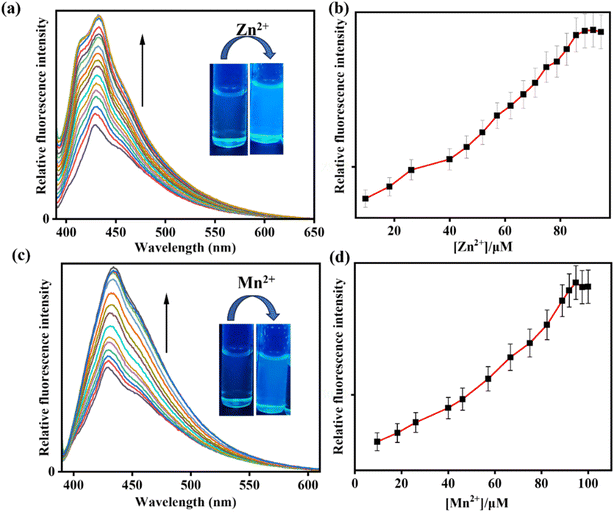
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH 7.4) (λex = 378 nm). Initially, IMA itself exhibited very weak fluorescence with an emission signal at 428 nm with a low quantum yield (Φ = 0.016). But, gradual addition of OCl− to IMA solution led to 21-fold increase in fluorescence intensity with a red shifted emission peak at 521 nm (Δλ = 93) ensuring a fluorescence change from colourless to greenish (Φ = 0.91) (Fig. 2).
3, v/v, pH 7.4) (λex = 378 nm). Initially, IMA itself exhibited very weak fluorescence with an emission signal at 428 nm with a low quantum yield (Φ = 0.016). But, gradual addition of OCl− to IMA solution led to 21-fold increase in fluorescence intensity with a red shifted emission peak at 521 nm (Δλ = 93) ensuring a fluorescence change from colourless to greenish (Φ = 0.91) (Fig. 2).
However, the selectivity of IMA towards Zn2+ and Mn2+ was studied by the fluorescence titration in CH3CN/HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH 7.4). The chemosensor IMA initially showed a weak fluorescence at 428 nm, but addition of Zn2+ and Mn2+ to receptor solution led to a bathochromic enhancement of emission signals at 432 nm (Δλ = 4) and 435 nm (Δλ = 7) by 4-fold and 3-fold respectively exhibiting an effective turn-on blue emission (Fig. 3). The quantum yield for IMA on binding with Zn2+ and Mn2+ is found to be 0.06 and 0.03 respectively.
3, v/v, pH 7.4). The chemosensor IMA initially showed a weak fluorescence at 428 nm, but addition of Zn2+ and Mn2+ to receptor solution led to a bathochromic enhancement of emission signals at 432 nm (Δλ = 4) and 435 nm (Δλ = 7) by 4-fold and 3-fold respectively exhibiting an effective turn-on blue emission (Fig. 3). The quantum yield for IMA on binding with Zn2+ and Mn2+ is found to be 0.06 and 0.03 respectively.
The detection limits of IMA for OCl−, Zn2+, and Mn2+ have been determined as 2.8 µM, 12.71 µM, and 17.34 µM, respectively by using the equation DL = K × Sb1/S, where K = 3, S is the slope and Sb1 is the standard deviation of the blank solution (Fig. S2–S4, ESI†).45 The fast response of IMA towards OCl− was determined by calculating the rate constant as 3.237 s−1 at 521 nm (Fig. S7†). Job's plot analysis established the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry of IMA with Zn2+ and Mn2+ (Fig. S5 and S6, ESI†) with the notable association constants (Ka) values of 3.3 × 102 M−1 and 2.85 × 103 M−1 respectively (Fig. S1, ESI†).46
1 binding stoichiometry of IMA with Zn2+ and Mn2+ (Fig. S5 and S6, ESI†) with the notable association constants (Ka) values of 3.3 × 102 M−1 and 2.85 × 103 M−1 respectively (Fig. S1, ESI†).46
3.2 Interference study
The interference studies of IMA for various interfering anions (such as Cl−, CH3COO−, Br−, F−, NO2−, C2O4−, SO42−, H2O2, NO3−) and cations (such as Al3+, Cd2+, Fe3+, Fe2+, Hg2+, Mn2+, Cu2+, Ni2+, Pb2+, and Zn2+) were carried out in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer (7
HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
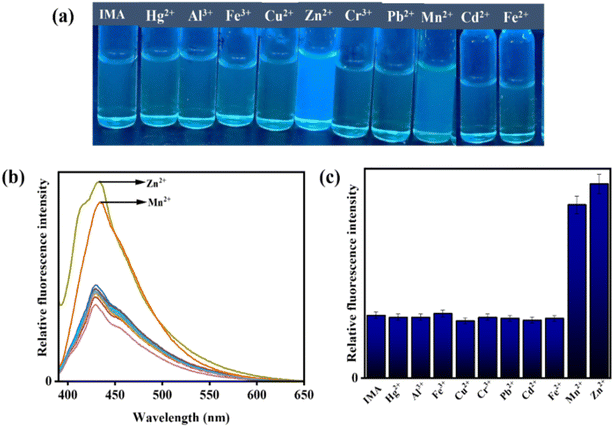
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, pH 7.4). Significantly, the selectivity of IMA towards OCl− was demonstrated by the appearance of emission peak at 521 nm but no significant fluorescence changes were observed upon addition of other interfering anions, which was shown also in bar diagram (Fig. 4).
3 v/v, pH 7.4). Significantly, the selectivity of IMA towards OCl− was demonstrated by the appearance of emission peak at 521 nm but no significant fluorescence changes were observed upon addition of other interfering anions, which was shown also in bar diagram (Fig. 4).
Nevertheless, the addition of Zn2+ and Mn2+ to IMA solution resulted in a significant emission enhancement. In contrast, IMA did not exhibit any fluorescence response when other interfering metal ions were added, indicating high selectivity and sensibility of IMA for Zn2+ and Mn2+ (Fig. 5). Thus, the comparison of emission spectra (Fig. 4b and 5b) and bar diagrams (Fig. 4c and 5c) demonstrates the selectivity of IMA towards OCl−/Zn2+/Mn2+. In the bar diagram representations, the highest intensities, identified by maroon and blue bars, indicate the selectivity and sensitivity of IMA towards OCl− and Zn2+ as well as Mn2+, respectively.
3.3 Binding pathway of IMA with OCl−, Zn2+ and Mn2+ in solution phase
The probable binding mode of IMA with OCl− and Zn2+, as well as Mn2+ in the solution phase, has been explained through the chemodosimetric and chelation pathways, respectively (Scheme 2), which result in changes in the absorption and emission wavelengths before and after the addition of the corresponding analytes (Table S2†).The initial absorbance IMA was high due to the delocalization of electrons across the molecule, which allow the molecule to absorb light in the visible or UV spectrum, leading to higher absorbance at 263 nm and 378 nm. But, the oxidative cleavage by gradual addition of OCl− results the loss of conjugation reducing the molecule's ability to absorb photons at certain energies, leading to a decrease in absorbance at those wavelengths (Fig. 1a).47 Similarly, the binding of Zn2+ and Mn2+ with IMA leads to a decrease in absorbance due to variations in the electronic structure of the imine–metal complex causing a shift or fall in the absorbance band. This change in the electronic environment can disrupt the conjugation and lower the intensity of electronic transitions, leading to reduced absorbance of IMA (Fig. 1c and e).47 On contrary, IMA exhibits weak fluorescence at 428 nm due to the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond that leads to non-radiative deactivation processes because the imine group introduces low-energy vibrational modes that facilitate the dissipation of energy.48 Moreover, the nitrogen lone pair of the imine moiety of IMA can interact with the excited state and facilitate non-radiative transitions, which results the appearance of very weak fluorescence of IMA.49 But, the selective detection of OCl− by IMA has been demonstrated by the oxidative breakage of imine bond by OCl−, leading to the formation of the corresponding indole-3-aldehyde (ICA), which may subsequently lose the diaminomaleonitrile unit. Significantly, this reaction is more enhanced through the resonance conjugation by the electron donation from indole-NH, facilitating nucleophilic attack by H2O and resulting in the removal of 1,2-diaminomaleonitrile, thereby forming indole-3-carbaldehyde (ICA). The proposed mechanism of interaction between IMA and OCl− is depicted in Scheme 3. IMA itself showed very weak fluorescence with an emission band at 428 nm with a low quantum yield. But the addition of OCl− to IMA solution results fluorescence enhancement at 521 nm corresponds to the formation of indole carbaldehyde by hypochlorite mediated oxidative cleavage of imine “C
N bond that leads to non-radiative deactivation processes because the imine group introduces low-energy vibrational modes that facilitate the dissipation of energy.48 Moreover, the nitrogen lone pair of the imine moiety of IMA can interact with the excited state and facilitate non-radiative transitions, which results the appearance of very weak fluorescence of IMA.49 But, the selective detection of OCl− by IMA has been demonstrated by the oxidative breakage of imine bond by OCl−, leading to the formation of the corresponding indole-3-aldehyde (ICA), which may subsequently lose the diaminomaleonitrile unit. Significantly, this reaction is more enhanced through the resonance conjugation by the electron donation from indole-NH, facilitating nucleophilic attack by H2O and resulting in the removal of 1,2-diaminomaleonitrile, thereby forming indole-3-carbaldehyde (ICA). The proposed mechanism of interaction between IMA and OCl− is depicted in Scheme 3. IMA itself showed very weak fluorescence with an emission band at 428 nm with a low quantum yield. But the addition of OCl− to IMA solution results fluorescence enhancement at 521 nm corresponds to the formation of indole carbaldehyde by hypochlorite mediated oxidative cleavage of imine “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N” bond producing a greenish fluorescence (Scheme 2). Furthermore, the oxidative cleavage of imine bond of IMA and the formation of indole-3-carbaldehyde has been proved by the presence of a mass peak at m/z = 145.800 (Fig. S10, ESI†). Upon examining the 1H-NMR spectra of the crude product formed by the reaction of OCl− with IMA, it was observed that the singlet peak corresponding to the –NH group of indole at δ 11.71 ppm shifted slightly downfield to δ 12.05 ppm due to the formation of free indole-3-aldehyde. Simultaneously, a new peak appeared at δ 9.93 ppm, corresponding to the proton signal of the aldehyde group in the indole moiety. Simultaneously, other aromatic proton signals shifted to downfield with higher δ values due to OCl− induced oxidative cleavage of IMA (Fig. S13, ESI†).
N” bond producing a greenish fluorescence (Scheme 2). Furthermore, the oxidative cleavage of imine bond of IMA and the formation of indole-3-carbaldehyde has been proved by the presence of a mass peak at m/z = 145.800 (Fig. S10, ESI†). Upon examining the 1H-NMR spectra of the crude product formed by the reaction of OCl− with IMA, it was observed that the singlet peak corresponding to the –NH group of indole at δ 11.71 ppm shifted slightly downfield to δ 12.05 ppm due to the formation of free indole-3-aldehyde. Simultaneously, a new peak appeared at δ 9.93 ppm, corresponding to the proton signal of the aldehyde group in the indole moiety. Simultaneously, other aromatic proton signals shifted to downfield with higher δ values due to OCl− induced oxidative cleavage of IMA (Fig. S13, ESI†).
On the other hand, the co-ordination with Zn2+ and Mn2+ with imine and amine nitrogen of IMA, leads to the production of CHEF (chelation-induced enhanced fluorescence) and enhanced ICT (intermolecular charge transfer), which results the enhancement of fluorescence showing a blue emission. Additionally, the formation of the Mn+–IMA complex caused a fluorescence enhancement via the chelation-enhanced fluorescence (CHEF) mechanism. This enhancement is attributed to increased conjugation between the indole-NH and the cyanide group of diaminomaleonitrile upon metal binding, which promotes ICT process across the π-systems, resulting in enhancement of fluorescence.50 To indicate donor and acceptor parts of IMA, which is responsible for ICT within the molecule, Molecular electrostatic potential (MEP) and natural bond orbital (NBO) analysis have been carried out. MEP surface is a significant technique to explain the physicochemical characteristics and molecular structure.51 The MEP analysis provides insight into the electrophilic (negative) and nucleophilic (positive) interactions of the IMA. It reveals critical information about the molecule's size, shape, charge density, and electronegativity. The MEP surface employs various colors to indicate regions of differing electrostatic potential: red for negative, blue for positive, and green for neutral. In the IMA molecule, the electrostatic potential ranges from −5.975 × 102 a.u. to +5.975 × 102 a.u., with a corresponding color gradient transitioning from red to blue.52 The changes in electrostatic potential on the molecular surface align with color variations, following the order red < orange < yellow < green < blue53 (Fig. S16†). On the other hand, NBO method offers an effective framework for examining electron density distribution and electron delocalization within the molecular orbital system of IMA.54 NBO analysis serves as a valuable tool for understanding inter- and intramolecular interactions by analyzing donor–acceptor orbital exchanges within the molecule IMA, based on second-order perturbation theory.55 The calculated donor–acceptor interactions and the key stabilization energies of IMA are presented in Table S3.† Therefore, upon complexation with Zn2+ and Mn2+ result the enhancement of CHEF and ICT exhibiting a strong ‘switch on’ of blue fluorescence (Fig. 3). The complex formation of IMA with Zn2+ and Mn2+ has been demonstrated through the appearance of a peak at m/z = 374.900 and 365.700 attributed to [Zn(IMA)(Cl)(CH3CN)] and [Mn(IMA)(Cl)(CH3CN)] respectively (Fig. S11 and S12, ESI†), which has also been verified by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometric ratio by Job's plot and DFT study.
1 binding stoichiometric ratio by Job's plot and DFT study.
3.4 Practical applications
3.5 Biological study
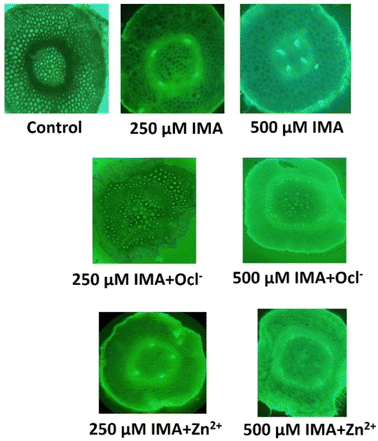
For the biological application, we have utilized the ligand IMA to detect metal ion Zn2+ and OCl− in living plant tissue like green gram (Vigna radiata) seedlings. Green gram is recognized as a cost-effective, user-friendly, and readily available plant model for the preliminary assessment of OCl−/Zn2+ binding compounds. The seeds were sourced from a local market, thoroughly washed with distilled water, and prepared for sprouting. Once the root radicals grew to approximately 3 cm, the sprouted seeds (about 10 per sample) were exposed to different experimental conditions for 3 hours. After treatment, the roots were sectioned transversely, subjected to the same conditions for an additional 10 minutes, and then observed using an epi-fluorescent microscope (LEICA DMi8 inverted microscope) equipped with a 490–570 nm green illumination filter at low (10×) magnification. The transverse root sections were further analyzed under fluorescence microscopy. The results indicated that both the control and individual IMA samples showed no significant fluorescence. However, the combination of IMA and analytes (OCl− and Zn2+) exhibited a marked increase in green fluorescence under microscopic observation (Fig. 8).3.6 Theoretical study
To explain the binding mechanism between IMA with metal ions Zn2+ and Mn2+, we have performed the structure optimization of IMA, IMA–Zn complex and IMA–Mn complex using DFT calculations at the B3LYP level (Fig. 9). The 6-31G(d,p) basis set was used for the simple receptor (IMA), and the LANL2DZ basis set was used for the metal complex, with the calculations performed using the Gaussian 09 program. We have also measured the spatial the electron cloud distribution and the orbital energies of HOMO and LUMO for IMA, IMA–Zn complex and IMA–Mn complex. In the optimized structures of IMA–Zn complex and IMA–Mn complex, metals show square planar coordination using four co-ordinations, consisting of two nitrogens from imine and amine groups of IMA, one nitrogen of acetonitrile solvent and one chlorine as shown in the optimized structures in Fig. 9. | ||
| Fig. 9 Geometry optimized molecular structures of (a) IMA and (b) IMA–Zn complex. (c) IMA–Mn complex. | ||
From the optimized structure of IMA–Zn complex, the calculated distances between Zn and nitrogens of imine and amine groups of IMA are 1.41 Å, 1.94 Å respectively. The bond distances of Zn and nitrogen of acetonitrile and Zn–Cl bond distances are 2.11 Å, 2.27 Å respectively. While the bond angles between imine nitrogen, Zn with amine nitrogen and chlorine are estimated as 84.60° and 117.58° respectively and the bond angles of acetonitrile nitrogen, Zn with amine nitrogen and chlorine are 106.25° and 99.05° respectively. For IMA–Mn complex, the calculated distances between Mn and nitrogens of imine and amine groups of IMA are 1.94 Å, 1.91 Å respectively. The bond distances of Mn and nitrogen of acetonitrile and Zn–Cl bond distances are 1.86 Å, 2.26 Å respectively. While the bond angles between imine nitrogen, Mn with amine nitrogen and chlorine are estimated as 89.25° and 118.26° respectively and the bond angles of acetonitrile nitrogen, Mn with amine nitrogen and chlorine are 110.56° and 105.34° respectively. The energy gap between HOMO (−4.952 eV) and LUMO (−2.204 eV) for IMA is 2.748 eV. The frontier energy gap for IMA–Zn complex is 3.237 eV (HOMO = −5.551 eV, LUMO = −2.313 eV) and for IMA–Mn complex is 2.667 eV (HOMO = −5.605 eV, LUMO = −2.938 eV). Significantly, it is found that the energy levels of HOMO and LUMO for IMA–Zn and IMA–Mn complexes are stabilized as compared to ligand IMA itself, which is responsible for the stabilization of the complex formation (Fig. 10). The ligand IMA itself exhibits absorption maxima at 378 nm experimentally, which is good supported by theoretical absorption band at 381 nm for S0 → S2 transition (E = 3.2548 eV, f = 0.5773). The changes of the absorbance of IMA occurs on binding with Zn2+ and Mn2+ at 378 nm and from the theoretical study, the corresponding estimated absorption band has been calculated at 402 nm S0 → S3 transition (E = 3.0844 eV, f = 0.0106). Thus, the experimental results are in good agreement with the theoretical computations.
 | ||
| Fig. 10 Frontier molecular orbital with energy difference of IMA, IMA–Zn complex and IMA–Mn complex. | ||
4. Conclusion
In conclusion, the indole-coupled diaminomaleonitrile-based fluorescent chemosensor IMA demonstrates significant potential for the selective detection of reactive oxygen species (OCl−) and metal ions (Zn2+ and Mn2+) through distinct sensing mechanisms. The sensor exhibits a strong fluorescence response towards OCl− via oxidative cleavage of imine bond and towards Zn2+ and Mn2+ via CHEF and enhanced ICT pathways, with excellent sensitivity and detection limits. The sensing mechanism of IMA with OCl−, Zn2+, and Mn2+ has been demonstrated by UV-vis spectroscopy, fluorescence, mass spectroscopic techniques, DFT study, and Job's plot analysis. The application of various analytical techniques effectively validates these interactions, and the sensor's practical utility is demonstrated through commercial sample analysis and bio-imaging studies. These findings suggest that IMA could serve as a valuable tool in environmental and biological sensing applications.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors express their gratitude to Christ University, Bangalore, for providing research facilities, and Center for Research, Christ University for the seed money grant (grant approval number CU-ORS-SM-24/09). Avijit Kumar Das specially acknowledges State University Research Excellence (SERB-SURE) of the Science and Engineering Research Board (SERB) (File Number: SUR/2022/002461) under Anusandhan National Research Foundation (ANRF) and Department of Science and Technology (DST), Government of India, for the financial support by the research grant. Malavika S. Kumar extends her appreciation to the University Grants Commission (UGC), Government of India, for the Savitribai Jyotirao Phule Fellowship for Single Child (SJSGC), F. No. 82-7/2022(SA-III).References
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- S.-P. Wu, K.-J. Du and Y.-M. Sung, Dalton Trans., 2010, 39, 4363–4368 RSC.
- V. Vajpayee, Y. H. Song, Y. J. Jung, S. C. Kang, H. Kim, I. S. Kim, M. Wang, T. R. Cook, P. J. Stang and K.-W. Chi, Dalton Trans., 2012, 41, 3046–3052 RSC.
- Y. Jiang, G. Zheng, Q. Duan, L. Yang, J. Zhang, H. Zhang, J. He, H. Sun and D. Ho, Chem. Commun., 2018, 54, 7967–7970 RSC.
- R. Ji, K. Qin, A. Liu, Y. Zhu and Y. Ge, Tetrahedron Lett., 2018, 59, 2372–2375 CrossRef CAS.
- D. Yun, J. B. Chae and C. Kim, J. Chem. Sci., 2019, 131, 38 CrossRef.
- S.-L. Shen, X.-F. Zhang, Y.-Q. Ge, Y. Zhu and X.-Q. Cao, RSC Adv., 2017, 7, 55296–55300 RSC.
- S. Yi, Z. Lu, Y. Lin, J. Wang, Z. Qiao, R. Shen, J. Zhang and L. Hou, Talanta, 2020, 209, 120516 CrossRef CAS PubMed.
- X. Xu and Y. Qian, New J. Chem., 2017, 41, 9607–9612 RSC.
- (a) S. Goswami, S. Paul and A. Manna, Dalton Trans., 2013, 42, 10682–10686 RSC; (b) Y. Feng, S. Li, D. Li, Q. Wang, P. Ning, M. Chen, X. Tian and X. Wang, Sens. Actuators, B, 2018, 254, 282–290 CrossRef CAS.
- S. C. Lee and C. Kim, Inorg. Chem. Commun., 2019, 108, 107545 CrossRef CAS.
- T. Sasikumar and M. Ilanchelian, Anal. Methods, 2017, 9, 3151–3158 RSC.
- R. Duan, C. Li, S. Liu, Z. Liu, Y. Li, J. Zhu and X. Hu, J. Taiwan Inst. Chem. Eng., 2015, 50, 43–48 CrossRef CAS.
- W.-C. Chen, P. Venkatesan and S.-P. Wu, Anal. Chim. Acta, 2015, 882, 68–75 CrossRef CAS PubMed.
- Y.-M. Zhang, H. Fang, W. Zhu, J.-X. He, H. Yao, T.-B. Wei, Q. Lin and W.-J. Qu, Dyes Pigm., 2020, 172, 107765 CrossRef CAS.
- X. Lin, W. Qin, Y. Chen, L. Bao, N. Li, S. Wang, K. Liu, F. Kong and T. Yi, Sens. Actuators, B, 2020, 324, 128732 CrossRef CAS.
- (a) K. Starzak, A. Matwijczuk, B. Creaven, A. Matwijczuk, S. Wybraniec and D. Karcz, Int. J. Mol. Sci., 2019, 20, 281 CrossRef PubMed; (b) Aruna, B. Rani, S. Swami, A. Agarwala, D. Behera and R. Shrivastava, RSC Adv., 2019, 9, 30599–30614 RSC.
- C. Chang, F. Wang, J. Qiang, Z. Zhang, Y. Chen, W. Zhang, Y. Wang and X. Chen, Sens. Actuators, B, 2017, 243, 22–28 CrossRef CAS.
- (a) X. Lou, Y. Zhang, Q. Li, J. Qin and Z. Li, Chem. Commun., 2011, 47, 3189–3191 RSC; (b) S. Vishnu, A. K. Das, S. Sogra, V. Aishwarya, P. S. Chaithra, L. Suchi and S. Abhishek, J. Fluoresc., 2024 DOI:10.1007/s10895-023-03552-1; (c) A. K. Das and S. Goswami, Sens. Actuators, B, 2017, 245, 1062–1125 CrossRef CAS.
- (a) C. Xu, Y. Qian, Z.-Q. Qi, C.-G. Lu and Y.-P. Cui, New J. Chem., 2018, 42, 6910–6917 RSC; (b) M. S. Kumar, S. Vishnu, M. Dolai, A. Nag, Y. Bylappa and A. K. Das, Anal. Methods, 2024, 16, 676–685 RSC; (c) S. Maity, A. C. Maity, A. k. Das and N. Bhattacharyya, Anal. Methods, 2022, 14, 2739–2744 RSC.
- (a) B. Zhu, L. Wu, M. Zhang, Y. Wang, Z. Zhao, Z. Wang, Q. Duan, P. Jia and C. Liu, Sens. Actuators, B, 2018, 263, 103–108 CrossRef CAS; (b) A. K. Das, N. Hayashi, Y. Shiraishi and T. Hirai, RSC Adv., 2017, 7, 30453–30458 RSC; (c) Y. Jiang, S. Wu, C. Jin, B. Wang and J. Shen, Sens. Actuators, B, 2018, 265, 365–370 CrossRef CAS.
- (a) B. Zhu, L. Wu, H. Zhu, Z. Wang, Q. Duan, Z. Fang, P. Jia, Z. Li and C. Liu, Sens. Actuators, B, 2018, 269, 1–7 CrossRef CAS; (b) S. Goswami, S. Maity, A. C. Maity and A. K. Das, Sens. Actuators, B, 2014, 204, 741–745 CrossRef CAS.
- (a) W. L. Wu, Z. M. Zhao, X. Dai, L. Su and B. X. Zhao, Sens. Actuators, B, 2017, 243, 22–28 CrossRef; (b) S. Vishnu, A. K. Das, Y. Bylappa, A. Nag and M. Dolai, Anal. Methods, 2024, 16, 8164–8178 RSC.
- S. Goswami, A. K. Das, A. Manna, A. K. Maity, P. Saha, C. K. Quah, H. K. Fun and H. A. Abdel-Aziz, Anal. Chem., 2014, 86, 6315–6322 CrossRef CAS PubMed.
- L. Zang, C. Liang, Y. Wang, W. Bu, H. Sun and S. Jiang, Sens. Actuators, B, 2015, 211, 164–169 CrossRef CAS.
- B. Guo, H. Nie, W. Yang, Y. Tian, J. Jing and X. Zhang, Sens. Actuators, B, 2016, 236, 459–465 CrossRef CAS.
- G. Wu, F. Zeng and S. Wu, Anal. Methods, 2013, 5, 5589–5596 RSC.
- B. Zhang, X. Yang, R. Zhang, Y. Liu, X. Ren, M. Xian, Y. Ye and Y. Zhao, Anal. Chem., 2017, 89, 10384–10390 CrossRef CAS PubMed.
- C. Wang, H. Ji, M. Li, L. Cai, Z. Wang, Q. Li and Z. Li, Faraday Discuss., 2017, 196, 427–438 RSC.
- S.-R. Liu and S.-P. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS PubMed.
- M. J. Cho, H. Ryu, H. J. Lee and S.-K. Chang, Sens. Actuators, B, 2017, 241, 285–291 CrossRef CAS.
- S. Goswami, A. Manna, S. Paul, C. K. Quah and H.-K. Fun, Chem. Commun., 2013, 49, 11656–11658 RSC.
- Y. Jiang, S. Wu, C. Jin, B. Wang and J. Shen, Sens. Actuators, B, 2018, 265, 365–370 CrossRef CAS.
- B. Zhu, L. Wu, M. Zhang, Y. Wang, C. Liu, Z. Wang, Q. Duan and P. Jia, Biosens. Bioelectron., 2018, 107, 218–223 CrossRef CAS PubMed.
- M. A. Zoroddu, J. Aaseth, G. Crisponi, S. Medici, M. Peana and V. M. Nurchi, J. Inorg. Biochem., 2019, 195, 120–129 CrossRef CAS PubMed.
- J. M. Berg and Y. Shi, Science, 1996, 271, 1081–1085 CrossRef CAS PubMed.
- J. M. McCord and I. Fridovich, J. Biol. Chem., 1969, 244, 6049–6055 CrossRef CAS PubMed.
- J. Crossgrove and W. Zheng, NMR Biomed., 2004, 17, 544–553 CrossRef CAS PubMed.
- B. L. Vallee and K. H. Falchuk, Physiol. Rev., 1993, 73, 79–118 CrossRef CAS PubMed.
- S. Rastegarzadeh and V. Rezaei, Sens. Actuators, B, 2008, 129, 327–331 CrossRef CAS.
- S. Kasana, J. Din and W. Maret, J. Trace Elem. Med. Biol., 2015, 29, 47–62 CrossRef CAS PubMed.
- (a) D. Sareen, P. Kaur and K. Singh, Coord. Chem. Rev., 2014, 265, 125–154 CrossRef CAS; (b) S. Goswami, A. K. Das, K. Aich, A. Manna, S. Maity, K. Khanra and N. Bhattacharyya, Analyst, 2013, 138, 4593–4598 RSC; (c) S. Goswami, A. K. Das, B. Pakhira, S. B. Roy, A. K. Maity, P. Saha and S. Sarkar, Dalton Trans., 2014, 43, 12689–12697 RSC; (d) G. C. Das, A. K. Das, D. Das, T. R. Maity, A. Samanta, F. A. Alasmary, A. S. Almalki, A. Iqbal and M. Dolai, J. Photochem. Photobiol., A, 2023, 440, 114663 CrossRef CAS.
- (a) M. B. Gumpu, S. Sethuraman, U. M. Krishnan and J. B. B. Rayappan, Sens. Actuators, B, 2015, 213, 515–533 CrossRef CAS; (b) S. Goswami, S. Maity, A. C. Maity, A. K. Das, K. Khanra, T. K. Mandal and N. Bhattacharyya, Tetrahedron Lett., 2014, 55, 5993–5997 CrossRef CAS; (c) S. Swami, A. Agarwala, D. Behera and R. Shrivastava, Sens. Actuators, B, 2018, 260, 1012–1017 CrossRef CAS.
- S. Goswami, S. Paul and A. Manna, Dalton Trans., 2013, 42, 10097–10101 RSC.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 8, 1414–1418 CrossRef PubMed.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS; (b) Y. Shiraishi, Y. Kohno and T. Hirai, Ind. Eng. Chem. Res., 2005, 44, 847–851 CrossRef CAS.
- M. E. Belowich and J. Fraser Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence: Principles and Applications, John Wiley & Sons, 2013 Search PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- S. Goswami, K. Aich, S. Das, C. Das Mukhopadhyay, D. Sarkar and T. K. Mondal, J. Chem. Soc., Dalton Trans., 2015, 44, 5763–5770 RSC.
- M. Hagar, H. A. Ahmed, G. Aljohani and O. A. Alhaddad, Int. J. Mol. Sci., 2020, 21, 3922 CrossRef CAS PubMed.
- H. Gokce, F. Sen, Y. Sert, B. F. Abdel-Wahab, B. M. Kariuki and G. A. El-Hiti, Molecules, 2022, 27, 2193–3014 CrossRef CAS PubMed.
- M. T. Gulluoglu, Y. Erdogu, J. Karpagam, N. Sundaraganesan and S. Yurdakal, J. Mol. Struct., 2011, 990, 14–20 CrossRef.
- S. Muthu, J. Uma Maheswari and T. Sundius, Spectrochim. Acta, Part A, 2013, 106, 299–309 CrossRef CAS PubMed.
- (a) J. A. Agwupuye, H. Louis, T. O. Unimuke, P. David, E. I. Ubana and Y. L. Moshood, J. Mol. Liq., 2021, 337, 116458 CrossRef CAS; (b) J. A. Agwupuye, P. A. Neji, H. Louis, J. O. Odey, T. O. Unimuke, E. A. Bisiong and T. N. Ntui, Heliyon, 2021, 7, e07544 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08191a |
| This journal is © The Royal Society of Chemistry 2025 |