Open Access Article
Open Access ArticleA hybrid scaffold of modified human amniotic membrane with gelatine/dendrimer-protected silver nanoparticles for skin wound healing applications
Mahdi Khalili a,
Aryan Ekhlasi
a,
Aryan Ekhlasi a,
Atefeh Solouk
a,
Atefeh Solouk *a,
Masoumeh Haghbin Nazarpak
*a,
Masoumeh Haghbin Nazarpak *b and
Somaye Akbari
*b and
Somaye Akbari c
c
aBiomedical Engineering Department, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran. E-mail: atefeh.solouk@aut.ac.ir
bNew Technologies Research Center (NTRC), Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran. E-mail: haghbin@aut.ac.ir
cDepartment of Textile Engineering, Amirkabir University of Technology (Polytechnic Tehran), Tehran, Iran
First published on 3rd March 2025
Abstract
The human amniotic membrane (hAM) is a biological material widely utilized to mimic the extracellular matrix in damaged skin. Despite its potential, clinical applications of hAM have been hindered by its poor mechanical properties. Furthermore, cryopreservation process used to store hAM could compromise its inherent bactericidal properties. This study explores an innovative approach by combining hAM with 2, 4, 6 and 8% w/v of gelatine (Gel) and incorporating 100, 500 and 1000 μL of poly(propylene imine) (PPI) dendrimer-protected silver nanoparticles (AgNPs) to create antibacterial-bolstered scaffolds using freeze-drying technique. Based on results, hAM/Gel2/S500 scaffold was identified as optimal specimen. It exhibited favorable properties, including an ultimate tensile strength of 16 kPa, an elastic modulus of 26.66 kPa, an elongation at break of 59.60%, an average pore size of 490 μm and a porosity of 52.93%. In vitro degradation indicated that degradation rate of the scaffold was 30% lower on the 1st day and 20% higher on the 21st day compared to commercial ChitoHeal dressing. It also demonstrated higher water absorbance of 100 and 139% at 1 and 48 hours, respectively, compared to ChitoHeal dressing. Additionally, uniform distribution of AgNPs throughout the scaffold and their release from 2.30 μg mL−1 on the 1st day to 10.40 μg mL−1 by the 3rd day, resulted in an elevated inhibition zone against S. aureus and E. coli. Finally, all antibacterial-bolstered scaffolds exhibited 85–89% cell viability after 24 hours and 80–83% after 72 hours. Consequently, hAM/Gel2/S500 scaffold showed promising results for application in wound healing.
1. Introduction
The skin is the largest organ in the human body, with a surface area of approximately 1.5 to 1.8 m2 and a thickness ranging from 0.5 (in the lower eyelid) to 15 mm (in the foot sole) in healthy adult. Its volume could vary from 7500 to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mm3, reflecting its diverse physiological functions, which include mechanistic, energetic, metabolic and immunological roles.1 In fact, skin creates a safety barrier against pathogens and defends the human body from external chemical, mechanical or physical insults. Skin loss could occur for various reasons, such as thermal trauma, genetic disorders, chronic wounds, burns or even surgical interventions.2 Skin grafts are the most common approach in the treatment of chronic wounds.3 However, in the case of deep or large wounds or extensive severe burns, due to low immunogenicity and the limited availability of donor skin, skin grafts often fail to provide complete recovery, rendering them unsuitable for widespread use.4 With the advent of skin tissue engineering, the therapeutic potential for healing chronic wounds has significantly developed, as this advanced strategy aims to fabricate skin substitutes that act as bioactive wound dressings, facilitating wound healing rather than simply covering it.5
000 mm3, reflecting its diverse physiological functions, which include mechanistic, energetic, metabolic and immunological roles.1 In fact, skin creates a safety barrier against pathogens and defends the human body from external chemical, mechanical or physical insults. Skin loss could occur for various reasons, such as thermal trauma, genetic disorders, chronic wounds, burns or even surgical interventions.2 Skin grafts are the most common approach in the treatment of chronic wounds.3 However, in the case of deep or large wounds or extensive severe burns, due to low immunogenicity and the limited availability of donor skin, skin grafts often fail to provide complete recovery, rendering them unsuitable for widespread use.4 With the advent of skin tissue engineering, the therapeutic potential for healing chronic wounds has significantly developed, as this advanced strategy aims to fabricate skin substitutes that act as bioactive wound dressings, facilitating wound healing rather than simply covering it.5
Human amniotic membrane (hAM), the innermost portion of the fetal membranes, is a biological tissue that surrounds the fetus in the mother's womb and has been applied as a biological scaffold in wound care.6,7 It consists of three main layers: an epithelial monolayer that is separated from the stroma layer by a basement membrane.8,9 The basement membrane is composed of various extracellular matrix components, such as different types of collagen, elastin, laminin, fibronectin, vitronectin and glycosaminoglycans. Additionally, it contains natural inhibitors and is rich in biologically active factors, including cytokines and growth factors, which have been shown to be promote wound healing and tissue regeneration.10 Moreover, hAM exhibited a range of beneficial properties, including anti-inflammatory, anti-microbial, anti-fibrotic, anti-scarring, non-immunogenic, non-tumorigenic and bacteriostatic effects, as well as enhanced epithelialization.11 Despite all these excellent properties, hAM has limited clinical use for wounds of varying dimensions due to three main limitations. First, its poor mechanical and handling characteristics hinder effective application.12 Second, it has been observed that neither fresh nor acellularized hAM exhibits potent bactericidal activity against multidrug-resistant (MDR) bacterial strains.13 This highlights the need for alternative treatment strategies to effectively address such infections.14–20 Finally, the limited accessibility of fresh hAM due to its short shelf life has led to the development of various preservation methods, such as cryopreservation in liquid nitrogen,17 which reduces its bactericidal properties after each cycle of cryopreservation.20
The poor mechanical and handling characteristics could be addressed by reinforcement with electrospun nanofibers that enhance its mechanical and handling properties.21 For instance, gelatine (Gel) nanofibers have been successfully applied to construct a composite membrane through interfacial bonding to address the issues of the fragile nature and rapid degradation rate of decellularized hAM.22 Gel is a natural mimic of the extracellular matrix found in human tissues and organs. The properties of Gel that have attracted the attention of most biomaterial researchers include excellent biocompatibility, good biodegradability, cell interactivity, non-immunogenicity, as well as its excellent processability, ready availability and cost-effectiveness.23
The insufficient protection against infections caused by MDR bacteria, along with the reduced bactericidal properties after each cycle of cryopreservation, could be addressed through antibacterial modification. One notable approach to combat bacteria and prevent wound infection is the incorporation of antimicrobial noble metals, with silver being the most popular choice.24 Silver nanoparticles (AgNPs) have been widely used in medical devices due to their high biocidal activity against a broad spectrum of microbes and organisms. Specifically, AgNPs could serve as an effective antibacterial agent targeting Pseudomonas aeruginosa (P. aeruginosa).25 Additionally, AgNPs exhibit lower toxicity to human cells compared to other metallic elements, making them a safer option for therapeutic applications. Their cost-effectiveness further enhances their appeal, allowing for widespread use in various medical and healthcare settings.26 There are various methods to synthesize AgNPs,27 including the dendritic-assisted method.28,29
Among various dendritic materials, those that are amine-terminated, such as poly(amidoamine) (PAMAM) and poly(propylene imine) (PPI) dendrimers, attract significant attention. PPI dendrimers have become one of the most popular polymers in biomedical engineering applications due to their spherical structure and high density of functional end groups. Furthermore, PPI dendrimers exhibit potent antibacterial properties due to their terminal amine groups, without inducing bacterial resistance.20,30–37
In this study, we explored the innovative combination of cryopreserved hAM with Gel and utilized dendrimer-protected AgNPs to develop an antibacterial biological scaffold tailored for the repair of partial and full-thickness wounds. Gel, renowned for its enzymatic biodegradability and biocompatibility in physiological environments, is used to enhance the mechanical and handling properties of the hAM. Furthermore, Gel serves as an ideal matrix due to its denatured collagen composition, which closely resembles the natural components of hAM. To counteract the reduction in antibacterial properties from cryopreserved hAM and to achieve sufficient protection against infections caused by MDR bacteria, the scaffold was fortified with AgNPs synthesized via in situ formation on PPI dendrimers, thereby enhancing its antibacterial efficacy.
2. Materials and methods
2.1. Materials
Commercial hAM was provided by Iranian Tissue Products Co., Iran. Gel, pepsin enzyme (obtained from porcine gastric mucosa), tetrahydrofuran (Mw = 72.11 g mol−1), phosphate-buffered saline (PBS) tablet, HA (Mw = 36.46 g mol−1), glutaraldehyde (GA) and glycine were purchased from Merck, Germany. The second generation of PPI (G2, Mw = 773.28 g mol−1) was purchased from SyMo-Chem Co., Netherlands. Silver nitrate (AgNO3) solution (0.1 M) was obtained from Titrachem Co., Iran. Sodium borohydride (Mw = 37.83 g mol−1) was bought from Alpha Co., India. Ultrapure water was acquired from Dacell, Korea. L929 mouse fibroblast cells were obtained from National Cell Bank, Pasteur Institute, Iran. Staphylococcus aureus (S. aureus, ATCC 29213) and Escherichia coli (E. coli, ATCC 25922) strains were obtained from the Pathobiological Laboratory of Lashgarak, Iran. Commercial ChitoHeal foam dressing, which was used as the control in the test for morphological, mechanical, porosity and pore size, in vitro degradation and equilibrium water absorbance (EWA), was purchased from Chitotech Co., Iran. Commercial AGICOAT silver nanocrystalline dressing, which was used as the positive control in the disc diffusion antibacterial test, purchased from Emad Pharmaceutical Co., Iran. All the chemical substances were used without any refinement.2.2. Synthesize AgNPs using PPI dendrimer-assistance
AgNPs were synthesized using dendrimer-assistance nanoparticle formation, where PPI dendrimers were employed as stabilizers. First, the PPI dendrimers were dissolved in tetrahydrofuran (THF) with a concentration of 1 mg mL−1. Secondly, sodium hydroxide solution (20 mM) as a reducing agent was prepared by using ultrapure water (18 MΩ cm). In the next step, aqueous AgNO3 (0.1 M) was added to the PPI dendrimers in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Simultaneously, all mixtures were stirred vigorously for approximately 1 hour under the ambient atmosphere. Additionally, 15 μL of reducing agent was added to 5 mL of the AgNO3/PPI solution to create AgNPs stabilized by PPI dendrimers.28,38 Finally, the clear orange colour of the AgNO3/PPI solution confirmed the production of AgNPs at a concentration of 100 ppm. In fact, metal ions that exhibit an affinity for amines are effectively adsorbed, primarily driven by mechanisms such as covalent bond formation, electrostatic interactions, complexation reactions, or a combination of these forces within PAMAM and PPI dendrimers.39 As illustrated in Fig. 1, which presents a schematic of AgNPs synthesized using PPI dendrimer-assistance, dendrimers could form interdendrimer complexes that lead to the formation of larger metal nanoclusters. These nanoclusters are protected by the exterior amine groups, which provide stabilizing activity. Moreover, the amine-terminated functional groups in PPI dendrimers also enhance of the reducing agent activity.40
1. Simultaneously, all mixtures were stirred vigorously for approximately 1 hour under the ambient atmosphere. Additionally, 15 μL of reducing agent was added to 5 mL of the AgNO3/PPI solution to create AgNPs stabilized by PPI dendrimers.28,38 Finally, the clear orange colour of the AgNO3/PPI solution confirmed the production of AgNPs at a concentration of 100 ppm. In fact, metal ions that exhibit an affinity for amines are effectively adsorbed, primarily driven by mechanisms such as covalent bond formation, electrostatic interactions, complexation reactions, or a combination of these forces within PAMAM and PPI dendrimers.39 As illustrated in Fig. 1, which presents a schematic of AgNPs synthesized using PPI dendrimer-assistance, dendrimers could form interdendrimer complexes that lead to the formation of larger metal nanoclusters. These nanoclusters are protected by the exterior amine groups, which provide stabilizing activity. Moreover, the amine-terminated functional groups in PPI dendrimers also enhance of the reducing agent activity.40
 | ||
| Fig. 1 Schematic of AgNPs synthesized using PPI dendrimer-assistance, illustrating how PPI and its surface amine groups act as stabilizing agents. | ||
2.3. Preparation of hAM/Gel blend scaffold (mechanical-reinforced scaffolds)
To achieve the desired composition of the hAM and Gel blend sponge, four different formulations were prepared. First, 1.5% w/v fragmentary hAM was dissolved in 0.1 M hydrochloric acid (HCL) and 1 mg mL−1 pepsin enzyme by stirring for 60 hours at room temperature (hAM solution). Second, aqueous solutions of 2, 4, 6 and 8% w/v Gel powder were prepared by stirring for about 30 minutes at 45 °C (Gel solutions). Third, 2 mL of hAM solution was added to 10 mL of each Gel solution to create a total of 12 mL of hAM/Gel constituent solutions (hAM/Gel solution). Next, the solutions were stirred and poured into four sealed vessels, then kept in the refrigerator for 15 minutes to provide a homogeneous gel phase. Afterward, the gels were kept in a freezer at −85 °C for 24 hours. The obtained freeze-gels were then freeze-dried for 24 hours to create spongy scaffolds. GA vapor was used for cross-linking the scaffolds and enhancing their mechanical properties.33,36,41 This procedure was performed in a sealed vacuum desiccator with 2 mL of 25% GA solution for 24 hours at 25 °C. Subsequently, the residual GA in the scaffold structure was neutralized with a 1 M glycine solution for 1–3 hours. Following this, the scaffolds were washed three times with PBS to eliminate any traces of glycine.332.4. Preparation of antibacterial hAM/Gel hybrid scaffold (antibacterial-bolstered scaffolds)
To enhance the bactericidal properties of the mechanical-reinforced scaffolds, various concentrations of PPI/AgNPs solution (100, 500 and 1000 μL) were added to the hAM/Gel solution containing 2% w/v of Gel. After stirring the solutions, the homogenized solutions were freeze-dried as previously described. Table 1 presents the specimen codes, and a schematic representation of the different steps of the work is shown in Fig. 2.| Treatment | Specimen code | hAM content (% w/v) | Gel content (% w/v) | PPI/AgNPs content (μL) |
|---|---|---|---|---|
| Plain scaffold | Plain hAM | 1.5 | — | — |
| Mechanical-reinforced scaffolds | hAM/Gel2 | 1.5 | 2 | — |
| hAM/Gel4 | 1.5 | 4 | — | |
| hAM/Gel6 | 1.5 | 6 | — | |
| hAM/Gel8 | 1.5 | 8 | — | |
| Antibacterial-bolstered scaffolds | hAM/Gel2/S100 | 1.5 | 2 | 100 |
| hAM/Gel2/S500 | 1.5 | 2 | 500 | |
| hAM/Gel2/S1000 | 1.5 | 2 | 1000 |
2.5. Characterization
 | (1) |
 | (2) |
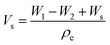 | (3) |
 | (4) |
Furthermore, the morphology of the cross-section and surface structure of the hAM/Gel scaffolds after 7 days of incubation at 37 °C was examined using the same SEM. The cross-sectioned scaffolds were prepared by fracturing them in liquid nitrogen.
 | (5) |
| A = εbc | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) ratio. After another 24 hours, the medium was removed, and the wells were washed with PBS. Subsequently, 0.5 mg mL−1 MTT reagent in PBS was added to each well, and the plate was incubated at 37 °C for 4 hours. After removing the MTT solution, the formazan crystals were dissolved in isopropyl alcohol in each well. Optical absorbance measurements were performed at 570 nm using an ELISA microplate reader (STAT FAX 2100, USA). The cell viability of the scaffolds was normalized to that of the control specimen (the plate floor) and calculated using eqn (7).49
10 (v/v) ratio. After another 24 hours, the medium was removed, and the wells were washed with PBS. Subsequently, 0.5 mg mL−1 MTT reagent in PBS was added to each well, and the plate was incubated at 37 °C for 4 hours. After removing the MTT solution, the formazan crystals were dissolved in isopropyl alcohol in each well. Optical absorbance measurements were performed at 570 nm using an ELISA microplate reader (STAT FAX 2100, USA). The cell viability of the scaffolds was normalized to that of the control specimen (the plate floor) and calculated using eqn (7).49
 | (7) |
(II) Cell morphology study: the sterilized hAM/Gel2/S500 specimen was placed in 12-well plates. Subsequently, the cells were seeded onto the specimen at a density of 1 × 104 cells per well and cultured in a complete medium composed of RPMI-1640 culture medium supplemented with 1% antibiotic penicillin/streptomycin and 10% v/v FBS in a humidified incubator at 37 °C and 5% CO2. The culture medium was exchanged every two days. After 24 hours of cell seeding, the cell-containing specimen was washed twice with PBS and then fixed using a 2.5% GA solution. The fixed cells were dehydrated in graded concentrations of ethanol (50, 60, 70, 80, 90 and 100% v/v). Cell attachment and morphology on the surface of the scaffold were studied by the same SEM.
2.6. Statistical analysis
Using the SPSS software, all results were reported as means ± standard deviations (SD), and a p-value of ≤0.05 was considered statistically significant. Moreover, a one-way analysis of variance (ANOVA) was performed to evaluate the differences between groups.3. Results and discussion
3.1. Characterization of AgNPs
The data characterization of the synthesized AgNPs is presented in Fig. 3. The FE-SEM image (Fig. 3(a)) illustrates a uniform spherical morphology and consistent size of the AgNPs. The UV-vis spectrophotometry analysis (Fig. 3(b)) reveals a distinct absorbance peak between 385 and 425 nm, which is a defining feature of AgNPs.28,40,50 This peak is indicative of the surface plasmon resonance (SPR) phenomenon, which is significantly influenced by the size of the nanoparticles. In fact, the position of the SPR could be finely tuned by adjusting the size of AgNPs, typically ranging from 60 to 100 nm. Furthermore, by varying the solution concentration during the growth phase, a red shift in the SPR wavelength has been observed, ranging from 395 to 450 nm.51 The presence of a single dipole SPR peak in the extinction spectra validate the spherical morphology of the synthesized nanoparticles, indicating a symmetric shape of the resulting nanoparticles.52,53 Finally, the DLS results (Fig. 3(c)) indicate an average particle size of 69 nm. This measurement aligns well with the expectations derived from UV-vis spectrophotometry, which shows an absorbance peak at 400 nm for the AgNPs.3.2. Characterization of the scaffolds
| Specimen | Ultimate tensile strength (kPa) | Elastic modulus (kPa) | Elongation at break (%) |
|---|---|---|---|
| hAM/Gel2 | 16 ± 2.10 | 26.66 ± 0.94 | 59.60 ± 1.40 |
| hAM/Gel4 | 23 ± 2.20 | 56.66 ± 1.25 | 44.78 ± 1.30 |
| hAM/Gel6 | 25 ± 2.40 | 73.33 ± 1.25 | 46.22 ± 1.50 |
| hAM/Gel8 | 34 ± 2.20 | 90.00 ± 1.43 | 42.39 ± 1.80 |
| ChitoHeal | 360 ± 10.0 | 1260.00 ± 22.34 | 33.96 ± 1.40 |
| Specimen | Average pore size (μm) | Porosity (%) |
|---|---|---|
| hAM/Gel2 | 490 ± 11 | 52.93 ± 4.13 |
| hAM/Gel4 | 420 ± 20 | 52.50 ± 4.14 |
| hAM/Gel6 | 210 ± 34 | 53.04 ± 2.41 |
| hAM/Gel8 | 110 ± 10 | 56.17 ± 2.42 |
| ChitoHeal | 410 ± 21 | 31.54 ± 1.86 |
Fig. 6 illustrates the cross-section and surface structure of the degradation morphology in hAM/Gel scaffolds following incubation for 7 days. As shown in Fig. 6(a)–(d), which shows the cross-section of the hAM/Gel scaffolds, the hAM/Gel2 scaffold retains bulk porosity during degradation. In contrast, scaffolds with higher Gel content (4, 6 and 8%) exhibit a reduction in bulk porosity. In Fig. 6(e)–(h), which shows the surface, it can be observed that, similar to bulk porosity, a higher Gel content (4, 6 and 8%) in the scaffold results in a reduction in surface porosity. The bulk and surface porosity are crucial, as these pores facilitate air supply during the wound healing process. Therefore, it is essential for the scaffolds to maintain porosity throughout degradation. The degradation is also crucial because it may control the release of AgNPs. As a result, the hAM/Gel scaffold containing 2% w/v Gel, which exhibits the highest bulk and surface porosity during degradation compared to the other hAM/Gel scaffolds, could be more efficient in facilitating air supply during the wound healing process while also controlling the release of AgNPs.
Based on the results of the morphological analysis, porosity and pore size analysis, in vitro degradation study and EWA study, the specimen containing 2% w/v Gel (hAM/Gel2) was identified as the optimal specimen among the hAM/Gel scaffolds. This designation is attributed to its superior pore size distribution, highest average pore size, greatest bulk and surface porosity during degradation and highest EWA. Consequently, the remaining characterizations were conducted using this specimen, incorporating it with 100 μL (hAM/Gel2/S100), 500 μL (hAM/Gel2/S500) and 1000 μL (hAM/Gel2/S1000) of PPI dendrimer-protected AgNPs.
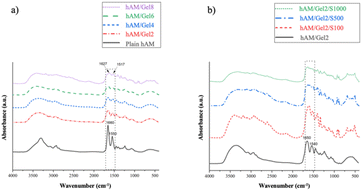
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode. Additionally, the peaks in the range of 1510–1560 cm−1 are attributed to the amide II protein absorption band, associated with C–N stretching and N–H bending modes. The absorption band within the ranges of 1210–1300 and 1070–1080 cm−1 are attributed not only to amide III protein but also to the phosphodiester group of nucleic acids, phospholipids and glycolipids. The absorption band attributed to amide III result from the interaction of N–H in plane-bending and C–N stretching modes, with some contributions from C
O stretching mode. Additionally, the peaks in the range of 1510–1560 cm−1 are attributed to the amide II protein absorption band, associated with C–N stretching and N–H bending modes. The absorption band within the ranges of 1210–1300 and 1070–1080 cm−1 are attributed not only to amide III protein but also to the phosphodiester group of nucleic acids, phospholipids and glycolipids. The absorption band attributed to amide III result from the interaction of N–H in plane-bending and C–N stretching modes, with some contributions from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending vibrations and C–C stretching.64 Proteins spectra are characterized by amide and carboxyl groups. A significant shift in the amide II band from 1550 to 1517 cm−1 correlates with collagen helix denaturation.65 It should be noted that adding Gel (as denatured collagen) to the compound caused this shift. This alteration highlights an enhanced separation of the amide I and amide II bands at 1650 and 1550 cm−1. In addition, the weaker peaks in the ranges of 1600–1640 cm−1, related to C
O bending vibrations and C–C stretching.64 Proteins spectra are characterized by amide and carboxyl groups. A significant shift in the amide II band from 1550 to 1517 cm−1 correlates with collagen helix denaturation.65 It should be noted that adding Gel (as denatured collagen) to the compound caused this shift. This alteration highlights an enhanced separation of the amide I and amide II bands at 1650 and 1550 cm−1. In addition, the weaker peaks in the ranges of 1600–1640 cm−1, related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and 1510–1560 cm−1 related to N–H, indicate that carboxyl and amine groups in hAM have reacted with amine and carboxyl groups in Gel, respectively. Furthermore, the peak at 2960 cm−1 may be attributed to the asymmetric stretching mode of the methyl (CH3) group. Based on Fig. 8(b), it can be observed that adding more bactericidal solution to the hAM/Gel2 scaffold attenuates the peaks assigned to C
O, and 1510–1560 cm−1 related to N–H, indicate that carboxyl and amine groups in hAM have reacted with amine and carboxyl groups in Gel, respectively. Furthermore, the peak at 2960 cm−1 may be attributed to the asymmetric stretching mode of the methyl (CH3) group. Based on Fig. 8(b), it can be observed that adding more bactericidal solution to the hAM/Gel2 scaffold attenuates the peaks assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H. Specifically, the increase in the antibacterial solution in the compound causes an interaction of these amine groups with unreacted carboxyl groups in the compound due to the presence of amine terminal groups in the PPI dendrimers. Therefore, the related peaks were reduced.63
O and N–H. Specifically, the increase in the antibacterial solution in the compound causes an interaction of these amine groups with unreacted carboxyl groups in the compound due to the presence of amine terminal groups in the PPI dendrimers. Therefore, the related peaks were reduced.63
The amount of AgNPs released from the hAM/Gel2/S scaffolds in the wound-simulated area is shown in Table 4. The amounts of free AgNPs varied from 1.02 to 2.67 μg mL−1 on the 1st day, and from 2.23 to 12.19 μg mL−1 on the 3rd day across all hAM/Gel2/S scaffolds. The results of this assay indicate a consistent release of AgNPs upon contact with the body. In fact, high concentrations of AgNPs (greater than 44.0 μg mL−1) could lead to rapid rupture of the cell membrane, resulting in cytotoxic effect.66 However, since small amounts of AgNPs were used in the scaffold formulation, it is anticipated that the side effects of AgNPs in the wound area will be negligible. Therefore, it is expected that all hAM/Gel2/S scaffolds demonstrate an acceptable level of toxicity.
| Specimen | AgNPs amount at 1st day (μg mL−1) | AgNPs amount at 3rd day (μg mL−1) |
|---|---|---|
| hAM/Gel2/S100 | 1.02 ± 0.30 | 2.23 ± 0.35 |
| hAM/Gel2/S500 | 2.30 ± 0.27 | 10.40 ± 0.46 |
| hAM/Gel2/S1000 | 2.67 ± 0.39 | 12.19 ± 0.81 |
| Specimen | Inhibition zone (mm) | |
|---|---|---|
| S. aureus | E. coli | |
| Plain hAM | 8.30 ± 0.42 | 11.18 ± 0.34 |
| hAM/Gel2 | 2.98 ± 0.26 | — |
| hAM/Gel/S100 | 3.16 ± 0.60 | 3.53 ± 0.53 |
| hAM/Gel/S500 | 3.44 ± 0.15 | 4.42 ± 0.36 |
| hAM/Gel/S1000 | 4.22 ± 0.22 | 5.41 ± 0.37 |
| AGICOAT | 1.67 ± 0.18 | 2.67 ± 0.58 |
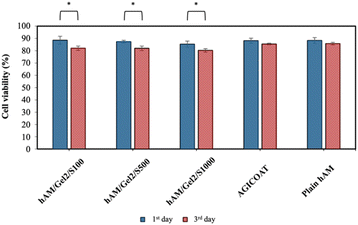 | ||
| Fig. 11 Cell viability of plain hAM, hAM/Gel2/S and AGICOAT scaffolds on 1st day and 3rd day (* for p-value ≤ 0.05). | ||
Based on the results of the antibacterial activity and biological evaluation, the specimen containing 2% w/v Gel and 500 μL of PPI dendrimer-protected AgNPs (hAM/Gel2/S500) was identified as the optimal specimens among the hAM/Gel2/S scaffolds. This designation is attributed to its uniform distribution of AgNPs throughout the scaffold, which demonstrates bactericidal activity against both microorganisms while maintaining the highest cell viability. Furthermore, in the realm of clinical scenarios, this scaffold could be competitive with ChitoHeal commercial dressing because it not only exhibits similar characteristics but also demonstrates superior properties such as enhanced antibacterial activity.
Finally, the fibroblast cell morphology from two different locations of hAM/Gel2/S500, identified as the optimum specimen, is shown in Fig. 12(a) and (b). This scaffold exhibits inherent properties that lead to improved cell adhesion. In fact, fibroblast cells were successfully attached to the surface of the scaffold, indicating a proper interaction between the scaffold and the cells. This finding aligns with the previous studies that demonstrated good adherence and viability of fibroblast cells on decellularized hAM.68 Moreover, it has been noted that collagen accumulation occurs specifically at the front of the cell protrusions after fibroblasts adhesion.69 The fibroblast protrusions depicted in this figure are the primary means by which fibroblast remodel their surrounding fibrous collagen matrices. Hence, hAM/Gel2/S500 scaffold may accelerate fibroblast adhesion compared to wounds without the scaffold, potentially leading to improved wound healing.66
4. Conclusions
In the present study, an effective skin regeneration scaffold was developed through a systematic approach that involved optimizing composition, enhancing antibacterial properties and evaluating scaffold performance. Initially, the optimization of hAM and Gel concentrations was performed. By mixing four different concentrations of Gel (2, 4, 6 and 8% w/v) with an equivalent amount of hAM solution (1.5% w/v), it was determined that the hAM/Gel2 scaffold exhibited uniform pore size, a high average pore size, excellent water absorbance and favourable degradability. Subsequently, the antibacterial properties of the hAM/Gel2 scaffold were enhanced. A potent bactericidal solution of dendrimer-protected AgNPs was prepared at a concentration of 100 ppm. Three different amounts (100, 500 and 1000 μL) of this antibacterial solution were tested to identify the minimum inhibitory concentration necessary for effective bacterial control. Finally, a comprehensive evaluation of the scaffolds was performed through AgNPs release assays, disc diffusion antibacterial tests, cell toxicity assessments and cell attachment studies. The results indicated that the hAM/Gel2/S500 scaffold was the optimal specimen, demonstrating significant potential for controlling infections in wounds while promoting wound healing with minimal toxicity. This study highlights the promising application of the hAM/Gel2/S500 scaffold in regenerative medicine, particularly in wound management, and suggests directions for future research to further enhance its efficacy and applicability in clinical settings.Data availability
All relevant experimental data supporting the findings of this study are included within the manuscript in the form of tables and figures. Additional inquiries regarding the data may be directed to the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The commercial hAM used in this study was prepared by the Iranian Tissue Products Co. (ITC), Iran. We extend our gratitude to Darman Andishan Marham (D&M) Co., Iran, for their support. Special thanks are also due to Dr Mazaher Gholipourmalekabadi for his insightful scientific comments regarding hAM. Finally, we would like to thank Ms. Anna-Katharina Schmid for her assistance in editing this manuscript.Notes and references
- M. C. O. Englund, P. Sartipy and J. Hyllner, Regenerative Medicine, Springer Netherlands, Dordrecht, 2011 Search PubMed.
- E. Taghiabadi, S. Nasri, S. Shafieyan, S. J. Firoozinezhad and N. Aghdami, Cell J., 2015, 16, 476–487 Search PubMed.
- A. Przekora, Cells, 2020, 9, 1–29 CrossRef PubMed.
- S. Dixit, D. R. Baganizi, R. Sahu, E. Dosunmu, A. Chaudhari, K. Vig, S. R. Pillai, S. R. Singh and V. A. Dennis, J. Biol. Eng., 2017, 11, 1–23 CrossRef PubMed.
- Y. M. Bello, A. F. Falabella and W. H. Eaglstein, Am. J. Clin. Dermatol., 2001, 2, 305–313 CrossRef CAS PubMed.
- S. Davis, Johns Hopkins Med. J., 1910, 15, 307–396 Search PubMed.
- J. H. Arrizabalaga and M. U. Nollert, ACS Biomater. Sci. Eng., 2018, 4, 2226–2236 CrossRef CAS PubMed.
- M. Fénelon, S. Catros, C. Meyer, J. C. Fricain, L. Obert, F. Auber, A. Louvrier and F. Gindraux, Membranes, 2021, 11, 1–27 CrossRef PubMed.
- S. Iranpour, N. Mahdavi-Shahri, R. Miri, H. Hasanzadeh, H. R. Bidkhori, H. Naderi-Meshkin, E. Zahabi and M. M. Matin, Cell Tissue Banking, 2018, 19, 357–371 CrossRef CAS PubMed.
- N. Fitriani, G. Wilar, A. C. Narsa, A. F. A. Mohammed and N. Wathoni, Pharmaceutics, 2023, 15, 1–22 CrossRef PubMed.
- G. Castellanos, Á. Bernabé-García, J. M. Moraleda and F. J. Nicolás, Placenta, 2017, 59, 146–153 CrossRef CAS PubMed.
- R. Sarvari, P. Keyhanvar, S. Agbolaghi, L. Roshangar, E. Bahremani, N. Keyhanvar, M. Haghdoost, S. H. Keshel, A. Taghikhani, N. Firouzi, A. Valizadeh, E. Hamedi and M. Nouri, J. Mater. Sci.:Mater. Med., 2022, 33(3), 32 CrossRef CAS PubMed.
- M. Gholipourmalekabadi, M. Bandehpour, M. Mozafari, A. Hashemi, H. Ghanbarian, M. Sameni, M. Salimi, M. Gholami and A. Samadikuchaksaraei, Burns, 2015, 41, 1488–1497 CrossRef CAS PubMed.
- K. Ramakrishnan and V. Jayaraman, Burns, 1997, 23, S33–S36 CrossRef PubMed.
- M. Parthasarathy, R. Sasikala, P. Gunasekaran and J. Raja, J. Acad. Ind. Res., 2014, 2, 545–547 Search PubMed.
- E. Inge, Y. P. Talmi, L. Sigler, Y. Finkelstein and Y. Zohar, Placenta, 1991, 12, 285–288 CrossRef PubMed.
- M. Gholipourmalekabadi, N. P. S. Chauhan, B. Farhadihosseinabad and A. Samadikuchaksaraei, in Perinatal Tissue-Derived Stem Cells, Springer, 2016, pp. 81–105 Search PubMed.
- N. G. Fairbairn, M. A. Randolph and R. W. Redmond, J. Plast. Reconstr. Aesthetic Surg., 2014, 67, 662–675 CrossRef CAS PubMed.
- A. Chopra and B. S. B. Thomas, J. Biomimetics, Biomater., Tissue Eng., 2013, 18, 1–8 Search PubMed.
- F. A. Tehrani, A. Ahmadiani and H. Niknejad, Cryobiology, 2013, 67, 293–298 CrossRef CAS PubMed.
- R. Ramakrishnan, L. K. Krishnan, R. P. Nair and V. Kalliyana Krishnan, Int. J. Polym. Mater. Polym. Biomater., 2020, 69, 810–819 CrossRef CAS.
- L. Chen, X. Song, Z. Yao, C. Zhou, J. Yang, Q. Yang, J. Chen, J. Wu, Z. Sun, L. Gu, Y. Ma, S.-J. Lee, C. Zhang, H.-Q. Mao and L. Sun, Biomaterials, 2023, 300, 122207 CrossRef CAS PubMed.
- S. P. Ndlovu, K. Ngece, S. Alven and B. A. Aderibigbe, Polymers, 2021, 13, 2959 CrossRef CAS PubMed.
- B. S. Atiyeh, M. Costagliola, S. N. Hayek and S. A. Dibo, Burns, 2007, 33(2), 139–148 CrossRef PubMed.
- S. Liao, Y. Zhang, X. Pan, F. Zhu, C. Jiang, Q. Liu, Z. Cheng, G. Dai, G. Wu, L. Wang and L. Chen, Int. J. Nanomed., 2019, 14, 1469–1487 CrossRef CAS PubMed.
- H. Shi, H. Liu, S. Luan, D. Shi, S. Yan, C. Liu, R. K. Y. Li and J. Yin, Compos. Sci. Technol., 2016, 127, 28–35 CrossRef CAS.
- A. Haider and I.-K. Kang, Adv. Mater. Sci. Eng., 2015, 2015, 1–16 CrossRef.
- M. Franckevičius, A. Gustainytė, R. Kondrotas, R. Juškėnas, M. Marcos, J. L. Serrano, R. Vaišnoras and V. Gulbinas, J. Nanopart. Res., 2014, 16, 2343 CrossRef.
- L. Balogh, D. R. Swanson, D. A. Tomalia, G. L. Hagnauer and A. T. McManus, Nano Lett., 2001, 1, 18–21 CrossRef CAS.
- A. P. Dias, S. da Silva Santos, J. V. da Silva, R. Parise-Filho, E. Igne Ferreira, O. El Seoud and J. Giarolla, Int. J. Pharm., 2020, 573, 118814 CrossRef CAS PubMed.
- M. A. Mintzer and M. W. Grinstaff, Chem. Soc. Rev., 2011, 40, 173–190 RSC.
- N. Wrońska, J. P. Majoral, D. Appelhans, M. Bryszewska and K. Lisowska, Molecules, 2019, 24, 2894 CrossRef PubMed.
- P. Rujitanaroj, N. Pimpha and P. Supaphol, Polymer, 2008, 49, 4723–4732 CrossRef CAS.
- S. Khaliliazar, S. Akbari and M. H. Kish, Appl. Surf. Sci., 2016, 363, 593–603 CrossRef CAS.
- H. Klasen, Burns, 2000, 26, 131–138 CrossRef CAS PubMed.
- A. G. Destaye, C.-K. Lin and C.-K. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 4745–4752 CrossRef CAS PubMed.
- M. A. Jebelli, E. Kalantar, A. Maleki, H. Izanloo, F. Gharibi, H. Daraei, B. Hayati, E. Ghasemi and A. Azari, Jundishapur J. Nat. Pharm. Prod., 2015, 10(3), e20621 Search PubMed.
- A. Castonguay and A. K. Kakkar, Adv. Colloid Interface Sci., 2010, 160, 76–87 CrossRef CAS PubMed.
- R. W. J. Scott, O. M. Wilson and R. M. Crooks, J. Phys. Chem. B, 2005, 109, 692–704 CrossRef CAS PubMed.
- X. Sun, S. Dong and E. Wang, Macromolecules, 2004, 37, 7105–7108 CrossRef CAS.
- G. Scott, Degradable Polymers, Springer, Dordrecht, Dordrecht, 2002 Search PubMed.
- Q. Tan, S. Li, J. Ren and C. Chen, Int. J. Mol. Sci., 2011, 12, 890–904 CrossRef CAS PubMed.
- Y. Pooshidani, N. Zoghi, M. Rajabi, M. Haghbin Nazarpak and Z. Hassannejad, J. Mater. Sci.:Mater. Med., 2021, 32(4), 46 CrossRef CAS PubMed.
- M. Pezeshki Modaress, H. Mirzadeh and M. Zandi, Iran. Polym. J., 2012, 21, 191–200 CrossRef CAS.
- F. Öfkeli, D. Demir and N. Bölgen, J. Appl. Polym. Sci., 2021, 138, 50337 CrossRef.
- P. T. Sudheesh Kumar, V.-K. Lakshmanan, T. V. Anilkumar, C. Ramya, P. Reshmi, A. G. Unnikrishnan, S. V. Nair and R. Jayakumar, ACS Appl. Mater. Interfaces, 2012, 4, 2618–2629 CrossRef CAS PubMed.
- P. Threepopnatkul, K. Vichitchote, S. Saewong, T. Tangsupa-Anan, C. Kulsetthanchalee and S. Suttiruengwong, J. Met., Mater. Miner., 2010, 20(3), 185–187 CAS.
- S. Patel, U. Jammalamadaka, L. Sun, K. Tappa and D. Mills, Bioengineering, 2015, 3, 1 CrossRef PubMed.
- S. Bahrami, A. Solouk, H. Mirzadeh and A. M. Seifalian, Composites, Part B, 2019, 168, 421–431 CrossRef CAS.
- M. Alim-Al-Razy, G. M. A. Bayazid, R. U. Rahman, R. Bosu and S. S. Shamma, J. Phys.:Conf. Ser., 2020, 1706(1), 12–20 CrossRef.
- A. Amirjani, N. N. Koochak and D. F. Haghshenas, J. Chem. Educ., 2019, 96, 2584–2589 CrossRef CAS.
- B. Rao and R. C. Tang, Adv. Nat. Sci.:Nanosci. Nanotechnol., 2017, 8, 015014 Search PubMed.
- A. Amirjani, F. Firouzi and D. F. Haghshenas, Plasmonics, 2020, 15, 1077–1082 Search PubMed.
- S. H. Salehi, K. As'adi, S. J. Mousavi and S. Shoar, Indian J. Surg., 2013, 1–5 Search PubMed.
- V. Vivcharenko, M. Wojcik, K. Palka and A. Przekora, Materials, 2021, 14, 1–21 CrossRef PubMed.
- M. Kéri, A. Forgács, V. Papp, I. Bányai, P. Veres, A. Len, Z. Dudás, I. Fábián and J. Kalmár, Acta Biomater., 2020, 105, 131–145 CrossRef PubMed.
- J. Xiao, Y. Ma, W. Wang, K. Zhang, X. Tian, K. Zhao, S. Duan, S. Li and Y. Guo, Food Chem., 2021, 345, 128802 CrossRef CAS PubMed.
- E. Chiellini, P. Cinelli, E. G. Fernandes, E.-R. S. Kenawy and A. Lazzeri, Biomacromolecules, 2001, 2, 806–811 CrossRef CAS PubMed.
- R. Lanza, R. Langer and J. P. Vacanti, Principles of Tissue Engineering, Elsevier, 4th edn, 2014 Search PubMed.
- A. Mirtaghavi, A. Baldwin, N. Tanideh, M. Zarei, R. Muthuraj, Y. Cao, G. Zhao, J. Geng, H. Jin and J. Luo, Int. J. Biol. Macromol., 2020, 164, 1949–1959 CrossRef CAS PubMed.
- S. Chen, Q. Zhang, T. Nakamoto, N. Kawazoe and G. Chen, Tissue Eng., Part C, 2016, 22, 189–198 CrossRef CAS PubMed.
- M. Sun, Y. Wang, L. Yao, Y. Li, Y. Weng and D. Qiu, Polymers, 2022, 14(7), 1400 CrossRef CAS PubMed.
- D. A. Long, J. Raman Spectrosc., 2004, 35, 905 CrossRef.
- S. Cavalu, G. Roiu, O. Pop, D. A. P. Heredea, T. O. Costea and C. F. Costea, Materials, 2021, 14, 1–19 CrossRef PubMed.
- R. Sripriya and R. Kumar, Prog. Biomater., 2016, 5, 161–172 CrossRef CAS PubMed.
- P. Gopinath, S. K. Gogoi, A. Chattopadhyay and S. S. Ghosh, Nanotechnology, 2008, 19, 075104 CrossRef CAS PubMed.
- A. Ekhlasi, A. Solouk, M. Haghbin and P. Pasbakhsh, Appl. Clay Sci., 2023, 243, 107083 CrossRef CAS.
- M. Mahmoudi-Rad, E. Abolhasani, H. Moravvej, N. Mahmoudi-Rad and Y. Mirdamadi, Clin. Exp. Dermatol., 2013, 38, 646–651 CrossRef CAS PubMed.
- D. Shakiba, F. Alisafaei, A. Savadipour, R. A. Rowe, Z. Liu, K. M. Pryse, V. B. Shenoy, E. L. Elson and G. M. Genin, ACS Nano, 2020, 14, 7868–7879 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |