Open Access Article
Open Access ArticleDesign, synthesis, molecular docking and anticancer activity of benzothiazolecarbohydrazide–sulfonate conjugates: insights into ROS-induced DNA damage and tubulin polymerization inhibition†
Najla A. Altwaijry a,
Mohamed A. Omarb,
Hanaa S. Mohamedc,
Marwa M. Mounierd,
Ahmed H. Afifid and
Aladdin M. Srour
a,
Mohamed A. Omarb,
Hanaa S. Mohamedc,
Marwa M. Mounierd,
Ahmed H. Afifid and
Aladdin M. Srour *c
*c
aDepartment of Pharmaceutical Sciences, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
bChemistry of Natural and Microbial Products Department, Pharmaceutical and Drug Industries Research Institute, National Research Centre, Dokki, 12622, Giza, Egypt
cDepartment of Therapeutic Chemistry, Pharmaceutical and Drug Industries Research Institute, National Research Centre, Dokki, 12622, Giza, Egypt. E-mail: aladdinsrour@gmail.com
dDepartment of Pharmacognosy, Pharmaceutical and Drug Industries Research Institute, National Research Centre, Dokki, 12622, Giza, Egypt
First published on 21st February 2025
Abstract
A series of novel benzothiazolecarbohydrazide–sulfonate conjugates 6a–l were designed, synthesized, and then assessed as potential antiproliferative agents in three distinct human cancer cell lines: MCF-7 (breast cancer), HCT-116 (colon cancer), and PC3 (prostate cancer), along with a normal cell line (BJ-1). The reference standard used was 5-fluorouracil. The results obtained reveal that the newly synthesized analogs demonstrate varying degrees of cytotoxicity against the targeted cell lines; however, compounds 6i and 6e exhibited the highest efficacy against MCF-7, HCT-116, and PC3 cells with IC50 values of 78.8 ± 2.6, 81.4 ± 1.9, and 90.6 ± 2.7 μM, respectively, compared to an IC50 of 78.4 ± 4.2 μM for 5-FU in MCF-7 cells, 29.2 ± 1.7 μM in HCT-116 cells and >200 μM in PC3 cells. Moreover, the most potent compounds demonstrated acceptable safety profiles when evaluated aganist BJ-1 cells. Consequently, compound 6i, which possesses no cytotoxicity towards BJ-1 cells and displays promising anticancer activity, was further investigated for its impact on tubulin polymerization compared to control MCF-7 cells, 210.3 and 632.9 pg ml−1, respectively. Compound 6i was found to significantly elevate the ROS levels in treated cancer cells, resulting in an 8.3-fold increase in DNA fragmentation compared to untreated cells. Additionally, it raised the percentage of accumulated cells in the G2 phase from 6.85% to 18.27% in MCF-7 cells. A molecular docking technique was conducted to elucidate the binding energy, binding pose, and binding interactions of compound 6i, revealing a strong fit within the active sites of the tubulin–colchicine binding site (CBS). This study provides valuable insights into the design and synthesis of novel anticancer agents targeting tubulin polymerization.
1. Introduction
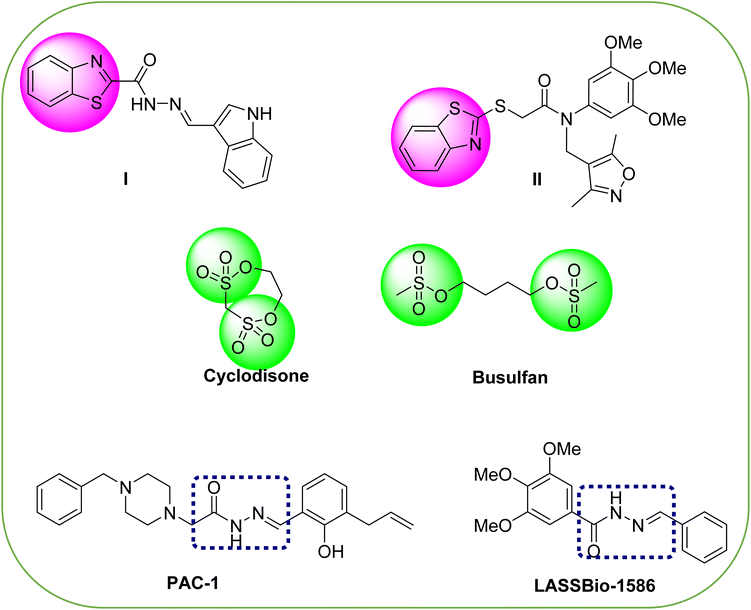
Cancer is regarded as among the most intricate and challenging illnesses that threaten human life, representing a severe disease burden. According to the latest World Cancer Report, an estimated 20 million new cases were recorded in the year 2020, a number projected to rise to about 30 million by 2030. Due to substantial demographic changes, including population aging and growth, the global number of cancer patients is expected to increase over the next five decades.1 Different regions will experience varying trends in cancer incidence, further contributing to this rise. Assuming that current incidence patterns for major cancer types continue, we anticipate that the overall cancer incidence will double by 2070 compared to 2020.2,3 Despite the discovery and approval of various methodologies, techniques, and drugs for cancer treatment, a large number of individuals still endure the burden of this illness each year.4,5 The adverse impact of off-target effects remains a prominent limitation of cancer therapeutics. As a result, current attention in cancer chemotherapy is directed toward creating highly selective anticancer agents that lack off-target effects. This approach aims to enhance the potency and efficacy specifically against cancer cells while minimizing potential side effects.5–8 Tubulin polymerization is a vital process for assembling microtubules and is crucial for preserving cell structure and enabling cell division. In various cancers, certain tubulin isoforms can be overexpressed, which may drive tumorigenesis and accelerate cancer progression.9–11 Certain cancers, including breast12,13 and prostate,14 frequently show overexpression of specific tubulin isoforms, suggesting their significant role in malignancy. Microtubules, essential components of the cytoskeleton with a tubular structure, participate in numerous cellular processes in eukaryotic cells, including processes such as cell growth, transport, communication, movement, replication, and mitotic phase. They are composed of α-tubulin and β-tubulin heterodimers and microtubules are crucial targets for anticancer drugs.15,16 Drugs that act on microtubules interact with three key regions on tubulin: the paclitaxel location, the vinca zone, and the colchicine region. Inhibitors that focus on the paclitaxel or vinca alkaloid binding areas exhibit multidrug resistance issues and dose-limiting toxicity.17–19 Consequently, researchers have been increasingly focusing on developing inhibitors that specifically target the colchicine binding site (CBSIs) in recent years.20,21For decades, medicinal chemists have dedicated their efforts to discovering more potent and safer chemotherapeutic agents.22–24 However, there remains a critical need for anti-cancer medicines that combine both safety and efficacy. Among various approaches, using heterocyclic scaffolds in drug development has proven highly effective in creating cytotoxic agents. Benzothiazolecarbohydrazide-containing scaffolds are widely recognized for their extensive chemotherapeutic potential, especially as anticancer agents (compound I Fig. 1),25–27 and as a well-known tubulin polymerization inhibitor which inhibits cell proliferation (compound II Fig. 1).28–30
 | ||
| Fig. 1 The structures of benzothiazole, sulfonates and N-acyl hydrazone derivatives as anticancer agents. | ||
Compounds featuring a sulfonate group have garnered considerable interest from researchers for their potential anticancer properties, as seen with agents like cyclodisone and busulfan (Fig. 1).31–35 Their unique physicochemical characteristics allow sulfonate-containing structures to effectively interact with lipid membranes, facilitating their passage through cell membranes to target sites.36,37 N-Acyl hydrazone (NAH) motifs are commonly used in the development of heterocyclic scaffolds of pharmaceutical interest.38,39 Recently, several compounds containing NAH, including PAC-1 (ref. 40) and LASSBio-1586 (ref. 41) (Fig. 1), have emerged as promising anticancer agents. The strategic incorporation of NAH as a linker is proposed as an inventive methodology to design new potent and versatile anticancer therapies.
Building on our previous studies,42,43 this research utilizes a structure-based molecular hybridization approach to efficiently create a series of derivatives with a multi-target action mechanism. We have designed and synthesized various NAH derivatives that incorporate benzothiazole and sulfonate components, aiming to create novel compounds with strong anticancer efficacy, while reducing potential side effects (Fig. 2).
2. Results and discussion
2.1. Chemistry
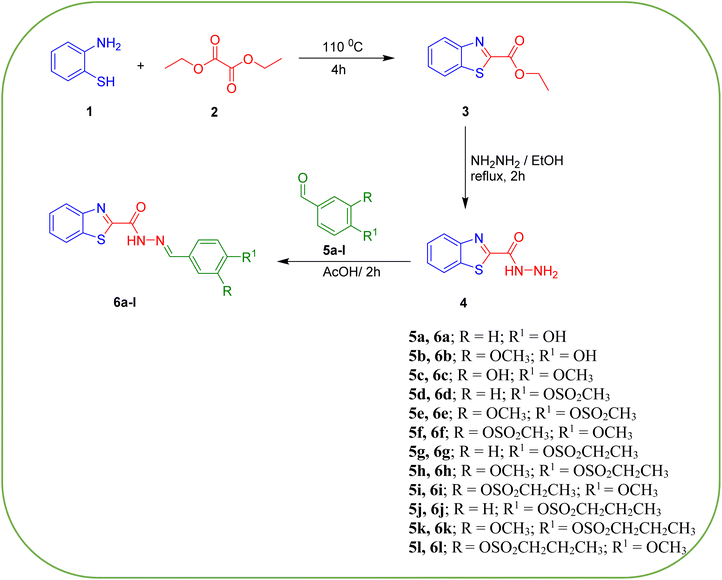
The synthetic pathway for the production of the desired benzothiazole–sulfonate analogs 6a–l started from the esterification reaction of 2-amino thiophenol 1 with diethyl oxalate 2 affording the corresponding ester 3, which is a precursor of benzothiazole carbohydrazide 4 when heated under reflux for 2 h in ethanol with hydrazine monohydrate. Benzothiazole carbohydrazide 4 was then allowed to interact with a variety of para-substituted alkane sulfonyl aryl aldehydes 5a–l to give the targeted benzylidene thiazole carbohydrazide analogs 6a–l in respectable yields (Scheme 1). The chemical structure of the new chemical entities was proved by employing various spectroscopic methods (IR, 1H NMR and 13C NMR) in addition to the elemental analysis data.The IR spectrum for derivatives 6a–l displays at 3371–3210 cm−1 strong stretching vibrational bands attributed to NH groups. Additionally, two stretching bands that appeared in the range of 1686–1661 cm−1, and 1665–1641 cm−1 are noticed for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively, whereas the band representing the sulfonate group SO2 is present in the 1383–1344 cm−1 range. The 1H NMR spectrum revealed that the alkane sulfonate functions (CH3, CH3CH2, and/or CH3CH2CH2) are represented by singlet peaks attributed to methyl groups, triplet and quartet signals attributed to ethyl groups, and triplet, sextet, and triplet signals depicting propyl side chains. The azomethine protons appear as singlet peaks between δH = 8.53 ppm and δH = 8.71 ppm. The NH protons corresponding to the hydrazine derivatives are observed as a singlet peak at δH = 12.78 and 12.46 ppm. The 13C NMR spectrum reveals characteristic peaks associated with the alkane sulfonate groups (CH3, CH3CH2, and/or CH3CH2CH2) in the aliphatic region at δC = 17.00–56.26 ppm. As a representative example of the para-substituted alkane sulfonyl analogs 6d–l, the IR (KBr) spectrum for derivative 6d disclosed stretching bands at 3224, 1661, 1650, and 1350 cm−1, corresponding to the functional groups NH, C
O groups, respectively, whereas the band representing the sulfonate group SO2 is present in the 1383–1344 cm−1 range. The 1H NMR spectrum revealed that the alkane sulfonate functions (CH3, CH3CH2, and/or CH3CH2CH2) are represented by singlet peaks attributed to methyl groups, triplet and quartet signals attributed to ethyl groups, and triplet, sextet, and triplet signals depicting propyl side chains. The azomethine protons appear as singlet peaks between δH = 8.53 ppm and δH = 8.71 ppm. The NH protons corresponding to the hydrazine derivatives are observed as a singlet peak at δH = 12.78 and 12.46 ppm. The 13C NMR spectrum reveals characteristic peaks associated with the alkane sulfonate groups (CH3, CH3CH2, and/or CH3CH2CH2) in the aliphatic region at δC = 17.00–56.26 ppm. As a representative example of the para-substituted alkane sulfonyl analogs 6d–l, the IR (KBr) spectrum for derivative 6d disclosed stretching bands at 3224, 1661, 1650, and 1350 cm−1, corresponding to the functional groups NH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and SO2, respectively; the 1H NMR spectrum disclosed a singlet peak at δH = 3.44 ppm corresponding to the methyl group and at δH = 7.46–8.28 ppm representing the aromatic region, while the azomethine proton CH
N, and SO2, respectively; the 1H NMR spectrum disclosed a singlet peak at δH = 3.44 ppm corresponding to the methyl group and at δH = 7.46–8.28 ppm representing the aromatic region, while the azomethine proton CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N was depicted at δH = 8.71 ppm, and the NH proton was depicted at δH = 12.76 ppm; 13C NMR exhibited a peak at δC = 37.57 ppm for the methyl group, at δC = 122.73–156.19 ppm representing aromatic carbons and at δC = 163.52 ppm for the C
N was depicted at δH = 8.71 ppm, and the NH proton was depicted at δH = 12.76 ppm; 13C NMR exhibited a peak at δC = 37.57 ppm for the methyl group, at δC = 122.73–156.19 ppm representing aromatic carbons and at δC = 163.52 ppm for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.
O group.
2.2. Anticancer activity
The cytotoxic assessment for the new chemical entities was conducted via an MTT assay in three distinct human cancer cell lines: MCF-7 (breast cancer), HCT-116 (colon cancer), and PC3 (prostate cancer), along with a normal cell line (BJ-1), beginning at a concentration of 100 μM. The reference drug 5-fluorouracil was included for comparison. The initial findings indicate that compounds 6c, 6d, 6g, 6i and 6k exhibit potent cytotoxicity against MCF-7 cells, with percentages of 58.1, 62.5, 62.3, 53.4, and 59.6, respectively. Similarly, compounds 6d, 6e, 6f, 6g, 6h, 6j and 6k demonstrated significant effects on HCT-116 cells, with growth inhibition percentages of 66.7, 61.1, 62.8, 68.6, 55.2, 68.5, and 77.2, respectively. For PC3 cells, the most promising compounds were 6d, 6e, 6g, 6h, 6j and 6k with cytotoxic percentages of 66.5, 60.8, 74.8, 63.4, 62.3, and 64.6, respectively.Conversely, compounds 6a, 6b, 6c, 6i, and 6l demonstrated a remarkable safety profile when evaluated in the normal cell line (BJ-1), exhibiting a cytotoxicity percent inhibition ranging from 35% to 10.8% (Table S1 and Fig. S1†).
Based on preliminary cytotoxicity data obtained from both cancer and normal cell lines, only compounds that demonstrated a cytotoxicity percent inhibition of over 50% for all tested cancer cell lines and simultaneously showed a safe profile in the normal cell line (BJ-1) were subjected to dose-dependent screening. The screening involved using a range of concentrations from 100 μM to 12.5 μM to determine their respective IC50 values (Table 1).
| Compd ID | IC50 values (μM) | ||
|---|---|---|---|
| MCF-7 | HCT-116 | PC3 | |
| 6a | — | 102 ± 2.1 | — |
| 6c | 80.9 ± 3.7 | — | — |
| 6e | — | 81.4 ± 1.9 | 90.6 ± 2.7 |
| 6i | 78.8 ± 2.6 | — | — |
| 5-FU | 78.4 ± 4.2 | 29.2 ± 1.7 | >200 |
The findings showed that compound 6i demonstrated the greatest potency within the tested series against MCF-7, with an IC50 value of 78.8 ± 2.6 μM, an almost similar response to the standard reference 5-FU (IC50 = 78.4 ± 4.2 μM). Derivative 6c showed an approximately close IC50 value (80.9 ± 3.7) to 5-FU (IC50 = 78.4 ± 4.2 μM), while derivatives 6a (IC50 = 102 ± 2.1 μM) and 6e (IC50 = 81.4 ± 1.9 μM) displayed lower activity in the HCT-116 cell line compared to 5-FU (IC50 = 29.2 ± 1.7 μM). Furthermore, compound 6e exhibited promising behavior in the PC3 cell line (IC50 = 90.6 ± 2.7 μM) compared to the reference standard 5-FU, which displayed an IC50 value exceeding 200 μM (Table 1).
Based on the findings that we observed and following the SAR (structure-activity relationship) rule, it was discovered that incorporating an alkane sulfonate moiety significantly enhanced the cytotoxic activity in all examined cell lines, except for compound 6l. Notably, an activity order was observed, with methane > ethane > propane, which holds promise for guiding the future design of potent anti-cancer candidates. This information will be valuable for developing effective strategies to pursue novel anti-cancer agents. Except for compounds 6d, 6f, 6g, 6h, 6j and 6k, the series demonstrated no to minimal cytotoxic activity detected against the used normal human cell line (BJ-1).
2.3. Tubulin evaluation of in vitro tubulin polymerization inhibitory activity
Antitubulin-targeting therapies are considered a good strategy for fighting cancer cells. Tubulin-microtubules hold a crucial role in cell survival. The polymerization of α- and β-tubulin dimers forms microtubules, while their depolymerization reverts them to tubulin dimers. Disrupting microtubule dynamics affects DNA segregation and cell mitosis, leading to the destruction of cancer cells.44 To explore the relationship between the newly synthesized derivatives and tubulin in terms of their antiproliferative activity, compound 6i, the most potent, was examined for its tubulin polymerization properties compared with the control MCF7 cells. The assay revealed that 6i influenced the microtubule mass protein tubulin B by preventing the formation of tubulin polymers in MCF-7 as compared to untreated breast cells with 210.3 and 632.9 pg ml−1, respectively (Fig. S2†).2.4. Reactive oxygen species (ROS) production
Extensive research has been conducted on generating intracellular oxidative stress in the form of reactive oxygen species (ROS) in various kinds of cancer.45 ROS-stimulating agents may enable specific cancer therapy. The maintenance of various cellular processes relies on redox homeostasis. Cancer cells generally display elevated levels of reactive oxygen species (ROS) while maintaining redox balance due to their antioxidant capacity. Recent studies have focused on targeting cancer cells by increasing ROS levels and disrupting redox balance, leading to significant damage to the cancer cells.46 The levels of intracellular ROS have been assessed to check whether compound 6i-mediated membrane damage can lead to oxidative stress induction. It was observed that 6i manages to elevate ROS levels in treated cancer cells, leading to the induction of DNA fragmentation by 8.3 fold compared to untreated cells (Fig. S3†).2.5. Cell cycle analysis
Most cytotoxic drugs produce cell cycle arrest, either directly through regulating cell cycle regulators or indirectly by modifying other cell components. To investigate the effects of derivative 6i on the cell cycle. The MCF-7 cells were subjected to a 24 h treatment with 6i. Following this treatment, the cells were marked with propidium iodide (PI) and subsequently examined via flow cytometry. The findings indicated an increase in the proportion of cells in the G2 phase, rising from 6.85% in the untreated control group to 18.27% in the group treated with 6i. This observation implies that compound 6i induces a cell cycle halt in MCF-7 cells at the G2/M stage (Fig. 3). | ||
| Fig. 3 Cell cycle analysis of the MCF-7 cell line after 24 hours of treatment with 6i. Analysis was done by annexin V/PI staining. | ||
2.6. Cell apoptosis
Mitochondria are involved in many vital cellular processes, like differentiation and apoptosis. Mitochondria-targeting therapy might be more operatively related to conventional chemotherapy. Mitochondrial dysfunction leads to apoptosis initiation.47 Apoptosis is a physiological process that holds a key role in maintaining tissue homeostasis, and it is considered to be the most appropriate way of eliminating undesirable cells. Most existing anticancer drugs are mediated by the initiation of apoptosis in cancer cells.To further inspect the impact of compound 6i against MCF-7 tumour cells, the capability of 6i to induce apoptosis was inspected by flow cytometry. Treatment of MCF-7 cells with the predetermined IC50 of 6i induced necrosis (Fig. 4). The early apoptosis increases from 0.37% (control) to 13.88% (treated cells) and late apoptosis increases from 0.14% (control) to 7.31% (treated cells). Compound 6i specifically induced necrosis in the treated MCF-7 cells, unlike the controls.
 | ||
| Fig. 4 Compound 6i induced cell apoptosis in an annexin V-FITC assay. It represents the total of early apoptotic (annexin V+/PI−) cell percentage and late apoptotic (annexin V+/PI+) cell percentage. | ||
2.7. Molecular docking study
Molecular docking simulation was used to investigate how derivative 6i interacts with the binding site of colchicine (CBS) on tubulin. Specifically, the compound's binding energy, spatial orientation, and interaction mechanisms at this unique pocket, which is strategically positioned at the interface between the α and β tubulin protein complex subunits, were analyzed. CBS is reported to encompass three distinct interaction zones. Zone 1 is the most proximal to the α–β tubulin interface and zone 2 is located next to zone 1 in the β-tubulin subunit, while zone 3 is extra severely submerged in β-tubulin. Zone 2 is considered the main zone which accommodates the major portion of the inhibitor structures. In contrast, both zone 1 and zone 3 act as “accessory zones” that help stabilize the inhibitor inside the binding pocket.48 Tubulin CBS inhibitors exhibit different orientations and localizations within CBS, corresponding to the three identified zones. Classical CBS inhibitors, such as colchicine, are found in zone 1 and zone 2 near the α-tubulin subunit, while non-classical CBS inhibitors, like nocodazole, penetrate more deeply into zone 2 and zone 3 (Fig. 5).49 To validate our docking protocol, we redocked the co-crystalized inhibitor (nocodazole) into the tubulin CBS and compared the docked pose with the original co-crystalized pose. The docking protocol managed to reproduce the co-crystalized pose as indicated by the perfect superposition and low RMSD value (0.16 Å) between the two poses (Fig. 6A). Compound 6i has demonstrated strong binding to tubulin CBS as shown by its docking score (−9.93 kcal mol−1), which surpasses that of nocodazole and colchicine (−8.53 and −8.32 kcal mol−1, respectively). Compound 6i has adopted a binding mode similar to nocodazole as it was placed in zone 2 extending in zone 3 deeply in β-tubulin (Fig. 5). It is important to note that the o-methoxy phenyl ethane sulfonate ring does not directly interact with the binding pocket; instead, it is located in the hydrophobic pocket of zone 2 forming several hydrophobic pi–alkyl interactions with Cys241, Leu248, Ala316, Ile318, and Ala354, this may potentially inhibit the normal dimerization of tubulin subunits. Conversely, the benzothiazole ring is deeply inserted into zone 3, forming two hydrogen bonds: one among the nitrogen atom of benzothiazole besides Tyr202, and another between hydrazone (NH) and Val238. Additionally, hydrophobic pi–alkyl interactions are observed between the benzothiazole ring and both Leu252 and Val238 (Fig. 6B and C).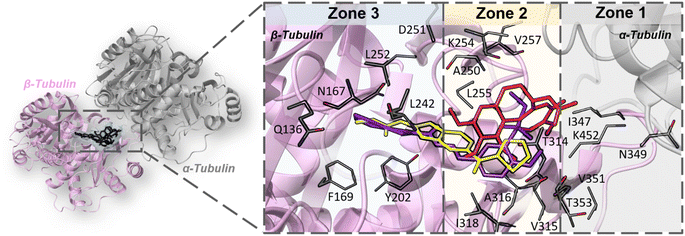 | ||
| Fig. 5 3D depiction illustrating different binding modes of colchicine (red sticks), nocodazole (yellow sticks) and compound 6i (purple sticks) inside tubulin CBS. | ||
3. Experimental
3.1. Chemistry
The ESI† file includes comprehensive details on the chemicals, various analytical equipment, and spectral charts displayed in Fig. S4–S27.†3.1.1.1. N′-(4-Hydroxybenzylidene)benzo[d]thiazole-2-carbohydrazide (6a). Yield: 98%; mp 285–257 °C; IR (KBr) cm−1, ν: 3400 (OH), 3312 (NH), 1686 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1650 (C
O), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR δ (ppm): 6.86 (d, 2H, J = 9.0 Hz, H-3′ and H-5′), 7.57 (d, 2H, J = 9.0 Hz, H-2′ and H-6′), 7.61–7.62 (m, 1H, H-5), 7.64–7.67 (m, 1H, H-6), 8.18 (d, 1H, J = 8.5 Hz, H-4), 8.26 (d, 1H, J = 8.5 Hz, H-7), 8.57 (s, 1H, CH
N); 1H NMR δ (ppm): 6.86 (d, 2H, J = 9.0 Hz, H-3′ and H-5′), 7.57 (d, 2H, J = 9.0 Hz, H-2′ and H-6′), 7.61–7.62 (m, 1H, H-5), 7.64–7.67 (m, 1H, H-6), 8.18 (d, 1H, J = 8.5 Hz, H-4), 8.26 (d, 1H, J = 8.5 Hz, H-7), 8.57 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 10.02 (brs, 1H, OH), 12.46 (s, 1H, NH); 13C NMR δ (ppm): 115.76 (C-3′ and C-5′), 122.97 (C-4), 123.98 (C-5), 124.96 (C-1′), 162.96 (C-6), 127.17 (C-7), 129.18 (C-2′ and C-6′), 135.99 (C-7a), 150.81 (C
N), 10.02 (brs, 1H, OH), 12.46 (s, 1H, NH); 13C NMR δ (ppm): 115.76 (C-3′ and C-5′), 122.97 (C-4), 123.98 (C-5), 124.96 (C-1′), 162.96 (C-6), 127.17 (C-7), 129.18 (C-2′ and C-6′), 135.99 (C-7a), 150.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.70 (C-3a), 155.78 (C-4′), 159.82 (C-2), 163.98 (C
N), 152.70 (C-3a), 155.78 (C-4′), 159.82 (C-2), 163.98 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C15H11N3O2S (297.33): C, 60.59; H, 3.73; N, 14.13. Found: C, 60.71; H, 3.51; N, 14.29.
O); anal. calcd for: C15H11N3O2S (297.33): C, 60.59; H, 3.73; N, 14.13. Found: C, 60.71; H, 3.51; N, 14.29.
3.1.1.2. N′-(4-Hydroxy-3-methoxybenzylidene)benzo[d]thiazole-2-carbohydrazide (6b). Yield: 91%; mp 232–234 °C; IR (KBr) cm−1, ν: 3488 (OH), 3304 (NH), 1656 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1641 (C
O), 1641 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR δ (ppm): 3.84 (s, 3H, OCH3), 6.87 (d, 1H, J = 8.0 Hz, H-5′), 7.11 (d, 1H, J = 8.0 Hz, H-6′), 7.33 (d, 1H, J = 1.0 Hz, H-2′), 7.58–7.60 (m, 1H, H-5), 7.63–7.66 (m, 1H, H-6), 8.17 (d, 1H, J = 8.0 Hz, H-4), 8.24 (d, 1H, J = 8.0 Hz, H-7), 8.57 (s, 1H, CH
N); 1H NMR δ (ppm): 3.84 (s, 3H, OCH3), 6.87 (d, 1H, J = 8.0 Hz, H-5′), 7.11 (d, 1H, J = 8.0 Hz, H-6′), 7.33 (d, 1H, J = 1.0 Hz, H-2′), 7.58–7.60 (m, 1H, H-5), 7.63–7.66 (m, 1H, H-6), 8.17 (d, 1H, J = 8.0 Hz, H-4), 8.24 (d, 1H, J = 8.0 Hz, H-7), 8.57 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.64 (brs, 1H, OH), 12.48 (brs, 1H, NH); 13C NMR δ (ppm): 55.61 (CH3), 109.29 (C-2′), 115.56 (C-5′), 122.74 (C-4), 122.80 (C-5), 124.04 (C-6′), 125.47 (C-6), 127.00 (C-7), 127.21 (C-1′), 136.07 (C-7a), 148.14 (C
N), 9.64 (brs, 1H, OH), 12.48 (brs, 1H, NH); 13C NMR δ (ppm): 55.61 (CH3), 109.29 (C-2′), 115.56 (C-5′), 122.74 (C-4), 122.80 (C-5), 124.04 (C-6′), 125.47 (C-6), 127.00 (C-7), 127.21 (C-1′), 136.07 (C-7a), 148.14 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 149.54 (C-3′), 151.17 (C-4′), 152.76 (C-3a), 155.95 (C-2), 163.96 (C
N), 149.54 (C-3′), 151.17 (C-4′), 152.76 (C-3a), 155.95 (C-2), 163.96 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C16H13N3O3S (327.36): C, 58.71; H, 4.00; N, 12.84. Found: C, 58.55; H, 4.22; N, 12.70.
O); anal. calcd for: C16H13N3O3S (327.36): C, 58.71; H, 4.00; N, 12.84. Found: C, 58.55; H, 4.22; N, 12.70.
3.1.1.3. N'-(3-Hydroxy-4-methoxybenzylidene)benzo[d]thiazole-2-carbohydrazide (6c). Yield: 78%; mp 250–252 °C; IR (KBr) cm−1, ν: 3461 (OH), 3210 (NH), 1676 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1649 (C
O), 1649 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR δ (ppm): 3.81 (s, 3H, OCH3), 6.98 (d, 1H, J = 8.5 Hz, H-5′), 7.07 (dd, 1H, J = 8.5, 1.5 Hz, H-6′), 7.31 (d, 1H, J = 2.0 Hz, H-2′), 7.58–7.61 (m, 1H, H-5), 7.64–7.67 (m, 1H, H-6), 8.18 (d, 1H, J = 8.5 Hz, H-4), 8.25 (d, 1H, J = 8.5 Hz, H-7), 8.53 (s, 1H, CH
N); 1H NMR δ (ppm): 3.81 (s, 3H, OCH3), 6.98 (d, 1H, J = 8.5 Hz, H-5′), 7.07 (dd, 1H, J = 8.5, 1.5 Hz, H-6′), 7.31 (d, 1H, J = 2.0 Hz, H-2′), 7.58–7.61 (m, 1H, H-5), 7.64–7.67 (m, 1H, H-6), 8.18 (d, 1H, J = 8.5 Hz, H-4), 8.25 (d, 1H, J = 8.5 Hz, H-7), 8.53 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.37 (s, 1H, OH), 12.50 (s, 1H, NH); 13C NMR δ (ppm): 55.56 (CH3), 111.85 (C-5′), 112.51 (C-2′), 120.80 (C-4), 122.94 (C-6′), 124.00 (C-5), 126.87 (C-6), 126.96 (C-7), 127.17 (C-1′), 136.04 (C-7a), 146.97 (C
N), 9.37 (s, 1H, OH), 12.50 (s, 1H, NH); 13C NMR δ (ppm): 55.56 (CH3), 111.85 (C-5′), 112.51 (C-2′), 120.80 (C-4), 122.94 (C-6′), 124.00 (C-5), 126.87 (C-6), 126.96 (C-7), 127.17 (C-1′), 136.04 (C-7a), 146.97 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.21 (C-3′), 150.71 (C-4′), 152.72 (C-3a), 155.89 (C-2), 163.93 (C
N), 150.21 (C-3′), 150.71 (C-4′), 152.72 (C-3a), 155.89 (C-2), 163.93 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C16H13N3O3S (327.36): C, 58.71; H, 4.00; N, 12.84. Found: C, 58.90; H, 4.21; N, 12.69.
O); anal. calcd for: C16H13N3O3S (327.36): C, 58.71; H, 4.00; N, 12.84. Found: C, 58.90; H, 4.21; N, 12.69.
3.1.1.4. 4-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)phenyl methanesulfonate (6d). Yield: 76%; mp 219–221 °C; IR (KBr) cm−1, ν: 3224 (NH), 1661 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1650 (C
O), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1350 (SO2); 1H NMR δ (ppm): 3.44 (s, 3H, CH3), 7.46 (d, 2H, J = 9.0 Hz, H-3′ and H-5′), 7.61–7.64 (m, 1H, H-5), 7.65–7.69 (m, 1H, H-6), 7.85 (d, 2H, J = 9.0 Hz, H-2′ and H-6′), 8.20 (d, 1H, J = 9.0 Hz, H-4), 8.28 (d, 1H, J = 8.5 Hz, H-7), 8.71 (s, 1H, CH
N), 1350 (SO2); 1H NMR δ (ppm): 3.44 (s, 3H, CH3), 7.46 (d, 2H, J = 9.0 Hz, H-3′ and H-5′), 7.61–7.64 (m, 1H, H-5), 7.65–7.69 (m, 1H, H-6), 7.85 (d, 2H, J = 9.0 Hz, H-2′ and H-6′), 8.20 (d, 1H, J = 9.0 Hz, H-4), 8.28 (d, 1H, J = 8.5 Hz, H-7), 8.71 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.76 (s, 1H, NH); 13C NMR δ (ppm): 37.57 (CH3), 122.73 (C-2′ and C-6′), 123.01 (C-4), 124.05 (C-6), 127.10 (C-7), 127.24 (C-5), 128.99 (C-3′ and C-5′), 133.04 (C-4′), 136.03 (C-7a), 149.13 (C
N), 12.76 (s, 1H, NH); 13C NMR δ (ppm): 37.57 (CH3), 122.73 (C-2′ and C-6′), 123.01 (C-4), 124.05 (C-6), 127.10 (C-7), 127.24 (C-5), 128.99 (C-3′ and C-5′), 133.04 (C-4′), 136.03 (C-7a), 149.13 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.29 (C-3a), 152.64 (C-1′), 156.19 (C-2), 163.52 (C
N), 150.29 (C-3a), 152.64 (C-1′), 156.19 (C-2), 163.52 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C16H13N3O4S2 (375.42): C, 51.19; H, 3.49; N, 11.19. Found: C, 51.25; H, 3.31; N, 11.09.
O); anal. calcd for: C16H13N3O4S2 (375.42): C, 51.19; H, 3.49; N, 11.19. Found: C, 51.25; H, 3.31; N, 11.09.
3.1.1.5. 4-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)-2-methoxyphenyl methanesulfonate (6e). Yield: 80%; mp 185–187 °C; IR (KBr) cm−1, ν: 3362 (NH), 1671 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1665 (C
O), 1665 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1361 (SO2); 1H NMR δ (ppm): 3.39 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 7.35 (d, 1H, J = 8.5 Hz, H-6′), 7.42 (d, 1H, J = 9.0 Hz, H-5′), 7.52 (s, 1H, H-3′), 7.61–7.64 (m, 1H, H-5), 7.66–7.69 (m, 1H, H-6), 8.21 (d, 1H, J = 9.0 Hz, H-4), 8.28 (d, 1H, J = 8.0 Hz, H-7), 8.67 (s, 1H, CH
N), 1361 (SO2); 1H NMR δ (ppm): 3.39 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 7.35 (d, 1H, J = 8.5 Hz, H-6′), 7.42 (d, 1H, J = 9.0 Hz, H-5′), 7.52 (s, 1H, H-3′), 7.61–7.64 (m, 1H, H-5), 7.66–7.69 (m, 1H, H-6), 8.21 (d, 1H, J = 9.0 Hz, H-4), 8.28 (d, 1H, J = 8.0 Hz, H-7), 8.67 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.78 (s, 1H, NH); 13C NMR δ (ppm): 38.46 (CH3), 56.08 (OCH3), 110.84 (C-3′), 120.74 (C-4), 123.01 (C-6′), 124.11 (C-6), 124.32 (C-7), 127.14 (C-5′), 127.27 (C-5), 134.11 (C-4′), 136.08 (C-7a), 139.36 (C-1′), 149.44 (C
N), 12.78 (s, 1H, NH); 13C NMR δ (ppm): 38.46 (CH3), 56.08 (OCH3), 110.84 (C-3′), 120.74 (C-4), 123.01 (C-6′), 124.11 (C-6), 124.32 (C-7), 127.14 (C-5′), 127.27 (C-5), 134.11 (C-4′), 136.08 (C-7a), 139.36 (C-1′), 149.44 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.79 (C-2′), 152.68 (C-3a), 156.29 (C-2), 163.52 (C
N), 151.79 (C-2′), 152.68 (C-3a), 156.29 (C-2), 163.52 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C17H15N3O5S2 (405.44): C, 50.36; H, 3.73; N, 10.36. Found: C, 50.21; H, 3.51; N, 10.49.
O); anal. calcd for: C17H15N3O5S2 (405.44): C, 50.36; H, 3.73; N, 10.36. Found: C, 50.21; H, 3.51; N, 10.49.
3.1.1.6. 5-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)-2-methoxyphenyl methanesulfonate (6f). Yield: 99%; mp 221–223 °C; IR (KBr) cm−1, ν: 3349 (NH), 1670 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1659 (C
O), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1363 (SO2); 1H NMR δ (ppm): 3.40 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 7.31 (d, 1H, J = 8.5 Hz, H-6′), 7.31 (d, 1H, J = 8.5 Hz, H-3′), 7.60–7.63 (m, 1H, H-5), 7.65–7.69 (m, 3H, H-6 + H4′ + H6′), 8.20 (d, 1H, J = 8.0 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.62 (s, 1H, CH
N), 1363 (SO2); 1H NMR δ (ppm): 3.40 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 7.31 (d, 1H, J = 8.5 Hz, H-6′), 7.31 (d, 1H, J = 8.5 Hz, H-3′), 7.60–7.63 (m, 1H, H-5), 7.65–7.69 (m, 3H, H-6 + H4′ + H6′), 8.20 (d, 1H, J = 8.0 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.62 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.68 (s, 1H, NH); 13C NMR δ (ppm): 38.38 (CH3), 56.26 (OCH3), 113.77 (C-3′), 121.94 (C-6′), 122.97 (C-7), 124.05 (C-4′), 127.05 (C-4 + C-5), 127.21 (C-6), 128.03 (C-5′), 136.05 (C-3a), 136.07 (C-1′), 149.12 (C
N), 12.68 (s, 1H, NH); 13C NMR δ (ppm): 38.38 (CH3), 56.26 (OCH3), 113.77 (C-3′), 121.94 (C-6′), 122.97 (C-7), 124.05 (C-4′), 127.05 (C-4 + C-5), 127.21 (C-6), 128.03 (C-5′), 136.05 (C-3a), 136.07 (C-1′), 149.12 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.69 (C-7a), 153.17 (C-1′), 156.08 (C-2), 163.67 (C
N), 152.69 (C-7a), 153.17 (C-1′), 156.08 (C-2), 163.67 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C17H15N3O5S2 (405.44): C, 50.36; H, 3.73; N, 10.36. Found: C, 50.29; H, 3.91; N, 10.22.
O); anal. calcd for: C17H15N3O5S2 (405.44): C, 50.36; H, 3.73; N, 10.36. Found: C, 50.29; H, 3.91; N, 10.22.
3.1.1.7. 4-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)phenyl ethanesulfonate (6g). Yield: 72%; mp 191–193 °C; IR (KBr) cm−1, ν: 3320 (NH), 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1660 (C
O), 1660 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1355 (SO2); 1H NMR δ (ppm): 1.39 (t, 3H, J = 7.0 Hz, CH3), 3.58 (q, 2H, J = 7.0 Hz, CH2), 7.44 (d, 2H, J = 8.5 Hz, H-3′ and H-5′), 7.60–7.63 (m, 1H, H-5), 7.65–7.68 (m, 1H, H-6), 7.85 (d, 2H, J = 8.5 Hz, H-2′ and H-6′), 8.20 (d, 1H, J = 8.0 Hz, H-4), 8.27 (d, 1H, J = 7.5 Hz, H-7), 8.70 (s, 1H, CH
N), 1355 (SO2); 1H NMR δ (ppm): 1.39 (t, 3H, J = 7.0 Hz, CH3), 3.58 (q, 2H, J = 7.0 Hz, CH2), 7.44 (d, 2H, J = 8.5 Hz, H-3′ and H-5′), 7.60–7.63 (m, 1H, H-5), 7.65–7.68 (m, 1H, H-6), 7.85 (d, 2H, J = 8.5 Hz, H-2′ and H-6′), 8.20 (d, 1H, J = 8.0 Hz, H-4), 8.27 (d, 1H, J = 7.5 Hz, H-7), 8.70 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.75 (s, 1H, NH); 13C NMR δ (ppm): 8.00 (CH3), 44.83 (CH2), 122.61 (C-2′ and C-6′), 122.99 (C-4), 124.04 (C-6), 127.08 (C-7), 127.22 (C-5), 128.99 (C-3′ and C-5′), 132.93 (C-4′), 136.04 (C-7a), 149.11 (C
N), 12.75 (s, 1H, NH); 13C NMR δ (ppm): 8.00 (CH3), 44.83 (CH2), 122.61 (C-2′ and C-6′), 122.99 (C-4), 124.04 (C-6), 127.08 (C-7), 127.22 (C-5), 128.99 (C-3′ and C-5′), 132.93 (C-4′), 136.04 (C-7a), 149.11 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.13 (C-3a), 152.64 (C-1′), 156.19 (C-2), 163.53 (C
N), 150.13 (C-3a), 152.64 (C-1′), 156.19 (C-2), 163.53 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C17H15N3O4S2 (389.44): C, 52.43; H, 3.88; N, 10.79. Found: C, 52.61; H, 3.61; N, 10.61.
O); anal. calcd for: C17H15N3O4S2 (389.44): C, 52.43; H, 3.88; N, 10.79. Found: C, 52.61; H, 3.61; N, 10.61.
3.1.1.8. 4-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)-2-methoxyphenyl ethanesulfonate (6h). Yield: 78%; mp 175–177 °C; IR (KBr) cm−1, ν: 3299 (NH), 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1659 (C
O), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1383 (SO2); 1H NMR δ (ppm): 1.40 (t, 3H, J = 6.5 Hz, CH3), 3.53 (q, 2H, J = 7.0 Hz, CH2), 3.92 (s, 3H, OCH3), 7.34 (d, 1H, J = 8.0 Hz, H-6′), 7.40 (d, 1H, J = 8.0 Hz, H-5′), 7.51 (s, 1H, H-3′), 7.61–7.64 (m, 1H, H-5), 7.66–7.69 (m, 1H, H-6), 8.21 (d, 1H, J = 7.5 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.67 (s, 1H, CH
N), 1383 (SO2); 1H NMR δ (ppm): 1.40 (t, 3H, J = 6.5 Hz, CH3), 3.53 (q, 2H, J = 7.0 Hz, CH2), 3.92 (s, 3H, OCH3), 7.34 (d, 1H, J = 8.0 Hz, H-6′), 7.40 (d, 1H, J = 8.0 Hz, H-5′), 7.51 (s, 1H, H-3′), 7.61–7.64 (m, 1H, H-5), 7.66–7.69 (m, 1H, H-6), 8.21 (d, 1H, J = 7.5 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.67 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.76 (s, 1H, NH); 13C NMR δ (ppm): 8.04 (CH3), 45.76 (CH2), 56.08 (OCH3), 110.80 (C-3′), 120.69 (C-4), 123.02 (C-6′), 124.09 (C-6), 124.26 (C-7), 127.13 (C-5′), 127.26 (C-5), 133.96 (C-4′), 136.07 (C-7a), 139.28 (C-1′), 149.42 (C
N), 12.76 (s, 1H, NH); 13C NMR δ (ppm): 8.04 (CH3), 45.76 (CH2), 56.08 (OCH3), 110.80 (C-3′), 120.69 (C-4), 123.02 (C-6′), 124.09 (C-6), 124.26 (C-7), 127.13 (C-5′), 127.26 (C-5), 133.96 (C-4′), 136.07 (C-7a), 139.28 (C-1′), 149.42 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.74 (C-2′), 152.67 (C-3a), 156.26 (C-2), 163.54 (C
N), 151.74 (C-2′), 152.67 (C-3a), 156.26 (C-2), 163.54 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C18H17N3O5S2 (419.47): C, 51.54; H, 4.09; N, 10.02. Found: C, 51.71; H, 4.21; N, 10.15.
O); anal. calcd for: C18H17N3O5S2 (419.47): C, 51.54; H, 4.09; N, 10.02. Found: C, 51.71; H, 4.21; N, 10.15.
3.1.1.9. 5-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)-2-methoxyph-enyl ethanesulfonate (6i). Yield: 65%; mp 215–217 °C; IR (KBr) cm−1, ν: 3367 (NH), 1678 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1656 (C
O), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1351 (SO2); 1H NMR δ (ppm): 1.41 (t, 3H, J = 7.5 Hz, CH3), 3.54 (q, 2H, J = 7.5 Hz, CH2), 3.91 (s, 3H, OCH3), 7.31 (d, 1H, J = 9.0 Hz, H-3′), 7.60–7.63 (m, 1H, H-5), 7.65–7.68 (m, 3H, H-6 + H-4′ + H6′), 8.20 (d, 1H, J = 8.0 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.61 (s, 1H, CH
N), 1351 (SO2); 1H NMR δ (ppm): 1.41 (t, 3H, J = 7.5 Hz, CH3), 3.54 (q, 2H, J = 7.5 Hz, CH2), 3.91 (s, 3H, OCH3), 7.31 (d, 1H, J = 9.0 Hz, H-3′), 7.60–7.63 (m, 1H, H-5), 7.65–7.68 (m, 3H, H-6 + H-4′ + H6′), 8.20 (d, 1H, J = 8.0 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.61 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.67 (s, 1H, NH); 13C NMR δ (ppm): 8.00 (CH3), 45.69 (CH2), 56.24 (OCH3), 113.68 (C-3′), 121.77 (C-6′), 122.98 (C-7), 124.02 (C-4′), 126.98 (C-4), 127.04 (C-5), 127.20 (C-6), 127.94 (C-5′), 136.01 (C-3a), 137.93 (C-1′), 149.10 (C
N), 12.67 (s, 1H, NH); 13C NMR δ (ppm): 8.00 (CH3), 45.69 (CH2), 56.24 (OCH3), 113.68 (C-3′), 121.77 (C-6′), 122.98 (C-7), 124.02 (C-4′), 126.98 (C-4), 127.04 (C-5), 127.20 (C-6), 127.94 (C-5′), 136.01 (C-3a), 137.93 (C-1′), 149.10 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.66 (C-7a), 153.10 (C-1′), 156.03 (C-2), 163.66 (C
N), 152.66 (C-7a), 153.10 (C-1′), 156.03 (C-2), 163.66 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C18H17N3O5S2 (419.47): C, 51.54; H, 4.09; N, 10.02. Found: C, 51.37; H, 4.18; N, 10.13.
O); anal. calcd for: C18H17N3O5S2 (419.47): C, 51.54; H, 4.09; N, 10.02. Found: C, 51.37; H, 4.18; N, 10.13.
3.1.1.10. 4-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)phenyl propane-1-sulfonate (6j). Yield: 65%; mp 176–178 °C; IR (KBr) cm−1, ν: 3371 (NH), 1679 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1658 (C
O), 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1359 (SO2); 1H NMR δ (ppm): 1.04 (s, 3H, J = 7.5 Hz, CH3), 1.86 (sextet, 2H, J = 7.5 Hz, CH2), 3.55 (t, 2H, J = 8.0 Hz, CH2), 7.43 (d, 2H, J = 9.0 Hz, H-3′ and H-5′), 7.60–7.63 (m, 1H, H-5), 7.65–7.69 (m, 1H, H-6), 7.84 (d, 2H, J = 9.0 Hz, H-2′ and H-6′), 8.20 (d, 1H, J = 8.5 Hz, H-4), 8.27 (d, 1H, J = 8.5 Hz, H-7), 8.70 (s, 1H, CH
N), 1359 (SO2); 1H NMR δ (ppm): 1.04 (s, 3H, J = 7.5 Hz, CH3), 1.86 (sextet, 2H, J = 7.5 Hz, CH2), 3.55 (t, 2H, J = 8.0 Hz, CH2), 7.43 (d, 2H, J = 9.0 Hz, H-3′ and H-5′), 7.60–7.63 (m, 1H, H-5), 7.65–7.69 (m, 1H, H-6), 7.84 (d, 2H, J = 9.0 Hz, H-2′ and H-6′), 8.20 (d, 1H, J = 8.5 Hz, H-4), 8.27 (d, 1H, J = 8.5 Hz, H-7), 8.70 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.75 (s, 1H, NH); 13C NMR δ (ppm): 12.29 (CH3), 16.94 (CH2), 51.52 (CH2), 122.62 (C-2′ and C-6′), 122.97 (C-4), 124.04 (C-6), 127.06 (C-7), 127.20 (C-5), 128.98 (C-3′ and C-5′), 132.92 (C-4′), 136.04 (C-7a), 149.11 (C
N), 12.75 (s, 1H, NH); 13C NMR δ (ppm): 12.29 (CH3), 16.94 (CH2), 51.52 (CH2), 122.62 (C-2′ and C-6′), 122.97 (C-4), 124.04 (C-6), 127.06 (C-7), 127.20 (C-5), 128.98 (C-3′ and C-5′), 132.92 (C-4′), 136.04 (C-7a), 149.11 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.11 (C-3a), 152.64 (C-1′), 156.18 (C-2), 163.53 (C
N), 150.11 (C-3a), 152.64 (C-1′), 156.18 (C-2), 163.53 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C18H17N3O4S2 (403.47): C, 53.58; H, 4.25; N, 10.41. Found: C, 53.81; H, 4.11; N, 10.60.
O); anal. calcd for: C18H17N3O4S2 (403.47): C, 53.58; H, 4.25; N, 10.41. Found: C, 53.81; H, 4.11; N, 10.60.
3.1.1.11. 4-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)-2-methoxyphenyl propane-1-sulfonate (6k). Yield: 66%; mp 150–152 °C; IR (KBr) cm−1, ν: 3340 (NH), 1674 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1651 (C
O), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1344 (SO2); 1H NMR δ (ppm): 1.04 (t, 3H, J = 7.5 Hz, CH3), 1.88 (sextet, 2H, J = 7.5 Hz, CH2), 3.50 (t, 2H, J = 8.0 Hz, CH2), 3.93 (s, 3H, OCH3), 7.34 (d, 1H, J = 8.0 Hz, H-6′), 7.40 (d, 1H, J = 8.0 Hz, H-5′), 7.51 (s, 1H, H-3′), 7.61–7.64 (m, 1H, H-5), 7.66–7.69 (m, 1H, H-6), 8.21 (d, 1H, J = 8.0 Hz, H-4), 8.28 (d, 1H, J = 8.0 Hz, H-7), 8.67 (s, 1H, CH
N), 1344 (SO2); 1H NMR δ (ppm): 1.04 (t, 3H, J = 7.5 Hz, CH3), 1.88 (sextet, 2H, J = 7.5 Hz, CH2), 3.50 (t, 2H, J = 8.0 Hz, CH2), 3.93 (s, 3H, OCH3), 7.34 (d, 1H, J = 8.0 Hz, H-6′), 7.40 (d, 1H, J = 8.0 Hz, H-5′), 7.51 (s, 1H, H-3′), 7.61–7.64 (m, 1H, H-5), 7.66–7.69 (m, 1H, H-6), 8.21 (d, 1H, J = 8.0 Hz, H-4), 8.28 (d, 1H, J = 8.0 Hz, H-7), 8.67 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.77 (s, 1H, NH); 13C NMR δ (ppm): 12.36 (CH3), 17.02 (CH2), 52.44 (CH2), 56.07 (OCH3), 110.78 (C-3′), 120.66 (C-4), 123.00 (C-6′), 124.06 (C-6), 124.29 (C-7), 127.10 (C-5′), 127.24 (C-5), 133.93 (C-4′), 136.04 (C-7a), 139.24 (C-1′), 149.39 (C
N), 12.77 (s, 1H, NH); 13C NMR δ (ppm): 12.36 (CH3), 17.02 (CH2), 52.44 (CH2), 56.07 (OCH3), 110.78 (C-3′), 120.66 (C-4), 123.00 (C-6′), 124.06 (C-6), 124.29 (C-7), 127.10 (C-5′), 127.24 (C-5), 133.93 (C-4′), 136.04 (C-7a), 139.24 (C-1′), 149.39 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.71 (C-2′), 152.65 (C-3a), 156.22 (C-2), 163.53 (C
N), 151.71 (C-2′), 152.65 (C-3a), 156.22 (C-2), 163.53 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C19H19N3O5S2 (433.50): C, 52.64; H, 4.42; N, 9.69. Found: C, 52.71; H, 4.29; N, 9.50.
O); anal. calcd for: C19H19N3O5S2 (433.50): C, 52.64; H, 4.42; N, 9.69. Found: C, 52.71; H, 4.29; N, 9.50.
3.1.1.12. 5-((2-(Benzo[d]thiazole-2-carbonyl)hydrazono)methyl)-2-methoxyphenyl propane-1-sulfonate (6l). Yield: 70%; mp 210–212 °C; IR (KBr) cm−1, ν: 3371 (NH), 1673 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1648 (C
O), 1648 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1353 (SO2); 1H NMR δ (ppm): 1.05 (t, 3H, J = 7.5 Hz, CH3), 1.88 (sextet, 2H, J = 7.5 Hz, CH2), 3.51 (t, 2H, J = 7.5 Hz, CH2), 3.91 (s, 3H, OCH3), 7.31 (d, 1H, J = 8.0 Hz, H-3′), 7.60–7.63 (m, 1H, H-5), 7.65–7.68 (m, 3H, H-6+H-4′ + H6′), 8.20 (d, 1H, J = 8.5 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.61 (s, 1H, CH
N), 1353 (SO2); 1H NMR δ (ppm): 1.05 (t, 3H, J = 7.5 Hz, CH3), 1.88 (sextet, 2H, J = 7.5 Hz, CH2), 3.51 (t, 2H, J = 7.5 Hz, CH2), 3.91 (s, 3H, OCH3), 7.31 (d, 1H, J = 8.0 Hz, H-3′), 7.60–7.63 (m, 1H, H-5), 7.65–7.68 (m, 3H, H-6+H-4′ + H6′), 8.20 (d, 1H, J = 8.5 Hz, H-4), 8.27 (d, 1H, J = 8.0 Hz, H-7), 8.61 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.68 (s, 1H, NH); 13C NMR δ (ppm): 12.36 (CH3), 17.00 (CH2), 52.35 (CH2), 56.26 (OCH3), 113.71 (C-3′), 121.83 (C-6′), 122.99 (C-7), 124.02 (C-4′), 126.97 (C-4), 127.05 (C-5), 127.21 (C-6), 127.93 (C-5′), 136.01 (C-3a), 137.91 (C-1′), 149.10 (C
N), 12.68 (s, 1H, NH); 13C NMR δ (ppm): 12.36 (CH3), 17.00 (CH2), 52.35 (CH2), 56.26 (OCH3), 113.71 (C-3′), 121.83 (C-6′), 122.99 (C-7), 124.02 (C-4′), 126.97 (C-4), 127.05 (C-5), 127.21 (C-6), 127.93 (C-5′), 136.01 (C-3a), 137.91 (C-1′), 149.10 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.66 (C-7a), 153.11 (C-1′), 156.03 (C-2), 163.65 (C
N), 152.66 (C-7a), 153.11 (C-1′), 156.03 (C-2), 163.65 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for: C19H19N3O5S2 (433.50): C, 52.64; H, 4.42; N, 9.69. Found: C, 52.51; H, 4.30; N, 9.84.
O); anal. calcd for: C19H19N3O5S2 (433.50): C, 52.64; H, 4.42; N, 9.69. Found: C, 52.51; H, 4.30; N, 9.84.
3.2. Anti-cancer activity
3.3. Human reactive oxygen species (ROS) estimation
More details are provided in the ESI† data.3.4. Tubulin beta enzyme-linked immunosorbent assay kit (TUBb)
This experiment was accomplished following the documented procedure.51 More details are provided in the ESI† data.3.5. Estimation of DNA fragmentation through DPA assay
DNA fragmentation assessment of the cells was performed according to the reported method.52 More details are provided in the ESI† data.3.6. Cell cycle analysis and apoptosis detection
According to the documented procedure employing flow cytometry,53 cell cycle analysis and apoptosis detection were conducted. More details are provided in the ESI† data.3.7. Docking
The structural data for the α/β-tubulin heterodimer complexed with nocodazole was retrieved from the Protein Data Bank (PDB entry: 7Z2P). Receptor preparation involved selective chain preservation (A and B), and comprehensive removal of water molecules, ions, and ancillary molecular components. Hydrogen atom modifications included polar hydrogen incorporation and non-polar hydrogen consolidation with corresponding heavy atoms. Kollman charges were systematically applied, and the receptor was subsequently converted to PDBQT format for molecular docking procedures. Compound 6i was graphically rendered using ChemBioDraw Ultra 14.0, underwent energy minimization via an MMFF94x Force Field in a gaseous environment, and was formatted to PDBQT. A grid box measuring 20 × 20 × 20 Å with 0.375 Å spacing was positioned centrally on the co-crystallized ligand's coordinates (X = 16.7, Y = 64.9, Z = 37.6). Molecular docking simulation was executed through Autodock 4.2 utilizing default computational parameters. Docking poses were hierarchically ranked based on their computational scores, with the energetically most favorable configuration selected. Interaction analysis and comprehensive visualization were conducted through Discovery Studio Visualizer v21.1.0.20298.544. Conclusion
The synthesized benzothiazolecarbohydrazide–sulfonate conjugates 6a–l demonstrated reasonable to low cytotoxicity against three distinct human cancer cell lines, and compounds 6e, and 6i showed the highest potency. Meanwhile, compound 6i exhibited no cytotoxicity towards normal cells and showed promising anticancer activity, inducing DNA fragmentation and affecting cell cycle progression in MCF-7 cells. It significantly increased ROS levels, leading to an 8.3-fold rise in DNA fragmentation and a G2 phase increase from 6.85% to 18.27% in MCF-7 cells. Molecular docking indicated a favorable interaction of derivative 6i with the tubulin–colchicine binding site. These findings suggest that benzothiazolecarbohydrazide–sulfonate conjugates, especially compound 6i, hold potential for development as anticancer agents targeting tubulin polymerization. Future studies should focus on detailed mechanistic exploration, in vivo efficacy, and optimization to enhance the therapeutic potential.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors extend their appreciation to the National Research Centre, Dokki, Cairo, Egypt for supporting this work, Project number (13010139) and Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R89), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this work.References
- C. P. Wild, E. Weiderpass and B. W. Stewart, World Cancer Report: Cancer Research for Cancer Prevention, 2020 Search PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, Ca-Cancer J. Clin., 2021, 71(3), 209–249, DOI:10.3322/caac.21660.
- I. Soerjomataram and F. Bray, Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070, Nat. Rev. Clin. Oncol., 2021, 18, 663–672, DOI:10.1038/s41571-021-00514-z.
- I. Jarak, C. L. Varela, E. Tavares da Silva, F. F. M. Roleira, F. Veiga and A. Figueiras, Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications, Eur. J. Med. Chem., 2020, 206, 112526–112551, DOI:10.1016/j.ejmech.2020.112526.
- H. H. Fahmy, A. M. Srour, M. A. Ismail, M. A. Khater, R. A. Serrya and M. A. El-Manawaty, Design and synthesis of some new tri-substituted pyrazole derivatives as anticancer agents, Res. Chem. Intermed., 2016, 42, 6881–6892, DOI:10.1007/s11164-016-2502-2.
- Y. Wan, G. Fang, H. Chen, X. Deng and Z. Tang, Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation, Eur. J. Med. Chem., 2021, 15(226), 113837–113858, DOI:10.1016/j.ejmech.2021.113837.
- N. G. Fawazy, S. S. Panda, A. Mostafa, B. M. Kariuki, M. S. Bekheit and Y. Moatasim, et al., Spiro-3-indolin-2-ones: Synthesis, biological properties and computational studies, Sci. Rep., 2022, 12, 13880–13934, DOI:10.21203/rs.3.rs-1660054/v1.
- A. M. Srour, H. H. Fahmy, M. A. Khater, M. A. El-Manawaty and E. M. Shalaby, Synthesis, characterization, and cytotoxic activity of some new 1,3,4-trisubstituted pyrazoles against diverse tumor cell lines, Monatsh. Chem., 2018, 149, 1137–1147, DOI:10.1007/s00706-018-2153-7.
- J. Howard and A. A. Hyman, Dynamics and mechanics of the microtubule plus end, Nature, 2003, 422(6933), 753–758, DOI:10.1158/1078-0432.CCR-05-2715.
- T. E. Hetland, E. Hellesylt, V. A. Flørenes, C. Tropé, B. Davidson and J. Kærn, Class III β-tubulin expression in advanced-stage serous ovarian carcinoma effusions is associated with poor survival and primary chemoresistance, Hum. Pathol., 2011, 42(7), 1019–1026, DOI:10.1016/j.humpath.2010.10.025.
- R. A. Mohamed-Ezzat and A. M. Srour, Design and Synthesis of Aspirin-chalcone Mimic Conjugates as Potential Anticancer Agents, Anti-Cancer Agents Med. Chem., 2024, 24(7), 544–557, DOI:10.2174/0118715206280025231213065519.
- P. Lebok, M. Öztürk, U. Heilenkötter, F. Jaenicke, V. Müller and P. Paluchowski, et al., High levels of class III β-tubulin expression are associated with aggressive tumor features in breast cancer, Oncol. Lett., 2016, 11(3), 1987–1994, DOI:10.3892/ol.2016.4206.
- X. Zhao, C. Yue, J. Chen, C. Tian, D. Yang and L. Xing, et al., Class III β-Tubulin in Colorectal Cancer: Tissue Distribution and Clinical Analysis of Chinese Patients, Med. Sci. Monit., 2016, 22, 3915–3924, DOI:10.12659/msm.901252.
- G. Ploussard, S. Terry, P. Maillé, Y. Allory, N. Sirab and L. Kheuang, et al., Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy, Cancer Res., 2010, 70(22), 9253–9264, DOI:10.1158/0008-5472.CAN-10-1447.
- J. Howard and A. A. Hyman, Dynamics and mechanics of the microtubule plus end, Nature, 2003, 422(6933), 753–758, DOI:10.1038/nature01600.
- F. Xu, W. Li, W. Shuai, L. Yang, Y. Bi and C. Ma, et al., Design, synthesis and biological evaluation of pyridine-chalcone derivatives as novel microtubule-destabilizing agents, Eur. J. Med. Chem., 2019, 173, 1–14, DOI:10.1016/j.ejmech.2019.04.008.
- M. A. Jordan and L. Wilson, Microtubules as a target for anticancer drugs, Nat. Rev. Cancer, 2004, 4(4), 253–265, DOI:10.1038/nrc1317.
- C. Dumontet and M. A. Jordan, Microtubule-binding agents: a dynamic field of cancer therapeutics, Nat. Rev. Drug Discovery, 2010, 9(10), 790–803, DOI:10.1038/nrd3253.
- E. Porcù, R. Bortolozzi, G. Basso and G. Viola, Recent advances in vascular disrupting agents in cancer therapy, Future Med. Chem., 2014, 6(13), 1485–1498, DOI:10.4155/fmc.14.104.
- S. D. Guggilapu, L. Guntuku, T. S. Reddy, A. Nagarsenkar, D. K. Sigalapalli and V. G. M. Naidu, et al., Synthesis of thiazole linked indolyl-3-glyoxylamide derivatives as tubulin polymerization inhibitors, Eur. J. Med. Chem., 2017, 138, 83–95, DOI:10.1016/j.ejmech.2017.06.025.
- G. R. Pettit, S. B. Singh, M. R. Boyd, E. Hamel, R. K. Pettit, J. M. Schmidt and F. Hogan, Antineoplastic agents. 291. Isolation and synthesis of combretastatins A-4, A-5, and A-6(1a), J. Med. Chem., 1995, 38(10), 1666–1672, DOI:10.1021/jm00010a011.
- A. M. Srour, D. H. Dawood and D. O. Saleh, Synthesis, 3D-pharmacophore modelling and 2D-QSAR study of new pyridine-3-carbonitriles as vasorelaxant active agents, New J. Chem., 2021, 45, 7731–7740, 10.1039/D0NJ06319C.
- A. Irfan, F. Batool, S. A. Zahra Naqvi, A. Islam, S. M. Osman and A. Nocentini, et al., Benzothiazole derivatives as anticancer agents, J. Enzyme Inhib. Med. Chem., 2020, 35(1), 265–279, DOI:10.1080/14756366.2019.1698036.
- X. H. Shi, Z. Wang, Y. Xia, T. H. Ye, M. Deng and Y. Z. Xu, et al., Synthesis and biological evaluation of novel benzothiazole-2-thiol derivatives as potential anticancer agents, Molecules, 2012, 17(4), 3933–3944, DOI:10.3390/molecules17043933.
- C. O. Leong, M. Suggitt, D. J. Swaine, M. C. Bibby, M. F. Stevens and T. D. Bradshaw, In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles, Mol. Cancer Ther., 2004, 3(12), 1565–1575 CrossRef CAS PubMed.
- K. Liu, Y. Ding and C. Kang, Synthesis and Antiproliferative Activity of New N-Acylhydrazone Derivatives Containing Benzothiazole and Indole Based Moiety, Pharm. Chem. J., 2020, 54, 345–352, DOI:10.1007/s11094-020-02215-w.
- C. G. Mortimer, G. Wells, J. P. Crochard, E. L. Stone, T. D. Bradshaw, M. F. Stevens and A. D. Westwell, Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines, J. Med. Chem., 2006, 49(1), 179–185, DOI:10.1021/jm050942k.
- A. V. S. Rao, B. B. Rao, S. Sunkari, S. P. Shaik, B. Shaik and A. Kamal, 2-Arylaminobenzothiazole-arylpropenone conjugates as tubulin polymerization inhibitors, MedChemComm, 2017, 8, 924–941, 10.1039/c6md00562d.
- D. J. Fu, S. M. Liu, F. H. Li, J. J. Yang and J. Li, Antiproliferative benzothiazoles incorporating a trimethoxyphenyl scaffold as novel colchicine site tubulin polymerisation inhibitors, J. Enzyme Inhib. Med. Chem., 2020, 35(1), 1050–1059, DOI:10.1080/14756366.2020.1753721.
- N. M. Kumar, S. K. Nukala, T. N. Swamy, M. Ravinder, T. M. Krishna and S. Narsimha, J. Mol. Struct., 2022, 1250, 131722–131734, DOI:10.1016/j.molstruc.2021.131722.
- S. Grindrod, S. Suy, S. Fallen, M. Eto, J. Toretsky and M. L. Brown, Effects of a fluorescent Myosin light chain phosphatase inhibitor on prostate cancer cells, Front. Oncol., 2011, 1, 27–44, DOI:10.3389/fonc.2011.00027.
- S. Fortin, X. Charest-Morin, V. Turcotte, C. Lauvaux, J. Lacroix, M. F. Côté, S. Gobeil and R. C-Gaudreault, Activation of Phenyl 4-(2-Oxo-3-alkylimidazolidin-1-yl)benzenesulfonates Prodrugs by CYP1A1 as New Antimitotics Targeting Breast Cancer Cells, J. Med. Chem., 2017, 60(12), 4963–4982, DOI:10.1021/acs.jmedchem.7b00343.
- A. B. Krueger, S. J. Dehdashti, N. Southall, J. J. Marugan, M. Ferrer and X. Li, et al., Identification of a selective small-molecule inhibitor series targeting the eyes absent 2 (Eya2) phosphatase activity, J. Biomol. Screening, 2013, 18(1), 85–96, DOI:10.1177/1087057112453936.
- N. W. Gibson, Characterization of DNA damage and cytotoxicity induced in two human colon carcinoma cell lines by cyclodisone, Cancer Res., 1989, 49(1), 154–157 CAS.
- R. Tamari, M. Scordo, B. M. Kunvarjee, A. Proli, A. Lin and J. Flynn, et al., Association between busulfan exposure and survival in patients undergoing a CD34+ selected stem cell transplantation, Blood Adv., 2023, 7(18), 5225–5233, DOI:10.1182/bloodadvances.2023009708.
- S. Chen, Y. Zhang, Y. Liu and Q. Wang, Design, Synthesis, Acaricidal Activities, and Structure-Activity Relationship Studies of Novel Oxazolines Containing Sulfonate Moieties, J. Agric. Food Chem., 2019, 67(49), 13544–13549, DOI:10.1021/acs.jafc.9b05547.
- S. S. Ragab, A. M. Sweed and A. M. Srour, Synthesis and Antiproliferative Properties of Some Spirocyclic Pyrimidine Hydrazones, ChemistrySelect, 2024, 9(8), e202400161, DOI:10.1002/slct.202400161.
- P. Mombelli, M. C. Witschel, A. W. van Zijl, J. G. Geist, M. Rottmann and C. Freymond, et al., Identification of 1,3-Diiminoisoindoline Carbohydrazides as Potential Antimalarial Candidates, ChemMedChem, 2012, 7, 151–158, DOI:10.1002/cmdc.201100441.
- M. Carcelli, D. Rogolino, A. Gatti, M. Sechi, G. Kumar and S. W. White, et al., N-Acylhydrazone inhibitors of influenza virus PA endonuclease with versatile metal binding modes, Sci. Rep., 2016, 6, 31500–31514, DOI:10.1038/srep31500.
- A. D. Joshi, R. C. Botham, L. J. Schlein, H. S. Roth, A. Mangraviti and A. Borodovsky, et al., Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients, Oncotarget, 2017, 8(46), 80124–80138, DOI:10.18632/oncotarget.19085.
- L. P. de Figueiredo, A. L. Ibiapino, D. N. do Amaral, L. S. Ferraz, T. Rodrigues and E. J. Barreiro, et al., Structural characterization and cytotoxicity studies of different forms of a combretastatin A4 analogue, J. Mol. Struct., 2017, 1147, 226–234, DOI:10.1016/j.molstruc.2017.06.093.
- (a) M. A. Omar, R. A. El-Shiekh, D. H. Dawood, A. Temirak and A. M. Srour, Hydrazone-sulfonate hybrids as potential cholinesterase inhibitors: design, synthesis and molecular modeling simulation, Future Med. Chem., 2023, 15(24), 2269–2287, DOI:10.4155/fmc-2023-0238; (b) A. F. Kassem, S. S. Ragab, M. A. Omar, N. A. Altwaijry, M. Abdelraof, A. Temirak, A. Saleh and A. M. Srour, New quinazolone-sulfonate conjugates with an acetohydrazide linker as potential antimicrobial agents: design, synthesis and molecular docking simulations, RSC Adv., 2025, 15(2), 1033–1048, 10.1039/d4ra07563c.
- A. F. Kassem, M. A. Omar, A. Temirak, R. A. El-Shiekh and A. M. Srour, Barbiturate–sulfonate hybrids as potent cholinesterase inhibitors: design, synthesis and molecular modeling studies, Future Med. Chem., 2024, 16(16), 1615–1631, DOI:10.1080/17568919.2024.2366158.
- S. Khwaja, K. Kumar, R. Das and A. S. Negi, Microtubule associated proteins as targets for anticancer drug development, Bioorg. Chem., 2021, 116, 105320–105336, DOI:10.1016/j.bioorg.2021.105320.
- H. Nakamura and K. Takada, Reactive oxygen species in cancer: current findings and future directions, Cancer Sci., 2021, 112(10), 3945–3952, DOI:10.1111/cas.15068.
- S. J. Kim, H. S. Kim and Y. R. Seo, Understanding of ROS-Inducing Strategy in Anticancer Therapy, Oxid. Med. Cell. Longevity, 2019, 2019, 5381692–5381712, DOI:10.1155/2019/5381692.
- T. Zaidieh, J. R. Smith, K. E. Ball and Q. An, ROS as a novel indicator to predict anticancer drug efficacy, BMC Cancer, 2019, 19(1), 1224–1338, DOI:10.1186/s12885-019-6438-y.
- A. Massarotti, A. Coluccia, R. Silvestri, G. Sorba and A. Brancale, The tubulin colchicine domain: a molecular modeling perspective, ChemMedChem, 2012, 7(1), 33–42, DOI:10.1002/cmdc.201100361.
- W. Li, H. Sun, S. Xu, Z. Zhu and J. Xu, Tubulin inhibitors targeting the colchicine binding site: a perspective of privileged structures, Future Med. Chem., 2017, 9(15), 1765–1794, DOI:10.4155/fmc-2017-0100.
- M. M. Ibrahim, M. M. Mounier and S. A. Bekheet, Targeting apoptotic anticancer response with natural glucosinolates from cell suspension culture of Lepidium sativum, J. Genet. Eng. Biotechnol., 2023, 21(1), 53–68, DOI:10.1186/s43141-023-00511-y.
- K. Liliom, A. Lehotzky, A. Molnár and J. Ovádi, Characterization of tubulin-alkaloid interactions by enzyme-linked immunosorbent assay, Anal. Biochem., 1995, 228(1), 18–26, DOI:10.1006/abio.1995.1309.
- K. Burton, A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid, Biochem. J., 1956, 62(2), 315–323, DOI:10.1042/bj0620315.
- S. Diab, T. Teo, M. Kumarasiri, P. Li, M. Yu and F. Lam, et al., Discovery of 5-(2-(phenylamino)pyrimidin-4-yl)thiazol-2(3H)-one derivatives as potent Mnk2 inhibitors: synthesis, SAR analysis and biological evaluation, ChemMedChem, 2014, 9(5), 962–972, DOI:10.1002/cmdc.201300552.
- M. A. Raslan and A. H. Afifi, In vitro wound healing properties, antioxidant activities, HPLC-ESI-MS/MS profile and phytoconstituents of the stem aqueous methanolic extract of Dracaena reflexa Lam, Biomed. Chromatogr., 2022, 36(6), e5352, DOI:10.1002/bmc.5352.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07810a |
| This journal is © The Royal Society of Chemistry 2025 |