Open Access Article
Open Access ArticlePreparation and structural optimization of vanadium oxide nanotubes as cathode materials for PIBs with improved performance†
Yuan Xiea,
Jia Wena,
Junyuan Huanga,
Rong Jianga,
Longjun Daia,
Yang Rena,
Zhu Liuab and
Xiaowei Zhou *a
*a
aDepartment of Physics, School of Physics and Astronomy, Yunnan University, Kunming, Yunnan 650504, China. E-mail: zhouxiaowei@ynu.edu.cn
bYunnan Key Laboratory of Micro/Nano-Materials and Technology, School of Materials and Energy, Yunnan University, Kunming, 650504, China
First published on 3rd February 2025
Abstract
In this work, we synthesized hollow multi-walled vanadium oxide nanotubes (VOx-NT) using a soft template technique under hydrothermal reaction conditions. By replacing organic templates, which do not contribute to electrochemical potassium storage, with K+ in a solution environment, we effectively retained the original hollow multi-walled structure of VOx-NT while obtaining the K-VOx-NT material. We conducted systematic characterizations of the microstructure, morphology, and composition of these materials and evaluated their potassium storage performance. Compared to the original VOx-NT, K-VOx-NT exhibited significantly enhanced cycling stability and rate performance when serving as the cathode in potassium-ion batteries (PIBs). It demonstrated a reversible discharge specific capacity of 75.7 mA h g−1 for the 1st cycle at a current density of 50 mA g−1 within a voltage range of 1.5–3.8 V (vs. K+/K) and retained 62.2 mA h g−1 after the 50th cycle. When a current density of 600 mA g−1 was applied, it could still deliver a capacity of 44.3 mA h g−1. Furthermore, the storage and degradation mechanisms of K+ in K-VOx-NT were elucidated. In addition, using hard carbon as the anode, the K-VOx-NT full-cell was tested to further evaluate its practical performance. This work provides insight into the design and modification of vanadium-based cathode materials for future PIBs.
1. Introduction
Nowadays, effective energy storage is a crucial aspect of industrial production and daily life. In recent decades, lithium-ion batteries (LIBs) have dominated the energy storage market owing to their high energy and power density.1–3 However, in view of the high cost and limited shortage of lithium resources as well as the need for sustainable development in large-scale energy storage for the future, there is an urgent need to explore new alternatives and supplements for LIBs.4,5 Notably, K+ exhibits chemical properties similar to those of Li+ and Na+ but with a smaller Stokes radius in the electrolyte than both, which could facilitate its ionic conductivity in the electrolyte.6,7 Moreover, K+ possesses a lower standard potential (−2.93 V vs. SHE) than Na+ (−2.71 V vs. SHE), which is closer to that of Li+ (−3.04 V vs. SHE).8,9 Thus, potassium-ion batteries (PIBs) can be considered promising candidates for future electrochemical energy storage devices with relatively high working voltage and energy density.5–10 At present, significant progress has been made in research on anode materials for PIBs, with a strong focus on carbon-based materials and their nanocomposites, such as various amorphous carbons, metal/C (Sb/C, Sn/C, etc.) and metal chalcogenide/carbon nanocomposites (Bi2S3/C, Sb2Se3/C, etc.).11,12 Generally, these materials deliver high capacity for potassium storage and display great potential for practical applications. However, advancements in cathode materials remain unsatisfactory, limiting the overall performance and future commercialization of PIBs.13,14In order to develop PIBs with high performance, it is crucial to investigate cost-effective cathodes with optimal electrochemical performance. The most studied cathode materials for PIBs are Prussian blue (PB) and its analogs (PBAs), whose unique three-dimensional channel structures can provide abundant active sites for K+.15,16 However, PB and PBAs suffer from some inherent drawbacks, such as poor conductivity, thermal stability and the presence of interstitial water, which can impact their K+ storage properties.17 Another type of transition metal oxide (TMO) (M = Mn, Co, Cr, V, etc.) has attracted the attention of researchers due to its easy preparation in air, stability and special layered structure, which could provide smooth pathways for fast transport and effective storage of K+.18,19 Among these TMOs, a typical representative is vanadium oxide, which has the advantages of facile synthesis and an inherent multi-channel framework, facilitating the rapid diffusion of K+.20 According to previous reports, Deng et al. prepared a kind of K0.5V2O5 nanobelt by hydrothermal reaction with K+ pre-intercalated into layered V2O5, which exhibited an initial capacity of 60 mA h g−1 under 100 mA g−1 between 1.5 and 3.8 V and retained 51.6 mA h g−1 after 100 cycles.21 Xu et al. reported NH4+ inserted NH4V4O10 with a flower-like architecture, which was synthesized via hydrothermal reaction utilizing V2O5 as the vanadium source and NH4Cl as the ammonium source. It delivered a relatively high capacity of 80 mA h g−1 at 50 mA g−1 for the 2nd discharge within a voltage range of 2–3.8 V and retained a value of 75 mA h g−1 after 200 cycles, indicating good cycling stability.22 Evidently, it is an effective strategy to adjust the interlayer spacing of vanadium oxide for enhancing K+ storage.
In particular, vanadium oxide nanotubes (VOx-NT) with a hollow and multi-walled microstructure were successfully fabricated under hydrothermal conditions using V2O5 as the precursor and suitable organic amines with long-chains as the soft template.23 Typically, Nordlinder et al. have applied this VOx-NT as the cathode material for lithium-ion batteries (LIBs), which exhibited a large capacity of up to 250 mA h g−1 for the 2nd discharge.24 The unique microstructure of VOx-NT can offer plentiful active sites, which is beneficial for the storage of Li+. It is expected that VOx-NT can also provide similar advantages for K+ storage. Therefore, it is necessary and meaningful to explore the potential of VOx-NT as the cathode for PIBs. In this study, VOx-NT was synthesized through a one-step hydrothermal process adopting V2O5 as the vanadium source and dodecylamine (C12H27N) as the soft template. In addition, the K+-exchange technique was performed and the organic template within VOx layers was effectively removed, resulting in the generation of K-VOx-NT. We conducted a comprehensive analysis and comparison between VOx-NT and K-VOx-NT by various characterization methods and electrochemical K+ storage tests. The relationship between their reversible electrochemical K+ storage performances, mechanisms and microstructures was systematically discussed and elucidated. We presented the intrinsic reasons and mechanism for the degradation of reversible K+ storage performance in VOx-NT. A feasible strategy to enhance the K+ storage properties of VOx-NT via the cation-exchange method was proposed. This research provides valuable insights to design vanadium-based cathode materials with high-performance for PIBs through interlayer microstructure regulation.
2. Experimental section
2.1 Preparation of VOx-NT and K-VOx-NT
Specifically, 0.91 g of V2O5 powder was dispersed in 70 mL of deionized (DI) water and stirred for 30 min. Next, 0.93 g of dodecylamine (C12H27N) was dissolved in 3 mL of ethanol. Then, this solution was added to the above V2O5 dispersion and stirred continuously for another 12 h to form a uniform yellow suspension. Subsequently, the suspension was transferred to a 100 mL Teflon-lined autoclave and sealed, which was subjected to hydrothermal treatment in an oven at 180 °C for 5 days. Finally, a black product was obtained via centrifugation, washing and drying, which was denoted as VOx-NT.For the preparation of K+-exchanged VOx-NT (K-VOx-NT), firstly, 0.4 g of as-prepared VOx-NT was dispersed in a mixed solution containing 80 mL of anhydrous ethanol and 20 mL of DI water with agitation for 30 min. Then, 8 g of KCl was dissolved into the above black suspension, which was stirred continuously for 12 h to conduct the K+ exchange process. The resulting product was washed, dried and labeled as K-VOx-NT.
2.2 Characterization
X-Ray Diffraction (XRD, TTRIII-18KW, Rigaku) was employed to study the crystalline structure of samples over a 2θ angle range from 2° to 60° using Cu Kα (λ = 1.5406 Å) radiation. Field emission scanning electron microscopy (FESEM, Nova Nano 450, FEI) and transmission electron microscopy (TEM, JEOL, JEM-F200) were utilized to observe the morphology and microstructure of target samples. TEM was equipped with high angle annular dark field (HAADF) imaging and energy dispersive X-ray spectroscopy (EDS). Raman spectra were collected by using a spectrometer (Renishaw inVia, λ = 514.5 nm) in the wave-number range of 100 to 1800 cm−1. Fourier transform infrared (FTIR) spectra were recorded through a spectrometer (BFRL, WQF-520A) in the wave-number range from 400 to 4000 cm−1 based on KBr tabletting. Thermogravimetric analysis-differential scanning calorimetry (TGA/DSC1/1600LF, Mettler Toledo) was performed in air environment with a heating rate of 10 °C min−1. X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Scientific) was carried out to determine the elemental components and valence information of samples.2.3 Electrochemical measurement
The cathode was fabricated by mixing the active material (VOx-NT or K-VOx-NT), a conductive agent (carbon black) and a binder (polyvinylidene fluoride, PVDF) with a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using N-methyl-2-pyrrolidone (NMP) as the solvent. The slurry formed was uniformly coated on Al foil and heated at 100 °C for 10 h under vacuum. Then, the cathode plate dried was cut into a circular wafer with a diameter of 8 mm, which was used as the working electrode. For galvanostatic charge–discharge (GCD) tests, the active mass of VOx-NT or K-VOx-NT on the working electrode was approximately 1.7 mg cm−2. The CR2025 coin-cell was assembled in an argon-filled glove box with both moisture and oxygen concentrations below 0.01 ppm. A porous glass fiber membrane (GF/D) was utilized as the separator and K metal as both counter and reference electrodes. The electrolyte consisted of 1 M potassium bis(fluorosulfonyl)imide (KFSI), which was dissolved in a mixed solvent of ethyl carbonate (EC) and diethyl carbonate (DEC) with a 1
1 using N-methyl-2-pyrrolidone (NMP) as the solvent. The slurry formed was uniformly coated on Al foil and heated at 100 °C for 10 h under vacuum. Then, the cathode plate dried was cut into a circular wafer with a diameter of 8 mm, which was used as the working electrode. For galvanostatic charge–discharge (GCD) tests, the active mass of VOx-NT or K-VOx-NT on the working electrode was approximately 1.7 mg cm−2. The CR2025 coin-cell was assembled in an argon-filled glove box with both moisture and oxygen concentrations below 0.01 ppm. A porous glass fiber membrane (GF/D) was utilized as the separator and K metal as both counter and reference electrodes. The electrolyte consisted of 1 M potassium bis(fluorosulfonyl)imide (KFSI), which was dissolved in a mixed solvent of ethyl carbonate (EC) and diethyl carbonate (DEC) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. An electrochemical workstation (CHI660) was utilized to conduct cyclic voltammetry (CV) within the voltage range of 1.5–3.8 V (vs. K/K+) under various scan rates. A LAND battery test system (CT2001A) was employed to measure the GCD performance of cathodes at different current densities between 1.5 and 3.8 V. The CHI660 was also adopted to perform electrochemical impedance spectroscopy (EIS) in a frequency range from 100 kHz to 0.01 Hz by applying an alternating current (AC) signal with an amplitude of 5 mV. The galvanostatic intermittent titration technique (GITT) was performed on a CT2001A through alternate application of pulse current (lasting 15 min with a current density of 20 mA g−1) and relaxation process (lasting 45 min in an open circuit state). All electrochemical tests were carried out under ambient temperature. To evaluate the practical performance of the K-VOx-NT cathode in a full-cell, we employed hard carbon (Kuraray, Type 1) as the standardized anode, which was slightly excessive when matched with K-VOx-NT (N/P ratio: ∼1.1). This hard carbon anode was pre-intercalated with K+. The GCD tests of the hard carbon half-cell were conducted in the voltage range of 0.01–2.5 V under 50 mA g−1. The GCD tests of the full-cell were performed in the voltage range of 1.0–3.6 V under 50 mA g−1. The rate capability of the full-cell was evaluated within the same voltage range with different N/P ratios of ∼1.1 and 4.0 (refer to the ESI†).
1 volume ratio. An electrochemical workstation (CHI660) was utilized to conduct cyclic voltammetry (CV) within the voltage range of 1.5–3.8 V (vs. K/K+) under various scan rates. A LAND battery test system (CT2001A) was employed to measure the GCD performance of cathodes at different current densities between 1.5 and 3.8 V. The CHI660 was also adopted to perform electrochemical impedance spectroscopy (EIS) in a frequency range from 100 kHz to 0.01 Hz by applying an alternating current (AC) signal with an amplitude of 5 mV. The galvanostatic intermittent titration technique (GITT) was performed on a CT2001A through alternate application of pulse current (lasting 15 min with a current density of 20 mA g−1) and relaxation process (lasting 45 min in an open circuit state). All electrochemical tests were carried out under ambient temperature. To evaluate the practical performance of the K-VOx-NT cathode in a full-cell, we employed hard carbon (Kuraray, Type 1) as the standardized anode, which was slightly excessive when matched with K-VOx-NT (N/P ratio: ∼1.1). This hard carbon anode was pre-intercalated with K+. The GCD tests of the hard carbon half-cell were conducted in the voltage range of 0.01–2.5 V under 50 mA g−1. The GCD tests of the full-cell were performed in the voltage range of 1.0–3.6 V under 50 mA g−1. The rate capability of the full-cell was evaluated within the same voltage range with different N/P ratios of ∼1.1 and 4.0 (refer to the ESI†).
3 Results and discussion
The cross-sectional microstructures of VOx-NT and K-VOx-NT are presented in Fig. 1(a), which can be depicted as rolled or concentric vanadate layers (VOx) intercalated by protonated dodecylamine (C12H25NH3+). In the hydrothermal reaction, C12H27N served as a soft template and at the same time a weak reducing agent, guiding the formation of VOx-NT by a curl formation mechanism. During the hydrothermal reaction that forms VOx-NT, the organic template (C12H25NH3+), which is located between the VOx layers, acts as a reducing agent. The reduction induces a partial conversion of V5+ to V4+ within the VOx layers, which consequently leads to the changes of internal stress in VOx layers. As a result, the VOx layers undergo directional curling, ultimately forming a multilayered tubular structure (VOx-NT).25–27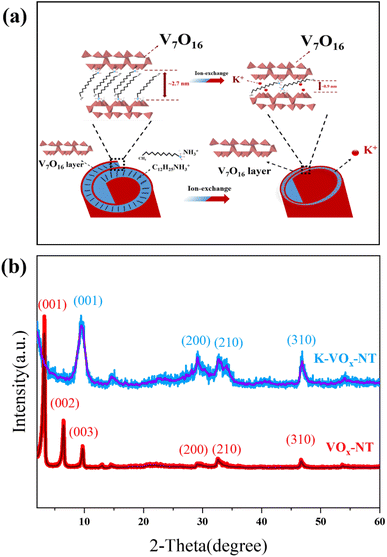 | ||
| Fig. 1 (a) Schematic of the microstructure of VOx-NT and illustration of the K+ exchange process; (b) XRD patterns and relevant fittings of VOx-NT and K-VOx-NT. | ||
With regard to the VOx layer, it resembles the triclinic crystal system of BaV7O16·nH2O bronze.23,25 C12H25NH3+ between VOx layers would be effectively removed by the K+ exchange process, resulting in an obvious reduction of distance between VOx layers. Simultaneously, K+ will enter the interlayer of VOx layers to maintain the overall electrical neutrality of VOx-NT, finally forming K-VOx-NT. It is worth noting that throughout this process, the two-dimensional microstructure of VOx layers is well preserved. XRD patterns and relevant fittings for the two samples are shown in Fig. 1(b), which reveal two series of diffraction peaks: (00l) series, which is in the small-angle region (2°–15°), reflects the periodic layered structure of VOx-NT and directly indicates the spacing of VOx layers via the corresponding calculation, and (hk0) series, which is in the large-angle region (15°–50°), demonstrates the microstructural features of the two-dimensional VOx layer itself.27 It can be observed that after K+ exchange, the diffraction peaks of K-VOx-NT are noticeably broader compared to those of VOx-NT, indicating an increase in its amorphous nature. The peak positions of the (hk0) series in the large angle region don't display obvious shifting, indicating that the K+ exchange process has hardly affected the microstructure of 2D VOx layers. The (00l) peak in the small-angle region shifts from 3.22° for VOx-NT to 9.5° for K-VOx-NT through K+ exchange. According to Bragg's law, it indicates that the interlayer spacing of VOx-NT decreases from ∼2.74 nm to ∼0.93 nm. It is because C12H25NH3+ with a long chain-length between VOx layers is replaced by K+ with a relatively small diameter, reducing the interlayer distance.
TEM and HR-TEM images of VOx-NT (a–c) and K-VOx-NT (d–f) are given in Fig. 2. It can be seen that both samples are hollow and multi-walled nanotubes. The dark lattice fringes represent VOx layers and the bright stripes correspond to the protonated dodecylamine template. The difference is that the interlayer distance between VOx layers for K-VOx-NT in Fig. 2(f) decreases from ∼2.7 nm for VOx-NT in Fig. 2(c) to ∼0.9 nm. This change corresponds well with XRD results and the K+ exchange mechanism proposed. It indicates that the protonated dodecylamine between the VOx layers of VOx-NT was effectively removed via K+ exchange. EDX mapping in Fig. 2(g) shows that K, N, O, V and C elements are evenly distributed in K-VOx-NT, which illustrates that K+ enters the interlayer of VOx layers successfully after the exchange process and there is still a small amount of residual dodecylamine in K-VOx-NT, as evidenced by the FTIR spectrum in Fig. S1† and the TGA-DSC test in Fig. 4(d). It should be noted that the clarity of C element in EDX mapping is due to the fact that K-VOx-NT was tested on a carbon-coated copper mesh. Therefore, it is difficult to avoid the interference from inherent carbon on carbon-coated copper mesh. Besides, EDX mapping is also sensitive to carbon element that still exists in residual organic dodecylamine. However, the original hollow and multi-walled microstructure of VOx-NT has been maintained.
 | ||
| Fig. 2 TEM and HR-TEM images of VOx-NT (a–c) and K-VOx-NT (d–f); HAADF image and the corresponding elemental mappings for K-VOx-NT (g). | ||
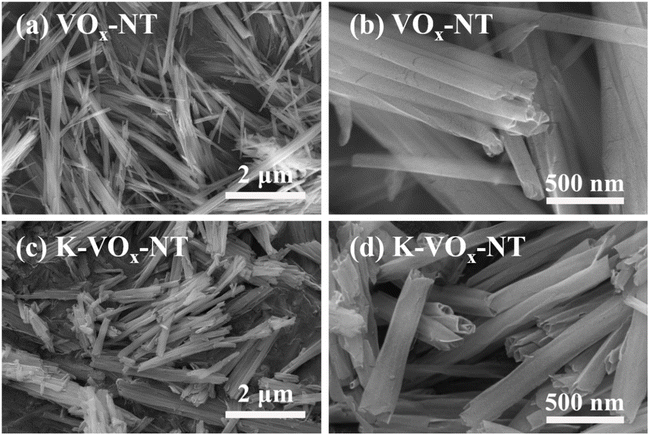
Fig. 3 shows the SEM images of VOx-NT (a, b) and K-VOx-NT (c, d). It clearly demonstrates that both samples possess a hollow tubular morphology and internal curling feature, especially as observed in Fig. 3(d). This phenomenon is consistent with the aforementioned TEM observations. The diameter and length for both samples are ∼100–200 nm and ∼2–5 μm, respectively. It is worth mentioning that the tube wall of K-VOx-NT has become thinner and its diameter becomes larger compared to VOx-NT, which may be caused by stress release when the curly structure relaxes during K+ exchange. TEM observations corroborate again the curl formation mechanism proposed earlier.
FTIR spectra for the two samples are depicted in Fig. 4(a), where the absorption peaks at ∼485 and ∼574 cm−1 correspond to V–O–V symmetric stretching and deforming vibrations of the vanadium oxygen polyhedron.14,28 The peak at ∼1001 cm−1 can be attributed to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching mode. The positions of these peaks, which could be designated as vibration modes of vanadium–oxygen bonds, have hardly shifted before and after K+ exchange. This illustrates that the microstructure of VOx layers in VOx-NT is basically not affected by K+ exchange. The absorption peaks at ∼1611 and 3400 cm−1 for both samples are caused by bending and stretching vibrations of O–H bonds in surface adsorbed and deep crystalline water, respectively.14,29,30 Due to K+ exchange, the peaks located at ∼1461, 2854 and 2919 cm−1 for K-VOx-NT, which can be ascribed to different stretching and bending vibration modes of C–H bonds in dodecylamine, significantly weaken compared with VOx-NT. Furthermore, the peak at ∼3160 cm−1, which can be assigned to the vibration mode of the N–H bond in dodecylamine,22,28,31 nearly disappears for K-VOx-NT. These changes suggest that most of the C12H25NH3+ within VOx-NT has been removed after K+ exchange. Raman spectra of the two samples are presented in Fig. 4(b). For K-VOx-NT, the characteristic peaks at ∼164 and 683 cm−1 can be assigned to the bending and stretching vibrations of the V–O bond, respectively. The peaks centered at ∼265 and 1010 cm−1 can be ascribed to the bending and stretching vibrations of the V
O asymmetric stretching mode. The positions of these peaks, which could be designated as vibration modes of vanadium–oxygen bonds, have hardly shifted before and after K+ exchange. This illustrates that the microstructure of VOx layers in VOx-NT is basically not affected by K+ exchange. The absorption peaks at ∼1611 and 3400 cm−1 for both samples are caused by bending and stretching vibrations of O–H bonds in surface adsorbed and deep crystalline water, respectively.14,29,30 Due to K+ exchange, the peaks located at ∼1461, 2854 and 2919 cm−1 for K-VOx-NT, which can be ascribed to different stretching and bending vibration modes of C–H bonds in dodecylamine, significantly weaken compared with VOx-NT. Furthermore, the peak at ∼3160 cm−1, which can be assigned to the vibration mode of the N–H bond in dodecylamine,22,28,31 nearly disappears for K-VOx-NT. These changes suggest that most of the C12H25NH3+ within VOx-NT has been removed after K+ exchange. Raman spectra of the two samples are presented in Fig. 4(b). For K-VOx-NT, the characteristic peaks at ∼164 and 683 cm−1 can be assigned to the bending and stretching vibrations of the V–O bond, respectively. The peaks centered at ∼265 and 1010 cm−1 can be ascribed to the bending and stretching vibrations of the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Besides, the peak at ∼501 cm−1 could be attributed to the stretching mode of the V–O–V bond.22,32,33 With respect to VOx-NT, it only exhibits two broadened peaks at ∼1390 and 1580 cm−1, which can be attributed to the vibration modes of C–H bonds in dodecylamine, indicating a substantial presence of C12H25NH3+ in VOx-NT.34 However, other characteristic peaks derived from vanadium–oxygen bond vibrations are not distinctly observed for VOx-NT. It is mainly because the relatively strong vibration modes of C–H bonds in dodecylamine cover the features of vanadium–oxygen bond vibration. Almost no characteristic peaks originating from C–H bond vibration were detected in K-VOx-NT, suggesting a sufficient exchange of C12H25NH3+ in VOx-NT by K+.
O bond. Besides, the peak at ∼501 cm−1 could be attributed to the stretching mode of the V–O–V bond.22,32,33 With respect to VOx-NT, it only exhibits two broadened peaks at ∼1390 and 1580 cm−1, which can be attributed to the vibration modes of C–H bonds in dodecylamine, indicating a substantial presence of C12H25NH3+ in VOx-NT.34 However, other characteristic peaks derived from vanadium–oxygen bond vibrations are not distinctly observed for VOx-NT. It is mainly because the relatively strong vibration modes of C–H bonds in dodecylamine cover the features of vanadium–oxygen bond vibration. Almost no characteristic peaks originating from C–H bond vibration were detected in K-VOx-NT, suggesting a sufficient exchange of C12H25NH3+ in VOx-NT by K+.
TGA-DSC analyses of VOx-NT (c) and K-VOx-NT (d) are illustrated in Fig. 4. For VOx-NT, a weight loss of ∼1.26% from room temperature to 200 °C happens, which is due to the evaporation of adsorbed water and a small quantity of dodecylamine on the surface of VOx-NT. There is a significant weight loss of ∼33.89% between 200 and 400 °C, which is primarily brought about by oxidative decomposition of dodecylamine intercalated in VOx layers. It should be noted that the remaining vanadium oxide will undergo oxidation and recrystallization at the same time. So, weight loss and gain occur simultaneously, but weight loss dominates within this temperature range. The DSC curve for VOx-NT displays two exothermic peaks: the first one at ∼269 °C is mainly due to the oxidative decomposition of dodecylamine; the second one at ∼389 °C is jointly caused by the decomposition of dodecylamine and oxidation of residual VOx. Above 400 °C, a slight increase in weight occurs, which may be owing to the complete oxidation of VOx (containing a certain amount of V4+) into V2O5.14 A downward endothermic peak at ∼673 °C is due to the melting of V2O5. In regard to K-VOx-NT, there is a weight loss of ∼3.22% from room temperature to 272 °C, which is mainly caused by the evaporation of adsorbed water and decomposition of residual dodecylamine, whereas a weight gain of ∼2.19% happens between ∼272 and 480 °C, which is chiefly due to further oxidation of residual KyVOx into potassium vanadate with higher V valence. A weak but wide exothermic peak in the temperature range of ∼150 to 450 °C can be observed. It can be ascribed to the oxidative reaction of K-VOx-NT. Similarly, a downward endothermic peak at ∼526 °C occurs, which is owing to the melting of remanent potassium vanadate.
 | ||
| Fig. 4 FTIR (a) and Raman (b) spectra of VOx-NT and K-VOx-NT; TGA-DSC curves of VOx-NT (c) and K-VOx-NT (d) under air condition. | ||
General XPS spectra of the two samples are shown in Fig. 5(a) and (c). Compared to VOx-NT, the relative intensity of the C1s peak for K-VOx-NT is significantly reduced and its N1s peak has disappeared. Besides, K2s and K2p peaks are detected in K-VOx-NT. Fig. S2† displays the K2p fine survey spectrum of K-VOx-NT. Both C1s and N1s mainly originate from dodecylamine, so it indicates that K+ has effectively replaced the protonated dodecylamine in VOx-NT to form K-VOx-NT. Fig. 5(b) and (d) display the V2p fine survey spectra of the two samples, which are characterized by V2p1/2 centered at ∼524.0 eV and V2p3/2 at ∼516.6 eV. Both V2p1/2 and V2p3/2 can be fitted with two peaks, which represent the existence of V4+ at a slightly lower energy state and V5+ at a slightly higher energy state, respectively.34–36 Fittings and subsequent calculations based on peak areas revealed that the average valence states of vanadium in VOx-NT and K-VOx-NT are +4.38 and +4.57, respectively. In comparison with VOx-NT, the proportion of V5+ in K-VOx-NT slightly increases after K+ exchange. This may be due to the fact that the presence of substantial dodecylamine on the surface and between the interlayers of VOx-NT could influence the accurate survey of vanadium valence state because XPS is a surface analysis technique. Based on the XPS results of V2p in K-VOx-NT, its vanadium valence state is very close to that in V7O16 reported in previous literature.23,25,37 So, it confirms the structural concept mentioned earlier for the VOx layer in samples.
Fig. 6(a) and (b) depict the first three cyclic voltammetry (CV) curves for VOx-NT and K-VOx-NT with a scan rate of 0.2 mV s−1 in the voltage range of 1.5–3.8 V. For VOx-NT, the initial cathodic scan reveals a distinct reduction peak at ∼1.7 V, which is significantly different from the 2nd scan and primarily due to the formation of an irreversible solid electrolyte interface (SEI) film on the surface of the electrode during the initial discharge process.7 This also leads to a lower initial coulombic efficiency (ICE). The 2nd and 3rd CV curves of VOx-NT lack clear redox peaks and show a certain degree of noncoincidence, implying its amorphous feature and subsequent poor cycling stability. By contrast, the initial cathodic scan for K-VOx-NT reveals less deviation from 2nd and 3rd scans, indicating the formation of a less obvious SEI film and higher ICE compared to VOx-NT. The CV curves for K-VOx-NT overlap well and exhibit two pairs of obvious redox peaks at ∼1.9/1.5 V and ∼2.2/2.0 V, suggesting its good cycling stability and reversible phase transition for K+ storage. Fig. 6(c) and (d) show the GCD profiles of the two samples at the 1st, 10th and 50th cycles. As VOx-NT and K-VOx-NT are initially in a charged state with insufficient K+, their first charging curves are incomplete. Their charge capacities are mainly attributed to self-discharge and the extraction of a small amount of structural K+. It can be observed that the specific capacity of VOx-NT significantly decreases over cycles without distinct voltage plateaus, which is consistent with its CV results. In comparison, K-VOx-NT maintains a high-capacity retention upon cycling with pronounced voltage plateaus, as corroborated by its CV results. To further investigate the K+ storage mechanism of K-VOx-NT during the discharge process, ex situ XRD tests at pristine and discharge to 2.0 V and 1.5 V states were performed, as illustrated in Fig. S3.† It indicates a slight contraction for the (001) plane of K-VOx-NT, which can be attributed to the enhanced interaction between intercalated K+ and negatively charged VOx layers as well as the reduced electrostatic repulsion between O2− in adjacent layers. Raman spectra were collected at different discharge states for K-VOx-NT and are shown in Fig. S4(a),† which illustrates the obvious transformation of V5+ to V4+ during discharge. The V2p fine spectrum in the fully discharged state is shown in Fig. S4(b),† which indicates that a portion of V4+ and V5+ is reduced to V3+ and V4+ upon K+ intercalation, respectively.
Cycling performances of the two samples at 50 mA g−1 between 1.5 and 3.8 V are depicted in Fig. 6(e). VOx-NT demonstrates a specific capacity of 103.7 mA h g−1, 70.2 mA h g−1 and 23.6 mA h g−1 for the 1st, 10th and 50th discharges, respectively. Under the same condition, K-VOx-NT delivers a capacity of 88.5 mA h g−1, 72.5 mA h g−1 and 59.2 mA h g−1, respectively. The result indicates that K-VOx-NT possesses significantly better cycling stability compared with VOx-NT, which can be attributed to the effective removal of the electrochemically inactive dodecylamine, thereby diminishing its possible side reactions with the electrode or electrolyte. Due to the introduction of the organic template during the synthesis process and the subsequent K+ exchange step, it is challenging to accurately determine the exact stoichiometric molecular formula of VOx-NT and K-VOx-NT. The slight shift observed in the (001) peak of the XRD pattern for discharged K-VOx-NT suggests the intercalation of K+ into the VOx layers of K-VOx-NT. The qualitative charge–discharge reactions of VOx-NT and K-VOx-NT can be described by the following equations:
| VOx-NT + yK+ + ye− ↔ KyVOx-NT | (1) |
| K-VOx-NT + zK+ + ze− ↔ K1+zVOx-NT | (2) |
At the beginning of the reversible potassium storage stage, y > z. However, as cycling progresses, z > y. Rate performances of the two samples at different current densities are presented in Fig. 6(f). VOx-NT displays a specific capacity of 71.0, 32.5, 17.4, 6.4, 4.7 and 46.8 mA h g−1 under 20, 50, 200, 400, 600 and 20 mA g−1 for the 10th discharge, respectively. Under the same conditions, the corresponding capacity values for K-VOx-NT are 89.0, 66.4, 56.9, 46.2, 44.3 and 84.1 mA h g−1, respectively. This indicates that K-VOx-NT, which profits from the effective removal of dodecylamine without electrochemical activity and meanwhile maintains its nanotubular structure, can exhibit significantly improved cycling and rate performance compared to pristine VOx-NT.
Fig. 7 gives the TEM images of VOx-NT (a, b) and K-VOx-NT (c, d) after 100 cycles at 50 mA h g−1. It can be observed that the hollow nanotubular structure of VOx-NT collapses severely, whereas K-VOx-NT retains its hollow nanotubular morphology well. This indicates that the good microstructure retention of K-VOx-NT can be responsible for its better cycling performance compared to VOx-NT.
 | ||
| Fig. 7 TEM images of VOx-NT (a, b) and K-VOx-NT (c, d) at different magnifications after 100 cycles. | ||
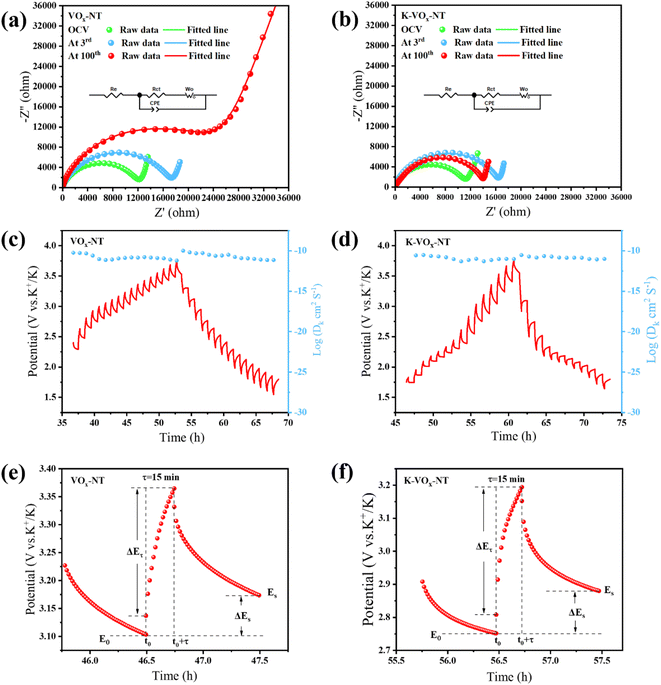
Nyquist plots (dot), relevant fitting results (line) and equivalent circuit (inset) of VOx-NT and K-VOx-NT at the open circuit voltage (OCV), 3rd cycle and 100th cycle are presented in Fig. 8(a) and (b), respectively. Nyquist plots are characterized by a semicircle in the middle and high frequency regions associated with charge transfer resistance (Rct) and a sloping line in the low frequency region related to Warburg impedance (Wo). Rct indicates the difficulty of K+ passing through the electrolyte/electrode interface, while Wo reflects the diffusion impedance of K+ within the electrode.38 Additionally, the intercept of the Nyquist plot with the real impedance (Z′) axis represents the electrolyte resistance (Re). The constant phase element (CPE) in the equivalent circuit stands for the capacitive behavior related to charge transfer at the electrolyte/electrode interface.39
Through fitting based on the equivalent circuit, the Rct values of VOx-NT at the OCV, 3rd and 100th cycles were 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 Ω, 17
190 Ω, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 Ω and 21
286 Ω and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 Ω, respectively. By contrast, the Rct values of K-VOx-NT at the corresponding states were 11
520 Ω, respectively. By contrast, the Rct values of K-VOx-NT at the corresponding states were 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 Ω, 16
230 Ω, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 Ω and 13
440 Ω and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 Ω, respectively. It is evident that the Rct value of VOx-NT increased with cycling, which correlates with its microstructural degradation upon repeated K+ insertion/extraction. Conversely, the Rct value of K-VOx-NT initially increased, but showed a descending trend as the cycling continued. On one hand, it is related to the good microstructural preservation of K-VOx-NT upon cycling. On the other hand, it may benefit from the gradual maturation of the SEI film on the surface of K-VOx-NT during cycling, facilitating K+ migration between the electrolyte and electrode. EIS tests indicate that the effective removal of dodecylamine intercalated in VOx-NT will be conducive to dynamic behavior of K+ at the electrode/electrolyte interface, maintaining lower Rct.
990 Ω, respectively. It is evident that the Rct value of VOx-NT increased with cycling, which correlates with its microstructural degradation upon repeated K+ insertion/extraction. Conversely, the Rct value of K-VOx-NT initially increased, but showed a descending trend as the cycling continued. On one hand, it is related to the good microstructural preservation of K-VOx-NT upon cycling. On the other hand, it may benefit from the gradual maturation of the SEI film on the surface of K-VOx-NT during cycling, facilitating K+ migration between the electrolyte and electrode. EIS tests indicate that the effective removal of dodecylamine intercalated in VOx-NT will be conducive to dynamic behavior of K+ at the electrode/electrolyte interface, maintaining lower Rct.
GITT tests were conducted to explore the K+ diffusion and transport properties in VOx-NT and K-VOx-NT, acquiring their K+ diffusion coefficients (DK+). GITT curves and log(DK+) vs. working potential for the two samples during charge/discharge processes are illustrated in Fig. 8(c) and (d). Their representative titration curves in a single pulse charge and relaxation are depicted in Fig. 8(e) and (f). Here, τ represents the pulse time, ΔES denotes the change of steady-state voltage before and after pulse stimulation and ΔEτ denotes the real-time potential variation under pulse stimulation. Based on formula (3), DK+ for the two samples can be calculated.30,39
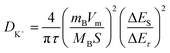 | (3) |
Fig. 9(a)–(c) give the typical CV curves for VOx-NT and K-VOx-NT at different scan rates from 0.2 to 2.0 mV s−1 between 1.5 and 3.8 V, respectively. Notably, K-VOx-NT exhibits obvious redox peaks, whereas those for VOx-NT are less pronounced, indicating that K-VOx-NT maintains good reversible K+ intercalation and deintercalation characteristics even under rapid scanning. The relationship between the peak current (i) of CV scan and scanning rate (v) is described by the following equations:39,42,43
| i = avb | (4) |
log(i) = log(avb) = log(a) + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) log(v)
| (5) |
By fitting the logarithmic relationship between anodic/cathodic peak currents (i) and scan rates (v), we obtain a linear correlation. The slope is the b value in eqn (4), which provides insights into the K+ storage mechanism within the electrode. Specifically, when b is close to 0.5, it indicates that the storage of K+ is predominantly controlled by internal diffusion. If the b value approaches 1, it suggests that surface pseudocapacitive behavior dominates the K+ storage.41,42 The fitting results of b values for VOx-NT and K-VOx-NT are depicted in Fig. 9(e), whose average values are ∼0.84 and 0.88, respectively. It indicates that K+ storage contributions for both samples are predominantly governed by surface pseudocapacitive behavior. Among them, K-VOx-NT demonstrates a slightly stronger pseudocapacitive contribution compared to VOx-NT.
To quantitatively describe the K+ storage contributions of the two samples, we divided the total current at specific voltages into surface-controlled and diffusion-controlled sections by the following formulas:28,41,43
| i(V) = k1v + k2v1/2 | (6) |
 | (7) |
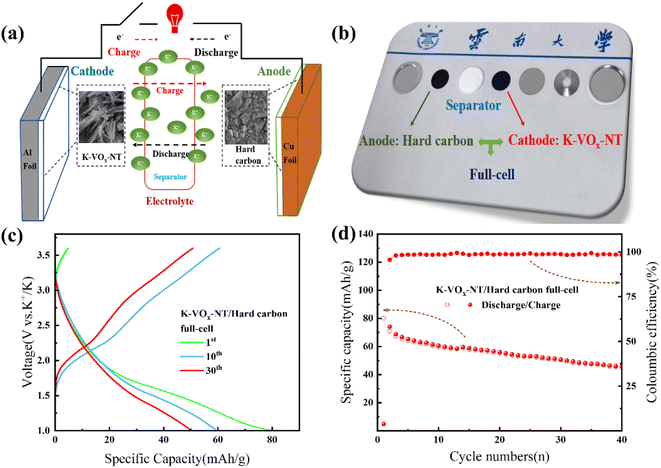
To evaluate the K+ storage performance of the K-VOx-NT cathode in practical applications, we assembled a full-cell using hard carbon as the anode. While standard anode materials for lithium-ion and sodium-ion batteries are well-established, a widely accepted anode for PIBs remains lacking. Hard carbon, which is known for its excellent potassium storage performance and low cost, is considered a promising anode material for PIBs.44 The details for the electrochemical performance of hard carbon in a half-cell configuration are illustrated in Fig. S6.† The configuration of the full-cell and actual structure of the coin-prototype cell in our laboratory are illustrated in Fig. 10(a) and (b), respectively. Fig. 10(c) and (d) present the initial three GCD profiles and cycling performance of the K-VOx-NT/Hard carbon full-cell with a N/P ratio of ∼1.1. The specific capacity of the full-cell was determined based on the mass of the active K-VOx-NT cathode. The results showed that this full-cell delivered an initial discharge specific capacity of 80.1 mA h g−1 at 50 mA g−1 between 1.0 and 3.6 V. For the 10th and 30th cycles, its discharge specific capacities decreased to 59.8 and 50.2 mA h g−1, respectively. The energy density of the full-cell is illustrated in Fig. S7.† For the evaluation of rate capability of the full-cell, we employed different N/P ratios of ∼1.1 and 4.0 (refer to Fig. S8†). It was observed that a higher N/P ratio (∼4.0) enables the K-VOx-NT cathode to fully demonstrate its ideal rate capability for K+ storage. This can be primarily attributed to the fact that, at the condition of high N/P ratio, the hard carbon anode mainly operates by capacitive behavior for K+ storage, thereby minimizing the polarization effects on the full-cell, as discussed in the ESI.†
4 Conclusion
In this study, a novel K-VOx-NT cathode with a hollow and multi-walled structure has been successfully fabricated by a soft template technique under hydrothermal conditions combined with a subsequent K+ exchange process. Systematic characterization confirmed that the dodecylamine intercalated between vanadium oxide layers, which lacks potassium storage activity, was effectively removed after K+ exchange, while the special hollow and multi-walled structure of VOx-NT was well preserved. K-VOx-NT as the cathode for PIBs exhibited a superior capacity retention (74.4%) after 50 cycles under a current density of 50 mA g−1 compared to VOx-NT (merely 17.9%) without the exchange of K+ under identical conditions. Besides, K-VOx-NT displayed significantly enhanced rate capability and lower charge transfer resistance compared with VOx-NT. This is attributed to the effective removal of the organic dodecylamine template without electrochemical activity in VOx-NT. On the other hand, K-VOx-NT maintained its special hollow and multi-walled nanotubular microstructure after K+ exchange, which can provide abundant active sites during repeated K+ insertion/extraction processes. The potassium storage performance test indicated that the capacitive contribution predominantly governs the potassiation/depotassiation processes for K-VOx-NT. Furthermore, the potassiation process in K-VOx-NT was accompanied by the valence state transition of vanadium from V5+ to V3+. This K-VOx-NT could be adopted as a promising cathode candidate for application in PIBs. Moreover, the practical performance of the K-VOx-NT full-cell was evaluated using hard carbon as the anode, providing a comprehensive assessment of its application potential. Our research provides valuable guidance for further exploration of vanadium-based cathodes used in PIBs.Data availability
The fundamental data are within the manuscript and its “ESI†” file. Other relevant data are available from the authors upon reasonable request.Author contributions
Yuan Xie: conceptualization, investigation, methodology, writing – original draft. Jia Wen: conceptualization; software. Junyuan Huang: investigation, visualization. Rong Jiang: visualization. Longjun Dai: formal analysis, software. Yang Ren: validation. Zhu Liu: methodology, data curation. Xiaowei Zhou: supervision, data curation, funding acquisition, writing – review & editing.Conflicts of interest
The authors declare that they have no competing financial interest.Acknowledgements
It is very grateful to financial supports from the National Natural Science Foundation of China (Grant No. 12264054, 12464016), General & Key Project of Applied Basic Research of Yunnan Science and Technology Department (Grant No. 202301AT070139, 202401AS070130), Double First Class Joint Special Key Project of Yunnan Science and Technology Department & Yunnan University (Grant No. 202401BF070001-012), Xingdian Talent Support Program for Youth Project, Postgraduate Quality Course (Nanomaterials and Nanotechnology) Construction Project of Yunnan Province in 2024 and Postgraduate Research Innovation Project of Yunnan University (Grant No. KC-23235277, KC-23235538).References
- J. Li, J. Fleetwood and W. B. Hawley, et al., From materials to cell: state-of-the-art and prospective technologies for lithium-ion battery electrode processing, Chem. Rev., 2021, 122(1), 903–956 CrossRef PubMed.
- L. Niu, R. Zhang and Q. Zhang, et al., Carbon-coated silicon/graphite oxide composites as anode materials for highly stable lithium-ion batteries, Phys. Chem. Chem. Phys., 2024, 26, 17292–17302 RSC.
- Y. Tong, Y. Wu and Z. Liu, et al., Fabricating multi-porous carbon anode with remarkable initial coulombic efficiency and enhanced rate capability for sodium-ion batteries, Chin. Chem. Lett., 2023, 34, 107443 CrossRef CAS.
- Y. Wang, L. Xie and R. Huang, et al., Theoretical investigation of Janus Ti2BST (T = O, Se) monolayers as anode materials for Na/K-ion batteries, Phys. Chem. Chem. Phys., 2024, 26, 18394–18401 RSC.
- Z. Chen, H. Lin and Y. Tan, et al., Anchoring multi-coordinated bismuth metal atom sites on honeycomb-like carbon rods achieving advanced potassium storage, Adv. Funct. Mater., 2024, 2407653 CrossRef CAS.
- Z. Chen, Y. Wu and X. Liu, et al., Bi/Bi3Se4 nanoparticles embedded in hollow porous carbon nanorod: High rate capability material for potassium-ion batteries, J. Energy Chem., 2023, 81, 462–471 CrossRef CAS.
- Y. Tan, H. Lin and Z. Chen, et al., Regulating the coordination microenvironment of atomic bismuth sites in nitrogen-rich carbon nanosheets as anode for superior potassium-ion batteries, J. Energy Chem., 2024, 99, 365–374 CrossRef CAS.
- N. Cheng, P. Xu and B. Lu, et al., Covalent sulfur as stable anode for potassium ion battery, J. Energy Chem., 2021, 62, 645–652 CrossRef CAS.
- J. Zhang, T. Liu and X. Cheng, et al., Development status and future prospect of non-aqueous potassium ion batteries for large scale energy storage, Nano Energy, 2019, 60, 340–361 CrossRef CAS.
- L. Wu, H. Fu and S. Li, et al., Phase-engineered cathode for super-stable potassium storage, Nat. Commun., 2023, 14(1), 644 CrossRef CAS PubMed.
- Y. Zhang, Y. Wang and L. Hou, et al., Recent Progress of Carbon-Based Anode Materials for Potassium Ion Batteries, Chem. Rec., 2022, 22(10), e202200072 CrossRef CAS PubMed.
- X. Liu, Z. Sun and Y. Sun, et al., Fast and long-lasting potassium-ion storage enabled by rationally engineering strain-relaxation Bi/Bi2O3 nanodots embedded in carbon sheets, Adv. Funct. Mater., 2023, 2307205 CrossRef CAS.
- Y. Lee, J. K. Yoo and H. Park, et al., An exceptionally large energy cathode with the K–SO 4–Cu conversion reaction for potassium rechargeable batteries, J. Mater. Chem. A, 2021, 9(9), 5475–5484 RSC.
- B. Tian, W. Tang and C. Su, et al., Reticular V2O5 0.6 H2O xerogel as cathode for rechargeable potassium ion batteries, ACS Appl. Mater. Interfaces, 2018, 10(1), 642–650 CrossRef CAS PubMed.
- L. Li, Z. Hu and Y. Lu, et al., A Low-strain potassium-rich prussian blue analogue cathode for high power potassium-ion batteries, Angew. Chem., Int. Ed., 2021, 133(23), 13160–13166 CrossRef.
- C. Gao, Y. Lei and Y. Wei, et al., Coexistence of two coordinated states contributing to high-voltage and long-life Prussian blue cathode for potassium ion battery, Chem. Eng. J., 2022, 431, 133926 CrossRef CAS.
- S. Liu, L. Kang and S. C. Jun, Challenges and strategies toward cathode materials for rechargeable potassium-ion batteries, Adv. Mater., 2021, 33(47), 2004689 CrossRef CAS.
- Y. S. Xu, S. Y. Duan and Y. G. Sun, et al., Recent developments in electrode materials for potassium-ion batteries, J. Mater. Chem. A, 2019, 7(9), 4334–4352 RSC.
- W. Li, Z. Bi and W. Zhang, et al., Advanced cathodes for potassium-ion batteries with layered transition metal oxides: a review, J. Mater. Chem. A, 2021, 9(13), 8221–8247 RSC.
- X. Xu, F. Xiong and J. Meng, et al., Vanadium-based nanomaterials: a promising family for emerging metal-ion batteries, Adv. Funct. Mater., 2020, 30(10), 1904398 CrossRef CAS.
- L. Deng, X. Niu and G. Ma, et al., Layered potassium vanadate K0.5V2O5 as a cathode material for nonaqueous potassium ion batteries, Adv. Funct. Mater., 2018, 28(49), 1800670 CrossRef.
- Y. Xu, H. Dong and M. Zhou, et al., Ammonium vanadium bronze as a potassium-ion battery cathode with high rate capability and cyclability, Small Methods, 2019, 3(8), 1800349 CrossRef.
- V. Petkov, P. Y. Zavalij and S. Lutta, et al., Structure beyond Bragg: Study of V2O5 nanotubes, Phys. Rev. B, 2004, 69(8), 085410 CrossRef.
- S. Nordlinder, K. Edström and T. Gustafsson, The performance of vanadium oxide nanorolls as cathode material in a rechargeable lithium battery, Electrochem. Solid-State Lett., 2001, 4, A129 CrossRef CAS.
- C. K. Christensen, E. D. Bøjesen and D. R. Sørensen, et al., Structural evolution during lithium-and magnesium-ion intercalation in vanadium oxide nanotube electrodes for battery applications, ACS Appl. Nano Mater., 2018, 1(9), 5071–5082 CrossRef CAS.
- J. Wei, Y. Zhu and J. Zhang, Effects of synthetic conditions on the structure and morphology of open-ended vanadium oxide nanotubes and study of their growth mechanism, Chin. Sci. Bull., 2007, 52, 1920–1924 CrossRef CAS.
- R. H. Kim, J. S. Kim and H. J. Kim, et al., Highly reduced VOx nanotube cathode materials with ultra-high capacity for magnesium ion batteries, J. Mater. Chem. A, 2014, 2(48), 20636–20641 RSC.
- Q. Zong, Q. Q. Wang and C. Liu, et al., Potassium ammonium vanadate with rich oxygen vacancies for fast and highly stable Zn-ion storage, ACS Nano, 2022, 16(3), 4588–4598 CrossRef CAS PubMed.
- Z. Feng, J. Sun and Y. Liu, et al., Polypyrrole-intercalation tuning lamellar structure of V2O5· nH2O boosts fast zinc-ion kinetics for aqueous zinc-ion battery, J. Power Sources, 2022, 536, 231489 CrossRef CAS.
- X. Niu, J. Qu and Y. Hong, et al., High-performance layered potassium vanadium oxide for K-ion batteries enabled by reduced long-range structural order, J. Mater. Chem. A, 2021, 9(22), 13125–13134 RSC.
- E. A. Esparcia, M. S. Chae and J. D. Ocon, et al., Ammonium vanadium bronze (NH4V4O10) as a high-capacity cathode material for nonaqueous magnesium-ion batteries, Chem. Mater., 2018, 30(11), 3690–3696 CrossRef CAS.
- H. Yang, Q. Li and L. Sun, et al., MXene-Derived Na+-Pillared Vanadate Cathodes for Dendrite-Free Potassium Metal Batteries, Small, 2024, 20(5), 2306572 CrossRef CAS.
- H. Zhou H, X. Yan and L. Ding, et al., Ultra-stable aqueous nickel-ion storage achieved by iron-ion pre-introduction assisted hydrated vanadium oxide cathode, Energy Storage Mater., 2024, 68, 103340 CrossRef.
- W. Chen, L. Mai and J. Peng, et al., Raman spectroscopic study of vanadium oxide nanotubes, J. Solid State Chem., 2004, 177(1), 377–379 CrossRef CAS.
- A. Wang, D. H. Liu and L. Yang, et al., Building stabilized Cu0.17Mn0.03V2O5– 2.16 H2O cathode enables an outstanding room-/low-temperature aqueous Zn-ion batteries, Carbon Energy, 2024, e512 CrossRef CAS.
- H. He, K. Cao and S. Zeng, et al., K2VOP2O7 as a novel high-voltage cathode material for potassium ion batteries, J. Power Sources, 2023, 587, 233715 CrossRef CAS.
- F. Yang, Y. Zhu and Y. Xia, et al., Fast Zn2+ kinetics of vanadium oxide nanotubes in high-performance rechargeable zinc-ion batteries, J. Power Sources, 2020, 451, 227767 CrossRef CAS.
- K. Yang, J. Tang and Y. Liu, et al., Controllable synthesis of peapod-like Sb@C and corn-like C@Sb nanotubes for sodium storage, ACS Nano, 2020, 14(5), 5728–5737 CrossRef CAS PubMed.
- C. Chen, Q. Deng and Q. Zhang, et al., PEDOT-intercalated NH4V3O8 nanobelts as high-performance cathode materials for potassium ion batteries, J. Colloid Interface Sci., 2023, 633, 619–627 CrossRef CAS PubMed.
- J. Kim, S. H. Lee and C. Park, et al., Controlling vanadate nanofiber interlayer via intercalation with conducting polymers: cathode material design for rechargeable aqueous zinc ion batteries, Adv. Funct. Mater., 2021, 31(26), 2100005 CrossRef CAS.
- Y. Zhang, X. Niu and L. Tan, et al., K0.83V2O5: a new layered compound as a stable cathode material for potassium-ion batteries, ACS Appl. Mater. Interfaces, 2020, 12(8), 9332–9340 CrossRef CAS PubMed.
- Y. Qi, M. Liao and Y. Xie, et al., Long-life vanadium oxide cathode for zinc battery enabled by polypyrrole intercalation and concentrated electrolyte, Chem. Eng. J., 2023, 470, 143971 CrossRef CAS.
- G. Xu, X. Liu and S. Huang, et al., Freestanding, Hierarchical, and Porous Bilayered NaxV2O5·nH2O/rGO/CNT Composites as High-Performance Cathode Materials for Nonaqueous K-Ion Batteries and Aqueous Zinc-Ion Batteries, ACS Appl. Mater. Interfaces, 2019, 12(1), 706–716 CrossRef.
- S. Alvin, H. S. Cahyadi and J. Hwang, et al., Revealing the intercalation mechanisms of lithium, sodium, and potassium in hard carbon, Adv. Energy Mater., 2020, 10(20), 2000283 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07672a |
| This journal is © The Royal Society of Chemistry 2025 |