Open Access Article
Open Access ArticleTerpene polymerization via a binary neodymium-based catalytic system with di-n-butylmagnesium as a co-catalyst†
Teresa Córdova a,
Francisco Javier Enriquez-Medranoa,
Ilse Magaña
a,
Francisco Javier Enriquez-Medranoa,
Ilse Magaña a,
Maricela García-Zamora
a,
Maricela García-Zamora a,
Nelson A. Jimenéz-Reyesa,
José M. Mata-Padillaa,
Edgar E. Cabrera-Álvarez
a,
Nelson A. Jimenéz-Reyesa,
José M. Mata-Padillaa,
Edgar E. Cabrera-Álvarez b,
Luis Valencia
b,
Luis Valencia *c and
Ramón Díaz de León*a
*c and
Ramón Díaz de León*a
aResearch Center for Applied Chemistry, Blvd Enrique Reyna 140, Saltillo 25294, Mexico. E-mail: Ramon.diazdeleon@ciqa.edu.mx
bCONAHCYT, Research Center for Applied Chemistry, Unidad Monterrey, Av. Alianza Sur 204, Apodaca, Nuevo León C.P. 66629, Mexico
cBiofiber Tech Sweden AB, Norrsken House, Birger Jarlsgatan 57 C, Stockholm, Sweden. E-mail: Luisalex_val@hotmail.com
First published on 9th January 2025
Abstract
The development of materials from renewable resources has been increasing, intending to reduce the consumption of fossil sources, with terpenes being one of the main families that reduce the consumption of isoprene. The study of the binary catalytic system neodymium versatate/dibutyl magnesium (NdV3/Mg(n-Bu)2), for the coordination homopolymerization of β-myrcene and β-farnesene, was carried out analysing different [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratios (between 4 and 10). Reporting conversions of 92% and 83% at an [Nd]
[Mg] ratios (between 4 and 10). Reporting conversions of 92% and 83% at an [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio of 8 for polymyrcene (PMy) and polyfarnesene (PFa), respectively, and microstructures comprising 1,4 content above 80% for both polymers (PMy, cis-59% and PFa, cis-83%). It was observed that PFa samples presented a higher 1,4-cis content in relation to PMy samples, presumably due to the size of the side group present in the monomer structure and due to steric hindrance; similarly, a 3,4 content of 14% (PMy) and 10% (PFa) was observed. The glass transition temperature of the PMy samples ranged from −63.7 °C to −66.5 °C, while for the PFa samples, it was between −75.4 °C and −75.5 °C. The binary [Nd]
[Mg] ratio of 8 for polymyrcene (PMy) and polyfarnesene (PFa), respectively, and microstructures comprising 1,4 content above 80% for both polymers (PMy, cis-59% and PFa, cis-83%). It was observed that PFa samples presented a higher 1,4-cis content in relation to PMy samples, presumably due to the size of the side group present in the monomer structure and due to steric hindrance; similarly, a 3,4 content of 14% (PMy) and 10% (PFa) was observed. The glass transition temperature of the PMy samples ranged from −63.7 °C to −66.5 °C, while for the PFa samples, it was between −75.4 °C and −75.5 °C. The binary [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] system used in the study predominantly exhibited a 1,4-cis content at [Nd]
[Mg] system used in the study predominantly exhibited a 1,4-cis content at [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratios of 8.
[Mg] ratios of 8.
Introduction
The advancement of sustainable and eco-friendly materials has emerged as a cornerstone in polymer science, motivated by increasing global concerns over environmental degradation and the depletion of fossil fuel reserves.1–3 This paradigm shift reflects a fundamental change in material science, emphasizing the development of innovative approaches to address sustainability challenges. Among these, the utilization of renewable resources as alternatives to petroleum-based raw materials has gained significant attention. Recent progress in polymerization techniques, such as visible-light-induced radical polymerization and light-controlled reversible addition–fragmentation chain transfer (RAFT) polymerization, has further expanded the potential for creating advanced materials. These methods, facilitated by quantum dot (QD) photocatalysts like cadmium selenide (CdSe), enable precise control over polymer architecture and open avenues for designing hybrid organic–inorganic nanocomposites.4,5 Such advancements not only contribute to reducing the environmental footprint but also demonstrate the versatility and functionality of next-generation polymeric materials, aligning with the broader goals of sustainability and innovation in material science.As a class of biobased monomers, terpenes have received considerable attention in this context.6–8 Their intrinsic renewable nature and wide prevalence in plant resins and essential oils make them an attractive alternative to some traditional petroleum-based monomers for polymer synthesis, like butadiene.9–12 In our previous study, we successfully synthesized high molecular weight polyterpenes via coordinative chain transfer polymerization using a ternary Ziegler–Natta catalyst system, resulting in a predominantly cis microstructure.13 This research, just like multiple others in the literature,10,14–20 has readily demonstrated the potential of terpenes as viable monomers for creating sustainable polymers.
Recent studies have further elucidated the role of monomer structure in determining polymer microstructure, particularly in systems utilizing magnesium-based co-catalysts.21 Notably, in the polymerization of isoprene and butadiene, the use of a binary system with [Mg(n-Bu)2] can lead to polymers with high trans isomer contents.22 It is crucial to elucidate the influence of this co-catalyst on the microstructural characteristics during the polymerization of renewable terpenes, such as β-myrcene and β-farnesene, particularly considering the impact of the larger side chains of these monomers.
The current study has two main objectives: first, to evaluate the effects of introducing [Mg(n-Bu)2] as a co-catalyst on critical parameters, including molecular weight, microstructure, and polymer yield, which refers to the consumption of monomer during the reaction; and second, to increase the understanding of how modified catalytic systems can be employed to produce tailored polymers from renewable resources for specific applications. We anticipate that the insights obtained from this research will contribute significantly to the advancement of sustainable polymer synthesis approaches.
Results and discussion
Polymerization behaviour and physical properties of polymers
The polymerization of β-myrcene using the neodymium-based catalytic system, in conjunction with di-n-butylmagnesium ([Mg(n-Bu)2]) as a co-catalyst was systematically explored in this research work, elucidating the effects of various reaction parameters on the polymerization behaviour and the properties of the resulting polymers. A conceptual schematic illustration of the monomers, catalysts, and co-catalysts used in this scientific work is shown in Fig. 1.The yield of the polyterpenes showed significant variations depending on the ratios used in the catalytic system. Specifically, the [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio, representing the molar proportion between neodymium and magnesium, and the [Mon]
[Mg] ratio, representing the molar proportion between neodymium and magnesium, and the [Mon]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] ratio, corresponding to the molar proportion between the monomer and neodymium, had a notable impact on the results obtained, as detailed in Table 1, where yield refers to the monomer consumption during the reaction, which is determined through gravimetric analysis. A higher [Nd]
[Nd] ratio, corresponding to the molar proportion between the monomer and neodymium, had a notable impact on the results obtained, as detailed in Table 1, where yield refers to the monomer consumption during the reaction, which is determined through gravimetric analysis. A higher [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio generally led to higher yields, indicating that the presence of [Mg(n-Bu)2] is favourable for the polymerization process. For instance, Run My2 with a 1
[Mg] ratio generally led to higher yields, indicating that the presence of [Mg(n-Bu)2] is favourable for the polymerization process. For instance, Run My2 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio of [Nd]
6 ratio of [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] achieved an 85.4% yield, which is notably higher than the 38% yield of Run My1 with a 1
[Mg] achieved an 85.4% yield, which is notably higher than the 38% yield of Run My1 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio. This variation can be attributed to the role of [Mg(n-Bu)2] in the catalytic system, which can engage in multiple functions: it can act as Lewis's acid to increase the electrophilicity of the neodymium catalyst, facilitate monomer insertion into the growing chain and also serve as chain transfer agent (CTA) to modulate molecular weight. Overall, it appears that a higher amount of [Mg(n-Bu)2] leads to higher yields, as it better activates the neodymium catalyst at higher ratios – by influencing the steric environment around the catalytic centre, facilitating monomer insertion by affecting the spatial arrangement around the active site.
4 ratio. This variation can be attributed to the role of [Mg(n-Bu)2] in the catalytic system, which can engage in multiple functions: it can act as Lewis's acid to increase the electrophilicity of the neodymium catalyst, facilitate monomer insertion into the growing chain and also serve as chain transfer agent (CTA) to modulate molecular weight. Overall, it appears that a higher amount of [Mg(n-Bu)2] leads to higher yields, as it better activates the neodymium catalyst at higher ratios – by influencing the steric environment around the catalytic centre, facilitating monomer insertion by affecting the spatial arrangement around the active site.
| Runa | [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg]b [Mg]b |
[Mon]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] [Nd] |
Time (h) | T (°C) | Yield (%) | Mnc (kg mol−1) | Mwc (kg mol−1) | PDIc | Np |
|---|---|---|---|---|---|---|---|---|---|
a Experimental conditions: cyclohexane = 50.9 mL and monomer = 10 mL.b [NdV3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg(n-Bu)2] molar ratio.c Determined by size exclusion chromatography using polystyrene standards. [Nd] = 2.29 × 10−4 mol, [Mon] = 5.73 × 10−2 mol, Np: average number of polymer chains produced by a single Nd atom. [Mg(n-Bu)2] molar ratio.c Determined by size exclusion chromatography using polystyrene standards. [Nd] = 2.29 × 10−4 mol, [Mon] = 5.73 × 10−2 mol, Np: average number of polymer chains produced by a single Nd atom. |
|||||||||
| My1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
250 | 140 | 50 | 38.0 | 32.0 | 112.1 | 3.5 | 0.40 |
| My2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
250 | 140 | 50 | 85.4 | 77.4 | 546.6 | 7.0 | 0.38 |
| My3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
250 | 140 | 50 | 92.0 | 62.9 | 550.0 | 8.7 | 0.50 |
| My4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
250 | 140 | 50 | 85.2 | 51.9 | 283.6 | 5.4 | 0.56 |
| My5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
250 | 114 | 60 | 50.3 | 44.9 | 289.6 | 6.4 | 0.38 |
| My6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
250 | 114 | 60 | 90.4 | 107.9 | 647.6 | 5.9 | 0.29 |
| My7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
250 | 114 | 60 | 88.2 | 82.3 | 528.0 | 6.4 | 0.36 |
| My8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
250 | 43 | 70 | 66.4 | 64.2 | 279.5 | 4.3 | 0.35 |
| My9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
250 | 48 | 70 | 84.2 | 92.8 | 560.2 | 6.0 | 0.31 |
| My10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
500 | 67 | 70 | 92.5 | 77.9 | 464.2 | 5.9 | 0.40 |
| My11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
500 | 67 | 70 | 86.3 | 47.7 | 335.4 | 7.0 | 0.62 |
Conversely, in Run My4, we observed a lower yield compared to Run My3 despite a higher [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio. This could be indicative of a more complex interplay of factors that affect the polymerization process. Several potential reasons could account for this deviation from the expected trend. For instance, at higher [Mg(n-Bu)2] concentrations, there may be competitive coordination to the neodymium centre, which could sterically hinder the approach of monomer molecules, thus reducing the polymerization rate and overall yield. It is possible that an excessive amount of co-catalyst forms stable complexes that are less active in the polymerization process or even impact the solubility and miscibility of the catalyst system complexes, which can adversely affect the yield. Additional experimentation would be necessary to elucidate this behaviour more thoroughly.
[Mg] ratio. This could be indicative of a more complex interplay of factors that affect the polymerization process. Several potential reasons could account for this deviation from the expected trend. For instance, at higher [Mg(n-Bu)2] concentrations, there may be competitive coordination to the neodymium centre, which could sterically hinder the approach of monomer molecules, thus reducing the polymerization rate and overall yield. It is possible that an excessive amount of co-catalyst forms stable complexes that are less active in the polymerization process or even impact the solubility and miscibility of the catalyst system complexes, which can adversely affect the yield. Additional experimentation would be necessary to elucidate this behaviour more thoroughly.
The molecular weights (Mn and Mw) exhibited considerable variability, suggesting that, indeed, the [Mg(n-Bu)2] co-catalyst is influencing the initiation and propagation steps of the polymerization. Initially, as the [Mg(n-Bu)2] concentration increases, it may enhance the activation of the neodymium catalyst, leading to more efficient monomer insertion and longer polymer chains, thus increasing Mn and Mw, as can be seen with Run My2 compared to My1. However, as the concentration of [Mg(n-Bu)2] continues to increase, the chain transfer activity may become predominant, effectively shortening the growing polymer chains and reducing the molecular weight, as observed for My4. This is because the chain transfer reaction competes with chain propagation, leading to the termination of the growing chains and the formation of new ones. The balance between propagation and termination rates is crucial. This would also explain the increase in polydispersity index (PDI) as the molecular weight distribution becomes broader with more frequent chain transfers reactions.
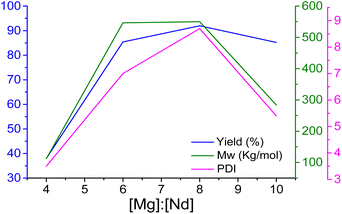
Fig. 2 shows more clearly the correlation between the [Mg]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] ratio and the yield, Mw, and PDI of the polymyrcene reactions, showing the behaviour and elucidating the catalytic system's capacity to modulate polymer characteristics and underpinning the design considerations for tailored material synthesis.
[Nd] ratio and the yield, Mw, and PDI of the polymyrcene reactions, showing the behaviour and elucidating the catalytic system's capacity to modulate polymer characteristics and underpinning the design considerations for tailored material synthesis.
The average number of polymer chains produced by a single Nd atom (Np) was calculated by dividing the total number of polymer chains formed by the total number of Nd atoms used in the reaction. This calculation helps to understand the efficiency of the catalytic system in terms of chain initiation and propagation.
Notably, at the optimal [Mg]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] ratio of 8, observed in Fig. 2, the average number of polymer chains produced per Nd atom (Np) also reaches its maximum. This suggests that the catalytic efficiency in terms of chain initiation and propagation is highest at this ratio. The enhanced Np at a ratio of 8 can be attributed to the co-catalyst [Mg(n-Bu)2] significantly improving the activation of the neodymium catalyst, thereby increasing the number of active sites available for polymer chain initiation. This results in more polymer chains being formed per Nd atom. Furthermore, the optimal ratio ensures a favorable spatial arrangement around the catalytic centre, facilitating efficient monomer insertion and reducing steric hindrance.
[Nd] ratio of 8, observed in Fig. 2, the average number of polymer chains produced per Nd atom (Np) also reaches its maximum. This suggests that the catalytic efficiency in terms of chain initiation and propagation is highest at this ratio. The enhanced Np at a ratio of 8 can be attributed to the co-catalyst [Mg(n-Bu)2] significantly improving the activation of the neodymium catalyst, thereby increasing the number of active sites available for polymer chain initiation. This results in more polymer chains being formed per Nd atom. Furthermore, the optimal ratio ensures a favorable spatial arrangement around the catalytic centre, facilitating efficient monomer insertion and reducing steric hindrance.
The polymerization of β-farnesene, as documented in Table 2, was also conducted under various conditions to investigate the influence of the [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg], and [Mon]
[Mg], and [Mon]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] ratios, time, and temperature on the yield, molecular weight, and PDI. Like the earlier findings with β-myrcene, the data from β-farnesene polymerization also presents an intricate interplay of these factors. The yield of β-farnesene polymers exhibited notable variations with changes in the [Nd]
[Nd] ratios, time, and temperature on the yield, molecular weight, and PDI. Like the earlier findings with β-myrcene, the data from β-farnesene polymerization also presents an intricate interplay of these factors. The yield of β-farnesene polymers exhibited notable variations with changes in the [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] and [Mon]
[Mg] and [Mon]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] ratios. For instance, Fa2 with a 1
[Nd] ratios. For instance, Fa2 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 [Nd]
8 [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio showed a significantly higher yield (83.9%) compared to Fa1 (19%) with a 1
[Mg] ratio showed a significantly higher yield (83.9%) compared to Fa1 (19%) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio. This suggests that, as observed with β-myrcene, the presence of [Mg(n-Bu)2] positively impacts the polymerization process, potentially enhancing catalyst activation and monomer insertion efficiency.
6 ratio. This suggests that, as observed with β-myrcene, the presence of [Mg(n-Bu)2] positively impacts the polymerization process, potentially enhancing catalyst activation and monomer insertion efficiency.
| Runa | [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg]b [Mg]b |
[Mon]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Nd] [Nd] |
Time (h) | T (°C) | Yield (%) | Mnc (kg mol−1) | Mwc (kg mol−1) | PDIc | Np |
|---|---|---|---|---|---|---|---|---|---|
a Experimental conditions: cyclohexane = 50.9 mL and monomer = 10 mL.b [NdV3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg(n-Bu)2] molar ratio.c Determined by size exclusion chromatography using polystyrene standards. [Nd] = 2.91 × 10−4 mol, [Mon] = 4.37 × 10−2 mol. Np: average number of polymer chains produced by a single Nd atom (Np). [Mg(n-Bu)2] molar ratio.c Determined by size exclusion chromatography using polystyrene standards. [Nd] = 2.91 × 10−4 mol, [Mon] = 4.37 × 10−2 mol. Np: average number of polymer chains produced by a single Nd atom (Np). |
|||||||||
| Fa1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
150 | 92 | 70 | 19.0 | 47.0 | 137.0 | 2.8 | 0.12 |
| Fa2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
150 | 92 | 70 | 83.9 | 178.3 | 852.7 | 4.7 | 0.14 |
| Fa3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
150 | 92 | 60 | 37.2 | 34.8 | 91.5 | 2.6 | 0.33 |
| Fa4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
150 | 92 | 60 | 55.6 | 172.0 | 982.5 | 5.7 | 0.10 |
| Fa5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
150 | 92 | 60 | 35.3 | 106.0 | 625.3 | 5.8 | 0.10 |
| Fa6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
250 | 92 | 60 | 20.0 | 16.0 | 213.5 | 13.3 | 0.38 |
The Mn and Mw displayed considerable variability across different runs. This aligns with the trends observed in β-myrcene polymerization, where increasing [Mg(n-Bu)2] concentrations initially lead to higher molecular weights. However, a further increase can result in reduced molecular weight due to enhanced chain transfer activity. For example, Fa3 shows a dramatic decrease in Mn compared to Fa2, likely due to this chain transfer effect. The PDI values in β-farnesene polymerization varied significantly, indicating the influence of [Mg(n-Bu)2] in chain transfer activities.
The Mn and Mw displayed considerable variability across different runs. This aligns with the trends observed in β-myrcene polymerization, where increasing [Mg(n-Bu)2] concentrations initially lead to higher molecular weights. However, a further increase can result in reduced molecular weight due to enhanced chain transfer activity. For example, Fa3 shows a dramatic decrease in Mn compared to Fa2, likely due to this chain transfer effect. The PDI values in β-farnesene polymerization varied significantly, indicating the influence of [Mg(n-Bu)2] in chain transfer activities. Higher PDIs, as seen in Fa6 (13.3), suggest a broader distribution of molecular weights, which can be attributed to the increased chain transfer activity at higher [Mg(n-Bu)2] concentrations.
The resulting polymyrcene and polyfarnesene samples' microstructure was analyzed using 1H and 13C NMR spectroscopy. The NMR data allowed for the assignment of key structural motifs, particularly the cis-1,4, trans-1,4, and 3,4 additions, which are crucial for determining the polymer's stereoregularity. Fig. 3 presents the 1H NMR spectrum of Run My2, highlighting the characteristic proton signals corresponding to the various isomers. Similarly, Fig. 4 shows the 13C NMR spectrum of Run My2, illustrating the carbon signals and facilitating the identification of stereoisomers in the polymer chain.
 | ||
| Fig. 3 1H NMR spectra, corresponding to Run My2, in order to illustrate the signal assignment in a polymyrcene sample. | ||
 | ||
| Fig. 4 13C NMR spectra, corresponding to Run My2, in order to illustrate the signal assignment in a polymyrcene sample. | ||
The microstructure of resulting polymyrcene and polyfarnesene samples, as analyzed by 1H and 13C NMR, predominantly exhibits cis-configurations, a finding that starkly contrasts with the high trans-content observed in the polymerization of 1,3-butadiene. This contrast is particularly highlighted in the work of Zheng et al.,22 where their system produced polybutadienes with around 96% trans-1,4 content, moderate molecular weights, and narrow polydispersity. The significant difference in stereoregularity between the terpene polymers and polybutadiene underscores the influential role of monomer side chains in determining polymer microstructure. The presence of larger side chains in β-myrcene and β-farnesene likely contributes to a sterically hindered environment that promotes cis configurations during polymerization. This steric effect appears to override the influence of [Mg(n-Bu)2], which, in the case of butadiene, promotes the formation of trans-14-polymers.
The Table 3 for example, examining specific β-myrcene runs reveals this trend: My1, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 [Nd]
4 [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio at 50 °C, displays a 53.3% cis content. My2, with a 1
[Mg] ratio at 50 °C, displays a 53.3% cis content. My2, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 [Nd]
6 [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio at the same temperature, shows an increased cis content of 59.9%. My3, with a 1
[Mg] ratio at the same temperature, shows an increased cis content of 59.9%. My3, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 [Nd]
8 [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio, continues this pattern, also exhibiting a cis content of 59.9%. Furthermore, My6, with a 1
[Mg] ratio, continues this pattern, also exhibiting a cis content of 59.9%. Furthermore, My6, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 [Nd]
8 [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio but at a higher temperature of 60 °C, demonstrates a further increase in cis content to 62%. These percentages for 1,4 cis and 1,4 trans correspond to the proportion of the 1,4 microstructure obtained from proton NMR. These results indicate that a higher co-catalyst content slightly increases the cis content in β-myrcene polymers, emphasizing the significant impact of monomer structure on polymer microstructure.
[Mg] ratio but at a higher temperature of 60 °C, demonstrates a further increase in cis content to 62%. These percentages for 1,4 cis and 1,4 trans correspond to the proportion of the 1,4 microstructure obtained from proton NMR. These results indicate that a higher co-catalyst content slightly increases the cis content in β-myrcene polymers, emphasizing the significant impact of monomer structure on polymer microstructure.
| Runa | [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg]b [Mg]b |
T (°C) | 3,4c (%) | 1,4c (%) | 1,4-cisd (%) | 1,4-transd (%) | Tge (°C) |
|---|---|---|---|---|---|---|---|
| a Experimental conditions: cyclohexane = 50.9 mL and monomer = 10 mL.b [NdV3]/[Mg(n-Bu)2] molar ratio.c Regioselectivity determined by 1H NMR.d Stereoselectivity determined by 13C NMR.e Glass transition temperature determined by DSC at 5 °C min−1. | |||||||
| My1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
50 | 17.4 | 82.6 | 55.8 | 26.8 | −65.5 |
| My2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
50 | 15.1 | 84.9 | 57.9 | 27 | −65.6 |
| My3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
50 | 14.5 | 85.5 | 59.9 | 25.6 | −65.7 |
| My6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
60 | 20 | 80 | 62.0 | 18.0 | −66.1 |
| My8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
70 | 18 | 82 | 53.8 | 28.2 | −63.1 |
| My10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
70 | 14.7 | 85.3 | 59.8 | 25.3 | −63.7 |
| Fa1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
70 | 10.5 | 89.5 | 83.8 | 5.7 | −75.4 |
| Fa2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
70 | 9.5 | 90.5 | 78.5 | 12.0 | −75.4 |
| Fa4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
60 | 8.6 | 91.4 | 74.8 | 16.6 | −75.5 |
| Fa5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
60 | 8.6 | 91.4 | 72.5 | 18.9 | −75.5 |
In β-farnesene polymers, this trend is consistently observed. Runs like Fa2 and Fa3, both conducted at a temperature of 70 °C but with different [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratios of 1
[Mg] ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively, show high cis contents of 83.8% and 78.5%. This consistency in both β-myrcene and β-farnesene polymers suggests a generalizable effect of monomer structure on the microstructure of the resulting polymers, highlighting the pivotal role of monomer side chains in the stereoregularity of polymers produced with [Mg(n-Bu)2] as part of the catalytic system.
10, respectively, show high cis contents of 83.8% and 78.5%. This consistency in both β-myrcene and β-farnesene polymers suggests a generalizable effect of monomer structure on the microstructure of the resulting polymers, highlighting the pivotal role of monomer side chains in the stereoregularity of polymers produced with [Mg(n-Bu)2] as part of the catalytic system.
The differential scanning calorimetry (DSC) analysis of the polymyrcene and polyfarnesene samples revealed glass transition temperatures (Tg) below −60 °C. In Fig. 5a, the effect of increasing the [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio is observed, showing that this increase does not significantly impact the Tg of polymyrcene, which remains within a narrow range between −65 °C and −66.1 °C. On the other hand, for polyfarnesene (Fig. 5c), an increase in the [Nd]
[Mg] ratio is observed, showing that this increase does not significantly impact the Tg of polymyrcene, which remains within a narrow range between −65 °C and −66.1 °C. On the other hand, for polyfarnesene (Fig. 5c), an increase in the [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio results in a significant decrease in Tg, with values between −75.4 °C and −75.5 °C, suggesting that polyfarnesene exhibits greater flexibility at low temperatures compared to polymyrcene. As shown in Fig. 5b, in polymyrcene samples, increasing the reaction temperature from 50 °C to 70 °C leads to a slight decrease in Tg, particularly in the My8 and My10 samples, which show values of −63.1 °C and −63.7 °C, respectively. This behavior suggests that the flexibility of the materials is directly related to their chemical and topological structure. In the present study, it was observed that polyfarnesene exhibits lower glass transition temperatures compared to polymyrcene, which can be attributed to its higher content of saturated groups and a more branched structure, promoting greater molecular mobility.
[Mg] ratio results in a significant decrease in Tg, with values between −75.4 °C and −75.5 °C, suggesting that polyfarnesene exhibits greater flexibility at low temperatures compared to polymyrcene. As shown in Fig. 5b, in polymyrcene samples, increasing the reaction temperature from 50 °C to 70 °C leads to a slight decrease in Tg, particularly in the My8 and My10 samples, which show values of −63.1 °C and −63.7 °C, respectively. This behavior suggests that the flexibility of the materials is directly related to their chemical and topological structure. In the present study, it was observed that polyfarnesene exhibits lower glass transition temperatures compared to polymyrcene, which can be attributed to its higher content of saturated groups and a more branched structure, promoting greater molecular mobility.
Experimental
Materials
All experimental manipulations were executed within an MBraun glove box under an inert argon atmosphere or via standard Schlenk line techniques. β-Myrcene (My, sourced from Aldrich) and β-farnesene (Fa, supplied by AMYRIS) were purified through distillation over sodium under an argon environment. Neodymium versatate (NdV3, procured from Strem Chemicals) and the co-catalyst dibutyl magnesium (Mg(n-Bu)2, obtained from Aldrich) were utilized as received. Cyclohexane, acquired from Aldrich, underwent distillation from sodium under an argon atmosphere to ensure purity.Catalyst activation and polymerization
In an MBraun glove box or under an inert nitrogen atmosphere, all catalyst manipulations were carried out in oven-dried bottles that were nitrogen-purged and sealed with a rubber septum for subsequent magnetic stirring. The catalyst components, namely [NdV3] and [Mg(n-Bu)2], were sequentially added to the reaction vessel. The catalyst mixture was then allowed to age at room temperature for 30 minutes prior to the polymerization reaction.Polymerizations were carried out in 30 mL glass vials, each equipped with a magnetic stir bar and secured to ensure a consistent nitrogen atmosphere. The reaction vial was charged with the monomer and cyclohexane, and the mixture was heated to the desired temperature while being stirred at 150 rpm. Upon reaching the target temperature, the pre-aged catalyst system was introduced into the reactor. Samples were taken at intervals to monitor the progress of the polymerization and to determine the polymer yield through gravimetric analysis. The reaction was quenched with acidified methanol, and the polymers were stabilized using Irganox 1076. The resulting materials were precipitated in methanol and dried under vacuum at 25 °C to constant weight.
Characterization techniques
The molecular weights of the samples were determined through size exclusion chromatography (SEC) employing a PLGel mixed column in a Hewlett-Packard instrument (HPLC series 1100) equipped with a refractive index detector. Calibration was performed with polystyrene standards, and tetrahydrofuran (HPLC grade from Aldrich) served as the eluent at a flow rate of 1 mL min−1.Differential scanning calorimetry (DSC) thermograms were acquired using a TA Instrument DSC 2000. The analyses were performed under an inert atmosphere with a heating rate of 5 °C min−1, and each sample was analyzed twice to eliminate thermal history effects.
Microstructure analyses of polymyrcene and polyfarnesene samples were conducted using 1H and 13C nuclear magnetic resonance (NMR) on a Bruker Ultrashield Plus 400 MHz spectrometer. CDCl3 was utilized as a solvent, and the analyses were carried out at room temperature. The isomer 3,4 in relation to the isomer 1,4 (cis + trans) was determined by the 1H NMR spectrum, integrating signals in the olefinic group region from 4.7 to 5.3 ppm. The cis/trans ratio was calculated by the 13C NMR spectrum (proton-gated decoupling no-NOE experiments), integrating signals of olefinic groups. The Fig. 6 show the microstructure schematics.
Conclusions
In conclusion, this study shows the intricate relationship between catalytic systems and monomer structure in the polymerization of terpenes. We have shown that the use of [Mg(n-Bu)2] as a co-catalyst in conjunction with a neodymium-based catalyst can significantly influence the yield, molecular weight, and microstructure of polyterpenes. Our research suggests that monomer side chains are crucial in defining the polymer microstructure, with the substantial side chains of β-myrcene and β-farnesene favouring a dominance of cis configurations. This effect is significant enough to even override the stereoselective tendencies of [Mg(n-Bu)2], observed in other systems like the polymerization of butadiene reported by W. Zheng et al.22 The Tg has reinforced our understanding of the polymers' thermal properties, revealing the impact of monomer side chains and the [Nd]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg] ratio on the polymers' flexibility and stability.
[Mg] ratio on the polymers' flexibility and stability.
Furthermore, our findings underscore the versatility of [Mg(n-Bu)2] in producing polymers with varied microstructures, an aspect significantly influenced by the inherent characteristics of the monomers. This accentuates the importance of monomer design in predicting polymer properties, which is crucial for the development of new catalytic systems aimed at sustainable polymer synthesis. The microstructural analysis of β-myrcene and β-farnesene polymers exemplifies the decisive role of monomer side chains in influencing stereoregularity, a factor that prevails over the catalytic system's inherent selectivity.
This study represents a contribution to the quest for sustainable material alternatives. By elucidating the effects of catalyst systems on terpene-based polymer synthesis, we pave the way for future advancements in renewable polymers tailored for specific applications.
Data availability
The data used and analyzed during the development of this work is available in the ESI files accompanying this document.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
To Consejo Nacional de Humanidades, Ciencia y Tecnologías (CONAHCYT) for the financial support provided for the completion of Córdova's postdoctoral fellowship. The authors gratefully acknowledge the financial support of CONAHCYT CF-2023-I-2151 “Estudio para controlar las propiedades mecánicas, antiflama y degradabilidad en el ambiente de nuevos biocompuestos poliméricos sustentables”, and (CONAHCyT) Mexico (grant no. 320630) “Descubrimiento y diseño de nuevos materiales termoeléctricos híbridos orgánicos/inorgánicos con alta eficiencia termoeléctrica”. And the financial support of the Research Center for Applied Chemistry (CIQA) through the internal project 6752.References
- A. Gandini and T. M. Lacerda, Molecules, 2022, 27(1), 159 CrossRef CAS PubMed.
- A. Kindler and O. Zelder, in Synthetic Biodegradable and Biobased Polymers: Industrial Aspects and Technical Products, ed, A. Künkel, G. Battagliarin, M. Winnacker, B. Rieger and G. Coates, Springer International Publishing, Cham, 2024, pp. 1–33 Search PubMed.
- I. Magaña, R. López, F. J. Enríquez-Medrano, S. Kumar, A. Aguilar-Sanchez, R. Handa, R. de León and L. Valencia, J. Mater. Chem. A, 2022, 10, 5019–5043 RSC.
- Y. Zhu and E. Egap, Polym. Chem., 2020, 11, 1018–1024 RSC.
- Y. Zhu, E. Ramadani and E. Egap, Polym. Chem., 2021, 12, 5106–5116 RSC.
- F. Della Monica and A. W. Kleij, Polym. Chem., 2020, 11, 5109–5127 RSC.
- M. E. G. Mosquera, G. Jiménez, V. Tabernero, J. Vinueza-Vaca, C. García-Estrada, K. Kosalková, A. Sola-Landa, B. Monje, C. Acosta, R. Alonso and M. Á. Valera, Sustainable Chem., 2021, 2, 467–492 CrossRef CAS.
- C. Wahlen and H. Frey, Macromolecules, 2021, 54, 7323–7336 CrossRef CAS.
- P. Sarkar and A. K. Bhowmick, RSC Adv., 2014, 4, 61343–61354 RSC.
- R. E. de León Gómez, F. J. Enríquez-Medrano, H. Maldonado Textle, R. Mendoza Carrizales, K. Reyes Acosta, H. R. López González, J. L. Olivares Romero and L. E. Lugo Uribe, Can. J. Chem. Eng., 2016, 94, 823–832 CrossRef.
- L. Valencia, F. J. Enríquez-Medrano, H. R. López González, R. Handa, H. S. Caballero, R. M. Carrizales, J. L. Olivares-Romero and R. E. de León Gómez, RSC Adv., 2020, 10, 36539–36545 RSC.
- R. Díaz de León, R. López, L. Valencia, R. Mendoza, J. Cabello and J. Enríquez, Key Eng. Mater., 2018, 779, 115–121 Search PubMed.
- T. Córdova, F. J. Enríquez-Medrano, E. M. Cartagena, A. B. Villanueva, L. Valencia, E. N. C. Álvarez, R. L. González and R. Díaz-de-León, Polymers, 2022, 14(14), 2907 CrossRef PubMed.
- P. Sahu and J. S. Oh, Ind. Eng. Chem. Res., 2022, 61, 11815–11824 CrossRef CAS.
- M. C. C. de Sá, T. Córdova, P. A. Melo, R. D. de León and J. C. Pinto, Can. J. Chem. Eng., 2023, 101, 5256–5269 CrossRef.
- A. Banda-Villanueva, J. L. González-Zapata, M. E. Martínez-Cartagena, I. Magaña, T. Córdova, R. López, L. Valencia, S. G. Medina, A. M. Rodríguez, F. Soriano and R. de León, Polymers, 2022, 14(7), 1406 CrossRef CAS PubMed.
- J. Liu, X. Fan, X. Min, X. Zhu, N. Zhao and Z. Wang, RSC Adv., 2018, 8, 21926–21932 RSC.
- P. Sarkar and A. K. Bhowmick, ACS Sustainable Chem. Eng., 2016, 4, 5462–5474 CrossRef CAS.
- J. Hilschmann and G. Kali, Eur. Polym. J., 2015, 73, 363–373 CrossRef CAS.
- J. Zhang, C. Aydogan, G. Patias, T. Smith, L. Al-Shok, H. Liu, A. M. Eissa and D. M. Haddleton, ACS Sustainable Chem. Eng., 2022, 10, 9654–9664 CrossRef CAS PubMed.
- A. Ventura, T. Chenal, M. Bria, F. Bonnet, P. Zinck, Y. Ngono-Ravache, E. Balanzat and M. Visseaux, Eur. Polym. J., 2013, 49, 4130–4140 CrossRef CAS.
- W. Zheng, N. Yan, Y. Zhu, W. Zhao, C. Zhang, H. Zhang, C. Bai, Y. Hu and X. Zhang, Polym. Chem., 2015, 6, 6088–6095 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra and gel permeation chromatographs. See DOI: https://doi.org/10.1039/d4ra07481e |
| This journal is © The Royal Society of Chemistry 2025 |