Open Access Article
Open Access ArticleA novel multifunctional fluorescent probe with ESIPT and AIE effects for the detection of Co2+ and HClO†
Chenxiang Lua,
Jiawei Xua,
Zhe Song*b,
Guoqin Zhu*c and
Zhenya Dai *a
*a
aDepartment of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing, 210009, P. R. China
bChina Pharmaceutical University Center for Analysis and Testing, 24 Tongjiaxiang, 210009, P. R. China. E-mail: daizhenya@hotmail.com
cDepartment of Geriatric Gastroenterology, Jiangsu Province Hospital, The First Affiliated Hospital of Nanjing Medical University, Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, P. R. China
First published on 6th February 2025
Abstract
We developed a novel fluorescent probe featuring excited-state intramolecular proton transfer (ESIPT) and aggregation-induced emission (AIE) effects, which displayed dual-channel fluorescence emission. The probe detected both Co2+ and HClO with naked eye under daylight as well as through a fluorescence spectrophotometer. The probe exhibited a low detection limit for Co2+ at 2.823 μM, while the detection limit for HClO was 11.55 μM. When the probe (10 μM) was mixed with Co2+, the fluorescence intensity at 556 nm rapidly decreased within 10 minutes and stabilized after 40 minutes, while for HClO, it took 960 min to observe the same decrease in intensity within 960 min. The probe (10 μM) achieved naked-eye detection of Co2+ recognition under daylight; however, achieving naked-eye detection of HClO under daylight necessitated higher concentrations (500 μM). Thus, this probe shows promising potential for environmental monitoring and water quality detection.
1. Introduction
HClO is prevalent in food preservation and sanitation practices, serving as a potent oxidizing agent, and effective in neutralizing harmful microorganisms, including bacteria. For living organisms, HClO is a dual-edged tool. It is indispensable in the fight against invading pathogens1 and the maintenance of redox balance.2–4 However, excessive HClO could become hazardous when reacted with biological macromolecules, such as proteins, DNA, RNA, amino acids, and cholesterol.5,6 These reactions could lead to oxidative stress, Alzheimer's disease,7,8 cardiovascular disease,9,10 inflammatory bowel disease,11 and organ transplant rejection.12Co2+ is naturally present in the Earth's crust, water bodies, flora, and fauna, and is ubiquitous within essential organs, such as the liver, bones, and kidneys, where it participates in complex signaling processes.13 Furthermore, Co2+ serves as a vital component of vitamin B12 and other cobalamins, playing crucial roles in iron metabolism and the synthesis of hemoglobin.14,15 However, both excessive accumulation and deficiency of Co2+ could lead to adverse health outcomes, including growth retardation, anemia, decreased appetite, reduced lactation,16 cardiovascular diseases,17–19 and pulmonary damage.20,21
Therefore, there is an urgent need to develop reliable methods to monitor HClO and Co2+ concentrations. Unlike traditional analytical techniques, such as electroanalysis, potentiometry, chemiluminescence, bioanalytical methods and chromatography,22–28 fluorescence probes offer several advantages, including high selectivity and sensitivity, rapid response rates, and the capability for real-time detection.29–36
Fluorescent probes designed for Co2+ commonly engage in chelation reactions, resulting in alterations to the probe's fluorescent characteristics. This chelation might lead to a decrease in the fluorescence intensity. However, in certain cases, that could trigger chelation-enhanced fluorescence (CHEF) or aggregation-induced emission enhancement (AIEE), resulting in an enhancement of fluorescence intensity. For instance, probe 1, which was designed by Ghazali et al.,37 complexed with Co2+ at a chelation ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
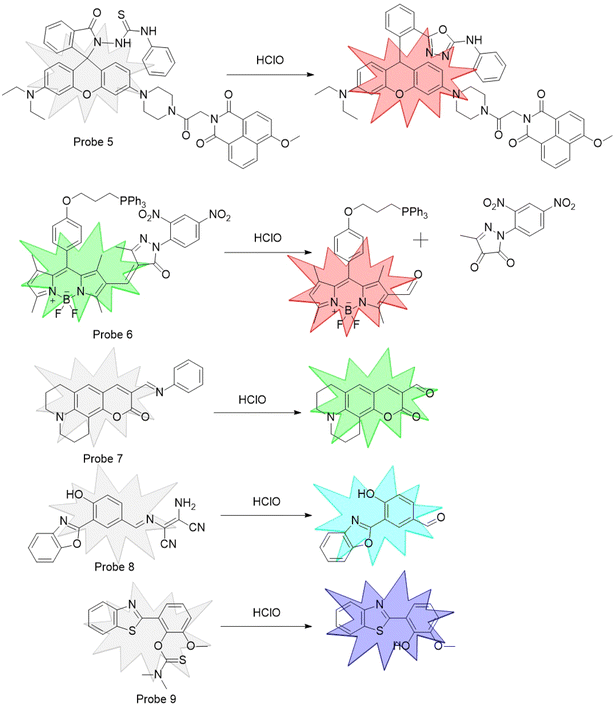
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. In the presence of Co2+, CHEF was activated, which increased the molecular rigidity and subsequently intensified the fluorescence intensity. In contrast, in probe 2, which was developed by Kim et al.,38 the fluorescence emission underwent a redshift following chelation with Co2+ (Fig. 1). The design strategy for HClO probes primarily involve a set of unique reaction, resulting in a change in the probe's fluorescence intensity. As shown in Fig. 2, leveraging the oxidizing nature of HClO, Shi et al.39 exploited the reductive properties of sulfur atoms to design a fluorescent probe for the detection of HClO, designated as Probe 3. The principle behind the probe 4 synthesized by Xia et al.40 for the recognition of HClO was similar to that of probe 3. Fluorescent probes designed based on the oxidizing nature of HClO are prone to interference in complex environments, while those relying on specific reactions for HClO recognition demonstrate enhanced specificity and interference resistance. As shown in Fig. 3, Zhu et al.41 utilized rhodamine as the fluorophore. In the presence of HClO, the 1,3,4-oxadiazole ring opened, which triggered the probe fluorescence emission. Xu et al.42 introduced an electron-deficient carbon–carbon double bond into probe 6, which reacted with HClO under mild conditions, causing the double bond to break. Yang et al.43 designed a Schiff base probe 7 that decomposed in the presence of HClO, leading to a change in the fluorescence peak and thus achieving the detection of HClO. Chen et al.44 synthesized probe 8 with diaminomalononitrile as the recognition site, which hydrolyzed in the presence of HClO to activate fluorescence. Wu et al.45 reported that N,N-dimethylthioformamide reacted with HClO, leading to its removal and exposure of the phenolic hydroxyl group. This activation of the ESIPT effect resulted in strong fluorescence emission.
3. In the presence of Co2+, CHEF was activated, which increased the molecular rigidity and subsequently intensified the fluorescence intensity. In contrast, in probe 2, which was developed by Kim et al.,38 the fluorescence emission underwent a redshift following chelation with Co2+ (Fig. 1). The design strategy for HClO probes primarily involve a set of unique reaction, resulting in a change in the probe's fluorescence intensity. As shown in Fig. 2, leveraging the oxidizing nature of HClO, Shi et al.39 exploited the reductive properties of sulfur atoms to design a fluorescent probe for the detection of HClO, designated as Probe 3. The principle behind the probe 4 synthesized by Xia et al.40 for the recognition of HClO was similar to that of probe 3. Fluorescent probes designed based on the oxidizing nature of HClO are prone to interference in complex environments, while those relying on specific reactions for HClO recognition demonstrate enhanced specificity and interference resistance. As shown in Fig. 3, Zhu et al.41 utilized rhodamine as the fluorophore. In the presence of HClO, the 1,3,4-oxadiazole ring opened, which triggered the probe fluorescence emission. Xu et al.42 introduced an electron-deficient carbon–carbon double bond into probe 6, which reacted with HClO under mild conditions, causing the double bond to break. Yang et al.43 designed a Schiff base probe 7 that decomposed in the presence of HClO, leading to a change in the fluorescence peak and thus achieving the detection of HClO. Chen et al.44 synthesized probe 8 with diaminomalononitrile as the recognition site, which hydrolyzed in the presence of HClO to activate fluorescence. Wu et al.45 reported that N,N-dimethylthioformamide reacted with HClO, leading to its removal and exposure of the phenolic hydroxyl group. This activation of the ESIPT effect resulted in strong fluorescence emission.
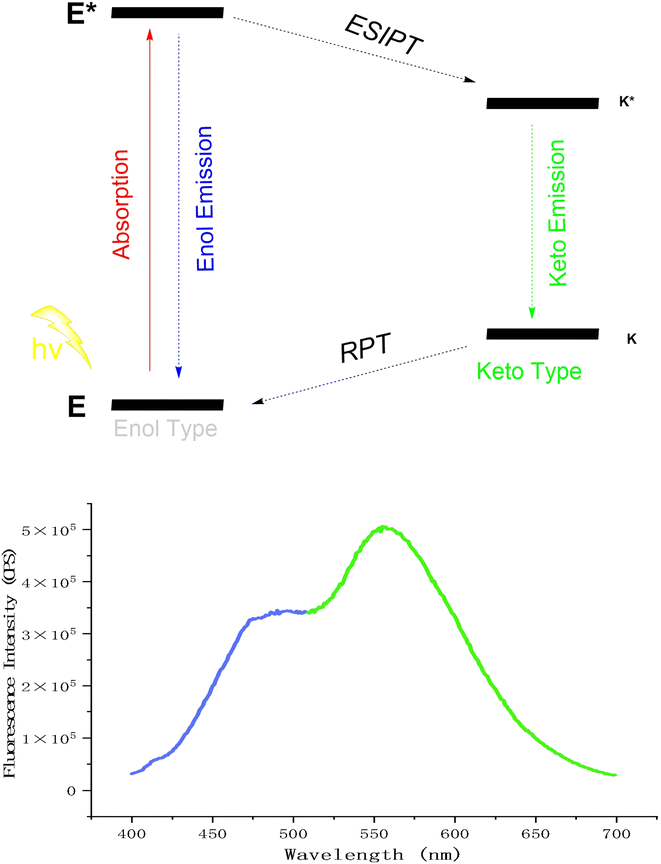
In the 1950s, Weller et al.46 first highlighted the benefits of the ESIPT (Excited-State Intramolecular Proton Transfer) effect, such as dual-channel fluorescence emission and large Stokes shifts, attracting significant interest. The ESIPT effect involved proton transfer from a donor (e.g., amino or hydroxyl groups) to an acceptor (e.g., nitrogen or oxygen atoms), exemplified by keto–enol tautomerism. This process followed a four-level cycle: a fluorescent molecule absorbs energy and transitions from the ground state (E) to the excited state (E*). Some excited molecules (E*) released enol fluorescence and returned to the ground state (E), while others transferred a proton to form a keto structure (K*), released keto fluorescence, fell back to the ground state (K), and then reverted to the enol form (E) via reverse proton transfer (RPT)47–50 (Fig. 6). ESIPT probes are highly valued in fields such as fluorescence,51 bioimaging,52,53 and luminescent materials54,55 due to their dual-channel emission, large Stokes shift, and extended fluorescence lifetime. However, their sensitivity to solvent microenvironments, especially protic solvents, severely constrains their practical utility.56 In 2001, Tang Benzhong's group first reported the AIE (Aggregation-Induced Emission) effect.57 AIE fluorescent molecules were non-emissive in the solution but emitted strong fluorescence when aggregated or in the solid state, offering a new platform for developing fluorescent probes.
The emergence of the AIE effect has provided new avenues for the development of ESIPT effects. Fluorescent probes, benefiting from the synergistic interplay of ESIPT and AIE effects, not only inherited the fluorescence advantages of these effects but also mutually enhanced each other, thereby improving the fluorescence performance of the probes. The AIE and ESIPT effects endowed probes with various advantages; for instance, large Stokes shifts could prevent self-absorption phenomena, multi-channel fluorescence emission could provide greater flexibility and operability for practical probe applications, and the ability for aggregate fluorescence emission could minimize the solvent's impact.
2. Experimental section
2.1 Materials and instrumentation
All chemicals were purchased directly through commercial channels and used without further purification. The specific product information sources are shown in the ESI.† The instruments used in the experiment included a spectrophotometer, ESI-MS, nuclear magnetic resonance (NMR) and Malvern Zetasizer. The details about the manufacturer and model of the instrument are shown in the ESI.†2.2 General procedure for analysis
The synthesis of the probe is shown in Fig. 4. Benzophenone and 4-hydroxybenzophenone were combined to form the TPE core through a McMurry coupling reaction. Subsequently, aldehyde groups were introduced at the ortho position of the phenolic hydroxyl through a Duff reaction, which was combined with 2-hydrazinobenzimidazole to construct the recognition site for HClO, thus completing the synthesis of the probe. The probe was rich in heteroatoms, endowing it with the ability to chelate metal ions.2.3 Optical studies
| LOD = 3σ/k |
The determination of the association constant was accomplished by employing the canonical Benesi–Hildebrand method.
| 1/(F − Fmin) = 1/Ka(Fmax − Fmin)/[M] + 1/(Fmax − Fmin) |
3. Results and discussion
3.1 Design and synthesis
To design a novel AIE and ESIPT probe for the detection of HClO and Co2+, we used TPE units to afford the AIE effect, the proton transfer of 2-(2-benzothiazolyl) hydrazone and phenol hydroxyl to provide ESIPT effect.The electron-rich groups of the Schiff base and phenolic hydroxyl group have the potential to chelate with metal ions. Attaching two Schiff base units adjacent to the phenolic hydroxyl group enhanced the formation of a stable multi-dentate chelation structure with metal ions. On one hand, the probe detected by chelating metal ions with Schiff base structures or even phenolic hydroxyl groups, quenching keto-form fluorescence and even enol-form fluorescence. On the other hand, 2-(2-benzothiazolyl) hydrazone is a classic recognition site for detecting HClO.58 In the presence of HClO, the hydrazone and benzothiazole ring cyclized to form a triazole. This particular reaction was used to detect HClO, as it converted the 2-(2-benzothiazolyl) hydrazone into triazole, quenching keto-form fluorescence or altering enol-form fluorescence.
3.2 AIE and ESIPT effect
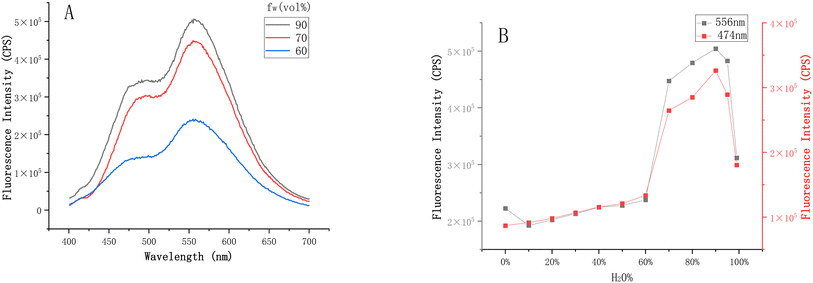
With AE-3 in hand, we discovered that it exhibited two-channel emission (Fig. 6) through fluorescence spectroscopy analyses, showing a typical ESIPT phenomenon. To demonstrate the AIE effect of AE-3, we incubated it in THF solutions with water content ranging from 0% to 99%. As shown in Fig. 7, the probe exhibited fluorescence emission peaks at 474 nm and 556 nm, corresponding to the enol-form and keto-form fluorescence, respectively. When the water content in the solvent increased from 60% to 95%, the intensity of the two emission peaks was significantly enhanced (Fig. S1–S2†).Upon meticulous analysis of the UV absorption spectra of the probe in the solution with varying water contents, we observed significant spectral alterations (Fig. S3†). Specifically, within the range of 10% to 70% water content, the probe exhibited distinct UV absorption peaks at 231 nm and 252 nm, which might be attributed to the benzothiazole. Additionally, the absorption peak at 370 nm corresponded to the π–π* transitions of the large conjugated benzene rings. As the water content increased, the polarity of the solvent heightened, leading to a redshift of the π–π* transition peaks of the benzene ring and the σ–π* transition peaks of benzothiazole displayed a blueshift. With further enhancement of the water content, the aggregation of the probe intensified, reducing the actual concentration of the probe dissolved in the solution, which ultimately led to a notable decrease in absorbance. Meanwhile, the probe features Schiff base structures and large conjugated benzene rings, which contributed to the poor solubility of the probe. These changes in the UV spectra also, to some extent, demonstrated the aggregation characteristics of the probe in different solvents.
In our investigation of the aggregation behavior of probes in the solution with varying water content using dynamic light scattering (DLS), we observed the following: when the solution contained 10% water, no significant particles were detected, indicating that the probe was completely dissolved in THF/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Upon increasing the solution water content to 50%, DLS measurements revealed that the average size of the aggregated probe was 159.5 nm, indicating a certain degree of probe aggregation. Elevating the aqueous fraction of the solution to 90% led to a significant enlargement of the aggregate dimensions, with the average size reaching 873.9 nm (Fig. 5). This phenomenon indicated that as the water content in the solvent increased, the probe gradually underwent aggregation.
1). Upon increasing the solution water content to 50%, DLS measurements revealed that the average size of the aggregated probe was 159.5 nm, indicating a certain degree of probe aggregation. Elevating the aqueous fraction of the solution to 90% led to a significant enlargement of the aggregate dimensions, with the average size reaching 873.9 nm (Fig. 5). This phenomenon indicated that as the water content in the solvent increased, the probe gradually underwent aggregation.
 | ||
| Fig. 5 Dynamic light scattering (DLS) study of probe AE-3 (10 μM) with different percentages of H2O: (A) 0%, (B) 50%, (C) 90%. | ||
3.3 Fluorescence response of AE-3 to Co2+
The initial phase of our study involved conducting selective fluorescence assays on AE-3 (10 μM) in the presence of various metal ions: Fe3+, Al3+, Cd3+, Mg2+, Cu2+, Co2+, Ba2+, Ca2+, Cr3+, Mn2+, Ni2+, Zn2+ and Fe2+ each at a concentration of 10 μM. Upon the addition of the above-mentioned metal ions to the AE-3 solution, the fluorescence spectrum remained largely unaffected, with the exception of notable changes observed with Co2+ and Cu2+ (Fig. S4†). The paramagnetic nature of Cu2+ resulted in quenching of the fluorescence peaks at 474 nm and 556 nm (Fig. 8). Subsequently, the probe's anti-interference ability during Co2+ recognition was evaluated (Fig. 8 and S5†).The limit of detection (LOD) for Co2+ was determined by the slope of the fluorescence intensity at F556 nm from continuous titration experiments with different equivalents of Co2+ and the standard deviation of 10 blank samples of AE-3 (10 μM). The absolute slope of the linear relationship between the fluorescence intensity at F556 nm and Co2+ (0.1–0.9 eq.) was found to be 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716.07 (Fig. S6†). Additionally, the standard deviation of the fluorescence intensity at F556 nm for the 10 blank samples was calculated as 29
716.07 (Fig. S6†). Additionally, the standard deviation of the fluorescence intensity at F556 nm for the 10 blank samples was calculated as 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 846.30 (Fig. S7†). The calculated limit of detection (LOD) using the formula 3σ/k was 2.823 μM.
846.30 (Fig. S7†). The calculated limit of detection (LOD) using the formula 3σ/k was 2.823 μM.
To investigate the binding mode of the probe and Co2+, the Job's plot curve method was employed. As shown in Fig. 9, when the total concentration of the probe and Co2+ was kept constant, the fluorescence intensity gradually decreased with an increase in the proportion of Co2+. When the concentration ratio of Co2+ to the total concentration exceeded 0.5, the fluorescence intensity tended to stabilize, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio between the probe and Co2+. By employing the Benesi–Hildebrand method, a linear regression equation was established, where Fmax denotes the fluorescence intensity of the probe at a 1
1 binding ratio between the probe and Co2+. By employing the Benesi–Hildebrand method, a linear regression equation was established, where Fmax denotes the fluorescence intensity of the probe at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 concentration ratio with Co2+. The calculated association constant (Ka) was determined to be 475
1 concentration ratio with Co2+. The calculated association constant (Ka) was determined to be 475![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579.02.
579.02.
To evaluate the sensitivity and stability of the probe in detecting Co2+ ions, we conducted fluorescence spectroscopy analysis at various incubation durations (Fig. 10). The fluorescence intensity of AE-3 at 556 nm exhibited a rapid decline within the first 10 minutes, followed by a gradual leveling off, and stabilization after 40 minutes.
 | ||
| Fig. 10 Point-line plot of the fluorescence intensity at 556 nm versus incubation time of the AE-3 (10 μM) with Co2+. | ||
3.4 Fluorescence response of AE-3 to HClO
Similarly, we first conducted selective tests on AE-3 (10 μM) using SCN−, NO2−, NO3−, SO42−, SO32−, Cl−, CH3COO−, H2O2 and HClO (50 μM). Upon the addition of HClO, the fluorescence intensities at 474 nm and 556 nm both decreased, while a new peak emerged at 594 nm. There were no significant changes in the fluorescence spectra upon the addition of other analytes (Fig. 11 and S8†). Then, the anti-interference ability of AE-3 recognition was evaluated (Fig. S9–S10†). AE-3 (10 μM) demonstrated robust anti-interference capacity in HClO detection and exhibited exceptional selectivity and anti-interference ability for HClO detection.The absolute slope of the linear relationship between the fluorescence intensity at 556 nm of AE-3 (10 μM) and HClO (0–6 eq.), measured by the continuous titration method, was found to be 7755.17 (Fig. S11†). The standard deviation of fluorescence intensity at 556 nm for the 10 blank specimens was calculated as 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 846.30 (Fig. S7†). The calculated limit of detection (LOD) using the formula 3σ/k was 11.55 μM.
846.30 (Fig. S7†). The calculated limit of detection (LOD) using the formula 3σ/k was 11.55 μM.
Similarly, to assess the sensitivity and stability of the probe in HClO recognition, fluorescence spectroscopy analysis was conducted at different incubation times (Fig. 12). We noted a gradual decrease in the fluorescence intensity at 556 nm of AE-3 (10 μM) over the 960 min incubation period, decreasing by 4-fold.
3.5 Naked-eye detection under daylight
Remarkably, the probe exhibited a distinct colorimetric response to Co2+ and HClO under daylight, enabling naked eye detection without the need for sophisticated instrumentation. The solution of AE-3 (10 μM) remained colorless upon the addition of the other eleven metal ions (10 μM) besides Co2+ but it turned yellow in the presence of Co2+ (10 μM) after incubating for 10 min, as observed with the naked eye under daylight (Fig. 13).At a low concentration, AE-3 (10 μM) was distinctly recognized Co2+ by the naked eye under daylight. When Co2+ and HClO were separately mixed with the probe and incubated for 10 min, the solution with HClO showed no significant change, while the solution with Co2+ turned straw-colored. At higher concentrations (500 μM), naked-eye differentiation between HClO and Co2+ was achievable; the solution with HClO turned grass green, while the solution with Co2+ turned yellow ochre (Fig. 14).
The development of a functional fluorescent probe capable of identifying specific analytes within environmental contexts is of paramount importance. The application necessitated a thorough investigation into the performance of fluorescent probes within environmental samples. Leveraging probe AE-3's excellent detective ability, to capitalize on the simplicity and timeliness of fluorescent probes, we selected natural water samples from China Pharmaceutical University (CPU), encompassing tap water (T-water), boiled water (B-water), and lake water (L-water). These samples were analyzed without any amendments. As shown in Fig. 15, the probe (10 μM) exhibited a straw-colored transformation in T-water and L-water, whereas B-water remained largely unchanged. This could be attributed to the low concentration of Co2+ in B-water, which was insufficient to induce visually perceptible alterations of the solution color. In the case of higher concentrations of the probe solution (100 μM), characteristic color changes indicative of HClO were not observed; the solution color alteration was overshadowed by the straw-colored distinctive of Co2+. This outcome might also stem from the insufficient concentration of HClO in the water to induce a noticeable color change in the probe solution. In summary, the probe exhibited higher sensitivity to Co2+ compared to HClO, as evidenced by the probe's lower detection limit.
3.6 Recognition mechanism study
The proposed identification mechanism is shown in Fig. 16. To further confirm the stoichiometry of AE-3 and Co2+, we performed a continuous titration method. Using the THF/phosphate buffer (1/9, v/v, 50 mM, pH = 7.4) as the solution, a series of gradient volumes of Co2+ stock solutions (10 μM) were added to the probe solution (10 μM). We observed that with the increase in Co2+ concentration, the fluorescence intensity at 556 nm gradually decreased, eventually reaching a plateau. When the Co2+ equivalent reached 1 eq., the fluorescence intensity remained essentially unchanged (Fig. 17). The results showed that the binding stoichiometry between the probe and Co2+ was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. ESI/MS confirmed this, with a peak at m/z 756.12 corresponding to [AE-3 + Co–H]+, indicating Co2+ chelation with the phenolic hydroxyl group and hydrogen atom replacement (Fig. 18). For HClO recognition, after incubating AE-3 with HClO for 12 hours, ESI/MS revealed peaks at m/z 695.17 ([AE-HClO-1 – H]−) and m/z 693.15 ([AE-HClO-2 – H]−), supporting the proposed recognition mechanism (Fig. S17–S21†).
1. ESI/MS confirmed this, with a peak at m/z 756.12 corresponding to [AE-3 + Co–H]+, indicating Co2+ chelation with the phenolic hydroxyl group and hydrogen atom replacement (Fig. 18). For HClO recognition, after incubating AE-3 with HClO for 12 hours, ESI/MS revealed peaks at m/z 695.17 ([AE-HClO-1 – H]−) and m/z 693.15 ([AE-HClO-2 – H]−), supporting the proposed recognition mechanism (Fig. S17–S21†).
The fluorescence response graphs of the probe to HClO and Co2+ further corroborated the recognition mechanism of AE-3. As shown in Fig. 19, HClO quenched the enol-form fluorescence peak at 474 nm and keto-form fluorescence peak at 556 nm, and induced a new fluorescence peak at 597 nm, suggesting that HClO might react with the ESIPT fluorophore of AE-3. Co2+quenched the enol-form and keto-form fluorescence peaks at 474 nm and 556 nm, respectively, likely due to the chelation of Co2+ with the probe, which blocked the ESIPT effect.
 | ||
| Fig. 19 Fluorescence spectra of AE-3 (10 μM) with the addition of Co2+ (10 μM), and HClO (50 μM) in THF/phosphate buffer (1/9, v/v, 50 mM, pH = 7.4). | ||
In the presence of HClO and Co2+, the UV absorption spectrum of the probe exhibited significant changes, which might be related to variations in solubility (Fig. S12†). Upon chelation with Co2+, the probe solubility in polar solvents increased, manifesting as two UV absorption peaks at approximately 221 nm and 206 nm, akin to the benzothiazole structure's original profile. Based on this, the decrease in fluorescence intensity of AE-3 after chelation with Co2+ might not only be due to the quenching effect of Co2+ but also because the increased solubility reduced the aggregation degree of the probe, thereby turning off the AIE effect. Conversely, when the probe was mixed with HClO, a single absorption peak was observed at 206 nm. This change might be attributed to the reaction between the probe and HClO, which disrupted the carbon–nitrogen double bond of the benzothiazole, leading to alterations in the σ–π* transitions. Additionally, the destruction of the Schiff base structure might also contribute to an improvement in the probe's solubility to some extent.
4. Conclusions
In conclusion, we successfully developed a novel fluorescent probe that offers combined ESIPT and AIE effects. This probe exhibited dual-channel fluorescence emission, which indicates its versatile application potential in sensing.The probe demonstrated high sensitivity and selectivity in detecting Co2+ by indicating a change fluorescence intensity at 556 nm. The detection limit for Co2+ was 2.823 μM, which was significantly lower than that for HClO, which was 11.55 μM. The association constant of the probe with Co2+ was determined to be 475![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579.02. Notably, the probe allowed for naked-eye recognition of Co2+ under daylight; however, higher concentrations were required for HClO detection with naked eye. The detection of HClO using the probe is interfered with by cobalt ions in practical applications. Overall, this developed probe holds a promising potential for applications in environmental monitoring and water quality detection.
579.02. Notably, the probe allowed for naked-eye recognition of Co2+ under daylight; however, higher concentrations were required for HClO detection with naked eye. The detection of HClO using the probe is interfered with by cobalt ions in practical applications. Overall, this developed probe holds a promising potential for applications in environmental monitoring and water quality detection.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful to the National Natural Science Foundation of China for their financial support (No. 21102180).References
- S. J. Klebanoff, Myeloperoxidase: friend and foe, J. Leukocyte Biol., 2005, 77(5), 598–625 CrossRef CAS.
- C. Liu, Y. Shang and T. Zhao, et al., Facile functionalized fluorescein derivative as a reversible fluorescence probe for selective monitor of the redox cycle between hypochlorous acid and cysteine, Sens. Actuators, B, 2021, 348, 130632 CrossRef CAS.
- J. C. Genereux, A. K. Boal and J. K. Barton, DNA-Mediated Charge Transport in Redox Sensing and Signaling, J. Am. Chem. Soc., 2010, 132(3), 891–905 CrossRef CAS.
- W. Qu, T. Guo and B. Yang, et al., Tracking HOCl by an incredibly simple fluorescent probe with AIE plus ESIPT in vitro and in vivo, Spectrochim. Acta, Part A, 2022, 121649 CrossRef CAS.
- Y. Yang, Y. Zhao and S. Wei, et al., Colorimetric fluorescence probe detecting Hg2+ and OCl – based on intramolecular charge transfer and excited-state intramolecular proton transfer mechanisms, J. Lumin., 2019, 209, 102–108 CrossRef CAS.
- W. A. Prütz, R. Kissner and W. H. Koppenol, et al., On the Irreversible Destruction of Reduced Nicotinamide Nucleotides by Hypohalous Acids, Arch. Biochem. Biophys., 2000, 380(1), 181–191 CrossRef.
- P. S. Green, A. J. Mendez and J. S. Jacob, et al., Neuronal expression of myeloperoxidase is increased in Alzheimer's disease, J. Neurochem., 2004, 90(3), 724–733 CrossRef CAS PubMed.
- Y. W. Yap, M. Whiteman and N. S. Cheung, Chlorinative stress: An under appreciated mediator of neurodegeneration?, Cell. Signal., 2007, 19(2), 219–228 CrossRef CAS PubMed.
- S. Sugiyama, K. Kugiyama and M. Aikawa, Hypochlorous Acid, a Macrophage Product, Induces Endothelial Apoptosis and Tissue Factor Expression, Arterioscler., Thromb., Vasc. Biol., 2004, 7(24), 1309–1314 CrossRef.
- K. C. Huang, C. C. Yang and K. T. Lee, et al., Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water, Kidney Int., 2003, 64(2), 704–714 CrossRef CAS PubMed.
- M. Hausmann, F. Obermeier and D. H. Paper, et al., In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis, Clin. Exp. Immunol., 2007, 148(2), 373–381 CrossRef CAS.
- G. M. Pieper, V. Nilakantan and T. K. Nguyen, Reactive Oxygen and Reactive Nitrogen as Signaling Molecules for Caspase 3 Activation in Acute Cardiac Transplant Rejection, Antioxid. Redox Signaling, 2008, 10, 1031–1039 CrossRef CAS.
- D. J. Eide, Zinc transporters and the cellular trafficking of zinc, Biochim. Biophys. Acta Mol. Cell Res., 2006, 1763(7), 711–722 CrossRef CAS.
- J. González-Montaña, F. Escalera-Valente and A. J. Alonso, Relationship between Vitamin B12 and Cobalt Metabolism in Domestic Ruminant: An Update, Animals, 2020, 10, 1855–1891 CrossRef PubMed.
- X. Wang, W. Zheng and H. Lin, et al., A new selective phenanthroline-based fluorescent chemosensor for Co2+, Tetrahedron Lett., 2009, 50(14), 1536–1538 CrossRef CAS.
- S. Goswami, B. Naskar and C. M. Das, A new diformyl phenol based chemosensor selectively detects Zn2+ and Co2+ in the nanomolar range in 100% aqueous medium and HCT live cells, New J. Chem., 2022, 11946–11955 Search PubMed.
- A. Linna, P. Oksa and K. Groundstroem, Exposure to cobalt in the production of cobalt and cobalt compounds and its effect on the heart, Occup. Environ. Med., 2004, 61(11), 877–885 CrossRef CAS.
- D. Maity, A. Raj and D. Karthigeyan, et al., Reaction-based probes for Co(ii) and Cu(i) with dual output modes: fluorescence live cell imaging, RSC Adv., 2013, 3(37), 16788–16794 RSC.
- A. R. Khorrami, T. Hashempur and A. Mahmoudi, et al., Determination of ultra trace amounts of cobalt and nickel in water samples by inductively coupled plasma-optical emission spectrometry after preconcentration on modified C18-silica extraction disks, Microchem. J., 2006, 84, 75–79 CrossRef CAS.
- Y. Saini, K. K. Greenwood and C. Merrill, et al., Acute Cobalt-Induced Lung Injury and the Role of Hypoxia-Inducible Factor 1α in Modulating Inflammation, Toxicol. Sci., 2010, 116(2), 673–681 CrossRef CAS PubMed.
- O. Karovic, I. Tonazzini and N. Rebola, et al., Toxic effects of cobalt in primary cultures of mouse astrocytes, Biochem. Pharmacol., 2007, 73(5), 694–708 CrossRef CAS PubMed.
- N. O. Soto, B. Horstkotte and J. G. March, et al., An environmental friendly method for the automatic determination of hypochlorite in commercial products using multisyringe flow injection analysis, Anal. Chim. Acta, 2008, 611(2), 182–186 CrossRef PubMed.
- A. P. Soldatkin, D. V. Gorchkov and C. Martelet, et al., New enzyme potentiometric sensor for hypochlorite species detection, Sens. Actuators, B, 1997, 43(1–3), 99–104 CrossRef CAS.
- T. M. Jeitner, H. Xu and G. E. Gibson, Inhibition of the a-ketoglutarate dehydrogenase complex by the myeloperoxidase products, hypochlorous acid and mono-N-chloramine, J. Neurochem., 2005, 92, 302–310 CrossRef CAS.
- M. Shahbaz, B. Dar and S. Sharif, et al., Recent advances in the fluorimetric and colorimetric detection of cobalt ions, RSC Adv., 2024, 14(14), 9819–9847 RSC.
- P. Xing, Z. Zhang and Y. Niu, et al., Water solubility is essential for fluorescent probes to image hypochlorous acid in live cells, Chem. Commun., 2018, 54(71), 9889–9892 RSC.
- D. Shi, S. Chen and B. Dong, Evaluation of HOCl-generating anticancer agents by an ultrasensitive dual-mode fluorescent probe, Chem. Sci., 2019, 10(13), 3715–3722 RSC.
- L. Yuan, L. Wang and B. K. Agrawalla, et al., Development of Targetable Two-Photon Fluorescent Probes to Image Hypochlorous Acid in Mitochondria and Lysosome in Live Cell and Inflamed Mouse Model, J. Am. Chem. Soc., 2015, 137(18), 5930–5938 CrossRef CAS PubMed.
- Y. Yang, Q. Zhao and W. Feng, et al., Luminescent Chemodosimeters for Bioimaging, Chem. Rev., 2013, 113(1), 192–270 CrossRef CAS PubMed.
- W. Xu, Z. Zeng and J. H. Jiang, et al., Discerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes, Angew. Chem., Int. Ed., 2016, 55(44), 13658–13699 CrossRef CAS PubMed.
- X. Chen, X. Tian and I. Shin, et al., Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species, Chem. Soc. Rev., 2011, 40(9), 4783–4804 RSC.
- X. Li, X. Gao and W. Shi, et al., Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes, Chem. Rev., 2014, 114(1), 590–659 CrossRef CAS PubMed.
- W. Sun, S. Guo and C. Hu, et al., Recent Development of Chemosensors Based on Cyanine Platforms, Chem. Rev., 2016, 116(14), 7768–7817 CrossRef CAS PubMed.
- Z. Xu, J. Chen and L. L. Hu, et al., Recent advances in formaldehyde-responsive fluorescent probes, Chin. Chem. Lett., 2017, 28(10), 1935–1942 CrossRef CAS.
- J. Chan, S. C. Dodani and C. J. Chang, Reaction-based small-molecule fluorescent probes for chemoselective bioimaging, Nat. Chem., 2012, 4(12), 973–984 CrossRef CAS PubMed.
- D. Wu, Y. Shen and J. Chen, et al., Naphthalimide-modified near-infrared cyanine dye with a large stokes shift and its application in bioimaging, Chin. Chem. Lett., 2017, 28(10), 1979–1982 CrossRef CAS.
- S. Ghazali, J. Wang and J. Fan, et al., Selective imaging of Co2+ in live cells with a “turn-on” fluorescent probe, Sens. Actuators, B, 2017, 239, 1237–1242 CrossRef CAS.
- D. Kim, A. Jo, B. K. Seo, et al., Colorimetric Detection of Transition Metal Ions with Azopyridine-based Probing Molecule in Aqueous Solution and in PMMA Film.
- L. Shi, S. Yang and H. J. Hong, A novel target and pH dual-activatable fluorescent probe for precisely detecting hypochlorite in lysosomes, Anal. Chim. Acta, 2020, 1094, 122–129 CrossRef CAS.
- Q. Xia, X. Wang and Y. Liu, An endoplasmic reticulum-targeted two-photon fluorescent probe for bioimaging of HClO generated during sleep deprivation, Spectrochim. Acta, Part A, 2020, 229, 117992 CrossRef CAS.
- Z. Zhu, H. Ding and Y. Wang, et al., Rational design of a FRET-based ratiometric fluorescent chemosensor for detecting ClO− with large Stokes based on rhodamine and naphthalimide fluorophores, Tetrahedron, 2020, 76(26), 131291 CrossRef CAS.
- C. Xu and Y. Qian, The α, β-unsaturated pyrazolone-based fluorescent sensor with red emission and its application for real-time monitoring hypochlorite in cancer cells and zebrafish, Dyes Pigm., 2019, 161, 303–312 CrossRef CAS.
- J. Yang, A near-infrared fluorescent probe based on phosphorus-substituted rhodamine for deep imaging of endogenous hypochlorous acid in vivo, Sens. Actuators, B, 2020, 307, 127652 CrossRef.
- L. Chen, S. J. Park and D. Wu, et al., A two-photon ESIPT based fluorescence probe for specific detection of hypochlorite, Dyes Pigm., 2018, 158, 526–532 CrossRef CAS.
- L. Wu, Q. Yang and L. Liu, et al., ESIPT-based fluorescence probe for the rapid detection of hypochlorite (HOCl/ClO−), Chem. Commun., 2018, 54(61), 8522–8525 RSC.
- A. Weller, Über die Fluoreszenz der Salizylsäure und verwandter Verbindungen, Naturwissenschaften, 1955, 42(7), 175–176 CrossRef CAS.
- H. Ren, F. Huo and X. Wu, et al., An ESIPT-induced NIR fluorescent probe to visualize mitochondrial sulfur dioxide during oxidative stress in vivo, Chem. Commun., 2020, 57, 655–657 RSC.
- A. Bi, M. Liu and S. Huang, et al., Construction and theoretical insights into the ESIPT fluorescent probe for imaging formaldehyde in vitro and in vivo, Chem. Commun., 2021, 57(28), 3496–3499 RSC.
- H. C. Zhang, D. H. Tian and Y. L. Zheng, et al., Designing an ESIPT-based fluorescent probe for imaging of hydrogen peroxide during the ferroptosis process, Spectrochim. Acta, Part A, 2021, 248, 119264 CrossRef CAS PubMed.
- C. Wu, H. Xu and Y. Li, et al., An ESIPT-based fluorescent probe for the detection of phosgene in the solution and gas phases, Talanta, 2019, 200, 78–83 CrossRef CAS PubMed.
- Y. Li, D. Dahal and C. S. Abeywickrama, et al., Progress in Tuning Emission of the Excited-State Intramolecular Proton Transfer (ESIPT)-Based Fluorescent Probes, ACS Omega, 2021, 6(10), 6547–6553 CrossRef CAS PubMed.
- V. V. Shynkar, A. S. Klymchenko and C. Kunzelmann, et al., Fluorescent Biomembrane Probe for Ratiometric Detection of Apoptosis, J. Am. Chem. Soc., 2007, 129(7), 2187–2193 CrossRef CAS PubMed.
- Y. Zhang, Y. Deng and N. Ji, et al., A rationally designed flavone-based ESIPT fluorescent chemodosimeter for highly selective recognition towards fluoride and its application in live-cell imaging, Dyes Pigm., 2019, 166, 473–479 CrossRef CAS.
- N. N. Mohd Yusof Chan, A. Idris and Z. H. Zainal Abidin, et al., White light employing luminescent engineered large (mega) Stokes shift molecules: a review, RSC Adv., 2021, 11(22), 13409–13445 RSC.
- V. S. Padalkar and S. Seki, Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters, Chem. Soc. Rev., 2016, 45(1), 169–202 RSC.
- C. Lu, J. Xu and Z. Song, et al., Advancements in ESIPT probe research over the past three years based on different fluorophores, Dyes Pigm., 2024, 224, 111994 CrossRef CAS.
- J. Luo, Z. Xie and J. W. Y. Lam, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC.
- K. Wang, D. Xi and C. Liu, et al., A ratiometric benzothiazole-based fluorescence probe for selectively recognizing HClO and its practical applications, Chin. Chem. Lett., 2020, 31(11), 2955–2959 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07451c |
| This journal is © The Royal Society of Chemistry 2025 |