Open Access Article
Open Access ArticleAdvancements of paper-based microfluidics and organ-on-a-chip models in cosmetics hazards
Sanidhya Pai†
 a,
Amanda Binu†b,
G. S. Lavanya†b,
Meenakshi Harikumar†b,
Srikrishna Kedlaya Herga†
a,
Amanda Binu†b,
G. S. Lavanya†b,
Meenakshi Harikumar†b,
Srikrishna Kedlaya Herga† c,
Marimuthu Citartan†
c,
Marimuthu Citartan† d and
Naresh Kumar Mani
d and
Naresh Kumar Mani *b
*b
aTechnical University of Munich, Campus Straubing for Biotechnology and Sustainability, Straubing, Germany
bMicrofluidics, Sensors and Diagnostics (μSenD) Laboratory, Centre for Microfluidics, Biomarkers, Photoceutics and Sensors (μBioPS), Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India. E-mail: naresh.mani@manipal.edu; maninaresh@gmail.com
cDepartment of Public Health Genomics, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India
dAdvanced Medical and Dental Institute, Universiti Sains Malaysia, Kepala Batas, Penang 13200, Malaysia
First published on 3rd April 2025
Abstract
Cosmetics have been used in society for centuries for beautification and personal hygiene maintenance. Modern cosmetics include various makeup, hair, and skincare products that range from moisturizers and shampoos to lipsticks and foundations and have become a quintessential part of our daily grooming activities. However, dangerous adulterants are added during the production of these cosmetics, which range from heavy metals to microbial contaminants. These adulterants not only reduce the quality and efficacy of cosmetic products but also pose a significant risk to human health. Detecting the presence of adulterants in cosmetics is crucial for regulating substandard cosmetic products in the industry. The conventional methods to detect such adulterants and quality testing are expensive and take a lot of effort, particularly when involving advanced analytical detection and clinical trials. Recently, efficient methods such as microfluidic methods have emerged to detect adulterants rapidly. In this review, we mainly focus on various adulterants present in cosmetics and their detection using paper-based microfluidic devices. In addition, this review also sheds light on the organ-on-a-chip model with the goal of developing a human tissue model for cosmetic testing. Combined, these approaches provide an efficient, inexpensive, and sustainable approach for quality testing in the cosmetics industry.
Introduction
Cosmetic products are used to enhance one's appearance, maintain our hair and skin, are utilizable for personal hygiene, and also possess anti-aging properties.1,2 The use of cosmetics has increased excessively over the years, and with the concomitant increase in their demands and price, the surge in fraudulent practices associated with cosmetics has also been recently reported.3 Cosmetic products contain a wide range of additives or adulterants that are used to enhance their properties, such as colour, fragrance, texture, and shelf life. These products could be harmful to the users due to the possible health hazards and can have detrimental effects at higher concentrations.4 According to A. Panico et al., 2019, adulterants can be classified as fragrances, preservatives, and other chemicals of concern. Fragrance ingredients (like limonene), when used excessively, cause skin irritation, sensitization, and allergies, which is the very reason for establishing a certain concentration limit for its utilization by the EU. Preservatives, including parabens and formaldehyde, cause cancer and hypersensitivity responses, whereas methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI) cause skin irritation and allergies. Chemicals such as benzophenone-3 and benzophenone-1 are used for protection against ultraviolet rays, but excessive quantities could lead to alterations in behavioural development and cause congenital malformations. On the other hand, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), cocamide diethanolamine (cocamide DEA) and toluene cause skin irritation. Additionally, toluene can also act as a teratogen and affect the central nervous system.5Common cosmetic products such as lightening creams, sunscreens, and lipsticks have been speculated to be contaminated with heavy metal residues of chromium (Cr), cadmium (Cd), nickel (Ni), iron (Fe), and lead (Pb) in various concentrations.6,7 For instance, lipsticks are reported to possess higher amounts of Fe, whereas sunscreens possess higher concentrations of Ni, Pb, and Cr.8 Another major harmful ingredient in the cosmetic industry is parabens, which have physicochemical properties compatible with cosmetic agents and can be used up to a limit of 0.8%.9 Despite its desirable characteristic use, it was observed to cause breast cancer.10 There have been incidences where adulterated skincare cosmetics had adverse effects on pregnant ladies, where adulterants like microplastics,11 parabens,2,12,13 benzophenones,14,15 phthalates,13,16,17 and some metals18,19 were linked to neurotoxic inborn defects like congenital enteric neuropathies in the children.20 There have also been references linking the effect of such adulterated cosmetics causing preterm birth and low birth weights.21 Cosmetic adulterants also pose a risk to children who use them. There have been incidences of cosmetic poisoning in children,22 including those linked with structural birth defects,23 adulterant-induced diseases like cancer,24 and cosmetic cytotoxicity leading to impaired growth and development.25 Since the excessive usage of adulterants can cause various toxicity-related issues, there is a pressing need for safety and compositional testing of cosmetic products at various stages, including formulations, pre-clinical, clinical, and post-clinical trial phases, and random regular testing of batches distributed in the market to ensure consumer health and safety.26 Various conventional methods are used to evaluate product quality to serve this purpose. Authorities such as the Food and Drug Administration (FDA) and the Central Drugs Standard Control Organization (CDSCO) are involved in the quality control and assurance of cosmetics.27 Conventional methods like high-performance liquid chromatography, gas chromatography, mass spectrometry, Raman spectroscopy, near infrared (NIR), infrared (IR) and ultraviolet (UV) spectroscopy are considered to be gold standard methods for analytical testing of cosmetics to detect adulterants.28 Although standard methods are accurate, they come with limitations like cost ineffectiveness, tedious preparations, and technical expertise. This potentially extends the duration of the analysis and increases the possibility of errors. As a consequence, the supply chain of the product is affected. Additionally, some of the conventional methods require destructive sample testing, which renders the product unsuitable for sale or use, leading to product wastage and financial losses. Hence, industries need to address the aforementioned issues using many feasible, robust, frugal, and easily deployable methods to ensure consumer safety and regulatory adherence.4
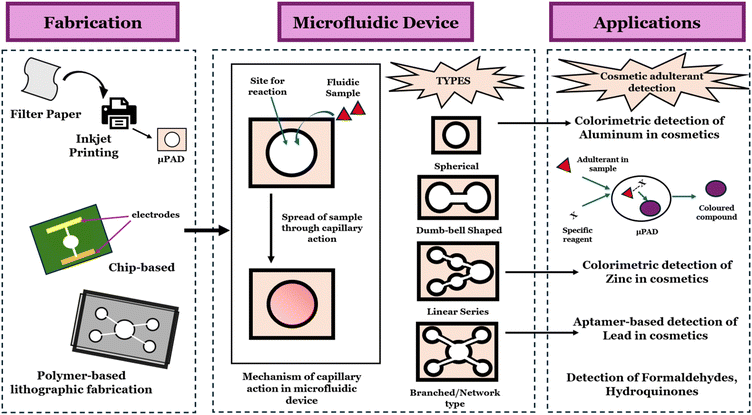
During the last two decades, microfluidics have gained traction in basic as well as applied research such as in medical and environmental-based applications.29,30 Microfluidic technologies employ the manipulation of small volumes of fluids, using channels that typically have dimensions within 10–100 microns, for testing and analysing fluidic interactions in small scale. However, its innumerable customizable properties, ease of use, and miniaturizations in microfluidics allow for the sustainable performance of experiments and the generation of efficient and reliable data, even with a small volume of reagents, effectively and rapidly.31–33 Paper-based microfluidics is one such subsidiary of microfluidics, where paper is used as a medium to perform microfluidic assays. Paper-based microfluidics operates based on the natural capillary action produced by the intermolecular interactions between the liquid and the cellulose fibres of the paper that allow the liquid to flow through the pores without the assistance of any additional pumps.34 The hydrophilic properties of the cellulose fibres and the surface tension of the liquids allow the liquids to spread and flow through the paper matrix. By using the material's porous network, which consists of interwoven cellulose fibres, the flow of the liquid can be directed and controlled through specific channels and patterns imprinted on the paper that also impart hydrophobic boundaries. This allows for minimal sample usage and reduces fluid travel time. Due to its flexibility, lightness, low cost, and widespread availability, paper is becoming a highly desirable substrate material for microfluidic applications.35,36 Moreover, paper can be easily modulated for microfluidics by draining an aqueous suspension of diluted cellulose fibres through a sieve, pressing, and drying the resulting sheet of randomly woven fibres.37
Recently, the development of 3D microfluidic paper-based analytical device (μPAD) systems has been exploited in paper-based microfluidics for efficient detecting accuracy and ease of applications. It provides automated sample distributions across the surface with mechanically activated valves to connect or disconnect channels for better detection of the target. μPADs can be combined with colorimetry, fluorimetry, chemiluminescence and other such analytical quantification techniques. It enhances the detecting accuracy of the target substance even in minute quantities. The primary purpose of μPADs is to provide a mobile, affordable, and user-friendly analytical platform for diagnostic assays.33 Inexpensive diagnostic tests can be produced in large quantities at a cost affordable to end-users.38,39 Recent diagnostic strategies emphasize the need for simple, affordable, and reliable tests that can be widely used, including in resource-limited countries. From this perspective, μPADs are considered the most practical alternative to traditional devices in the developing world.40
After analytical testing, the next step in cosmetic product quality check is the clinical and dermatological study to determine product compatibility and toxicity profile for humans. It ensures compliance with regulatory standards before market release. The conventional approach to these safety trials includes testing on cell cultures, patch tests, cutometric and corneometric testing, and toxicity analysis in live animal models.26 This process takes a lot of time and resources, making this a dark and hectic phase before releasing the product into the market. The use of certain conventional methods for safety testing is bound to ethical issues, which further delays the process.41 As an alternative to this, the latest organ-on-a-chip (OOAC) technology presents a facile approach to conducting safety tests.42 As compared to the microfluidics, OOAC technology aims at creating a live 3D microenvironment of the cells, with continuous exchange of growth factors and media using microfluidic technology.43–45 OOAC technology can be used to create human organs-on-a-chip models, mimicking actual organs, which offers real-time analysis of toxicity and compatibility profiles.46–48 Also, OOAC provides a human-specific microenvironment for testing,49 rather than animal models in traditional approaches, aiding in obtaining a more precise safety analysis of the cosmetic product.50 These technologies align with the United Nations Sustainable Development Goal (SDG) 3 (Good Health and Well-being) by promoting safer consumer products and SDG 12 (Responsible Consumption and Production) through sustainable testing methods. They also address SDG 8 by sustainable testing, SDG 9 (Industry, Innovation, and Infrastructure), which supports and paves the way for innovation, SDG 15 (Life on Earth) by reducing animal use in safety trials, and SDG 16 (Peace, Justice, and Strong Institutions) by ensuring ethical practices.51–53
In this review, we have compiled and analysed recent advances in microfluidics and OOAC technology for cosmetic adulterant detection and safety testing (Fig. 1). First, we expound on the characteristics of paper-based microfluidics and OOAC. As for the paper-based microfluidics, we explore the targets that have been subjected to the detection based on paper-based microfluidics, ranging from small molecules such as nitrosamines to biological contaminants. Other facets of our study also encompass the significance, reliance, benefits, ease of use, and efficiency of the paper-based microfluidics and OOAC as compared to the conventional approaches. The integration of artificial intelligence (AI) into microfluidics technology is also emphasized given the paramount significance of the former in advancing the latter. We prophesied that this microfluidics technology is a welcoming boon to the public, healthcare sectors, industries, and regulatory bodies in conducting robust cosmetic product testing and quality analysis.
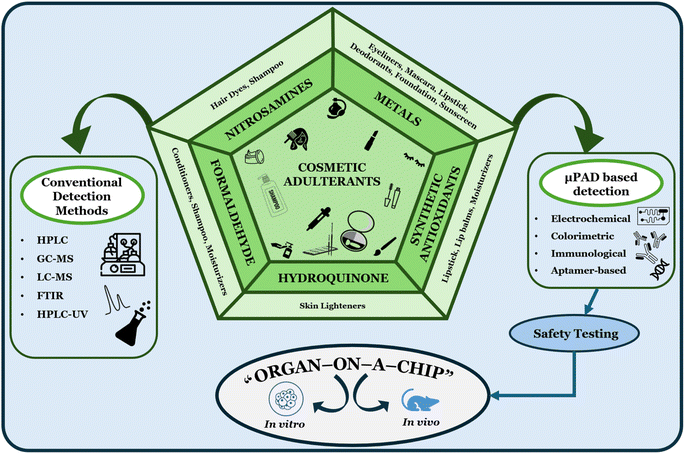 | ||
| Fig. 1 A general schematic of the cosmetic adulterants and utility of μPADs and OOAC in their detection and safety testing. | ||
Advances in paper-based microfluidics
Paper-based microfluidics are cheap, sensitive, easy to assemble, operable using minimal sample volumes, and have a biodegradable and disposable nature. Due to their inherent capillary action, they do not require any external equipment to control the flow of liquids. Papers as the substrates of microfluidics are widely available, of low cost, light, of various thicknesses, and able to confer capillary action to transport fluid effectively.36 Capillary action refers to the spontaneous flow of the liquid without the assistance of external factors or forces. The surface tension effects that stem from the geometry and surface chemistry of a microchannel initiate this capillary action without the need for any peripheral equipment.54 Multiple target detection can be carried out on a single piece of paper to create a simple, cost-effective, and self-contained device. As such, they are widely applied to detect adulterants in cosmetics.55To expedite point-of-care (POC) applications, the chosen signal transduction assays must be seamlessly integrated into paper-based microfluidic devices. There are various signal production strategies such as colorimetric detection, electrochemical sensing (EC), fluorescence and chemiluminescence (CL) sensing, and electrochemiluminescence sensing (ECL). Among all these methods, colorimetric assays are the most common due to their ability to allow naked-eye observation, which infers the presence or absence of the target molecules. Small samples of cosmetics move through the paper channels impregnated with special reagents (Fig. 2). As the sample moves through the microfluidic channel by capillary action, the subsequent reaction with these reagents results in colour change. The naked eye observation is based on the colour changes that take place against the backdrop of paper substrates, which are clear and colourless. The high contrast of the colour changes against the white-coloured background permits a clear observation. In fact, the near-white background of the paper-based microfluidics offers significant advantages over spectrophotometry. Besides naked eye observation, colorimetric detection also offers semi-quantitative analyses. The colorimetric sensor can detect and quantify analytes down to the nanomolar range due to the high sensitivity and selectivity of the sensor. This is possible by comparing the colour intensity produced by a known analyte concentration with the colour intensity of an unknown analyte in the test region.56
Portable devices like smartphones and digital cameras can be used for detection in microfluidic assays.56–59 They allow for rapid on-site product safety assessment which is mainly useful in areas with limited access to advanced and sophisticated analytical equipment.60 Electrochemical sensing is also widely utilized as the output signal is stable even in the presence of sample contaminants and light conditions. In addition, the high porosity and rough texture of the paper substrate increases the surface area of the deposited material, thereby improving the response of the electrochemical sensor.61 These outcomes make paper-based microfluidics a recent and advanced approach for rapidly detecting and quantifying any target molecules in diverse settings.
Paper-based microfluidics for adulterant detection
Paper-based microfluidic devices are developed to detect various commonly found adulterants in cosmetics, including chemical adulterants like nitrosamines,62 hydroquinone,63 formaldehyde,64 antioxidants,65 heavy metals,66–69 and even biological contaminants.37,70 These μPADs offer instantaneous and low-cost detection of adulterants. These can also be used during the early formulation stage, as they require micro volumes of samples for detection. This helps in the early detection of adulterants and prevents product loss or wastage at a later stage. Also, these devices could be used to monitor and identify sources of contamination without necessitating bulky analytical methods. μPADs also offer an easy approach for regulatory bodies and customers to detect adulterants in substandard cosmetics. This helps in immediate detection of non-compliant products in market and helps in informed decision making. This helps strengthen public trust in cosmetic safety regulations. There are various studies on utilizing microfluidic devices for adulterant detections. A detailed summary of common cosmetic adulterants, microfluidic device to detect them and its fabrications, working principles and limits of has been discussed in the Table 1. Various cosmetic adulterants for which μPADs detection has been implemented are as follows.| Adulterants | Present in/as | Side effects | Sample | Detection mode | Fabrication | Device structure | Device materials | Limit of detection | Range | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrosamines | Hair dyes, shampoos | Carcinogenic | Cosmetics | Paper spray ionization, filter cone spray ionization | — | Paper-based ionization method | Paper substrate and filter cone | As low as 1.0 ppm by FCSI method and 5.0 ng by PSI method | — | — | 62 |
| Arsenic | Lipstick, whitening toothpaste, eyeliner, and nail color | Toxic vapors can cause scabbing, blisters, and sores. Prolonged exposure can lead to organ failure | Water | Colorimetric assay | — | Paper-based microfluidics | Cysteine-capped Ag NPrs, and methionine-capped Ag NPrs | 0.0005 ppm | 0.0005 to 1 ppm | — | 71 |
| Aluminium | Aerosol, roll-on antiperspirants products | Aluminium may cause gene instability, alter gene expression or enhance oxidative stress | Deodorant | Colorimetric assay | — | Paper-based microfluidics | — | 3.07 and 10.2 mg l−1 | 10.0 to 125.0 mg l−1 | — | 71 |
| Mercury | Skin lightening agents and preservative | Kidney and nervous system damage | Food | Chemical based colorimetric method | Filter paper and acrylic | Porous fibre | AgNPs, filter paper, acrylic, glycerol | — | 0.1–3 ppb | — | 69 |
| Lead | Nitrosamines | Poisoning the nervous system causes learning impairment, language, behaviour | Water | Aptamer sensor | Whatman No. 1 and nylon filter papers | The aptamer works on the principle of interaction of gold nanoparticles with NaCl | — | 1.2 nM and 0.7 nM | — | — | 72 |
| Cobalt | Make-up, hair dye | Asthma-like allergy, heart, thyroid, liver, and kidney damage | Lipstick samples | Colorimetric/spectrophotometric method | — | μPAD platform | DMG reagent | 0.11 mg l−1 | — | — | 73 |
| Nickel | Foundations and lip product | Rashes and itching | Lipstick samples | Colorimetric/spectrophotometric method | — | μPAD platform | DMG reagent | 0.36 mg l−1 | — | — | 74 |
| Zinc | Sunscreen, skin whitening agent in foundation | Abdominal pain, nausea, and vomiting | Urine sample | Colorimetric | — | Circular sampling zone with two identical tables | — | 35.9 μg l−1 | — | 75 | |
| BHA and BHT | Lipsticks and moisturizers | Endocrine disruption and organ-system toxicity | Food sample | Paper chromatography | Chromatography paper | μPAD platform | Chromatography paper | BHA-0.001–0.02%, BHT-0.0002–0.8% | — | — | 74 |
| Hydroquinone | Whitening creams | Carcinogenic | Whitening creams | Colorimetric assays | Whatman No. 1 chromatography paper | A hydrophilic reaction zone and a hydrophobic barrier layer | Hydrophobic wax ink made of bisamide and maleic anhydride waxes | — | — | Close to 100% | 63 |
| Formaldehyde | Preservatives in cosmetic products | Redness, itching, and scaling of the skin, irritation to the eyes | Food sample | CMOS camera | Filter paper-based | — | μPAD and a portable detection system comprising a power source, an LED UV light source, a chip holder, a hot plate, a CMOS camera | — | — | — | 45 |
| Heavy metals (Ag+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Hg2+, La2+, Mn2+, Ni2+, Pb2+, Ti2+, Zn2+, Al3+, As3+, Fe3+, V4+) | Herbal supplements, cosmetics | Toxicity, organ damage | Liquid samples | Distance-based detection (DbD) using paper-based microfluidics | Ion-exchange reagent immobilization on anion-exchange filter paper | Ion-exchange interaction for reagent immobilization and metal binding | Anion-exchange filter paper, 5-Br-PAPS | Even in low mg per mL range | 0.25–1 mM (5-Br-PAPS), 20–500 mM buffer concentration | Enhanced reagent retention and clear detection endpoints | 76 |
Nitrosamines
Nitrosamines are a class of organic compounds that contain a nitroso group (–NO) bonded to an amine group (–NH2). These compounds are formed naturally due to certain environmental processes like the aging of food items or microbial activity in soil or water.77,78 They are even formed during industrial processes like rubber manufacturing and pesticides production.79,80 Nitrosamines are potentially harmful to human health and are associated with posing a high risk of cancer specially in organs such as the liver, lung, stomach, and oesophagus.81 Nitrosamines may be formed when cosmetics containing amines are subjected to certain environmental factors such as heat, humidity and light or when they come into contact with nitrosating agents.78 Amines such as diethanolamine (DEA) and triethanolamine (TEA) are the additives commonly used in cosmetics as these amines help in adjusting the pH levels and act as wetting agents. Nitrosamines are also found in cosmetic products such as hair dye, shampoos, and other personal care products.12 Due to their carcinogenic properties, regulatory authorities have set the limit of 50 ppb for nitrosamine in cosmetics.82,83According to Trevor J. McDaniel et al., 2023, the two paper-based methods used for the detection of nitrosamine in cosmetics are paper spray ionization (PSI) and filter cone spray ionization (FCSI), as described in Fig. 3.62 Paper spray ionization is a relatively simple and rapid ionization technique where paper is used as a substrate. First, sample is placed directly on the paper substrate and an electric current is then supplied to induce the ionisation of analytes present in the sample. These charged ions are then analysed using a mass spectrometer. PSI is a suitable choice for quick screening of nitrosamine in cosmetics because it requires minimal sample preparation.84 Filter cone spray ionisation is another ionisation technique where a filter cone is used as an ionisation source. The sample is placed on the tip of the filter cone and a suitable solvent is used to extract the analytes. Additionally, charged droplets are formed upon the application of electric fields leading to ionisation of analytes and these ions are later analysed using a mass spectrometer. FCSI is suitable in identifying minute amounts of nitrosamine in cosmetics due to its good selectivity and sensitivity.85 Due to their advantages, both PSI and FCSI can be incorporated into microfluidic devices to create integrated analytic systems. Some of the microfluidic devices that can be integrated with PSI and FCSI are microfluidic sample preparation chips for mixing, extracting and purifying samples, microfluidic analyte separation devices for chromatographic separations, microfluidic spray devices for controlled ionisation, microfluidic droplet generators for precise electrospray ionisation and microfluidic mixing chambers for increased extraction and ionisation efficiency. The integration of PSI AND FCSI with microfluidic devices allows better control of sample introduction process, improves sensitivity. Moreover, they can function using less sample with improved overall efficiency of mass spectrometric analysis and thereby the detection of nitrosamine in cosmetics.62,86
 | ||
| Fig. 3 Paper spray ionization and filter cone spray ionization working: the first part depicts paper spray ionization, which is used to identify nitrosamines in cosmetics; an electric current is applied to the sample directly on the paper substrate, causing the analytes within to ionize. After that, a mass spectrometer is used to analyse these charged ions. Paper spray ionization design modified and adapted from ref. 84, licensed under CC BY 4.0. In filter cone spray ionization, an additional ionization technique, charged droplets are formed on the application of electric fields, leading to the ionization of analytes, and these ions are subsequently analysed using a mass spectrometer. The sample is placed on the tip of the filter cone and an appropriate solvent is used to extract the analytes. Filter cone spray ionization modified and adapted from ref. 85, with permissions under Copyright ©2020, the American Chemical Society Publications. These methods were tested and adopted for nitrosamine detection using the same principle.62 | ||
Metals
Various metals (light and heavy metals) have been detected in cosmetics. Though their presence in small quantities is considered safe, exceeding certain limits may cause toxicity. Metals can be absorbed into the blood through the skin, accumulate over continuous use, and harm various organs. Detection of various metals using microfluidic devices has been described in Fig. 3 and summarized in Table 1. | ||
| Fig. 4 Detection of various metals using paper-based microfluidics. (a) Aluminum hydrochloride in anti-perspirant samples using a colorimetric technique based on paper. The purple tint of Alizarin S was the colorimetric agent utilized. The figure design is modified and adapted from ref. 71, with permissions under Copyright ©2018, Elsevier. (b) Scheme showing using μPAD integrated colorimetric method based on aptamer, to detect lead in water samples. Due to differences in localized surface plasmon resonance (LSPR), the aptamers split apart when they bind to the target, accumulating AuNPs and changing color from red to blue or purple. Due to AuNP aggregation, the interaction of gold particles with NaCl causes a color shift from red to purple when lead is present. The figure design is modified and adapted from ref. 68, with permissions under Copyright © 2018, the Royal Society of Chemistry. (c) Scheme showing the design of a microfluidic device to detect zinc. The circular sample zone of the microfluidic paper-based device is surrounded by two similar arms, each of which has a circular pre-treatment zone and a circular detecting zone. Zinc and 1,2 naphthol reagent (PAN) react at room temperature and pH 6.0. Pink chelates are formed by zinc. Modified and adapted from ref. 92, licensed under CC BY 4.0. | ||
Xiaolu Xiong et al. 2020 have developed paper-based microfluidics combined with a colorimetric assay for the detection of nickel. The platform was designed to contain a core zone, 10 reaction zones and ten detection zones. Each zone is designed to harbour a particular chromogenic reaction. By maximising the photoresist layer thickness and spin coating speed, the flow passage volume is controlled during the fabrication process. The reaction solution injection interval time is designed for simultaneous detection of Nickel. The intensity of the colour is used to determine Ni(II)concentration based on a standard curve. For Ni(II) the detection threshold was found to be 0.5 Mm.73
Synthetic antioxidants
Butylated hydroxytoluene (BHT) and butylated hydroxy anisole (BHA) are the two synthetic antioxidants used in various industries such as cosmetics, food, and pharmaceuticals. BHA is made of a mixture of organic compounds, which are isomeric in nature such as 3-tert-butyl-4-hydroxyanisole and 2-tert-butyl-4-hydroxyanisole. BHT is derived from the reaction of isobutylene and 4-methoxyphenol, catalysed by sulphuric acid. BHA and BHT are usually found in cosmetic products such as lipsticks, lip balms, moisturisers, and personal care products. These compounds act as preservatives and thereby increase the durability and stability of the products by preventing the spoilage and oxidation of products by inhibiting the formation of free radicals. These compounds are carcinogenic in nature and can cause other harmful effects such as endocrine disruption and organ-system toxicity when used in excess quantity.65 The safety limit of BHA and BHT to be used in dermally or sprayable cosmetic product type is 0.001–0.02% and 0.0002–0.8%, respectively.99Paper-based microfluidics is a cost-effective and straightforward analytical method for the detection of BHA and BHT. Using an appropriate solvent, a cosmetic sample can be extracted and applied as a small spot on the chromatography paper before placing this in a container with solvent. The components in the sample are separated upon interaction with the paper and solvent. As the solvent goes up carrying the various dissolved components with it, BHA and BHT will travel at different rates and form separate spots on the paper. Based on the location and appearance of spots on the paper, the presence of BHA and BHT can be determined. Paper chromatography can only be useful for qualitative analysis to determine the presence or absence of compounds. For quantitative analysis, HPLC or GC-MS should be used. According to Maryam Nejadmansouri et al., 2021 samples loaded onto paper-based microfluidics can be transported without the need for an external pump. Based on the affinity of BHA and BHT to the paper matrix, they are separated as they move up the paper channel through capillary action. The microfluidic property of the paper-based microfluidics helps in the controlled movement of the sample, thereby increases the separation efficiency and reduces the analysis time. Once the separation is complete, visual detection or chemical detection can be used to determine the presence or absence of the target. In a visual detection, dyes are used to indicate the presence of BHA and BHT in a sample while chemical detection involves the use of various reagents that react with BHA and BHT to produce products that infer the presence of absence of the sample to detect their presence in a sample. Paper-based microfluidics help in both quantitative and qualitative analysis and have numerous other advantages such as minimal sample requirement, cost-effectiveness, reduced analysis time which makes it a suitable tool for the detection of BHA and BHT in cosmetics.74
Hydroquinone
Hydroquinone is a widely used chemical in creams for depigmentation of the skin for more than 50 years. It is generally used to treat discolouration and pigmentation disorders of the skin such as freckles, melasma, chloasma, solar lentigines, and post inflammatory hyperpigmentation. The hydroquinone cream is applied as a thin layer over the depigmented skin. It is suggested by medical professionals to discontinue hydroquinone treatment after a few months to avoid any side-effects. Although it is not significantly toxic, some studies have suggested long-term usage can create malignancies.93 In a study conducted by Fahmi, et al., 2019, spectrophotometric method hydroquinone. The orange-coloured final product is produced upon the interaction between hydroquinone and phloroglucinol under alkaline conditions, which can be observed by naked eye. This reaction principle can be crucially planned and modified to devise a microfluidic approach for the detection of hydroquinones in cosmetics.100In general, risk to consumer health was determined using systemic exposure dosage (SED) which is the amount of a substance that enters the bloodstream following absorption and this dosage is compared with the safety limit and concluded whether the exposure is safe or harmful, margin of safety (MoS) which is the difference between the exposure level of the substance and its harmfulness threshold and a higher margin will indicate lower risk to human health, hazard quotient (HQ) is the ratio of actual exposure level of a substance to the level deemed safe and if the ratio is greater than 1, there is a potential impact on human health, hazard index is the summation of hazard quotients for various substances and if the value is greater than 1, it may cause harmful effects on human health and lifetime cancer risk (LCR) which helps in determining the possibility of developing cancer due to the exposure to carcinogenic substances.101
Organ-on-a-chip (OOAC) technology for cosmetic safety
Organ-on-a-chip (OOAC) is a device that mimics different organs or organ systems. OOAC is prepared by containing and sustaining the cells to create a microenvironment that mimics the real state of the body. OOAC can also be interwoven with microfluidics platform to accurately emulate in vivo environment. Microfluidics can help to drive the flow of the liquid at certain flow rates and introduce mechanical stress, which mimics the physiological conditions of the complex matrices present in the in vivo environment. Microfluidics technology also allows the transportation of nutrients and waste product from the cells on OOAC. Moreover, these organs are used as the disease model or for toxicity testing including the analysis of the response to certain stimuli. Mimicking the actual organ or organ systems in the human body, OOAC is a much safer and feasible alternative as compared to the usage of animal to carry out similar functions. Such microenvironments created using OOAC help scientists to bridge in vitro and in vivo studies.45 Two different types of OOAC single- and multi-organ systems, are depending on the focus of study. While multi-organ systems aid in understanding the interaction of at least two organs through metabolites, reactions, or signalling, single-organ systems are used to study the response of a single organ to compounds or reactions.102 Integrating microfluidics into OOAC enables the usage of low sample volumes ranging from microliters to femtoliters reducing its Reynolds number, which is indicative of the laminar flow whereby high value of Reynold signifies turbulent flow. Other additional benefits of microfluidics on OOAC are rapid mixing rates for faster responses and tighter control of the liquid volumes. The chief feature of OOAC is the better regulation of the cell microenvironment, which refers to the cellular environment that is characterized by a complex interplay of cellular factors, extracellular matrix and other cells. OACC facilitates the precise control of the culture chamber geometry, physical and chemical phenomena of fluids as compared to two-dimensional (2D) cell culture systems that could not simulate the microenvironment milieu.103Deciding the approach for forming the functional tissues is important while designing an OOAC. There are two types of approaches that can be used: top-down and bottom-up approaches. A primary tissue is integrated into the system using a top-down technique while a bottom-up approach involves culturing of primary tissue cells in a microfluidic environment to aid in the remodelling of the tissues into a new environment. There are two types of OOAC device architectures, barrier tissue chips and solid organ chips. In solid organ chips, cells are cultured as three-dimensional tissue masses where they can interact with the culture medium and each other, but in barrier tissue chips, the cells are supported to form a natural barrier between fluid compartments. The choice of materials for the device depends on the functionality, stage of product development, etc. The most often utilised materials are glass, thermoplastics like PS, poly (methyl methacrylate) (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC), and silicone rubbers like poly(dimethyl siloxane) (PDMS).45
To ensure cell growth and constant medium flow, the tissue framework needs a dense pore network and high porosity. The network offers nutrition, gas exchange, and a pathway for removing cellular waste and by-products of scaffold breakdown. In order to ensure that protein exchange between cells is facilitated through the network of interconnected pores, the architecture and porosity of the matrix must be carefully balanced to maintain the mechanical integrity and stability. The scaffold's mechanical and architectural characteristics are influenced by the manufacturing process used to make it.104 For an industrial scale, the manufacturing process must be affordable and allows scaling up. In order to fabricate complicated structures, industrial technologies including electrospinning, 3D printing, and injection moulding are applicable. To ensure that the various scaffolds may be utilised for pre-clinical OOAC system testing, good fabrication criteria must be established. Techniques such as stereolithography and fused deposition modelling are well-suited printing techniques to prepare a scaffold of optimum porosity, which can be extended for OOAC development.105 Collagen is a widely used material to develop scaffolds due to its excellent biocompatible properties such as low immunogenicity, high porosity and permeability.106
Eye-on-a-chip in cosmetics
Eye-on-a-chip model is an advanced microfluidic device that mimics the physiological and functional characteristics of the various components of the eye including the retina, optic nerve, lens, cornea, aqueous and vitreous humour, and blood vessels. Researchers use this model to study ocular biology and to test the effects of various components such as cosmetics and medications on ocular tissues thereby reducing the dependency on animal testing by creating a controlled environment.107In a prominent study by Z., Yu et al., 2022, “Human cornea-on-a-chip model” was constructed by creating a bond between the glass substrates (Fig. 5). Polydimethylsiloxane (PDMS) layers and a polycarbonate (PC) membrane coated with extracellular matrix were sandwiched between the glass substrates. SU-8, which is an epoxy-based negative photoresist is created using soft lithography and was used as the base to form the top and bottom PDMS layers. Microfluidic channels were created when the PDMS duplicates were peeled off from the mould. Access holes of the microfluidics' inlets and outlets were punched, and a circular hole was punched in the middle of the microfluidic channels in each PDMS slab. The exposed surface on the top was used to mimic the ocular surface by creating an air–liquid interface and the hole was used to mimic the human corneal structure. The PC membrane was embedded between the two microfluidic channels after cleaning the glass membrane and PDMS. The “Trans-epithelial electrical resistance (TEER) zone is a designated area in a biological or microfluidic device used to measure the electrical resistance between layers of epithelial cells. This measurement, which indicates how well the cells create a protective barrier, is essential for assessing the integrity and barrier function of these cell layers. The TEER zone is typically integrated into in vitro models, such as organ-on-a-chip devices, which enables real-time, non-invasive evaluation of tissue health and function and thereby is integrated into the device and connected to the cell culture region helps in in situ measurement of “TEER”, which is a recognized technique for assessing the corneal barrier function in vitro.108 “Eye-on-a chip model” has the potential to dramatically alter cosmetic testing by providing a more accurate, moral, and ethical way to study the effects of cosmetic products on ocular tissue by reducing the dependency on animal testing. It also helps in studying the safety, absorption, compatibility, and the long-term effects of cosmetic products on ocular tissues.107
 | ||
| Fig. 5 Eye-on-a-chip and skin-on-a-chip device structure: the first part depicts EOAC. The epithelium and endothelium cells derived from a human cornea are cultured on two scaffolds. There exists an extra-cellular membrane. There are inlet channels connected to both levels. The two cell layers interact with each other. The TEER (trans-epithelial electrical resistance) zone is connected to a computer to evaluate the corneal barrier function. Skin-on-a-chip (in the second part) consists of three layers: epidermis, dermis, and endothelium cells derived from the skin, which are grown one over the other, and are separated by an ECM. There are inlet channels which can be used for different treatments. Vertically stacked cell layers with ECM in between are used to study the interaction and inflammatory markers in between the layers. Eye on a chip modified and adapted from ref. 108, licensed under CC BY 4.0., and skin on a chip modified and adapted from ref. 109, licensed under CC BY 4.0. | ||
Skin-on-a-chip in cosmetics
Skin-on-a-chip are another type of OOAC models can be used for toxicity testing, disease modelling and to study skin responses to various stimuli. They can be used for drug development and are able to replace animals for testing.43 Skin-on-a-chip models have increasingly gained widespread interest in the cosmetic industry by providing a morally sound, precise, and effective ways to evaluate the efficacy, interactions, and safety of cosmetic products. These models mimic the human skin more accurately compared to the traditional 2D cultures, which in turn helps in understanding the adverse response predictions, mechanisms, and customization of various skin types. The increased use of OOAC in cosmetic industry has moral and ethical benefits over animal testing, improved predictive capabilities, reduced product development time and cost, potential for various formulations based on consumer needs and ensures compliance with more stringent regulatory requirements. Moreover, OOAC reduces the negative impact of using animal for testing and allow precise study on the interactions of skin with various cosmetic products and substances.110,111Two approaches have been used to create microfluidic skin models, which are. Direct introduction to skin fragments and in situ skin model generation. Direct interaction approach involves the direct introduction of skin fragment onto the microfluidic chip. While this model helps in studying specific interactions, it can also incorporate other organs, such as the liver, hair, etc., to create multi-organ systems. In the in situ skin model generation, the skin model is made within the device with multiple channels, which deliver nutrients to the skin model. This model helps investigate the toxicity and immunological responses as this model creates skin models that exhibit immune responses. Wufuer et al., 2016 developed a microfluidic platform based on an in vitro skin model consisting of dermal, endothelial, and epidermal layers (Fig. 5).109 These layers are separated using a transparent membrane to allow interactions between these layers. This model can be used to test the toxicity of cosmetics.
“You-on-a-chip” and clinical-trial-on-chip
Drug efficacy and adverse effects might vary depending on the genetic makeup of the population, highlighting the need for and importance of patient-centred strategies. In the case of uncommon diseases, the current “one-size-fits-all” philosophy in healthcare is frequently less effective for treating these disorders, as familial genetic variants frequently cause large variations in the efficacy and safety of experimental medications. Before administering the experimental drug to the patient, cells from the patient could be utilised to conduct a clinical trial on a chip to determine the efficacy and toxicity profile of the substance. Moreover, different tissues taken from a patient with rare disorders could also be fabricated on a chip to provide more insights into these diseases. Organ-on-chips and other microfluidic devices are essential to fulfill this need. Using “you-on-a-chip” models built using the patient's own cells, vital information about the diseases, medication toxicity, and metabolic profiles can be acquired. This knowledge informs dose choices and treatment plans, removing uncertainty and improving patient safety. Using the patient's cells, clinical-trial-on-chip models can also predict treatment responses for uncommon diseases without the need for volunteers. This model can also garner insight on pharmacogenomic polymorphisms and probable susceptibility to adverse events.44OOAC as an alternative to animal testing to assess the safety of cosmetics
Any drugs, chemical food additives, or cosmetic products must undergo pre-clinical and clinical testing before they reach the market to ensure the safety of the product on humans. Before human trials, the cosmetics are tested on animals, primarily on rodents, then progressing to higher animals to test their adverse reaction and evaluate the safety of the product's formulation. In this process, according to the People for the Ethical Treatment of Animals (PETA), over 110 million animals, including mice, rats, frogs, dogs, cats, rabbits, hamsters, guinea pigs, monkeys, fish, and birds, are sacrificed per year, in the US alone.112 The global statistics are tremendously high. But the tragedy is that about 90% of the animal tests fail in human testing, leading to unnecessary loss of these animals' life.113 This provokes ethical consideration for the lives of animals, making the safety testing of new products challenging. To avoid this reckless sacrifice and torture on animal's lives, in vitro methods are preferred for the preliminary safety testing of the products. However, in vitro methods are unsuitable to comprehend safety assessment as they don't mimic the complex human physiology.45 As a result, toxicity impact of the product on other organs, the metabolic fates and side effects cannot be comprehended by in vitro testing alone. Also, the predictivity of drug safety based on animal testing is poor, as there is high chance for drug disposition in animals compared to humans.114 Due to these challenges, there is a tremendous need to find alternative options for testing product safety before letting it to the market.The OOAC model emerges as the one stop solution for all these issues. Recently, OOAC models are being used to design drugs and pharmaceuticals. OOAC models are emerging to be successful at this, because the organs that are used would be human derived itself. This overcomes the limitations of animal testing that animals do not mimic human physiology.115 Thus, OOAC could be used to effectively predict the effects of the drugs and comprehend the safety of the products on humans efficiently and with broader clinical clarifications. There are some studies on adopting OOAC and multi-OOAC for pharmaceutical testing. Ewart et al., 2022 have evaluated the use of liver-on-a-chip for studying toxicology in drug development processes. They have tested 870 liver-chips to check how these models predict drug induced liver damage. Their study highlighted the potential of this technique, as the outcome was found to be excellent, with the predictivity of this OOAC-based technique exceptionally better than the traditional animal model testing.116 However, to the best of our knowledge, no studies in the literature comprehend the safety of any cosmetics on humans predicted by OOAC models. A recent study conducted by Rhee et al., 2024 proposes a 3D perfused skin-on-a-chip model fabricated using micro-precision 3D printing for in vivo-like drug response studies for cosmetics.117 Similar to this approach, Mohamadali et al., 2023 have developed a skin chip, as an in vitro model, consisting of normal skin tissue with epidermis and dermis layer, which was later scaled up with PDMS microchannel-based perfusion feeding system to develop an in vivo-like skin-on-a-chip model.118 Both studies suggest the reliability of the OOAC technology as an excellent alternative for in vivo testing, even better than animal-based testing. We anticipate that pilot studies on this approach might be in progress to determine the efficacy of this approach in industrial settings.
Integration of AI and machine learning (AI-ML) in microfluidics
Microfluidic technology, with its capability to manipulate small fluid volumes in precisely controlled environments, has transformed biomedical research and diagnostics in recent decades. But, in the recent years, the integration of Artificial Intelligence (AI) and Machine Learning (ML) concepts have further restructured this technology with smart and innovative approaches, providing enhanced functionalities. Recent studies on integrating AI for efficient biomarker detection using chips,119 ML for data processing and analysis have paved the way for the development of “digital microfluidics” and “intelligent microfluidics” which will certainly change the dimensions of microfluid based diagnostics and microfluidic assays.120,121Recently, AI-assisted digital microfluidic platform, the μDropAI system, was developed by Guo et al., in their 2024 study. This platform incorporates the state-of-the-art semantic segmentation models for multistate droplet operations, such as generation, splitting and merging.120 These features are critical when considering the complex workflows required in organ-on-a-chip (OOAC) platforms, which will permit multi-organ, high-throughput assays of biomarkers of inflammation and toxicity associated with cosmetic hazards and adulterants. In addition, the self-adaptive feedback systems of the integrated system decrease user intervention and produce high reproducibility, which is critical during hazard detection of cosmetic formulations, to be ethical and scalable. Another major advancement is wearable AI-powered microfluidic sensors, and these can be used in detecting non-invasive biomarkers. Wang et al. (2024) developed a multiplexed colorimetric microfluidic sensor for tears with an AI-enabled colour interpretation feature, which can monitor the concentrations of various biomarkers such as pH and proteins in real time.122 Error correction is enabled by the use of deep-learning algorithms, resulting in an accurate performance across varying environmental conditions. These potentially find their applications in the skin-like OOAC platforms enabling real-time data acquisition for cosmetic safety assessments.
The concept of AI, ML and microfluidics is not limited only to droplet control and smart sensors. In a study, Sekhwama et al., 2024 have discussed how AI and ML based deep learning techniques could be used and adapted in microfluidic technologies for precise sample control, miniaturization of the study, efficient resource utilization with automation.119 This provides valuable cues to adopt and devise novel microfluidic based assays and experiments for the detection of cosmetic hazards, to study how cosmetics interact with the body cells, the biomarkers released in response and their toxicological analysis, without the need of animals, and even with less use of chemicals and reagents. This forms the core of sustainability, wherein we can integrate AI systems to reduce the sample and reagent volumes and conduct physiochemical interaction studies without the need of animal models. These approaches pave the way for improved data outcomes and precisions, along with which, hold a significance in ethical and sustainable considerations.
In another strategy, the transformer and pretrained models have also been adopted for much precise AI-based image analysis. Li et al. have developed a system for parasite counting in fecal samples without the need for any user intervention. By means of an inexpensive, portable, robotic microscope, the entire McMaster chamber can be scanned to capture high resolution bright field images without the involvement of any personnel. Convolutional neural network (CNN) algorithm used is able to distinguish the image of parasite eggs and those that are derived from background signals. It is this feat that AI is able to achieve that can never be paralleled by human intervention, whereby automated discerning of the bona fide specific image from the background image can facilitated rapidly within a short period of time. Similarly, this can be extrapolated for the detection of adulterants in cosmetics on microfluidics platform for a much accurate and hassle-free analysis.59
Challenges and limitations
Paper-based microfluidics are not without their challenges and limitations. The pressing challenges include sample preparation, batch-to-batch variations, limited shelf life, the need for a specific readout system and the requirement of a suitable conjugation method to link the molecular recognition elements to the surface of the platform. Moreover, the choice of a suitable etching method is needed to ensure a smooth and directed flow of the liquid samples. On the other hand, the challenges of OOAC are the availability of a standard, universally accepted protocol and materials needed for fabrication. These often result in reproducibility crises, which hamper large-scale and cost-effective production. As such, the protocol and materials used for the fabrication of OACC must be standardized across laboratories worldwide. Another pressing challenge is the difficulty in uniformizing the inter-organ transportation rates as well as ensuring uniform organ sizes. This, together with the disparities in the liquid-to-cell ratio, further aggravates the reproducibility issue. The exact mimicry of the physical attributes of the organ is also a tall order, which calls for the meticulous design of the experiment and the need for appropriate control.Future perspectives
Establishing and consolidating international guidelines for the acceptance of microfluidic and organ-on-a-chip (OOAC) systems as part of preclinical safety assessments are crucial for establishing practicability of these technologies. Efforts on the part of researchers, regulatory agencies, and industry to create such a framework should be taken to ensure compliance and encourage innovation in the use of microfluidics and OOAC platforms in routine adulterant detection and safety assessment, paving the way for marketability of these technologies. The key elements such as point-of-care and artificial intelligence-driven features of paper-based microfluidics and organ-on-a-chip must be accentuated to entice more researchers to increasingly adopt these technologies for much more feasible monitoring of cosmetics hazards.Conclusion
Cosmetics have been an integral part of self-expression, enhancement of physical features, and art for hundreds of centuries. Although cosmetics in the early days contained natural substances, present-day cosmetics consist of a lot of adulterants. Low manufacturing qualities lead to impurities in the product, which creates a need for easier detection methods. Although the adulterants are present to enhance certain cosmetic properties, they can potentially harm at higher and more frequent doses, making their detection significant. Microfluidics and point-of-care devices have overcome the shortcomings of conventional detection methods as they are much more time-saving and efficient and require microlitres of samples without compromising sensitivity and specificity. As such, paper-based microfluidics are used widely to detect adulterants in cosmetics. Organ-on-a-chip technology is another robust microfluidic milestone that can be efficiently used for the safety testing of cosmetic products. Together these can be used to empower both consumers and regulatory bodies to screen products efficiently. These methods also allow for ethical and scientific validity in safety testing. The integration of these technologies can proceed in the future with artificial intelligence and automation tools for point-of-care detection of adulterants using computerized scanners. This promises even more efficient high-throughput testing. As such, this will further ensure safe consumer products, regulatory compliance, and safer cosmetic products in the future.Paper-based microfluidics and OOAC technology are gaining traction for cosmetic adulterant detection and safety testing due to their salient features, including portability, low cost, and minimal sample volume. The ultimate goal of any diagnostic assay is a point-of-care-based approach, which is ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid/Robust, Equipment-free, and Deliverable – ASSURED). Paper-based microfluidic and OOAC technology has all the features that are able to fulfill these criteria. For a seamless transition from bench-site to point-of-care diagnostics of cosmetic adulterant detection and safety testing, dodged determination and active engagement of multiple stakeholders in transdisciplinary research initiatives is necessitated.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Sanidhya Pai – writing – original draft, methodology, Amanda Binu – writing – original draft, methodology, Meenakshi Harikumar – writing – original draft, methodology, Srikrishna Kedlaya Herga – writing – original draft, methodology, Citartan Marimuthu – writing – review & editing, methodology, conceptualization, Naresh Kumar Mani – writing – review & editing, validation, supervision, project administration, funding acquisition, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Dr Naresh Kumar Mani thanks Dr Anusha Prabhu for her fruitful discussions and inputs.Notes and references
- M. Nayak, D. Sreedhar, S. S. Prabhu and V. S. Ligade, Cosmetics, 2021, 8(3), 75 CAS.
- K. Nowak, E. Jabłońska and W. Ratajczak-Wrona, Environ. Res., 2021, 198, 110488 CAS.
- M. Bilal and H. M. N. Iqbal, Cosmetics, 2020, 7, 13 CAS.
- A. A. Aziz, F. N. M. Nordin, Z. Zakaria and N. K. Abu Bakar, J. Cosmet., Dermatol., 2022, 21, 71–84 CrossRef PubMed.
- A. Panico, F. Serio, F. Bagordo, T. Grassi, A. Idolo, M. DE Giorgi, M. Guido, M. Congedo and A. De Donno, J. Prev. Med. Hyg., 2019, 60, E50–E57 CAS.
- N. M. Hepp, W. R. Mindak, J. W. Gasper, C. B. Thompson and J. N. Barrows, J. Cosmet. Sci., 2014, 65, 125–145 CAS.
- M. Almukainzi, L. Alotaibi, A. Abdulwahab, N. Albukhary and A. M. El Mahdy, Sci. Rep., 2022, 12, 18299 CrossRef CAS PubMed.
- M. Irfan, A. Shafeeq, U. Siddiq, F. Bashir, T. Ahmad, M. Athar, M. T. Butt, S. Ullah, A. Mukhtar, M. Hussien and S. S. Lam, J. Hazard. Mater., 2022, 433, 128806 CrossRef CAS PubMed.
- P. Cherian, J. Zhu, W. F. Bergfeld, D. V Belsito, R. A. Hill, C. D. Klaassen, D. C. Liebler, J. G. Marks, R. C. Shank, T. J. Slaga, P. W. Snyder and B. Heldreth, Internet J. Toxicol., 2020, 39, 5S–97S CrossRef.
- L. K. Al-Halaseh, S. Al-Adaileh, A. Mbaideen, M. N. A. Hajleh, A. Al-Samydai, Z. Z. Zakaraya and W. A. Dayyih, J. Cosmet., Dermatol., 2022, 21, 3265–3271 CrossRef PubMed.
- M. Prüst, J. Meijer and R. H. S. Westerink, Part. Fibre Toxicol., 2020, 17, 24 CrossRef.
- R. E. Dodson, M. Nishioka, L. J. Standley, L. J. Perovich, J. G. Brody and R. A. Rudel, Environ. Health Perspect., 2012, 120, 935–943 CAS.
- S. Yesumanipreethi, N. Nirmal Magadalenal and R. Moses Inbaraj, Proc. Zool. Soc., 2021, 74, 572–590 Search PubMed.
- A. Wnuk, J. Rzemieniec, W. Lasoń, W. Krzeptowski and M. Kajta, Mol. Neurobiol., 2018, 55, 5059–5074 CAS.
- Y. Jiang, H. Zhao, W. Xia, Y. Li, H. Liu, K. Hao, J. Chen, X. Sun, W. Liu, J. Li, Y. Peng, C. Hu, C. Li, B. Zhang, S. Lu, Z. Cai and S. Xu, Environ. Int., 2019, 126, 413–421 CAS.
- M. R. Holahan and C. A. Smith, Neurotoxicology, 2015, 48, 21–34 CAS.
- S. M. Engel, H. B. Patisaul, C. Brody, R. Hauser, A. R. Zota, D. H. Bennet, M. Swanson and R. M. Whyatt, Am. J. Public Health, 2021, 111, 687–695 Search PubMed.
- M. S. Abed, A. A. Moosa and M. A. Alzuhairi, J. Hazard. Mater. Adv., 2024, 13, 100390 CAS.
- T. A. Olasehinde and A. O. Olaniran, Environ. Toxicol., 2024, 40, 128–139 CrossRef PubMed.
- K. Jones, L. M. Wessel, K.-H. Schäfer and M. Á. Tapia-Laliena, Biomolecules, 2024, 14, 984 CAS.
- H. Li, J. Zheng, H. Wang, G. Huang, Q. Huang, N. Feng and J. Xiao, Sci. Rep., 2019, 9, 8030 CrossRef PubMed.
- F. Brodhead, Am. J. Nurs., 2019, 119, 14 CrossRef PubMed.
- J. E. Evangelista, D. J. B. Clarke, Z. Xie, G. B. Marino, V. Utti, S. L. Jenkins, T. M. Ahooyi, C. G. Bologa, J. J. Yang, J. L. Binder, P. Kumar, C. G. Lambert, J. S. Grethe, E. Wenger, D. Taylor, T. I. Oprea, B. de Bono and A. Ma’ayan, Commun. Med., 2023, 3, 98 Search PubMed.
- F. J. Salles, F. P. Paniz, B. L. Batista, A. C. Nardocci and K. P. K. Olympio, Int. J. Environ. Res. Public Health, 2022, 20, 531 CrossRef.
- E. A. Medley, K. E. Kruchten, M. J. Spratlen, M. Ureño, A. Cole, R. Joglekar and J. B. Herbstman, Int. J. Environ. Res. Public Health, 2023, 20, 2114 CrossRef PubMed.
- M. Barthe, C. Bavoux, F. Finot, I. Mouche, C. Cuceu-Petrenci, A. Forreryd, A. Chérouvrier Hansson, H. Johansson, G. F. Lemkine, J.-P. Thénot and H. Osman-Ponchet, Cosmetics, 2021, 8, 50 CrossRef.
- L. M. Katz, K. M. Lewis, S. L. Spence and N. Sadrieh, Dermatol. Clin., 2022, 40, 307–318 CrossRef CAS.
- F. Mohammed, D. Guillaume, J. Warland and N. Abdulwali, Microchem. J., 2021, 168, 106501 CrossRef CAS.
- Y. Zhang, J. Li, S. Jiao, Y. Li, Y. Zhou, X. Zhang, B. Maryam and X. Liu, Sci. Total Environ., 2024, 929, 172734 CAS.
- P. Aryal, C. Hefner, B. Martinez and C. S. Henry, Lab Chip, 2024, 24, 1175–1206 CAS.
- P. Pattanayak, S. K. Singh, M. Gulati, S. Vishwas, B. Kapoor, D. K. Chellappan, K. Anand, G. Gupta, N. K. Jha, P. K. Gupta, P. Prasher, K. Dua, H. Dureja, D. Kumar and V. Kumar, Microfluid. Nanofluid., 2021, 25, 99 Search PubMed.
- A. Enders, A. Grünberger and J. Bahnemann, Mol. Biotechnol., 2024, 66, 365–377 CAS.
- S. F. Berlanda, M. Breitfeld, C. L. Dietsche and P. S. Dittrich, Anal. Chem., 2021, 93, 311–331 CAS.
- C. E. Hefner, P. Aryal, E. Brack, T. Alexander and C. S. Henry, Analyst, 2024, 149, 5684–5692 CAS.
- S. Nishat, A. T. Jafry, A. W. Martinez and F. R. Awan, Sens. Actuators, B, 2021, 336, 129681 CAS.
- A. Sinha, M. Basu and P. Chandna, Prog. Mol. Biol. Transl. Sci., 2022, 109–158 CAS.
- R. Ray, A. Prabhu, D. Prasad, V. kumar Garlapati, T. M. Aminabhavi, N. K. Mani and J. Simal-Gandara, Food Chem., 2022, 390, 133173 CrossRef CAS PubMed.
- A. Prabhu, M. S. Giri Nandagopal, P. Peralam Yegneswaran, H. R. Singhal and N. K. Mani, Cellulose, 2020, 27, 7691–7701 CrossRef CAS.
- H. R. Singhal, A. Prabhu, M. S. Giri Nandagopal, T. Dheivasigamani and N. K. Mani, Microchem. J., 2021, 165, 106126 CrossRef CAS.
- T. Akyazi, L. Basabe-Desmonts and F. Benito-Lopez, Anal. Chim. Acta, 2018, 1001, 1–17 CrossRef CAS.
- A. M. Alnuqaydan, Front. Public Health, 2024, 12 DOI:10.3389/fpubh.2024.1439027.
- C. G. Alver, E. Drabbe, M. Ishahak and A. Agarwal, Nat. Commun., 2024, 15, 5118 CrossRef CAS.
- F. Zheng, F. Fu, Y. Cheng, C. Wang, Y. Zhao and Z. Gu, Small, 2016, 12, 2253–2282 CrossRef CAS PubMed.
- R. Driver and S. Mishra, BioChip J., 2023, 17, 1–23 CrossRef CAS.
- C. M. Leung, P. de Haan, K. Ronaldson-Bouchard, G.-A. Kim, J. Ko, H. S. Rho, Z. Chen, P. Habibovic, N. L. Jeon, S. Takayama, M. L. Shuler, G. Vunjak-Novakovic, O. Frey, E. Verpoorte and Y.-C. Toh, Nat. Rev. Methods Primers, 2022, 2, 33 CrossRef CAS.
- Q. Wu, J. Liu, X. Wang, L. Feng, J. Wu, X. Zhu, W. Wen and X. Gong, Biomed. Eng. Online, 2020, 19, 9 CrossRef PubMed.
- Y. Wang, Y. Gao, Y. Pan, D. Zhou, Y. Liu, Y. Yin, J. Yang, Y. Wang and Y. Song, Acta Pharm. Sin. B, 2023, 13, 2483–2509 CrossRef.
- J. E. Sosa-Hernández, A. M. Villalba-Rodríguez, K. D. Romero-Castillo, M. A. Aguilar-Aguila-Isaías, I. E. García-Reyes, A. Hernández-Antonio, I. Ahmed, A. Sharma, R. Parra-Saldívar and H. M. N. Iqbal, Micromachines, 2018, 9, 536 CrossRef.
- M. Mansouri, J. Lam and K. E. Sung, Lab Chip, 2024, 24, 1293–1306 RSC.
- J. H. Sung and J. J. Kim, Expert Opin. Drug Metab. Toxicol., 2023, 19, 249–267 CrossRef CAS.
- S. Morton, D. Pencheon and N. Squires, Br. Med. Bull., 2017, 1–10 Search PubMed.
- United Nations Department of Economic and Social Affairs, The 17 Goals – Sustainable Development, https://sdgs.un.org/goals, accessed 20 November 2024.
- J. F. Feitor, L. C. Brazaca, A. M. Lima, V. G. Ferreira, G. Kassab, V. S. Bagnato, E. Carrilho and D. R. Cardoso, ACS Biomater. Sci. Eng., 2023, 9, 2220–2234 Search PubMed.
- A. Olanrewaju, M. Beaugrand, M. Yafia and D. Juncker, Lab Chip, 2018, 18, 2323–2347 RSC.
- B. G. S. Guinati, L. R. Sousa, K. A. Oliveira and W. K. T. Coltro, Anal. Methods, 2021, 13, 5383–5390 Search PubMed.
- F. Shalileh, H. Sabahi, M. Golbashy, M. Dadmehr and M. Hosseini, Anal. Chim. Acta, 2023, 1284, 341935 Search PubMed.
- G. Xing, J. Ai, N. Wang and Q. Pu, TrAC, Trends Anal. Chem., 2022, 157, 116792 CAS.
- E. B. Strong, S. A. Schultz, A. W. Martinez and N. W. Martinez, Sci. Rep., 2019, 9, 7 Search PubMed.
- B. Wang, Y. Li, M. Zhou, Y. Han, M. Zhang, Z. Gao, Z. Liu, P. Chen, W. Du, X. Zhang, X. Feng and B.-F. Liu, Nat. Commun., 2023, 14, 1341 Search PubMed.
- I. Berasarte, A. Bordagaray, R. Garcia-Arrona, M. Ostra, W. Reis de Araujo and M. Vidal, Talanta, 2024, 276, 126254 CrossRef CAS PubMed.
- L. Bezinge, C. Shih, D. A. Richards and A. J. deMello, Small, 2024, 20(38), 2401148 Search PubMed.
- T. J. McDaniel, J. M. Holtz, E. H. Bondzie, M. Overfelt, P. W. Fedick and C. C. Mulligan, Rapid Commun. Mass Spectrom., 2023, 37, e9493 CrossRef CAS PubMed.
- H. Sulistyarti, P. A. Puspitaloka, B. I. Putra, R. Retnowati and H. Tolle, Makara J. Sci., 2021, 25, 7 Search PubMed.
- J. M. C. C. Guzman, L. L. Tayo, C.-C. Liu, Y.-N. Wang and L.-M. Fu, Sens. Actuators, B, 2018, 255, 3623–3629 CAS.
- S. A. B. Galal, E. S. Elzanfaly, E. M. Hussien, E. A. H. Amer and H. E. Zaazaa, Sci. Rep., 2024, 14, 4498 Search PubMed.
- A. Saadati, F. Farshchi, M. Hasanzadeh, Y. Liu and F. Seidi, RSC Adv., 2022, 12, 21836–21850 RSC.
- A. Radwan, I. M. El-Sewify and H. M. E.-S. Azzazy, ACS Omega, 2022, 7, 15739–15750 CrossRef CAS.
- N. Fakhri, M. Hosseini and O. Tavakoli, Anal. Methods, 2018, 10, 4438–4444 Search PubMed.
- L.-M. Fu, M.-K. Shih, C.-W. Hsieh, W.-J. Ju, Y.-L. Tain, K.-C. Cheng, J.-H. Hsu, Y.-W. Chen and C.-Y. Hou, Biosensors, 2021, 11, 491 Search PubMed.
- A. Hasandka, A. R. Singh, A. Prabhu, H. R. Singhal, M. S. G. Nandagopal and N. K. Mani, Anal. Bioanal. Chem., 2022, 414, 847–865 CrossRef CAS PubMed.
- A. L. P. Silvestre, M. I. Milani, E. L. Rossini, L. Pezza and H. R. Pezza, Spectrochim. Acta, Part A, 2018, 204, 432–435 CrossRef CAS.
- Q. ul A. Zahra, Z. Luo, R. Ali, M. I. Khan, F. Li and B. Qiu, Nanomaterials, 2021, 11, 840 Search PubMed.
- X. Xiong, J. Zhang, Z. Wang, C. Liu, W. Xiao, J. Han and Q. Shi, BioChip J., 2020, 14, 429–437 Search PubMed.
- M. Nejadmansouri, M. Majdinasab, G. S. Nunes and J. L. Marty, Sensors, 2021, 21, 1176 Search PubMed.
- E. Mavrakis and S. A. Pergantis, Anal. Chim. Acta, 2021, 1179, 338830 Search PubMed.
- Y. Manmana, M. Macka and N. Nuchtavorn, RSC Adv., 2024, 14, 36142–36151 Search PubMed.
- H. Akkaraju, R. Tatia, S. S. Mane, A. B. Khade and S. J. Dengale, Regul. Toxicol. Pharmacol., 2023, 139, 105355 Search PubMed.
- J. C. Beard and T. M. Swager, J. Org. Chem., 2021, 86, 2037–2057 Search PubMed.
- W. Wichitnithad, S. Nantaphol, K. Noppakhunsomboon and P. Rojsitthisak, Saudi Pharm. J., 2023, 31, 295–311 Search PubMed.
- P. Sheth and R. N. Desai, Nitrosamine Generating Accelerators in Curing of Rubber, 2013, vol. 1 Search PubMed.
- A. Agüera, M. J. Martínez Bueno and A. R. Fernández-Alba, Environ. Sci. Pollut. Res., 2013, 20, 3496–3515 Search PubMed.
- S. Horne, M. D. Vera, L. R. Nagavelli, V. A. Sayeed, L. Heckman, D. Johnson, D. Berger, Y. Y. Yip, C. L. Krahn, L. O. Sizukusa, N. F. M. Rocha, R. N. Bream, J. Ludwig, D. A. Keire and G. Condran, J. Pharm. Sci., 2023, 112, 1166–1182 Search PubMed.
- SCCS European Commission, Opinion on NDELA in Cosmetic Products and Nitrosamines in Balloons, European Commission, 2012 Search PubMed.
- E. M. McBride, P. M. Mach, E. S. Dhummakupt, S. Dowling, D. O. Carmany, P. S. Demond, G. Rizzo, N. E. Manicke and T. Glaros, TrAC, Trends Anal. Chem., 2019, 118, 722–730 Search PubMed.
- W. L. Fatigante, S. Mukta, Z. E. Lawton, A. M. Bruno, A. Traub, A. J. Gasa, A. R. Stelmack, C. R. Wilson-Frank and C. C. Mulligan, J. Am. Soc. Mass Spectrom., 2020, 31, 336–346 CrossRef CAS PubMed.
- H. M. Brown, T. J. McDaniel, C. P. West, E. H. Bondzie, M. R. Aldeman, B. T. Molnar, C. C. Mulligan and P. W. Fedick, Int. J. Mass Spectrom., 2022, 474, 116781 CrossRef CAS.
- C. Exley, Mol. Med. Today, 1998, 4, 107–109 CrossRef CAS PubMed.
- R. Bonfiglio, M. Scimeca and A. Mauriello, Arch. Toxicol., 2023, 97, 2997–2998 CAS.
- J. Nie, Adv. Exp. Med. Biol., 2018, 99–111 CAS.
- P. Nayak, Environ. Res., 2002, 89, 101–115 Search PubMed.
- K. Klotz, W. Weistenhöfer, F. Neff, A. Hartwig, C. van Thriel and H. Drexler, Dtsch Arztebl Int., 2017, 114, 653–659 Search PubMed.
- M. Pérez-Rodríguez and M. d. P. Cañizares-Macías, Talanta Open, 2023, 7, 100178 Search PubMed.
- O. D. Bamidele, B. A. Kayode, O. I. Eniayewu, A. J. Adegbola, R. S. Olatoye, N. S. Njinga, S. T. Abdullahi and M. T. Bakare-Odunola, Sci. Rep., 2023, 13, 20992 Search PubMed.
- Lead in Cosmetics, FDA Search PubMed.
- L. Zhang, J. Wu, M. Xiao, S. Zhang, S. Ren, D. Luo, F. Xi, H. Liu, Y. Li, Q. Li and Y. Jing, Int. J. Electrochem. Sci., 2024, 19, 100858 Search PubMed.
- K.-B. Kim, Y. W. Kim, S. K. Lim, T. H. Roh, D. Y. Bang, S. M. Choi, D. S. Lim, Y. J. Kim, S.-H. Baek, M.-K. Kim, H.-S. Seo, M.-H. Kim, H. S. Kim, J. Y. Lee, S. Kacew and B.-M. Lee, J. Toxicol. Environ. Health, Part B, 2017, 20, 155–182 Search PubMed.
- L. M. Plum, L. Rink and H. Haase, Int. J. Environ. Res. Public Health, 2010, 7, 1342–1365 Search PubMed.
- E. A. Abdulkareem and J. O. Abdulsattar, Baghdad Sci. J., 2022, 19, 1286–1296 Search PubMed.
- SCCS, Scientific Committee on Consumer Safety, SCCS OPINION on Butylated Hydroxytoluene (BHT), 2021 Search PubMed.
- M. I. Fahmi, H. Sulistyarti, A. Mulyasuryani and A. Wiryawan, J. Pure Appl. Chem. Res., 2019, 8, 53–61 Search PubMed.
- H. Arshad, M. Z. Mehmood, M. H. Shah and A. M. Abbasi, Saudi Pharm. J., 2020, 28, 779–790 Search PubMed.
- Y. Huang, T. Liu, Q. Huang and Y. Wang, ACS Sens., 2024, 9, 3466–3488 CAS.
- N. Farhang Doost and S. K. Srivastava, Biosensors, 2024, 14, 225 CrossRef PubMed.
- S. Liu, S. Kumari, H. He, P. Mishra, B. N. Singh, D. Singh, S. Liu, P. Srivastava and C. Li, Biosens. Bioelectron., 2023, 231, 115285 CrossRef CAS PubMed.
- L. A. Osório, E. Silva and R. E. Mackay, Bioengineering, 2021, 8, 113 CrossRef.
- M. Horue, J. M. Silva, I. R. Berti, L. R. Brandão, H. d. S. Barud and G. R. Castro, Pharmaceutics, 2023, 15, 424 CAS.
- Z. Peng, L. Zhou, J. K. W. Wong and Y. K. Chan, Expert Rev. Ophthalmol., 2020, 15, 259–261 CrossRef CAS.
- Z. Yu, R. Hao, J. Du, X. Wu, X. Chen, Y. Zhang, W. Li, Z. Gu and H. Yang, iScience, 2022, 25, 104200 CAS.
- M. Wufuer, G. Lee, W. Hur, B. Jeon, B. J. Kim, T. H. Choi and S. Lee, Sci. Rep., 2016, 6, 37471 CAS.
- W. Sun, Z. Liu, J. Xu, Y. Cheng, R. Yin, L. Ma, H. Li, X. Qian and H. Zhang, Chin. Chem. Lett., 2023, 34, 107819 CAS.
- E. Filaire, R. Nachat-Kappes, C. Laporte, M. Harmand, M. Simon and C. Poinsot, Int. J. Cosmet. Sci., 2022, 44, 604–613 CAS.
- PETA, Facts and Statistics about Animal Testing, https://www.peta.org/issues/animals-used-for-experimentation/animals-used-experimentation-factsheets/animal-experiments-overview/#:%7E:text=Eachyear%2Cmorethan110millionanimals—includingmice%2C,experimentation%2Candchemical%2Cdrug%2Cfood%2Candcosmet, accessed 24 November 2024.
- A. Laybourne, Animal Testing Facts – Why Should Animal Experimentation Be Stopped?, https://worldanimalfoundation.org/advocate/animal-testing-facts/, accessed 24 November 2024.
- D. van Berlo, E. van de Steeg, H. E. Amirabadi and R. Masereeuw, Curr. Opin. Toxicol., 2021, 27, 8–17 CrossRef CAS.
- Y. Zhao, S. Landau, S. Okhovatian, C. Liu, R. X. Z. Lu, B. F. L. Lai, Q. Wu, J. Kieda, K. Cheung, S. Rajasekar, K. Jozani, B. Zhang and M. Radisic, Nat. Rev. Bioeng., 2024, 2, 588–608 CrossRef CAS.
- L. Ewart, A. Apostolou, S. A. Briggs, C. V. Carman, J. T. Chaff, A. R. Heng, S. Jadalannagari, J. Janardhanan, K.-J. Jang, S. R. Joshipura, M. M. Kadam, M. Kanellias, V. J. Kujala, G. Kulkarni, C. Y. Le, C. Lucchesi, D. V. Manatakis, K. K. Maniar, M. E. Quinn, J. S. Ravan, A. C. Rizos, J. F. K. Sauld, J. D. Sliz, W. Tien-Street, D. R. Trinidad, J. Velez, M. Wendell, O. Irrechukwu, P. K. Mahalingaiah, D. E. Ingber, J. W. Scannell and D. Levner, Commun. Med., 2022, 2, 154 CrossRef PubMed.
- S. Rhee, C. Xia, A. Chandra, M. Hamon, G. Lee, C. Yang, Z. Guo and B. Sun, Bioengineering, 2024, 11, 1055 CrossRef CAS PubMed.
- M. Mohamadali, A. Ghiaseddin, S. Irani, M. A. Amirkhani and M. Dahmardehei, Sci. Rep., 2023, 13, 8861 CrossRef CAS PubMed.
- M. Sekhwama, K. Mpofu, S. Sudesh and P. Mthunzi-Kufa, Discover Appl. Sci., 2024, 6, 458 CrossRef CAS.
- K. Guo, Z. Song, J. Zhou, B. Shen, B. Yan, Z. Gu and H. Wang, Microsyst. Nanoeng., 2024, 10, 138 CrossRef PubMed.
- G. Antonelli, J. Filippi, M. D'Orazio, G. Curci, P. Casti, A. Mencattini and E. Martinelli, Biosens. Bioelectron., 2024, 263, 116632 CrossRef CAS.
- Z. Wang, Y. Dong, X. Sui, X. Shao, K. Li, H. Zhang, Z. Xu and D. Zhang, npj Flexible Electron., 2024, 8, 35 CrossRef CAS.
Footnote |
| † Contributed Equally. |
| This journal is © The Royal Society of Chemistry 2025 |