 Open Access Article
Open Access ArticleFormation of self-assembly aggregates in traditional Chinese medicine decoctions and their application in cancer treatments
Chunqiu Fang a,
Yinghang Wang
a,
Yinghang Wang *b and
Zhi Pan
*b and
Zhi Pan *a
*a
aJilin Ginseng Academy, Changchun University of Chinese Medicine, Changchun 130117, P.R. China. E-mail: 13596030117@126.com; Tel: +8613596030117
bThe Affiliated Hospital to Changchun University of Chinese Medicine, Changchun 130117, P.R. China. E-mail: panzhiwyh@sohu.com; Tel: +8613844993950
First published on 18th February 2025
Abstract
Traditional Chinese Medicine (TCM) formulas, based on the principles of Chinese medicine, have a long history and are widely applied in the treatment of diseases. Compared to single-component drugs, TCM formulas demonstrate superior therapeutic efficacy and fewer side effects owing to their synergistic effects and mechanisms of detoxification and efficacy enhancement. However, various drawbacks, such as the uncertainty of functional targets and molecular mechanisms, poor solubility of components, and low bioavailability, have limited the global promotion and application of TCM formulas. To overcome these limitations, self-assembled aggregate (SA) nanotechnology has emerged as a promising solution. SA nanotechnology significantly enhances the bioavailability and anti-tumor efficacy of TCM by improving its absorption, distribution, and precise targeting capabilities, thereby providing an innovative solution for the modernization and internationalization of TCM. This review delves into the nature and common interactions of SAs based on the latest research developments. The structural characteristics of SAs in TCM formulas, paired-herb decoctions, and single-herb decoctions are analyzed and their self-assembly mechanisms are systematically elucidated. In addition, this article elaborates on the advantages of SAs in cancer treatment, particularly in enhancing the bioavailability and targeting capabilities. Furthermore, this review aims to provide new perspectives for the study of TCM compatibility and its clinical applications, thereby driving the innovative development of nanomaterials in this field. On addressing the technological challenges, SAs are expected to further promote the global application and recognition of TCM in the healthcare sector.
1 Introduction
Traditional Chinese Medicine (TCM), which uses valuable resources from the nature, has been widely used across Asia and surrounding regions, and it plays a crucial role in improving human health.1,2 In recent years, owing to its clinical efficacy, TCM has gradually gained acceptance within modern medicine.3 TCM decoctions represent a concentrated embodiment of the holistic view and syndrome differentiation principles of TCM.4 Their efficacy is based on the “Monarch–Minister–Assistant–Guide” (Jun–Chen–Zuo–Shi in Chinese) theory, where the “Monarch herb” directly targets the root cause of the disease, the “Minister herb” enhances the effect, the “Assistant herb” reduces the toxicity, and the “Guide herb” directs the formula to the precise target. This formulation strategy not only considers efficacy but also emphasizes on the synergy and safety among the herbs. Nevertheless, the uncertainty regarding the functional targets and molecular mechanisms of TCM formulas, along with issues such as poor solubility and bioavailability of certain components, has limited their widespread global application. To overcome these limitations, the introduction of self-assembled aggregate nanotechnology in TCM has become a crucial approach for improving drug absorption and distribution. Self-assembled aggregate nanotechnology in TCM not only enhances the solubility of its components but also increases their targeting capability within the body, thereby improving the therapeutic efficacy, especially in the context of anti-tumor treatments. The self-assembly of TCM compounds not only enhances anti-cancer activity but also uncovers specific interactions between different components, providing critical insights into the optimization of drug combinations.5Although modern medicine has attempted to address the challenges of inconvenient administration and low utilization of herbal materials by converting decoctions into new dosage forms, such as granules and extracts, these methods tend to overemphasize single components, thereby reducing the therapeutic efficacy. In addition, certain excipients, such as the hydrogen peroxide residues in Tween 80 and lysophospholipids in lecithin, pose safety concerns; it is reported that the synthetic surfactants induces gastrointestinal irritation.6,7 In contrast, TCM formulas leverage the synergistic effects of multiple herbs, eliminating the need for excipients or complex equipment, thereby avoiding these safety issues. In recent years, research has revealed that TCM formulas undergo complex physicochemical changes after decoction, particularly the widespread presence of SAs in aqueous decoctions, a phenomenon that is quite intriguing.8 These SAs include spontaneously formed monomolecular aggregates, metabolite intermediate aggregates, and complex structures that they form, such as gels, fibers, micelles, and nanoparticles.9–11 These structures exhibit various biological activities such as hypoglycemic, antioxidant, anti-inflammatory, toxicity-reducing, and antibacterial effects. They can also serve as natural carriers for drug delivery, significantly enhancing the bioavailability and therapeutic efficacy of medications.12–14 For example, research has shown that through self-assembly techniques, the lipophilic components of herbs can be emulsified during heating, forming stable SAs such as precipitates, colloidal particles, and suspensions.15,16 The potential of these SAs in TCM-based anti-tumor therapies is particularly noteworthy. The formation of SAs is closely related to the pharmacological efficacy of TCM formulas. For instance, after decoction, Baihu decoction, a formula mentioned in “Treatise on Cold Pathogenic Diseases”, has also been found to contain SAs that significantly impact biological activity.8,17 The stability of medicinal components can be effectively improved, toxicity reduced, and therapeutic efficacy enhanced by these SA structures, thereby making the application prospects of TCM formulas in the field of anti-tumor therapy even broader. In addition, supramolecular chemistry, pioneered by Jean-Marie Lehn, the 1973 Nobel Laureate in Chemistry, and further explored by subsequent researchers, has gradually unveiled the macroscopic behavior of SAs.2,18 Supramolecular chemistry explores self-assembly processes driven by non-covalent bonding forces, leading to the formation of SAs.18 Supramolecular chemistry methods are widely applied in TCM research, helping to uncover the complex molecular mechanisms of TCM formulas.19 These methods help explain their unique pharmacological mechanisms at the microscopic level, opening new avenues for their modernization and application. Hou19 et al. also suggested that multi-component complexes formed spontaneously through self-assembly have the greatest potential to enhance the therapeutic efficacy, as free drugs often lack the full bioactivity of complete TCM formulations. Specifically, interactions between the various components within the complex play a crucial role in determining therapeutic effects. For instance, the processes of thermal reflux and water decoction inadvertently create optimal conditions for the formation of these natural complexes, simultaneously reducing the toxicity of individual components and enhancing the water solubility of bioactive compounds.5
As research on SAs progresses, scientists have identified that they are widely present in nature and biological systems, including DNA double helices, cell membranes, and even in fish soup.20,21 SAs can form naturally from food components under heat induction. In fish soup, spherical SAs primarily consist of triglycerides, which contain carbohydrates and are coated with phospholipids.22 They exhibit antioxidant, antibacterial, and hepatoprotective effects.22–24 The formation of these SAs in decoctions suggests that similar structural transformations may occur in the active components of TCM during the decoction process. This provides a new perspective for the further exploration of the molecular mechanisms of TCM formulas. Integrating the self-assembly technology with the anti-tumor properties of TCM formulas offers innovative tools for modern medical applications while establishing a robust foundation for their global advancement. Therefore, building upon previous research, this paper delves into the nature of SAs, their common interactions, and the structural characteristics found in TCM decoctions, including complex formulas, paired-herb decoctions, and single-herb decoctions. It systematically elucidates their mechanisms of action from the perspective of self-assembly involving both identical and different TCM components. Additionally, this paper provides a detailed explanation of the applications and advantages of SAs in cancer treatment, particularly their impact on enhancing bioavailability and targeting capabilities. The aim is to integrate existing research findings and offer a reference for further studies on SAs in TCM decoctions.
2 Essence of SAs and their common forces
Supramolecular self-assembly is the process whereby molecules spontaneously form multi-molecular aggregates ranging from nanometer to micrometer scale, with specific macroscopic and microscopic structures, through weak intermolecular interactions via non-covalent bonds.18,25,26 It is widely believed that non-covalent forces between molecules, such as hydrogen bonding and π–π stacking, are the main drivers of molecular self-assembly.27,28 These forces lead to the formation of multi-molecular aggregates with various microstructures, such as nanoparticles, gels and so on.13,25,29 Once SAs are formed, their components retain their original properties while exhibiting unique overall functions through interactions.30 The arrangement of molecules in self-assembly was determined by non-covalent forces, where hydrogen bonds initially aggregate molecules into dimers, which then are stacked axially or helically. Further aggregation is achieved through π–π stacking and van der Waals forces, ultimately forming higher-order structures and one-dimensional nanostructures. These fragile and reversible non-covalent forces allow SAs to respond specifically to external stimuli, making them widely studied in fields such as organ regeneration, repair, drug delivery, and cell culture.28,31,32 TCM, the concept of holistic treatment is regarded as essential. The efficacy of TCM is often attributed to the synergistic effects of multiple components rather than a single component. The formation of SAs illustrates this synergy, with key benefits such as improved bioavailability, enhanced stability, optimized drug release, and synergistic enhancement.16,33 The self-assembled structures formed by the interactions of multiple components in TCM decoctions can be seen as a way to explain the significant efficacy of TCM formulas in treating complex diseases. This holistic approach not only aids modern scientific understanding of TCM but also provides theoretical support for its modernization and internationalization. Research has shown that the components in TCM decoctions are able to spontaneously assemble into SAs under the influence of various non-covalent forces. These SAs are tightly and orderly structured, possessing unique biophysical and biochemical properties, as illustrated in Table 1 and Fig. 1, 2.| Primary source | Main components of self-assembly | Driving forces | Morphologies | Therapeutic action | Structure | Ref. |
|---|---|---|---|---|---|---|
| a ↓: Reduced; ↑: improved. | ||||||
| Coptis chinensis Franch. and Scutellaria baicalensis Georgi co-decoction | Berberine | Hydrophobic interactions and electrostatic interactions | Nano particles or nano fibers | Antibacterial effect |  |
37 |
| Baicalin |  |
|||||
| Wogonoside | 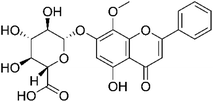 |
|||||
| Coptis chinensis Franch. and Rheum tanguticum Maxim co-decoction | Berberine | Hydrogen bond, π–π stacking, electrostatic interactions | Nano particles | Antibacterial effect |  |
27 |
| Rhein |  |
|||||
| Rheum tanguticum Maxim decoction | Rhein | Hydrogen bond, π–π stacking (pH: 8.0–9.4) | Nanofibers | Neuro inflammatory prevention activity |  |
14 |
| Coptis chinensis Franch. and Aristolochia debilis Sieb. et Zucc. co-decoction | Berberine | π–π stacking, electrostatic interactions | Cross-linked network structure | ↓Acute nephrotoxicity of aristolochic acid |  |
41 |
| Aristolochic acid |  |
|||||
| Coptis chinensis Franch. and Cinnamomum cassia (L.) J. Presl co-decoction | Berberine | Hydrogen bond and π–π stacking | Nano particles | Bacteriostatic activity |  |
13 |
| Cinnamic acid |  |
|||||
| Huanglian Jiedu decoction | Berberine | electrostatic interactions | Spherical-like microparticles (precipitate) | Neuroprotective activity; regulate glucose uptake; antibacterial effect |  |
72 and 73 |
| Baicalin |  |
|||||
| Maxing Shigan decoction | Ephedrine | Hydrogen bond, van der Waals forces and electrostatic interactions | Nano particles | Anti-influenza-virus activity |  |
84 and 85 |
| Pseudoephedrine |  |
|||||
| Baihu decoction | Polysaccharides; Fe2+, Fe3+, Ca2+, Mg2+, Zn2+ | Coordination interactions | Nano particles | Antipyretic effect; ↓inflammatory factors levels | 17, 90 and 91 | |
| Neomangiferin |  |
|||||
| Mangiferin |  |
|||||
| Glycyrrhizic acid |  |
|||||
| Paeonia lactiflora Pall. and Glycyrrhiza uralensis Fisch. co-decoction | Paeoniflorin | Hydrophilic interactions and hydrophobic interactions | Micelles | ↑Gastrointestinal absorption; ↑anti-inflammatory and immunomodulatory effects |  |
100 and 101 |
| Glycyrrhizic acid |  |
|||||
| Astragalus membranaceus (Fisch.) Bunge and Angelica sinensis (Oliv.) Diels co-decoction | Astragaloside IV | Hydrophobic interactions | Nano particles | Anti-myocardial fibrosis |  |
105 |
| Z-ligustilide |  |
|||||
| Glycyrrhiza uralensis Fisch.–Coptis chinensis Franch. co-decoction | Glycyrrhizic acid | Hydrophobic interactions and electrostatic interactions | Nano particles | Antibacterial effect |  |
14 and 112 |
| Berberine |  |
|||||
| Dushen decoction | Rg1 | Alkyl–alkyl interactions and hydrogen bonds | Nano micelles | Anti-tumor activity |  |
117 and 118 |
| Rb1 |  |
|||||
| Re |  |
|||||
| Coptis chinensis Franch. decoction | Coptis chinensis Franch. polysaccharide | Hydrogen bond | Nano particles | Antibacterial activity | 59 | |
| Berberine | ||||||
| Angelica sinensis (Oliv.) Diels | Angelica sinensis (Oliv.) Diels protein | Hydrophobic interactions | Spherical particles | Free radical scavenging effect | 128 | |
 | ||
| Fig. 1 Different self-assembly forces of SAs in decoction. (A) CA-BBR NPs formed by hydrogen bonding and π–π stacking as the main self-assembly forces. Copyright © 2019 the American Chemical Society; (B) Ber-Rhe NPs formed by π–π stacking and hydrogen bond interactions as the main self-assembly forces. Copyright © 2020 Elsevier B.V.; (C) WOG-BBR NFs and (D) BA-BBR NPs formed through hydrophobic interactions as the main self-assembly forces. Copyright © 2019 the American Chemical Society. (E) UA-PTX NPs formed by hydrophobic interactions as the main self-assembly forces. Copyright © 2020 the American Chemical Society; (F) A−B formed by electrostatic and π–π stacking interactions as the main self-assembly forces. Copyright © 2021 the American Chemical Society; (G) Rhe hydrogel formed by π–π interactions as the main self-assembly forces. Copyright © The Author(s) 2019; (H) Chemo-photothermal agent formed by coordination interactions as the main self-assembly forces. Copyright © 2020 Elsevier B.V. adapted with permission from ref. 13, 14, 27, 37, 38, 41 and 45. | ||
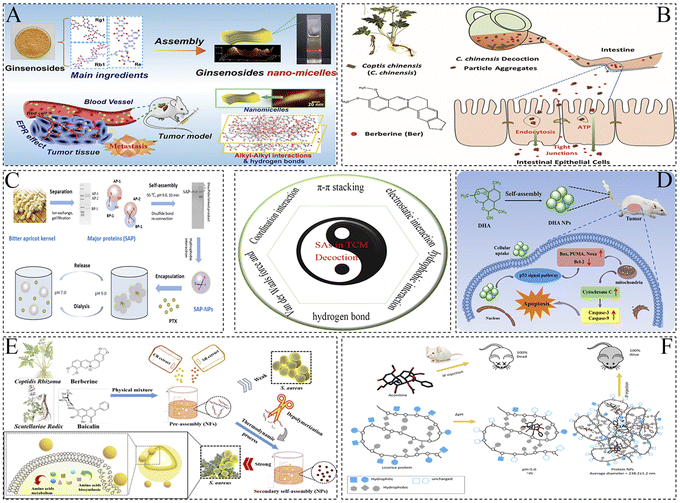 | ||
| Fig. 2 Different self-assembly forces of SAs in decoction. (A) Ginsenosides nano-micelles formed by hydrogen bonding forces as the main self-assembly forces. Copyright © 2022 Elsevier B.V.; (B) C. chinensis decoction particle aggregates formed by hydrogen bonding forces as the main self-assembly forces. Copyright © The Royal Society of Chemistry 2020; (C) PTX-ASP-NPs formed by hydrophobic interactions as the main self-assembly forces. Copyright © 2020 Elsevier B.V.; (D) DHA NPs formed by hydrogen bonding and hydrophobic interactions as the main self-assembly forces. Copyright © 2023, The Royal Society of Chemistry; (E) BBR-BA NPs formed by electrostatic forces as the main self-assembly forces. Copyright © 2022, BMC; (F) GP-AC NPs formed by hydrophobic interactions as the main self-assembly forces. © 2015 Ke et al.; adapted with permission from ref. 59, 118, 127, 139, 141 and 144. | ||
2.1 Hydrogen bonding forces
Hydrogen bonds are intermolecular interactions that occur between a hydrogen atom and an electronegative atom (such as oxygen, nitrogen, or fluorine). It is characterized by its directional nature and moderate strength, playing a crucial role in various chemical and biological systems. It plays a critical role in supramolecular self-assembly. As non-covalent interactions, they enable molecules to spontaneously aggregate into specific structures, drive molecular binding, and offer high selectivity in the assembly process. For example, the double-helix structure of DNA relies on hydrogen bonds for stability, while in nanomaterials, hydrogen bonds can be utilized to design self-assembled functional materials.20,34 To date, SA structures have been identified in numerous decoctions.8,16 Berberine (BBR) is a commonly used medication for treating bacterial diarrhea. It also plays a role in reducing the toxicity of other harmful drugs and enhancing their safety. Huang13 et al. in their study on the SAs formed during the co-decoction of Coptis chinensis Franch. and Cinnamomum cassia (L.) J. Presl found that the active components berberine (BBR) and cinnamic acid (CA) could spontaneously self-assemble into CA–BBR NPs with a size of 65.99 ± 0.43 nm (Fig. 1A). Spectroscopy and XRD analyses revealed that the butterfly-shaped 1D structural units of the CA–BBR NPs spontaneously form, driven by hydrogen bonding and π–π stacking. These 1D units continuously accumulate to construct a layered 3D spatial configuration. The study further validated the exceptional antibacterial activity and biofilm removal capability of CA–BBR NPs against multidrug-resistant Staphylococcus aureus through in vitro experiments. Additionally, in vivo tests using cell-based assays and a zebrafish model confirmed their low toxicity and excellent biocompatibility, highlighting the potential for developing novel antibacterial nanomedicines. During the study of the compatibility between Coptis chinensis Franch. and Rheum tanguticum Maxim. ex Balf. Lin.35 et al. discovered that when Coptis chinensis Franch. and Rheum tanguticum Maxim. ex Balf. are decocted together, they spontaneously undergo an exothermic reaction, resulting in the formation of SA Ber–Rhe NPs. Under the influence of hydrogen bonding, rhein (Rhe) forms a layered framework, within which BBR is embedded through electrostatic and π–π stacking interactions, resulting in a stable three-dimensional structure14 (Fig. 1B). Pharmacological experiments have revealed that untreated bacteria maintain smooth surfaces with intact nucleoid centers. The structure of Rhe contains multiple alternating carbonyl, phenolic hydroxyl, and carboxyl groups, which can form hydrogen bonds with the carboxyl and amide groups at the termini of peptidoglycans on the bacterial cell membrane. Scientists have inferred that when bacteria are co-incubated with Ber-Rhe NPs, a significant number of these nanoparticles adhere to the bacterial surface. The high concentration of BBR and Rhe disrupts the cell membrane ion channel proteins, causing membrane contraction, fusion, and cytoplasm leakage, ultimately leading to bacterial lysis and death. Importantly, the experiments have shown that the synergistic antibacterial action of BBR and Rhe enhances the efficacy of the nanoparticles compared to the free drugs. Furthermore, Zheng27 et al. studied Rhe to explore its application in slow-release hydrogels for the treatment of neuroinflammation. The research revealed that Rhe can self-assemble into a hydrogel through π–π stacking and hydrogen bond interactions. Under conditions of pH 8.0–9.4 and heat, some Rhe is deprotonated to form sodium rheinate. Through π–π stacking and hydrogen bonding, sodium rheinate and Rhe monomers aggregate in a J-type manner to form J-type dimers. Due to electrostatic repulsion between carboxylate ions, the molecules tend to align in an antiparallel fashion, with Rhe molecules joining the aggregate in a left-handed helical arrangement, leading to the formation of left-handed nanofibers. These fibers are further cross-linked to form a three-dimensional network structure. Notably, the three-dimensional network structure of the Rhe hydrogel demonstrates superior sustained release properties, thixotropy, and stability compared to free drugs, showing significant efficacy in the prevention of neuritis. Research suggests that ursolic acid (UA) molecules, pentacyclic triterpenoids derived from TCM such as Prunella vulgaris L. and Hedyotis diffusa Willd., can self-assemble with shiitake mushroom polysaccharides under the influence of hydrogen bonds and van der Waals forces.292.2 Hydrophobic interactions
Hydrophobic interactions refer to the tendency of hydrophobic groups to aggregate in water to avoid contact with water molecules. This phenomenon is widely observed in the self-assembly of TCM, aiding in the formation of stable structures, enhancing efficacy and bioavailability, and offering potential values in nanomedicine delivery applications. In many studies involving self-assembly processes, hydrophobic interactions serve as the primary driving forces, while other forces, such as electrostatic interactions and hydrogen bonds, balance these effects, collectively contributing to the formation of stable structures.36,37 Furthermore, Li37 et al. found that under the influence of electrostatic and hydrophobic interactions, BBR can spontaneously form SAs with flavonoid glycosides—namely, baicalin (BA) and wogonoside (WOG)—derived from Scutellariae radix in aqueous solutions (Fig. 1C). The self-assembly of two-component systems with similar molecular skeletons can result in the formation of distinct nanomorphologies. BA–BBR, due to the proximity of its hydrophobic flavonoid sites, is formed into one-dimensional composite units, which then are self-assembled into three-dimensional BA–BBR NPs of approximately 100 nm through hydrophobic interactions. The structure of the BA–BBR NPs is arranged with hydrophilic parts facing outward and hydrophobic parts inward (Fig. 1D). The WOG–BBR unit is a structural unit with hydrophobic ends and a hydrophilic center, capable of forming an I-shaped hydrophobic planar structure. Due to its lack of hydrophobicity, the basic unit is unable to form nanoparticles but can spontaneously assemble in aqueous solutions under strong hydrophobic interactions, resulting in nanofibers with a diameter of 50–100 nm and a length of about 10 μm. More importantly, compared to BBR, the self-assembled BA–BBR NPs exhibit stronger antibacterial effects and biofilm clearance capabilities against Staphylococcus aureus, whereas the efficacy of the self-assembled WOG–BBR NFs is less than that of BBR. More importantly, in vitro experiments demonstrated that NPs significantly outperformed free BBR in antibacterial activity and biofilm removal, whereas NFs exhibited weaker antibacterial effects. In vivo zebrafish models and cytotoxicity tests further revealed that both NPs and NFs possess excellent biocompatibility and low toxicity. This natural small-molecule self-assembly strategy based on NPs and NFs offers significant potential for the development of highly effective and low-toxicity nanoscale antibacterial drugs, particularly in the field of self-delivering antibacterial therapies. Wang38 et al. in their exploration of the drug delivery process using natural active small molecules and their potent synergistic anti-tumor effects discovered that the phenyl rings between UA molecules and paclitaxel (PTX) exhibit strong hydrophobic interactions. These interactions encourage the formation of stable nanocomposites through the coupling of two methyl groups in UA with two phenyl rings connected by amide bonds in PTX, thereby enhancing the drug loading capacity (Fig. 1E). Experiments have demonstrated that the composite material of UA–PTX NPs can prolong the plasma half-life, prevent rapid drug leakage, and preserve the anti-tumor and hepatic protective effects of UA.2.3 π–π stacking
π–π stacking interactions are important spatial interactions between aromatic compounds, involving weak interactions between electron-rich and electron-poor aromatic rings. This mechanism is equally important as hydrogen bonding in non-covalent interactions, though its underlying principles are more complex.39 Alkaloids and flavonoids contain aromatic rings, which readily undergo π–π stacking interactions, leading to self-assembly with themselves or other aromatic-ring-containing molecules. These interactions have become some of the primary mechanisms for their assembly.40 This process has a significant impact on the molecular structure, stability, and functionality. Aristolochic acid (AA), a principal component of TCM such as Asarum heterotropoides F. Schmidt and Aristolochia contorta Bunge. However, studies have shown that it possesses nephrotoxicity, which can lead to serious health issues such as AA nephropathy and cancer.41,42 In recent years, scholars have revealed the detoxification mechanism of BBR combined with AA through multicomponent self-assembly studies.41 Research indicates that under electrostatic and π–π stacking interactions, BBR and AA can self-assemble into linear heterogeneous SAs (A–B), featuring hydrophobic groups on the exterior and hydrophilic groups on the interior. In in vitro experiments with HK-2 cells, as well as in zebrafish embryo and C57BL/6 mouse models, the mechanism of the linear heterogeneous SA (A–B) structure and its ability to inhibit the formation of toxic metabolites were investigated. The results indicated that this structure significantly mitigates acute kidney injury induced by AA, while having no significant detrimental effects on gut microbiota homeostasis. These findings provide scientific support for the detoxification potential of TCM combinations (Fig. 1F). Cheng43 et al. co-assembled betulinic acid with Chlorin e6 (Ce6) through hydrogen bonding to form amphiphilic prodrug molecules. These molecules, via π–π stacking, interact with the photosensitizer Ce6, resulting in the formation of a hollow shell network structure for photochemotherapy nanoparticles. The nanoparticles exhibit good biocompatibility and low toxicity, and they can synergistically enhance anti-tumor effects when combined with anti-PD-L1 antibodies. Betulonic acid, a derivative of betulinic acid, also co-assembles with Ce6 via π–π stacking and hydrophobic interactions. This process enhances its water solubility and boosts its chemical/photodynamic anti-tumor effects.44 This study developed a photosensitive nanoplatform regulated by natural small molecules, which significantly enhanced the efficiency of type I photochemical reactions and the synergistic anti-tumor effects. In vitro experiments confirmed its high efficiency in generating ROS and its potent tumor cell-killing effects. In vivo experiments demonstrated excellent tumor inhibition rates and good biosafety, providing new insights for the application of photochemical therapy in hypoxic tumor environments. In addition, quinone compounds typically possess planar structures, where aromatic rings form parallel π-electron systems. This structural feature facilitates intermolecular self-assembly through π–π interactions.27 Such interactions can enhance intermolecular binding forces, promote the formation of ordered aggregates, and endow quinone compounds with significant potential for applications in materials science and drug delivery. Studies have shown that under mildly alkaline conditions with a pH range of 8.0 to 9.4, Rhe can self-assemble into a hydrogel based on a nanofiber network. This hydrogel facilitates the slow release of Rhe, thereby enhancing its anti-inflammatory effects.27 Ultraviolet spectroscopy, circular dichroism, X-ray diffraction analysis, and theoretical calculations indicate that Rhe molecules undergo deprotonation under mild alkaline conditions, forming Rhe sodium salts. These monomers and sodium salts interact through π–π stacking to form dimers. Due to electrostatic repulsion between carboxylate ions, the dimers arrange in opposite orientations and further assemble into higher-order aggregates, eventually crosslinking into a three-dimensional (3D) network structure. In vitro experiments confirm that this hydrogel demonstrates effective drug release control, low toxicity, anti-inflammatory activity, and efficient cellular uptake, as shown in Fig. 1G.2.4 Coordination
In addition, some common forces for self-assembly also include hydrophobic interactions and coordination.45,46 Coordination interactions are significant intermolecular forces, often stronger than hydrogen bonds and comparable to weaker covalent bonds, and they can be adjusted by environmental conditions. Research indicates that various natural flavonoid constituents of TCM can self-assemble into nanoparticles via coordination with metal ions, such as iron (Fe3+), forming nanoparticles like those created by luteolin found in Chrysanthemum morifolium Ramat. and Lonicera japonica Thunb., which possess photothermal properties45 (Fig. 1H). In vitro experiments have demonstrated that these nanoparticles exhibit significantly enhanced solubility under physiological conditions and show an efficient photothermal effect under 808 nm laser irradiation, with a photothermal conversion efficiency of 26.0%. Additionally, their combined chemotherapy and photothermal therapy exert a notable inhibitory effect on U87MG cells, reducing the IC50 value from 30.8 μg mL−1 in standalone chemotherapy to 6.6 μg mL−1. In vivo experiments further confirmed that the nanoparticles exhibit excellent tumor suppression effects in animal models, with the combined photothermal therapy group showing significantly better efficacy than single therapy approaches. The coordination self-assembly strategy effectively modulates the structure and properties of natural flavonoids, enabling their application as chemotherapeutic agents or photothermal agents. In addition, BA, an active component of Scutellaria baicalensis Georgi, exhibits anti-inflammatory and antibacterial properties. The functional groups within its molecule, including phenolic hydroxyl, hydroxyl, carbonyl, and carboxyl groups, can interact with metal ions (such as Al3+) via hydrogen bonding and coordination bonding, promoting the self-assembly into nanoparticles. Based on this, Jia47 et al. developed an oral insulin delivery system. Their research revealed that this system could protect insulin in the acidic environment of the stomach and release it into the alkaline environment of the intestine, thereby prolonging its action time. Additionally, BA enhances insulin absorption via a paracellular pathway, reduces blood glucose levels, and co-assembles with berberine to improve its solubility.2.5 Electrostatic forces
In the self-assembly process of TCM, electrostatic interactions serve as crucial non-covalent forces, enabling molecules to form stable structures through charge attraction or repulsion.48 Charged TCM molecules or their derivatives facilitate self-assembly via electrostatic interactions, enhancing the stability and biocompatibility of nanomaterials, thereby optimizing drug delivery and release performance. Electrostatic interactions, combined with other non-covalent forces such as hydrogen bonding and π–π stacking, further improve the efficiency of self-assembly and the therapeutic performance of TCM components. Alkaloids, as nitrogen-containing heterocyclic compounds, can self-assemble with negatively charged groups (such as carboxyl groups) via electrostatic interactions, thereby constructing stable drug delivery systems. Research has shown that UA can self-assemble into nanoparticles with a diameter of approximately 150 nm, driven by electrostatic and hydrophobic interactions. In vitro studies have demonstrated that these nanoparticles exhibit stronger anti-proliferative activity compared to free UA and significantly enhance the activation of CD4+ T cells, indicating their potential for immunotherapy.49 In addition, UA self-assemblies have been employed for the delivery of various drugs including doxorubicin, PTX, and aspirin.50–52 Wang53 et al. reported that inulin and gelatin co-assemble to form core nanoparticles, which are subsequently stabilized into core–shell nanogels (CSNGs) through chemical crosslinking. The study encapsulated the antimicrobial peptide Cath30 into CSNGs via electrostatic interactions for the treatment of aerobic vaginitis. Under the action of gelatinase secreted by pathogenic bacteria, the nanogels gradually released Cath30 and inulin, achieving a dual therapeutic effect.Many active components of TCM possess self-assembly capabilities, forming stable nanostructures via non-covalent interactions such as hydrogen bonding, π–π stacking, hydrophobic interactions, and coordination. These interaction forces have distinct characteristics and play different roles in the self-assembly process, but each also has its limitations. Many active components in TCM can self-assemble due to their unique structures. However, their ordered structures are influenced by external environmental factors such as pH and protein concentration, which can lead to variations in drug efficacy. Hydrogen bonding and coordination interactions are relatively strong; however, they are highly sensitive to environmental factors such as solvents. π–π stacking and van der Waals forces are weaker; nonetheless, they facilitate the formation of ordered structures. Hydrophobic interactions are notably significant in aqueous solutions; electrostatic forces, meanwhile, are easily influenced by medium conditions. Hydrophilic interactions play a critical role in stabilizing polar systems. In future research, establishing standardized characterization and quantification methods for self-assembly will be crucial. Such advancements will enhance research consistency and provide deeper insights into the pivotal roles of these forces in the self-assembly of TCM. The self-assembled nanoparticles within the Isatis indigotica Fortune decoction system exhibit reversible responses to changes in pH and temperature.54 During the decoction process, the protein components of Glycyrrhiza uralensis Fisch. spontaneously self-assemble into protein nanoparticles, which not only promote liver cell proliferation but also enhance the solubility and bioavailability of insoluble astragaloside IV through encapsulation.55 However, the particle size distribution of Glycyrrhiza uralensis Fisch. protein self-assembled nanoparticles is influenced by heating temperature and pH changes. Notably, the critical impact of different decoction conditions and environmental factors on the structure and function of the aggregates must be recognized, as these are key considerations in the development of SAs.
3 Study on the structure of SAs in TCM decoctions
TCM decoctions are complex dispersed systems in which the active components form SAs via interactions such as hydrogen bonding and π–π stacking. This process increases the solubility of poorly soluble components, encapsulates toxic substances to reduce toxicity, and enhances the stability, absorption, bioavailability, and efficacy of the medication.34,56–59 The structure of SAs in TCM decoctions tends to aggregate and form “precipitates” due to their high surface free energy, as observed in the decoction of Liu Jun Zi Tang.60 TCM holds that the precipitate should be consumed along with the decoction to ensure its therapeutic effects. Studies dating back to the 1980s revealed that decoctions remain in a suspended state after boiling, and removing the precipitate significantly diminishes their efficacy.16 Subsequent research has revealed that these SAs are typically composed of proteins, polysaccharides, and surfactants, often forming nanoscale particles. By encapsulating or adsorbing active components, they significantly enhance drug stability, absorption, and bioavailability, serving as a critical material basis for the therapeutic efficacy of TCM.61 However, during the pharmaceutical process, the precipitate is often filtered out and discarded along with the drugs in an attempt to obtain a “clear liquid”. This practice leads to the loss of certain water-soluble and lipid-soluble active components, ultimately reducing the therapeutic efficacy of the decoction.62 Experiments indicate that co-decoction is a primary condition for the formation of self-assembly aggregates (SAs). For instance, during the co-decoction of Sanhuang Xiexin Tang and Hong Jing Tian decoction, active components from different herbs undergo thorough interactions, resulting in the formation of more stable SAs with smaller particle sizes. Compared to the simple combination of individual decoctions, these co-decocted formulations demonstrate significantly improved antipyretic, analgesic, and anti-inflammatory effects.63,64 In conclusion, the formation of SAs is a key material basis for the therapeutic effects of decoctions. The investigation of this phenomenon can further uncover the compatibility principles of TCM and provide scientific guidance for optimizing clinical formulations. This approach helps enhance drug efficacy consistency, reduce the waste of active ingredients, and foster the development and application of modern TCM.3.1 Research on the structure of SAs in prescription decoctions
In summary, the self-precipitates of HJD, formed through acid–base complexation and self-assembly, exhibit significant antibacterial, anti-inflammatory, and neuroprotective pharmacological effects. The self-precipitation mechanism enhances drug stability and improves the overall efficacy, showing particular potential in the treatment of complex diseases. However, the differences in activity due to component interactions and the complex formation mechanisms require further research and optimization. Furthermore, extensive clinical validation is essential to support its broader application.
In conclusion, the SAs in MSD decoctions exhibit antiviral properties, which hold significant implications for the study of TCM decoctions. Pharmacological studies have indicated that active components in TCM decoctions may exist in the form of SAs, suggesting that research on TCM efficacy should broaden its perspective to consider aggregates typically viewed as “insoluble”.85 Additionally, nano-scale aggregates may possess physiological and pharmacological activities, thus experimental designs should be adjusted accordingly. In vivo activity experiments should take into account the impact of aggregates on the delivery, absorption, and action of active components. In vitro cell experiments must evaluate the effects of ultrafiltration and filtration on sample activity, as demonstrated in the aforementioned study on MSD antiviral activity, where 0.45 μm membrane filtration may have influenced the results by removing aggregates.
In summary, the nano-phase of BD significantly enhances drug solubility and efficacy, particularly excelling in antipyretic and anti-inflammatory effects, offering innovative approaches for the modernization of TCM. However, despite its clear advantages in improving solubility and bioavailability, further research is needed to explore its long-term safety and the metabolic mechanisms of nanoparticles in the body. Additionally, challenges remain in achieving standardization and quality control during production. A more comprehensive evaluation is required to ensure its safety and feasibility before clinical application.
3.2 Research on the structure of SAs in medicinal-pair decoction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combination of Paeonia lactiflora Pall and Glycyrrhiza uralensis Fisch. and demonstrates significant clinical efficacy.94,95 The “monarch” herb, Paeonia lactiflora Pall., is rich in active compounds such as paeoniflorin, which exhibits anti-inflammatory, immunomodulatory, and analgesic effects. However, due to its high polarity, these compounds have poor absorption in the gastrointestinal tract when taken orally.96–98 Research found that the amphiphilic compounds in Glycyrrhiza uralensis Fisch. such as glycyrrhizic acid and liquiritin, self-assemble into nanoparticles during the water decoction process. These nanoparticles significantly enhance the absorption efficiency of the active components in Paeonia lactiflora Pall.99,100 Shen101 et al. prepared self-assembling nanoparticles based on paeoniflorin and glycyrrhizic acid using an ultrasonic dispersion method, with a particle size of approximately 200 nm. This nanoparticle system effectively encapsulated paeoniflorin, significantly enhancing its oral bioavailability and controlling its release rate. In vivo pharmacokinetic studies showed that compared to paeoniflorin solution, the encapsulated nanoparticles increased paeoniflorin's Cmax by 2.17 times and AU C0–t by 3.64 times, indicating a significant improvement in both absorption rate and efficacy.101
1 combination of Paeonia lactiflora Pall and Glycyrrhiza uralensis Fisch. and demonstrates significant clinical efficacy.94,95 The “monarch” herb, Paeonia lactiflora Pall., is rich in active compounds such as paeoniflorin, which exhibits anti-inflammatory, immunomodulatory, and analgesic effects. However, due to its high polarity, these compounds have poor absorption in the gastrointestinal tract when taken orally.96–98 Research found that the amphiphilic compounds in Glycyrrhiza uralensis Fisch. such as glycyrrhizic acid and liquiritin, self-assemble into nanoparticles during the water decoction process. These nanoparticles significantly enhance the absorption efficiency of the active components in Paeonia lactiflora Pall.99,100 Shen101 et al. prepared self-assembling nanoparticles based on paeoniflorin and glycyrrhizic acid using an ultrasonic dispersion method, with a particle size of approximately 200 nm. This nanoparticle system effectively encapsulated paeoniflorin, significantly enhancing its oral bioavailability and controlling its release rate. In vivo pharmacokinetic studies showed that compared to paeoniflorin solution, the encapsulated nanoparticles increased paeoniflorin's Cmax by 2.17 times and AU C0–t by 3.64 times, indicating a significant improvement in both absorption rate and efficacy.101In summary, the self-assembled nanoparticles in the Paeonia lactiflora Pall.–Glycyrrhiza uralensis Fisch. co-decoction significantly enhance the absorption and efficacy of Paeonia lactiflora Pall., components, showing great clinical potential by improving intestinal absorption and strengthening anti-inflammatory and immunomodulatory effects, thereby providing better protection for the body. However, challenges such as the complexity of preparation, long-term safety concerns, and potential risks arising from component interactions hinder its widespread application. Thus, while the technological innovations show promise, further research and optimization are necessary for broader clinical adoption.
In conclusion, the SAs of Astragalus membranaceus (Fisch.) Bunge and Angelica sinensis (Oliv.) Diels offer a potential new mechanism for treating myocardial fibrosis, along with the advantage of sustained drug release. However, the underlying mechanism remains unclear, clinical research is limited, and the preparation process is complex. Despite its innovation and therapeutic potential, further research and optimization are necessary to enable broader clinical applications.
In conclusion, the self-assembled precipitates formed during the co-decoction of Glycyrrhiza uralensis Fisch. and Coptis chinensis Franch. are key components responsible for their pharmacological effects and demonstrate significant antibacterial efficacy. However, current research remains largely at the experimental stage, with limited clinical validation. Moreover, the preparation process is complex, and although the potential for application is promising, further research and extensive clinical trials are necessary.
3.3 Research on the structure of SAs in single-herb decoctions
In conclusion, after decoction, the ginsenosides in Dushen decoction self-assemble into micelles, encapsulating drugs and precisely targeting tumor cells, thereby enhancing their antitumor effects.
4 Mechanism of SA formation in TCM decoctions
4.1 SAs of TCM with the same components
In conclusion, polysaccharide-based SADs show significant potential in drug delivery, particularly for improving the solubility and absorption of poorly soluble drugs. However, despite these promising findings, challenges remain in their practical application. First, the self-assembly mechanisms and stability of polysaccharide-based nanostructures may be influenced by varying conditions such as pH, temperature, and ion concentration, which could limit their effectiveness in complex physiological environments. Second, most current research is still at the laboratory stage, with a lack of systematic in vivo and clinical data to validate their safety and efficacy. Therefore, future research should focus on exploring the long-term biocompatibility and pharmacological stability of polysaccharide-based SADs to ensure their practical value in drug delivery systems.
In conclusion, TCM-derived protein-based SAs show significant potential in drug delivery, especially in encapsulating poorly soluble drugs and synergistically enhancing their therapeutic effects. Their excellent biocompatibility offers a new approach for developing drug carriers. However, despite the promising results from experimental studies, there are still challenges in their practical application. First, more in vivo and clinical studies are needed to understand the mechanisms of protein self-assembly, nanoparticle stability, and the long-term safety of these drug carriers. Additionally, how to maintain the stability and efficacy of protein nanoparticles in complex physiological environments requires further exploration.
In conclusion, although terpenoids, particularly triterpenoids, exhibit promising self-assembly properties for drug delivery, challenges remain in their clinical application. Although self-assembly systems such as glycyrrhizic acid have made progress in improving drug solubility, their stability, large-scale production, and cost-effectiveness still require further investigation. Additionally, while DHA nanoparticles exhibit significant anti-cancer effects, their mechanisms, safety, and performance in humans have not yet been fully validated, limiting their clinical translation.
4.2 SAs of TCM with different components
In summary, nanoparticles formed by the self-assembly of flavonoids and alkaloids hold great potential for drug development and delivery, especially in improving drug solubility, stability, and controlled release. Additionally, these self-assembled nanostructures demonstrate good biocompatibility and enhanced antibacterial activity. However, current research remains focused primarily on laboratory simulations and in vitro studies, lacking systematic in vivo validation and clinical research. Future efforts should be directed toward investigating their stability and efficacy under complex physiological conditions to ensure their safety and effectiveness in clinical applications.
In conclusion, encapsulating toxic alkaloids in herbal protein-based self-assembling nanoparticles is a key mechanism for toxicity reduction and efficacy enhancement, as evidenced in the combination of Glycyrrhiza uralensis Fisch. and Aconitum kusnezoffii Reichb. This self-assembly strategy not only reduces toxicity but also enhances the therapeutic effects of the drugs. However, current research is mainly focused on animal models, and there is a lack of human clinical data. Future studies should further explore the biocompatibility of these nanoparticles and assess their safety and efficacy in humans to ensure their widespread clinical application.
5 Application and advantages of SAs in cancers treatment with TCM
5.1 Applications of SAs in TCM for antitumor therapy
Malignant tumors remain a primary cause of premature mortality worldwide. Although significant progress has been made in treatment, the incidence of new cancer cases and the proportion of cancer-related deaths are reported to be increasing, particularly in China and other regions.145–147 The pathogenesis of tumors is complex and influenced by multiple risk factors, and effective treatment options for advanced-stage tumors remain lacking. Current treatments primarily rely on surgical resection combined with chemotherapy and/or radiotherapy. However, challenges such as the side effects of chemotherapy and the development of drug resistance persist.148–152 In this context, plant-based TCM formulations are increasingly recognized as potential alternative cancer treatments, owing to their synergistic therapeutic effects, minimal side effects, and lower likelihood of inducing drug resistance.153,154 SAs, formed through non-covalent interactions such as hydrogen bonding, π–π stacking, and hydrophobic interactions, create nanostructures that demonstrate significant potential in TCM for antitumor therapy. Natural small molecules found in TCM, such as UA, flavonoids, and terpenoids, are capable of self-assembling into stable nanoparticles. This self-assembly enhances drug solubility, bioavailability, and targeting specificity while simultaneously reducing side effects and strengthening antitumor activity.5 TCM can also enhance the activity of immune cells, such as NK cells and T cells, thereby further improving the antitumor efficacy.155 In recent years, combination therapy strategies involving active components of TCM and chemotherapeutic drugs have attracted increasing attention, particularly for their remarkable efficacy in reversing tumor drug resistance, enhancing therapeutic outcomes, and reducing side effects.156 The application of SAs in different cancers is summarized in Table 2. For example, UA, a natural compound derived from herbs such as Mespilus japonica Thunb., Prunella vulgaris L., and Hedyotis diffusa Willd., has been shown to exhibit significant anticancer effects against various cancers, including NSCLC, colorectal cancer, breast cancer, liver cancer, and colorectal carcinoma.29,157–160 UA exerts its anticancer effects through various mechanisms, including the induction of apoptosis and the inhibition of cell proliferation and migration. Furthermore, its self-assembly into nanoparticles significantly enhances both its stability and therapeutic efficacy. Fan49 et al. found that UA self-assembles into carrier-free UA NPs via hydrophobic interactions and hydrogen bonding between molecules (Fig. 3). In a lung cancer model, these UA NPs showed stronger anticancer effects than free UA, inhibiting the proliferation of A549 human lung adenocarcinoma cells, inducing apoptosis, reducing COX-2/VEGFR2/VEGFA expression, and enhancing immune-stimulatory activities of TNF-α, IL-6, and IFN-β. UA also combines with astragaloside IV (AS-IV) to form multifunctional nanoparticles, UA/(AS-IV)@PDA–HA, which fuse chemotherapy, PTT, and immunotherapy to further enhance the antitumor effect (Fig. 4). This combination is especially effective in treating NSCLC, significantly increasing cytotoxicity and inhibiting NSCLC metastasis while optimizing AS-IV's immune response to suppress NSCLC growth and metastasis.160 Additionally, UA combined with Sora forms US NPs, which protect liver cells, exhibiting excellent particle size, dispersibility, and stability. These nanoparticles synergistically inhibit the proliferation of multiple liver cancer cell lines, suppress migration, and reduce colony formation161 (Fig. 5).| Cancer type | SAs preparation | Drug composition | Effects | Drug structure | Ref. |
|---|---|---|---|---|---|
| a ↓: Reduced or inhibited; ↑: improved. | |||||
| Lung cancer | UA NPs | UA (ursolic acid) | ↑Immunostimulatory activity of TNF-α, IL-6 and IFN-β, ↑activation of CD4+ T cells, ↑cells apoptosis; ↓expression of COX-2/VEGFR2/VEGFA, ↓the activity of STAT-3 | 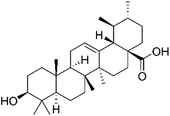 |
49 |
| Lung cancer | UA/(AS-IV) @PDA–HA NPs | UA (ursolic acid) | ↓Tumor proliferation and metastasis; ↑tumor targeting ability 3 | 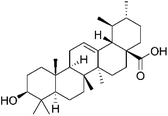 |
160 |
| AS-IV (astragaloside IV) |  |
||||
| DA (dopamine hydrochloride) |  |
||||
| HA (hyaluronic acid) |  |
||||
| Lung cancer | PTX-ss-BBR NPs | PTX (paclitaxel) | ↑Level of ROS in cancer cells, ↑block the cell cycle and cells apoptosis; ↓mitochondria membrane potential |  |
164 |
| BBR (berberine) |  |
||||
| Liver cancer | US NPs | UA (ursolic acid) | ↓Proliferation of HepG2 cells, SMMC7721 cells and H22 cells, ↓the migration of HepG2 cells and SMMC7721 cells, ↓membrane potential of mitochondrial; ↑cells apoptosis |  |
161 |
| Sora (sorafenib) |  |
||||
| Liver cancer | OA–GA–PTX NPs | OA (oleanolic acid) | ↑Antitumor activity; ↑drug releasing capacity and cellular uptake, ↑SOD, GSH; ↓ALT, AST, LDH, CK, MDA, ↓liver damage caused by PTX |  |
52 |
| GA (glycyrrhetinic acid) |  |
||||
| PTX (paclitaxel) |  |
||||
| Colorectal cancer | LNT–UA NPs | LNT (lentinan) | ↓Tumor growth and metastasis; ↑antitumor immunity; ↑drug anticancer and bioavailability performance |  |
29 |
| UA (ursolic acid) |  |
||||
| Breast cancer | UA–PTX NPs | UA (ursolic acid) | ↑Encapsulation efficiency and drug-loading, ↑block the cell cycle and cells apoptosis; ↓proliferation of breast tumor |  |
38 |
| PTX (paclitaxel) |  |
||||
| Breast cancer | OC | Ce6 (chlorin e6) | Improved solubility, efficacy and safety of the OA |  |
165 |
| OA (oleanolic acid) |  |
||||
| Ovarian cancer | PTX-ss-TMP NPs | PTX (paclitaxel) | ↑Block the cell cycle and cell apoptosis, ↑cytotoxicity, ↑cellular uptake and rapid intracellular drug release; ↓the expression levels of p-VEGFR2/VEGFR2, p-AKT/AKT, p-mTOR/mTOR, and p-p38/p38, ↓the VEGFR2-AKT/mTOR/p38 signaling axis |  |
167 |
| TMP (tetramethylpyrazine) |  |
||||
 | ||
| Fig. 3 Schematic of the preparation of UA NPs with a carrier-free, self-delivery system and its anticancer mechanism, Copyright © 2018 the American Chemical Society, adapted with permission from ref. 49. | ||
 | ||
| Fig. 4 Schematic of the synthesis of the core–shell UA/(AS-IV)@PDA–HA nanomedicine, which combined chemo-, immuno-, and photothermal-therapy in inhibiting the growth and metastasis of lung cancer, Copyright © The Royal Society of Chemistry 2023, adapted with permission from ref. 160. | ||
 | ||
| Fig. 5 Schematic of the preparation and antitumor effect of US NPs, Copyright © 2023 Published by Elsevier B.V, adapted with permission from ref. 161. | ||
5.2 Combined applications of SAs with chemotherapy, photothermal therapy (PTT), and immunotherapy
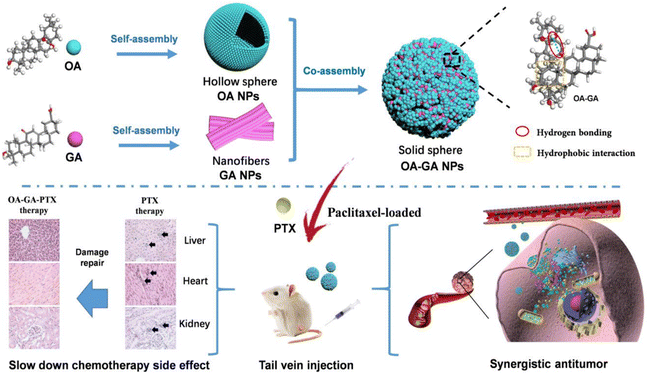 | ||
| Fig. 6 Schematic of the preparation of OA–GA NPs with a high drug loading and self-delivery system, and its antitumor mechanism, Copyright © the American Chemical Society, adapted with permission from ref. 52. | ||
 | ||
| Fig. 7 Schematic of the preparation procedures of self-assembled nanoparticles and their application for synergistic chemo-/sono-photodynamic anti-tumor therapy. Copyright © 2021, Elsevier, adapted with permission from ref. 165. | ||
 | ||
| Fig. 8 Schematic of LNT–UA preparation and its combination with αCD47 for CRC immunotherapy via modulating the tumor immunosuppressive microenvironment, Copyright © 2022 Ivyspring International Publisher, adapted with permission from ref. 29. | ||
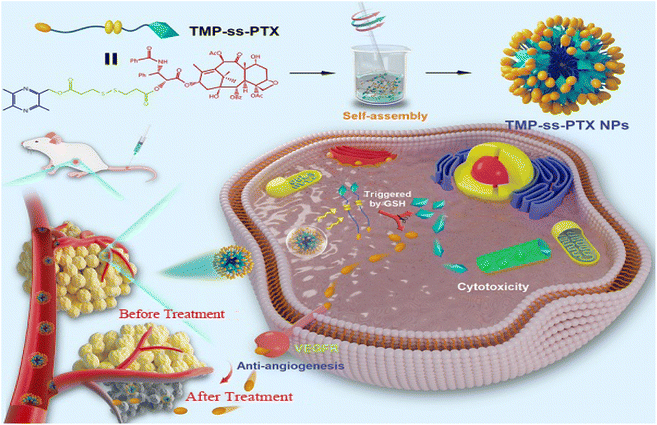 | ||
| Fig. 9 Schematic of the preparation of PTX-ss-TMP NPs with a redox-responsive carrier-free nanosystem and its mechanism for suppressing ovarian carcinoma growth, Copyright © 2021 Ivyspring International Publisher, adapted with permission from ref. 167. | ||
 | ||
| Fig. 10 Schematic of a representative small molecule, betulonic acid (BC)-meditated co-assembled synergistic antitumor BC–Ce6 NP for significantly enhanced chemo-photodynamic combination. Copyright © American Chemical Society, adapted with permission from ref. 44. | ||
In conclusion, by utilizing nanoparticles to enhance permeability and modify active targeting functions, SAs can effectively address the challenges of poor solubility and low bioavailability in antitumor active compounds, enabling more precise targeting of tumor cells. These self-assembly systems not only enhance the antitumor effects of active components but also synergize with other chemotherapy drugs, improving therapeutic outcomes while reducing toxicity and side effects. The self-assembly of active components from TCM with chemical drugs into nanomedicines offers an innovative strategy for cancer treatment, surpassing the efficacy of single drugs or simple combination therapies. These nanomedicines not only significantly enhance drug stability and targeting capabilities but also provide a foundation for constructing multifunctional diagnostic and therapeutic platforms, demonstrating broad potential applications in cancer diagnosis and treatment.168 Additionally, through modification, nanomedicines can be equipped with photothermal or magnetic functionalities, enabling integrated tumor diagnosis and treatment. With their efficiency, versatility, and safety, SAs hold great promise for future applications in tumor diagnosis and therapy.
6 Conclusion
TCM decoctions, a vital component of TCM, are rooted in the holistic perspective of compound formulations, reflecting the scientific principles of the “Monarch–Minister–Assistant–Guide” theory. In recent years, the SA behavior of secondary metabolites, such as flavonoids and saponins, in decoctions has received widespread attention, offering molecular-level explanations for the pharmacological mechanisms of TCM. Studies have found that SAs are driven by non-covalent interactions such as hydrogen bonding, hydrophobic interactions, and π–π stacking to form stable supramolecular structures. These structures not only enhance the solubility, stability, and bioavailability of active components but also prolong the duration of therapeutic effects, providing a scientific basis for the “synergistic enhancement” observed in TCM compound formulations. For example, the synergistic self-assembly of glycyrrhizic acid and BA significantly improves solubility, while the self-assembled system of BBR and Rhe enhances antibacterial activity by increasing the drug concentration.14,136 These findings support the traditional wisdom of “appropriate formulation” in TCM, providing a new scientific perspective for modern TCM research.Nevertheless, current research on SAs in TCM is still in its early stages. Many aspects, such as their formation mechanisms, structure–function relationships, and in vivo pharmacodynamics, remain unresolved. The complexity of components in decoctions further complicates the identification of key driving forces and the dynamic structural changes of SAs. Moreover, certain self-assembled products may lead to potential side effects due to inadequate release control or extended half-life, raising the need for more rigorous safety evaluations. Future research, integrating TCM theories with modern scientific approaches, can be developed in the following directions: (1) Deepening the molecular study of the “Monarch–Minister–Assistant–Guide” theory in TCM: using modern supramolecular chemistry techniques to analyze the formation mechanisms of SAs in decoction formulations. This approach aims to uncover the roles and interactions of different medicinal components in the self-assembly process, providing quantitative evidence to support the scientific rationale behind TCM formulations. (2) Establishing a multiscale research framework for TCM decoctions: leveraging molecular dynamics simulations and omics technologies to systematically analyze the behavior of SAs from the microscopic level (molecular assembly behavior) to the macroscopic level (overall pharmacological effects). This approach aims to build a comprehensive bridge between “TCM efficacy” and “molecular structure”. (3) Enhancing the stability and targeting efficiency of SAs: using technologies such as liposomes, polyethylene glycol (PEG) modification, and biomaterial coatings to improve the stability and biocompatibility of SAs. Additionally, aligning with the TCM principle of “treatment based on syndrome differentiation”, targeted self-assembled nanomedicines can be developed for specific diseases.169 (4) Innovating TCM formulations and clinical translation: building on the traditional advantage of TCM decoctions being “convenient for consumption”, developing novel liquid or nanoformulations based on SAs to enhance the quality and stability of these preparations, and enabling the modernization and broader application of traditional medicines. (5) Scientific explanation for the internationalization of TCM: using SAs as a focal point to elucidate the molecular mechanisms by which TCM decoctions enhance drug activity, reduce toxicity, and improve efficacy. This approach aims to advance the internationalization of TCM and provide unique solutions for the global health industry. (6) New explorations in intelligent and precision medicine: integrating advanced intelligent nanotechnology to develop multifunctional self-assembled drugs that meet the precision treatment needs of TCM principles such as “different treatments for the same disease” and “similar treatments for different diseases”. This approach further highlights the unique value of TCM in the management of complex diseases.
In conclusion, research on SAs in TCM decoctions has, on a theoretical level, deepened the scientific understanding of the core principles behind TCM formulations and, on a practical level, advanced the modernization and internationalization of TCM. By integrating supramolecular chemistry with TCM theories, studying the formation mechanisms and pharmacological effects of SAs in decoctions not only optimizes the modern clinical application of TCM but also offers promising scientific prospects for the development of novel TCM formulations and contributions to global health governance.
Data availability
No new data were used during the preparation of this review.Author contributions
Chunqiu Fang and Zhi Pan conceived and designed the research. Chunqiu Fang wrote the paper. Yinghang Wang created figures and tables. Zhi Pan revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (82074324), the Natural Science Foundation of Jilin Province (20210101235JC), and the Jilin Province Science and Technology Development Plan Project (20230508065RC).References
- T.-L. Lin, C.-C. Lu, W.-F. Lai, T.-S. Wu, J.-J. Lu, Y.-M. Chen, C.-M. Tzeng, H.-T. Liu, H. Wei and H.-C. Lai, Protein Cell, 2020, 12, 394–410 CrossRef PubMed.
- Z. Wang, W. Li, J. Lu, Z. Yuan, W. Pi, Y. Zhang, H. Lei, W. Jing and P. Wang, J. Ethnopharmacol., 2023, 300115704 Search PubMed.
- H.-H. Zhu, D.-P. Wu, X. Du, X. Zhang, L. Liu, J. Ma, Z.-H. Shao, H.-Y. Ren, J.-D. Hu, K.-L. Xu, J.-W. Wang, Y.-P. Song, M.-Y. Fang, J. Li, X.-Y. Yan and X.-J. Huang, Lancet Oncol., 2018, 19, 871–879 CrossRef CAS.
- X. Luan, L.-J. Zhang, X.-Q. Li, K. Rahman, H. Zhang, H.-Z. Chen and W.-D. Zhang, J. Ethnopharmacol., 2020, 254, 112687 CrossRef CAS PubMed.
- Q. Li, Y. Lianghao, G. Shijie, W. Zhiyi, T. Yuanting, C. Cong, Z. Chun-Qin and F. Xianjun, Biomater. Sci., 2024, 12, 1662–1692 RSC.
- T. Du, R. Sun, S. Du, S. Gao, M. Hu, Y. Zhang, J. Chen and G. Yang, J. Chromatogr. B, 2019, 1128, 121767 CrossRef CAS PubMed.
- W. Jia, J. Liu, R. Hu, A. Hu, W. Tang, L. Li and J. Li, Front. Pharmacol, 2020, 11, 382 CrossRef CAS PubMed.
- Y. Zhuang, J. Yan, W. Zhu, L. Chen, D. Liang and X. Xu, J. Ethnopharmacol., 2008, 117, 378–384 CrossRef PubMed.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- B. Rybtchinsk, ACS Nano, 2011, 5, 6791–6818 CrossRef PubMed.
- M. e. Malík, J. r. Velechovský and P. Tlustǒs, Fitoterapia, 2021, 151, 104845 CrossRef PubMed.
- J. Hu, Z. Wu, J. Yan, W. Pang, D. Liang and X. Xu, J. Ethnopharmacol., 2009, 123, 267–274 CrossRef PubMed.
- X. Huang, P. Wang, T. Li, X. Tian, W. Guo, B. Xu, G. Huang, D. Cai, F. Zhou, H. Zhang and H. Lei, ACS Appl. Mater. Interfaces, 2020, 12, 227–237 CrossRef CAS PubMed.
- X. Tian, P. Wang, T. Li, X. Huang, W. Guo, Y. Yang, M. Yan, H. Zhang, D. Cai, X. Jia, F. Li, B. Xu, T. Ma, C. Yan and H. Lei, Acta Pharm. Sin. B, 2020, 10, 1784–1795 CrossRef CAS PubMed.
- A. H. Elcock, Curr. Opin. Struct. Biol., 2010, 20, 196–206 CrossRef CAS.
- Y. Gao, Y. Dong, Q. Guo, H. Wang, M. Feng, Z. Yan and D. Bai, Molecules, 2022, 27, 3268 CrossRef CAS.
- Y. Ping, Y. Li, S. Lü, Y. Sun, W. Zhang, J. Wu, T. Liu and Y. Li, Biomed. Pharmacother., 2020, 124, 109826 CrossRef CAS.
- F. Huang and E. V. Anslyn, Chem. Rev., 2015, 115, 6999–7000 CrossRef CAS PubMed.
- Y. Hou, L. Zou, Q. Li, M. Chen, H. Ruan, Z. Sun, X. Xu, J. Yang and G. Ma, Mater. Today Bio, 2022, 15, 100327 CrossRef CAS PubMed.
- E. Mahon, T. Aastrup and M. Barboiu, Dynamic Nanoplatforms in Biosensor and Membrane Constitutional Systems, in Constitutional Dynamic Chemistry, 2011, pp. 139–163 Search PubMed.
- X. Dou, N. Mehwish, C. Zhao, J. Liu, C. Xing and C. Feng, Acc. Chem. Res., 2020, 53, 852–862 CrossRef CAS PubMed.
- X. Qian, X. Fan, H. Su, J. Zhang, N. Tao, J. Zhong, X. Wang and B. Han, LWT, 2019, 111, 69–76 CrossRef CAS.
- Z. Yu, G. Gao, H. Wang, L. Ke, J. Zhou, P. Rao, T. Chen, Z. Peng, J. Zou and S. Luo, Int. J. Biol. Macromol., 2020, 151, 781–786 CrossRef CAS PubMed.
- G. Gao, H. Wang, J. Zhou, P. Rao, L. Ke, J. J. Lin, B. S. Pan, Y. Zhang and Q. Wang, J. Agric. Food Chem., 2021, 69, 1610–1618 CrossRef CAS PubMed.
- Q. Zhao, X. Luan, M. Zheng, X.-H. Tian, J. Zhao, W.-D. Zhang and B.-L. Ma, Pharmaceutics, 2020, 12, 128 CrossRef CAS PubMed.
- L. Qiao, H. Yang, S. Gao, L. Li, X. Fu and Q. Wei, J. Mater. Chem. B, 2022, 10, 1908–1922 RSC.
- J. Zheng, R. Fan, H. Wu, H. Yao, Y. Yan, J. Liu, L. Ran, Z. Sun, L. Yi, L. Dang, P. Gan, P. Zheng, T. Yang, Y. Zhang, T. Tang and Y. Wang, Nat. Commun., 2019, 10, 1604 CrossRef CAS.
- L. Cai, S. Liu, J. Guo and Y.-G. Jia, Acta Biomater., 2020, 113, 84–100 CrossRef CAS.
- Q. Mao, J. Min, R. Zeng, H. Liu, H. Li, C. Zhang, A. Zheng, J. Lin, X. Liu and M. Wu, Theranostics, 2022, 12, 6088–6105 CrossRef CAS PubMed.
- E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed.
- L. Li, R. Sun, R. Zheng and Y. Huang, Mater. Des., 2021, 205, 109759 CrossRef CAS.
- J. Xu, G. Qi, W. Wang and X. S. Sun, NPJ Sci. Food, 2021, 5, 14 CrossRef PubMed.
- L. Li, R. Zheng and R. Sun, Pharmacol. Res., 2022, 4, 100158 Search PubMed.
- J. Huang, Y. Zhu, H. Xiao, J. Liu, S. Li, Q. Zheng, J. Tang and X. Meng, Chin. Med., 2023, 18, 66 CrossRef CAS PubMed.
- X. Lin, X. Huang, X. Tian, Z. Yuan, J. Lu, X. Nie, P. Wang, H. Lei and P. Wang, ACS Omega, 2022, 7, 43510–43521 CrossRef CAS PubMed.
- C. M. A. Leenders, M. B. Baker, I. A. B. Pijpers, R. P. M. Lafleur, L. Albertazzi, A. R. A. Palmans and E. W. Meijer, Soft Matter, 2016, 12, 2887–2893 RSC.
- T. Li, P. Wang, W. Guo, X. Huang, X. Tian, G. Wu, B. Xu, F. Li, C. Yan, X.-J. Liang and H. Lei, ACS Nano, 2019, 13, 6770–6781 Search PubMed.
- J. Wang, H. Zhao, K. Zhi and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 6827–6839 CrossRef CAS.
- D. Yang, S. Gao, Y. Fang, X. Lin, X. Jin, X. Wang, L. Ke and K. Shi, Nanomedicine, 2018, 13, 3159–3177 CrossRef CAS PubMed.
- N. Han, X. Huang, X. Tian, T. Li, X. Liu, W. Li, S. Huo, Q. Wu, Y. Gu, Z. Dai, B. Xu, P. Wang and H. Lei, Curr. Drug Delivery, 2021, 18, 914–921 CrossRef CAS PubMed.
- P. Wang, W. Guo, G. Huang, J. Zhen, Y. Li, T. Li, L. Zhao, K. Yuan, X. Tian, X. Huang, Y. Feng, H. Lei and A. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 32729–32742 CrossRef CAS.
- S. Das, S. Thakur, M. Korenjak, V. S. Sidorenko, F. F.-L. Chung and J. Zavadil, Nat. Rev. Cancer, 2022, 22, 576–591 CrossRef CAS.
- J. Cheng, H. Zhao, B. Li, H. Zhang, Q. Zhao, S. Fu, Y. Han, W. Lu, J. Shi and X. Yang, Acta Pharm. Sin. B, 2023, 13, 879–896 CrossRef CAS PubMed.
- J. Cheng, H. Zhao, J. Wang, Y. Han and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 43488–43500 CrossRef CAS PubMed.
- Y. Liu, L. Zhao, G. Shen, R. Chang, Y. Zhang and X. Yan, Colloids Surf., A, 2020, 598, 124805 CrossRef CAS.
- S. Garde, Nat. Commun., 2015, 517, 277–279 CrossRef CAS PubMed.
- X. Jia, Z. Yuan, Y. Yang, X. Huang, N. Han, X. Liu, X. Lin, T. Ma, B. Xu, P. Wang and H. Lei, J. Nanobiotechnol., 2022, 20, 116 CrossRef CAS.
- S. Song, Q. Zheng, A. Song and J. Hao, Langmuir, 2011, 28, 219–226 CrossRef.
- L. Fan, B. Zhang, A. Xu, Z. Shen, Y. Guo, R. Zhao, H. Yao and J.-W. Shao, Mol. Pharm., 2018, 15, 2466–2478 CrossRef CAS PubMed.
- K. Jiang, L. Han, Y. Guo, G. Zheng, L. Fan, Z. Shen, R. Zhao and J. Shao, J. Mater. Chem. B, 2017, 5, 9121–9129 RSC.
- C. Li, J. Lin, P. Wu, R. Zhao, J. Zou, M. Zhou, L. Jia and J. Shao, Bioconjugate Chem., 2018, 29, 3495–3502 CrossRef CAS PubMed.
- J. Wang, H. Zhao, W. Qiao, J. Cheng, Y. Han and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 42537–42550 CrossRef CAS PubMed.
- X. Wang, Y. Wang, M. Tang, X. Wang, W. Xue, X. Zhang, Y. Wang, W.-H. Lee, Y. Wang, T.-Y. Sun, Y. Gao and L.-L. Li, Adv. Healthcare Mater., 2023, 12, 2202432 CrossRef CAS PubMed.
- J. Zhou, J. Liu, D. Lin, G. Gao, H. Wang, J. Guo, P. Rao and L. Ke, J. Tradit. Complementary Med., 2017, 7, 178–187 CrossRef PubMed.
- J. Zhou, J. Zhang, G. Gao, H. Wang, X. He, T. Chen, L. Ke, P. Rao and Q. Wang, J. Agric. Food Chem., 2019, 67, 9354–9361 Search PubMed.
- K. Matsuoka, R. Miyajima, Y. Ishida, S. Karasawa and T. Yoshimura, Colloids Surf., A, 2016, 500, 112–117 CrossRef CAS.
- D. Lin, Q. Du, H. Wang, G. Gao, J. Zhou, L. Ke, T. Chen, C. Shaw and P. Rao, BioMed Res. Int., 2017, 2017, 1–8 Search PubMed.
- Q. Weng, X. Cai, F. Zhang and S. Wang, Food Chem., 2019, 274, 796–802 CrossRef CAS PubMed.
- J. Wu, Y. Yang, X. Yuan, H. Xu, Q. Chen, Q. Zhang, Z. Hou, F. Jiao and D. Yin, Food Funct., 2020, 11, 10480–10492 RSC.
- E. Shang, X. Ma, X. Yu, S. Sun and G. Shen, J. PharmTech Res., 2021, 40, 216–220 Search PubMed.
- M. Wan, L. Liu, H. Wu, C. Lu, Y. Fan and H. Huang, J. Chin. Mater. Med., 2011, 34, 455–458 Search PubMed.
- L.-M. Qin, Z. Zheng, F.-L. Niu, R.-Y. Dong, Y.-F. Yam, F.-H. Lang and G.-P. Fan, Chin. J. Exp. Tradit. Med. Formulae, 2000, 6, 3–5 Search PubMed.
- Z.-J. Chen, J. Wang, S. Gan, J. Li, X. Wang, Y.-F. Cheng and W. Li, Pharm. Clin. Chin. Mater. Med., 2018, 9, 23–27 Search PubMed.
- X. Li, Z. Liang, J. Du, Z. Wang, S. Mei, Z. Li, Y. Zhao, D. Zhao, Y. Ma, J. Ye, J. Xu, Y. Zhao, J. Chang, Y. Qin, L. Yu, C. Wang and C. Jiang, Sci. China Life Sci., 2019, 62, 333–348 CrossRef CAS PubMed.
- X.-J. Zhang, Y.-X. Deng, Q.-Z. Shi, M.-Y. He, B. Chen and X.-M. Qiu, Phytomedicine, 2014, 21, 615–623 CrossRef PubMed.
- X. Li, H. Tang, Q. Tang and W. Chen, Front. Cell Dev. Biol., 2021, 9, 638366 CrossRef PubMed.
- R. Tian, X. Liu, Y. Xiao, L. Jing, H. Tao, L. Yang and X. Meng, J. Ethnopharmacol., 2024, 323, 117686 CrossRef CAS PubMed.
- M. Chen, P. Wang, T. Li, L. Li, J. Li, H. Bai, H. Lei and Q. Ma, J. Pharm. Biomed. Anal., 2021, 195, 113820 CrossRef CAS PubMed.
- J.-Y. Zheng, X.-X. Li, W.-Y. Lin, S. Su, H.-C. Wu, R.-D. Hu, H.-F. Pan, J.-H. Ye, Y.-F. Cai and S.-J. Zhang, J. Ethnopharmacol., 2023, 315, 116658 CrossRef CAS PubMed.
- Y. Chen, Q. Li, X. Yang, W. Wu, Z. Jin and J. Liaoning, Univ. China Med., 2023, 25(07), 214–220 Search PubMed.
- L. Pan, J. Fu, H. Zhu and L. Guo, China J. Chin. Mater. Med., 2010, 35, 40–43 Search PubMed.
- H. Wang, T. Li, H. Xiang, X. Zhang, K. Fang, G. Wu, M. Yan, N. Xue, M. Chen, T. Xie, Y. Zhang, P. Wang and H. Lei, Molecules, 2017, 22, 1456 CrossRef.
- K. Fang, G.-R. Wu, H. Wang, Z. Rui, X.-Y. Zhang, N.-N. Xue, M. Chen, W.-B. Guo, F.-H. Chu, B. Xu, P.-L. Wang and H.-M. Lei, Chin. Tradit. Herb. Drugs, 2017, 48, 3714–3719 Search PubMed.
- T. Li, H. Wang, H. Zhang, X.-H. Tian, Q.-H. Chen, K. Fang, G.-R. Wu, B. Xu, F.-H. Chu, P.-L. Wang and H.-M. Lei, Chin. Tradit. Herb. Drugs, 2017, 48, 3505–3510 Search PubMed.
- D.-M. Liu, S.-M. Liu, J.-X. Zu, F. Lu, L. Jing, W. He and Z.-H. Huang, Chin. Tradit. Pat. Med., 2012, 34, 74–78 CAS.
- L.-F. Lin, G.-S. Chen, H. Ll and H.-J. Yang, China J. Chin. Mater. Med., 2023, 48, 5790–5797 Search PubMed.
- X. Ke, L. Zhang, L. Xin, D. Zhang, L. Han and C. Chuan, Chin. Tradit. Pat. Med., 2020, 42, 2192–2195 Search PubMed.
- X. An, C. Shi, Y. Han, X. Li, L. Dong, Y. Li, H. Chen, Y. Wang, J. Li, G. Liu, F. Lian, R. Ma and X. Tong, Front. Pharmacol, 2023, 14, 1279519 CrossRef PubMed.
- Y. Xu, L. Bao, S. Cao, B. Pang, J. Zhang, Y. Zhang, M. Chen, Y. Wang, Q. Sun, R. Zhao, S. Guo, J. Sun and X. Cui, J. Ethnopharmacol., 2024, 320, 117424 CrossRef CAS PubMed.
- L.-Q. Ma, C.-S. Pan, N. Yang, Y.-Y. Liu, L. Yan, K. Sun, X.-H. Wei, K. He, M.-M. Xiao, J.-Y. Fan and J.-Y. Han, Microcirculation, 2014, 21, 649–663 CrossRef PubMed.
- Q. Li, C. Bai, R. Yang, W. Xing, X. Pang, S. Wu, S. Liu, J. Chen, T. Liu and X. Gu, Front. Pharmacol, 2020, 11, 581691 CrossRef CAS PubMed.
- T. Guo, Y. Guo, Q. Liu, Y. Xu, L. Wei, Z. Wang, S. Chen, C. Wang, Y. Tian, J. Cui, Y. Wang, Y. Wang and L. Sun, J. Ethnopharmacol., 2021, 275, 114133 CrossRef CAS PubMed.
- H.-Z. Qiao, L.-Q. Di, Q.-N. Ping and L.-H. Hu, China J. Chin. Mater. Med., 2021, 46, 2443–2448 Search PubMed.
- C. He and Y. Pu, Chin. J. Exp. Tradit. Med. Formulae, 2022, 28(15), 259–266 Search PubMed.
- Q. Du, Y. Huang, H.-Q. Wang, G.-Z. Gao, J.-W. Zhou, L.-J. Ke and P.-F. Rao, China J. Tradit. Chin. Med. Pharm., 2014, 29, 3746–3750 CAS.
- Y.-X. Zhu, W. Chen, Z.-Z. Wang, H.-Z. Qiao and L.-Q. Di, Acta Pharmacol. Sin., 2021, 56, 2112–2118 Search PubMed.
- X. Wang, H. Long, M. Chen, Z. Zhou, Q. Wu, S. Xu, G. Li and Z. Lu, Front. Physiol., 2022, 13, 1023453 CrossRef PubMed.
- T. Lu, L. Li, Y. Li and X. Li, J. Poult. Sci., 2023, 60, 2023012 Search PubMed.
- S.-W. Lv, Y.-Q. Wu, Y.-P. Li, Y.-H. Wang, Z.-X. Yang, R. Wang, Q.-X. Guan and Y.-J. Li, Chin. J. Exp. Tradit. Med. Formulae, 2020, 26, 154–160 Search PubMed.
- J.-W. Hu, G.-X. Jia, Y.-Q. Dong, Q. Lu, S.-Y. Tian, S.-S. Yang and Y.-B. Li, Chin. Tradit. Herb. Drugs, 2022, 53, 7307–7316 CAS.
- S. Lü, H. Su, S. Sun, Y. Guo, T. Liu, Y. Ping and Y. Li, Sci. Rep., 2018, 8, 12209 CrossRef PubMed.
- S.-W. Lv, Y.-Q. Wu, Y.-P. Li, S. Sun, D.-Y. Yang, Y.-Y. Guo, H. Su and Y.-J. Li, J. Int. Pharm. Res., 2020, 47, 870–875 Search PubMed.
- J. Wu, Preliminary Study on the Antipyretic Effect and Mechanism of Bai-hu Decoction and its Nanometer Phase, Heilongjiang University of Chinese Medicine, 2018 Search PubMed.
- R. He, S. Wang, S. Yang, R. Liu, N. Nan, X. Lu, M. Gong and J. Li, J. Ethnopharmacol., 2023, 309, 116300 CrossRef CAS PubMed.
- R. He, Y. Xu, J. Peng, T. Ma, J. Li and M. Gong, J. Nat. Med., 2016, 71, 198–207 Search PubMed.
- Z. Q. Liu, Z. H. Jiang, L. Liu and M. Hu, Pharm. Res., 2006, 23, 2768–2780 Search PubMed.
- C. Wang, J. Yuan, L. L. Zhang and W. Wei, Xenobiotica, 2016, 46, 1142–1150 Search PubMed.
- B. Yan, M. Shen, J. Fang, D. Wei and L. Qin, J. Pharm. Biomed. Anal., 2018, 160, 276–288 CrossRef CAS PubMed.
- P. Gan, M. Zhong, X. Huang, M. Sun, Y. Wang, Y. Xiao, C. Zeng, Q. Yuan, Z. Liu and H. Zhou, Planta Med., 2011, 78, 237–243 Search PubMed.
- H. Iitsuka, K. Koizumi, A. Inujima, M. Suzaki, Y. Mizuno, Y. Takeshita, T. Eto, Y. Otsuka, R. Shimada, M. Liu, K. Ikeda, M. Nakano, R. Suzuki, K. Maruyama, Y. Zhou, H. Sakurai and N. Shibahara, Biochem. Biophys. Res., 2018, 16, 62–68 Search PubMed.
- C. Shen, B. Shen, J. Zhu, J. Wang, H. Yuan and X. Li, Drug Dev. Ind. Pharm., 2020, 47, 207–214 Search PubMed.
- K. K. L. Kwan, T. T. X. Dong and K. W. K. Tsim, Phytomedicine, 2021, 88, 153605 CrossRef CAS PubMed.
- G.-s. Chai, J. Gong, J.-j. Wu, R.-k. Ma, J. Zhu, D.-d. Jia, Y.-q. Zhang, X.-r. Zhai, H.-x. Sun, Y. j. Nie, P. Zhao, Y.-l. Xu and H. t. Yu, J. Ethnopharmacol., 2023, 313, 116554 Search PubMed.
- Y. Gao, Y. Zhang, W. Liu, N. Zhang, Q. Gao, J. Shangguan, N. Li, Y. Zhao and Y. Jia, Pharm. Biol., 2023, 61, 710–721 CrossRef CAS PubMed.
- P. Liang, T. Bi, Y. Zhou, Y. Ma, X. Liu, W. Ren, S. Yang and P. Luo, ACS Appl. Mater. Interfaces, 2023, 15, 47939–47954 Search PubMed.
- C. Zhang, N. Li, G.-S. Zhong, L.-L. Xiu, H.-Y. Liu, S.-H. Chen, F. Chen, M. Li, W.-Y. Liao and Y.-N. Ren, Chin. Tradit. Herb. Drugs, 2021, 52, 6425–6430 Search PubMed.
- Y. Yang, C. T. Vong, S. Zeng, C. Gao, Z. Chen, C. Fu, S. Wang, L. Zou, A. Wang and Y. Wang, J. Ethnopharmacol., 2021, 268, 113573 CrossRef CAS PubMed.
- B. Chen, L. Zhang, D. Lu, J. Yan, Z. Sun and J. Hunan, Univ. China Med., 2021, 41, 224–229 Search PubMed.
- W. Li, Z.-J. Wang, X.-Y. Lin, X.-J. Liu, N.-N. Han, W.-M. Pi, Z.-H. Yuan, H.-M. Lei and P.-L. Wang, Acta Pharmacol. Sin., 2022, 57, 1901–1908 Search PubMed.
- J. Deng, Chemical Changes of Berberine-Containing Traditional Chinese Medicine and Glycyrrhiza Uralensis Fisch during Decocting – Study on Precipitation Reaction Mechanism of Berberine and Glycyrrhizic Acid, Hunan University of Chinese Medicine, 2007 Search PubMed.
- R. Wang, Mechanisms underlying the pharmacokinetic influence of Glycyrrhizae Radix et Rhizoma on berberine in Coptidis Rhizoma, Shanghai University of Traditional Chinese Medicine, 2019 Search PubMed.
- W. Li, Z.-J. Wang, X.-J. Liu, N.-N. Han, T. Li, H.-M. Lei and P.-L. Wang, Acta Pharmacol. Sin., 2021, 56, 2119–2126 Search PubMed.
- S.-J. Chu, Y.-N. Dong, Q. Zhang, L.-J. Xle, J.-H. Ll, F. F. Xu, J. Yu, H.-L. Feng, Z.-Q. Co and Z.-H. Lou, Ginseng Res., 2022, 34, 34–39 Search PubMed.
- D. T.-K. Yun, Lancet Oncol., 2001, 2, 49–55 CrossRef PubMed.
- J. Xiong, J. Guo, L. Huang, B. Meng and Q. Ping, Int. J. Pharm., 2008, 360, 191–196 CrossRef CAS PubMed.
- M. Li, L. Lin, Y. Wang, J. Shi, C. Wu and C. Zhu, Chin. Tradit. Herb. Drugs, 2024, 55, 688–696 CrossRef.
- C. Li, Doxorubicin Nanomedicine Based on Ginsenoside Rg1 with Alleviated Cardiotoxicity and Enhanced Antitumor Activity, Tianjin University of Technology, 2022 Search PubMed.
- X.-R. Tan, C. Li, K.-K. Feng, J.-Q. Le, J.-W. Shen and J.-W. Shao, J. Ind. Eng. Chem., 2022, 116, 303–309 CrossRef CAS.
- F. Chen, G. Huang, Z. Yang and Y. Hou, Int. J. Biol. Macromol., 2019, 138, 673–680 Search PubMed.
- T. Khan, A. Date, H. Chawda and K. Patel, Carbohydr. Polym., 2019, 210, 412–428 CrossRef CAS PubMed.
- X. Mei, W. Yang, G. Huang and H. Huang, Int. J. Biol. Macromol., 2020, 142, 232–236 CrossRef CAS PubMed.
- N. S. Chandel, Cold Spring Harbor Perspect. Biol., 2021, 13, a040568 CrossRef CAS PubMed.
- M. Guo, S. Shao, D. Wang, D. Zhao and M. Wang, Food Funct., 2021, 12, 494–518 RSC.
- X.-F. Yan, Y. Yang and D.-K. Yin, Chin. J. Biochem. Pharm., 2014, 34, 175–178 CAS.
- C. Cao, C. K. Wong, R. P. Kuchel, S. D. Luca, J. M. Hook, C. J. Garvey, S. Smith, J. Ho and M. H. Stenzel, Nat. Commun., 2019, 10, 582 CrossRef PubMed.
- Y. Zhao, P. Wan, J. Wang, P. Li, Q. Hu and R. Zhao, Carbohydr. Polym., 2020, 229, 115473 CrossRef CAS PubMed.
- D. Lin, W. Lin, G. Gao, J. Zhou, T. Chen, L. Ke, P. Rao and Q. Wang, Int. J. Biol. Macromol., 2020, 159, 850–858 Search PubMed.
- X. Li, Self-assembly Effect of Angelica Sinensis Protein Andits Application, Fuzhou University, 2018 Search PubMed.
- L. Wang, B. Yang, X.-P. Lin, X.-F. Zhou and Y. Liu, Nat. Prod. Rep., 2013, 30, 455–473 RSC.
- Y. Zhuo, M. Li, Q. Jiang, H. Ke, Q. Liang, L.-F. Zeng and J. Fang, Front. Endocrinol., 2022, 13, 901545 CrossRef PubMed.
- P. B. G. Bag and D. R. Majumdar, Chem. Rec., 2017, 17, 841–873 CrossRef PubMed.
- K. Zhi, H. Zhao, X. Yang, H. Zhang, J. Wang, J. Wang and J. M. Regenstein, Nanoscale, 2018, 10, 3639–3643 RSC.
- X. Yang, C. Ma, Z. Chen, J. Liu, F. Liu, R. Xie, H. Zhao, G. Deng, A. T. Chen, N. Gong, L. Yao, P. Zuo, K. Zhi, J. Wang, X. Gao, J. Wang, L. Fan and J. Zhou, Nano Res., 2019, 12, 2468–2476 CrossRef CAS PubMed.
- X. Li, S. Lee and J. Yoon, Chem. Soc. Rev., 2018, 47, 1174–1188 RSC.
- D. A. Saha, D. J. Adamcik, D. S. Bolisetty, S. Handschin and P. D. R. Mezzenga, Angew Chem. Int. Ed. Engl., 2015, 54, 5408–5412 CrossRef PubMed.
- G. You, T. Feng, G. Zhang, M. Chen, F. Liu, L. Sun, M. Wang and X. Ren, Int. J. Pharm., 2021, 601, 120546 CrossRef CAS PubMed.
- O. Y. Selyutina and N. E. Polyakov, Int. J. Pharm., 2019, 559, 271–279 CrossRef CAS PubMed.
- F.-H. Yang, Q. Zhang, Q.-Y. Liang, S.-Q. Wang, B.-X. Zhao, Y.-T. Wang, Y. Cai and G.-F. Li, Molecules, 2015, 20, 4337–4356 CrossRef CAS PubMed.
- Y. Li, W. Zhang, N. Shi, W. Li, J. Bi, X. Feng, N. Shi, W. Zhu and Z. Xie, Biomater. Sci., 2023, 11, 2478–2485 RSC.
- C. Zhang, R. Zhao, W. Yan, H. Wang, M. Jia, N. Zhu, Y. Zhu, Y. Zhang, P. Wang and H. Lei, Molecules, 2016, 21, 1094 CrossRef PubMed.
- X. Huang, X. Liu, X. Lin, Z. Yuan, Y. Zhang, Z. Wang, W. Pi, H. Zhao, H. Lei and P. Wang, J. Nanobiotechnol., 2022, 20, 1662–1692 Search PubMed.
- B. Zhou, J. Zhang, S. Wu, Q. Zhuo, W. Gao, J. Hao and S. Man, J. Ethnopharmacol., 2015, 169, 1–7 CrossRef CAS PubMed.
- J.-M. Zhang, C.-M. Fu, Y.-X. He, J.-R. Lu, F. Gao, J.-S. Wang and W. Liao, Chin. Tradit. Herb. Drugs, 2013, 44, 165–169 CAS.
- L.-J. Ke, G.-Z. Gao, Y. Shen, J.-W. Zhou and P.-F. Rao, Nanoscale Res. Lett., 2015, 10, 449 CrossRef PubMed.
- R. Pirlog, P. Chiroi, I. Rusu, A. M. Jurj, L. Budisan, C. Pop-Bica, C. Braicu, D. Crisan, J.-C. Sabourin and I. Berindan-Neagoe, Int. J. Mol. Sci., 2022, 23, 5346 CrossRef CAS PubMed.
- F. Bray, M. Laversanne, H. Sung, J. Ferlay and R. L. Siegel, Ca - Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed.
- B. Han, R. Zheng, H. Zeng, S. Wang, K. Sun, R. Chen, L. Li, W. Wei and J. He, J. Natl. Cancer Cent., 2024, 4, 47–53 CrossRef PubMed.
- A. Sancar and R. N. V. Gelde, Science, 2021, 371, eabb0738 CrossRef CAS PubMed.
- B. Li, H. Shao, L. Gao, H. Li, H. Sheng and L. Zhu, Drug Deliv., 2022, 29, 2130–2161 CrossRef CAS PubMed.
- A. J. K. Phillips, A. J. Lawther and A. K. Walker, Brain Behav. Immun., 2022, 100, 172–173 CrossRef PubMed.
- J. Kim, S. Lee, Y. Kim, M. Choi, I. Lee, E. Kim, C. G. Yoon, K. Pu, H. Kang and J. S. Kim, Nat. Rev. Mater., 2023, 8, 710–725 CrossRef.
- Y. Hu, J. Zou, Q. Wang, Y. Chen, H. Wang and J. Li, Eur. J. Pharm. Biopharm., 2024, 196, 114184 CrossRef CAS PubMed.
- A. Mandal, R. Bisht, I. D. Rupenthal and A. K. Mitra, J. Contr. Release, 2017, 248, 96–116 CrossRef CAS PubMed.
- J. Sun, Y. Wei, J. Wang, M. Hou and L. Su, Front. Pharmacol, 2024, 15, 1377592 CrossRef CAS PubMed.
- Y. Liu, S. Yang, K. Wang, J. Lu, X. Bao, R. Wang, Y. Qiu, T. Wang and H. Yu, Cell Prolif., 2020, 53, e12894 CrossRef PubMed.
- Q. Gao, J. Feng, W. Liu, C. Wen, Y. Wu, Q. Liao, L. Zou, X. Sui, T. Xie, J. Zhang and Y. Hu, Adv. Drug Deliv. Rev., 2022, 188, 114445 CrossRef CAS PubMed.
- R. Yin, T. Li, J. X. Tian, P. Xi and R. H. Liu, Crit. Rev. Food Sci. Nutr., 2017, 58, 568–574 CrossRef PubMed.
- G.-H. Kim, S.-Y. Kan, H. Kang, S. Lee, H. M. Ko, J. H. Kim and J.-H. Lim, Int. J. Mol. Sci., 2019, 20, 4767 CrossRef CAS PubMed.
- J.-L. Zheng, S.-S. Wang, K.-P. Shen, L. Chen, X. Peng, J.-F. Chen, H.-M. An and B. Hu, BMC Complementary Med. Ther., 2021, 21, 52 CrossRef CAS PubMed.
- F. Xu, M. Li, Z. Que, M. Su, W. Yao, Y. Zhang, B. Luo, Y. Li, Z. Zhang and J. Tian, J. Mater. Chem. B, 2023, 11, 3453–3472 RSC.
- L.-W. Tong, J.-Q. Le, X.-H. Song, C.-L. Li, S.-J. Yu, Y.-Q. Lin, Y.-F. Tu and J.-W. Shao, Colloids Surf. B Biointerfaces, 2024, 234, 113724 CrossRef CAS PubMed.
- J. Chen, Z.-y. Ding, S. Li, S. Liu, C. Xiao, Z. Li, B.-x. Zhang, X.-p. Chen and X. Yang, Theranostics, 2021, 11, 1345–1363 CrossRef CAS PubMed.
- A. E. Pomeroy, E. V. Schmidt, P. K. Sorger and A. C. Palmer, Trends Cancer, 2022, 8, 915–929 CrossRef CAS PubMed.
- Y. Cheng and Y. Ji, J. Contr. Release, 2020, 318, 38–49 CrossRef CAS PubMed.
- Y. Zheng, Z. Li, Y. Yang, H. Shi, H. Chen and Y. Gao, Phytomedicine, 2021, 93, 153788 CrossRef CAS PubMed.
- M. M. S. Lee, L. Zheng, B. Yu, W. Xu, R. T. K. Kwok, J. W. Y. Lam, F. Xu, D. Wang and B. Z. Tang, Mater. Chem. Front., 2019, 3, 1454–1461 RSC.
- L. Zou, X. Liu, J. Li, W. Li, L. Zhang, C. Fu, J. Zhang and Z. Gu, Theranostics, 2021, 11, 4171–4186 CrossRef CAS PubMed.
- M. M. S. Lee, E. Y. Yu, J. H. C. Chau, J. W. Y. Lam, R. T. K. Kwok, D. Wang and B. Z. Tang, Biomaterials, 2022, 288, 121712 CrossRef CAS PubMed.
- J. Liu, Mechanistic Insight into the Interaction of Gastrointestinal Mucus with Oral Administration of Nanopolymeric Carriers, Qingdao university, 2018 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
