Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Room temperature sensing of CO2 using C3-symmetry pyridinium-based porous ionic polymers with triazine or benzene cores†
Maha A. Alshubramy*a,
Khalid A. Alamry a,
Hajar S. Alorfia,
Sameh H. Ismailb,
Nadjet Rezki
a,
Hajar S. Alorfia,
Sameh H. Ismailb,
Nadjet Rezki c,
Mohamed Reda Aouad
c,
Mohamed Reda Aouad c,
Salsabeel Al-Sodiesac and
Mahmoud A. Hussein
c,
Salsabeel Al-Sodiesac and
Mahmoud A. Hussein *ad
*ad
aChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia. E-mail: maha.alshubramy@gmail.com; malshubramy@stu.kau.edu.sa; maabdo@kau.edu.sa
bEgypt Nanotechnology Center, Cairo University, El–Sheikh Zayed, 6th October, Giza, Egypt
cDepartment of Chemistry, Taibah University, 30002, Al-Madina Al-Mounawara, Saudi Arabia
dChemistry Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt. E-mail: mahussein74@yahoo.com
First published on 3rd February 2025
Abstract
A new class of ionic polymers tethering triazine (benzene) core hybrids with three dipyridinium as cationic counterparts combined with bromide and/or chloride anions PPyBz-OBr and PPyTri-OCl were successfully prepared via the alkylation of 4,4′-dipyridyl derivatives 4,4′-bp-O with 1,3,5-tris(bromomethyl)benzene BB and/or cyanuric chloride CC. The precursor, 4,4′-bp-O,was synthesized through the condensation of 4-pyridine carboxaldehyde and 4,4′-oxydianiline. The resulting ionic polymers, PPyBz-OBr and PPyTri-OCl, underwent metathetical anion exchange, forming new ionic polymers bearing LiTFSI and KPF6 as anions. Characterization of the synthesized hybrid molecules was performed through FTIR, 1H NMR, and 13C NMR analyses. PXRD and SEM showed semi-crystalline structures and a homogenous distribution of micro-/or nanoparticles. TGA and DTA displayed high thermal stability of the synthesized polymer. The sensing activity of the modified ionic polymers was examined using a quartz crystal nanobalance (QCN) for CO2 detection. The resulting sensor demonstrated the ability to provide precise, selective, and reproducible CO2 measurements.
1. Introduction
The detection of carbon dioxide (CO2) is pivotal across diverse domains, including fire detection,1 greenhouse gas surveillance,2 air-quality monitoring,3,4 health,5–7 marine and environmental studies,8 and food supply chain management.9,10 Traditional CO2 sensing methods, such as gas chromatography,11 infrared spectrometry,12 fluorescence,13–15 photoacoustic spectroscopy,16 and severinghaus electrodes,17 offer high selectivity and sensitivity. However, they often suffer from drawbacks such as high costs, bulkiness, elevated power consumption, and susceptibility to electromagnetic interference.16,18 The development of highly sensitive, efficient, and cost-effective sensors is critical for on-site analysis and reducing CO2 emissions. The quartz crystal nanobalance (QCN) is a device that utilizes a quartz crystal microbalance (QCM) to detect mass changes at the nanogram level by measuring changes in the resonator frequency. By incorporating an appropriate chemical interface layer or recognition element, the QCN exhibits significant potential for CO2 sensing.19CO2-responsive polymers have garnered significant attention due to their high flexibility and sensitivity at room temperature.20–24 These polymers undergo changes in physical attributes, such as electrical resistance, volume, and transparency, when exposed to CO2.25 Among the functional groups in these polymers, amidine,26,27 guanidine,28 and amines,29,30 especially tertiary amines, have been extensively studied for their CO2 responsiveness. Tertiary amines, with moderate base properties, exhibit good switchability between neutral and charged states. In contrast, primary and secondary amines form carbamate salts in the presence of CO2, making them less suitable for sensing applications.31–33
In recent years, considerable efforts have been made dedicated to the design, synthesis, and development of focused macromolecules with significant electrochemical features, which are the aims associated with various applications in organic synthesis. A wide range of porous materials such as covalent organic frameworks (COFs),34 porous organic polymers (POPs),35 metal–organic frameworks (MOFs),36 organic–inorganic hybrid materials37 etc., are attractive scaffolds for designing and developing various applications such as gas storage,38–41 photovoltaics,42 conductivity,43–45 batteries,46–50 catalysis,51–54 optoelectronic devices,55 and electrochemical sensing.56
While various advanced porous materials such as zeolites,57 metal–organic frameworks (MOFs),58 ionic liquids,59,60 phenolic resins, activated carbons, and carbon composites61 have been explored, POPs, particularly those with porous frameworks containing C–N linkages, have emerged as promising candidates for CO2 sensing. The dipole–dipole interaction between CO2 and N atoms in these frameworks enhances their effectiveness.62–64 One of the effective methods for incorporating C–N functional groups is to synthesize triazine with the rigid-shaped structure and C3-symmetry, utilizing a simple synthetic method not only without the formation of byproducts but also without the need for any kind of catalyst. Moreover, the other N-rich functionalities, such as pyridine, amine, amide, carbazole, or porphyrin units, need to be designed and synthesized reasonably, which have been proven to be efficient functional materials for gas storage/separation, heterogeneous catalysis, and sensing.65,66
However, the performance of QCM sensors depends significantly on the properties of the coating material used. Previous studies have explored a range of materials, including acrylonitrile–styrene copolymers (AS), tetramethylammonium fluoride tetrahydrate (TMAF), and polyethyleneimine (PEI) coatings. For example, AS-coated QCM sensors showed sensitivity to CO2 but were also highly affected by humidity, limiting their specificity for CO2 in mixed-gas environments.67 Similarly, TMAF-coated QCM sensors demonstrated sensitivity to multiple gases such as SO2, NH3, and CO2, with limited selectivity for CO2.68 More recently, multilayered systems involving materials such as PEI and nitrogen-doped tungsten carbide (WC@NC) have shown promise in mixed-gas classification but are complex to fabricate and optimize.69 Despite these advances, challenges remain in achieving high sensitivity, selectivity, and thermal stability for CO2 detection using QCM-based sensors. In this context, the design of porous materials with tailored functionalities presents a promising approach. Porous ionic polymers (PIPs), particularly those combining nitrogen-rich frameworks and electrostatic interaction sites, offer significant potential for CO2 sensing. However, previous work in this area has been limited by the lack of synergistic design elements that enhance both adsorption and sensor performance. In this study, we report a new class of porous ionic polymers (PIPs) designed for CO2 sensing, incorporating triazine (benzene) cores and pyridinium cations with PF6 and TFSI anions. The unique combination of triazine and pyridinium units introduces several advantages. The triazine core provides a nitrogen-rich, semi-crystalline framework with high thermal stability, enabling robust performance in harsh conditions. Pyridinium cations introduce electrostatic interaction sites, increasing affinity for CO2 molecules while maintaining selectivity over NH3 and H2S. Furthermore, the incorporation of PF6 and TFSI anions influences the material's morphology and crystallinity, optimizing gas diffusion and adsorption.
2. Experimental
2.1. Synthesis of N,N′-(oxybis(4,1-phenylene))bis(1-(pyridin-4-yl)methanimine) 4,4′-bp-O
A mixture of 4-pyridine carboxaldehyde (2 mmol) and 4,4′-oxydianiline (1 mmol) was dissolved in ethanol (25 mL) with drops of conc. HCl as a catalyst. The reaction mixture was heated under reflux for 3 h. Then, the precipitate formed was filtered, washed thoroughly with ethanol, dried, and recrystallized from acetone to give the desired Schiff bases 4,4′-bp-O. It was obtained as white crystals in 90%, Mp = 250 °C. FTIR (ν, cm−1): 3016 (s, Ar–H stretch), 1622 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, imine), 1596 (C
N, imine), 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, pyridine), 1460 (Ar C
N, pyridine), 1460 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1082 (Ar C–N bending), 829 (Ar C
C bending), 1082 (Ar C–N bending), 829 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending). 1H NMR (400 MHz, DMSO-d6): δH = 7.14 (d, 4H, J = 5.48 Hz, Ar–
C bending). 1H NMR (400 MHz, DMSO-d6): δH = 7.14 (d, 4H, J = 5.48 Hz, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.45 (d, 4H, J = 5.84 Hz, Ar–
), 7.45 (d, 4H, J = 5.84 Hz, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.86 (d, 4H, J = 3.96 Hz, Ar–
), 7.86 (d, 4H, J = 3.96 Hz, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 8.75 (s, 2H,
), 8.75 (s, 2H, ![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.76 (d, 4H, J = 3.96 Hz, Ar–
N), 8.76 (d, 4H, J = 3.96 Hz, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ). 13C NMR (DMSO-d6, 200 MHz): δC = 158, 156, 150, 146, 143, 123, 122, 119.
). 13C NMR (DMSO-d6, 200 MHz): δC = 158, 156, 150, 146, 143, 123, 122, 119.
2.2. Synthesis of dipyridinium cationic polymers-based triazine and/or benzene core PPyBz-OBr and PPyTri-OCl
To a solution of 4,4′-dipyridyl, 4,4′-bp-O (1.5 mmol) in acetonitrile (20 mL) was added dropwise a solution 1,3,5-tris(bromomethyl)benzene BB and/or cyanuric chloride CC in acetonitrile (20 mL) under stirring. The reaction mixture was then refluxed for 72 h. The precipitate formed was collected by filtration, washed with chloroform (3 × 15 mL) dried to the desired ionic polymers PPyBz-OBr and PPyTri-OCl.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending). 1H NMR (850 MHz, DMSO): δ (ppm) 8.77 (s, 1H), 8.49 (m, 2H), 8.29 (m, 2H), 8.02 (s, 1H), 7.94–7.33 (m, 4H), 5.80 (m, 2H). 13C NMR (400 MHz, DMSO): δ(ppm) 160.21, 149.96, 147.92, 145.22, 144.47, 140.07, 134.23, 132.76, 130.71, 129.54, 126.76, 66.06.
C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending). 1H NMR (850 MHz, DMSO): δ (ppm) 8.77 (s, 1H), 8.49 (m, 2H), 8.29 (m, 2H), 8.02 (s, 1H), 7.94–7.33 (m, 4H), 5.80 (m, 2H). 13C NMR (400 MHz, DMSO): δ(ppm) 160.21, 149.96, 147.92, 145.22, 144.47, 140.07, 134.23, 132.76, 130.71, 129.54, 126.76, 66.06.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1384 (C–N bending) 1460 (Ar C
N), 1384 (C–N bending) 1460 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending). 1H NMR (850 MHz, DMSO): δ (ppm) 8.81 (m, 2H), 8.59 (m, 2H), 8.34 (m, 1H), 8.25–8.01 (m, 2H), 7.62–7.28 (m, 2H). 13C NMR (400 MHz, DMSO): δ (ppm) 171.86, 166.97, 155.94, 152.24, 141.35, 138.27, 133.66, 122.06.
C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending). 1H NMR (850 MHz, DMSO): δ (ppm) 8.81 (m, 2H), 8.59 (m, 2H), 8.34 (m, 1H), 8.25–8.01 (m, 2H), 7.62–7.28 (m, 2H). 13C NMR (400 MHz, DMSO): δ (ppm) 171.86, 166.97, 155.94, 152.24, 141.35, 138.27, 133.66, 122.06.2.3. Metathesis synthesis of ionic polymers PPyBz-OPF6, PPyBz-OTFSI, PPyTri-OPF6 and PPyTri-OTFSI
To a solution of ionic polymers PPyBz-OBr and PPyTri-OCl (1 mmol) in acetonitrile was added a solution of lithium bis(trifluoromethane)sulfonimide (LiTFSI) and/or potassium hexafluorophosphate (KPF6) (1.2 mmol) and the solution was refluxed for 24 h. The solid formed was collected by filtration, washed with deionized water and dried at 100 °C.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending), 863 (PF6−). 1H NMR (850 MHz, DMSO): δ (ppm) 8.85 (s, 1H), 8.59 (m, 2H), 8.41 (m, 2H), 8.15 (s, 1H), 8.03–7.37 (m, 4H), 5.92 (m, 2H). 13C NMR (400 MHz, DMSO): δ(ppm) 160.21, 148.24, 147.71, 145.16, 144.92, 140.31, 134.28, 132.98, 130.84, 129.83, 128.49, 126.87, 67.06. 31P NMR (162 MHz, DMSO) δ −131.01 to −157.36 (sep). 19F NMR (377 MHz, DMSO) δ −69.18 (s), −71.07 (s).
C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending), 863 (PF6−). 1H NMR (850 MHz, DMSO): δ (ppm) 8.85 (s, 1H), 8.59 (m, 2H), 8.41 (m, 2H), 8.15 (s, 1H), 8.03–7.37 (m, 4H), 5.92 (m, 2H). 13C NMR (400 MHz, DMSO): δ(ppm) 160.21, 148.24, 147.71, 145.16, 144.92, 140.31, 134.28, 132.98, 130.84, 129.83, 128.49, 126.87, 67.06. 31P NMR (162 MHz, DMSO) δ −131.01 to −157.36 (sep). 19F NMR (377 MHz, DMSO) δ −69.18 (s), −71.07 (s).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending), 1207 (SO2, TFSI) and 1353 (CF3, TFSI). 1H NMR (850 MHz, DMSO): δ (ppm) 8.85 (s, 1H), 8.59 (m, 2H), 8.41 (m, 2H), 8.15 (s, 1H), 8.03–7.37 (m, 4H), 5.92 (m, 2H). 13C NMR (400 MHz, DMSO): δ(ppm) 160.05, 148.16, 147.81, 145.36, 144.64, 140.23, 134.16, 132.74, 130.03, 128.65, 126.76, 66.98. 19F NMR (377 MHz, DMSO): δ(ppm) −73.49 (s).
C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending), 1207 (SO2, TFSI) and 1353 (CF3, TFSI). 1H NMR (850 MHz, DMSO): δ (ppm) 8.85 (s, 1H), 8.59 (m, 2H), 8.41 (m, 2H), 8.15 (s, 1H), 8.03–7.37 (m, 4H), 5.92 (m, 2H). 13C NMR (400 MHz, DMSO): δ(ppm) 160.05, 148.16, 147.81, 145.36, 144.64, 140.23, 134.16, 132.74, 130.03, 128.65, 126.76, 66.98. 19F NMR (377 MHz, DMSO): δ(ppm) −73.49 (s).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1384 (C–N+ bending)1460 (Ar C
N), 1384 (C–N+ bending)1460 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending), 863 (PF6−). 1H NMR (850 MHz, DMSO): δ (ppm) 8.87 (m, 2H), 8.67 (m, 2H), 8.39 (s, 1H), 8.33–8.19 (m, 2H), 7.73–7.38 (m, 2H). 13C NMR (400 MHz, DMSO): δ (ppm) 171.79, 167.11 154.94, 152.24, 141.18, 138.41, 133.76, 121.99. 31P NMR (162 MHz, DMSO) δ −131.01 to −157.36 (sep). 19F NMR (377 MHz, DMSO) δ −69.17 (s), −71.06 (s).
C bending), 1160 (Ar C–N bending), 829 (Ar C–C bending), 863 (PF6−). 1H NMR (850 MHz, DMSO): δ (ppm) 8.87 (m, 2H), 8.67 (m, 2H), 8.39 (s, 1H), 8.33–8.19 (m, 2H), 7.73–7.38 (m, 2H). 13C NMR (400 MHz, DMSO): δ (ppm) 171.79, 167.11 154.94, 152.24, 141.18, 138.41, 133.76, 121.99. 31P NMR (162 MHz, DMSO) δ −131.01 to −157.36 (sep). 19F NMR (377 MHz, DMSO) δ −69.17 (s), −71.06 (s).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1384 (s, C–N+ bending)1460 (s, Ar C
N), 1384 (s, C–N+ bending)1460 (s, Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending), 1160 (s, Ar C–N bending), 829 (vs., Ar C–C bending), 1207 (SO2, TFSI) and 1353 (CF3, TFSI). 1H NMR (850 MHz, DMSO): δ (ppm) 8.77 (m, 2H), 8.55 (m, 2H), 8.30 (s, 1H), 8.21–8.06 (m, 2H), 7.60–7.25 (m, 2H). 13C NMR (400 MHz, DMSO): δ (ppm) 171.93, 167.04 155.94, 152.31, 141.03, 138.41, 133.76, 122.06. 19F NMR (377 MHz, DMSO) δ −73.49 (s).
C bending), 1160 (s, Ar C–N bending), 829 (vs., Ar C–C bending), 1207 (SO2, TFSI) and 1353 (CF3, TFSI). 1H NMR (850 MHz, DMSO): δ (ppm) 8.77 (m, 2H), 8.55 (m, 2H), 8.30 (s, 1H), 8.21–8.06 (m, 2H), 7.60–7.25 (m, 2H). 13C NMR (400 MHz, DMSO): δ (ppm) 171.93, 167.04 155.94, 152.31, 141.03, 138.41, 133.76, 122.06. 19F NMR (377 MHz, DMSO) δ −73.49 (s).2.4. Fabrication of PPyTri-O and PPyBz-O CO2 sensors
The QCN was used to evaluate the PPyTri-O and PPyBz-O activity toward CO2 detection. In order to ensure a clean surface, the piranha solution (a blend of sulfuric acid and hydrogen peroxide) was used to clean the QCN chips, and the samples were rinsed with deionized water and dried using nitrogen. To create conductive pathways for the PIP sensors, the gold electrode was deposited on the QCN chip using a sputtering deposition method. The PIP layers were then applied using the spin coating technique and connected to the measurement system using bond wires. The CO2 chamber was prepared by installing inlets and outlets for gas flow and a temperature control system of 20 °C, 25 °C, and 30 °C using Peltier temperature baths. CO2 gas was then introduced into the chamber, and the sensor responses were analyzed to evaluate the performance of the PIP sensors under different temperature conditions.3. Results and discussion
3.1. Synthesis and characterization
The design of C3-symmetry porous ionic polymers (PIPs) involved careful consideration of the core structure and linkers. Thus, in the present work, new ionic polymers with a C3-symmetry structure were synthesized and investigated for their CO2 detection. The benzene and/or triazine core, viologen as linkers, and PF6− and TFSI− as counter anions were combined in the same framework of the tailored polymers in order to improve the electrostatic and aromatic properties impacting their overall behavior as well as CO2 sensing. The synthesis of the targeted C3-symmetry pyridinium ionic polymers PPyBz-OBr, PPyBz-OPF6, PPyBz-OTFSI, PPyTri-OCl, PPyTri-OPF6, and PPyTri-OTFSI was accomplished using the synthetic methodology depicted in Schemes 1 and 2. The first step involved the synthesis of the precursor's Schiff base 4,4′-bp-O through thermal condensation of the 4-pyridine carboxaldehyde with 4,4′-oxydianiline in refluxing ethanol and in the presence of the catalytic amount of acid. The 1H NMR spectra of the monomer 4,4′-bp-O showed characteristic signals at 7.14–8.76 ppm and 7.09–8.76 ppm attributed to the aromatic protons of the phenyl and pyridine rings, respectively. While the imine proton (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) appeared as a distinct singlet at 8.75 ppm.
N) appeared as a distinct singlet at 8.75 ppm.
 | ||
| Scheme 1 Synthesis of the precursor Schiff base 4,4′-bp-O and targeted C3-symmetry porous ionic polymers PPyBz-OBr, PPyTri-OCl. | ||
The Menshutkin reaction was adopted as a common method for generating polymers-based quaternary pyridinium as ammonium salt. Thus, the appropriate 1,3,5-tris(bromomethyl)benzene BB and/or cyanuric chloride CC were used in the polymerization process through their conjunction with the synthesized bis(1-(pyridin-4-yl)methanimine) Schiff base 4,4′-bp-O as linkers to furnish the desired cationic polymers PPyBz-OBr and PPyTri-OCl bearing chloride and bromide as counter anions, as elucidated in Scheme 1. The success of the synthesis of the targeted C3-symmetry ionic polymers was supported by the investigation of their spectral data based on FTIR and NMR results. Thus, the FTIR spectrum of the polymer PPyTri-OCl (Fig. 1(A)) clearly showed the disappearance of the stretching vibration near 850 cm−1 attributed to the C–Cl band of the starting material CC, supporting the quaternization of pyridine ring in the monomer 4,4′-bp-O. A new intense band was observed near 1645 cm−1 belonging to pyridinium cations, which is another piece of evidence for the success of the quaternization reaction and formation of the targeted ionic polymers PPyTri-OCl. The spectra also showed a shift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N absorption bands of the triazine ring (1508 cm−1) compared to their precursor CC. The C
N absorption bands of the triazine ring (1508 cm−1) compared to their precursor CC. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N absorption of the azomethine linkage and pyridine ring were observed at 1630 and 1650 cm−1, respectively. Similarly, no stretching bands near 690 cm−1 were recorded in the FTIR spectra of the polymers tethering to the benzene core PPyBz-OBr (Fig. 1(B)), confirming the absence of the C–Br bond in their structure and confirming their involvement in the alkylation of the pyridine nucleus yielding the desired adduct PPyBz-OBr, with the quaternary nitrogen atoms (N+) exhibiting an intense band around 1645 cm−1, evidencing the proposed structures. In addition, the band observed at 1640 cm−1 confirmed the presence of the imine linkage in the framework of the resulting PPyBz-OBr ionic polymer.
N absorption of the azomethine linkage and pyridine ring were observed at 1630 and 1650 cm−1, respectively. Similarly, no stretching bands near 690 cm−1 were recorded in the FTIR spectra of the polymers tethering to the benzene core PPyBz-OBr (Fig. 1(B)), confirming the absence of the C–Br bond in their structure and confirming their involvement in the alkylation of the pyridine nucleus yielding the desired adduct PPyBz-OBr, with the quaternary nitrogen atoms (N+) exhibiting an intense band around 1645 cm−1, evidencing the proposed structures. In addition, the band observed at 1640 cm−1 confirmed the presence of the imine linkage in the framework of the resulting PPyBz-OBr ionic polymer.
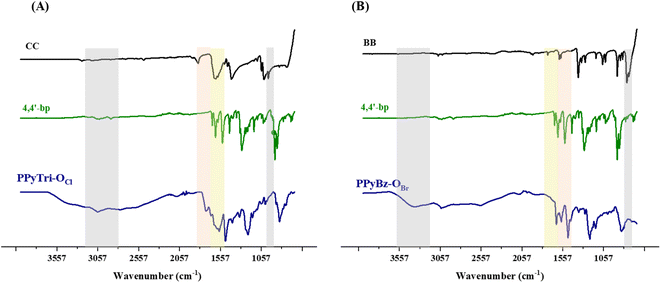 | ||
| Fig. 1 FTIR spectra of the precursor Schiff base 4,4′-bp-O and targeted C3-symmetry porous ionic polymers. (A) Triazine core and (B) benzene core. | ||
On the other hand, the structures of the synthesized polymers PPyBz-OBr, PPyBz-OPF6, PPyBz-OTFSI, PPyTri-OCl, PPyTri-OPF6, and PPyTri-OTFSI were elucidated based on their NMR spectra. The 1H NMR spectra of the developed polymers-based triazine core PPyTri-OCl and their analogs PPyTri-OPF6 and PPyTri-OTFSI (Fig. 2(A)) exhibited a broad signal in the aromatic area ranging from 7.25 to 8.87 ppm due to the polymeric backbone effect. The imine protons resonated around 8.30–8.39 ppm. Moreover, the carbons of the triazine ring and imine were recorded at 171.86–171.93 ppm and 166.97–167.04 ppm, respectively, in their 13C NMR spectra. The remaining aromatic carbons were observed in their appropriate area (121.80 to 155.94 ppm) and are detailed in the experimental section.
 | ||
| Fig. 2 1H NMR spectra of the precursor Schiff base 4,4′-bp-O and the targeted C3-symmetry porous ionic polymers. (A) Triazine core and (B) benzene core. | ||
The 1H NMR of the polymers comprising the benzene nucleus, PPyBz-OBr, PPyBz-OPF6, and PPyBz-OTFSI (Fig. 2(B)), revealed additional signals at 5.80 to 5.94 ppm attributed to methylene protons (CH2), confirming the incorporation of the benzene core in the polymer structures resulting from the quaternization reaction. Their NMR spectra revealed the same broadness as appeared on the first set of the triazine-ionic polymers. The spectra also displayed characteristic singlets at 8.02, 8.19, and 8.15 ppm belonging to the imine protons (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) with the same shift impact that seemed in the triazine core results. Extra aromatic protons resonated at 8.77 and 8.85 ppm confirming the presence of the benzene rings. The 13C NMR spectra showed new signals ranging from 66.06 to 66.98 ppm, revealing the presence of the methylene carbons (CH2).
N) with the same shift impact that seemed in the triazine core results. Extra aromatic protons resonated at 8.77 and 8.85 ppm confirming the presence of the benzene rings. The 13C NMR spectra showed new signals ranging from 66.06 to 66.98 ppm, revealing the presence of the methylene carbons (CH2).
The resulting cationic polymers, PPyBz-OBr and PPyTri-OCl, which incorporate chloride (Cl−) and bromide (Br−) as counter anions, undergo a metathesis reaction. This anion exchange process occurs when the polymers are treated with a saturated brine solution of LiTFSI and KPF6, yielding the cationic polymers with PF6− and TFSI− as counter anions: PPyBz-OPF6, PPyBz-OTFSI, PPyTri-OPF6 and PPyTri-OTFSI. The success of the metathesis reaction was confirmed and supported by the investigation of the spectral data of the resulting polymers PPyBz-OPF6, PPyBz-OTFSI, PPyTri-OPF6 and PPyTri-OTFSI tethering the PF6 and TFSI as counter anions. Their FTIR spectra (Fig. S1†) showed the appearance of the absorption bands at 863 cm−1, 1207 cm−1, and 1353 cm−1, confirming the presence of PF6−, SO2, and CF3, respectively, of TFSI− anions. The analysis of 19F NMR and 31P NMR (Fig. S2†) of the polymers PPyBz-OPF6, PPyBz-OTFSI, PPyTri-OPF6, and PPyTri-OTFSI were in accordance with their structures. The polymers PPyBz-OPF6 and PPyTri-OPF6 displayed distinguished two singlets at −69.17 and −71.06 ppm and −69.18 and −71.07 ppm, respectively. In addition, the PF6− anion exchange was also confirmed from the resonance of distinct septet in the range of −131.01 to −157.36 ppm appearing in the 31P NMR spectra of PPyBz-OPF6 and PPyTri-OPF6 polymers. The TFSI− anions appeared as a singlet near −73.49 ppm in the 19F NMR spectra of PPyBz-OTFSI and PPyTri-OTFSI polymers supporting their structures (Fig. S2†).
3.2. Morphology study
Powder X-ray diffraction (PXRD) was utilized to analyze the crystalline nature of the synthesized polymers (Fig. 3). The PXRD patterns showed semi-crystalline behavior for all formed polymers, with four main characteristic peaks at 2θ = 19.3°, 26.4°, 54.4°, and 72.9°. The diffraction peaks at 2θ = 10°–30° and 2θ = 35°–50° were the confirmation of the (002) and (101) planes of the carbon materials, respectively. The peak at 10°–30° indicated the fragmented and devolved crystallite aromatic structure, while a broad and weak peak at 35°–50° indicated an amorphous structure with minimal graphitization degree.70The exchange anions affected the crystallinity of the designed two core polymers due to the flexible structure via the imine and aliphatic bridges between the rigid aromatic rings. The anions displacement by the TFSI anion in PPyBz-OTFSI and PPyTri-OTFSI polymers caused an increase in the crystallinity in the PXRD patterns and showed diffraction peaks at 19.5°, 28.9°, 31.7°, 39.0°, 45.1° that matched (201), (114), (015), (116), (125) crystal planes, respectively, and were consistent with the data from the JCPDS card (no: 01-082-2341) of TFSI. The bulky size of the TFSI ion affected the natural crystal packing due to the formation of the bilayer structure with no significant change in the crystallinity of PF6. This structural flexibility has also been reported in porous materials.71–73
To obtain in-depth insight into the impact of the anions on the morphology of the C3-symmetry porous ionic polymers, the synthesized polymers were examined using scanning electron microscopy (SEM) at a magnification of 20 kx, as shown in (Fig. 4(A–F)). In the case of the polymers PPyTri-O and PPyBz-O, distinct and well-defined morphologies were observed, featuring sphere-shaped structures with sizes ranging from nanosized. Conversely, both PPyTri-OPF6 and PPyBz-OPF6 exhibited aggregated clusters, coarse surfaces, and loosely arranged irregular particles. Similarly, the polymers PPyTri-OTFSI and PPyBz-OTFSI showed aggregated and irregular particles in the micron-scale dimensions. These SEM images vividly depict the significant impact of bulky anions on the polymer morphology. This observation aligns with findings reported by E. Maya et al.74
 | ||
| Fig. 4 SEM images of the synthesized porous ionic polymers with C3-symmetry (A) PPyBz-OBr, (B) PPyTri-OCl, (C) PPyBz-OPF6, (D) PPyTri-OPF6, (E) PPyBz-OTFSI and (F) PPyTri-OTFSI. | ||
3.3. Thermal study
The thermal properties of the synthesized C3-symmetry ionic polymers were studied using thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) in the temperature range of 25–1000 °C at a heating rate of 10 °C min−1 (Fig. S3†). The TGA/DTG curves exhibited three-step weight loss. Most synthesized polymers were not hygroscopic in nature, and the first stage of weight loss occurred between 200 °C and 600 °C, while the second stage was observed between 630 °C and 840 °C owing to the degradation of the polymer backbone.70,75–77Table 1 presents a comprehensive comparison of T10, T25, and T50, illustrating thermal decomposition at 10%, 25%, and 50%, respectively, for the polymers. The values of T10, T25, and T50 in the case of triazine core, the polymer with PF6 anion has the highest thermal stability, while in the case of benzene core, the anion with TFSI has the highest thermal stability. Moreover, Table 1 outlines the final temperature for the polymer degradation (PDTfinal) and the maximum polymer decomposition temperature (PDTmax). As exemplified by Table 1, the PDTfinal values ranged from 680 °C to 830 °C, while the DTG revealed PDTmax in the range of 670–800 °C. The bulky anion TFSI with triazine core shows the highest lowest values of PDTfinal and PDTmax in comparison with the other anions. While the polymers with PF6 anion have the same value of PDTfinal in two cores; however, the PDTmax value shows the highest thermal stability in the triazine core. From the polymers under investigation, it was found that the triazine core had more thermal stability, which may be due to less flexibility, and the bulky anion TFSI also increases the thermal stability by increasing the electrostatic effect.
| T10% | T25% | T50% | Tfinal | Tmax | |
|---|---|---|---|---|---|
| PPyTri-OCl | 280 | 320 | 380 | 740 | 690 |
| PPyTri-OPF6 | 270 | 370 | 470 | 680 | 700 |
| PPyTri-OTFSI | 220 | 360 | 400 | 830 | 800 |
| PPyBz-OBr | 230 | 275 | 360 | 765 | 730 |
| PPyBz-OPF6 | 160 | 190 | 300 | 685 | 670 |
| PPyBz-OTFSI | 300 | 370 | 460 | 770 | 720 |
3.4. Nitrogen adsorption analysis
Nitrogen adsorption–desorption isotherms, as shown in Fig. S4,† were taken to characterize the pores in the C3-symmetry ionic polymer. All the polymers exhibited type IV nitrogen adsorption–desorption isotherms.78 The adsorption equilibrium leads to the growth of the H3 hysteresis loop, associated with capillary condensation in mesoporous structures, including the accumulation of the slit pore formation.79 Table 2 shows the specific surface area calculated using the Bruner–Emmett–Teller (BET) theory model and density functional theory (DFT), as well as pore volume and average pore size calculated using the BJH model and DFT. Accordingly, the pore-size distributions were evaluated through BJH and DFT, approved mesopores character (more than 2 nm pores) for all the pore-size distribution patterns (Fig. S4†). Table 2 shows that the polymers with the triazine core have a higher specific surface area when compared with polymers with benzene cores for the same anion. That may be due to the flexibility of methylene in the benzene core that reduces the surface area.80 However, when the anion is exchanged by PF6 or TFSI for the same core, it is found that TFSI has a lower surface area compared to PF6. The same pattern is found in the benzene and triazine cores, and that may affect the bulky anions to reduce the surface area. Notably, the counter anions occupy some spaces within the networks of these porous polymers, more or less leading to reduced surface areas.81 On the other hand, for the ionic porous polymers with C3-symmetry, the rigidity of skeletons was more or less weakened by the tritopic building blocks comprising phenyl and/or triazine rings, which could create much more complex conformational torsions by free rotation around single carbon–carbon bonds, and also tends to cause the interpenetration of chain segments. Correspondingly, these three porous polymers show lower specific surface areas.82,83| Sample code | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | ||||
|---|---|---|---|---|---|---|---|
| BET | BJH | DFT | BJH | DFT | BJH | DFT | |
| PPyTri-OCl | 54.73 | 58.40 | 52.83 | 0.17 | 0.17 | 5.7 | 6.9 |
| PPyTri-OPF6 | 60.57 | 64.66 | 57.73 | 0.18 | 0.18 | 5.7 | 6.3 |
| PPyTri-OTFSI | 35.00 | 26.76 | 33.78 | 0.11 | 0.10 | 5.7 | 6.3 |
| PPyBz-OBr | 50.76 | 34.18 | 54.49 | 0.19 | 0.19 | 5.7 | 6.3 |
| PPyBz-OPF6 | 53.84 | 56.30 | 56.61 | 0.21 | 0.19 | 5.7 | 6.3 |
| PPyBz-OTFSI | 46.15 | 37.60 | 46.35 | 0.15 | 0.15 | 5.7 | 6.3 |
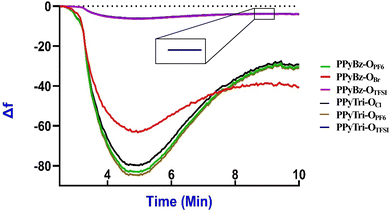
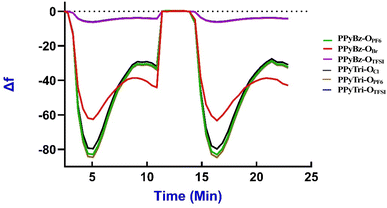
3.5. QCN study
 | ||
| Fig. 6 The average frequency shift (Δf) and standard deviation for each PIP sensor at 20 °C, 25 °C, and 30 °C. | ||
The reproducibility test was undertaken through multiple repeated measurements of the frequency shift (Δf) for randomly chosen PIPs at three temperatures (20 °C, 25 °C, and 30 °C). The relative standard deviations (RSD) of the Δf values were computed to evaluate the sensors' reproducibility. Table S1† and Fig. (8) exhibit the frequency shift measurements (Δf) and RSD (%) values for the samples, respectively. The RSD values in the table indicate that the frequency shifts between the triplicate measurements demonstrate a high level of reproducibility, and the outcomes in the figures for each run were stable and indistinguishable, as there were no noticeable differences in the response. These results demonstrate the dependability of the designed sensors when exposed to CO2 gas under various temperature conditions.
Evaluating the efficiency of the formed sensors on the QCN chips as CO2 gas sensors at various temperatures provides vital information regarding the capabilities of the sensors. Sensitivity can be represented as the change in the frequency (Δf) per unit concentration of CO2 and can be calculated by dividing the average frequency shift (Δf) by CO2 concentration.
| Δf = −(2·f02·Δm)/(A·ρ·√μ) |
The designed PIP sensors on QCN chips enable precise, selective, and reproducible CO2 measurements due to the stability of the physisorption process. This process forms weak intermolecular bonds between the CO2 molecules and the PIP-coated QCN chip surface, ensuring stable and continuous linkages during gas adsorption and desorption.
To contextualize the performance of the synthesized porous ionic polymers for CO2 sensing, a comparison was made with the previously reported QCM-based sensing materials. Table 3 summarizes the frequency shifts observed for CO2 detection using various materials, alongside their selectivity toward interfering gases such as NH3 and H2S. Ethyl cellulose (EC) exhibited strong CO2 responsiveness, while polypyrrole (PPy) demonstrated limited sensitivity. Tetramethylammonium fluoride tetrahydrate (TMAF) and polyethyleneimine (PEI) showed substantial CO2 responses; however, their cross-sensitivity to NH3 and SO2 limits their practical applicability. Similarly, nitrogen-doped tungsten carbide (WC@NC) provided excellent selectivity for H2S but showed minimal sensitivity to CO2. In comparison, the newly developed porous ionic polymers (e.g., PPyTri-OTFSI, PPyBz-OTFSI, and PPyTri-OPF6) exhibited high CO2 sensitivity with frequency shifts ranging from 264.96 Hz to 284.63 Hz, demonstrating superior performance compared to the previously reported materials. Furthermore, these polymers exhibited exceptional selectivity for CO2, with minimal interference from NH3 and H2S, which is critical for real-world applications. The tunable performance of these polymers, achieved through structural modifications and tailored anion incorporation, further underscores their potential as next-generation materials for precise and selective CO2 sensing.
| Material | Frequency shift for CO2 (Hz) | Selectivity CO2 over H2S | Selectivity CO2 over NH3 | Notes | Ref. |
|---|---|---|---|---|---|
| Ethyl cellulose (EC) | 438.95 | Moderate | Low | Strong CO2 response; limited cross-responsiveness | 88 |
| Polypyrrole (PPy) | 59.00 | Low | Low | Modest sensitivity to CO2, mainly used in gas mixture detection | |
| Tetramethylammonium fluoride (TMAF) | 300.80 | Moderate | Low | Good CO2 sensitivity but notable responses to SO2 and NH3 | |
| Polyethyleneimine (PEI) | 980.78 | Low | Low | Exceptional response to NH3, making it less selective for CO2 | |
| WC@NC (nitrogen-doped tungsten carbide) | 11.78 | Low | Low | Excellent H2S selectivity but minimal CO2 sensitivity | |
| PPyTri-OTFSI | 264.96 | High | High | High CO2 sensitivity with excellent selectivity over H2S and NH3; thermally stable | This work |
| PPyBz-OTFSI | 269.10 | High | High | ||
| PPyBz-OBr | 271.17 | High | High | ||
| PPyTri-OCl | 277.38 | High | High | ||
| PPyBz-OPF6 | 280.49 | High | High | ||
| PPyTri-OPF6 | 284.63 | High | High |
4. Conclusions
An efficient quaternization of the designed 4,4′-dipyridyl derivative 4,4′-bp-O with the appropriate 1,3,5-tris(bromomethyl)benzene BB and/or cyanuric chloride CC resulted in the effective synthesis of a new class of porous ionic polymers tethering triazine (benzene) nucleus conjugated to three pyridiniums as cationic counterparts tethered bromide/chloride anions PPyBz-O and PPyTri-O. LiTFSI and KPF6 anions were incorporated into the core of such polymers through a metathesis protocol furnished on the formation of new ionic polymers. The structures of the formed polymers were investigated by FTIR, 1H NMR, and 13C NMR analyses. The synthesized polymer was analyzed using PXRD, which showed the presence of semi-crystalline structures. The SEM examination demonstrated that micro or nanoparticles were uniformly distributed within the polymer. Furthermore, the TGA and DTA analyses indicated that the synthesized polymers exhibited good thermal stability. The modified porous ionic polymer was tested for CO2 gas sensing using quartz crystal nanobalance (QCN), which demonstrated precise, selective, and reproducible measurements.Data availability
The authors confirm that all data are included in the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (GPIP: 1193-130-2024). The authors, therefore, acknowledge with thanks DSR for technical and financial support.References
- S.-J. Chen, D. C. Hovde, K. A. Peterson and A. W. Marshall, “Fire detection using smoke and gas sensors”, Fire Saf. J., 2007, 42(8), 507–515, DOI:10.1016/j.firesaf.2007.01.006.
- A. De Pinto, et al., “Low Emission Development Strategies in Agriculture. An Agriculture, Forestry, and Other Land Uses (AFOLU) Perspective”, World Dev., 2016, 87, 180–203, DOI:10.1016/j.worlddev.2016.06.013.
- C. Jiang, M. K. Masood, Y. C. Soh and H. Li, “Indoor occupancy estimation from carbon dioxide concentration”, Energy Build., 2016, 131, 132–141, DOI:10.1016/j.enbuild.2016.09.002.
- Z. A. Siefker, et al., “Manipulating polymer composition to create low-cost, high-fidelity sensors for indoor CO2 monitoring”, Sci. Rep., 2021, 11(1), 13237, DOI:10.1038/s41598-021-92181-4.
- M. Chatterjee, et al., “A rate-based transcutaneous CO2 sensor for noninvasive respiration monitoring”, Physiol. Meas., 2015, 36(5), 883–894, DOI:10.1088/0967-3334/36/5/883.
- C. G. Cooney, B. C. Towe and C. R. Eyster, “Optical pH, oxygen and carbon dioxide monitoring using a microdialysis approach”, Sens. Actuators, B, 2000, 69(1–2), 183–188, DOI:10.1016/S0925-4005(00)00544-X.
- A. Mills, A. Lepre and L. Wild, “Breath-by-breath measurement of carbon dioxide using a plastic film optical sensor”, Sens. Actuators, B, 1997, 39(1–3), 419–425, DOI:10.1016/S0925-4005(96)02116-8.
- G. Neurauter, I. Klimant and O. S. Wolfbeis, “Fiber-optic microsensor for high resolution pCO2 sensing in marine environment”, Fresenius. J. Anal. Chem., 2000, 366(5), 481–487, DOI:10.1007/s002160050097.
- S. Neethirajan, D. S. Jayas and S. Sadistap, “Carbon Dioxide (CO2) Sensors for the Agri-food Industry—A Review”, Food Bioprocess Technol., 2009, 2(2), 115–121, DOI:10.1007/s11947-008-0154-y.
- P. Puligundla, J. Jung and S. Ko, “Carbon dioxide sensors for intelligent food packaging applications”, Food Control, 2012, 25(1), 328–333, DOI:10.1016/j.foodcont.2011.10.043.
- A. Bulbul and H. Kim, “A bubble-based microfluidic gas sensor for gas chromatographs”, Lab Chip, 2015, 15(1), 94–104, 10.1039/C4LC00892H.
- T.-V. Dinh, I.-Y. Choi, Y.-S. Son and J.-C. Kim, “A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction”, Sens. Actuators, B, 2016, 231, 529–538, DOI:10.1016/j.snb.2016.03.040.
- L. Yu, et al., “A fluorescence probe for highly selective and sensitive detection of gaseous ozone based on excited-state intramolecular proton transfer mechanism”, Sens. Actuators, B, 2018, 266, 717–723, DOI:10.1016/j.snb.2018.03.175.
- Y. Yan, et al., “Sensitive detection of sulfide based on the self-assembly of fluorescent silver nanoclusters on the surface of silica nanospheres”, Talanta, 2017, 174, 387–393, DOI:10.1016/j.talanta.2017.06.027.
- M. Sun, et al., “Palladacycle Based Fluorescence Turn-On Probe for Sensitive Detection of Carbon Monoxide”, ACS Sens., 2018, 3(2), 285–289, DOI:10.1021/acssensors.7b00835.
- T. Yang, W. Chen and P. Wang, “A review of all-optical photoacoustic spectroscopy as a gas sensing method”, Appl. Spectrosc. Rev., 2021, 56(2), 143–170, DOI:10.1080/05704928.2020.1760875.
- S. Neethirajan, M. S. Freund, D. S. Jayas, C. Shafai, D. J. Thomson and N. D. G. White, “Development of carbon dioxide (CO2) sensor for grain quality monitoring”, Biosyst. Eng., 2010, 106(4), 395–404, DOI:10.1016/j.biosystemseng.2010.05.002.
- N. B. Borchert, J. P. Kerry and D. B. Papkovsky, “A CO2 sensor based on Pt-porphyrin dye and FRET scheme for food packaging applications”, Sens. Actuators, B, 2013, 176, 157–165, DOI:10.1016/j.snb.2012.09.043.
- A. Mirmohseni, K. Farhadi and S. Jahangiri, “Application of polydimethylsiloxane/acrylic resins coated quartz crystal nano balance sensor for detection of glyphosate pesticide”, Int. J. Environ. Anal. Chem., 2020, 100(7), 733–745, DOI:10.1080/03067319.2019.1639687.
- G. Barandun, et al., “Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases”, ACS Sens., 2019, 4(6), 1662–1669, DOI:10.1021/acssensors.9b00555.
- R. Alrammouz, J. Podlecki, P. Abboud, B. Sorli and R. Habchi, “A review on flexible gas sensors: From materials to devices”, Sens. Actuators, A, 2018, 284, 209–231, DOI:10.1016/j.sna.2018.10.036.
- S. Demirci, “Crosslinked-Polymer Brushes with Switchable Capture and Release Capabilities”, Polymers, 2018, 10(9), 956, DOI:10.3390/polym10090956.
- A. Darabi, J. Glasing, P. G. Jessop and M. F. Cunningham, “Preparation of CO2-switchable latexes using N-[3-(dimethylamino)propyl]-methacrylamide (DMAPMAm)”, J. Polym. Sci., Part A:Polym. Chem., 2017, 55(6), 1059–1066, DOI:10.1002/pola.28466.
- T. C. D. Doan, J. Baggerman, R. Ramaneti, H. D. Tong, A. T. M. Marcelis and C. J. M. Van Rijn, “Carbon dioxide detection with polyethylenimine blended with polyelectrolytes”, Sens. Actuators, B, 2014, 201, 452–459, DOI:10.1016/j.snb.2014.05.023.
- M. F. Cunningham and P. G. Jessop, “An introduction to the principles and fundamentals of CO2-switchable polymers and polymer colloids”, Eur. Polym. J., 2016, 76, 208–215, DOI:10.1016/j.eurpolymj.2016.01.036.
- Z. Guo, Y. Feng, Y. Wang, J. Wang, Y. Wu and Y. Zhang, “A novel smart polymer responsive to CO2”, Chem. Commun., 2011, 47(33), 9348, 10.1039/c1cc12388b.
- Q. Yan, R. Zhou, C. Fu, H. Zhang, Y. Yin and J. Yuan, “CO2-Responsive Polymeric Vesicles that Breathe”, Angew. Chem., Int. Ed., 2011, 50(21), 4923–4927, DOI:10.1002/anie.201100708.
- J. Y. Quek, P. J. Roth, R. A. Evans, T. P. Davis and A. B. Lowe, “Reversible addition–fragmentation chain transfer synthesis of amidine-based, CO2-responsive homo and AB diblock (Co)polymers comprised of histamine and their gas-triggered self-assembly in water”, J. Polym. Sci., Part A:Polym. Chem., 2013, 51(2), 394–404, DOI:10.1002/pola.26397.
- H. Liu, Y. Zhao, C. A. Dreiss and Y. Feng, “CO2-switchable multi-compartment micelles with segregated corona”, Soft Matter, 2014, 10(34), 6387–6391, 10.1039/C4SM01207K.
- Q. Yan and Y. Zhao, “CO2-Stimulated Diversiform Deformations of Polymer Assemblies”, J. Am. Chem. Soc., 2013, 135(44), 16300–16303, DOI:10.1021/ja408655n.
- H. Liu, S. Lin, Y. Feng and P. Theato, “CO2-Responsive polymer materials”, Polym. Chem., 2017, 8(1), 12–23, 10.1039/C6PY01101B.
- A. K. Alshamrani, J. R. Vanderveen and P. G. Jessop, “A guide to the selection of switchable functional groups for CO2-switchable compounds”, Phys. Chem. Chem. Phys., 2016, 18(28), 19276–19288, 10.1039/C6CP03302D.
- M. F. Cunningham and P. G. Jessop, “Carbon Dioxide-Switchable Polymers: Where Are the Future Opportunities?”, Macromolecules, 2019, 52(18), 6801–6816, DOI:10.1021/acs.macromol.9b00914.
- Y. Shi, J. Yang, F. Gao and Q. Zhang, “Covalent Organic Frameworks: Recent Progress in Biomedical Applications”, ACS Nano, 2023, 17(3), 1879–1905, DOI:10.1021/acsnano.2c11346.
- Y. Zhu, P. Xu, X. Zhang and D. Wu, “Emerging porous organic polymers for biomedical applications”, Chem. Soc. Rev., 2022, 51(4), 1377–1414, 10.1039/D1CS00871D.
- M. Al Sharabati, R. Sabouni and G. A. Husseini, “Biomedical Applications of Metal–Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review”, Nanomaterials, 2022, 12(2), 277, DOI:10.3390/nano12020277.
- L. Yue, K. Yang, X.-Y. Lou, Y.-W. Yang and R. Wang, “Versatile Roles of Macrocycles in Organic–Inorganic Hybrid Materials for Biomedical Applications”, Matter, 2020, 3(5), 1557–1588, DOI:10.1016/j.matt.2020.09.019.
- H. Ma, et al., “Microstructure Manipulation of Covalent Organic Frameworks (COFs)-based Membrane for Efficient Separations”, Chem. Res. Chin. Univ., 2022, 38(2), 325–338, DOI:10.1007/s40242-022-1474-6.
- Z. Wang, S. Zhang, Y. Chen, Z. Zhang and S. Ma, “Covalent organic frameworks for separation applications”, Chem. Soc. Rev., 2020, 49(3), 708–735, 10.1039/C9CS00827F.
- K. Geng, et al., “Covalent Organic Frameworks: Design, Synthesis, and Functions”, Chem. Rev., 2020, 120(16), 8814–8933, DOI:10.1021/acs.chemrev.9b00550.
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, “A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework”, J. Am. Chem. Soc., 2009, 131(13), 4570–4571, DOI:10.1021/ja8096256.
- V. Stavila, A. A. Talin and M. D. Allendorf, “MOF-based electronic and opto-electronic devices”, Chem. Soc. Rev., 2014, 43(16), 5994–6010, 10.1039/C4CS00096J.
- F. Yu, W. Liu, S.-W. Ke, M. Kurmoo, J.-L. Zuo and Q. Zhang, “Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch”, Nat. Commun., 2020, 11(1), 5534, DOI:10.1038/s41467-020-19315-6.
- S. Xu and Q. Zhang, “Recent progress in covalent organic frameworks as light-emitting materials”, Mater. Today Energy, 2021, 20, 100635, DOI:10.1016/j.mtener.2020.100635.
- F. Yu, W. Liu, B. Li, D. Tian, J. Zuo and Q. Zhang, “Photostimulus-Responsive Large-Area Two-Dimensional Covalent Organic Framework Films”, Angew. Chem., Int. Ed., 2019, 58(45), 16101–16104, DOI:10.1002/anie.201909613.
- V. Singh, et al., “Thiazole-Linked Covalent Organic Framework Promoting Fast Two-Electron Transfer for Lithium-Organic Batteries”, Adv. Energy Mater., 2021, 11(17), 2003735, DOI:10.1002/aenm.202003735.
- X. Yang, et al., “Mesoporous Polyimide-Linked Covalent Organic Framework with Multiple Redox-Active Sites for High-Performance Cathodic Li Storage”, Angew. Chem., Int. Ed., 2022, 61(31), e202207043, DOI:10.1002/anie.202207043.
- X. Luo, et al., “Covalent Organic Framework with Highly Accessible Carbonyls and π-Cation Effect for Advanced Potassium-Ion Batteries”, Angew. Chem., Int. Ed., 2022, 61(10), e202117661, DOI:10.1002/anie.202117661.
- S. Zheng, et al., “Orthoquinone–Based Covalent Organic Frameworks with Ordered Channel Structures for Ultrahigh Performance Aqueous Zinc–Organic Batteries”, Angew. Chem., 2022, 134(12), e202117511, DOI:10.1002/ange.202117511.
- L. Zhong, Z. Fang, C. Shu, C. Mo, X. Chen and D. Yu, “Redox Donor–Acceptor Conjugated Microporous Polymers as Ultralong-Lived Organic Anodes for Rechargeable Air Batteries”, Angew. Chem., Int. Ed., 2021, 60(18), 10164–10171, DOI:10.1002/anie.202016746.
- F. R. Fortea-Pérez, et al., “The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry”, Nat. Mater., 2017, 16(7), 760–766, DOI:10.1038/nmat4910.
- Q. Sun, Z. Dai, X. Meng and F.-S. Xiao, “Porous polymer catalysts with hierarchical structures”, Chem. Soc. Rev., 2015, 44(17), 6018–6034, 10.1039/C5CS00198F.
- X. Wang, X. Han, J. Zhang, X. Wu, Y. Liu and Y. Cui, “Homochiral 2D Porous Covalent Organic Frameworks for Heterogeneous Asymmetric Catalysis”, J. Am. Chem. Soc., 2016, 138(38), 12332–12335, DOI:10.1021/jacs.6b07714.
- W. Tu, Y. Xu, S. Yin and R. Xu, “Rational Design of Catalytic Centers in Crystalline Frameworks”, Adv. Mater., 2018, 30(33), 1707582, DOI:10.1002/adma.201707582.
- R. K. Sharma, et al., “Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications”, Mater. Horiz., 2020, 7(2), 411–454, 10.1039/C9MH00856J.
- K. Xu and N. Huang, “Recent Advances of Covalent Organic Frameworks in Chemical Sensing”, Chem. Res. Chin. Univ., 2022, 38(2), 339–349, DOI:10.1007/s40242-022-1476-4.
- Y. Li, L. Li and J. Yu, “Applications of Zeolites in Sustainable Chemistry”, Chem, 2017, 3(6), 928–949, DOI:10.1016/j.chempr.2017.10.009.
- C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, “The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion”, Nat. Rev. Mater., 2017, 2(8), 17045, DOI:10.1038/natrevmats.2017.45.
- S. Zeng, et al., “Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process”, Chem. Rev., 2017, 117(14), 9625–9673, DOI:10.1021/acs.chemrev.7b00072.
- Q. Sun, Y. Jin, B. Aguila, X. Meng, S. Ma and F. Xiao, “Porous Ionic Polymers as a Robust and Efficient Platform for Capture and Chemical Fixation of Atmospheric CO2”, ChemSusChem, 2017, 10(6), 1160–1165, DOI:10.1002/cssc.201601350.
- A. E. Creamer and B. Gao, “Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review”, Environ. Sci. Technol., 2016, 50(14), 7276–7289, DOI:10.1021/acs.est.6b00627.
- L. Tao, F. Niu, J. Liu, T. Wang and Q. Wang, “Troger's base functionalized covalent triazine frameworks for CO2 capture”, RSC Adv., 2016, 6(97), 94365–94372, 10.1039/C6RA21196H.
- L. Zou, et al., “Porous Organic Polymers for Post-Combustion Carbon Capture”, Adv. Mater., 2017, 29(37), 1700229, DOI:10.1002/adma.201700229.
- M. Carta, M. Croad, K. Bugler, K. J. Msayib and N. B. McKeown, “Heterogeneous organocatalysts composed of microporous polymer networks assembled by Tröger’s base formation”, Polym. Chem., 2014, 5(18), 5262, 10.1039/C4PY00608A.
- S. Das, P. Heasman, T. Ben and S. Qiu, “Porous Organic Materials: Strategic Design and Structure–Function Correlation”, Chem. Rev., 2017, 117(3), 1515–1563, DOI:10.1021/acs.chemrev.6b00439.
- N. Chaoui, M. Trunk, R. Dawson, J. Schmidt and A. Thomas, “Trends and challenges for microporous polymers”, Chem. Soc. Rev., 2017, 46(11), 3302–3321, 10.1039/C7CS00071E.
- S. Muraoka, Y. Kiyohara, H. Oue and S. Higashimoto, “A CO2 Sensor Using a Quartz Crystal Microbalance Coated with a Sensitive Membrane”, Electron. Commun. Jpn., 2014, 97(2), 60–66, DOI:10.1002/ecj.11601.
- M. T. S. R. Gomes, P. S. T. Nogueira and J. A. B. P. Oliveira, “Quantification of CO2, SO2, NH3, and H2S with a single coated piezoelectric quartz crystal”, Sens. Actuators, B, 2000, 68(1–3), 218–222, DOI:10.1016/S0925-4005(00)00432-9.
- X. Liu, G. He, X. Huang, Y. Zhuang and J. Hou, “Detection of CO2, H2S, NH3, and gas mixtures using a quartz crystal microbalance sensor array with five sensitive materials”, Measurement, 2025, 242, 115891, DOI:10.1016/j.measurement.2024.115891.
- K. Cai, et al., “Construction of bifunctional triazine-based imidazolium porous ionomer polymers by a post-crosslinking tactic for efficient CO2 capture and conversion”, Chem. Eng. J., 2023, 451, 138946, DOI:10.1016/j.cej.2022.138946.
- Y. Li, et al., “A composite solid polymer electrolyte incorporating MnO2 nanosheets with reinforced mechanical properties and electrochemical stability for lithium metal batteries”, J. Mater. Chem. A, 2020, 8(4), 2021–2032, 10.1039/C9TA11542K.
- A. A. Raja and C. T. Yavuz, “Charge induced formation of crystalline network polymers”, RSC Adv., 2014, 4(104), 59779–59784, 10.1039/C4RA10594J.
- Y. Biswas, P. Banerjee and T. K. Mandal, “From Polymerizable Ionic Liquids to Poly(ionic liquid)s: Structure-Dependent Thermal, Crystalline, Conductivity, and Solution Thermoresponsive Behaviors”, Macromolecules, 2019, 52(3), 945–958, DOI:10.1021/acs.macromol.8b02351.
- E. M. Maya, E. Verde-Sesto, D. Mantione, M. Iglesias and D. Mecerreyes, “New poly(ionic liquid)s based on poly(azomethine-pyridinium) salts and its use as heterogeneous catalysts for CO2 conversion”, Eur. Polym. J., 2019, 110, 107–113, DOI:10.1016/j.eurpolymj.2018.07.030.
- W. Lin, et al., “Novel triazine-based cationic covalent organic polymers for highly efficient and selective removal of selenate from contaminated water”, J. Hazard. Mater., 2022, 436, 129127, DOI:10.1016/j.jhazmat.2022.129127.
- S. Hou, M. Meng, D. Liu and P. Zhang, “Mechanochemical Process to Construct Porous Ionic Polymers by Menshutkin Reaction”, ChemSusChem, 2021, 14(15), 3059–3063, DOI:10.1002/cssc.202101093.
- W. Li, et al., “Design of Thiazolo[5,4-d]thiazole-Bridged Ionic Covalent Organic Polymer for Highly Selective Oxygen Reduction to H 2 O 2”, Chem. Mater., 2020, 32(19), 8553–8560, DOI:10.1021/acs.chemmater.0c02843.
- K. S. W. Sing, “Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity”, 1984, DOI:10.1351/pac198557040603.
- M. Wang, Z. Li, Z. Liang, Z. Jiang and W. Wu, “Method Selection for Analyzing the Mesopore Structure of Shale—Using a Combination of Multifractal Theory and Low-Pressure Gas Adsorption”, Energies, 2023, 16(5), 2464, DOI:10.3390/en16052464.
- S. E. Fazli, M. Mirzaei, S. Mohammadinejad, and H. Fazli, “Impact of flexibility on the aggregation of polymeric macromolecules | The European Physical Journal E”, doi: DOI:10.1140/epje/s10189-023-00324-4.
- K. Cai, et al., “Imidazolium- and triazine-based ionic polymers as recyclable catalysts for efficient fixation of CO2 into cyclic carbonates”, J. CO2 Util., 2021, 51, 101658, DOI:10.1016/j.jcou.2021.101658.
- J. Lin, et al., “A metal-free approach to bipyridinium salt-based conjugated porous polymers with olefin linkages”, Polym. Chem., 2021, 12(11), 1661–1667, 10.1039/D0PY01743D.
- A. Liu, J. Zhang and X. Lv, “Novel hydrazine-bridged covalent triazine polymer for CO2 capture and catalytic conversion”, Chin. J. Catal., 2018, 39(8), 1320–1328, DOI:10.1016/S1872-2067(18)63040-2.
- R. Li, Y. Fan, Z. Ma, D. Zhang, Y. Liu and J. Xu, “Controllable preparation of ultrathin MXene nanosheets and their excellent QCM humidity sensing properties enhanced by fluoride doping”, Microchim. Acta, 2021, 188(3), 81, DOI:10.1007/s00604-021-04723-2.
- B. Sun, G. Xie, Y. Jiang and X. Li, “Comparative CO2-Sensing Characteristic Studies of PEI and PEI/Starch Thin Film Sensors”, Energy Procedia, 2011, 12, 726–732, DOI:10.1016/j.egypro.2011.10.098.
- M. Berouaken et al., “CO2 Gas Sensors Based on Hydrophilic Vanadium Oxide Thin Film Coated QCM”, in Springer Proceedings in Energy, ICREEC 2019, ed. A. Belasri and S. A. Beldjilali, Springer, Singapore, 2020, pp. 633–638, DOI:10.1007/978-981-15-5444-5_79.
- S. Gu, et al., “1,3,5-Triazine-Based Microporous Polymers with Tunable Porosities for CO2 Capture and Fluorescent Sensing”, Macromolecules, 2017, 50(21), 8512–8520, DOI:10.1021/acs.macromol.7b01857.
- X. Liu, G. He, X. Huang, Y. Zhuang and J. Hou, “Detection of CO2, H2S, NH3, and gas mixtures using a quartz crystal microbalance sensor array with five sensitive materials”, Measurement, 2025, 242, 115891, DOI:10.1016/j.measurement.2024.115891.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07062c |
| This journal is © The Royal Society of Chemistry 2025 |