Open Access Article
Open Access ArticleHolistic analysis of Ganga basin water quality: a statistical approach with WQI, HMCI, HMQI and HRI indices
Dipti Tiwaria,
Rajendra Kumara,
Monika Yadava,
Gopal Kumar Guptab,
Santosh kumar Singhc,
Nishikant Kishor Dhapekard,
Majed A. Alotaibie and
Renuka Sharma *f
*f
aDepartment of Applied Sciences, Faculty of Engineering and Technology, Rama University, Kanpur 209217, Uttar Pradesh, India
bSymbiosis Institute of Technology Nagpur Campus, Symbiosis International (Deemed University), Pune 440008, Maharashtra, India
cDepartment of Mechanical Engineering, United Institute of Technology, Prayagraj 211010, Uttar Pradesh, India
dDepartment of Civil Engineering, MATS University, Raipur 493441, Chhattisgarh, India
eDepartment of Electrical Engineering, College of Engineering, King Saud University, Riyadh 11451, Saudi Arabia
fDepartment of Commerce and Management, Banasthali Vidyapith, Tonk 304022, Rajasthan, India. E-mail: sharma.renuka30@gmail.com
First published on 31st January 2025
Abstract
The Ganga river, one of the largest and most culturally significant rivers in India, supports millions of people living along its banks. However, extensive use and untreated wastewater discharge have led to significant contamination. This study utilizes land use and land cover (LULC) analysis, along with regular water sampling from 2021 to 2222, to assess variations in physical, chemical, and biological characteristics and evaluate health risks posed by heavy metals across eight monitoring sites in the Ganga and Yamuna rivers, Prayagraj, India. Results were compared with drinking water standards established by the Bureau of Indian Standards (BIS) and the World Health Organization (WHO). The Water Quality Index (WQI) indicated substantial water quality degradation at sites S2 (Ganga) and S8 (Yamuna). Although heavy metal levels (Cu, Fe, Cd, Pb, Mn, Cr) fluctuated across sites, Pb and Cd frequently exceeded permissible limits. Health Risk Assessment (HRI) findings pointed to potential health risks at sites S4 (Ganga) and S8 (Yamuna) due to elevated Pb and Cd levels. The Heavy Metal Contamination Index (HMCI) ranged from 733.78 to 981.33, classifying all samples as highly polluted, with Heavy Metal Quality Index (HMQI) values also indicating high risk, especially at sites S4 and S8. Further analysis using principal component analysis (PCA) and cluster analysis highlighted correlations among water quality parameters, while Pearson's correlation matrix and heat maps indicated positive relationships for DO, pH, alkalinity, and SO4, with most heavy metals (except Zn and Mg) showing strong inter-correlations. These findings underline the urgent need for pollution control measures to safeguard public health in the region.
1. Introduction
The cultural history of India is extensive and diverse. The history of the world comprises tributes to its numerous ancient civilizations. The Ganga river is said to be the world's oldest river. This river is significant for religious as well as economic reasons. Water is a component that is necessary not only for the existence of human beings but also for the health of our environment. It is impossible for there to be life on our planet if there is not enough water. Human beings and other living organisms perish, and agricultural activities and corporate operations are rendered impossible.1 Because it travels across hilly and undulating terrain, it has a great deal of energy that could potentially be turned into power and is put to use in several states. Water is further put to use in agriculture, as well as for drinking and many industrial purposes. Surface runoff is a periodic occurrence mostly controlled by climate, but wastewater from homes and businesses is a continuous source of pollution.2 Diverse demographic settlements have expanded along its banks. Due to all of these human activities, garbage that contains a significant number of toxic and dangerous compounds has been released into the environment, polluting the Ganga river's water. Today, large-scale farming relies heavily on pesticides and fertilizers to get high yields. For numerous reasons, such as the widespread use of chemical fertilizers, the industry's poor waste management practices, mass bathing during festivities, and other similar activities, have put the aquatic ecosystem under environmental stress.3 These chemicals are then thrown into the water, which eventually finds its way into the river. However, factory effluents are not treated prior to being discharged into the environment. Sewage flowing into the river is another factor contributing to water contamination. It is essential that the Ganga river be kept free of pollutants and dangerous materials since both the water in the river and humankind as a whole benefit from it. Many researchers have looked at a variety of physical and chemical properties present in water bodies to assess the quality of the water.4–6Given that the Ganges basin is home to one of the densest populations on Earth, the current analysis's goal was to assess the water quality of the river across the Prayagraj region, with a particular emphasis on the Yamuna and Ganges river confluence. In addition to drinking water, the residents of the riverbank community use it for numerous residential, commercial, agricultural, and industrial uses. After usage, water is frequently released into the river by sewage, agricultural, and industrial systems. The Central Pollution Control Board's (CPCB) study states that Prayagraj's total sewage generation (208.00 MLD) is only 42.8% of what can be treated in sewage treatment plants.7,8 In addition, open defecation, runoff from rural areas, corpse dumping, and dead body disposal all contribute to the rising level of pollution. The greatest tributary of the Ganga, the Yamuna, is found to be tainted with wastewater discharged from the 69 stream drains in the national capital. Furthermore, research revealed that 72% of the cow population in the Yamuna river watershed bathed and cleaned themselves immediately with flowing water.9 The likelihood of risks to human health increases when people utilize river water frequently. About 82% of all diseases that pose a negative impact on human health are triggered by consuming contaminated water, according to the WHO.9 Rivers that have become eutrophic due to an overabundance of nutrients and surface water contaminated by harmful chemicals are major global environmental concerns. Excessive release of biologically accessible nutrients and hazardous substances into rivers can result in fish mortality, toxic algal blooms, oxygen depletion, the loss of aquatic plant beds and coral reefs, and, ultimately, a decline in biodiversity.10
Additionally, heavy metals are a major factor in surface water pollution. “Heavy metals” is a general phrase for a group of compounds that are often associated with environmental harm and toxicity. In the majority of terrestrial ecosystems, the parent material under the soil and the atmosphere are the two main sources of heavy metals. The weathering of the bedrock and metal imports from the atmosphere have an impact on the quantity of heavy metals in the soil.11 Through human activities like mining and industry, heavy metal deposition in soil, air, and water is known to result from emissions of hazardous heavy metals like Pb, Cd, and others, including Cr, Fe, Zn, Cu, and Co.12
The stability and bioaccumulative properties of heavy metals allow them to remain in soil and water for extended periods of time. They become part of the ecological food chain when plants absorb them through the soil and water. Through large entrances into the food chain, soil and water absorption by plants, and accumulation in biological systems, including people, they accumulate. Eating food grown nearby is a major way to get exposed to different metals.13 They may function as cumulative, slow poisons that affect public health because they have lengthy biological half-lives and are difficult for humans to remove from the body.14 Furthermore, positively toxic heavy metals like lead, cadmium, chromium (+6), arsenic, and others are included in the category of all hazardous heavy metals, even if they serve no useful purpose. On the other hand, necessary heavy metals are those that are needed in trace amounts for the preservation of metabolic processes; they include iron, cobalt, manganese, chromium (+3), zinc, copper, and so on. Although important, these metals have the potential to become toxic if their concentration rises above a certain point.15 When evaluating the quality of water, heavy metal contaminants, in particular, present health risks. Assessing a source's potential to release pollutants into the environment, evaluating the quality of risk agents that come into contact with people, animals, and plants, and evaluating the health effects of exposure or contact are all important components of effective risk assessment.16 These routes of heavy metal exposure may provide both non-carcinogenic and carcinogenic health risks. Certain heavy metals can be harmful to one's health if they are internalized. The toxicity and accumulation of a metal in the body are determined by its chemical form. Lead (Pb) absorption rates are 15% for inorganic forms and 80% for organic forms when consumed. While its organic derivative is a potent nerve poison, inorganic mercury (Hg) is harmful to the kidneys.17 Lead is extremely dangerous and can cause a number of health problems, including mental retardation, birth abnormalities, migraines, nausea, hypertension, lung cancer, and kidney damage. Because it is widely distributed in the kidneys and bones, cadmium (Cd), a carcinogen, has a substantial effect on these organs. Mercury is a hazardous metal that may induce mental deterioration and joint problems.18 Numerous studies have examined the potential hazards associated with human exposure to contaminated water sources in order to quantify the health implications of that exposure.19
As a result, another popular method for identifying and assessing water contamination is the Water Quality Index or WQI. “A rating reflecting the composite impact of various quality boundaries on the overall physical characteristics of water” is one definition for this index.20 The quality of the water determines whether using it for various reasons is appropriate. Effluent discharges containing hazardous compounds, whether from natural sources or intentionally generated, can have adverse effects on human health and the communities living in the aquatic system they enter.21 Therefore, in order to prevent disease and bad health among the public, evaluations of the river's water quality in connection with its position along the stretch and under different weather conditions are crucial. As suggested by ref. 22, published research has established the usefulness of water quality Indices as a water quality indicator. There are now WQIs available for many rivers around the globe, including several Indian rivers like Tamilnadu's Cauvery river;23 the Mahanadi and Atharabanki rivers, Paradip area;24 the Ram Ganga river, U.P.;25 the Ganges river, Haridwar26 Massive databases on water quality may be easily comprehensible by WQI, enabling reliable public reporting. The fundamental theory behind these investigations was that the water quality may be impacted by a range of human interventions, including urbanization, and population pressures at different locations, as well as geographic and temporal variance. The overarching hypothesis of this study was that the water quality might be impacted by a range of intervening human activities, substantial urbanization and population pressures at different locations, as well as geographical and temporal fluctuation. The water quality showed a seasonal “turning-back” pattern that varied dramatically from spring to summer before returning in the winter. Seasonal variations in water temperature and dissolved oxygen levels may have influenced this outcome.27 The material industries, farming, material producers, and printing facilities located close to or within the river's catchment region are the sources of contaminants. The river's overexploitation and the release of untreated or insufficient industrial effluents pose serious threats to the ecosystem's ability to retain its typical features. Another fact is the health risk that comes with drinking water from the river and the food chain for inhabitants in the study zone. This led to the completion of some relevant studies on heavy metals in the water of several comparable river systems in Bangladesh. But little is known about the health concerns that inhabitants of the Gomti river endure as a result of heavy metal poisoning in their water.28 The hazard degree is used by the health risk assessment tool to quantify the link between human health and the environment, as depicted in Fig. 1. This study looked at the health effects associated with drinking river water with certain heavy metals found in Prayagraj water sources.
Even though the literature review mentioned above addresses all the relevant factors in determining the Ganga basin's water quality and how it affects the local population, it falls short of linking the different factors to produce a comprehensive method for evaluating water quality and how it affects human health.
This study uniquely integrates traditional water quality assessment methods such as the Water Quality Index (WQI), Heavy Metal Contamination Index (HMCI), Heavy Metal Quality Index (HMQI), and Health Risk Index (HRI) with advanced spatial and statistical analyses, providing a multi-faceted understanding of water quality dynamics in the Ganga and Yamuna rivers. Including LULC, PCA, and CA strengthens the study's ability to address complex environmental challenges, offering novel insights into anthropogenic activities' hydrological and ecological consequences on the watershed.
This study highlights significant impacts on the Ganga and Yamuna watersheds, where anthropogenic activities and land use changes elevate heavy metal concentrations and nutrient loads. Hydrologically, pollutants compromise the rivers' self-purification capacity, and ecologically, reduced dissolved oxygen levels, toxic metal accumulation, and habitat degradation threaten aquatic biodiversity, disrupt food webs, and pose risks to ecosystems and human health. This underscores the urgent need for sustainable watershed management.
2. Material and methodology
2.1 Area for study
With a land area of 5480 × 106 square meters, the Prayagraj district is situated at 24° 47 minutes north latitude and 81° 09 minutes east longitude. The district has 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954
954![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391 residents overall, according to the 2011 census figures. Sand, gravel, kankar, and clay are alluvial deposits, and thus make, the area is considered to have an alluvial aspect. Temperatures within the sub-humid environment range from 10 to 45 °C. However, 744.1 millimetres of precipitation on average every year29 Fig. 2 provides a map of the research region as well as the sampling sites. The samples were taken from the upper, middle, and lower streams of the Ganga and Yamuna. Even though the literature review mentioned above addresses all the relevant factors in determining the Ganga basin's water quality and how it affects the local population, it falls short of linking the different factors to produce a comprehensive method for evaluating water qualty and how it affects human health. The current study uses a comprehensive methodology that includes almost all of the measurement parameters needed to evaluate the water quality in the Ganga rivers basin. The current investigation shows a holistic approach to finding the water quality of Ganga by using WQI, HMCI, HMQI, and HRI as indicators in determining the alterations in physical, chemical, and biological characteristics and elevation in the heavy metals level in Ganga and Yamuna rivers in Pryagraj; furthermore, it also assesses the effect of water pollution load on the vicinity population. Table 1 displays the sampling sites.
391 residents overall, according to the 2011 census figures. Sand, gravel, kankar, and clay are alluvial deposits, and thus make, the area is considered to have an alluvial aspect. Temperatures within the sub-humid environment range from 10 to 45 °C. However, 744.1 millimetres of precipitation on average every year29 Fig. 2 provides a map of the research region as well as the sampling sites. The samples were taken from the upper, middle, and lower streams of the Ganga and Yamuna. Even though the literature review mentioned above addresses all the relevant factors in determining the Ganga basin's water quality and how it affects the local population, it falls short of linking the different factors to produce a comprehensive method for evaluating water qualty and how it affects human health. The current study uses a comprehensive methodology that includes almost all of the measurement parameters needed to evaluate the water quality in the Ganga rivers basin. The current investigation shows a holistic approach to finding the water quality of Ganga by using WQI, HMCI, HMQI, and HRI as indicators in determining the alterations in physical, chemical, and biological characteristics and elevation in the heavy metals level in Ganga and Yamuna rivers in Pryagraj; furthermore, it also assesses the effect of water pollution load on the vicinity population. Table 1 displays the sampling sites.
| Location | Site symbol | Latitude | Longitude | Description of site | Small and large-scale industries in Prayagraj |
|---|---|---|---|---|---|
| Sobatiyabagh | S1 | 25°29′55.0′′N | 81°49′02.1′′E | Before the Ganges enters Prayagraj, there is a ghat; other activities include boating, fishing, dumping of solid trash from homes and businesses along the riverbank, and the release of garbage and industrial wastewater | Registered industrial unit: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 type of industry: clothing made of artificial thread, wool, silk, soda water, cotton textiles, and agro-based materials. Chemical and chemical-based, rubber, plastic, and petroleum-based, metal-based (steel fabrication), engineering units, repairing and maintaining, clothing made of wool, silk, and synthetic thread, (source: DIC, Prayagraj) 047 type of industry: clothing made of artificial thread, wool, silk, soda water, cotton textiles, and agro-based materials. Chemical and chemical-based, rubber, plastic, and petroleum-based, metal-based (steel fabrication), engineering units, repairing and maintaining, clothing made of wool, silk, and synthetic thread, (source: DIC, Prayagraj) |
| Draupadi Ghat | S2 | 25°28′33.07′′N | 81°49′7.52′′E | Prayagraj's Ganges Ghat is mostly used for fishing, boating, disposing of trash and municipal solid waste beside rivers, and discharging waste and sewage from homes and businesse, agricultural runoff | |
| Rasoolabad | S3 | 25°30′1482′N | 81°51′3175′E | Human cremation, religious rituals, organic waste discharge | |
| Daraganj | S4 | 25°26′7282′N | 81°53′3840′E | Runoff from irrigation systems, boating, fishing, piles of garbage and municipal and household solid waste along riverbanks, and the discharge of industrial and domestic wastewater | |
| Prior to Sangam | S5 | 25°25′5564′N | 81°52′9738′E | Sangam before convergence in the Ganges | |
| Sangam | S6 | 25°25′5836′N | 81°52.9347′E | Junction of Ganges and Yamuna | |
| Chatanag | S7 | 25°25′31.0′′N | 81°54′45.2′′E | Agricultural runoff, boating, fishing, riverbank trash, and home sewage discharge | |
| Yamuna-Arail Kachar | S8 | 25°23′58.9′′N | 81°49′20.1′′E | Agricultural runoff, boating, fishing, a pile of municipal and home solid waste disposal, residential sewage discharge at the riverbank, and a small factory nearby all contribute to the crowded Yamuna riverbank |
2.2 Taking samples
Water samples were collected from the riverbanks as well as the middle of the channel using a corrosion-resistant steel bucket. Afterwards, they were placed in a plastic container that had been well-cleaned. An ice box was used for the transportation of the samples to the testing lab following their collection. Following that, the samples were filtered using Whatman GF/F fibre glass filters (with a 47 mm diameter and 0.7 m pore size), and the specimens were kept at 4 °C until the analysis was completed. One hundred twenty samples were gathered from a total of twenty-four separate sampling events. The sampling events that occurred and the total number of samples that were collected at each event are shown in Fig. 4. Samples were cleaned, acid-washed, and stored in ice boxes at 4 °C or below to determine the presence of heavy metals. The bottles were then sent to a laboratory for further investigation. After the materials were run through glass filter paper (Whatman 42), nitric acid was added to further break them down. By using blanks and doing duplicate sample analysis on about 10% of the sample, however, quality assurance was maintained. The device was calibrated with Merck standard solutions, and for every heavy metal examined, the precision was above 2%. Using the serial dilution method, standard solutions with varying concentrations were created, and a blank solution was created. After every ten water samples.2.3 Land use land cover study
The Land Use and Land Cover (LULC) data for each sampling site (S1–S8) provides valuable context for identifying contaminant sources and understanding the environmental factors influencing heavy metal mobilization, as shown in Fig. 3. The data enables correlations between land use patterns and metal concentrations observed in the water samples by categorising land cover into agricultural, urban, industrial, forest, and water bodies. For example, sites with high proportions of industrial or urban land cover, such as S1 (Sobatiyabagh) and S4 (Daraganj), demonstrate elevated lead (Pb) and cadmium (Cd) levels, likely from vehicular emissions and industrial runoff. Similarly, sites near intensive agricultural areas like S2 (Draupadi Ghat) and S5 (before Sangam) show increased levels of zinc (Zn) and copper (Cu), consistent with agricultural runoff containing fertilisers and pesticides.The LULC data also allows us to interpret changes in key physicochemical parameters such as pH, dissolved oxygen (DO), electrical conductivity (EC), and total dissolved solids (TDS), along with variations in anions (like nitrate, sulfate, and phosphate) and cations (such as calcium, magnesium, sodium, and potassium). Sites with significant urban or industrial land cover, including S1 and S4, often display higher EC and TDS due to elevated ion concentrations from industrial discharge and urban runoff. Agricultural runoff at sites like S2 and S5 increases anion concentrations, mainly nitrate and phosphate from fertilizers, resulting in nutrient enrichment and potential eutrophication. Cations such as potassium and sodium, commonly found in fertilizers, are often present in elevated levels at agricultural sites this increase contribute to changes in water hardness, which can further influence metal solubility and mobility in the river system. Additionally, as seen at S7 (Chatnag Ghat), forested areas or vegetative cover can mitigate contamination spread by acting as natural filters and reducing anthropogenic metal contamination through bioaccumulation. Water bodies classified in the LULC data provide insights into sediment deposition patterns, which is essential for understanding heavy metal accumulation in the riverbed. These sediment patterns give a clearer picture of the contaminant retention processes. Integrating LULC data with physicochemical, anion, and cation analyses provides a comprehensive understanding of how land use influences water quality. And how urban, industrial, and agricultural activities increase specific heavy metals, nutrients, and ions, impacting water chemistry, while forested areas and water bodies help buffer contaminant spread. This holistic approach underscores the dynamic relationship between land use patterns and water quality in the Ganga and Yamuna rivers. The above study figure and the graph are powered by ESri sentinel-2 land use/land cover data by Esri and Impact Observatory software av, which is available as a free source to share and adapt.
2.4 Analysis of the physicochemical and microbiological properties
Samples were collected, and pH, total dissolved solids (TDS), and electrical conductivity (EC) tests were performed using the Labtronics Soil and Water Testing Kit (LT-59). Alkalinity was measured by titration with sulfuric acid. DO was calculated by Winkler's method. Chloride (Cl) was measured by a precipitate titration. Fluoride is measured by the fluoride test kit (AE210). Sulfate (SO42−) was analyzed by an argentometric method utilizing silver nitrate as the precipitating agent. Using a systronics spectrophotometer (type 168), phosphate (PO42−) was tested using the stannous chloride technique. Nitrate (NO3−) was tested using the brucine method. Analysis of cations was performed using a Digital Flame Photometer (LT-671), specifically on Na+, Ca++, Mg++, and K+. The MPN count technique was used in order to measure the total coliform. Total coliform was measured by growing duplicate batches of liquid broth in ten-fold solutions, where the highest possible measurement was up to 1800 MPN/100 ml.30 For the purpose of measuring the aforementioned parameters, the standard techniques of storage and analysis of the samples recommended by the American Public Health Association31 were adhered to throughout the process.2.5 Analysis of statistical data
The possible cause of contamination in rivers water at different places was identified by calculating the Pearson's correlation coefficient between WQI and observed water quality metrics.32 In order to look into the several possible reasons why rivers water is contaminated in different locations. Finding the pollution's source was the aim of this study.Step-1: use the formula to determine the unit weight (Wa) factor for each parameter.
| Wa = K÷Sa |
Step-2 – calculate the sub index (Qa) by this formula
| Qa = [(Va − Vi)]/[(Sa − Vi)] × 100 |
| QpH = [(Va − 7)]/[(Sa − 7)] × 100 |
| QDO = [(Va − 14.6)]/[(Sa − 14.6)] × 100 |
Step-3: by addition of step 1 and step 2 WQI is calculated as32
| WQI = ∑WaQa/∑Wa | (1) |
2.6 Statistical evaluation of drinking water heavy metals
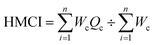 | (1) |
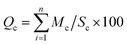 | (2) |
The quality of the water is indicated by the ratio of the content of heavy metals to the standard permissible level. Based on the quantity of heavy metals present, the sample regions are divided into 8 zones in order to assess the water quality of the rivers.
The HMCI, whose cutoff value is below 100 for security and well-being reasons, is the main index for measuring the content of heavy metals in water.34 The high HMCI values are caused by wastewater discharges from industrial and municipal sources into the rivers. To determine the pollution load and evaluate the water quality for these zones, the HMCI of 8 separate sites were compared. The HMCI readings are divided into three groups in order to determine the amount of contamination:
(i) Low (HMCI value <15);
(ii) Medium (HMCI value = 15–30)
(iii) High (HMCI value >30).35,36
 | (3) |
2.7 Human health risk assessment (HRI)
Assessment of exposure: calculation of oral water intake.The daily oral intake of metals transmitted from water was calculated using the following method, as mentioned in ref. 10.
| (DIM) = (Cm × Df)/(Bab) |
Where Cm= represents the heavy metal concentrations in water (wet weight in mg l−1), Df= represents daily intake of a water. Bab = represents the average bodyweight.
According to ICMR2010,37 the average daily water consumption in the current study was 2 liters, and the average body weight was 60 kg.
| HRI = RfD/DIM |
For lead (Pb), chromium (Cr), cobalt (Co), copper (Cu), zinc (Zn), iron (Fe), and cadmium (Cd), the reference oral doses (RfDs) were 0.015, 0.1, 0.005, 1.3, 5, and 0.005 mg per kg per day, respectively as per.39 An index greater than 1 is considered unsafe for human health.10 Dietary essential metals for which recommended intakes37 have been established include iron, zinc, copper, cobalt, and copper, which may have negative consequences in excess of RfD. Their HRI was thus also calculated.
3. Result and discussion
The samples were collected from the study area and analysed for their physical, chemical, biological properties and potential health risk by evaluating level of heavy metal for determining their designated best use. Where physical, chemical and biological parameters are pH, electrical conductivity (EC), temperature, total dissolved solids (TDS),dissolved oxygen (DO),cations (such as K+, Na+, Ca2+, Mg2+), significant anions (such as Cl−, F−, PO4−2,NO3−, SO42−, alkalinity), biological parameter like total coliform and fecal coliform, and others have all been measured. Concentration of heavy metals like Fe, Mn, Cr, Zn, Pb, Cu, Cd are also examined at eight locations which are S1-Sobatiyabagh, S2-Draupadi Ghat, S3-Rasoolabad, S4-Daraganj, S5-Prior to Sangam, S6-Sangam, S7-chatanag at Ganga and S8-Yamuna-Arail Kachar at Yamuna rivers in a roughly 55 kilometer radius. The findings of research were not in prescribed limit provided by BIS40 and WHO.20 Using the measured physical and chemical data, we have computed WQI and by measured concentration of heavy metal we also calculated HCI, HEMI, HRI. And all findings are correlated with Pearson correlation matri and heatmap.3.1 Physical and chemical factors
Experimental observations of physical factors like pH, DO, TDS, and EC are explained below.| Factors | Locations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sobatiyabagh (S1) | Draupadi Ghat (S2) | Rasoolabad (S3) | Daraganj (S4) | Prior to Sangam (S5) | Sangam (S6) | Chatnag Ghat (S7) | Yamuna (S8) | Standard value by BIS | |||||||||
| AV | SD | AV | SD | AV | SD | AV | SD | AV | SD | AV | SD | AV | SD | AV | SD | NS | |
| a NS stands for no health-based recommendations. | |||||||||||||||||
| Temp. | 26 | ±0.07 | 24 | ±0.0 7 | 22 | ±0.0 8 | 24 | ±0.13 | 26 | ±0.14 | 27 | ±0.0 8 | 24 | ±0.0 1 | 25 | ±0.0 2 | 6.5–8.5 |
| pH | 8.85 | ±0.1 7 | 8.04 | ±0.1 3 | 8.72 | ±0.1 7 | 8.65 | ±0.1 0 | 8.67 | ±0.05 | 8.75 | ±0.03 | 8.77 | ±0.02 | 8.69 | ±0.03 | 5 |
| DO (Mg L−1) | 9.5 | ±0.46 | 5.25 | ±0.37 | 7.62 | ±0.18 | 8.82 | ±0.21 | 9.54 | ±0.18 | 8.5 | ±1.18 | 7.2 | ±0.06 | 6.5 | ±0.21 | 500 |
| TDS (Mg l−1) | 334.8 | ±11.39 | 603 | ±6.44 | 335.2 | ±11.44 | 328.5 | ±5.91 | 334 | ±15.47 | 340.4 | ±13.68 | 326.4 | ±15.1 | 466.8 | ±11.31 | 300 |
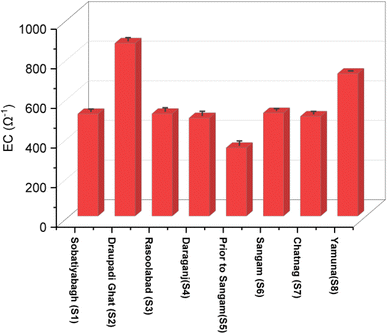
| EC | 515.6 | ±11.72 | 872.2 | ±15.13 | 516.3 | ±17.32 | 494.9 | ±20.84 | 344.6 | ±21.35 | 520.3 | ±9.08 | 503 | ±12.23 | 718.9 | ±1.31 | 120 |
| Alkalinity (Mg L−1) | 178.3 | ±10.68 | 269.5 | ±20.32 | 182.4 | ±12.74 | 182.3 | ±11.75 | 180.4 | ±12.76 | 175.4 | ±10.77 | 176.4 | ±12.78 | 182.4 | ±0.14 | 200 |
| Na+ | 47.32 | ±5.76 | 77.01 | ±3.68 | 74.77 | ±6.73 | 74.77 | ±8.74 | 40.77 | ±3.75 | 74.76 | ±8.26 | 66.38 | ±3.77 | 42.77 | ±2.78 | 55 |
| K+ | 4.98 | ±0.14 | 9.01 | ±0.15 | 8.98 | ±1.16 | 6.8 | ±0.17 | 6.91 | ±0.58 | 5.91 | ±0.50 | 0.31 | ±0.31 | 5.91 | ±0.42 | 75 |
| Ca+ | 21.3 | ±2.26 | 32.02 | ±1.8 | 29.03 | ±1.9 | 28.3 | ±2.5 | 26.4 | ±3.11 | 35.3 | ±2.22 | 28.5 | ±2.5 | 18.3 | ±1.8 | 30 |
| Mg+ | 13.01 | ±0.31 | 18.05 | ±0.21 | 18.02 | ±0.34 | 17.02 | ±0.2.35 | 17.64 | ±1.36 | 15.02 | ±0.87 | 16.1 | ±2.38 | 10.21 | ±0.79 | 1 |
| F− | 0.13 | ±0.005 | 0.4 | ±0.02 | 0.13 | ±0.07 | 0.3 | ±0.01 | 0.39 | ±0.01 | 0.125 | ±0.01 | 0.285 | ±0.01 | 0.54 | ±0.05 | 250 |
| Cl− | 23.01 | ±1.88 | 16.7 | ±1.59 | 22.01 | ±2.5 | 19.12 | ±1.91 | 21.21 | ±1.72 | 63.03 | ±2.93 | 22.01 | ±1.94 | 66.02 | ±3.95 | 20 |
| NO42− | 3.25 | ±0.16 | 3.4 | ±0.21 | 3.25 | ±0.18 | 3.22 | ±0.19 | 3.29 | ±0.20 | 3.35 | ±0.22 | 3.25 | ±0.26 | 3.04 | ±0.23 | 150 |
| SO42− | 7.66 | ±0.68 | 12.22 | ±0.34 | 3.25 | ±0.19 | 3.15 | ±0.20 | 3.35 | ±21 | 3.4 | ±0.22 | 3.31 | ±0.33 | 3.05 | ±0.24 | 12 |
| PO42− | 0.87 | ±0.10 | 1.27 | ±0.09 | 0.89 | ±0.12 | 0.75 | ±0.08 | 0.67 | ±0.04 | 0.7 | ±0.02 | 1.01 | ±0.14 | 1.56 | ±0.23 | NS |
| Total coliform × 103(MPN/100 ml) | 5.7 | ±1.02 | 8.5 | ±0.98 | 5.6 | ±1.23 | 5.3 | ±1.65 | 5.4 | ±1.67 | 6.2 | ±1.98 | 5.8 | ±0.96 | 7.2 | ±2.01 | NS |
| Fecal coliform x103(MPN/100 ml) | 4.6 | ±0.56 | 4.9 | ±0.96 | 4.4 | ±0.43 | 4 | ±1.01 | 3.6 | ±1.04 | 3.2 | ±0.98 | 3.4 | ±1.05 | 4.7 | ±1.67 | |
It must be pointed out that a river's buffering capacity for neutralising acidic contaminants from rainwater or wastewater is often determined by the alkalinity of the water. The very high level of alkalinity in Draupadi Ghat shows that the water is dangerous to consume and might result in digestive issues.
3.2 Measurement of major ions
At 8 locations along the Ganges rivers and Yamuna, the concentration of each measured ion is described in Table 2.Sewage discharge, which mostly adds Na+ and Cl− ions to river water, seems to be the cause of the observed greater concentration of Na+ throughout the whole Ganges stretch in Prayagraj, including Yamuna42 as shown in Table 2 and Fig. 8.
3.3 Measurement of total and fecal coliform
People often refer to the entire coliform category as total coliform bacteria. These microbes may be found in large numbers in untreated surface water, decaying plant and animal debris, and soil. Deep groundwater and well-treated surface water often do not contain them. This term covers not just fecal coliform bacteria but also a subset of coliform bacteria that may be found in the intestines of animals, including humans. E. coli is the primary fecal coliform of interest. Serious concern should be given if these bacteria are detected in drinking water since they may be accompanied by disease-causing germs that are spread by animal waste. The value of total coliform in the present study was highest 8.5 ± 1.31 mg l−1, on Draupti Ghat in water of Ganges whereas in water of Yamuna rivers it was 7.2 ± 2.02 mg l−1. In the present study, the maximum number of MPN 4.9 ± 0.56 mg l−1, on Draupadi Ghat in the water of Ganges whereas in water of Yamuna rivers it was 4.7 ± 1.67 mg l−1.aa The minimum number was recorded in winter. The high value in the present study may be attributed to the presence of bacterial load from the nearby surrounding areas (Fecal matter), and due to this reason, the river Ganga is absolutely unfit for drinking and unhealthy for bathing. Such higher value of MPN is also supported by the studies of other researchers44 as shown in Table 2 and Fig. 10.3.4 Principal component analysis (PCA) and hierarchical cluster analysis
The water quality assessment of the Ganga and Yamuna rivers using principal component analysis (PCA) and hierarchical clustering analysis for physical, chemical, cation, anion and biological factors reveals intricate spatial and compositional variations across sampling sites, shedding light on pollution sources and key environmental pressures. PCA identifies two main components capturing the largest variance in water quality data: the first principal component (PC1), explaining 43.46% of the variance, is associated with parameters such as total dissolved solids (TDS), electrical conductivity (EC), alkalinity, chloride (Cl−), and sulfate (SO42−), indicating an ion-rich gradient characteristic of pollution from urban runoff, industrial discharges, and agricultural practices. High positive loadings for TDS and EC on PC1 suggest that sites with elevated PC1 scores experience higher salinity, possibly from mineral-rich runoff and wastewater inputs, marking the influence of industrial and agricultural activities on water quality (Fig. 1). In contrast, the second principal component (PC2), explaining 26.25% of the variance, correlates strongly with dissolved oxygen (DO) and sodium (Na+), capturing variations due to biological and chemical processes influenced by organic matter decomposition and sodium inputs, possibly from agricultural or urban sources. Low DO levels are critical for aquatic life, and significant loadings on PC2 suggest that certain sites may face oxygen depletion due to high biological oxygen demand (Fig. 2). Hierarchical clustering further supports these findings by grouping the sites into three clusters based on physico-chemical similarities: Cluster 1, containing sites S1, S3, S4, and S7, represents areas with similar pollution profiles, likely from urban runoff or industrial discharge; Cluster 2, with sites S5 and S6, shows moderate similarity, reflecting shared but distinct environmental pressures, possibly from localized agricultural runoff; and Cluster 3, consisting of sites S2 and S8, highlights unique pollution sources or natural variations (Fig. 3). Sites in Cluster 1 may benefit from integrated pollution control strategies due to high TDS, EC, and chloride, while Cluster 3 may require targeted interventions for distinct pollution characteristics. Together, PCA and cluster analysis provide a comprehensive understanding of water quality across the study area, emphasizing the need for site-specific management (Fig. 11 and 12). | ||
| Fig. 11 PCA biplot showing sample distribution (S1–S8) and environmental parameter influence along PC1 (43.46%) and PC2 (26.25%). | ||
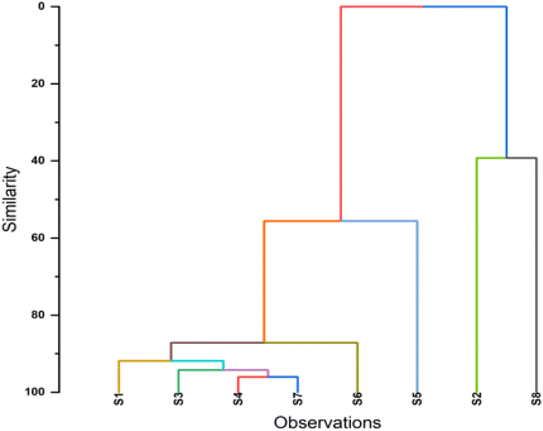 | ||
| Fig. 12 Hierarchical clustering dendrogram showing similarity relationships among observations S1 to S8. | ||
3.5 WQI of water from the Ganges and Yamuna river
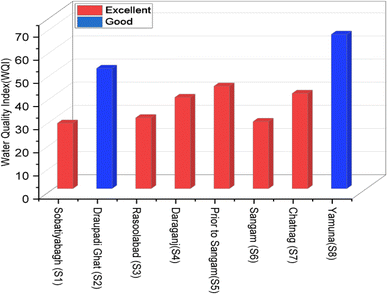
Table 3 (S1–S8) shows that the measured water quality indices for the Ganges rivers in Prayagraj which ranges from 28.27 to 66.98. Ramakrishnaiah et al.45 categorized the whole Ganga and Yamuna rivers sites in the table as good and excellent, where good water quality indicates a minor degree of threat or impairment, whereas excellent water quality is protected with the virtual absence of threat or impairment close to natural or pristine lable. S2 and S8 were stated as good where, as the rest of the sites were stated as excellent. Furthermore, WQI values where also categorized in accordance with the rating system of Brown et al.32 as shown in Tables 4 and 5. Water Quality Index (WQI) ratings at sampling sites using two different scales and as a result, a more precise understanding of the rivers in Prayagraj's water quality has been attained. Based on this classification, we can raSangam water quality of Sobatiyabagh, Rasoolabad, Daraganj, before Sangam, Sangam, Chatnag, as good quality (WQI ranges 26–50), while the water quality of Draupadi Ghat (S2) and the Yamuna rivers in Arail Kachar (S8)were rated as poor (WQI ranges 51–75), as shown in (Table 4, Fig. 13 and 14), poor water quality is always threatened or impaired, which deviates from the desirable label due to heavy pollution load. However, the dumping of corpses, livestock bathing, agricultural runoff, nearly stagnant water, and open defecation all seem to be contributing factors to the unsatisfactory quality of the water. Due to customs and beliefs involving the burning and discarding of dead body ashes into the river, it was discovered that the water quality in Rasoolabad and Daraganj was extremely low, and the water quality at Sangam was almost acceptable.| Parameter | Bis standard (Sa) | 1/Sa | ∑1/Sa | K = 1/(∑1/Sa) | Wa = k/Sa | Ideal value (Vi) | Mean conc. value (Va) | Va/Sa | Va/Sa × 100 = Qa | WaQa | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.6472 | 0.0761 | 7 | 8.85 | 1.2643 | 123.33 | 9.3905 | ||
| DO | 5 | 0.2 | 1.5452 | 0.6472 | 0.1294 | 14.6 | 9.5 | 0.6507 | 53.125 | 6.8763 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.6472 | 0.0013 | 0 | 334.75 | 0.6695 | 66.95 | 0.0867 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.6472 | 0.0022 | 0 | 515.63 | 1.7188 | 171.88 | 0.3708 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.6472 | 0.0054 | 0 | 178.28 | 1.4857 | 148.57 | 0.8012 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.6472 | 0.0032 | 0 | 47.32 | 0.2366 | 23.66 | 0.0766 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.6472 | 0.0118 | 0 | 4.98 | 0.0905 | 9.0545 | 0.1065 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.6472 | 0.0086 | 0 | 21.3 | 0.284 | 28.4 | 0.2451 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.6472 | 0.0216 | 0 | 13.01 | 0.4337 | 43.367 | 0.9355 | ||
| F− | 1 | 1 | 1.5452 | 0.6472 | 0.6472 | 0 | 0.13 | 0.13 | 13 | 8.4134 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.6472 | 0.0026 | 0 | 23.01 | 0.092 | 9.204 | 0.0238 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.6472 | 0.0324 | 0 | 3.25 | 0.1625 | 16.25 | 0.5258 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.6472 | 0.0043 | 0 | 7.66 | 0.0511 | 5.1067 | 0.022 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.6472 | 0.0539 | 0 | 0.87 | 0.0725 | 7.25 | 0.391 | ||
| 1.545 | 1 | WQI | 28.27 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S2 | ||||||||||||
| Ph | 8.5 | 0.12 | 1.55 | 0.65 | 0.08 | 7 | 8.64 | 1.23 | 109 | 8.3245 | ||
| DO | 5 | 0.2 | 1.55 | 0.65 | 0.13 | 14.6 | 5.25 | 0.36 | 97.4 | 12.607 | ||
| TDS | 500 | 0 | 1.55 | 0.65 | 0 | 0 | 603 | 1.21 | 121 | 0.1561 | ||
| EC | 300 | 0 | 1.55 | 0.65 | 0 | 0 | 872 | 2.91 | 291 | 0.6272 | ||
| Alkalinity | 120 | 0.01 | 1.55 | 0.65 | 0.01 | 0 | 269 | 2.25 | 225 | 1.211 | ||
| Na+ | 200 | 0.01 | 1.55 | 0.65 | 0 | 0 | 77 | 0.39 | 38.5 | 0.1246 | ||
| K+ | 55 | 0.02 | 1.55 | 0.65 | 0.01 | 0 | 9.01 | 0.16 | 16.4 | 0.1928 | ||
| Ca+ | 75 | 0.01 | 1.55 | 0.65 | 0.01 | 0 | 32 | 0.43 | 42.7 | 0.3684 | ||
| Mg+ | 30 | 0.03 | 1.55 | 0.65 | 0.02 | 0 | 18.1 | 0.6 | 60.2 | 1.298 | ||
| F− | 1 | 1 | 1.55 | 0.65 | 0.65 | 0 | 0.4 | 0.4 | 40 | 25.887 | ||
| Cl− | 250 | 0 | 1.55 | 0.65 | 0 | 0 | 16.7 | 0.07 | 6.68 | 0.0173 | ||
| NO42− | 20 | 0.05 | 1.55 | 0.65 | 0.03 | 0 | 3.4 | 0.17 | 17 | 0.5501 | ||
| SO42− | 150 | 0.01 | 1.55 | 0.65 | 0 | 0 | 12.2 | 0.08 | 8.15 | 0.0351 | ||
| PO42− | 12 | 0.08 | 1.55 | 0.65 | 0.05 | 0 | 1.27 | 0.11 | 10.6 | 0.5708 | ||
| 1.55 | 1 | WQI | 51.97 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S3 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.6472 | 0.0761 | 7 | 8.72 | 1.2457 | 114.67 | 8.7306 | ||
| DO | 5 | 0.2 | 1.5452 | 0.6472 | 0.1294 | 14.6 | 7.62 | 0.5219 | 72.708 | 9.4111 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.6472 | 0.0013 | 0 | 335.17 | 0.6703 | 67.034 | 0.0868 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.6472 | 0.0022 | 0 | 516.33 | 1.7211 | 172.11 | 0.3713 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.6472 | 0.0054 | 0 | 182.4 | 1.52 | 152 | 0.8198 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.6472 | 0.0032 | 0 | 74.77 | 0 | 0 | 0 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.6472 | 0.0118 | 0 | 8.98 | 0.1633 | 16.327 | 0.1921 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.6472 | 0.0086 | 0 | 29.03 | 0.3871 | 38.707 | 0.334 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.6472 | 0.0216 | 0 | 18.02 | 0.6007 | 60.067 | 1.2958 | ||
| F− | 1 | 1 | 1.5452 | 0.6472 | 0.6472 | 0 | 0.13 | 0.13 | 13 | 8.4134 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.6472 | 0.0026 | 0 | 22.01 | 0.088 | 8.804 | 0.0228 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.6472 | 0.0324 | 0 | 3.25 | 0.1625 | 16.25 | 0.5258 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.6472 | 0.0043 | 0 | 3.25 | 0.0217 | 2.1667 | 0.0093 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.6472 | 0.0539 | 0 | 0.89 | 0.0742 | 7.4167 | 0.4 | ||
| 1.5452 | 1 | WQI | 30.613 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S4 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.6472 | 0.0761 | 7 | 8.65 | 1.2357 | 110 | 8.3753 | ||
| DO | 5 | 0.2 | 1.5452 | 0.6472 | 0.1294 | 14.6 | 8.82 | 0.6041 | 60.208 | 7.7931 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.6472 | 0.0013 | 0 | 328.54 | 0.6571 | 65.708 | 0.085 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.6472 | 0.0022 | 0 | 494.93 | 1.6498 | 164.98 | 0.3559 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.6472 | 0.0054 | 0 | 182.3 | 1.5192 | 151.92 | 0.8193 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.6472 | 0.0032 | 0 | 74.77 | 0 | 0 | 0 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.6472 | 0.0118 | 0 | 6.8 | 0.1236 | 12.364 | 0.1455 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.6472 | 0.0086 | 0 | 28.3 | 0.3773 | 37.733 | 0.3256 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.6472 | 0.0216 | 0 | 17.02 | 0.5673 | 56.733 | 1.2239 | ||
| F− | 1 | 1 | 1.5452 | 0.6472 | 0.6472 | 0 | 0.3 | 0.3 | 30 | 19.415 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.6472 | 0.0026 | 0 | 19.12 | 0.0765 | 7.648 | 0.0198 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.6472 | 0.0324 | 0 | 3.22 | 0.161 | 16.1 | 0.521 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.6472 | 0.0043 | 0 | 3.15 | 0.021 | 2.1 | 0.0091 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.6472 | 0.0539 | 0 | 0.75 | 0.0625 | 6.25 | 0.3371 | ||
| 1.545 | 1 | WQI | 39.43 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S5 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.6472 | 0.0761 | 7 | 8.67 | 1.2386 | 111.33 | 8.4768 | ||
| DO | 5 | 0.2 | 1.5452 | 0.6472 | 0.1294 | 14.6 | 9.54 | 0.6534 | 52.708 | 6.8224 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.6472 | 0.0013 | 0 | 334 | 0.668 | 66.8 | 0.0865 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.6472 | 0.0022 | 0 | 344.57 | 1.1486 | 114.86 | 0.2478 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.6472 | 0.0054 | 0 | 180.4 | 1.5033 | 150.33 | 0.8108 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.6472 | 0.0032 | 0 | 40.77 | 0.2039 | 20.385 | 0.066 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.6472 | 0.0118 | 0 | 6.91 | 0.1256 | 12.564 | 0.1478 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.6472 | 0.0086 | 0 | 26.4 | 0.352 | 35.2 | 0.3037 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.6472 | 0.0216 | 0 | 17.64 | 0.588 | 58.8 | 1.2685 | ||
| F− | 1 | 1 | 1.5452 | 0.6472 | 0.6472 | 0 | 0.39 | 0.39 | 39 | 25.24 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.6472 | 0.0026 | 0 | 21.21 | 0.0848 | 8.484 | 0.022 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.6472 | 0.0324 | 0 | 3.29 | 0.1645 | 16.45 | 0.5323 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.6472 | 0.0043 | 0 | 3.35 | 0.0223 | 2.2333 | 0.0096 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.6472 | 0.0539 | 0 | 0.67 | 0.0558 | 5.5833 | 0.3011 | ||
| 1.545 | 1 | WQI | 44.34 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S6 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.6472 | 0.0761 | 7 | 8.75 | 1.25 | 116.67 | 8.8829 | ||
| DO | 5 | 0.2 | 1.5452 | 0.6472 | 0.1294 | 14.6 | 8.5 | 0.5822 | 63.542 | 8.2246 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.6472 | 0.0013 | 0 | 340.43 | 0.6809 | 68.086 | 0.0881 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.6472 | 0.0022 | 0 | 520.33 | 1.7344 | 173.44 | 0.3742 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.6472 | 0.0054 | 0 | 175.4 | 1.4617 | 146.17 | 0.7883 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.6472 | 0.0032 | 0 | 74.76 | 0 | 0 | 0 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.6472 | 0.0118 | 0 | 5.91 | 0.1075 | 10.745 | 0.1264 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.6472 | 0.0086 | 0 | 35.3 | 0.4707 | 47.067 | 0.4061 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.6472 | 0.0216 | 0 | 15.02 | 0.5007 | 50.067 | 1.0801 | ||
| F− | 1 | 1 | 1.5452 | 0.6472 | 0.6472 | 0 | 0.125 | 0.125 | 12.5 | 8.0898 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.6472 | 0.0026 | 0 | 63.03 | 0.2521 | 25.212 | 0.0653 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.6472 | 0.0324 | 0 | 3.35 | 0.1675 | 16.75 | 0.542 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.6472 | 0.0043 | 0 | 3.4 | 0.0227 | 2.2667 | 0.0098 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.6472 | 0.0539 | 0 | 0.7 | 0.0583 | 5.8333 | 0.3146 | ||
| 1.545 | 1 | WQI | 28.99 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S7 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.64718 | 0.0761 | 7 | 8.77 | 1.2529 | 118 | 8.9844 | ||
| DO | 5 | 0.2 | 1.5452 | 0.64718 | 0.1294 | 14.6 | 7.2 | 0.4932 | 77.08333 | 9.9774 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.64718 | 0.0013 | 0 | 326.4 | 0.6528 | 65.28 | 0.0845 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.64718 | 0.0022 | 0 | 503 | 1.6767 | 167.6667 | 0.3617 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.64718 | 0.0054 | 0 | 176.44 | 1.4703 | 147.0333 | 0.793 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.64718 | 0.0032 | 0 | 66.38 | 0 | 0 | 0 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.64718 | 0.0118 | 0 | 0.31 | 0.0056 | 0.563636 | 0.0066 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.64718 | 0.0086 | 0 | 28.5 | 0.38 | 38 | 0.3279 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.64718 | 0.0216 | 0 | 16.1 | 0.5367 | 53.66667 | 1.1577 | ||
| F− | 1 | 1 | 1.5452 | 0.64718 | 0.6472 | 0 | 0.285 | 0.285 | 28.5 | 18.445 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.64718 | 0.0026 | 0 | 22.01 | 0.088 | 8.804 | 0.0228 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.64718 | 0.0324 | 0 | 3.25 | 0.1625 | 16.25 | 0.5258 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.64718 | 0.0043 | 0 | 3.31 | 0.0221 | 2.206667 | 0.0095 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.64718 | 0.0539 | 0 | 1.01 | 0.0842 | 8.416667 | 0.4539 | ||
| 1.545 | 1 | WQI | 41.15 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| S8 | ||||||||||||
| Ph | 8.5 | 0.1176 | 1.5452 | 0.6472 | 0.0761 | 7 | 8.69 | 1.2414 | 112.6667 | 8.5783 | ||
| DO | 5 | 0.2 | 1.5452 | 0.6472 | 0.1294 | 14.6 | 6.5 | 0.4452 | 84.375 | 10.921 | ||
| TDS | 500 | 0.002 | 1.5452 | 0.6472 | 0.0013 | 0 | 466.75 | 0.9335 | 93.35 | 0.1208 | ||
| EC | 300 | 0.0033 | 1.5452 | 0.6472 | 0.0022 | 0 | 718.88 | 2.3963 | 239.6267 | 0.5169 | ||
| Alkalinity | 120 | 0.0083 | 1.5452 | 0.6472 | 0.0054 | 0 | 182.4 | 1.52 | 152 | 0.8198 | ||
| Na+ | 200 | 0.005 | 1.5452 | 0.6472 | 0.0032 | 0 | 42.77 | 0.2139 | 21.385 | 0.0692 | ||
| K+ | 55 | 0.0182 | 1.5452 | 0.6472 | 0.0118 | 0 | 5.91 | 0.1075 | 10.74545 | 0.1264 | ||
| Ca+ | 75 | 0.0133 | 1.5452 | 0.6472 | 0.0086 | 0 | 18.3 | 0.244 | 24.4 | 0.2105 | ||
| Mg+ | 30 | 0.0333 | 1.5452 | 0.6472 | 0.0216 | 0 | 10.21 | 0.3403 | 34.03333 | 0.7342 | ||
| F− | 1 | 1 | 1.5452 | 0.6472 | 0.6472 | 0 | 0.54 | 0.54 | 54 | 34.948 | ||
| Cl− | 250 | 0.004 | 1.5452 | 0.6472 | 0.0026 | 0 | 66.02 | 0.2641 | 26.408 | 0.0684 | ||
| NO42− | 20 | 0.05 | 1.5452 | 0.6472 | 0.0324 | 0 | 3.04 | 0.152 | 15.2 | 0.4919 | ||
| SO42− | 150 | 0.0067 | 1.5452 | 0.6472 | 0.0043 | 0 | 3.05 | 0.0203 | 2.033333 | 0.0088 | ||
| PO42− | 12 | 0.0833 | 1.5452 | 0.6472 | 0.0539 | 0 | 1.56 | 0.13 | 13 | 0.7011 | ||
| 1.545 | 1 | WQI | 58.32 | |||||||||
| WQI | |||
|---|---|---|---|
| Site | Value | Employing the scale indicated by (Ramakrishnaiah et al. 2009) | Employing the scale indicated by (Brown et al. 2010) |
| Sobatiyabagh (S1) | 28.27 | Excellent | Good |
| Draupadi Ghat (S2) | 51.97 | Good | Poor |
| Rasoolabad (S3) | 30.61 | Excellent | Good |
| Daraganj (S4) | 39.43 | Excellent | Good |
| Prior to Sangam (S5) | 44.27 | Excellent | Good |
| Sangam (S6) | 28.99 | Excellent | Good |
| Chatnag Ghat (S7) | 41.15 | Excellent | Good |
| Yamuna-Arail Kachar (S8) | 66.98 | Good | Poor |
| Water quality | Employing the scale indicated by (Ramakrishnaiah et al. 2009) | Employing the scale indicated by (Brown et al. 2010) |
|---|---|---|
| Excellent | <50 | 0–25 |
| Good | 50–100 | 26–50 |
| Poor | 100–200 | 51–75 |
| Very poor | 200–300 | 76–100 |
| Unsuitable | >300 | Above 100 |
3.6 Heavy metal analysis
Table 7 and Fig. 15 and displays the levels of heavy metal contamination Cr, Mn, discharge Fe, Cd, Pb, and Cu in river water from the Ganga Basin and Yamuna at all the 8 sample locations. Wherever the Ganga and Yamuna rivers received wastewater from Sewage, municipality, and many industries, there was a higher quantity of heavy metals was found. The lithological impacts, hydrological processes, distinct man-made sources, and existing vegetation might all be contributing factors in the amounts of distinct heavy metals.46Cadmium: throughout the whole Ganga and Yamuna study area, the content of cadmium was found to be higher than the allowable level. Site 4, or Daraganj, has the highest concentration of Cd enrichment. Here, municipal wastewater and sewage disposal plants may have been the source of the Cd. Additionally, several minor businesses like those that produce engineering and electrical items, textiles, and chemicals, as well as those that are located along the Yamuna rivers across the region, may be responsible for the region's high concentration. Cd causes kidney and liver disorders, if the level of Cd content exceeded BIS guidelines, which poses risks to human health.46
Chromium: in all eight sites under investigation, the concentration of Cr exceeded the allowable limit. Approximately 90% of all samples had high Cr contents, above the allowed limit of.05 ppm, particularly at site S4. Cr is regarded as a particular contaminant that shows signs of contamination from industries such as metal surface processing, semiconductor packing, and electroplating.46 It has been demonstrated that human activity caused a high concentration of Cr (0.34) in the Yamuna rivers at site S8. Comparable results have been seen in the water of the Ganga rivers.46 They discovered that the concentration of Cr in over 55% of the sample was higher than allowed. People who live near the Ganga and Yamuna rivers at a risk due to long-term exposure to contaminated water through ingestion can cause major health problems such as kidney and liver damage, stomach ulcers, and lung cancer, gall bladder cancer due to the higher-than-average percentage of Cr in the water. It may affect the general health of aquatic ecosystems, interfere with reproductive processes, and result in genetic mutation.47
Copper: there were significant swings in the content of copper in almost all the sites. Site S4 has shown the greatest concentration, which may be related to the region's operations for the paper, textile, and shoe industries. Yamuna site S8,48 also showed the same results, which has been linked to discharges from the pulp, and electroplating manufacturing unit. Even though practically all living things require copper, a high quantity of the metal is regarded as a pollution. Abdominal pain, nausea, and vomiting can result from consuming water containing high amounts of copper. Long-term exposure can harm the kidneys and liver and the central nervous system may be impacted. It may play a role in the emergence of persistent illnesses like Wilson's disease. Through the food chain, copper can build up in aquatic organisms and have harmful effects.47
Lead: range of Pb(lead) lables varies between 0.44 to 0.62 ppm in the various segments under study, surpassing the permissible limit of BIS.40 High Pb content in site S5 Rasoolabad may be caused by nearby businesses such footwear, pharmacy, and tanneries as well as e-waste and lead battery-based units' effluents.46 Present research also revealed high lead content in the Yamuna rivers at site S8 (0.62 ppm). Finally it was concluded that roughly 65% of water samples from the Ganga rivers basin found to have beyond the WHO and BIS approved limits. Drinking water tainted with high lead concentrations can have detrimental effects on aquatic life as well as people. Lead is a hazardous heavy metal that can harm the central nervous system, impair cognitive function, cause cardiovascular disease in adults, damage the kidneys, and cause anemia. Aquatic organisms that accumulate lead may experience reduced growth, reproduction, and survival rates, as well as disturbances to their physiological processes.49
Manganese: the significant variations in manganese (Mn) concentration in the surface water of Yamuna rivers particularly in the downstream sites S2 and S4 compare to other sites in the Ganga Region indicates a potential issue with pollution and industrial activities in the Yamuna rivers basin, meanwhile the increased concentration of Mn at Arail Kachar(0.56 ppm) in the Yamuna rivers region might be attributed to a number of enterprises, effluents, and municipalities waste from small scale industrial units. Human health can suffer grave consequences from excessive manganese in contaminated water, including inhaling manganese dust or fumes is linked to respiratory issues, which can result in hepatic dysfunction. Prolonged exposure to high manganese levels has been linked to neurotoxicity and sabotage aquatic environments.47
Iron: all the samples in this investigation had iron contents over the BIS allowable level. However site S5 of Ganga Region exhibited maximum concentration of Fe which may be due to run-off from soil and human sources. Additionally, it was33 discovered that roughly 67% of the samples in the Ganga rivers basin had higher Fe concentrations. With some exceptions like site S8 of Yamuna rivers basin where the concentration of iron, which is detected as 0.789 ppm, greater than that of the Ganga rivers in the current research. The high content of Fe in the soil may be the cause of these elevated Fe readings, however anthropogenic Fe sources from steel and metal companies' effluents cannot be completely ruled out. When water is utilized for domestic purposes, the high iron concentration can cause corrosion of supply line pipes and the release of oxide strains on sanitary goods and laundry. The effects of drinking water contaminated with high levels of iron can vary in what happens to humans and aquatic life. Gastrointestinal issues like nausea, vomiting, and stomach discomfort can be brought on by high iron content in water. Iron overload illnesses such hemochromatosis, liver damage, and heart disease may be exacerbated by prolonged exposure to high iron levels in drinking water. Elevated iron levels can be hazardous to aquatic creatures, which can have an impact on their growth, reproduction, and ability to survive. In severe circumstances, fish deaths may result from an abrupt rise in iron concentration.47
Zinc: the main sources of zinc in rivers water may be the various electroplating and brass production businesses as well as the agrochemical industries, which include the fertilizer and pesticide sectors50 However, it was discovered that the Zn content throughout the whole Ganga and Yamuna rivers stretch, from its source to the site of confluence, was below the allowable limits. However overconsumption of zinc in water can have negative impacts on aquatic life as well as human health. It's crucial to remember that zinc is a trace element that is necessary for many physiological functions, but prolonged exposure may weaken the immune system and effects on the neurological system, such as impaired cognitive function and nerve damage. It may pose also impacts on aquatic creatures like fish, crustaceans, and algae. Fish deaths and disturbances to the aquatic environment may result from this ref. 46 also prevalence of contaminants such as nutrients, heavy metals, and emerging pollutants in wastewater, underscoring the ongoing challenges these pose to water quality and environmental health.51
This expanded discussion integrates geological, pedological, and site-specific anthropogenic sources to comprehensively understand the factors shaping water quality and heavy metal indices in the Ganga and Yamuna basins.
| Location | Fe | SD | Mn | SD | Cr | SD | Zn | SD | Pb | SD | Cu | SD | Cd | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sobatiya Bagh (S1) | 0.74 | 0.06 | 0.47 | 0.04 | 0.31 | 0.16 | 4.03 | 1.25 | 0.54 | 0.02 | 2.04 | 0.47 | 0.48 | 0.14 |
| Draupadi Ghat (S2) | 0.64 | 0.05 | 0.43 | 0.03 | 0.32 | 0.18 | 4.25 | 1.17 | 0.44 | 0.03 | 1.98 | 0.48 | 0.45 | 0.18 |
| Rasoolabad (S3) | 0.75 | 0.04 | 0.44 | 0.05 | 0.33 | 0.16 | 4.5 | 1.16 | 0.58 | 0.04 | 2.22 | 0.48 | 0.48 | 0.16 |
| Daraganj (S4) | 0.75 | 0.06 | 0.51 | 0.04 | 0.42 | 0.27 | 4.94 | 1.10 | 0.53 | 0.02 | 2.32 | 0.62 | 0.56 | 0.19 |
| Prior to Sangam (S5) | 0.64 | 0.02 | 0.34 | 0.04 | 0.32 | 0.25 | 5 | 1.09 | 0.6 | 0.04 | 2.01 | 0.53 | 0.47 | 0.15 |
| Sangam (S6) | 0.73 | 0.07 | 0.4 | 0.02 | 0.4 | 0.26 | 5.25 | 0.86 | 0.54 | 0.01 | 2.03 | 0.49 | 0.48 | 0.12 |
| Chatnag Ghat (S7) | 0.7 | 0.03 | 0.37 | 0.05 | 0.32 | 0.14 | 5.05 | 1.00 | 0.5 | 0.04 | 2.04 | 0.28 | 0.44 | 0.14 |
| Yamuna-Arail Ghat (S8) | 0.79 | 0.06 | 0.49 | 0.02 | 0.34 | 0.15 | 5.42 | 1.05 | 0.62 | 0.05 | 2.52 | 0.44 | 0.68 | 0.17 |
| Permissible limit (PL)in ppm | Fe(PL) = 0.3 | Mn(PL) = 0.1 | Cr(PL) = 0.05 | Zn(PL) = 5 | Pb(PL) = 0.05 | Cu(PL) = 0.05 | Cd(PL) = 0 | |||||||
| M | Mc | Sc | Wc(1/Sc) | Qc(Mc/Sc × 100) | Wc × Qc | HMCI | |
|---|---|---|---|---|---|---|---|
| S1 | |||||||
| Cu | 2.04 | 1 | 1.0000 | 204.0000 | 204.000000 | 806.01828 | |
| Pb | 0.54 | 0.05 | 20.0000 | 1080.0000 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.000000 600.000000 |
||
| Zn | 4.03 | 5 | 0.2000 | 80.6000 | 16.120000 | ||
| Fe | 0.744 | 1 | 1.0000 | 74.4000 | 74.400000 | ||
| Mn | 0.47 | 0.1 | 10.0000 | 470.0000 | 4700.000000 | ||
| Cd | 0.48 | 0.05 | 20.0000 | 960.0000 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| Cr | 0.31 | 0.05 | 20.0000 | 620.0000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400.00000 400.00000 |
||
| 8.614 | 7.25 | 72.2000 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 194.520000 194.520000 |
||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S2 | |||||||
| Cu | 1.98 | 1 | 1.0000 | 198.0000 | 198.000000 | 733.78393 | |
| Pb | 0.44 | 0.05 | 20.0000 | 880.0000 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.000000 600.000000 |
||
| Zn | 4.25 | 5 | 0.2000 | 85.0000 | 17.000000 | ||
| Fe | 0.642 | 1 | 1.0000 | 64.2000 | 64.200000 | ||
| Mn | 0.43 | 0.1 | 10.0000 | 430.0000 | 4300.000000 | ||
| Cd | 0.45 | 0.05 | 20.0000 | 900.0000 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.000000 000.000000 |
||
| Cr | 0.32 | 0.05 | 20.0000 | 640.0000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S3 | |||||||
| Cu | 2.22 | 1 | 1.0000 | 222.0000 | 222.000000 | 835.38227 | |
| Pb | 0.58 | 0.05 | 20.0000 | 1160.0000 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| Zn | 4.5 | 5 | 0.2000 | 90.0000 | 18.000000 | ||
| Fe | 0.746 | 1 | 1.0000 | 74.6000 | 74.600000 | ||
| Mn | 0.44 | 0.1 | 10.0000 | 440.0000 | 4400.000000 | ||
| Cd | 0.48 | 0.05 | 20.0000 | 960.0000 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| Cr | 0.33 | 0.05 | 20.0000 | 660.0000 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| 72.2000 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 314.600000 314.600000 |
||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S4 | |||||||
| Cu | 2.32 | 1 | 1.0000 | 232.0000 | 232.000000 | 911.72798 | |
| Pb | 0.53 | 0.05 | 20.0000 | 1060.0000 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| Zn | 4.94 | 5 | 0.2000 | 98.8000 | 19.760000 | ||
| Fe | 0.75 | 1 | 1.0000 | 75.0000 | 75.000000 | ||
| Mn | 0.51 | 0.1 | 10.0000 | 510.0000 | 5100.000000 | ||
| Cd | 0.56 | 0.05 | 20.0000 | 1120.0000 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400.000000 400.000000 |
||
| Cr | 0.42 | 0.05 | 20.0000 | 840.0000 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
| 72.2000 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 826.760000 826.760000 |
||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S5 | |||||||
| Cu | 2.01 | 1 | 1.0000 | 201.0000 | 201.000000 | 821.12604 | |
| Pb | 0.6 | 0.05 | 20.0000 | 1200.0000 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.000000 000.000000 |
||
| Zn | 5 | 5 | 0.2000 | 100.0000 | 20.000000 | ||
| Fe | 0.643 | 1 | 1.0000 | 64.3000 | 64.300000 | ||
| Mn | 0.34 | 0.1 | 10.0000 | 340.0000 | 3400.000000 | ||
| Cd | 0.47 | 0.05 | 20.0000 | 940.0000 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
| Cr | 0.32 | 0.05 | 20.0000 | 640.0000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
| 72.2000 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 285.300000 285.300000 |
||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S6 | |||||||
| Cu | 2.03 | 1 | 1.0000 | 203.0000 | 203.000000 | 857.30471 | |
| Pb | 0.54 | 0.05 | 20.0000 | 1080.0000 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.000000 600.000000 |
||
| Zn | 5.25 | 5 | 0.2000 | 105.0000 | 21.000000 | ||
| Fe | 0.734 | 1 | 1.0000 | 73.4000 | 73.400000 | ||
| Mn | 0.4 | 0.1 | 10.0000 | 400.0000 | 4000.000000 | ||
| Cd | 0.48 | 0.05 | 20.0000 | 960.0000 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| Cr | 0.42 | 0.05 | 20.0000 | 840.0000 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
| 72.2000 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 897.400000 897.400000 |
||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S7 | |||||||
| Cu | 2.04 | 1 | 1.0000 | 204.0000 | 204.000000 | 753.38504 | |
| Pb | 0.5 | 0.05 | 20.0000 | 1000.0000 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.000000 000.000000 |
||
| Zn | 5.05 | 5 | 0.2000 | 101.0000 | 20.200000 | ||
| Fe | 0.702 | 1 | 1.0000 | 70.2000 | 70.200000 | ||
| Mn | 0.37 | 0.1 | 10.0000 | 370.0000 | 3700.000000 | ||
| Cd | 0.44 | 0.05 | 20.0000 | 880.0000 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.000000 600.000000 |
||
| Cr | 0.32 | 0.05 | 20.0000 | 640.0000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
| 72.2000 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 394.400000 394.400000 |
||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S8 | |||||||
| Cu | 2.52 | 1 | 1.0000 | 252.0000 | 252.000000 | 981.33767 | |
| Pb | 0.62 | 0.05 | 20.0000 | 1240.0000 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.000000 800.000000 |
||
| Zn | 5.42 | 5 | 0.2000 | 108.4000 | 21.680000 | ||
| Fe | 0.789 | 1 | 1.0000 | 78.9000 | 78.900000 | ||
| Mn | 0.49 | 0.1 | 10.0000 | 490.0000 | 4900.000000 | ||
| Cd | 0.68 | 0.05 | 20.0000 | 1360.0000 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.000000 200.000000 |
||
| Cr | 0.34 | 0.05 | 20.0000 | 680.0000 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.000000 600.000000 |
||
| 72.2000 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 852.580000 852.580000 |
||||||
| HMCI of 8 sites | |||
|---|---|---|---|
| Location | HMCI | Result | Reference |
| Sobatiyabagh (S1) | 806.01 | High | 36 |
| Draupadi Ghat (S2) | 733.78 | High | |
| Rasoolabad (S3) | 753.38 | High | |
| Daraganj (S4) | 911.7 | High | |
| Prior to Sangam (S5) | 821.12 | High | |
| Sangam (S6) | 857.3 | High | |
| Chatnag (S7) | 753.38 | High | |
| Yamuna-Arail Kachar (S8) | 981.33 | High | |
| Location | HMQI |
|---|---|
| Sobatiyabagh (S1) | 34.89 |
| Draupadi Ghat (S2) | 31.97 |
| Rasoolabad (S3) | 36.06 |
| Daraganj (S4) | 39.35 |
| Prior to Sangam (S5) | 34.85 |
| Sangam (S6) | 36.61 |
| Chatnag Ghat (S7) | 32.65 |
| Yamuna-Arail Kachar (S8) | 42.09 |
3.7 Human health risk assessment
Assessment of human health risk comprises the estimation of the nature and degree of adverse health effects in humans due to exposure to toxic substances. In the present study health risks assessment through ingestion rivers water were done following the protocol as outlined by the USEPA (2004).| Daily intake matel | |||||||
|---|---|---|---|---|---|---|---|
| Location | Fe | Mn | Cr | Zn | Pb | Cu | Cd |
| Sobatiya Bagh (S1) | 0.0248 | 0.01567 | 0.01033 | 0.13433 | 0.018 | 0.068 | 0.016 |
| Draupadi Ghat (S2) | 0.0214 | 0.01433 | 0.01067 | 0.14167 | 0.01467 | 0.066 | 0.015 |
| Rasoolabad (S3) | 0.02487 | 0.01467 | 0.01067 | 0.15 | 0.01933 | 0.074 | 0.016 |
| Daraganj (S4) | 0.025 | 0.017 | 0.01067 | 0.16467 | 0.01767 | 0.07733 | 0.01867 |
| Prior to Sangam (S5) | 0.02143 | 0.01133 | 0.01067 | 0.16667 | 0.02 | 0.067 | 0.01567 |
| Sangam (S6) | 0.02447 | 0.01333 | 0.01067 | 0.175 | 0.018 | 0.06767 | 0.016 |
| Chatnag Ghat (S7) | 0.0234 | 0.01233 | 0.01067 | 0.16833 | 0.01667 | 0.068 | 0.01467 |
| Yamuna-Arail Ghat (S8) | 0.0263 | 0.01633 | 0.01133 | 0.18067 | 0.02067 | 0.084 | 0.02267 |
| Location | Health risk index | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Mn | Cr | Zn | Pb | Cu | Cd | |
| Sobatiya Bagh (S1) | 0.08267 | 0.31333 | 0.10333 | 0.02687 | 1.2 | 0.05231 | 3.2 |
| Draupadi Ghat (S2) | 0.07133 | 0.28667 | 0.10667 | 0.02833 | 0.97778 | 0.05077 | 3 |
| Rasoolabad (S3) | 0.08289 | 0.29333 | 0.10667 | 0.03 | 1.28889 | 0.05692 | 3.2 |
| Daraganj (S4) | 0.08333 | 0.34 | 0.10667 | 0.03293 | 1.17778 | 0.05949 | 3.733 |
| Prior to Sangam (S5) | 0.07144 | 0.22667 | 0.10667 | 0.03333 | 1.33333 | 0.05154 | 3.1333 |
| Sangam (S6) | 0.08156 | 0.26667 | 0.10667 | 0.035 | 1.2 | 0.05205 | 3.2 |
| Chatnag Ghat (S7) | 0.078 | 0.24667 | 0.10667 | 0.03367 | 1.11111 | 0.05231 | 2.9333 |
| Yamuna-Arail Ghat (S8) | 0.08767 | 0.32667 | 0.11333 | 0.03613 | 1.37778 | 0.06462 | 4.5333 |
4 Water parameter correlation
4.1 Correlation of water parameters with WQI
Table 12 and Fig. 19 displays the Pearson correlation matrix for several factors along with WQI. The results showed a substantial positive correlation between dissolved oxygen and pH, suggesting that pH may possibly be a component of the increased alkaline medium in water that facilitates oxygen solubility. Raising the pH in a reasonable way within predetermined bounds may prevent the growth of bacteria that sustain high DO levels. Because of the somewhat low pH of the water in Draupadi Ghat, which encourages bacterial enrichment, the amount of DO was found to be extremely low there. Furthermore, we found a significant positive correlation between alkalinity and SO42−. A possible explanation for this connection is that alkalinity is formed when bacteria that decrease sulfur convert sulfurate ions into bicarbonate ions. Moreover, it was noted that chemical reactions that reduce sulphate are the means by which alkalinity is formed. Phosphate also shows a positive correlation with alkalinity, which shows that a higher level of sediment increases alkalinity. Ca2+ association With Na+, it may be indicated that there are salts of Na+ and Mg2+ ions in the water as a result of common natural weathering sources and human activities, both of which raise the water's hardness. The outcomes of our research indicate that there is a substantial negative association between pH and TDS, which implies that the growing TDS in water is mostly caused by organic matter, Cl−, SO42−, and NO3− anions. An even stronger negative association between DO and SO42− was discovered, which might indicate that elevated SO42− ion and carbonate salt concentrations impede oxygen solubility. The majority of TDS is made up of hydroxide, carbonate, and bicarbonate ions, as well as chloride, sulfate, and nitrate anions at all locations, according to TDS readings, which also show strong positive connections with alkalinity, EC, and Na+. Anions and cations, particularly K+, NO3−, SO42−, and alkalinity as a physical component, were discovered to be the controlling variables for all other water quality metrics in river water.| Para | WQI | Ph | DO | TDS | EC | Alkalinity | Na+ | K+ | Ca+ | Mg+ | F− | Cl− | NO42− | SO42− | PO42− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a *Correlation is significant at 0.05 level/P corr.-Pearson coefficient/2 tailed test of significance is used. | ||||||||||||||||
| WQI | P corr. | 1 | −0.43066 | −0.61661 | 0.66071 | 0.5657 | 0.35743 | −0.3565 | 0.05629 | −0.44364 | −0.36468 | 0.97093* | 0.28964 | −0.49512 | 0.09615 | 0.81827* |
| p-Value | — | 0.2868 | 0.10348 | 0.07449 | 0.14388 | 0.3847 | 0.38604 | 0.89468 | 0.27088 | 0.37443 | 6.01 × 10−5 | 0.48654 | 0.21221 | 0.82083 | 0.01303 | |
| Ph | P corr. | −0.4307 | 1 | 0.70161 | −0.88244* | −0.74335* | −0.97793* | −0.3777 | −0.5177 | −0.35158 | −0.41035 | −0.4299 | 0.27444 | −0.47494 | −0.76303* | −0.39273 |
| p-Value | 0.2868 | — | 0.05244 | 0.00371 | 0.03455 | 2.64 × 10−5 | 0.3563 | 0.18882 | 0.39309 | 0.3126 | 0.28775 | 0.51068 | 0.23434 | 0.02763 | 0.33585 | |
| DO | P corr. | −0.6166 | 0.70161 | 1 | −0.82942* | −0.88212* | −0.70695* | −0.3467 | −0.1422 | −0.11338 | 0.00661 | −0.4802 | −0.10511 | −0.0034 | −0.45026 | −0.8094* |
| p-Value | 0.10348 | 0.05244 | — | 0.01088 | 0.00374 | 0.0499 | 0.40021 | 0.73689 | 0.78923 | 0.9876 | 0.22846 | 0.80437 | 0.99363 | 0.26293 | 0.01493 | |
| TDS | P corr. | 0.66071 | −0.88244* | −0.82942* | 1 | 0.92946* | 0.89609* | 0.1202 | 0.41433 | 0.01877 | −0.04307 | 0.58216 | 0.0608 | 0.14497 | 0.73857* | 0.73821* |
| p-Value | 0.07449 | 0.00371 | 0.01088 | — | 8.32 × 10−4 | 0.00259 | 0.77679 | 0.30747 | 0.96481 | 0.91935 | 0.13 | 0.88628 | 0.73196 | 0.03637 | 0.03651 | |
| EC | P corr. | 0.5657 | −0.74335* | −0.88212* | 0.92946* | 1 | 0.78855* | 0.26246 | 0.29677 | −0.00375 | −0.197 | 0.42248 | 0.17217 | 0.02335 | 0.67552 | 0.81125* |
| p-Value | 0.14388 | 0.03455 | 0.00374 | 8.32 × 10−4 | — | 0.02005 | 0.53003 | 0.47536 | 0.99297 | 0.64007 | 0.29706 | 0.68351 | 0.95624 | 0.06597 | 0.01452 | |
| Alka | P corr. | 0.35743 | −0.97793* | −0.70695* | 0.89609* | 0.78855* | 1 | 0.35855 | 0.47683 | 0.30144 | 0.3515 | 0.33448 | −0.30864 | 0.50104 | 0.87193* | 0.42316 |
| p-Value | 0.3847 | 2.64 × 10−5 | 0.0499 | 0.00259 | 0.02005 | — | 0.38312 | 0.23222 | 0.46811 | 0.39322 | 0.41806 | 0.45699 | 0.20594 | 0.00476 | 0.29621 | |
| Na+ | P corr. | −0.3565 | −0.37769 | −0.34666 | 0.1202 | 0.26246 | 0.35855 | 1 | 0.21117 | 0.8101* | 0.57681 | −0.4102 | −0.20333 | 0.53518 | 0.18426 | −0.1774 |
| p-Value | 0.38604 | 0.3563 | 0.40021 | 0.77679 | 0.53003 | 0.38312 | — | 0.61567 | 0.01477 | 0.13442 | 0.31279 | 0.62914 | 0.17168 | 0.66225 | 0.67429 | |
| K+ | P corr. | 0.05629 | −0.51769 | −0.14223 | 0.41433 | 0.29677 | 0.47683 | 0.21117 | 1 | 0.17741 | 0.33392 | 0.0475 | −0.09049 | 0.22991 | 0.32542 | 0.011 |
| p-Value | 0.89468 | 0.18882 | 0.73689 | 0.30747 | 0.47536 | 0.23222 | 0.61567 | — | 0.67427 | 0.41888 | 0.91108 | 0.83125 | 0.58387 | 0.43155 | 0.97938 | |
| Ca+ | P corr. | −0.4436 | −0.35158 | −0.11338 | 0.01877 | −0.00375 | 0.30144 | 0.8101* | 0.17741 | 1 | 0.70578 | −0.41947 | −0.13869 | 0.81908* | 0.13732 | −0.46447 |
| p-Value | 0.27088 | 0.39309 | 0.78923 | 0.96481 | 0.99297 | 0.46811 | 0.01477 | 0.67427 | — | 0.05045 | 0.30088 | 0.74328 | 0.01287 | 0.74574 | 0.24627 | |
| Mg+ | PCorr. | −0.3647 | −0.41035 | 0.00661 | −0.04307 | −0.197 | 0.3515 | 0.57681 | 0.33392 | 0.70578 | 1 | −0.24806 | −0.71559* | 0.71443* | 0.17222 | −0.51902 |
| p-Value | 0.37443 | 0.3126 | 0.9876 | 0.91935 | 0.64007 | 0.39322 | 0.13442 | 0.41888 | 0.05045 | — | 0.55361 | 0.04594 | 0.04646 | 0.68342 | 0.18748 | |
| F− | P corr. | 0.97093* | −0.4299 | −0.4802 | 0.58216 | 0.42248 | 0.33448 | −0.4102 | 0.0475 | −0.41947 | −0.24806 | 1 | 0.16387 | −0.42939 | 0.06986 | 0.671 |
| p-Value | 6.01 × 10−5 | 0.28775 | 0.22846 | 0.13 | 0.29706 | 0.41806 | 0.31279 | 0.91108 | 0.30088 | 0.55361 | — | 0.6982 | 0.28838 | 0.86943 | 0.0685 | |
| Cl− | P corr. | 0.28964 | 0.27444 | −0.10511 | 0.0608 | 0.17217 | −0.30864 | −0.2033 | −0.0905 | −0.13869 | −0.71559* | 0.16387 | 1 | −0.41982 | −0.36706 | 0.32779 |
| p-Value | 0.48654 | 0.51068 | 0.80437 | 0.88628 | 0.68351 | 0.45699 | 0.62914 | 0.83125 | 0.74328 | 0.04594 | 0.6982 | — | 0.30044 | 0.37108 | 0.428 | |
| NO4−2 | P corr. | −0.4951 | −0.47494 | −0.0034 | 0.14497 | 0.02335 | 0.50104 | 0.53518 | 0.22991 | 0.81908* | 0.71443* | −0.42939 | −0.41982 | 1 | 0.53625 | −0.48435 |
| p-Value | 0.21221 | 0.23434 | 0.99363 | 0.73196 | 0.95624 | 0.20594 | 0.17168 | 0.58387 | 0.01287 | 0.04646 | 0.28838 | 0.30044 | — | 0.17066 | 0.22388 | |
| SO42− | P corr. | 0.09615 | −0.76303* | −0.45026 | 0.73857* | 0.67552 | 0.87193* | 0.18426 | 0.32542 | 0.13732 | 0.17222 | 0.06986 | −0.36706 | 0.53625 | 1 | 0.29923 |
| p-Value | 0.82083 | 0.02763 | 0.26293 | 0.03637 | 0.06597 | 0.00476 | 0.66225 | 0.43155 | 0.74574 | 0.68342 | 0.86943 | 0.37108 | 0.17066 | — | 0.47154 | |
| PO4−2 | P corr. | 0.81827* | −0.39273 | −0.8094* | 0.73821* | 0.81125* | 0.42316 | −0.1774 | 0.011 | −0.46447 | −0.51902 | 0.671 | 0.32779 | −0.48435 | 0.29923 | 1 |
| p-Value | 0.01303 | 0.33585 | 0.01493 | 0.03651 | 0.01452 | 0.29621 | 0.67492 | 0.97983 | 0.24627 | 0.18748 | 0.0685 | 0.428 | 0.22388 | 0.47154 | ||
4.2 Correlation among heavy metals
Table 13 and Fig. 20 Illustrate that there exist a positive correlation between heavy metals, with the exception of zinc and magnesium, where a negative correlation of p < 0.05 was noted. Moreover, while evaluating the heavy metal concentration in water in the Ganga and Yamuna rivers, despite all site-specific variations, the following order was established: Zn > Cu > Fe > Pb > Cd > Mn > Cr > Ni. Due to the high concentration of cities along this route, the rivers in these sections receives significant volumes of wastewater from several small-scale industries as well as municipal discharge effluents. Many manufacturing facilities, including those for packaging, plastics, tanneries, electroplating, and thermal power plants, were situated along this stretch. One possible explanation for the elevated concentration of heavy metals in this segment could be the municipal discharge from nearby residential and business sectors. Due to the heavy metals in the lower portions of the rivers, the water is generally highly contaminated. Due to anthropogenic activities, the Gang and Yamuna rivers water has a higher concentration of heavy metals than recommended by the BIS for drinking water quality. This could have an impact on the biological system by getting into the food chain.| Parameters | Fe | Mn | Cr | Zn | Pb | Ni | Cu | Cd | |
|---|---|---|---|---|---|---|---|---|---|
| a 2 Tailed test of significance is used/*correlation is significant in 0.05 level. | |||||||||
| Fe | Pearson corr. | 1 | 0.70712* | 0.37784 | 0.27583 | 0.48039 | 0.69591 | 0.76463* | 0.69353 |
| p-value | — | 0.04982 | 0.35609 | 0.50845 | 0.22826 | 0.05524 | 0.02712 | 0.05643 | |
| Mn | Pearson corr. | 0.70712* | 1 | 0.3661 | −0.15751 | 0.05741 | 0.76* | 0.69471 | 0.65616 |
| p-value | 0.04982 | — | 0.37243 | 0.70952 | 0.89259 | 0.02864 | 0.05584 | 0.07722 | |
| Cr | Pearson corr. | 0.37784 | 0.3661 | 1 | 0.46358 | 0.01499 | 0.06652 | 0.31655 | 0.30464 |
| p-value | 0.35609 | 0.37243 | — | 0.24729 | 0.9719 | 0.87565 | 0.44492 | 0.46316 | |
| Zn | Pearson corr. | 0.27583 | −0.15751 | 0.46358 | 1 | 0.46947 | 0.11683 | 0.45578 | 0.508 |
| p-value | 0.50845 | 0.70952 | 0.24729 | — | 0.24053 | 0.78292 | 0.25639 | 0.19868 | |
| Pb | Pearson corr. | 0.48039 | 0.05741 | 0.01499 | 0.46947 | 1 | 0.57891 | 0.56737 | 0.57762 |
| p-value | 0.22826 | 0.89259 | 0.9719 | 0.24053 | — | 0.13268 | 0.14243 | 0.13375 | |
| Ni | Pearson corr. | 0.69591 | 0.76* | 0.06652 | 0.11683 | 0.57891 | 1 | 0.91516* | 0.86289* |
| p-value | 0.05524 | 0.02864 | 0.87565 | 0.78292 | 0.13268 | — | 0.00143 | 0.0058 | |
| Cu | Pearson corr. | 0.76463* | 0.69471 | 0.31655 | 0.45578 | 0.56737 | 0.91516* | 1 | 0.9346* |
| p-value | 0.02712 | 0.05584 | 0.44492 | 0.25639 | 0.14243 | 0.00143 | — | 6.66 × 10−4 | |
| Cd | Pearson corr. | 0.69353 | 0.65616 | 0.30464 | 0.508 | 0.57762 | 0.86289* | 0.9346* | 1 |
| p-value | 0.05643 | 0.07722 | 0.46316 | 0.19868 | 0.13375 | 0.0058 | 6.66 × 10−4 | — | |
 | ||
| Fig. 20 Heat map showing Pearson's correlation various heavy metal concentrations in the Ganga and Yamuna river water. | ||
5. Conclusion
In this study, the water quality of the Ganga and Yamuna rivers was assessed over the period 2021–22 using various indices, including the Water Quality Index (WQI), Heavy Metal Contamination Index (HMCI), Heavy Metal Quality Index (HMQI), and Health Risk Index (HRI). To validate the findings, additional analyses—Land Use Land Cover (LULC), Principal Component Analysis (PCA), and Cluster Analysis (CA)—were conducted, all of which confirmed similar results, lending robustness to the conclusions.The study's findings indicate that water quality at key sites in both rivers is significantly below desirable standards. Specifically, the WQI value at the Ganga site S2 (Draupadi Ghat) was 51.97, while at the Yamuna site S8 (Arail Kachar), it was 66.98, both indicating poor quality. Analysis of heavy metal concentrations revealed even more concerning results: the HMCI values were 806.01 at the Ganga site S1 (Sobatiya Bagh) and 981.33 at the Yamuna site S8. These high HMCI values reflect significant levels of toxic metals, particularly lead (Pb) and cadmium (Cd), which are linked to heightened health risks for local populations. The HRI further corroborated this, showing increased health risks due to these metals at both Ganga site S1 and Yamuna site S8.
Moreover, the study identified elevated levels of specific anions and cations, such as Na+ and Cl− as primary contributors to pollution in these rivers. The deterioration in water quality is largely attributed to human activities, including agricultural runoff, water extraction for irrigation and drinking, washing clothes and utensils, sewage discharge, industrial effluents, and the improper disposal of municipal solid waste along riverbanks. Furthermore, religious practices, like those linked to Kumbh Mela, contribute to the pollution load.
These findings emphasize the urgent need for remediation to address heavy metal pollution and other contaminants that threaten water quality in the Ganga and Yamuna river basins. This study is crucial because both the Ganga and Yamuna support diverse flora and fauna, and understanding its water quality helps identify major causes of contamination and its impact on aquatic biota and overall ecosystem health. However, millions of people rely on these rivers for drinking, irrigation, irrigation and other daily activities, so monitoring their quality is essential to ensuring safety and preventing health hazards caused by the use of chlorinated water. At the same time, both rivers hold immense cultural and religious importance, like Kumbh Mela; therefore, their pollution can disrupt religious practices and cultural activities. Its water quality can also affect various sectors like agriculture, fisheries, and tourism, so it could lead to economic losses and affect the livelihoods of communities that directly depend on it.
Finally, the study comes to the conclusion that the rivers are greatly threatened by heavy metal pollution from nearby industries and that appropriate remediation measures must be taken to lower the metallurgical effluent load, stop further degradation of the river's water quality, and avert a catastrophe for human health. Results suggest that water filtration could be required for drinking and irrigation purposes for the inhabitants of the vicinity zone, like sailors and other members of the public, for their livelihood. This research also makes the important recommendation that Prayagraj's Ganga basin water be continuously monitored in order to identify the variables influencing pollution and how it affects water quality. Which helps develop global water control management programs.
Data availability
The necessary data used in the manuscript are present in the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors extend their Arabia, for funding this research work through the project number RSP2025R278.References
- B. Haxhibeqiri, F. Maloku and F. Brahushi, Physical-Chemical and Bacteriological Analysis of the River Drini I Bardhe, Eur. Sci. J., 2014, 3, 1857–7881 Search PubMed.
- G. Singh, N. Patel, T. Jindal, P. Srivastava and A. Bhowmik, Assessment of spatial and temporal variations in water quality by the application of multivariate statistical methods in the Kali River, Uttar Pradesh, India, Environ. Monit. Assess., 2020, 192, 394, DOI:10.1007/s10661-020-08307-0.
- S. Manoj Kumar and A. Shukdeo Prasad, Comparative Physico-Chemical Analysis of River Water and Underground Water in Winter Season of Rewa City, MP, India, Int. Res. J. Environ. Sci., 2014, 3, 59–61 Search PubMed.
- S. Azami-Aghdash, M. Ghojazadeh, F. Pournaghi Azar, M. Naghavi-Behzad, M. Mahmoudi and Z. Jamali, Fluoride concentration of drinking waters and prevalence of fluorosis in Iran: a systematic review, J. Dent. Res. Dent. Clin. Dent. Prospects, 2013, 7, 1–7, DOI:10.5681/joddd.2013.001.
- M. Ruhela, P. Kumar, V. Tyagi, F. Ahamad and K. Ram, Assessment of water quality of River Ganga at Haridwar with reference to Water Quality Index, Environ. Conserv. J., 2018, 19, 47–58, DOI:10.36953/ecj.2018.19306.
- R. Sarkar, A. R. Ghosh and N. K. Mondal, Comparative study on physicochemical status and diversity of macrophytes and zooplanktons of two urban ponds of Chandannagar, WB, India, Appl. Water Sci., 2020, 10, 1–8, DOI:10.1007/s13201-020-1146-y.
- CPCB, Water Quality Status of Yamuna River, Assessment and Development of River Basin, 2006, pp. 1–115 Search PubMed.
- P. W. Stackhouse, D. Westberg, J. M. Hoell, W. S. Chandler, T. Zhang, J. G. Arnold, J. R. Kiniry, R. Srinivasan, J. R. Williams, E. B. Haney, S. L. Neitsch, N. R. C. D. Moef, CPCB (Central Pollution Control Board), V. Tare, G. Roy, P. Bose, A. Desktop, O. Ministry, N. Resources, O. I. Hydrology, O. Arcmap, A. Data, U. Workspace, C. Workspace, S. Workspace, and X. Liu, Prediction of Worldwide Energy Resource (POWER) Agroclimatology Methodology (1.0 Latitude by 1.0 Longitude Spatial Resolution), Texas Water Resources Institute, TR-439, 2013, p. 134 Search PubMed.
- I. Programme, and O. N. Chemical, Guidelines for Drinking-Water Quality - Second Edition – Volume 2 – Health Criteria and Other Supporting Information, 2, 1996 Search PubMed.
- S. Rai, S. Gupta and P. C. Mittal, Dietary Intakes and Health Risk of Toxic and Essential Heavy Metals through the Food Chain in Agricultural, Industrial, and Coal Mining Areas of Northern India, Hum. Ecol. Risk Assess., 2015, 21, 913–933, DOI:10.1080/10807039.2014.946337.
- S. M. A. Reichman, The Response of Plant to Metal Toxicity: A Review of Focusing on Copper, Magnase and Zinc, 2002 Search PubMed.
- M. Aggarwal, S. Anbukumar, and T. Vijaya Kumar, Heavy Metals Concentrations and Risk Assessment in the Sediment of Ganga River between Kanpur and Prayagraj, Sadhana – Academy Proceedings in Engineering Sciences, U.P., India, 47, 2022, DOI:10.1007/s12046-022-01972-6.
- Q. Liu, X. Li and L. He, Health risk assessment of heavy metals in soils and food crops from a coexist area of heavily industrialized and intensively cropping in the Chengdu Plain, Sichuan, China, Front. Chem., 2022, 10, 1–11, DOI:10.3389/fchem.2022.988587.
- I. Gergen and M. Harmanescu, Application of principal component analysis in the pollution assessment with heavy metals of vegetable food chain in the old mining areas, Chem. Cent. J., 2012, 6, 1–13, DOI:10.1186/1752-153X-6-156.
- E. D. Anyanwu and O. G. Onyele, Human Health Risk Assessment of Some Heavy Metals in a Rural, Afr. J. Environ. Nat. Sci. Res., 2018, 1, 15–23 Search PubMed.
- F. Begum, M. Z. Khan and S. Mumtaz, A Human Health Risk Assessment of Heavy Metals in Drinking a Human Health Risk Assessment of Heavy, Fresenius Environ. Bull., 2019, 28, 2269–2277 Search PubMed.
- F. B. Masok, P. L. Masiteng, R. D. Mavunda, and P. P. Maleka, An integrated health risk evaluation of toxic heavy metals in water from Richards Bay, South Africa, Journal of Environment and Analytical Toxicology, 2017, vol. 7, pp. 487–493, DOI:10.4172/2161-0525.1000487.
- L. Jarup, Hazards of heavy metal contamination, Br. Med. Bull., 2003, 68, 167–182, DOI:10.1093/bmb/ldg032.
- G. Y. Hadzi, D. K. Essumang and J. K. Adjei, Distribution and Risk Assessment of Heavy Metals in Surface Water from Pristine Environments and Major Mining Areas in Ghana, J. Health Pollut., 2015, 5, 86–99, DOI:10.5696/2156-9614-5-9.86.
- R. Isaac, S. Siddiqui, P. Higgins, A. S. Paul, N. A. Lawrence, A. S. Lall, A. Khatoon, A. Singh, P. A. Majeed, S. Massey and A. Prasadd, Assessment of seasonal impacts on Water Quality in Yamuna river using Water Quality Index and Multivariate Statistical approaches, Bulletin, 2024, 2, 145–153, DOI:10.1016/j.wmb.2024.07.006.
- A. K. Dua, Anish, Water Quality Index for Assessment of Water Quality of River Ravi At Madhopur (India), Global J. Environ. Sci., 2009, 8, 10 Search PubMed.
- N. T. Giao, P. K. Anh and H. T. H. Nhien, Spatiotemporal Analysis of Surface Water Quality in Dong, Water, 2021, 13, 336 CrossRef CAS.
- S. Kalavathy, T. Rakesh Sharma, and P. Sureshkumar, Water Quality Index of River Cauvery in Tiruchirappalli district, Tamilnadu, Aes.Asia.Edu.Tw, 5, 2011, 55–61.
- P. Samantray, B. K. Mishra, C. R. Panda and S. P. Rout, Assessment of Water Quality Index in Mahanadi and Atharabanki Rivers and Taldanda Canal in Paradip Area, India, J. Hum. Ecol., 2009, 26, 153–161, DOI:10.1080/09709274.2009.11906177.
- M. Alam and J. K. Pathak, Rapid Assessment of Water Quality Index of Ramganga River, Western Uttar Pradesh (India) Using a Computer Programme, Nat. Sci., 2010, 8, 1–8 Search PubMed.
- D. M. Joshi, A. Kumar and N. Agrawal, Studies on physicochemical parameters to assess the water quality of river ganga for drinking purpose in Haridwar district, Rasayan J. Chem., 2009, 2, 195–203 CAS.
- M. Jehanzaib, S. A. Shah, J. Yoo and T. W. Kim, Investigating the impacts of climate change and human activities on hydrological drought using non-stationary approaches, J. Hydrol., 2020, 588, 125052, DOI:10.1016/j.jhydrol.2020.125052.
- A. S. Shafiuddin Ahmed, M. B. Hossain, S. M. O. F. Babu, M. M. Rahman and M. S. I. Sarker, Human health risk assessment of heavy metals in water from the subtropical river, Gomti, Bangladesh, Environ. Nanotechnol. Monit. Manag., 2021, 15, 100416, DOI:10.1016/j.enmm.2020.100416.
- D. Tiwari, M. Yadav, R. Kumar and S. K. Singh, The impact of organized bathing on ganga basin water quality during KUMBH-2022 by spatial and temporal analysis, Int. J. Health Sci., 2022, 3198–3212, DOI:10.53730/ijhs.v6ns3.6327.
- P. Sharma, P. K. Meher, A. Kumar, Y. P. Gautam and K. P. Mishra, Changes in water quality index of Ganges river at different locations in Allahabad, Sustain. Water Qual. Ecol., 2014, 3, 67–76, DOI:10.1016/j.swaqe.2014.10.002.
- APHA, Standard Methods for the Examination of Water and Waste Water, American Public Health Association, American Water Works Association, Water Environment Federation 33R, 22nd edn, 2012, n.d Search PubMed.
- R. M. Brown, N. I. Mcclelland, R. A. Deininger and M. F. O'connor, a Water Quality Index – Crashing the Psychological Barrier, Adv. Water Pollut. Res., 1973, 787–797, DOI:10.1016/b978-0-08-017005-3.50067-0.
- A. Singh, S. Chaudhary and B. S. Dehiya, Spatial Variations of Heavy Metal Content in the Surface Water of Yamuna River, India, Appl. Ecol. Environ. Sci., 2020, 8, 244–253, DOI:10.12691/aees-8-5-9.
- H. M. Zakir, S. Sharmin, A. Akter and M. S. Rahman, Assessment of health risk of heavy metals and water quality indices for irrigation and drinking suitability of waters: a case study of Jamalpur Sadar area, Bangladesh, Environ. Adv., 2020, 2, 100005, DOI:10.1016/j.envadv.2020.100005.
- S. Dange, K. Arumugam and S. S. Vijayaraghavalu, Geochemical Insights into Heavy Metal Contamination and Health Hazards in Palar River Basin: A Pathway to Sustainable Solutions, Ecol. Indic., 2024, 166, 112568, DOI:10.1016/j.ecolind.2024.112568.
- R. Singh, A. S. Venkatesh, T. H. Syed, A. G. S. Reddy, M. Kumar and R. M. Kurakalva, Assessment of potentially toxic trace elements contamination in groundwater resources of the coal mining area of the Korba Coalfield, Central India 54, Environ. Earth Sci., 2017, 76, 566, DOI:10.1007/s12665-017-6899-8.
- A. Manual, Dietary Guidelines for Indians – A Manual, National Institute of Nutrition, Hyderabad 500, 1998 Search PubMed.
- B. W. Huntsberry, News & Observer Prepares Pay Wall to Charge Users for Online Access, 2015, pp. 12–14 Search PubMed.
- EPA, U.S. Environmental Protection Agency 2021 Climate Adaptation Action Plan, 2021 Search PubMed.
- BIS, Indian Standard Drinking Water Specification (Second Revision), Bureau of Indian Standards IS 10500, 2012, pp. 1–11 Search PubMed.
- M. Tripathi and S. K. Singal, Use of Principal Component Analysis for parameter selection for development of a novel Water Quality Index: A case study of river Ganga India, Ecol. Indic., 2019, 96, 430–436, DOI:10.1016/j.ecolind.2018.09.025.
- R. M. Singh, and A. Gupta, Water Pollution-Sources , Effects and Control Water Pollution-Sources , Effects and Control, Research Gate, vol. 5, 2017, pp. 1–17 Search PubMed.
- V. S. Adithya, S. Chidambaram, C. Thivya, R. Thilagavathi, M. V. Prasanna, M. Nepolian and N. Ganesh, A study on the impact of weathering in groundwater chemistry of a hard rock aquifer, Arabian J. Geosci., 2016, 9, 1–11, DOI:10.1007/s12517-015-2073-3.
- R. Rawat, and A. R. Siddiqui, Physiochemical in Allahabad Metropolitan, vol. 19, 2019, pp. 121–135, DOI:10.1177/0972558X19835368.
- C. R. Ramakrishnaiah, C. Sadashivaiah and G. Ranganna, Assessment of water quality index for the groundwater in Tumkur taluk, Karnataka state, India, E-J. Chem., 2009, 6, 523–530, DOI:10.1155/2009/757424.
- E. Siddiqui and J. Pandey, Assessment of heavy metal pollution in water and surface sediment and evaluation of ecological risks associated with sediment contamination in the Ganga River: a basin-scale study, Environ. Sci. Pollut. Res., 2019, 26, 10926–10940, DOI:10.1007/s11356-019-04495-6.
- G. Yeh, H. G. Hoang, C. Lin, X. T. Bui, H. T. Tran, C. C. Shern and C. T. Vu, Assessment of heavy metal contamination and adverse biological effects of an industrially affected river, Environ. Sci. Pollut. Res., 2020, 27, 34770–34780, DOI:10.1007/s11356-020-07737-0.
- R. Bhardwaj, A. Gupta and J. K. Garg, Evaluation of heavy metal contamination using environmetrics and indexing approach for River Yamuna, Delhi stretch, India, Water Sci., 2017, 31, 52–66, DOI:10.1016/j.wsj.2017.02.002.
- M. S. Bhuyan and M. A. Bakar, Seasonal variation of heavy metals in water and sediments in the Halda River, Chittagong, Bangladesh, Environ. Sci. Pollut. Res., 2017, 24, 27587–27600, DOI:10.1007/s11356-017-0204-y.
- A. M. Huffman, and A. M. Sikder, Assessment of Heavy Metal Pollution in the Sediments of the Roanoke River, 2017, DOI:10.1130/abs/2017se-290796.
- J. Jayaraman, J. Kumaraswamy, Y. K. S. S. Rao, M. Karthick, S. Baskar, M. Anish, A. Sharma, A. S. Yadav, T. Alam and M. I. Ammarullah, Wastewater treatment by algae-based membrane bioreactors: a review of the arrangement of a membrane reactor, physicochemical properties, advantages and challenges, RSC Adv., 2024, 14, 34769–34790, 10.1039/D4RA04417G.
| This journal is © The Royal Society of Chemistry 2025 |