 Open Access Article
Open Access ArticleChitin nanofibers: recent advances in preparation and applications in biomedical and beyond
M. Ariful Islam†
 ab,
M. Nahid Hasan†
ab,
M. Nahid Hasan† a,
M. Sadik Hussain Evan
a,
M. Sadik Hussain Evan a,
M. Jalal Uddin
a,
M. Jalal Uddin a,
Wahid Salekin Tulin
a,
Wahid Salekin Tulin a,
M. Saydul Islama,
Mayeen Uddin Khandaker
a,
M. Saydul Islama,
Mayeen Uddin Khandaker cde,
Ismail M. M. Rahman
cde,
Ismail M. M. Rahman *f and
Faisal I. Chowdhury
*f and
Faisal I. Chowdhury *a
*a
aNanotechnology, Renewable Energy and Catalysis Laboratory, Department of Chemistry, University of Chittagong, Chattogram 4331, Bangladesh. E-mail: faisal@cu.ac.bd
bGraduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan
cApplied Physics and Radiation Technologies Group, CCDCU, Faculty of Engineering and Technology, Sunway University, Bandar Sunway 47500, Selangor, Malaysia
dDepartment of Physics, College of Science, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Republic of Korea
eFaculty of Graduate Studies, Daffodil International University, Daffodil Smart City, Birulia, Savar, Dhaka 1216, Bangladesh
fInstitute of Environmental Radioactivity, Fukushima University, 1 Kanayagawa, Fukushima City, Fukushima 960-1296, Japan. E-mail: immrahman@ipc.fukushima-u.ac.jp
First published on 19th May 2025
Abstract
Chitin and chitosan-based nanofibers (ChNFs), derived from renewable sources, have emerged as promising biomaterials due to their unique properties such as high surface area, porosity, biocompatibility, and biodegradability. This review provides a comprehensive overview of ChNF extraction and synthesis, focusing on both top-down and bottom-up approaches. A comparative analysis of these methods is presented, highlighting the challenges, opportunities, environmental impact, cost-effectiveness, and quality consistency associated with each. The advantages of ChNFs over similar nanomaterials are elucidated, emphasizing their diverse applications in biomedical and environmental fields. Biomedical applications include drug delivery, tissue engineering, cancer treatment, wound healing, and biosensing. Environmental applications encompass water treatment, air filtration, agriculture, and biodegradable packaging. Despite their potential, challenges remain, including low solubility, unstable mechanical properties, and inconsistent quality, which limit their widespread use. This review also examines recent advancements in ChNF research, aiming to guide the development of efficient and environmentally friendly synthesis methods. By encouraging innovation in ChNF-based nanotechnologies, this research contributes to a more sustainable future.
1 Introduction
Chitin and its deacetylated derivative, chitosan, are renowned cellulose analogs characterized by a repeating (1,4)-N-acetyl glucosamine structure.1 As the second most abundant biopolymer after cellulose,2,3 chitin is predominantly found in the exoskeletons of crustaceans and the cell walls of fungi.4 Despite being biosynthesized at a rate of 1010 to 1011 tons annually, most chitin is discarded as waste.5 Therefore, the efficient utilization of chitin as a sustainable green material is paramount. Chitin's linear structure, featuring two hydroxyl groups and an acetamide group, contributes to its high crystallinity, strong hydrogen bonding, and organization into antiparallel nano-sized chitin nanofibers (ChNFs) (Fig. 1).6 The ChNFs, typically 2–5 nm in diameter and 300 nm in length, are embedded within a protein matrix.7–10 The hierarchical ChNF-based structure of crab and prawn shells suggests that the isolation methods employed for cellulose nanofibers could be applied to other chitin-containing biomass sources.10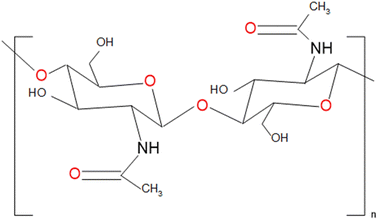 | ||
| Fig. 1 Structure of the chitin molecule, showing two N-acetylglucosamine units that repeat to form long chains in the β-(1 → 4)-linkage. | ||
ChNFs, defined as fibers with diameters below 100 nm and aspect ratios exceeding 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6,11 are fundamental building blocks in natural biological materials. Their assembly occurs in various biopolymers, including polypeptides like silk fibroin12,13 collagen,14 keratin,15 and polysaccharides like cellulose and chitin.16 The unique properties of ChNFs, distinct from those of micro-sized fibers, arise from their exceptionally high surface-to-volume ratio17 and the formation of highly porous meshes (Fig. 2). Due to their distinctive dimensional, optical,18 mechanical,19 and other properties, the preparation of ChNFs is a critical endeavor. While electrospinning is a common artificial method for producing ChNFs from polymer solutions,20 it has a significant environmental impact. Consequently, there has been growing interest in deriving ChNFs from biopolymers due to their environmentally friendly attributes, such as renewability, biocompatibility, biodegradability, and sustainability.1
6,11 are fundamental building blocks in natural biological materials. Their assembly occurs in various biopolymers, including polypeptides like silk fibroin12,13 collagen,14 keratin,15 and polysaccharides like cellulose and chitin.16 The unique properties of ChNFs, distinct from those of micro-sized fibers, arise from their exceptionally high surface-to-volume ratio17 and the formation of highly porous meshes (Fig. 2). Due to their distinctive dimensional, optical,18 mechanical,19 and other properties, the preparation of ChNFs is a critical endeavor. While electrospinning is a common artificial method for producing ChNFs from polymer solutions,20 it has a significant environmental impact. Consequently, there has been growing interest in deriving ChNFs from biopolymers due to their environmentally friendly attributes, such as renewability, biocompatibility, biodegradability, and sustainability.1
Nature produces a diverse array of ChNFs, including collagen triple helix fibers, keratin fibrils, and fibroin fibrils. Natural ChNFs are extracted by downsizing the structures of biomass-derived organizations, a process considered a “top-down” approach.11 In contrast, electrospinning is deemed a “bottom-up” approach, as it involves bundling molecules into ChNFs.
The ChNFs can be prepared through top-down and bottom-up processes (Fig. 3).11 However, compared to the abundant research on the bottom-up preparation of homologous NFs from chitin and cellulose, there have been fewer reports on the top-down production of ChNFs. Acidolysis of amorphous domains in semicrystalline chitin has resulted in the formation of chitin nanocrystals,22 which are suitable for reinforcing polymer nanocomposites. However, their low aspect ratio does not align with the natural fibril form of chitin found in crabs and prawns.
 | ||
| Fig. 3 Extraction of ChNFs via different routes of ‘bottom-up & top-down’ approach (**recreated from the text and other informations). | ||
2 Strategies for preparation of ChNFs from chitin
2.1 Top-down
The ChNFs hold significant promise in various applications due to their unique properties. However, the inherent insolubility of chitin necessitates a top-down approach for their production, primarily utilizing crustacean shells as the starting material. This approach involves breaking down bulk chitin into its nanoscale building blocks.The initial step typically involves the purification of chitin from crustacean shells through demineralization and deproteinization using acid and alkali treatments.23–25 Subsequently, the purified chitin undergoes acid hydrolysis, which cleaves the non-crystalline regions. The acidic environment protonates the primary amine groups, leading to a more stable colloidal suspension with smaller nanofibers.25,26
Goodrich et al.27,28 developed a method involving high-speed blending of a neutralized chitin suspension followed by lyophilization to produce ChNFs. However, this method often results in nanofibers larger than their naturally occurring counterparts (∼3 nm) and with a broad diameter distribution. Alternatively, grinding the chitin suspension at neutral pH yields smaller nanofibers (10–20 nm). This approach, however, requires the chitin to be directly extracted from crustacean shells and maintained in a hydrated state to prevent strong hydrogen bonding between fiber bundles upon drying.5,28
High-pressure homogenization offers a milder alternative. In this method, a pristine chitin dispersion at pH 4.1 is passed multiple times through a high-pressure homogenizer, resulting in a chitin/water dispersion that can be cast into thin films with an average nanofiber diameter of 20 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 (Fig. 4).
29 (Fig. 4).
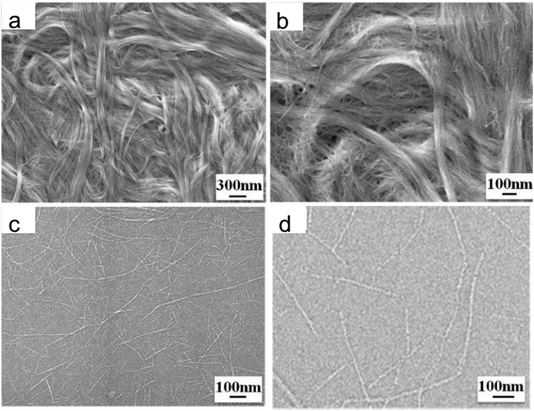 | ||
| Fig. 4 (a and c) SEM images of the cationized chitin and homogenized chitin with a pH of 4.1, respectively; (b and d) SEM images with higher magnification in comparison to (a) and (c), respectively.30 | ||
Another top-down approach involves the mediated oxidation of chitin. This method utilizes 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) along with sodium hypochlorite (NaClO) as a co-oxidant to selectively oxidize the primary hydroxyl groups of chitin to carboxylate groups.30–32 By controlling the NaClO concentration, the extent of oxidation can be adjusted, influencing the water-insoluble content while preserving the degree of N-acetylation (Fig. 5).29 The negatively charged carboxylate groups on the chitin crystallite surfaces facilitate the breakdown into ChNFs.
 | ||
| Fig. 5 (a) Relationships between the amount of NaClO added in the TEMPO-mediated oxidation of chitin and either the total reaction time or the weight ratio of the water-insoluble fraction. (b) Relationships between the amount of NaClO added in the TEMPO-mediated oxidation of chitin and either the carboxylate or aldehyde content of the water-insoluble fraction. The degree of N-acetylation is also plotted. (c) TEM images of TEMPO-oxidized chitin nanocrystals prepared under different conditions.29 | ||
Partial deacetylation of chitin to chitosan, followed by protonation of the resulting primary amines, is another strategy for ChNF production. This method exploits electrostatic repulsion to achieve individual nanofiber separation. Pristine crab shell chitin is treated with NaOH at elevated temperatures to increase the number of primary amines.33 Subsequent mechanical disintegration yields individual ChNFs with diameters of approximately 6 nm and higher aspect ratios compared to TEMPO-mediated ChNFs.34
Oh et al.35 reported a environmentally friendly method for disintegrating chitin using calcium ions and solvent exchange. This innovative approach produces a hierarchical chiral nematic phase, mimicking the Bouligand structure found in nature (Fig. 6). Chitin is dissolved in a Ca-saturated methanol solution, where Ca2+ ions disrupt intramolecular hydrogen bonding. Solvent exchange with methanol, isopropanol, and deionized water then removes the Ca2+ ions, yielding ChNFs that exhibit nematic or liquid crystalline phases in alcohol or chiral nematic phases in hydrogels.
 | ||
| Fig. 6 (a) Calcium-saturated methanol disintegrates ChNFs with minimal chemical modification, generating a Ca-methanol gel (disordered) (bottom-left panel). Ca2+ is removed from the Ca-methanol gel by washing with alcohol (methanol or IPA) and DI water, thus generating alcohol gels (methanol gel or IPA gel) in the N phase (bottom-middle panel) and a hydrogel in the N* phase (bottom-right panel). The yellow, pink, and blue beads represent three types of solvent molecules: methanol-solvated Ca2+, alcohol (methanol or IPA), and water. (b) TEM images show a morphological change in chitin nanowires by solvent exchange of Ca-methanol gel, (c) IPA gel, and (d) hydrogel.35 | ||
The various top-down approaches for ChNF synthesis are illustrated in a flowchart (Fig. 9).36
2.2 Bottom-up
In contrast to the top-down approach, the bottom-up approach to ChNF production involves assembling nanofibers from individual molecules into organized structures. This approach requires the dissolution of chitin molecules, posing a challenge due to chitin's limited solubility in water and most organic solvents.37Electrospinning of depolymerized chitin solutions is a widely employed bottom-up technique.38,39 In electrospinning, a high voltage applied to a solution-filled capillary creates an electric field that overcomes surface tension, forming a polymer jet. Upon contact with a substrate, the jet solidifies into nanofibers. Chitin is often depolymerized with gamma radiation and dissolved in solvents like 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to improve solubility. Alternatively, ionic liquids40–42 like 1-butyl-3-methylimidazolium chloride40 or 1-allyl-3-methyl-imidazolium bromide41,43 can be used to dissolve chitin at elevated temperatures. The dry-jet-wet-spinning method has also been employed to spin chitin dissolved in acetate salt solutions into microscale fibers.43 Huang et al.44 demonstrated the direct spinning of pure chitin microfibers from NaOH–urea solutions after freeze–thaw cycles (Fig. 7).
 | ||
| Fig. 7 Photograph of (a) freshly spun chitin fibers in water and (b) air-dried fibers. SEM images of the chitin fibers (c) lyophilized and fractured in liquid nitrogen and (d) air-dried.44 | ||
Self-assembly methods for producing ChNFs from chitin solutions in organic solvents without electrospinning have been developed45–47 (Fig. 8). These methods involve dissolving squid pen β-chitin in either HFIP or LiCl/N,N-dimethylacetamide (DMAC) to disrupt hydrogen bonds. Self-assembly is then initiated by solvent evaporation (HFIP) or precipitation (LiCl/DMAC). While LiCl/DMAC yields nanofibers of various sizes (3, 6, and 10 nm), HFIP-chitin solutions form monodispersed ChNFs with diameters of 3 nm (Fig. 8a–c). Interestingly, these self-assembled nanofibers exhibit the α-chitin crystal structure, which is more energetically favorable. Notably, these 3 nm α-ChNFs closely resemble those found in crustacean shells and arthropod cuticles, serving as versatile building blocks for biomimetic ChNF assemblies. The flowchart in Fig. 936 illustrates various bottom-up synthesis procedures for ChNFs. Table 1 provides a comparative analysis of these different bottom-up synthesis methods, outlining their respective challenges, applications, and cost-effectiveness.
 | ||
| Fig. 8 The morphology and diameter distribution of ChNFs. In the AFM images, the apparent nanofiber width is larger due to tip convolution. (a–c) 3 nm nanofibers prepared from HFIP solution (5 mL, 0.01 wt%): (a) AFM height image, (b) bright field TEM image, (c) AFM phase image of two fibers. (d) Thin transparent chitin film fabricated from HFIP solution drop-casting45 (a–c) and47 (d). | ||
 | ||
| Fig. 9 The various top-down and bottom-up approaches for ChNF synthesis.36 | ||
| Method | Advantages | Challenge | Principal application | Environmental impact | Cost | Ref. |
|---|---|---|---|---|---|---|
| Electrospinning | High surface area, scalable, fine control | Specific solvent requirements, costly | Textiles, filtration, biomedical | Moderate (solvent use, energy) | Moderate to high (equipment costs) | 48–50 |
| Self-assembly | Low-cost, green, simple process | Limited control over fiber morphology | Sustainable applications, bio-based products | Very low (green process) | Low (simple process, less equipment) | 51 and 52 |
| Hydrothermal/solvothermal | High crystallinity, controlled morphology | High-energy requirements, expensive | Large-scale production, high-performance materials | Moderate (energy-intensive) | High (specialized equipment, energy) | 53 and 54 |
| Biomimetic synthesis | Precision, functionalization, biocompatibility | High cost, scalability issues, complexity | Biomedical, sustainable packaging, cosmetics | Low | Hight | 55 and 56 |
| Enzymatic hydrolysis | Biodegradable, environmentally friendly | High cost of enzymes, slow process | Biodegradable applications, small-scale | Very low (biodegradable enzymes) | High (enzyme cost, slow process) | 57 and 58 |
3 Preparation of ChNFs from different sources
Several methods are employed to prepare ChNFs. The ionic gelation method involves dissolving chitosan in an acidic solution and adding a cross-linking agent like sodium tripolyphosphate (TPP) to induce nanoparticle formation through ionic interactions. In the emulsion-droplet coalescence method, a chitosan solution is mixed with an oil phase and subjected to ultrasonication to produce chitosan nanoparticles. The reverse microemulsion method involves stimulating a water-in-oil microemulsion containing chitosan and a cross-linking agent to form nanoparticles. Lastly, the coacervation method entails mixing chitosan with another polymer in water and inducing nanoparticle formation through a pH change or salt addition.3.1 ChNFs from crab shell
Crab shells, composed primarily of 25–30% chitin, 25% protein, and 40–50% calcium carbonate,59 exhibit a hierarchical structure with multiple layers (Fig. 10). ChNFs are encased in protein layers, forming a crystalline structure. Chitin synthesis can be achieved through traditional chemical or enzymatic approaches.60 ChNFs have been successfully extracted from crab shells using a disintegration method.5,61 Following purification through various conventional chemical treatments, the shells underwent mechanical processing to yield ChNFs. These NFs exhibited remarkable homogeneity, with a width of approximately 10 nm (Fig. 11). | ||
| Fig. 10 Schematic presentation of the exoskeleton structure of crab shells.1 | ||
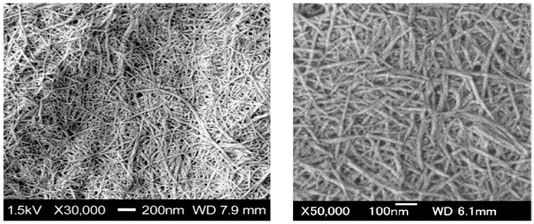 | ||
| Fig. 11 SEM images of ChNFs from crab shell after grinder treatment.1 | ||
 | ||
| Fig. 12 Schematic illustration of the preparation of ChNFs and dChNFs from speckled swimming crab shells.24 | ||
Initially, the crab shells are physically pulverized into fine powders using a high-speed rotor mill. These powders are then subjected to sequential treatments with NaOH, HCl, and ethanol solutions to eliminate proteins, minerals, and pigments. This process yields chitin with a 12 wt% yield. Subsequently, ChNFs are produced from the purified chitin through mechanical disintegration methods, such as wet grinding and high-pressure homogenization. The purified wet chitin is diluted to a 1 wt% suspension in deionized water under vigorous mechanical stirring. This suspension is then processed using a grinder equipped with grinding stones at room temperature, followed by passage through a high-pressure homogenizer with Z-shaped interaction chambers. High-pressure homogenization is repeated at controlled temperatures, ensuring the preservation of the ChNFs' original chemical and crystalline structures. Partial deacetylation of the ChNFs is achieved through treatment with a NaOH solution under vigorous mechanical stirring at elevated temperatures. The resulting ChNF and deacetylated ChNF (dChNF) suspensions are dialyzed against deionized water until a neutral pH is attained. Finally, the suspensions are diluted and stored for future use. This straightforward yet effective process enables the extraction of substantial quantities of homogeneous ChNFs from crab shells.5,24
The enzymatic method begins with physically grinding crab gills and mixing them with water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio). The mixture is heated to 50 °C under stirring, followed by the addition of an enzyme preparation in a 1
2 ratio). The mixture is heated to 50 °C under stirring, followed by the addition of an enzyme preparation in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 ratio. Enzymatic hydrolysis proceeds for 6 h at 50 °C, maintaining a pH of 6.5–7.0. The degree of protein hydrolysis (DH) is monitored, and upon reaching the maximum DH, enzyme activity is inhibited by heating the mixture to 95 °C, followed by cooling to 30 °C. Centrifugation is employed to separate the precipitate, which is then washed multiple times. The resulting precipitate serves as the starting material for chitin preparation, following the steps outlined in the chemical approach, with the exception of the initial deproteinization using a 4% NaOH solution.
0.06 ratio. Enzymatic hydrolysis proceeds for 6 h at 50 °C, maintaining a pH of 6.5–7.0. The degree of protein hydrolysis (DH) is monitored, and upon reaching the maximum DH, enzyme activity is inhibited by heating the mixture to 95 °C, followed by cooling to 30 °C. Centrifugation is employed to separate the precipitate, which is then washed multiple times. The resulting precipitate serves as the starting material for chitin preparation, following the steps outlined in the chemical approach, with the exception of the initial deproteinization using a 4% NaOH solution.
Wet chitin samples obtained through both methods are dried at a maximum temperature of 60 °C. The transparent protein hydrolysate solution resulting from the enzymatic method is further dried using a lyophilizer.60
Crab waste fermentation was performed under previously established conditions using the Bacillus strains. Inocula were routinely cultured in a Luria-Bertani (LB) broth medium.62,69 Fermentation occurred in 500 mL jars containing 100 mL of medium of 3% (w/v) crab shell waste, with or without 5% (w/v) glucose. The initial pH of the broth was adjusted to 7.0. Following sterilization, inoculums were added, and cultures were incubated for 5 days at 37 °C with shaking (200 rpm). Cultures were centrifuged, and the resulting fermented crab supernatants (FCSs) were freeze-dried and stored at −20 °C for further analysis. The FCSs were evaluated for composition, antioxidant activity, and antibacterial properties. Fermented crab waste was separated, washed, and dried for chitin recovery.62
3.2 ChNFs from prawn & shrimp shell
Crustacean shells primarily consist of chitin, calcium carbonate, proteins, lipids, and pigments. Chitin extraction involves deproteinization, demineralization, and removal of lipids and pigments. These steps, along with the subsequent conversion to chitosan, can be achieved through chemical or biological methods, such as microbial fermentation and enzymatic reactions. Chitosan, a partially acetylated form of chitin with a degree of deacetylation (DDA) around 50%, becomes soluble in acidic aqueous solutions.1,70The hierarchical structure of prawn shells allows for the application of ChNF extraction methods similar to those used for crab shells. The ChNFs with a uniform structure and high viscosity can be obtained from various prawn species, including Penaeus monodon, Marsupenaeus japonicas, and Pandalus eous Makarov. These nanofibers, derived from prawn shells, exhibit a thickness of 10–20 nm, similar to crab shell nanofibers (Fig. 13). The predominance of the finer exocuticle in prawn shells, compared to the coarser endocuticle in crab shells, facilitates easier fibrillation of chitin from prawn shells.71
 | ||
| Fig. 13 (a) FE-SEM micrograph of ChNFs from black tiger prawn shells; (b) FE-SEM micrograph of the surface of the black tiger prawn shell after removing the matrices.28 | ||
Chitin can also be synthesized from shrimp shells (Fig. 14). This process involves demineralization using a diluted HCl solution, followed by deproteinization with NaOH. The resulting crude chitin is dehydrated with ethanol and dried. Crude chitosan is then prepared by treating the chitin with NaOH at elevated temperatures.72 The purification of chitosan from prawn exoskeletons for potential medicinal use involves the removal of insolubles, reprecipitation with NaOH, and demetallization. This approach has successfully yielded chitosan with a 35.49% yield, demonstrating its potential as a pharmaceutical excipient.73 Chitin nanofibers can be derived from these chitins using various top-down and bottom-up processes, which are explored in this article.
 | ||
| Fig. 14 Synthesis of chitin from shrimp and prawn.74 | ||
Another method for producing ChNFs from shrimp involves dissolving chitin and subsequently electrospinning it into NF materials. Electrospinning, a simple, cost-effective, and high-throughput technique, creates nanofibrous scaffolds that mimic the natural extracellular matrix.75,76 It is the most widely used method for NF production due to its ease of operation. The electrospinning system typically consists of a grounded collector, a spinneret, and a high-voltage power source. A precursor solution is fed into the spinneret, forming a pendant drop that transforms into a Taylor Cone under high voltage. The resulting liquid jet stretches and whips, leading to solvent evaporation and fiber collection on the target. Electrospinning equipment is rapidly being commercialized,74,77 and various modifications to the traditional method have been developed to overcome its limitations.74,78
3.3 ChNFs from mushroom
Mushroom cell walls also contain ChNFs, forming complexes with glucans.78–80 A variety of mushroom species, namely Pleurotus eryngii (king trumpet mushroom), Agaricus bisporus (common mushroom), Lentinula edodes (shiitake), Grifola frondosa (maitake), Hypsizygus marmoreus (bunashimeji), Armillariella mellea (honey mushroom), and Morchella esculenta (yellow morel), underwent a systematic series of chemical treatments. These treatments were specifically designed to eliminate inherent proteins, pigments, glucans, and minerals from the fungal biomass.6,81 Following the purification process, the resultant material was subjected to nano-fibrillation utilizing an acetic acid-impregnated grinder, yielding uniform and thin ChNFs (Fig. 15). | ||
| Fig. 15 FE-SEM micrographs of ChNFs from (a) Pleurotus eryngii, (b) Agaricus bisporus, (c) Lentinula edodes, (d) Grifola frondosa, and (e) Hypsizygus marmoreus. The scale bars are 200 nm in length.6 | ||
Mushroom-derived ChNFs, unlike to those from crab and prawn shells, are characterized by the formation of complexes with glucans on their surface. Due to the carbohydrate nature of both glucan and chitin, the complete removal of glucans from chitin is challenging through chemical treatment alone. The glucan content and resulting nanofiber width varied among mushroom species, ranging from 20 to 28 nm. Despite exhibiting typical α-chitin crystal patterns in X-ray diffraction, the relative crystallinity indices of these nanofibers decreased with increasing amorphous glucan content. These findings expand the repertoire of dietary nanofibers and highlight the potential of mushroom-derived nanofibers for various applications, from novel food ingredients to medical applications.82–84
Zhang et al.85 adapted the abovementioned process with minor modifications to extract ChNFs from shiitake stripes, achieving a purity of over 98% and a diameter of 9 nm. Alternative methods have been reported to extract ChNFs from mushrooms without acid treatment. For instance, mechanical agitation and hot water treatment, followed by alkali treatment with NaOH, were employed to extract ChNFs from Agaricus bisporus, yielding 25.4% and 15% from the stalk and cap, respectively.86,87 This method has been extended to other mushroom species, including Pleurotus ostreatus, Lentinula edodes, and Flammulina velutipes.87 Aitor Larrañaga et al.88 utilized a top-down approach involving mechanical fibrillation, removal of water-soluble components, and deproteinization to isolate ChNFs from Agaricus bisporus with a crystallinity of 59.1%.
Another method (Fig. 16) involves extracting ChNFs from Agaricus bisporus cultivated in a liquid culture medium supplemented with various salts, hormones, and trace metals.89 After removing unwanted materials, the resulting chitin is ground to produce ChNFs.
 | ||
| Fig. 16 Schematic representation of the extraction of ChNFs from the commonly cultivated Agaricus bisporus: (a) extraction from 3 kg of whole mushrooms resulted in approximately 42 g of nanofibers. (b) A 3% w/v extract was obtained through chemical extraction. (c) A 0.8% w/v whole mushroom suspension, dispersed within one minute of blending and never dried, was used for nanopaper production. (d) Stability assessment of the 0.8% w/v whole mushroom chitin suspension after 7 days: left – the never-dried suspension; middle – resuspension of a freeze-dried sample after rapid freezing with liquid nitrogen; right – resuspension of a freeze-dried sample after slow freezing in a conventional freezer.86 | ||
3.4 ChNFs from squid pen
Two distinct crystalline forms of chitin exist in nature: α- and β-chitin, differentiated by antiparallel and parallel chain-packing modes, respectively.90,91 While α-chitin is widely distributed in nature, β-chitin is uniquely found in squid pens. Fan and Isogai et al.91 pioneered the development of ChNFs from squid pen β-chitin, obtaining NFs with a 3–4 nm length and a high aspect ratio without the need for chemical modification. The lower crystallinity, parallel chain packing, and relatively weak intermolecular forces of β-chitin contribute to the facile preparation of ChNFs.Various methods have been employed to synthesize β-ChNFs from squid pens. A common approach involves cleaning, cutting, and grinding the squid pen, followed by deproteinization and pH neutralization to extract pure chitin and lower the high pH resulting from the alkalization process. Another procedure involves creating a slurry by mixing and oxidizing the squid pen with ammonium persulfate and heating it, followed by suspension in water, pH adjustment, and ultrasonication to extract ChNFs.92,93
A distinct method utilizes high-temperature water treatment to extract β-ChNFs. In this approach, the squid pen content, water, and molten KNO3–NaNO3 salts are combined in a reactor at high temperatures (150 °C, 200 °C, 250 °C, and 300 °C) and then cooled. Subsequently, the residue is converted into a ChNF dispersion and disintegrated by a ‘Starburst’ system, which is a high-pressure water jet system.94,95
Alternatively, the ‘Starburst’ system can be used to prepare a slurry from the solid residue obtained through high-temperature water treatment and disintegrate it into nanofibers. This system has proven effective in converting acid-to-base and base-to-acid treated β-chitins into ChNFs, demonstrating its versatility in handling different pretreatment sequences.96
3.5 ChNFs from commercial chitin powder
A challenge in ChNF production is maintaining extracted chitin in a wet state to prevent inter-fibrillar coagulation, which hinders commercialization efforts. To address this, researchers have developed a simplified method for producing ChNFs from commercially available dry chitin powder. This method involves dissolving the dry chitin in acidic water and subjecting it to grinding. The commercial chitin, composed of NF aggregates, readily disintegrates into homogeneous NFs due to electrostatic repulsion between the cationized amino groups on the crystalline surfaces. Various organic acids can further enhance this disintegration process. The advantage of commercial chitin is the ability to obtain large quantities of ChNFs rapidly.In addition to grinding, the Star Burst instrument, a high-pressure waterjet system, has proven effective in nano-fibrillating dry chitin.97,98 This system, equipped with a ball-collision chamber, subjects chitin in aqueous acetic acid to high-pressure ejection through a small nozzle. Multiple treatments progressively reduce the thickness of the resulting NFs.
4 Comparison of ChNFs with other similar nanofibers
The ChNFs, nanocellulose (NFC), and silk fibroin (SF) are three naturally occurring biopolymers attracting significant interest due to their potential for creating sustainable and high-performance materials. Each possesses unique structural characteristics and functionalities, making them ideal candidates for various applications, particularly in the development of biocomposites. This section will explore their individual properties and the synergies achieved by combining them. Chitin, the second most abundant natural polymer after cellulose, boasts a semi-crystalline structure stabilized by hydrogen bonding and van der Waals interactions. Processing it into nanofibers via techniques like acid hydrolysis and high-pressure homogenization enhances its mechanical properties (strength and stiffness) and expands its applicability in composites. Derived from cellulose, a major component of plant cell walls, nanocellulose exhibits a highly crystalline structure, translating to exceptional mechanical strength and flexibility. It can be processed into two primary forms: nanofibrils (elongated, high-aspect-ratio) and nanocrystals (rod-like, high crystallinity). These forms are crucial in enhancing composite materials' mechanical and optical properties. Silk fibroin, a protein-based biopolymer extracted from silk cocoons, possesses a unique hierarchical structure for its excellent mechanical properties (high tensile strength) and biocompatibility. Its ability to transition from a random coil conformation to a β-sheet structure is crucial for its efficient integration into composite materials. Combining these biopolymers offers significant advantages when compared to their individual use.When incorporated into an SF matrix, ChNFs significantly improve the composite's mechanical performance by reinforcing the structure and enhancing strength and flexibility. The addition of cellulose nanocrystals to composites can significantly improve tensile strength and elongation at break. Silk fibroin contributes to biocomposites overall ductility and toughness, making it an ideal matrix material for embedding other nanofibers.
Due to their unique properties, these biopolymers hold promise for various applications. Their biocompatibility and mechanical properties make them suitable for biomedical applications like wound dressings and drug delivery systems. Additionally, their renewability and biodegradability make them attractive for use in textiles, medicine, and even environmental decontamination.99 Chitin and chitosan nanofibers are highly usable, except for thermal and some chemical limitations, they carry excellent properties. A concise comparison of ChNFs, NCs, and silk fibroin is presented in Table 2.
| Type | Properties | ChNFs | Nanocellulose | Silk fibroin | Ref. |
|---|---|---|---|---|---|
| Structural properties | Size | 2–5 nm | 10–90 nm | 150–400 nm | 5, 100 and 101 |
| Surface area to volume ratio | High surface area due to the nanoscale size and fibrillar structure | Slightly higher due to narrower fibers | Lower than cellulose or chitin due to larger aggregates | 102 and 103 | |
| Crystal structure | α-Chitin crystal structure with antiparallel arrangement | Cellulose Iβ structure in native cellulose | β-sheet crystalline regions in its native form | 104–106 | |
| Mechanical properties | Tensile strength | Partially deacetylated ChNF films exhibit the highest tensile strength of ∼140 MPa | Cellulose nanofiber green composites can achieve tensile strengths up to 90 MPa, comparable to glass-fiber-reinforced plastics | Ultrathin silk fibroin films have high tensile strength and toughness due to their self-reinforcing microstructure | 107, 108 and 109 |
| Young's modulus | Chitin nanopapers from mushroom extract have a Young's modulus of around 7 GPa | The Young's modulus of cellulose nanofibers from different sources ranges from 102 to 131 GPa, as measured by atomic force microscopy | Uniaxial extension of regenerated silk fibroin films increases their Young's modulus from 2.7 to 3.5 GPa | 110 and 111 | |
| Electrical properties | Electrical conductivity or resistivity | Insulating but can be modified for conductivity using composites | Insulator; conductive properties enhanced when hybridized with graphene | Limited conductivity but can function as a dielectric layer | 112, 113 and 114 |
| Thermal properties | Thermal conductivity | Chitin nanofiber films exhibited in-plane thermal conductivity of 0.73–0.82 W m−1 K−1, with surface amino groups influencing conductivity | Nanocellulose filaments fabricated by flow-focusing can exhibit thermal conductivity up to 14.5 W m−1 K−1, much higher than cellulose nanopaper or nanocrystals | Single silk fibroin fibers exhibit an axial thermal conductivity of approximately 0.775 W m−1 K−1 at room temperature, which is significantly higher than most textile fibers | 115–117 |
| Heat capacity | Not favourable | Moderate | Moderate | 118–120 | |
| Thermal stability | ChNFs start decomposing at 33 °C | Chemical pretreatments can enhance thermal properties, with NaOH/urea/thiourea-treated nanofibers demonstrating thermal degradation onset at 270 °C and maximum degradation at 370 °C | Silk fibroin decomposes at around 348 °C | 121 and 122 | |
| Thermal expansion coefficient | Low | Low | Slightly higher | 123 | |
| Biological properties | Biocompatibility | ChNFs demonstrate excellent biocompatibility, promoting cell proliferation and collagen deposition, which are crucial for wound healing | Critical biocompatibility | Superior biocompatibility | 124 and 125 |
| Antibacterial or antiviral activity | Chitin-based materials demonstrate over 99.95% bacteriostasis against pathogens like Staphylococcus aureus and Escherichia coli, making them effective in medical and civil applications | Its large surface area and porous structure facilitate effective interactions with bacteria, disrupting their membranes and inhibiting proliferation | Silk fibroin membranes combined with polyhexamethylene biguanide (PHMB) or silver oxide nanoparticles effectively inhibit Staphylococcus aureus and Escherichia coli | 126–128 | |
| Biodegradability | Chitin is highly biodegradable, breaking down into simple organic acids, which supports bacterial growth due to its favorable carbon![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen ratio nitrogen ratio |
Nanocellulose, derived from cellulose, is also biodegradable and exhibits excellent mechanical properties, making it suitable for various applications | Silk fibroin is known for its biocompatibility and biodegradability, although its degradation rate can be slower compared to chitin | 129 and 130 | |
| Surface properties | Adsorption and desorption behavior | High for all-purpose | Medium to high | High for heavy metals | 131 and 132 |
| Environmental properties | Photocatalytic degradation of pollutants | Chitin-based composites, particularly when integrated with TiO2, exhibit improved photocatalytic activity due to reduced band gap energy and enhanced reactive sites | The three-dimensional structure of these aerogels provides a large surface area, promoting effective photocatalytic reactions through increased active sites | Combined with metal oxide nanoparticles like ZnO and TiO2, the degradation efficiency of various organic pollutants, including pesticides and dyes, is enhanced under solar irradiation | 133–136 |
| Water purification potential | Excellent adsorption properties for heavy metals in water treatment | Strong capability for dye and heavy metal removal | Moderate potential for water purification, enhanced with chemical modifications | Water purification potential is medium | 137 and 138 |
| Environmental stability | Stable in mildly acidic, basic, and oxidative conditions but degrade under extreme environments, such as strong oxidants or high temperatures | Highly stable in aqueous and neutral environments. Stability decreases under strongly acidic or basic conditions but can be enhanced by cross-linking | Stable in physiological environments but prone to enzymatic degradation in biological systems. Combining it with nanocellulose enhances its environmental stability | Environmental stability | 139 and 140 |
5 Versatile applications of ChNFs
The ChNFs, derived from the second most abundant natural polymer, chitin, have emerged as a promising biomaterial due to their attractive properties such as biodegradability, biocompatibility, and high mechanical strength.112 The applications of ChNFs span various fields, including packaging, wastewater treatment, food, agriculture, cosmetics, and biomedicine.141 In the biomedical domain, ChNFs are particularly suitable for tissue engineering, drug delivery, wound dressing, and cancer diagnostics due to their non-toxicity, biocompatibility, and biodegradability. Other applications include cosmetics, food, agriculture, paper finishing, and solid-state batteries. Despite its versatility, chitin's poor solubility presents a challenge, often addressed through modification or functionalization. While traditionally focused on non-biomedical fields, recent research has increasingly highlighted the potential of chitin and ChNFs in biomedical applications.375.1 Biomedical applications
ChNFs, due to their unique properties of biocompatibility, biodegradability, and non-toxicity, have become highly desirable for biomedical applications. In the past fifty years, a wide range of chitin-based materials, including gels, membranes, scaffolds, nanofibers, microfibers, and nanoparticles, have been developed for use in tissue engineering, wound dressing, drug delivery, and cancer recognition.37,141 A study depicted the combination of FfAA11 with a chemical method designed to transform resistant chitins into functionalized materials. The approach employs oxyma-assisted click chemistry using ethyl (hydroxyimino) cyanoacetate, enabling rapid surface modifications. These modifications facilitate the incorporation of a fluorescent probe, a peptide, and gold nanoparticles, thereby enhancing the functionalization of chitins for various applications in materials science and biomedicine. This methodology demonstrates the versatility of click chemistry in surface engineering and its potential for developing advanced materials with tailored properties. The process is environmentally friendly, producing no toxic by-products or waste organic solvents, representing a greener approach to producing chitin-based biomaterials.1425.2 Tissue engineering
ChNFs have garnered significant attention in tissue engineering due to their structural and functional resemblance to the natural extracellular matrix (ECM).37 These nanofibers have been extensively explored as scaffoldings to support the regeneration of human tissues. Tissue engineering aims to restore, replace, maintain, or enhance the function of damaged tissues or organs by utilizing living cells to create biological substitutes.143,144 While chitin possesses low mechanical properties, its potential for bone tissue engineering can be improved by incorporating biomaterials such as hydroxyapatite (HA) or bioactive glass ceramics.141 Carboxymethyl chitin (CMC)/PVA blend nanofibrous scaffolds and α-chitin/nano bioactive glass ceramics (nBGC) composite scaffolds have also demonstrated promise for tissue engineering applications due to their bioactivity and non-toxicity.145 Recent research has highlighted the potential of β-chitin hydrogel/nano-hydroxyapatite (n-HAp) nanocomposite scaffolds, synthesized via freeze-drying, for bone tissue engineering. These scaffolds exhibit high porosity (70–80%), controlled biodegradation (30–40%), and improved protein adsorption.146 The incorporation of nano ZrO2 into chitin-chitosan scaffolds has also been shown to enhance osteogenesis, further expanding the possibilities for bone tissue engineering.147Chitin's biocompatibility and bioactivity can be augmented by the addition of silica. Chitin/nano-silica composite scaffolds have displayed bioactivity in simulated body fluid (SBF), biocompatibility with MG63 cell lines, and enhanced swelling ability, making them suitable for bone tissue engineering.148 Furthermore, α and β-chitin/gelatin membranes have been developed for tissue engineering applications. Chitin regenerated hydrogel (RG) and swelling hydrogel (SG) were prepared using α-chitin and β-chitin, respectively. These hydrogels were then mixed with gelatin and N-acetyl-D-(+)-glucosamine to create chitin/gelatin membranes. Thermal stability studies revealed that RG-based membranes exhibited superior thermal stability compared to SG-based membranes. Both types of membranes supported the growth of NIH/3T3 fibroblast cells, highlighting their potential in tissue engineering (Fig. 17).149,150
 | ||
| Fig. 17 (a) The growth of NIH/3T3 fibroblast cells on the chitin membrane prepared from RG and (b–d) the growth of NIH/3T3 fibroblast cells on different samples of chitin membranes prepared from SG.149 | ||
Chitin–chitosan/nano TiO2 composite scaffolds, synthesized through lyophilization, have also shown promise. Increasing TiO2 content led to reduced pore size, non-toxicity to various cell lines (MG-63, L929, and hMSCs), enhanced thermal stability, bioactivity, swelling, and degradation. These nanocomposite scaffolds can improve cell seeding and tissue growth, making them valuable for bone regeneration.147 Hydroxyapatite (HA)–chitin materials, fabricated by incorporating HA into chitin solutions, have demonstrated non-cytotoxicity, porosity, and enhanced degradation. These properties make them suitable for bone substitution due to their ability to promote the in-growth of surrounding tissues (Fig. 18a and b).146
 | ||
| Fig. 18 (a) SEM image of osteoblasts proliferated on the surface of a 25% HA–chitin thin film after 2 weeks. (b) Micrograph image of cell-free porous HA–chitin matrix after 2 months implantation (Rabbit femur model).151 | ||
In tissue engineering, engineered tissues require enhanced cellular and ECM organization for optimal function.152,153 Cell reorganization is influenced by factors such as topography, mechanical properties (stiffness, elasticity, viscosity), and interactions with the ECM.154 Concentration gradients of immobilized growth factors and ECM molecule alignment also play crucial roles in cellular organization.153 Substrates with controlled micro and nanopatterns have been developed to promote cellular organization, elongation, and orientation in engineered tissue.155 These substrates enable the production of functional and highly ordered cell sheets that can be easily detached and delivered to host tissues via enzymatic degradation or thermal stimulus.156 However, engineered cell sheets are often mechanically weak and challenging to handle, necessitating the use of a support platform for delivery to host tissues.157 These substrates need to be thin, flexible, robust, and easy to handle to ensure conformal contact with the target tissue.158 Structural biopolymers such as chitin, chitosan, and collagen are gaining popularity as support platforms due to their biocompatibility, nontoxicity, cytocompatibility, tunable biodegradability, and mechanical strength.159
Transparent, robust, and ultra-thin ChNF substrates with tunable and superior mechanical properties demonstrate significant promise as potential substrates in tissue engineering.160 Hassanzadeh et al.160 prepared and investigated both supported and free-standing micropatterned substrates composed of self-assembled ChNFs. These substrates, being mechanically robust, flexible, biodegradable, and easy to manipulate, show potential in creating complex tissue structures for regenerative medicine and tissue engineering applications, including myocardial repair, where the chitin substrate could provide mechanical support to damaged tissue during regeneration.152 The fabrication process involved pouring a chitin/HFIP solution onto a mold covered with a glass slide and drying it overnight for supported substrates, while free-standing substrates were obtained from more concentrated solutions.160 The resulting cell sheets were mechanically robust, flexible, and easily manipulated.160 Fig. 19 illustrates approaches to producing transparent, ultra-thin (<10 μm), mechanically robust, and flexible self-assembled ChNF microplate substrates for tissue engineering.160
 | ||
| Fig. 19 ChNF-based micropatterned substrate fabrication process. (a) Chitin/HFIP solution (0.1% w/w) was poured on top of the (b) mold covered with a glass slide to create a supported substrate after drying overnight and substrate optical image with the diffraction pattern. (c) Thicker films were obtained from more concentrated solutions (0.2% w/w) to create free-standing substrates, which were robust and easy to handle. The optical image demonstrated the diffraction pattern on the free-standing chitin film. (d) ChNF substrates are transparent and afford optical inspection (Inset). Fluorescence images of the actin cytoskeleton of the cells on G2 show the entire coverage and alignment of cells within the direction of the patterned features. The white arrow on the right corner of the inset image indicates the direction of the patterns. Scale bar 100 mm.160 | ||
NIH-3T3 fibroblast cells were seeded on the substrates with varying groove sizes to evaluate their behavior for tissue engineering applications.160 The organization of fibroblasts within the extracellular matrix (ECM) of native myocardial tissue is crucial for cell alignment, which influences the heart's electrical and mechanical properties.161 However, fibroblast attachment to chitin substrates is low, potentially due to the lack of reactive species and positive charges on the chitin surface.162 To improve cell attachment, chitin substrates can be partially deacetylated and coated with fibronectin, an ECM protein vital for growth, migration, cell adhesion, and differentiation.163 Deacetylation to 30% enhances crystallinity and intramolecular hydrogen bonding from the remaining acetyl groups.164
Post-deacetylation, cells spread and covered the entire film, aligning along the micropattern's major axis on the glass-supported transparent chitin substrates (Fig. 19d).160 The optical transparency of these substrates also suggests potential applications in retinal regeneration. The biocompatibility, nontoxicity, cytocompatibility, tunable biodegradability, and mechanical strength of ChNFs make them highly promising for regenerative medicine and tissue engineering.
ChNFs in biologically active matrices and TE applications are given in Table 3.
| Matrix | Chitin origin & isoform | Additives | Biomaterials | Role of CHNFs | TE application | Ref. |
|---|---|---|---|---|---|---|
| Chitin | Electrospun nano-fiber mats | No-inflammation in vivo improve cell attachment and spreading | Oral mucosa | 38 | ||
| Alpha isoforms | Nanofiber mat | Surface deacetylation increases cell adhesion and proliferation | Biomedical materials | 165 | ||
| Crab alpha isoform | Wet-spinning microfibers | High water sorption capacity supports the proliferation of rat cardiac myoblasts and mouse bone osteoblasts | Cardiac bone | 21 | ||
| Alpha isoform | Carbon nanotubes | Hydrogels | Enhance mechanical properties hemocompatible biocompatible to neuronal, and Schwann cells improve neuronal cell behavior | Nerve | 166 | |
| Alpha isoform | Hydroxyapatite crystals | Microsphere scaffolds | Promote cell adhesion in vivo bone healing | Bone | 167 | |
| Alkaline phosphatase | Mineralized nanopaper | Enhance mechanical properties homogenous and spatial controlled mineralization | Bone | 167 | ||
| Hydroxypeptide | Hydrogel/cryogel | Enhance mechanical properties | Cartilage | 168 | ||
| Chitosan | Alpha isoform | Films | Enhance mechanical properties. Promote cell adhesion and proliferation | Skin | 169 | |
| Alpha isoform | Films | Enhance mechanical properties to improve biological activities | Dermal tissue | 170 | ||
| Shrimp alpha isoform | Porous microspheres | Enhance mechanical properties. Allow hESCs multi-lineage differentiation | 3D cell culture | 171 | ||
| Alpha isoform | Films | Enhance mechanical properties orientation of structural elements increase specific conductivity promote skin fibroblast adhesion, viability, and proliferation | Skin | 172 | ||
| Cellulose silk | Hydrogel | Induce hMSCs osteogenic differentiation | Bone | 173 | ||
| Alpha Isoform | Hydroxyapatite | Hydrogel multilayer films or membrane | Enhance mechanical properties self-bonding function | Biomedical materials | 174 | |
| Collagen | Shrimp alpha isoform | Microfibers composite | Enhance mechanical properties and induce cell alignment | Vascular muscle | 174 | |
| Squid pen beta isoform | Films | Complement collagen biocompatibility on fibroblast growth | Biomedical materials | 175 | ||
| Gelatin | Crab alpha isoform | Films | Enhance wound healing process | Biomedical materials | 124 | |
| Crab alpha isoform | Nanocomposite films | Enhance mechanical properties | Biomedical materials | 176 | ||
| Crab alpha isoform | Hydrogels | Enhance mechanical properties | Tendons ligament | 177 | ||
| Gelatin methacrylate | Squid pen beta isoform | Hydrogels | Enhance mechanical properties | Vascular | 178 | |
| Lignin | Alpha isoform | Microcapsule like system | Biocompatible anti-inflammatory activity | Skin | 179 |
5.3 Wound dressing
Chitin-based membranes exhibit properties conducive to wound dressing applications, including good biocompatibility, high durability, low toxicity, antibacterial activity, and liquid adsorption. These properties can be further enhanced by incorporating polymers such as alginate, hyaluronic acid, poly(vinyl alcohol), α-poly(glutamic acid), polyethylene glycol diacrylate, and 2-hydroxyethyl methacrylate (Fig. 20).180 | ||
| Fig. 20 Chitin scaffold (left) and chitin/nano Ag composite scaffold (right) are suitable for wound healing applications.180 | ||
Studies have shown that α-chitin/nanosilver and β-chitin/nanosilver composite scaffolds exhibit antibacterial activity against Escherichia coli and Staphylococcus aureus, suggesting their potential as wound dressings.181 Further, β-chitin-based composites have demonstrated favorable antibacterial, swelling, cell attachment, blood clotting, and cytotoxicity properties, supporting their suitability for wound dressing applications. Research also indicates that modified ChNFs derived from crab shells can improve clinical symptoms and control ulcerative colitis.182
Electrospinning has been employed to fabricate chitosan (CS) blended with ethylene diamine tetraacetic acid (EDTA) and polyvinyl alcohol (PVA) (CS-EDTA/PVA) nanofiber scaffolds. These scaffolds exhibit good antibacterial activity and promote wound healing (Fig. 21).183
 | ||
| Fig. 21 Healings of the wound treated with three kinds of wound dressings at days 1, 4, 7, and 23 (a) gauze (negative control), (b) 30/70 CS-EDTA/PVA nanofiber scaffold and (c) commercial wound dressing (Sofra-tulle-register) (positive control).183 | ||
A comparison between electrospun ChNFs and commercial chitin microfibers (ChMs) revealed that ChNFs, with an average diameter of 163 nm, facilitated superior cell attachment and spreading of normal human keratinocytes and fibroblasts compared to ChM (average diameter: 8.77 μm). ChNFs also exhibited a faster degradation rate. The high surface-to-volume ratios and three-dimensionality of ChNFs contribute to their potential for skin regeneration and wound healing applications.38,184
5.4 Drug delivery system and cancer diagnosis
Chitosan, a biocompatible biopolymer, has garnered extensive attention for its diverse applications in drug delivery, tissue engineering, and more. In cancer therapy, chitosan-based nanoparticles demonstrate promising capabilities for targeted drug delivery, minimizing side effects and enhancing therapeutic efficacy. These nanoparticles can either passively accumulate at tumor sites via the Enhanced Permeability and Retention (EPR) effect or target tumor cells using ligands specific to tumor receptors.184,185 The nano chitin-fiber drug system has a higher specific surface area, shorter diffusion channels, and a greater release rate than bulk materials. Geetha et al.186 investigated amorphous chitin nanoparticles (AC-NPs) loaded with curcumin (CUR), docetaxel (DOC), and 5-fluorouracil (5-FU). They found entrapment efficiencies of 98.1% for CUR-AC-NPs, 77.2% for DOC-AC-NPs, and 47.12% for 5-FU-AC-NPs. CUR-AC-NPs demonstrated superior drug uptake and increased cell death in gastric adenocarcinoma (AGS) cells, making them the most effective carrier among the studied AC-NPs.Controlled-release drug delivery systems, enabled by chitosan, offer heightened safety and reliability in cancer treatment.185 This technology facilitates a predictable release of therapeutic agents, reducing adverse effects while maximizing efficacy. For instance, chitin nanogels (ChNGs), prepared through controlled regeneration techniques, have exhibited increased swelling and biodegradability compared to chitin.187,188 These ChNGs, combined with rhodamine-123 dye, demonstrate sound cellular localization without harming cells, suggesting their potential in drug delivery and tissue engineering. Moreover, carboxymethyl chitin (CM-chitin), a pH-sensitive derivative of chitin, can function as a hydrophilic matrix for controlled drug release. Recent studies have shown that CM-chitin releases aspirin at a slower rate in simulated gastric fluid than simulated intestinal fluid, indicating its potential for drug delivery applications, although further research is necessary to ensure safety and clinical viability.187,188 The ChNGs have also been investigated for the delivery of anticancer drugs. Doxorubicin-loaded ChNGs, for example, have demonstrated biodegradability, biocompatibility, and toxicity toward various cancer cells, making them suitable for treating prostate, breast, lung, and liver cancer.189 Chitin oligomers have shown potential as tumor growth inhibitors in mouse models, suggesting host-mediated effects.190
ChNFs, incorporated with anticancer agents like ellagic acid, have been explored for breast cancer treatment. Modified injectable hydrogels containing ChNFs have shown promise in anti-tumor drug delivery. ChNFs also find applications in bone regenerative engineering, a vital field in orthopedics for repairing damaged bones. Additionally, ChNFs synthesized with UV-absorbing chromophores, such as urocanic acid, have demonstrated a protective effect against UV radiation-induced damage in mice. ChNFs have significant cytotoxic effects on various cancer cells while exhibiting low toxicity to non-cancer cells and can induce apoptosis.191 Solairaj et al.192 evaluated the toxicity of chitin nanoparticles (CNP), silver nanoparticles (AgNP), copper nanoparticles (CuNP), and their nanocomposites on MCF-7 breast cancer cells. They found that CNP combined with metal nanocomposites showed more significant cytotoxicity than AgNP or CuNP alone, with CNP/AgNP notably inducing apoptosis. Additionally, the toxicity of AgNP and CuNP to non-cancerous HEK-293T cells decreased with CNP, indicating higher activity against cancer cells.
Chitosan nanocomposites effectively retain and protect anticancer drugs like doxorubicin and dexamethasone, increasing their therapeutic efficacy. Chitosan-coated nanoparticles, including those loaded with doxorubicin, exhibit potent antitumor activity against various cancers, including ovarian cancer.184 Furthermore, pH-sensitive polymers like chitosan facilitate controlled drug release in tumor tissues due to their responsiveness to the acidic tumor microenvironment.
Chitosan's inherent properties, such as biodegradability and pH sensitivity, make it an auspicious material for drug delivery systems. Understanding the molecular interactions between chitosan and drugs like doxorubicin is critical for optimizing drug delivery efficiency. Computer simulations, such as molecular dynamics, offer valuable insights into these atomic-level interactions, aiding in developing effective chitosan-based drug delivery.193
5.5 Biosensing applications
ChNFs possess numerous advantageous properties, including biodegradability, biocompatibility, commercial availability, affordability, abundance, flexibility, transparency, and remarkable mechanical and physicochemical attributes. Leveraging these beneficial features, researchers112 have developed transparent, flexible, biocompatible, lightweight, and efficient optical sensing bioplatforms by embedding various plasmonic nanoparticles (NPs), such as silver and gold NPs, within ChNF paper. ChNFs can be derived from the abundant and cost-effective raw material of shrimp shells. In contrast, the production of bacterial cellulose (BC) nano paper, also employed in biosensing, is both time-consuming and expensive, whereas ChNF production is rapid, straightforward, and inexpensive (Fig. 22).112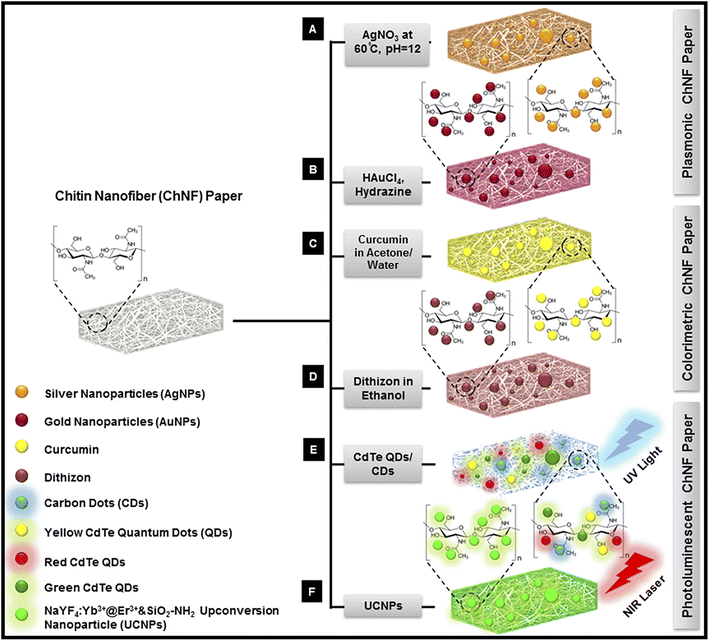 | ||
| Fig. 22 Schematic representation of the fabrication of various ChNF paper-based nanocomposites: (A and B) Plasmonic nanocomposites: (A) silver nanoparticle-ChNF paper (AgNPs-ChNF paper); (B) gold nanoparticle-ChNF paper (AuNPs-ChNF paper). (C and D) Colorimetric nanocomposites: (C) curcumin-ChNF paper (Cur-ChNF paper); (D) Dithizone-ChNF paper (DTZ-ChNF paper). (E and F) Photoluminescent nanocomposites: (E) CdTe@ZnS quantum dot-ChNF paper (QDs-ChNF paper) and carbon dot-ChNF paper (CDs-ChNF paper); (F) aminosilica-coated NaYF4:Yb3+@Er3+ upconversion nanoparticle-ChNF paper (UCNPs-ChNF paper).112 | ||
The fabrication, sensing mechanisms, and characterization of various ChNF paper-based nanocomposites have been investigated.112 Techniques such as UV-visible spectroscopy (UV-vis), field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDX), and thermogravimetric analysis (TGA) have demonstrated the creation of transparent, flexible, biocompatible, compact, portable, and efficient ChNF paper-based optical sensing bioplatforms. These platforms align with the World Health Organization's (WHO) ASSURED criteria for ideal diagnostic devices, emphasizing sensitivity, specificity, user-friendliness, affordability, rapidity, robustness, equipment-free operation, and deliverability to end-users (Fig. 23 and 24).
 | ||
| Fig. 23 Pictures of the fabricated ChNF paper-based sensing platforms with (A) 2D multi-wall, (B) 2D cuvette, and (C) spot patterns. (D) Picture showing the flexibility of the fabricated ChNF paper-based sensing platform. (E) Picture of the fabricated ChNF paper-based sensing platform.112 | ||
 | ||
| Fig. 24 Scanning electron micrographs of the fabricated AgNPs-ChNF paper: (a) in situ synthesized AgNPs-ChNF; (b) AgNPs-ChNF in the presence of 100 ppm MTZ; (c) AgNPs-ChNF paper in the presence of 3 ppm CN−. The inset image (upper left) shows a digital photograph of the fabricated AgNPs-ChNF paper, and (d) displays the AgNPs-ChNF paper upon the addition of MTZ (0–200 ppm).112 | ||
Chitin, a polymer of N-acetyl glucosamine prevalent in fungal cell walls, insect exoskeletons, and other natural structures, serves as the precursor for chitosan. With its enhanced solubility and hydrogel-forming properties, Chitosan is more extensively utilized in biosensor applications.194 Notably, the use of tyrosinase as a model enzyme immobilized on chitosan has demonstrated the creation of a sensitive phenol biosensor with a wide dynamic range, highlighting the adaptability of this approach for various sensing applications. Among bio-based polymers, chitin is the second most abundant natural polymer after cellulose. With an annual biosynthesis of approximately 1010–1011 tons, this natural amino-polysaccharide, composed of β-(1,4)-2-acetamido-2-deoxy-D-glucose, is primarily found in the exoskeletons of marine shrimp and crabs, insects, fungi, and yeasts. Chitin has garnered significant attention in various fields due to its inherent advantages over conventional polymeric materials, including low cost, abundance, non-toxicity, renewability, biodegradability, sustainability, and biocompatibility.112
A highly sensitive and selective label-free electrochemical immunosensor has been successfully developed to detect prostate-specific antigen (PSA). This sensor utilizes a composite of chitosan, graphene, ionic liquid, and ferrocene (CS-GR-IL-Fc) drop-cast onto a screen-printed carbon electrode (SPCE) and subsequently frozen to generate a 3D porous cryogel layer (CS-GR-IL-Fc cry). The cryogel is further decorated with gold nanoparticles (AuNPs).195 Additionally, glucose-treated rGO-activated carbon (rGO/AC) composites have enabled the detection of glucose within a range of 0.002 to 10 mM, exhibiting a sensitivity of 61.06 A mM cm−2, a response time of 4 seconds and a low detection limit of 2 μM, further highlighting the potential of these materials in biosensor applications.196
5.6 Water treatment
Environmental protection has become a global priority, driving industries to seek cleaner technologies. Chitin and its derivatives, like hydroxyl methyl chitin, demonstrate potential in wastewater treatment due to their low cost, non-toxicity, and ability to bind pollutants.185 Chitin's heavy metal chelating capacity has been demonstrated for metals including copper, iron, nickel, chromium, mercury, lead, zinc, cadmium, silver, and cobalt, with the strongest binding affinity observed for mercury and the weakest for cobalt.197Biocomposites incorporating chitin with nano-hydroxyapatite (n-HAp) have effectively removed Fe(III) from aqueous solutions. The Fe(III) sorption capacity of n-HAp/chitin biocomposites (5800 mg kg−1) surpassed that of n-HAp alone (4238 mg kg−1), following a Langmuir isotherm and characterized as endothermic and spontaneous, suggesting applicability in water treatment. Similar experiments demonstrated the sorbents' selectivity for metal ions in the order Fe(III) < Cu(II) < Cr(VI).198,199
In the context of mining-influenced water (MIW) with its high concentration of toxic metals, both Chitorem SC-20 (raw crushed crab shell) and Chitorem SC-80 (chitin polymer) have been investigated. SC-20 effectively removed iron, lead, and zinc and partially removed copper, cobalt, and manganese. SC-80 partially removed cobalt, manganese, lead, and cadmium without precipitation.200 Chitin/cellulose composite membranes, leveraging their microporous structure, have efficiently removed heavy metal ions like mercury, copper, and lead. The adsorption capacity followed the Hg2+ > Pb2+ > Cu2+, increasing with higher chitin content. These membranes offer a “green” approach to wastewater treatment due to their regenerability.201 Chitin hydrogels also exhibit promise in wastewater treatment due to their microporous structure, large surface area, and affinity for dye adsorption.202
Chitin and chitosan nanoparticles have numerous advantages in water filtration, including biodegradability, biocompatibility, and flexibility. Their performance is further enhanced when incorporated as nanofillers in biopolymer-based nanocomposites, offering improved surface area, mechanical stability, and customizable functionality. The unique properties of biopolymers enable the effective removal of heavy metals, organic pollutants, and microorganisms from water, making them valuable additions to membrane technologies and expanding their potential applications in water purification.203
5.7 Nanostructured film
The successful preparation of nanostructured films from chitin underscores its potential for creating value-added products. These films, being biodegradable and biocompatible, hold promise for applications as biomedical interfaces visually represent the nanostructured film production process from crab shells (Fig. 25). | ||
| Fig. 25 Photographic presentation of nanostructured film production from crab shells.59 | ||
ChNFs, with sizes ranging from 12 to 30 nm, were synthesized from chitin powder using a steam explosion technique followed by mild hydrolysis with oxalic acid. The CHNFs were then incorporated into natural rubber (NR) latex to create NR/CB/CHNF composites. It involved the uniform dispersion of ChNFs within the latex, followed by drying and mixing with carbon black (CB) using a two-roll mill. Remarkably, at a CHNF loading of 1 phr (parts per hundred rubber), the NR/CB/CHNF composite exhibited a substantial increase in mechanical properties compared to neat NR. Tensile strength improved by approximately 47%, and tear strength saw an even more significant enhancement of 160%. Furthermore, dynamic mechanical analysis revealed a 50% reduction in loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at 60 °C for the NR/CB/CHNF 1.0 composite compared to the NR/CB50 composite. This study successfully demonstrated the development of an innovative and eco-friendly tire tread formulation, contributing to advancing a circular economy and sustainable practices.204,205
δ) at 60 °C for the NR/CB/CHNF 1.0 composite compared to the NR/CB50 composite. This study successfully demonstrated the development of an innovative and eco-friendly tire tread formulation, contributing to advancing a circular economy and sustainable practices.204,205
5.8 Food and nutrition
Chitin and its derivatives find extensive applications in the food industry, encompassing the creation of value-added food products, preservation against microbial spoilage, waste recovery from food processing, development of biodegradable films, water purification, acid removal and clarification of fruit juices, and color stabilization. They also serve as food additives, thickening agents, emulsifiers, and natural flavor extenders. Chitin's ability to control moisture transfer, antioxidant release, heat transfer, respiration rate, and enzymatic browning in fruits further expands its utility in edible film production. Research suggests that chitin oligomers' physiological activities and functional properties are influenced by their degree of polymerization (DP), with high DP oligomers exhibiting greater functionality than low DP oligomers.206 These characteristics make them promising candidates for developing healthcare products, biopesticides, food, and additives.207 ChNFs also have applications in the sugar industry for adsorbing melanoidins, complex biopolymers of amino-carbonyl compounds, from sugar syrup.208Recent advancements include the development of chitin/chitosan whisker rectorite ternary films, demonstrating excellent water resistance and anti-bacterial properties, making them highly suitable for food packaging applications.209 Studies on kelp grouper and E. bruneus have shown that diets enriched with chitin and chitosan can increase phagocytic activity, complement activity, red blood cell count, hematocrit, hemoglobin levels, and white blood cell count.210 Additionally, dietary chitin supplements (5%) have been shown to modulate gut bacteria, including Bacillus thuringiensis, which exhibits bactericidal properties against fish pathogens, potentially offering a means to enhance disease resistance in fish.211 Carboxymethyl chitin (CM-chitin) has also demonstrated anti-obesity and anti-adipogenic effects, assessed by measuring lipid accumulation.212 These findings collectively highlight the significant potential of chitin-based materials in developing and processing novel food products.
ChNFs hold promise as antibacterial nanocomposite materials due to their inherent biocompatibility, organic nature, amino-containing macromolecular structure, and nano-size effects. In a recent study, molybdenum disulfide quantum dots (MoS2QDs) were successfully anchored onto partially dChNFs through aqueous reactions. The resulting MoS2QDs/dChNF nanocomposite demonstrated remarkable antibacterial activity against Escherichia coli under various conditions. At a concentration of 200 μg mL−1, the survival rates of bacteria were significantly reduced compared to DEChN alone, particularly under near-neutral conditions (pH ≈ 6) with a bacteriostatic rate exceeding 90%. In contrast, MoS2QDs/TOCN (prepared with TEMPO-oxidized cellulose nanofibers) exhibited no apparent antibacterial activity, underscoring the crucial role of DEChN and its amino groups. The MoS2QDs/DEChN composite film shows potential for preserving meat by delaying spoilage.213
5.9 Cosmetic and toiletries
Chitin and its primary derivative, chitosan, hold promise in cosmetics and toiletries due to their unique fungistatic, fungicidal, and solubility properties in organic acids. Their applications span hair, oral, and skin care.214 Notably, chitin and chitosan's properties make them ideal for treating acne, maintaining skin moisture, and enhancing hair suppleness and skin tone. They are found in various products like creams, lotions, permanent waving lotions, chewing gums, nail enamel, foundation, eye shadow, lipstick, cleansing materials, toothpaste, and bath agents. Certain derivatives also serve as nail lacquers.16,215 In oral care, they are found to be used as dental fillers, false teeth cleaners, and agents that prevent plaque formation and tooth damage.216Derived from insects, chitin and its derivatives are gaining traction in cosmetics for their biocompatibility and sustainability. They offer antioxidant and antimicrobial benefits, proving effective in diverse cosmetic and cosmeceutical applications.217 Developing sustainable and innovative hair products can address the growing consumer demand for natural and eco-friendly cosmetics catering to skin and environmental health. Several abundant biopolymers, like chitin, chitosan, and lignin, exhibit specific functionalities (antimicrobial, antioxidant, anti-inflammatory, etc.). They can be combined in nanostructured tissues, powders, and coatings to create advanced cosmeceuticals with potential applications in other sectors, such as biomedical, personal care, and packaging. The cosmetics and wellness market is projected to grow significantly in the coming years. This trend, alongside advancements in nanobiotechnology, suggests the need for a shift towards a circular economy. This model prioritizes redesigning, reducing, recycling, and reusing products, promoting sustainability and responsible consumption.218
5.10 Agriculture
Chitin and its derivatives exhibit various properties, such as bactericidal and fungicidal activity, making them attractive candidates for agricultural applications. They have been used to assess mold contamination in agricultural products215 and have shown potential to enhance plant growth and defense mechanisms. For instance, chitin-treated seeds exhibit accelerated growth due to decreased insect and fungal penetration.219 Chitin oligosaccharides, derived from chitin/chitosan hydrolysis, can act as antioxidants, antimicrobials, and biofertilizers.218 Additionally, chitin functions as a safe elicitor in controlled peanut cultures, inducing the production of trans-resveratrol and trans-piceatannol.220 Chitin and its derivatives can also trigger defense responses in various plant species by inducing fungal microbe-associated molecular patterns.221 Chitin and its derivatives find applications as fertilizers, soil conditioners, plant disease control agents, antitranspirants, natural product retardants, and seed coatings. They bolster natural plant defenses and act as plant growth regulators, growth stimulants, anti-stress agents, and elicitors for secondary metabolite production. While numerous reviews exist on chitosan in agriculture, this mini-review focuses specifically on the agricultural applications of chitin, not chitosan.2225.11 Energy storage devices
Renewable and green energy sources like tidal, solar, geothermal, wind, and biofuels are increasingly prominent in the scientific community. These sources drive the rapid development of advanced energy storage systems with high energy densities.223 As the demand for reliable electricity storage grows, advanced devices like fuel cells, supercapacitors, and separators for Li/Na-ion batteries are gaining importance. To ensure the sustainability of renewable energy on a large scale, developing low-cost, eco-efficient, and high-energy-density Li/Na-ion batteries is crucial.224 Separators are critical in these batteries, impacting their safety, performance, and sustainability.225Currently, commercial separators are made from polyolefins like polyethylene and polypropylene. While these materials offer advantages like high ionic conductivity and chemical/electrochemical stability, they have limitations, including low thermal stability and weak mechanical strength. These weaknesses can lead to high-temperature safety issues, potentially causing battery ignition or explosion. Moreover, their production from petrochemicals raises environmental concerns. ChNFs derived from prawn and crab shells present a potential alternative separator material. These nanofibers possess good mechanical strength, thermal stability, and sustainability.226 Chitin is a naturally abundant resource with annual biosynthesis estimated at 1010 to 1011 tons![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 sufficient for large-scale separator production for Li/Na-ion batteries (Fig. 26).226 Studies have shown that ChNF separators exhibit electrochemical performance comparable or superior to commercial polypropylene separators in LiFePO4/Li and Na3V2(PO4)3/Na half cells.226 Moreover, ChNFs can be used to produce low-cost, nitrogen-doped porous carbon electrodes with large surface areas and open hierarchical porous nanostructures, serving as a conductive substrate for zeolitic imidazolate frameworks (ZIF-8) derived porous carbon.227 Nitrogen hierarchical porous carbon (N-HPC) electrodes derived from ChNC have demonstrated excellent capacitive performance in supercapacitors due to their high specific surface area, nanostructures, rich surface functional groups, and nitrogen content covalently bound with carbon atoms (Fig. 27).227 These findings suggest that ChNF is a promising material that can contribute significantly to the future sustainable development of energy storage devices. Piezoelectric ChNFs are a new type of biodegradable sensor material. Chitin polymers dissolve with the chitinase enzyme in eight days without releasing toxic substances, making them promising for environmentally friendly piezoelectric materials. Hoque et al.228 reported two high-performance piezoelectric nanogenerators (PENG) based on ChNFs: a pure ChNFs PENG (CPENG) and a composite ChNFs/PVDF PENG (PCPENG). Both devices effectively conduct electricity from mechanical energy sources, including light touch and sonication, and have a short capacitance charging time, making them viable alternative energy sources for portable medical equipment. Chen et al.229 developed a highly conductive film made of ChNFs and multiwalled carbon nanotubes (MWCNT) for foldable electronic devices. After hydrogel treatment, the ChNFs/MWCNT gel film became denser and exhibited nearly double the conductivity compared to the untreated film—9.3 S cm−1 for the gel film versus 4.7 S cm−1 for the original. This enhanced conductivity is promising for applications in electrodes or collectors.
226 sufficient for large-scale separator production for Li/Na-ion batteries (Fig. 26).226 Studies have shown that ChNF separators exhibit electrochemical performance comparable or superior to commercial polypropylene separators in LiFePO4/Li and Na3V2(PO4)3/Na half cells.226 Moreover, ChNFs can be used to produce low-cost, nitrogen-doped porous carbon electrodes with large surface areas and open hierarchical porous nanostructures, serving as a conductive substrate for zeolitic imidazolate frameworks (ZIF-8) derived porous carbon.227 Nitrogen hierarchical porous carbon (N-HPC) electrodes derived from ChNC have demonstrated excellent capacitive performance in supercapacitors due to their high specific surface area, nanostructures, rich surface functional groups, and nitrogen content covalently bound with carbon atoms (Fig. 27).227 These findings suggest that ChNF is a promising material that can contribute significantly to the future sustainable development of energy storage devices. Piezoelectric ChNFs are a new type of biodegradable sensor material. Chitin polymers dissolve with the chitinase enzyme in eight days without releasing toxic substances, making them promising for environmentally friendly piezoelectric materials. Hoque et al.228 reported two high-performance piezoelectric nanogenerators (PENG) based on ChNFs: a pure ChNFs PENG (CPENG) and a composite ChNFs/PVDF PENG (PCPENG). Both devices effectively conduct electricity from mechanical energy sources, including light touch and sonication, and have a short capacitance charging time, making them viable alternative energy sources for portable medical equipment. Chen et al.229 developed a highly conductive film made of ChNFs and multiwalled carbon nanotubes (MWCNT) for foldable electronic devices. After hydrogel treatment, the ChNFs/MWCNT gel film became denser and exhibited nearly double the conductivity compared to the untreated film—9.3 S cm−1 for the gel film versus 4.7 S cm−1 for the original. This enhanced conductivity is promising for applications in electrodes or collectors.
 | ||
| Fig. 26 Microstructural characterization of the CNM separators fabricated by the ChNFs from prawn shells and commercialized PP separator. (a–e) SEM images of CNM separators with various amounts of SDCA in ChNF suspension (0, 20 wt%, 30 wt%, 40 wt%, and 50 wt%, respectively) and corresponding cross-sectional SEM images (Insets). (f) The SEM image of the commercialized PP separator and corresponding cross-section image (Inset).226 | ||
 | ||
| Fig. 27 Schematic illustration of the preparation of N-doped hierarchically porous carbon electrodes for high-performance supercapacitors.227 | ||
While chitin- and chitosan-derived HCs have similar d-spacings and crystallite sizes, their pyrolysis results in different carbon structures. Chitin yields micro–mesoporous carbon with a high specific surface area, while chitosan produces nonporous carbon. Despite this, both materials initially exhibit a comparable specific capacity of 280 mA h g−1 (at C/10 rate). However, their electrochemical performance diverges upon prolonged cycling at higher rates. Inorganic contaminants in chitosan-derived HC may hinder sodium ion diffusion, slowing electrochemical reactions and leading to polarization buildup. Optimizing chitosan-derived HC through acid treatment can unblock micropores, increase carbon content, and enhance the active surface area, thus mitigating capacity fading.230
5.12 Other relevant applications
Novel research has explored the potential of various chitin nanowhiskers/nanofibers, including TEMPO-oxidized α-chitin nanowhisker, HCl-hydrolyzed chitin nanowhisker, partially deacetylated α-chitin nanowhisker/nanofiber mixture, and squid pen β-ChNFs. These materials have demonstrated promising oxygen barrier properties, suggesting their suitability for biocompatible and biodegradable films in various applications.107 Furthermore, chitin-based molecularly imprinted polymers (MIPs), such as cholesteryl chitin carbonate, have been developed using covalent and non-covalent imprinting techniques. These MIPs exhibit high binding capacity with cholesterol in non-polar solvents through hydrogen bonding, making them potential candidates for sensing, separation, and delivery applications.231A facile method employing the TEMPO/NaClO2/NaClO system has enabled the fabrication of zwitterionic ChNFs through deacetylation and oxidation. The ChNFs facilitate chitosan immobilization of anthocyanin and enhance the rheological properties and printability of chitosan/anthocyanin/ChNF-based smart inks. Screen-printed labels incorporating these inks have been successfully used as fish freshness indicators, providing a convenient and visual strategy for assessing fish quality. The use of degradable, edible, and environmentally friendly ingredients in these labels further underscores their appeal.219
Active films composed of chitosan, esterified ChNFs, and rose essential oil (REO) have been developed in food preservation. The combined effects of ChNFs and REO on chitosan films' structural and physicochemical properties were investigated. Results showed enhanced water resistance, mechanical properties, UV resistance, increased oxygen permeability, and antioxidant activity.232 Zou et al.233 prepared ChNFs using low-intensity ultrasound and subsequently developed antibacterial finishing agents. Cotton fabrics treated with ChNFs showed less color difference than untreated fabrics, achieving an antibacterial rate of 99%. The study suggests good potential for using ChNFs in the textile industry. Another study explored the use of chitosan and esterified ChNFs with varying proportions of scallion flower extract (SFE) to create active packaging for banana preservation. Incorporating CF improved barrier properties and mechanical strength, while SFE enhanced physical and biological activity. The CF-4% SFE film demonstrated superior oxygen barrier performance, antibacterial efficacy, and antioxidant activity. Fresh-cut bananas stored in CF-4% SFE exhibited reduced weight loss, starch deterioration, color alteration, and visual changes compared to those in polyethylene film, highlighting its potential as a sustainable alternative to conventional plastic packaging.234
Finally, sustainable ChNF-coatings have been investigated to extend the storage life of fresh cucumbers and inhibit bacterial growth.235 The degree of acetylation and deacetylation time influenced the morphology and antibacterial properties of ChNFs. ChNFs deacetylated for 120 and 240 min, exhibiting a fibril-like structure, significantly reduced moisture loss and bacterial growth on cucumber surfaces,236 demonstrating their potential for reducing food waste and dependence on petroleum-based packaging materials.
6 Challenges and future outlook
Although the synthesis of chitin and chitosan nanofibers from various sources, such as crab, shrimp, and mushrooms, utilizing top-down and bottom-up approaches poses challenges to environmental sustainability and cost-effectiveness, the principal advantage of ChNFs over cellulose and silk fibroin lies in their hydrophilic properties and sensitivity to environmental conditions. This unique characteristic enhances their potential applications in various fields, including biomedicine and material science, where tailored interactions with environmental factors are essential. Synthesis methods such as mechanical disintegration and acid hydrolysis are being investigated. However, their environmental impacts require thorough evaluation through life cycle assessments (LCAs). Energy-intensive mechanical disintegration processes require optimization. Acid hydrolysis requires the management and recycling of significant amounts of acid, which raises environmental concerns. Industrial production of nano chitin must meet the quality of lab-synthesized materials. Batch-to-batch variations should be minimized through standardized testing protocols. Differences in raw chitin sources, such as crustaceans and fungi, can impact production consistency. The low solubility of chitin and chitosan nanofibers at neutral pH and the variability of their antimicrobial effectiveness under different conditions highlight the need for further research.By enhancing its solubility, boosting its antimicrobial activity, and deepening our understanding of its mechanisms, chitosan, and its derivatives could revolutionize fields such as sustainable agriculture, medical wound care, and food preservation. Nanochitin demonstrates considerable potential in the realms of bio-composites and high-barrier packaging films. However, the relationship between the dimensions of nanochitin and its material properties remains inadequately understood. Future investigations should focus on optimizing the reinforcement capabilities of nanochitin in nanocomposite formulations while elucidating the interplay between filler dimensions and matrix interactions. Besides, the application of techniques like TEMPO oxidation facilitates the introduction of zwitterionic properties to nano-chitin. This modification enhances its ability to interact effectively across diverse environments, making it particularly advantageous for various biomedical applications. The zwitterionic characteristics contribute to improved biocompatibility and reduced protein adsorption, thereby enhancing the functionality of nano-chitin in areas such as drug delivery, tissue engineering, and biosensing. Further research into the mechanisms of these interactions could expand the potential uses of nano-chitin in innovative biomedical solutions. Furthermore, the thermodynamic analysis of highly optimized ChNFs structure integrated with diverse nanomaterials, catalysts, and drugs can be studied in future research. This analysis aims to yield insights into the efficacy of ChNFs, thereby enhancing our understanding of their potential applications in cancer therapy and other biomedical domains. Such studies could provide binding affinity for targeted treatment methods and the effectiveness of drugs interacting with ChNFs.
Apart from this, the moisture sensitivity inherent to nanochitin poses significant challenges to its application. Strategies such as thermal annealing and the incorporation of superhydrophobic surfaces, particularly through SLIPS (Slippery Liquid-Infused Porous Surfaces) technology, may provide viable solutions. The prospective applications of self-assembled nanochitin structures span across various fields, including optical systems, load-bearing materials, environmental management, and electrochemical devices. Nonetheless, challenges of fabrication and consistency remain prevalent within the field.
In addition, the exploration of chitin-derived carbon materials for electrochemical and environmental applications, including energy storage and catalytic functions, is still in its primary phase. There is a need for further research aimed at optimizing these materials for targeted applications.
7 Conclusion
This comprehensive review examined the methods for preparing chitin and ChNFs, along with their diverse applications. Numerous preparation methods have been documented, with ChNFs extracted from various sources, including crab shells, prawn shells, shrimp shells, squid pens, and mushrooms, using techniques like grinding or high-pressure water jet disintegration. The resulting ChNFs exhibit fine, uniform structures (≈10–20 nm in width) with a high aspect ratio. Unlike traditional chitin, which is insoluble and precipitates in water, ChNFs disperse homogeneously, facilitating handling and molding for various applications. This study explores their utilization in biomedical applications (tissue engineering, wound dressing, drug delivery systems, cancer diagnostics), biosensing, water treatment, food and nutrition, cosmetics and toiletries, agriculture, energy storage devices, and other fields. Chitin and its derivatives, owing to their nontoxicity, biocompatibility, and biodegradability, are attractive natural materials for biomedical applications. This review aims to raise awareness of the importance of chitin, the second most abundant natural polymer, and its derivatives by discussing various aspects, including biological properties and applications. Despite progress, the production and applications of chitin, chitosan, and ChNFs derived from crab shells, prawn shells, squid pens, and mushrooms remain limited, necessitating further research to address knowledge gaps. Moreover, most studies to date have been conducted in vitro or in vivo, highlighting the need for clinical investigations to establish the true potential of chitin and ChNFs in clinical practice.Data availability
No new data were created or analyzed during this study. Data sharing is not applicable to this article.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was partially supported by grants from (a) the Research and Publication Cell, University of Chittagong, Bangladesh, for the project 'Extraction and characterization of chitin and chitosan from shrimp and prawn wastes and their applications'; (b) the Environmental Radioactivity Research Network Center (ERAN: I-24-21) at Fukushima University, Japan; and (c) Grants-in-Aid for Scientific Research (24K15337) from the Japan Society for the Promotion of Science (JSPS). While preparing this work, the author(s) used Gemini Advanced, QuillBot, and Grammarly to paraphrase and edit the language. After using those tools, the author(s) reviewed and revised the content as needed and take(s) full responsibility for the publication's content.References
- S. Ifuku, Chitin and chitosan nanofibers: Preparation and chemical modifications, Molecules, 2014, 19(11), 18367–18380, DOI:10.3390/molecules191118367.
- N. A. Z. Abidin, F. Kormin, N. A. Z. Abidin, N. A. F. M. Anuar and M. F. A. Bakar, The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources, Int. J. Mol. Sci., 2020, 21(14), 4978, DOI:10.3390/ijms21144978 , 32679639.
- G. Cabrera-Barjas, C. González, A. Nesic, K. P. Marrugo, O. Gómez, C. Delattre, O. Valdes, H. Yin, G. Bravo and J. Cea, Utilization of marine waste to obtain β-chitin nanofibers and films from giant Humboldt squid Dosidicus gigas, Mar. Drugs, 2021, 19(4), 184, DOI:10.3390/md19040184 , 33810536.
- M.-C. Li, Q. Wu, K. Song, H. N. Cheng, S. Suzuki and T. Lei, Chitin nanofibers as reinforcing and antimicrobial agents in carboxymethyl cellulose films: Influence of partial deacetylation, ACS Sustain. Chem. Eng., 2016, 4(8), 4385–4395, DOI:10.1021/acssuschemeng.6b00981.
- S. Ifuku, M. Nogi, K. Abe, M. Yoshioka, M. Morimoto, H. Saimoto and H. Yano, Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells, Biomacromolecules, 2009, 10(6), 1584–1588 CrossRef CAS PubMed.
- S. Ifuku and H. Saimoto, Chitin nanofibers: preparations, modifications, and applications, Nanoscale, 2012, 4(11), 3308–3318, 10.1039/C2NR30383C.
- D. Raabe, P. Romano, C. Sachs, H. Fabritius, A. Al-Sawalmih, S.-B. Yi, G. Servos and H. G. Hartwig, Microstructure and crystallographic texture of the chitin–protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus, Mater. Sci. Eng., A, 2006, 421(1), 143–153, DOI:10.1016/j.msea.2005.09.115.
- P.-Y. Chen, A. Y.-M. Lin, J. McKittrick and M. A. Meyers, Structure and mechanical properties of crab exoskeletons, Acta Biomater., 2008, 4(3), 587–596, DOI:10.1016/j.actbio.2007.12.010.
- M. M. Giraud-Guille, Fine structure of the chitin-protein system in the crab cuticle, Tissue Cell, 1984, 16(1), 75–92, DOI:10.1016/0040-8166(84)90020-X.
- D. Raabe, C. Sachs and P. Romano, The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material, Acta Mater., 2005, 53(15), 4281–4292, DOI:10.1016/j.actamat.2005.05.027.
- X. Zhang and M. Rolandi, Engineering strategies for chitin nanofibers, J. Mater. Chem. B, 2017, 5(14), 2547–2559, 10.1039/C6TB03324E.
- H.-J. Jin and D. L. Kaplan, Mechanism of silk processing in insects and spiders, Nature, 2003, 424(6952), 1057–1061, DOI:10.1038/nature01809.
- F. Vollrath and D. P. Knight, Liquid crystalline spinning of spider silk, Nature, 2001, 410(6828), 541–548, DOI:10.1038/35069000.
- P. Fratzl and R. Weinkamer, Nature's hierarchical materials, Prog. Mater. Sci., 2007, 52(8), 1263–1334, DOI:10.1016/j.pmatsci.2007.06.001.
- M. A. Meyers, P.-Y. Chen, A. Y.-M. Lin and Y. Seki, Biological materials: Structure and mechanical properties, Prog. Mater. Sci., 2008, 53(1), 1–206, DOI:10.1016/j.pmatsci.2007.05.002.
- M. Rinaudo, Chitin and chitosan: Properties and applications, Prog. Polym. Sci., 2006, 31(7), 603–632, DOI:10.1016/j.progpolymsci.2006.06.001.
- Z.-M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, A review on polymer nanofibers by electrospinning and their applications in nanocomposites, Compos. Sci. Technol., 2003, 63(15), 2223–2253, DOI:10.1016/S0266-3538(03)00178-7.
- H. Yano, J. Sugiyama, A. Nakagaito, M. Nogi, T. Matsuura, M. Hikita and K. Handa, Optically transparent composites reinforced with networks of bacterial nanofibers, Adv. Mater., 2005, 17(2), 153–155, DOI:10.1002/adma.200400597.
- A. N. Nakagaito, S. Iwamoto and H. Yano, Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites, Appl. Phys. A, 2005, 80(1), 93–97, DOI:10.1007/s00339-004-2932-3.
- T. J. Sill and H. A. von Recum, Electrospinning: Applications in drug delivery and tissue engineering, Biomaterials, 2008, 29(13), 1989–2006, DOI:10.1016/j.biomaterials.2008.01.011.
- L. Wang, N. Z. Ezazi, L. Liu, R. Ajdary, W. Xiang, M. Borghei, H. A. Santos and O. J. Rojas, Microfibers synthesized by wet-spinning of chitin nanomaterials: Mechanical, structural and cell proliferation properties, RSC Adv., 2020, 10(49), 29450–29459, 10.1039/D0RA06178F.
- J.-B. Zeng, Y.-S. He, S.-L. Li and Y.-Z. Wang, Chitin whiskers: An overview, Biomacromolecules, 2012, 13(1), 1–11, DOI:10.1021/bm201564a.
- A. Percot, C. Viton and A. Domard, Optimization of chitin extraction from shrimp shells, Biomacromolecules, 2003, 4, 12–18, DOI:10.1021/bm025602k.
- H. K. No, S. P. Meyers and K. S. Lee, Isolation and characterization of chitin from crawfish shell waste, J. Agric. Food Chem., 1989, 37(3), 575–579, DOI:10.1021/jf00087a001.
- J. Li, J.-F. Revol and R. H. Marchessault, Effect of degree of deacetylation of chitin on the properties of chitin crystallites, J. Appl. Polym. Sci., 1997, 65(2), 373–380, DOI:10.1002/(SICI)1097-4628(19970711)65:2<373::AID-APP18>3.0.CO;2-0.
- J. F. Revol and R. H. Marchessault, In vitro chiral nematic ordering of chitin crystallites, Int. J. Biol. Macromol., 1993, 15(6), 329–335, DOI:10.1016/0141-8130(93)90049-R.
- J. D. Goodrich and W. T. Winter, α-Chitin nanocrystals prepared from shrimp shells and their specific surface area measurement, Biomacromolecules, 2007, 8(1), 252–257, DOI:10.1021/bm0603589.
- S. Ifuku, M. Nogi, K. Abe, M. Yoshioka, M. Morimoto, H. Saimoto and H. Yano, Simple preparation method of chitin nanofibers with a uniform width of 10–20nm from prawn shell under neutral conditions, Carbohydr. Polym., 2011, 84(2), 762–764, DOI:10.1016/j.carbpol.2010.04.039.
- Y. Fan, T. Saito and A. Isogai, Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin, Biomacromolecules, 2008, 9(1), 192–198, DOI:10.1021/bm700966g.
- J. Wu, K. Zhang, N. Girouard and J. C. Meredith, Facile route to produce chitin nanofibers as precursors for flexible and transparent gas barrier materials, Biomacromolecules, 2014, 15(12), 4614–4620, DOI:10.1021/bm501416q.
- Y. Kato, J. Kaminaga, R. Matsuo and A. Isogai, TEMPO-mediated oxidation of chitin, regenerated chitin and N-acetylated chitosan, Carbohydr. Polym., 2004, 58(4), 421–426, DOI:10.1016/j.carbpol.2004.08.011.
- R. Muzzarelli, C. Muzzarelli, A. Cosani and M. Terbojevich, 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins, Carbohydr. Polym., 1999, 39(4), 361–367, DOI:10.1016/S0144-8617(99)00027-2.
- Y. Fan, T. Saito and A. Isogai, Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization, Carbohydr. Polym., 2010, 79(4), 1046–1051, DOI:10.1016/j.carbpol.2009.10.044.
- Y. Zhang, J. Jiang, L. Liu, K. Zheng, S. Yu and Y. Fan, Preparation, assessment, and comparison of α-chitin nano-fiber films with different surface charges, Nanoscale Res. Lett., 2015, 10, 1–11, DOI:10.1186/s11671-015-0926-z.
- D. X. Oh, Y. J. Cha, H.-L. Nguyen, H. H. Je, Y. S. Jho, D. S. Hwang and D. K. Yoon, Chiral nematic self-assembly of minimally surface damaged chitin nanofibrils and its load bearing functions, Sci. Rep., 2016, 6(1), 23245, DOI:10.1038/srep23245.
- X. Yang, J. Liu, Y. Pei, X. Zheng and K. Tang, Recent progress in preparation and application of nano-chitin materials, Energy Environ. Mater., 2020, 3(4), 492–515, DOI:10.1002/eem2.12079.
- R. Jayakumar, M. Prabaharan, S. Nair and H. Tamura, Novel chitin and chitosan nanofibers in biomedical applications, Biotechnol. Adv., 2010, 28(1), 142–150, DOI:10.1016/j.biotechadv.2009.11.001.
- H. K. Noh, S. W. Lee, J.-M. Kim, J.-E. Oh, K.-H. Kim, C.-P. Chung, S.-C. Choi, W. H. Park and B.-M. Min, Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts, Biomaterials, 2006, 27(21), 3934–3944, DOI:10.1016/j.biomaterials.2006.03.016.
- B.-M. Min, S. W. Lee, J. N. Lim, Y. You, T. S. Lee, P. H. Kang and W. H. Park, Chitin and chitosan nanofibers: electrospinning of chitin and deacetylation of chitin nanofibers, Polymer, 2004, 45(21), 7137–7142, DOI:10.1016/j.polymer.2004.08.048.
- H. Xie, S. Zhang and S. Li, Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2, Green Chem., 2006, 8(7), 630–633, 10.1039/B517297G.
- S. Yamazaki, A. Takegawa, Y. Kaneko, J.-i. Kadokawa, M. Yamagata and M. Ishikawa, An acidic cellulose–chitin hybrid gel as novel electrolyte for an electric double layer capacitor, Electrochem. Commun., 2009, 11(1), 68–70, DOI:10.1016/j.elecom.2008.10.039.
- Y. Wu, T. Sasaki, S. Irie and K. Sakurai, A novel biomass-ionic liquid platform for the utilization of native chitin, Polymer, 2008, 49(9), 2321–2327, DOI:10.1016/j.polymer.2008.03.027.
- Y. Qin, X. Lu, N. Sun and R. D. Rogers, Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers, Green Chem., 2010, 12(6), 968–971, 10.1039/C003583A.
- Y. Huang, Z. Zhong, B. Duan, L. Zhang, Z. Yang, Y. Wang and Q. Ye, Novel fibers fabricated directly from chitin solution and their application as wound dressing, J. Mater. Chem. B, 2014, 2(22), 3427–3432, 10.1039/C4TB00098F.
- C. Zhong, A. Cooper, A. Kapetanovic, Z. Fang, M. Zhang and M. Rolandi, A facile bottom-up route to self-assembled biogenic chitin nanofibers, Soft Matter, 2010, 6(21), 5298–5301, 10.1039/C0SM00450B.
- C. Zhong, A. Kapetanovic, Y. Deng and M. Rolandi, A chitin nanofiber ink for airbrushing, replica molding, and microcontact printing of self-assembled macro-, micro-, and nanostructures, Adv. Mater., 2011, 23(41), 4776–4781, DOI:10.1002/adma.201102639.
- P. Hassanzadeh, W. Sun, J. P. d. Silva, J. Jin, K. Makhnejia, G. L. W. Cross and M. Rolandi, Mechanical properties of self-assembled chitin nanofiber networks, J. Mater. Chem. B, 2014, 2(17), 2461–2466, 10.1039/C3TB21550D.
- M. A. Bonakdar and D. Rodrigue, Electrospinning: Processes, structures, and materials, Macromol, 2024, 4(1), 58–103, DOI:10.3390/macromol4010004.
- N. A. N. Dzolkifle and W. M. F. W. Nawawi, A review on chitin dissolution as preparation for electrospinning application, Int. J. Biol. Macromol., 2024, 265, 130858, DOI:10.1016/j.ijbiomac.2024.130858.
- J. Liu, Z. Dong, K. Huan, Z. He, Q. Zhang, D. Deng and L. Luo, Application of the electrospinning technique in electrochemical biosensors: An overview, Molecules, 2024, 29(12), 2769, DOI:10.3390/molecules29122769.
- Y. L. Tan, Y. Leow, J. H. M. Wong, X. J. Loh and R. Goh, Exploring stimuli-responsive natural processes for the fabrication of high-performance materials, Biomacromolecules, 2024, 25(9), 5437–5453, DOI:10.1021/acs.biomac.4c00718.
- D. A. Solomonov, D. A. Kozell and D. U. Shimanovich, Designing multifunctional biomaterials via protein self-assembly, Angew. Chem., Int. Ed., 2024, 63(14), e202318365, DOI:10.1002/anie.202318365.
- M. Wysokowski, S. Kaiser and T. Jesionowski, Hydrothermal synthesis of advanced chitin-based materials, in: Extreme Biomimetics, ed. H. Ehrlich, 2016, pp. 223–249, DOI:10.1007/978-3-319-45340-8_9.
- X. Dong, L. Shi, S. Ma, X. Chen, S. Cao, W. Li, Z. Zhao, C. Chen and H. Deng, Chitin/chitosan nanofibers toward a sustainable future: From hierarchical structural regulation to functionalization applications, Nano Lett., 2024, 24(39), 12014–12026, DOI:10.1021/acs.nanolett.4c02632.
- K. E. Park, S. Y. Jung, S. J. Lee, B.-M. Min and W. H. Park, Biomimetic nanofibrous scaffolds: Preparation and characterization of chitin/silk fibroin blend nanofibers, Int. J. Biol. Macromol., 2006, 38(3–5), 165–173, DOI:10.1016/j.ijbiomac.2006.03.003.
- J. Zhang, F. M. Said, N. F. S. Daud and Z. Jing, Present status and application prospects of green chitin nanowhiskers: A comprehensive review, Int. J. Biol. Macromol., 2024, 278, 134235, DOI:10.1016/j.ijbiomac.2024.134235.
- P. L. Chee, T. Sathasivam, Y. C. Tan, W. Wu, Y. Leow, Q. R. T. Lim, P. Y. M. Yew, Q. Zhu and D. Kai, Nanochitin for sustainable and advanced manufacturing, Nanoscale, 2024, 16(7), 3269–3292, 10.1039/D3NR05533G.
- N. Yamamoto, M. Totani and J.-I. Kadokawa, Hydrophobization of chitin nanofibers by grafting of partially 2-deoxygenated amyloses through enzymatic approach, Molecules, 2025, 30(1), 16, DOI:10.3390/molecules30010016.
- S. Pandharipande and P. H. Bhagat, Synthesis of chitin from crab shells and its utilization in preparation of nanostructured film, Int. J. Eng. Res. Sci. Technol., 2016, 5(5), 1378–1383 Search PubMed.
- V. Y. Novikov, K. S. Rysakova, N. V. Shumskaya, A. M. Mukhortova and K. A. Kesarev, King crab gills as a new source of chitin/chitosan and protein hydrolysates, Int. J. Biol. Macromol., 2023, 232, 123346, DOI:10.1016/j.ijbiomac.2023.123346.
- D. Li and Y. Xia, Electrospinning of nanofibers: Reinventing the wheel?, Adv. Mater., 2004, 16(14), 1151–1170, DOI:10.1002/adma.200400719.
- S. Hajji, O. Ghorbel-Bellaaj, I. Younes, K. Jellouli and M. Nasri, Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants, Int. J. Biol. Macromol., 2015, 79, 167–173, DOI:10.1016/j.ijbiomac.2015.04.027.
- R. Agrebi, A. Haddar, N. Hmidet, K. Jellouli and L. Manni, BSF1 fibrinolytic enzyme from a marine bacterium Bacillus subtilis A26: Purification, biochemical and molecular characterization, Process Biochem., 2009, 44(11), 1252–1259, DOI:10.1016/j.procbio.2009.06.024.
- N. Hmidet, H. Maalej and A. Haddar, A novel α-amylase from Bacillus mojavensis A21: Purification and biochemical characterization, Appl. Biochem. Biotechnol., 2010, 162, 1018–1030, DOI:10.1007/s12010-009-8902-7.
- N. Fakhfakh, N. Hmidet, A. Haddar and S. Kanoun, A novel serine metallokeratinase from a newly isolated Bacillus pumilus A1 grown on chicken feather meal: Biochemical and molecular characterization, Appl. Biochem. Biotechnol., 2009, 162, 329–344, DOI:10.1007/s12010-009-8774-x.
- R. Agrebi, N. Hmidet, H. Mohamed, N. Ktari, A. Haddar and N. Fakhfakh, Fibrinolytic serine protease isolation from Bacillus amyloliquefaciens An6 grown on Mirabilis jalapa tuber powders, Appl. Biochem. Biotechnol., 2009, 162, 75–88, DOI:10.1007/s12010-009-8800-z.
- N. Hadj-Ali, R. Agrebi, B. Ghorbel, A. Sellami-Kamoun and S. Kanoun, Biochemical and molecular characterization of detergent stable alkaline serine protease from a newly Bacillus lichenniformis NH1, Enzyme Microb. Technol., 2007, 40, 515–523, DOI:10.1016/j.enzmictec.2006.05.007.
- B. Ghorbel, A. Sellami-Kamoun, N. Fakhfakh, A. Haddar and L. Manni, Production and purification of a calcium-dependent protease from Bacillus cereus BG1, J. Ind. Microbiol. Biotechnol., 2005, 32, 186–194, DOI:10.1007/s10295-005-0228-z.
- H. Wang, J. Guo, X. Chen and H. He, The metabolomics changes in Luria–Bertani broth medium under different sterilization methods and their effects on Bacillus growth, Metabolites, 2023, 13, 958, DOI:10.3390/metabo13080958.
- S. Ngasotter, K. A. M. Xavier, M. M. Meitei, D. Waikhom, Madhulika, J. Pathak and S. K. Singh, Crustacean shell waste derived chitin and chitin nanomaterials for application in agriculture, food, and health – A review, Carbohydr. Polym. Technol. Appl., 2023, 6, 100349, DOI:10.1016/j.carpta.2023.100349.
- P. Y. Chen, A. Lin and J. McKittrick, Structure and mechanical properties of crab exoskeletons, Acta Biomater., 2008, 4, 587–596, DOI:10.1016/j.actbio.2007.12.010.
- F. Hisham, M. Akmal, F. Ahmad and K. Ahmad, Facile extraction of chitin and chitosan from shrimp shell, Mater. Today, 2021, 42(5), 2369–2373, DOI:10.1016/j.matpr.2020.12.329.
- H. Hoqani, N. Al-Shaqsi, M. Hossain and M. Sibani, Isolation and optimization of the method for industrial production of chitin and chitosan from Omani shrimp shell, Carbohydr. Res., 2020, 492, 108001, DOI:10.1016/j.carres.2020.108001.
- G. P. Tamilarasi, S. Govindaraj, K. Manikandan, S. Gouthaman, V. Alagarsamy and V. R. Solomon, Advances in electrospun chitosan nanofiber biomaterials for biomedical applications, Adv. Mater., 2023, 4, 3114–3139, 10.1039/D3MA00010A.
- V. Zubillaga, A. M. Salaberria, T. Palomares, A. Alonso-Varona, S. Kootala, J. Labidi and S. C. M. Fernandes, Chitin nanoforms provide mechanical and topological cues to support growth of human adipose stem cells in chitosan matrices, Biomacromolecules, 2018, 19(7), 3000–3012, DOI:10.1021/acs.biomac.8b00570.
- P. S. Barber, C. S. Griggs, J. R. Bonner and R. D. Rogers, Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells, Green Chem., 2013, 15, 601–607, 10.1039/C2GC36582K.
- T. Maschmeyer, R. Luque and M. Selva, Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials, Chem. Soc. Rev., 2020, 49, 4527–4563, 10.1039/C9CS00653B.
- S. Ifuku, R. Nomura, M. Morimoto and H. Saimoto, Preparation of chitin nanofibers from mushrooms, Materials, 2011, 43390, 1417–1425, DOI:10.3390/ma4081417.
- G. Michalenko, H. Hohl and D. Rast, Chemistry and architecture of the mycelial wall of Agaricus bisporus, Microbiol., 1976, 92, 251–262, DOI:10.1099/00221287-92-2-251.
- S. Zivanovic, R. Büscher and S. K. Kim, Mushroom texture, cell wall composition, color, and ultrastructure as affected by pH and temperature, J. Food Sci., 2006, 68, 1860–1865, DOI:10.1111/j.1365-2621.2003.tb12343.x.
- T. Ivshina, S. Artamonova, V. Ivshin and F. Sharnina, Isolation of the chitin-glucan complex from the fruiting bodies of mycothallus, Appl. Biochem. Microbiol., 2009, 45, 313–318, DOI:10.1134/S0003683809030132.
- M. Kujundzic, S. John and A. Bismarck, Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment, Mar. Drugs, 2020, 18(1), 64, DOI:10.3390/md18010064.
- C. A. Munro, Chitin and glucan, the Yin and Yang of the fungal cell wall, implications for antifungal drug discovery and therapy, Adv. Appl. Microbiol., 2013, 83, 145–172, DOI:10.1016/B978-0-12-407678-5.00004-0.
- M. Ayser, W. Tonny, I. S. Hernandez, R. Kuriakose, J. D. Smith, S. J. Wallaert, A. Karim, M. L. Robertson and V. Balan, Fractionating chitin-glucan complex and coproducts from Pleurotus Ostreatus mushrooms, Waste Biomass Valorization, 2024, 15, 2897–2910, DOI:10.1007/s12649-023-02364-5.
- M. Zhang, Y. Li, W. Wang, Y. Yang, X. Shi, M. Sun, Y. Hao and Y. Li, Comparison of physicochemical and rheology properties of Shiitake stipes-derived chitin nanocrystals and nanofibers, Carbohydr. Polym., 2020, 244, 116468, DOI:10.1016/j.carbpol.2020.116468.
- W. Nawawi, K.-Y. Lee, E. Kontturi, R. J. Murphy and A. Bismarck, Chitin nanopaper from mushroom extract: Natural composite of nanofibers and glucan from a single biobased source, ACS Sustain. Chem. Eng., 2019, 7(7), 6492–6496, DOI:10.1021/acssuschemeng.9b00721.
- M. I. M. Zin, D. N. Jimat and W. M. F. W. Nawawi, Physicochemical properties of fungal chitin nanopaper from shiitake (L. edodes), enoki (F. velutipes) and oyster mushrooms (P. ostreatus), Carbohydr. Polym., 2022, 281, 119038, DOI:10.1016/j.carbpol.2021.119038.
- A. Larrañaga, C. Bello-Álvarez and E. Lizundia, Cytotoxicity and inflammatory effects of chitin nanofibrils isolated from fungi, Biomacromolecules, 2023, 24(12), 5737–5748, DOI:10.1021/acs.biomac.3c00710.
- B. Bahrami, T. Behzad, F. Salehinik, A. Zamani and P. Heidarian, Incorporation of extracted Mucor Indicus fungus chitin nanofibers into starch biopolymer: Morphological, physical, and mechanical evaluation, Starch Staerke, 2021, 73, 2000218, DOI:10.1002/star.202000218.
- A. Dutta, H. Izawa, M. Morimoto, H. Saimoto and S. Ifuku, Simple preparation of chitin nanofibers from dry squid pen β-chitin powder by the star burst system, J. Chitin Chitosan Sci., 2013, 1, 186–191, DOI:10.1166/jcc.2013.1023.
- Y. Fan, T. Saito and A. Isogai, Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions, Biomacromolecules, 2008, 9, 1919–1923, DOI:10.1021/bm800178b.
- Q. Ma, K. Pang, K. Wang, S. Huang, B. Ding, Y. Duan and J. Zhang, Ultrafine and carboxylated β-chitin nanofibers prepared from squid pen and its transparent hydrogels, Carbohydr. Polym., 2019, 211, 118–123, DOI:10.1016/j.carbpol.2019.02.001.
- L. Luis, G. Alexander, A. Lilian and T. Cristian, Manufacture of β-chitin nano- and microparticles from jumbo squid pen (Dosidicus gigas) and evaluation of their effect on mechanical properties and water vapour permeability of polyvinyl alcohol/chitosan films, J. Food Eng., 2021, 290, 110230, DOI:10.1016/j.jfoodeng.2020.110230.
- M. Osada, M. Nishiwaki and T. Watanabe, Environment-friendly utilization of squid pen with water: Production of β-chitin nanofibers and peptides for lowering blood pressure, Int. J. Biol. Macromol., 2021, 189, 921–929, DOI:10.1016/j.ijbiomac.2021.08.190.
- A. K. Dutta, N. Kawamoto, G. Sugino, H. Izawa, M. Morimoto, H Saimoto and S. Ifuku, Simple preparation of chitosan nanofibers from dry chitosan powder by the Star Burst system, Carbohydr. Polym., 2013, 97(2), 363–367, DOI:10.1016/j.carbpol.2013.05.010.
- S. Suenaga, N. Nikaido, K. Totani, K Kawasaki, Y. Ito, K. Yamashita and M. Osada, Effect of purification method of β-chitin from squid pen on the properties of β-chitin nanofibers, Int. J. Biol. Macromol., 2016, 91, 987–993, DOI:10.1016/j.ijbiomac.2016.06.060.
- S. Ifuku, K. Yamada, M. Morimoto and H. Saimoto, Nanofibrillation of dry chitin powder by star burst system, J. Nanomater., 2012, 2012, 645624, DOI:10.1155/2012/645624.
- A. Dutta, K. Yamada, H. Izawa, M. Morimoto, H. Saimoto and S. Ifuku, Preparation of chitin nanofibers from dry chitin powder by star burst system: Dependence on number of passes, J. Chitin Chitosan Sci., 2013, 1, 59–64, DOI:10.1166/jcc.2013.1008.
- H. Yadav, R. Malviya and N. Kaushik, Chitosan in biomedicine: A comprehensive review of recent developments, Carbohydr. Polym. Technol. App., 2024, 8, 100551, DOI:10.1016/j.carpta.2024.100551.
- S. Maiti, J. Jayaramudu, K. Das, S. M. Reddy, R. Sadiku, S. S. Ray and D. Liu, Preparation and characterization of nano-cellulose with new shape from different precursor, Carbohydr. Polym., 2013, 98(1), 562–567, DOI:10.1016/j.carbpol.2013.06.029.
- H.-Y. Kweon, J.-H. Yeo, S.-O. Woo, S.-M. Han, Y.-Y. Jo and K.-G. Lee, Preparation and characterization of silk fibroin nanoparticles, Int. J. Indust. Entomol., 2010, 20(1), 25–28 Search PubMed.
- L. Panariello, M.-B. Coltelli, M. Buchignani and A. Lazzeri, Chitosan and nano-structured chitin for biobased anti-microbial treatments onto cellulose based materials, Eur. Polym. J., 2019, 113, 328–339, DOI:10.1016/j.eurpolymj.2019.02.004.
- J. Zhao, W. Zhang, X. Zhang, C. Lu and Y. Deng, Extraction of cellulose nanofibrils from dry softwood pulp using high shear homogenization, Carbohydr. Polym., 2013, 97(2), 695–702, DOI:10.1016/j.carbpol.2013.05.050.
- A. Wei, J. Fu and F. Guo, Mechanical properties of chitin polymorphs: A computational study, J. Mater. Sci., 2021, 56, 12048–12058, DOI:10.1007/s10853-021-06086-8.
- M. C. Jarvis, Structure of native cellulose microfibrils, the starting point for nanocellulose manufacture, Philos. Trans. R. Soc., A, 2017, 376(2112), 20170045, DOI:10.1098/rsta.2017.0045.
- Y. Cheng, L.-D. Koh, D. Li, B. Ji, M.-Y. Han and Y.-W. Zhang, On the strength of β-sheet crystallites of Bombyx mori silk fibroin, J. R. Soc. Interface, 2014, 11(96), 20140305, DOI:10.1098/rsif.2014.0305.
- Y. Fan, H. Fukuzumi, T. Saito and A. Isogai, Comparative characterization of aqueous dispersions and cast films of different chitin nanowhiskers/nanofibers, Int. J. Biol. Macromol., 2012, 50(1), 69–76, DOI:10.1016/j.ijbiomac.2011.09.026.
- C. Jiang, X. Wang, R. Gunawidjaja, Y.-H. Lin, M. K. Gupta, D. L. Kaplan, R. R. Naik and V. V. Tsukruk, Mechanical properties of robust ultrathin silk fibroin films, Adv. Funct. Mater., 2007, 17(13), 2229–2237, DOI:10.1002/adfm.200601136.
- Z. Fang, B. Li, Y. Liu, J. Zhu, G. Li, G. Hou, J. Zhou and X. Qiu, Critical role of degree of polymerization of cellulose in super-strong nanocellulose films, Matter, 2020, 2(4), 1000–1014, DOI:10.1016/j.matt.2020.01.016.
- J. Yin, E. Chen, D. Porter and Z. Shao, Enhancing the toughness of regenerated silk fibroin film through uniaxial extension, Biomacromolecules, 2010, 11(11), 2890–2895, DOI:10.1021/bm100643q.
- W. M. F. W. Nawawi, K.-Y. Lee, E. Kontturi, R. J. Murphy and A. Bismarck, Chitin nanopaper from mushroom extract: Natural composite of nanofibers and glucan from a single biobased source, ACS Sustainable Chem. Eng., 2019, 7(7), 6492–6496, DOI:10.1021/acssuschemeng.9b00721.
- T. Naghdi, H. Golmohammadi, H. Yousefi, M. Hosseinifard, U. Kostiv, D. Horák and A. Merkoçi, Chitin nanofiber paper toward optical (bio)sensing applications, ACS Appl. Mater. Interfaces, 2020, 12(13), 15538–15552, DOI:10.1021/acsami.9b23487.
- H. Yang, H. Zheng, Y. Duan, T. Xu, H. Xie, H. Du and C. Si, Nanocellulose-graphene composites: Preparation and applications in flexible electronics, Int. J. Biol. Macromol., 2023, 253, 126903, DOI:10.1016/j.ijbiomac.2023.126903.
- C. M. Costa, A. Reizabal, R. S. I. Serra, A. A. Balado, L. Pérez-Álvarez, J. L. G. Ribelles, J. L. Vilas-Vilela and S. Lanceros-Méndez, Broadband dielectric response of silk fibroin/BaTiO3 composites: Influence of nanoparticle size and concentration, Compos. Sci. Technol., 2021, 213, 108927, DOI:10.1016/j.compscitech.2021.108927.
- J. Wang, K. Kasuya, H. Koga, M. Nogi and K. Uetani, Thermal conductivity analysis of chitin and deacetylated-chitin nanofiber films under dry conditions, Nanomaterials, 2021, 11(3), 658, DOI:10.3390/nano11030658.
- G. Wang, M. Kudo, K. Daicho, S. Harish, B. Xu, C. Shao, Y. Lee, Y. Liao, N. Matsushima, T. Kodama, F. Lundell, L. D. Söderberg, T. Saito and J. Shiomi, Enhanced high thermal conductivity cellulose filaments via hydrodynamic focusing, Nano Lett., 2022, 22(21), 8406–8412, DOI:10.1021/acs.nanolett.2c02057.
- T. Tong, Y. Li, C. Wu, C. Ma, J. Yang and Z. Wei, Thermal conductivity of single silk fibroin fibers measured from the 3ω method, Int. J. Therm. Sci., 2023, 185, 108057, DOI:10.1016/j.ijthermalsci.2022.108057.
- G. Wang, M. Kudo, K. Daicho, S. Harish, B. Xu, C. Shao, Y. Lee, Y. Liao, M. Matsushima, G. Wang, L. Lundell, L. D. Söderberg, T. Saito and J. Shiomi, Enhanced high thermal conductivity cellulose filaments via hydrodynamic focusing, Nano Lett., 2022, 22(21), 8406–8412, DOI:10.1021/acs.nanolett.2c02057.
- J. Wang, K. Kasuya, H. Koga, M. Nogi and K. Uetani, Thermal conductivity analysis of chitin and deacetylated-chitin nanofiber films under dry conditions, Nanomaterials, 2021, 11(3), 658, DOI:10.3390/nano11030658.
- M. Pyda, X. Hu and P. Cebe, Heat capacity of silk fibroin based on the vibrational motion of poly(amino acid)s in the presence and absence of water, Macromolecules, 2008, 41(13), 4786–4793, DOI:10.1021/ma8003357.
- Y. Li, H. Zhu, M. Xu, Z. Zhuang, M. Xu and H. Dai, High yield preparation method of thermally stable cellulose nanofibers, Bioresources, 2014, 9, 1986–1997, DOI:10.15376/biores.9.2.1986-1997.
- H. Ishikawa, M. Tsukada, I. Toizume, A. Konda and K. Hirabayashi, DSC thermograms of silk fibroin, Sen'i Gakkaishi, 1972, 28, 91–98, DOI:10.2115/fiber.28.4-5_91.
- O. A. El Seoud, K. Jedvert, M. Kostag and S. Possidonio, Cellulose, chitin and silk: The cornerstones of green composites, Emergent Mater., 2022, 5, 785–810, DOI:10.1007/s42247-021-00308-0.
- Y. Ogawa, K. Azuma, H. Izawa, M. Morimoto, K. Ochi, T. Osaki, N. Ito, Y. Okamoto, H. Saimoto and S. Ifuku, Preparation and biocompatibility of a chitin nanofiber/gelatin composite film, Int. J. Biol. Macromol., 2017, 104, 1882–1889, DOI:10.1016/j.ijbiomac.2017.02.041.
- C. Lujerdean, G.-M. Baci, A.-A. Cucu and D. S. Dezmirean, The contribution of silk fibroin in biomedical engineering, Insects, 2022, 13(3), 286, DOI:10.3390/insects13030286.
- A. R. Egorov, O. M. Khubiev, R. A. Golubev, D. I. Semenkova, A. A. Nikolaev, A. M. Maharramov, G. Z. Mammadova, W. Liu, A. G. Tskhovrebov and A. S. Kritchenkov, New antibacterial and antioxidant chitin derivatives: Ultrasonic preparation and biological effects, Polymers, 2024, 16, 2509, DOI:10.3390/polym16172509.
- J. Li, Q. Ding, Y. Zha, J. Xie, F. Li, R. Li and N. Ao, The silk fibroin nanofibrous membrane loaded with polyhexamethyl biguanide for promoting infected wound healing, Eur. Polym. J., 2024, 202, 112666, DOI:10.1016/j.eurpolymj.2023.112666.
- A. Bakhshi, S. M. Naghib and N. Rabiee, Antibacterial and antiviral nanofibrous membranes, in: Antibacterial and Antiviral Functional Materials, ed. K. Deshmukh, 2024, vol. 147, pp. 47–88, DOI:10.1021/bk-2024-1472.ch002.
- Z. Yu, Y. Ji, V. Bourg, M. Bilgen and J. Meredith, Chitin- and cellulose-based sustainable barrier materials: A review, Emerg. Mater., 2020, 3, 919–936, DOI:10.1007/s42247-020-00147-5.
- J. Jin, P. Hassanzadeh, G. Perotto, W. Sun, M. A. Brenckle, D. Kaplan, F. G. Omenetto and M. Rolandi, A biomimetic composite from solution self-assembly of chitin nanofibers in a silk fibroin matrix, Adv. Mater., 2013, 25, 4482–4487, DOI:10.1002/adma.201301429.
- Y. Zheng, H. Zhang, Z. Wang, A. Lu, A. Yu and B. Duan, Chitin nanofibrils assisted 3D printing all-chitin hydrogels for wound dressing, Carbohydr. Polym., 2024, 334, 122028, DOI:10.1016/j.carbpol.2024.122028.
- S. Haijia, W. Lijuan and T. Tianwei, Adsorption of heavy metal ions by adsorbent from waste Mycelium Chitin, Chin. J. Chem. Eng., 2002, 10(6), 650–652 CAS.
- B. Yan, Y. Dai, L. Xin, M. Li, H. Zhang, H. Long and X. Gao, Research progress in the degradation of printing and dyeing wastewater using chitosan based composite photocatalytic materials, Int. J. Biol. Macromol., 2024, 263(2), 130082, DOI:10.1016/j.ijbiomac.2024.130082.
- Y. Zhang, F. Liu, L. Zhong, Z. Dong, C. Chen and Z. Xu, Reusable and environmentally friendly cellulose nanofiber/titanium dioxide/chitosan aerogel photocatalyst for efficient degradation of tetracycline, Appl. Surf. Sci., 2023, 641, 158425, DOI:10.1016/j.apsusc.2023.158425.
- I. Garrido, S. Aznar-Cervantes, M. Aliste, M. J. Yáñez-Gascón, N. Vela, J. L. Cenis, S. Navarro and J. Fenoll, Photocatalytic performance of electrospun silk fibroin/ZnO mats to remove pesticide residues from water under natural sunlight, Catalysts, 2020, 10(1), 110, DOI:10.3390/catal10010110.
- K. Bhuvaneswari, R. D. Bharathi and T. Pazhanivel, Silk fibroin linked Zn/Cd-doped SnO2 nanoparticles to purify the organically polluted water, Mater. Res. Express, 2018, 5(2), 024004, DOI:10.1088/2053-1591/aaaa35.
- R. Das, T. Lindström, P. R. Sharma, K. Chi and B. S. Hsiao, Nanocellulose for sustainable water purification, Chem. Rev., 2022, 122(9), 8936–9031, DOI:10.1021/acs.chemrev.1c00683.
- S. Pilley, H. Kaur, G. Hippargi, P. Gonde and S. Rayalu, Silk fibroin: A promising bio-material for the treatment of heavy metal-contaminated water, adsorption isotherms, kinetics, and mechanism, Environ. Sci. Pollut. Res., 2022, 29, 56606–56619, DOI:10.1007/s11356-022-19833-4.
- D. J. Fortman, J. P. Brutman, G. X. De Hoe, R. L. Snyder, W. R. Dichtel and M. A. Hillmyer, Approaches to sustainable and continually recyclable cross-linked polymers, ACS Sustain. Chem. Eng., 2018, 6(9), 11145–11159, DOI:10.1021/acssuschemeng.8b02355.
- E. M. Pritchard, P. B. Dennis, F. Omenetto, R. R. Naik and D. L. Kaplan, Physical and chemical aspects of stabilization of compounds in silk, Biopolymers, 2012, 97(6), 479–498, DOI:10.1002/bip.22026.
- M. Barikani, E. Oliaei, H. Seddiqi and H. Honarkar, Preparation and application of chitin and its derivatives: A review, Iran. Polym. J., 2014, 23, 307–326, DOI:10.1007/s13726-014-0225-z.
- D. Wang, J. Li, G. Salazar-Alvarez, L. S. McKee, V. Srivastava, J. A. Sellberg, V. Bulone and Y. S. Y. Hsieh, Production of functionalised chitins assisted by fungal lytic polysaccharide monooxygenase, Green Chem., 2018, 20, 2091–2100, 10.1039/C8GC00422F.
- G. G. Flores-Rojas, B. Gómez-Lazaro, F. López-Saucedo, R. Vera-Graziano, E. Bucio and E. Mendizábal, Electrospun scaffolds for tissue engineering: A review, Macromol, 2023, 3, 524–553, DOI:10.3390/macromol3030031.
- H. Omidian and E. J. Gill, Nanofibrous scaffolds in biomedicine, J. Compos. Sci., 2024, 8, 269, DOI:10.3390/jcs8070269.
- R. Jayakumar, D. Menon, K. Manzoor, S. V. Nair and H. Tamura, Biomedical applications of chitin and chitosan based nanomaterials—A short review, Carbohydr. Polym., 2010, 82(2), 227–232, DOI:10.1016/j.carbpol.2010.04.074.
- P. S. Kumar, S. Srinivasan, V.-K. Lakshmanan, H. Tamura, S. Nair and R. Jayakumar, β-Chitin hydrogel/nano hydroxyapatite composite scaffolds for tissue engineering applications, Carbohydr. Polym., 2011, 85(3), 584–591, DOI:10.1016/j.carbpol.2011.03.018.
- R. Jayakumar, R. Ramachandran, P. T. S. Kumar, V. V. Divyarani, S. Srinivasan, K. P. Chennazhi, H. Tamura and S. V. Nair, Fabrication of chitin–chitosan/nano ZrO2 composite scaffolds for tissue engineering applications, Int. J. Biol. Macromol., 2011, 49(3), 274–280, DOI:10.1016/j.ijbiomac.2011.04.020.
- K. Madhumathi, P. T. S. Kumar, K. C. Kavya, T. Furuike, H. Tamura, S. V. Nair and R. Jayakumar, Novel chitin/nanosilica composite scaffolds for bone tissue engineering applications, Int. J. Biol. Macromol., 2009, 45(3), 289–292, DOI:10.1016/j.ijbiomac.2009.06.009.
- H. Nagahama, N. Nwe, R. Jayakumar, S. Koiwa, T. Furuike and H. Tamura, Novel biodegradable chitin membranes for tissue engineering applications, Carbohydr. Polym., 2008, 73(2), 295–302, DOI:10.1016/j.carbpol.2007.11.034.
- H. Nagahama, T. Kashiki, N. Nwe, R. Jayakumar, T. Furuike and H. Tamura, Preparation of biodegradable chitin/gelatin membranes with GlcNAc for tissue engineering applications, Carbohydr. Polym., 2008, 73(3), 456–463, DOI:10.1016/j.carbpol.2007.12.011.
- Z. Ge, S. Baguenard, L. Y. Lim, A. Wee and E. Khor, Hydroxyapatite-chitin materials as potential tissue engineered bone substitutes, Biomaterials, 2004, 25(6), 1049–1058, DOI:10.1016/s0142-9612(03)00612-4.
- A. Khademhosseini, R. Langer, J. Borenstein and J. P. Vacanti, Microscale technologies for tissue engineering and biology, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(8), 2480–2487, DOI:10.1073/pnas.0507681102.
- T. Dvir, B. P. Timko, D. S. Kohane and R. Langer, Nanotechnological strategies for engineering complex tissues, Nat. Nanotechnol., 2011, 6, 13–22, DOI:10.1038/nnano.2010.246.
- D. E. Discher, P. Janmey and Y.-l. Wang, Tissue cells feel and respond to the stiffness of their substrate, Science, 2005, 310(5751), 1139–1143, DOI:10.1126/science.1116995.
- S. Kaihara, J. Borenstein, R. Koka, S. Lalan, E. R. Ochoa, M. Ravens, H. Pien, B. Cunningham and J. P. Vacanti, Silicon micromachining to tissue engineer branched vascular channels for liver fabrication, Tissue Eng., 2000, 6(2), 105–117, DOI:10.1089/107632700320739.
- T. Shimizu, M. Yamato, A. Kikuchi and T. Okano, Cell sheet engineering for myocardial tissue reconstruction, Biomaterials, 2003, 24(13), 2309–2316, DOI:10.1016/S0142-9612(03)00110-8.
- H. Obokata, M. Yamato, S. Tsuneda and T. Okano, Reproducible subcutaneous transplantation of cell sheets into recipient mice, Nat. Protoc., 2011, 6(7), 1053–1059, DOI:10.1038/nprot.2011.356.
- A. W. Feinberg, A. Feigel, S. S. Shevkoplyas, S. Sheehy, G. M. Whitesides and K. K. Parker, Muscular thin films for building actuators and powering devices, Science, 2007, 317(5843), 1366–1370, DOI:10.1126/science.1146885.
- A. Francesko, P. Petkova and T. Tzanov, Hydrogel dressings for advanced wound management, Curr. Med. Chem., 2018, 25(41), 5782–5797, DOI:10.2174/0929867324666170920161246.
- P. Hassanzadeh, M. Kharaziha, M. Nikkhah, S. R. Shin, J. Jin, S. He, W. Sun, C. Zhong, M. R. Dokmeci, A. Khademhosseini and M. Rolandi, Chitin nanofiber micropatterned flexible substrates for tissue engineering, J. Mater. Chem. B, 2013, 1, 4217–4224, 10.1039/C3TB20782J.
- M. Papadaki, N. Bursac, R. Langer, J. Merok, G. Vunjak-Novakovic and L. E. Freed, Tissue engineering of functional cardiac muscle: Molecular, structural, and electrophysiological studies, Am. J. Physiol. Heart Circ. Physiol., 2001, 280(1), H168–H178, DOI:10.1152/ajpheart.2001.280.1.H168.
- A. Cooper, C. Zhong, Y. Kinoshita, R. S. Morrison, M. Rolandi and M. Zhang, Self-assembled chitin nanofiber templates for artificial neural networks, J. Mater. Chem., 2012, 22(7), 3105–3109, 10.1039/C2JM15487K.
- J. R. Couchman, M. R. Austria and A. Woods, Fibronectin-cell interactions, J. Invest. Dermatol., 1990, 94(6), s7–s14, DOI:10.1111/1523-1747.ep12874973.
- K. Zhang, A. Geissler, S. Fischer, E. Brendler and E. Bäucker, Solid-state spectroscopic characterization of α-chitins deacetylated in homogeneous solutions, J. Phys. Chem. B, 2012, 116(15), 4584–4592, DOI:10.1021/jp210469x.
- K. Goto and Y. Teramoto, Distribution of the degree of deacetylation of surface-deacetylated chitin nanofibers: Effects on crystalline structure and cell adhesion and proliferation, ACS Appl. Bio Mater., 2020, 3(12), 8650–8657, DOI:10.1021/acsabm.0c01040.
- S. Wu, B. Duan, A. Lu, Y. Wang, Q. Ye and L. Zhang, Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates, Carbohydr. Polym., 2017, 174, 830–840, DOI:10.1016/j.carbpol.2017.06.101.
- B. Duan, K. Shou, X. Su, Y. Niu, G. Zheng, Y. Huang, A. Yu, Y. Zhang, H. Xia and L. Zhang, Hierarchical microspheres constructed from chitin nanofibers penetrated hydroxyapatite crystals for bone regeneration, Biomacromolecules, 2017, 18(7), 2080–2089, DOI:10.1021/acs.biomac.7b00408.
- F. Chen, Y. Liu, Y. Zou, J. Zhu, L. Liu and Y. Fan, Preparation of nanochitin hydrogel with adjustable inter-structure by sequencial genipin crosslinking and ice-templating under acid condition, Int. J. Biol. Macromol., 2022, 221, 1022–1030, DOI:10.1016/j.ijbiomac.2022.08.201.
- V. V. Kiroshka, T. A. Yurchuk, N. V. Repin, V. A. Petrova, I. V. Gofman, Y. A. Skorik, E. V. Kiroshka and T. P. Bondarenko, Adhesion, growth, and proliferation of endothelial cells on biopolymer extracellular film matrices, Bull. Exp. Biol. Med., 2014, 158(1), 153–158, DOI:10.1007/s10517-014-2712-9.
- N. V. Smirnova, K. A. Kolbe, E. N. Dresvyanina, S. F. Grebennikov, I. P. Dobrovolskaya, V. E. Yudin, T. Luxbacher and P. Morganti, Effect of chitin nanofibrils on biocompatibility and bioactivity of the chitosan-based composite film matrix intended for tissue engineering, Materials, 2019, 12, 1874, DOI:10.3390/ma12111874.
- X. Su, M. Tan, B. Duan, J. Cai, W. Jiang and L. Zhang, Hierarchical microspheres with macropores fabricated from chitin as 3D cell culture, J. Mater. Chem. B, 2019, 7(34), 5190–5198, 10.1039/C9TB01046G.
- E. N. Dresvyanina, V. V. Kodolova-Chukhontseva, S. G. Bystrov, I. P. Dobrovolskaya, G. V. Vaganov, N. V. Smirnova, K. A. Kolbe, A. M. Kamalov, E. M. Ivan’kova, P. Morganti and V. E. Yudin, Influence of surface morphology of chitosan films modified by chitin nanofibrils on their biological properties, Carbohydr. Polym., 2021, 262, 117917, DOI:10.1016/j.carbpol.2021.117917.
- J. G. Torres-Rendon, T. Femmer, L. D. Laporte, T. Tigges, K. Rahimi, F. Gremse, S. Zafarnia, W. Lederle, S. Ifuku, M. Wessling, J. G. Hardy and A. Walther, Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering, Adv. Mater., 2015, 27(19), 2989–2995, DOI:10.1002/adma.201405873.
- Y. Huang, Y. Wang, L. Chen and L. Zhang, Facile construction of mechanically tough collagen fibers reinforced by chitin nanofibers as cell alignment templates, J. Mater. Chem. B, 2018, 6(6), 918–929, 10.1039/C7TB02945D.
- M. Barbalinardo, M. Biagetti, F. Valle, M. Cavallini, G. Falini and D. Montroni, Green biocompatible method for the synthesis of collagen/chitin composites to study their composition and assembly influence on fibroblasts growth, Biomacromolecules, 2021, 22(8), 3357–3365, DOI:10.1021/acs.biomac.1c00463.
- C. Chen, Y. Wang, Y. Yang, M. Pan, T. Ye and D. Li, High strength gelatin-based nanocomposites reinforced by surface-deacetylated chitin nanofiber networks, Carbohydr. Polym., 2018, 195, 387–392, DOI:10.1016/j.carbpol.2018.04.095.
- C. Chen, D. Li, H. Yano and K. Abe, Insect cuticle-mimetic hydrogels with high mechanical properties achieved via the combination of chitin nanofiber and gelatin, J. Agric. Food Chem., 2019, 67(19), 5571–5578, DOI:10.1021/acs.jafc.9b00984.
- P. Hassanzadeh, M. Kazemzadeh-Narbat, R. Rosenzweig, X. Zhang, A. Khademhosseini, N. Annabi and M. Rolandi, Ultrastrong and flexible hybrid hydrogels based on solution self-assembly of chitin nanofibers in gelatin methacryloyl (GelMA), J. Mater. Chem. B, 2016, 4(15), 2539–2543, 10.1039/C6TB00021E.
- S. Danti, L. Trombi, A. Fusco, B. Azimi, A. Lazzeri, P. Morganti, M.-B. Coltelli and G. Donnarumma, Chitin nanofibrils and nanolignin as functional agents in skin regeneration, Int. J. Mol. Sci., 2019, 20(11), 2669, DOI:10.3390/ijms20112669.
- R. Jayakumar, M. Prabaharan, P. T. Sudheesh Kumar, S. V. Nair and H. Tamura, Biomaterials based on chitin and chitosan in wound dressing applications, Biotechnol. Adv., 2011, 29(3), 322–337, DOI:10.1016/j.biotechadv.2011.01.005.
- P. T. S. Kumar, S. Abhilash, K. Manzoor, S. V. Nair, H. Tamura and R. Jayakumar, Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications, Carbohydr. Polym., 2010, 80(3), 761–767, DOI:10.1016/j.carbpol.2009.12.024.
- K. Azuma, T. Osaki, T. Wakuda, S. Ifuku, H. Saimoto, T. Tsuka, T. Imagawa, Y. Okamoto and S. Minami, Beneficial and preventive effect of chitin nanofibrils in a dextran sulfate sodium-induced acute ulcerative colitis model, Carbohydr. Polym., 2012, 87(2), 1399–1403, DOI:10.1016/j.carbpol.2011.09.036.
- N. Charernsriwilaiwat, T. Rojanarata, T. Ngawhirunpat and P. Opanasopit, Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing, Int. Wound J., 2014, 11(2), 215–222, DOI:10.1111/j.1742-481X.2012.01077.x.
- A. Kumar and A. Kumar, Chitosan-based drug conjugated nanocomposites: Advances and innovation in cancer therapy, Regen. Eng. Transl. Med., 2024, 10(1), 1–8, DOI:10.1007/s40883-023-00310-4.
- M. N. V. Ravi Kumar, A review of chitin and chitosan applications, React. Funct. Polym., 2000, 46(1), 1–27, DOI:10.1016/S1381-5148(00)00038-9.
- P. Geetha, A. J. Sivaram, R. Jayakumar and C. G. Mohan, Integration of in silico modeling, prediction by binding energy and experimental approach to study the amorphous chitin nanocarriers for cancer drug delivery, Carbohydr. Polym., 2016, 142, 240–249, DOI:10.1016/j.carbpol.2016.01.059.
- X. Huang, Y. Wu, S. Wei, Q. Chen and C. Liu, The effect of carboxymethyl chitin on sustained drug release of aspirin tablet, Mater. Lett., 2012, 66(1), 206–208, DOI:10.1016/j.matlet.2011.08.061.
- N. S. Rejinold, A. Nair, M. Sabitha, K. P. Chennazhi, H. Tamura, S. V. Nair and R. Jayakumar, Synthesis, characterization and in vitro cytocompatibility studies of chitin nanogels for biomedical applications, Carbohydr. Polym., 2012, 87(1), 943–949, DOI:10.1016/j.carbpol.2011.08.044.
- R. Jayakumar, A. Nair, N. S. Rejinold, S. Maya and S. V. Nair, Doxorubicin-loaded pH-responsive chitin nanogels for drug delivery to cancer cells, Carbohydr. Polym., 2012, 87(3), 2352–2356, DOI:10.1016/j.carbpol.2011.10.040.
- K. Suzuki, T. Mikami, Y. Okawa, A. Tokoro, S. Suzuki and M. Suzuki, Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose, Carbohydr. Res., 1986, 151, 403–408, DOI:10.1016/S0008-6215(00)90359-8.
- K. Azuma, S. Ifuku, T. Osaki, Y. Okamoto and S. Minami, Preparation and biomedical applications of chitin and chitosan nanofibers, J. Biomed. Nanotechnol., 2014, 10, 2891–2920, DOI:10.1166/jbn.2014.1882.
- D. Solairaj, P. Rameshthangam and G. Arunachalam, Anticancer activity of silver and copper embedded chitin nanocomposites against human breast cancer (MCF-7) cells, Int. J. Biol. Macromol., 2017, 105, 608–619, DOI:10.1016/j.ijbiomac.2017.07.078.
- J. Li, S. Ying, H. Ren, J. Dai, L. Zhang, L. Liang, Q. Wang, Q. Shen and J.-W. Shen, Molecular dynamics study on the encapsulation and release of anti-cancer drug doxorubicin by chitosan, Int. J. Pharm., 2020, 580, 119241, DOI:10.1016/j.ijpharm.2020.119241.
- W. Suginta, P. Khunkaewla and A. Schulte, Electrochemical biosensor applications of polysaccharides chitin and chitosan, Chem. Rev., 2013, 113(7), 5458–5479, DOI:10.1021/cr300325r.
- J. Choosang, S. Khumngern, P. Thavarungkul, P. Kanatharana and A. Numnuam, An ultrasensitive label-free electrochemical immunosensor based on 3D porous chitosan–graphene–ionic liquid–ferrocene nanocomposite cryogel decorated with gold nanoparticles for prostate-specific antigen, Talanta, 2021, 224, 121787, DOI:10.1016/j.talanta.2020.121787.
- R. Ikram, B. Mohamed Jan, M. Abdul Qadir, A. Sidek, M. M. Stylianakis and G. Kenanakis, Recent advances in chitin and chitosan/graphene-based bio-nanocomposites for energetic applications, Polymers, 2021, 13, 3266, DOI:10.3390/polym13193266.
- G. Camci-Unal and N. L. B. Pohl, Quantitative determination of heavy metal contaminant complexation by the carbohydrate polymer chitin, J. Chem. Eng. Data, 2010, 55(3), 1117–1121, DOI:10.1021/je900552w.
- G. N. Kousalya, M. Rajiv Gandhi, C. Sairam Sundaram and S. Meenakshi, Synthesis of nano-hydroxyapatite chitin/chitosan hybrid biocomposites for the removal of Fe(III), Carbohydr. Polym., 2010, 82(3), 594–599, DOI:10.1016/j.carbpol.2010.05.013.
- M. Rajiv Gandhi, G. N. Kousalya and S. Meenakshi, Removal of copper(II) using chitin/chitosan nano-hydroxyapatite composite, Int. J. Biol. Macromol., 2011, 48(1), 119–124, DOI:10.1016/j.ijbiomac.2010.10.009.
- P. X. Pinto, S. R. Al-Abed and D. J. Reisman, Biosorption of heavy metals from mining influenced water onto chitin products, Chem. Eng. J., 2011, 166(3), 1002–1009, DOI:10.1016/j.cej.2010.11.091.
- H. Tang, C. Chang and L. Zhang, Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway, Chem. Eng. J., 2011, 173(3), 689–697, DOI:10.1016/j.cej.2011.07.045.
- H. Tang, W. Zhou and L. Zhang, Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels, J. Hazard. Mater., 2012, 209–210, 218–225, DOI:10.1016/j.jhazmat.2012.01.010.
- S. C. Mamah, P. S. Goh, B. C. Ng, M. S. Abdullah, A. F. Ismail, Z. Samavati, N. A. Ahmad and Y. O. Raji, The utilization of chitin and chitosan as green modifiers in nanocomposite membrane for water treatment, J. Water Proc. Eng., 2024, 62, 105394, DOI:10.1016/j.jwpe.2024.105394.
- P. Bashpa, K. Bijudas, P. Dileep, M. Singh, S. Elanthikkal and T. Francis, Natural rubber composites reinforced with sodium sulfate waste from pigment industry: A circular economy approach for solid waste management, Sustain. Chem. Pharm., 2024, 40, 101628, DOI:10.1016/j.scp.2024.101628.
- M. Dadkhah and M. Messori, A comprehensive overview of conventional and bio-based fillers for rubber formulations sustainability, Mater. Today Sustain., 2024, 27, 100886, DOI:10.1016/j.mtsust.2024.100886.
- F. Shahidi, J. K. V. Arachchi and Y.-J. Jeon, Food applications of chitin and chitosans, Trends Food Sci. Technol., 1999, 10(2), 37–51, DOI:10.1016/S0924-2244(99)00017-5.
- D. A. I. De-hui, L. I. Wei, H. U. Wei-lian and S. A. Xiao-ying, Effect of medium composition on the synthesis of chitinase and chitin deacetylase from thermophilic Paenibacillus sp. Hul, Procedia Environ. Sci., 2011, 8, 620–628, DOI:10.1016/j.proenv.2011.10.096.
- R. Dolphen and P. Thiravetyan, Adsorption of melanoidins by chitin nanofibers, Chem. Eng. J., 2011, 166(3), 890–895, DOI:10.1016/j.cej.2010.11.063.
- X. Li, X. Li, B. Ke, X. Shi and Y. Du, Cooperative performance of chitin whisker and rectorite fillers on chitosan films, Carbohydr. Polym., 2011, 85(4), 747–752, DOI:10.1016/j.carbpol.2011.03.040.
- R. Harikrishnan, J.-S. Kim, C. Balasundaram and M.-S. Heo, Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection, Aquaculture, 2012, 326–329, 46–52, DOI:10.1016/j.aquaculture.2011.11.034.
- F. Askarian, Z. Zhou, R. E. Olsen, S. Sperstad and E. Ringø, Culturable autochthonous gut bacteria in Atlantic salmon (Salmo salar L.) fed diets with or without chitin. Characterization by 16S rRNA gene sequencing, ability to produce enzymes and in vitro growth inhibition of four fish pathogens, Aquaculture, 2012, 326–329, 1–8, DOI:10.1016/j.aquaculture.2011.10.016.
- C.-S. Kong, J.-A. Kim, S.-S. Bak, H.-G. Byun and S.-K. Kim, Anti-obesity effect of carboxymethyl chitin by AMPK and aquaporin-7 pathways in 3T3-L1 adipocytes, J. Nutr. Biochem., 2011, 22(3), 276–281, DOI:10.1016/j.jnutbio.2010.02.005.
- S. Zhang, H. Chen, H. Ma, J. Yu, L. Liu and Y. Fan, A MoS2QDs/chitin nanofiber composite for improved antibacterial and food packaging, Int. J. Biol. Macromol., 2022, 209, 737–746, DOI:10.1016/j.ijbiomac.2022.04.016.
- A. M. Neyrinck, S. Possemiers, W. Verstraete, F. De Backer, P. D. Cani and N. M. Delzenne, Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice, J. Nutr. Biochem., 2012, 23(1), 51–59, DOI:10.1016/j.jnutbio.2010.10.008.
- P. K. Dutta, J. Dutta and V. S. Tripathi, Chitin and chitosan: Chemistry, properties and applications, J. Sci. Ind. Res., 2004, 63, 20–31 CAS.
- M. Triunfo, E. Tafi, A. Guarnieri, C. Scieuzo, T. Hahn, S. Zibek, R. Salvia and P. Falabella, Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals, Cosmetics, 2021, 8, 40, DOI:10.3390/cosmetics8020040.
- P. Morganti, G. Morganti and M.-B. Coltelli, Natural polymers and cosmeceuticals for a healthy and circular Life: The examples of chitin, chitosan, and lignin, Cosmetics, 2023, 10(2), 42, DOI:10.3390/cosmetics10020042.
- F. Shahidi and R. Abuzaytoun, Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects, Adv. Food Nutr. Res., 2005, 49, 93–135, DOI:10.1016/S1043-4526(05)49003-8.
- S.-L. Wang, T.-W. Liang and Y.-H. Yen, Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials, Carbohydr. Polym., 2011, 84(2), 732–742, DOI:10.1016/j.carbpol.2010.06.022.
- M.-H. Yang, C.-H. Kuo, W.-C. Hsieh and K.-L. Ku, Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor, J. Agric. Food Chem., 2010, 58(17), 9537–9541, DOI:10.1021/jf1022725.
- T. Shimizu, T. Nakano, D. Takamizawa, Y. Desaki, N. Ishii-Minami, Y. Nishizawa, E. Minami, K. Okada, H. Yamane, H. Kaku and N. Shibuya, Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice, Plant J., 2010, 64(2), 204–214, DOI:10.1111/j.1365-313X.2010.04324.x.
- J. Shamshina, A. Kelly, T. Oldham and R. Rogers, Agricultural uses of chitin polymers, Environ. Chem. Lett., 2019, 18, 1–8, DOI:10.1007/s10311-019-00934-5.
- W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood and E. E. Kwon, Recent advancements in supercapacitor technology, Nano Energy, 2018, 52, 441–473, DOI:10.1016/j.nanoen.2018.08.013.
- S. Chu, Y. Cui and N. Liu, The path towards sustainable energy, Nat. Mater., 2017, 16(1), 16–22, DOI:10.1038/nmat4834.
- K. Park, J. H. Cho, K. Shanmuganathan, J. Song, J. Peng, M. Gobet, S. G. Greenbaum, C. J. Ellison and J. B. Goodenough, New battery strategies with a polymer/Al2O3 separator, J. Power Sources, 2014, 263, 52–58, DOI:10.1016/j.jpowsour.2014.04.017.
- T.-W. Zhang, T.-W. Zhang, B. Shen, H.-B. Yao, T. Ma, L.-L. Lu, F. Zhou and S.-H. Yu, Prawn shell derived chitin nanofiber membranes as advanced sustainable separators for Li/Na-ion batteries, Nano Lett., 2017, 17(8), 4894–4901, DOI:10.1021/acs.nanolett.7b01875.
- Z. Shang, X. An, L. Liu, J. Yang, W. Zhang, H. Dai, H. Cao, Q. Xu, H. Liu and Y. Ni, Chitin nanofibers as versatile bio-templates of zeolitic imidazolate frameworks for N-doped hierarchically porous carbon electrodes for supercapacitor, Carbohydr. Polym., 2021, 251, 117107, DOI:10.1016/j.carbpol.2020.117107.
- N. A. Hoque, P. Thakur, P. Biswas, M. M. Saikh, S Roy, B. Bagchi, S. Das and P. P. Ray, Biowaste crab shell-extracted chitin nanofiber-based superior piezoelectric nanogenerator, J. Mater. Chem. A, 2018, 6(28), 13848–13858, 10.1039/C8TA04074E.
- C. Chen, C. Yang, S. Li and D. Li, A three-dimensionally chitin nanofiber/carbon nanotube hydrogel network for foldable conductive paper, Carbohydr. Polym., 2015, 134, 309–313, DOI:10.1016/j.carbpol.2015.08.004.
- J. Conder, C. Vaulot, C. Marino, C. Villevieille and C. M. Ghimbeu, Chitin and chitosan—structurally related precursors of dissimilar hard carbons for Na-ion battery, ACS Appl. Energy Mater., 2019, 2(7), 4841–4852, DOI:10.1021/acsaem.9b00545.
- Y. Tong, H. Guan, S. Wang, J. Xu and C. He, Syntheses of chitin-based imprinting polymers and their binding properties for cholesterol, Carbohydr. Res., 2011, 346(4), 495–500, DOI:10.1016/j.carres.2010.12.013.
- Y. Liu, R. Liu, J. Shi, R. Zhang, H. Tang, C. Xie, F. Wang, J. Han and L. Jiang, Chitosan/esterified chitin nanofibers nanocomposite films incorporated with rose essential oil: Structure, physicochemical characterization, antioxidant and antibacterial properties, Food Chem.: X, 2023, 18, 100714, DOI:10.1016/j.fochx.2023.100714.
- H. Zou, B. Lin, C. Xu, M. Lin and W. Zhan, Preparation and characterization of individual chitin nanofibers with high stability from chitin gels by low-intensity ultrasonication for antibacterial finishing, Cellulose, 2018, 25, 999–1010, DOI:10.1007/s10570-017-1634-x.
- S. Tanpichai, L. Pumpuang, Y. Srimarut, W. Woraprayote and Y. Malila, Development of chitin nanofiber coatings for prolonging shelf life and inhibiting bacterial growth on fresh cucumbers, Sci. Rep., 2023, 13(1), 13195, DOI:10.1038/s41598-023-39739-6.
- J. Liao, R. Wen, Y. Wang, Y. Zhou and J. Zhang, Film-forming capability and antibacterial activity of surface-deacetylated chitin nanocrystals: Role of degree of deacetylation, Biomacromolecules, 2024, 25(8), 5138–5148, DOI:10.1021/acs.biomac.4c00528.
- J. Sun, J. Li, R. Ren, L. Yao, L. Tong, J. Yuan and D. Wang, Effect of chitosan and hyperbranched poly-L-lysine treatment on quality of cucumber (Cucumis sativus L.) during storage, Foods, 2024, 13(9), 1354, DOI:10.3390/foods13091354.
Footnote |
| † Co-first author. |
| This journal is © The Royal Society of Chemistry 2025 |

