 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Study about the effect of cellulose nanocrystals on a polyacrylate miniemulsion
Zeping Wang a,
Lionel O'Younga,
Sajid Mahmoodab,
George Zheng Chen
a,
Lionel O'Younga,
Sajid Mahmoodab,
George Zheng Chen c,
Yitao Zhengd and
Binjie Hu*d
c,
Yitao Zhengd and
Binjie Hu*d
aGreen Chemicals & Energy Process Development Laboratory, China Beacons Institute, University of Nottingham Ningbo China, 199 Taikang East Road, Ningbo, 315000, China
bLow Dimensional Materials Research Center, Khazar University, Baku, AZ1096, Azerbaijan
cDepartment of Chemical and Environmental Engineering, Advanced Materials Research Group, Faculty of Engineering, The University of Nottingham, Nottingham, NG7 2RD, UK
dDepartment of Chemical & Environmental Engineering, University of Nottingham Ningbo China, 199 Taikang East Road, Ningbo, 315000, China. E-mail: Binjie.HU@nottingham.edu.cn
First published on 6th March 2025
Abstract
Cellulose nanocrystals (CNC) are widely used due to their biodegradability, high strength, large surface area, and functional versatility. This study investigates the interaction between CNC and acrylate emulsions, which mainly focuses on their impact on emulsion characteristics, polymerization behaviour, and storage stability. CNC was incorporated into an acrylate miniemulsion system at varying concentrations, followed by the systematic study of its effects on particle size, interfacial tension, zeta potential, yield, and viscosity. The morphology of CNC-acrylate systems was analysed using infrared spectroscopy and scanning electron microscopy (SEM). The results demonstrated that CNC effectively co-stabilized acrylate miniemulsions and enhanced their stability before polymerization. Although CNC did not directly participate in polymerization or affect yield or reaction rates, it slowed the diffusion of free radicals. However, CNC concentrations higher than 1 wt% negatively impacted post-polymerization storage stability and caused aggregation of droplets. These findings reveal the dual role of CNC as both a stabilizing and aggregating agent, offering new insights into its potential for the design of advanced polymer systems.
1. Introduction
In the coating industry, there is a potential to transform through sustainability, improved performance, and multifunctionality. Waterborne coatings are increasingly favoured due to their reduced environmental impact in lower volatile organic compound (VOC) emissions an alignment with global sustainability goals and regulatory requirements.1 These coatings utilize water as the primary solvent, thus offering an eco-friendly solution. However, challenges like suboptimal mechanical strength, stability in waterborne systems have necessitated the development of innovative additives to improve performance.2To solve those challenges, seeking more novel potential additive materials could be a solution. Cellulose as the most abundant biopolymer on earth, has drawn more attention recently on its various additive function due to its ease of altering, tailoring and manufacture.3,4 Cellulose nanocrystal (CNC), a rod-shaped nanomaterial extracted from cellulose microfibrils, has gained significant attention for their unique properties like high crystallinity and strong mechanical properties, and diverse applications.5 Compared with cellulose nanofiber (CNF), CNC is shorter in length and has a higher ratio of crystalline structure, exhibiting high specific strength, modulus, large surface area, gas impermeability, and unique liquid crystalline properties.6,7 In addition, CNC also shares advantages of cellulose, which are biodegradable, biocompatible, renewable and environmentally friendly.8 Attracted by these characteristics, CNC is widely applied in nanocomposites films and membranes for food packaging,9 conductive materials,10 water treatment,11 biomedical use,12 and waterborne coatings,13–16 especially. Moreover, the enhancement of CNC on film strength, water resistance, and environmental sustainability was also proven inside a waterborne polyacrylate system in architectural, automotive, and anticorrosive coatings.17–19
Despite these developments on CNC, previous research mainly focused on the applications of CNC, not much systematic research has been carried out. Especially the mechanisms and behaviour of CNC within coatings is yet understood. To address this gap, this study systematically investigates the effects of CNCs within a waterborne polyacrylate coating, providing deeper insights into their role and interactions between CNC and polymers within the coating.
2. Experimental methods and materials
2.1. Materials
Methyl methacrylate (MMA, CP), sodium dodecyl sulfonate (SDSO, CP), L-ascorbic acid (AAc, AR) and hydrogen peroxide (H2O2, AR) were supplied by Sinopharm Chemical Reagent Co., Ltd. Hexadecane (HD, 98%) and n-butyl acrylate (BA, 99%) were offered by Aladdin Industrial Inc. Cellulose nanocrystal (CNC) was purchased from ScienceK Co., Ltd. Ultra-pure water (18.2 MΩ cm) was obtained from a ultrapure water system (Milli-Q® IQ 7000).2.2. Preparation of waterborne acrylate coating
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 at 40 °C and 285 W.
7 at 40 °C and 285 W.2.3. Miniemulsion polymerization
After homogenization of oil phase and water phase, the mixture was loaded into a 250 mL four-necked flask equipped with a stirrer, a reflux condenser, a thermometer, and a nitrogen inlet. The temperature was controlled at 40 °C with a water bath. After stirring at 200 rpm and nitrogen bubbling for 1.5 h to remove oxygen, H2O2/AAc solution (0.1 mol% of the oil phase) was introduced to initiate the polymerization.2.4. Characterization techniques
3. Results and discussion
3.1. Effect of the CNC on the o/w interface of acrylate miniemulsion
The polyacrylate emulsion was composed of an oil phase, a water phase, and surfactants, which was proven to be an effective coating formula in our previous studies.20,21 Initially, to study the effect of CNC on the o/w interface, the interfacial tension between the oil and water phase at different concentrations of CNC without SDSO was measured and presented in Fig. 1. The interfacial tension remarkably decreased from 14.2 mN m−1 to 10.2 mN m−1 after introducing 0.1 wt% CNC. The value further decreased to 9.2 mN m−1 as the CNC content increased to 1 wt%, then remained relatively constant with further increases in CNC content up to 2 wt%. This validated that the CNC could effectively reduce the interfacial tension between the oil and water phase. However, the mixture of oil and water phases after ultrasonication could not achieve a homogeneous emulsion without SDSO. In other words, the CNC as received could not fully stabilize the o/w interface. Therefore, SDSO was introduced as the main stabilizer into this formulation. 30 mM was the cost-effective concentration for SDSO in an emulsion.22After adding 30 mM SDSO, the emulsion was supposed to be homogenous without CNC. However, there was still a small portion of the emulsion remained unstable. As shown in Fig. 2, two peaks were observed in the size distribution plot of emulsion without CNC, where the main peak distributed in the range of 0.1–1 μm, and the other small peak located between 1–10 μm. The main peak represented the stable emulsion, while the smaller peak could be ascribed to the flocculation or partial coalescence of a small quantity of oil droplets.23,24 In comparison, the emulsion with 0.1 wt% CNC presented only a single, tight peak in the size distribution plot, which indicated that the introduced CNC could effectively prevent flocculation or partial coalescence of oil droplet. In other words, CNC could act as a co-stabilizer in this coating formula. Therefore, the Sauter mean diameter (D3,2) of oil droplet decreased from 248 nm to 235 nm after introducing 0.1 wt% CNC.
 | ||
| Fig. 2 Variation in initial droplet size distribution with or without 0.1wt% CNC in water phase (30 mM SDSO). | ||
To prove the co-stabilization effect of CNC, the interfacial tension and droplet size at different CNC contents with 30 mM SDSO was measured and presented in Fig. 3. The interfacial tension reduced from 3.7 mN m−1 to 3.4 mN m−1 after the CNC content reaching 0.1 wt%. With increasing the CNC content to 1 wt%, the interfacial tension further reduced to 3.3 mN m−1, despite that the reduction was limited, Similar reductions in the interfacial tension were recorded by Hu, et al.,25 which was attributed to the intermediate wettability of CNC. The interfacial tension and particle size were positively correlated.26 This was proven by the change of initial droplet size in Fig. 3. More particles could nucleate at lower interfacial tension.27 For a fixed volume of oil phase, more particles indicated the smaller size in singe droplet.
 | ||
| Fig. 3 Effect of CNC content on the initial droplet size and interfacial tension between oil and water phase (30 mM SDSO). | ||
CNC was reported as a Pickering agent to stabilize the oil-water interface in emulsions due to intermediate wettability and nanometric size.28–32 Pickering emulsions were also known as particle-stabilized emulsions. Like other Pickering agents, CNC could prevent coalescence and sedimentation by forming a tightly packed layer.33 Although there was still a debate on whether particles could also decrease the interfacial tension like surfactants.34 Some literature insisted claiming that they found the reduction of interfacial tension with particles as stabilizers.35–39
To further validate the co-stabilization effect, zeta potential of each sample was measured and presented in Fig. 4. Empirically, the emulsion was considered as a stable system if the potential values higher than +30 mV or lower than −30 mV, which indicated that particles repelled each other and form a stable dispersion.40 Moreover, a higher absolute value of zeta potential referred to a higher stable state of colloidal systems.41 In this way, all the samples with or without CNC were stable. After introducing 0.1 wt% CNC, the absolute value of zeta potential increased from 40.2 to 45.1 mV, indicating the stability enhancement. This is another evidence of CNC to be the co-stabilizer.
3.2. Effect of the CNC on the acrylate miniemulsion polymerization
In addition to the droplet size, the yield of miniemulsion polymerization at different CNC contents was calculated and displayed in Fig. 7. The yield of each sample was similar, approximately 96% at the end of the experiment. Most of samples achieved the highest yield within first hour, except for the 1.5 wt% and 2 wt% CNC samples. The difference in termination time might be affected by the rate of miniemulsion polymerization Rp and diffusion coefficient D of initiator from water phase to monomer droplet. Bechthold and Landfester45 proposed an equation to calculate miniemulsion polymerization rate Rp (eqn (1)).
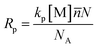 | (1) |
![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) was the average effective radical number per droplet, N was the number of droplets per liter of water phase, and NA was the Avogadro's number.
was the average effective radical number per droplet, N was the number of droplets per liter of water phase, and NA was the Avogadro's number.
In this study, the reaction temperature, monomer and initiator amount concentration were fixed, so the value of kp, [M] and ![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) remained constant. As for the number of droplets per liter of water phase N, it could be calculated with eqn (2).
remained constant. As for the number of droplets per liter of water phase N, it could be calculated with eqn (2).
 | (2) |
However, the reaction initiation point was different, which could be influenced by the diffusion of free radicals from initiator to monomer. The Stokes–Einstein equation in rotational diffusion46 was given as listed,
 | (3) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O in ester group, respectively, validating the existence of PMMA-co-PBA.49,50 For CNC, the peak at 613 cm−1 was caused by the stretching vibration of aromatic –CH.51 The peak at 896 cm−1 could be the characteristics of the C–O–C symmetric stretching vibration of β-glycosidic linkages.52 Additionally, peaks at 1161 cm−1 and 1060 cm−1 corresponded to the stretching vibration of C–O–C in the pyranose ring and the bending vibration of C–O–C in β-glycosidic linkages, respectively.53–56 For the ease of comparation, these characteristic peaks were summarized and listed in Table 1.
O and C–O in ester group, respectively, validating the existence of PMMA-co-PBA.49,50 For CNC, the peak at 613 cm−1 was caused by the stretching vibration of aromatic –CH.51 The peak at 896 cm−1 could be the characteristics of the C–O–C symmetric stretching vibration of β-glycosidic linkages.52 Additionally, peaks at 1161 cm−1 and 1060 cm−1 corresponded to the stretching vibration of C–O–C in the pyranose ring and the bending vibration of C–O–C in β-glycosidic linkages, respectively.53–56 For the ease of comparation, these characteristic peaks were summarized and listed in Table 1.
| Peak location (cm−1) | Origin of peak | Reference |
|---|---|---|
| 2997 | C–H stretching vibration of –CH3 groups | 47 |
| 2952 | C–H stretching vibration of –CH2- groups | 47 |
| 2899 | C–H asymmetric stretching vibration in glucose units | 56 and 57 |
| 2845 | C–H symmetric stretching vibration in glucose units | 57 |
| 1732 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration O stretching vibration |
49 and 50 |
| 1444 | C–H bending vibration of –CH3 and –CH2- groups | 48 |
| 1250, 1150 | C–O stretching vibrations of ester groups | 49 and 50 |
| 1161 | C(O)–O stretching vibration in pyranose ring | 53–55 |
| 1060 | C–O–C bending vibration of β-glycosidic linkages | 56 |
| 896 | C–O–C symmetric stretching vibration of β-glycosidic linkages | 52 |
| 613 | Aromatic –CH stretching vibration | 51 |
As shown in Fig. 9, FTIR spectra of polyacrylate samples with different CNC contents were presented. In the spectra, the peaks at 1161, 1060, 896, and 613 cm−1 became more apparent when CNCs content exceeded 0.5 wt%, which indicated the successful addition of CNC. Moreover, peaks at 2899 cm−1 and 2845 cm−1 were also observed in the samples with higher CNC contents (>0.5 wt%). These two peaks were categorized to C–H asymmetric and symmetric stretching vibration in glucose units, respectively.56,57 Meanwhile, CNC contained glucose units. In other words, this was another evidence that CNC was successfully incorporated into the polyacrylate samples. However, no other new peaks were observed, which indicated that no new substance formed. Therefore, CNC was estimated to exist between PMMA-co-PBA without attending the reaction.
To further evaluate the interaction between CNC and PMMA-co-PBA, the morphology of polyacrylate samples with or without CNC and CNC as received was investigated with SEM, which was shown in Fig. 10. For the polyacrylate sample without CNC, both the single droplet and coalesced droplets could be observed. After adding 0.1 wt% CNC, the polyacrylate sample was dispersed, and CNC located near the droplet. Similar phenomenon was observed in the 0.5 wt% sample, while the coalescence of droplets was more severe. With further increasing the CNC content to 1 wt%, the coagulation happened. Then the aggregation was found in 1.5 wt% and 2 wt% samples, where the aggregation of 2 wt% is relatively harder.
The interaction between CNC and PMMA-co-PBA was further evaluated using SEM to analyse the morphology of polyacrylate samples with varying CNC concentration (Fig. 10). Fig. 10g confirmed the rod-like shape of CNC. In the absence of CNC. In the absence of CNC (Fig. 10a), the polyacrylate sample exhibited the coexist of individual droplets and droplets coalescence, which suggests the instability in such system. Adding 0.1 wt% CNC (Fig. 10b) resulted in noticeable uniform dispersion within the polymer matrix, with CNC particles predominantly localized near the droplets. This suggested that presence of CNC at lower concentrations could prevent droplets coalescence even. This further suggests that CNC could acted as a stabilizing agent in emulsion formation. However, further increase CNC concentration to 0.5 wt%, the droplet coalescence became more pronounced. As the CNC content increased to 1 wt%, coagulation was observed. At higher CNC concentrations (1.5 wt% and 2 wt%), significant aggregation occurred, with the most severe aggregation seen in the 2 wt% sample. This phenomenon the function of CNC as co stabiliser is limited at lower concentration. At higher concentration, CNC particle–particle interactions become dominant to induce droplets coalescence hence jeopardise stability of emulsion.58
These results highlighted the dual role of CNC as both a stabilizing and aggregating agent, depending on its concentration. As shown in Fig. 11, the interaction between CNC and PMMA-co-PBA was illustrated. Without CNC, the acrylate emulsion can be initiated by free radicals to polyacrylate emulsion with reducing droplet size, proven by the droplet size change in Fig. 5. The addition of suitable amount of CNC can help the dispersion of acrylate and polyacrylate emulsion. After introducing excessive amount of CNC, the dispersion of acrylate droplets in emulsion might still be enhancing, proven by the droplet size, interfacial tension (Fig. 3), and zeta potential (Fig. 4). However, the stability of polyacrylate emulsion would not be as stable as the one without CNC, coagulation and aggregation happened during the polymerization and influenced the storage stability of emulsion.
3.3. Effect of the CNC on storage stability of polyacrylate miniemulsion
As discussed in Section 3.2.2, the storage stability of polyacrylate miniemulsion might be influenced by the CNC contents. According to Anton and Vandamme,59 changes in droplet size over time could indicate the storage stability of the emulsion. To observe the effect of CNC on the storage stability of emulsion, the droplet size of acrylate miniemulsions was measured every 7 days. Although the miniemulsion appeared visually stable, droplet sizes shown in Fig. 12 increased after 4 weeks of storage at room temperature. For the 0 wt% CNC sample, the miniemulsion was relatively stable, with initial and final droplet sizes of 192 nm and 198 nm, respectively. Similarly, the samples with CNC content lower than 0.5 wt% were also stable. This might be caused by the interactions between anionic surfactants and CNC.60 Without CNC, the emulsion was only stabilized by SDSO with hydrophobic site in the oil phase and hydrophilic site in the water phase. After introducing CNC, it might cooperate with SDSO, where SDSO stabilized the O/W interface, and CNC formed a stable network under hydrogen bonding and electrostatic repulsion.61,62 However, when the content exceeded 1 wt%, CNC would apply the adverse effect on the storage stability of the miniemulsion. Especially for 2 wt% CNC sample, the droplet size increased significantly from 202 nm to 230 nm, which indicated the coagulation and aggregation of droplets.63 This was proven by the SEM images (Fig. 10) as a sign of deterioration in storage stability.4. Conclusion
This study offers a comprehensive analysis of the interactions between CNC and PMMA-co-PBA in an emulsion system, both before and after polymerization. It provides valuable insights into CNC's functional roles. The results demonstrate that CNC effectively co-stabilizes acrylate miniemulsions before polymerization. While CNC did not directly participate in polymerization or affect yield or reaction rates, it slowed the diffusion of free radicals during the process. For certain concentrations, such as 1 wt%, CNC enhanced emulsion stability before polymerization but negatively impacted storage stability afterward. These findings highlight the dual role of CNC as both a stabilizing and aggregating agent, emphasizing its potential for tailoring polymer properties for industrial applications. Future research should focus on modifying CNC to improve its stabilizing effects on both the initial emulsion and the final polymerized system, while further elucidating the underlying mechanisms of its interactions with polymer matrices.Data availability
The data are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research is financially supported by Zhejiang Yuxi Corrosion Control Corporation, grant number (01.03.07.01.2015.06.002).References
- A. Arjmandi, H. Bi, S. U. Nielsen and K. Dam-Johansen, From Wet to Protective: Film Formation in Waterborne Coatings, ACS Appl. Mater. Interfaces, 2024, 16, 58006–58028 CrossRef CAS PubMed.
- H. Yao, L. Li, W. Li, D. Qi, W. Fu and N. Wang, Application of nanomaterials in waterborne coatings: A review, Resour. Chem. Mater., 2022, 1(2), 184–200 Search PubMed.
- T. Li, et al., Developing fibrillated cellulose as a sustainable technological material, Nature, 2021, 590(7844), 47–56 CrossRef CAS PubMed.
- G. Jiang, et al., A scalable bacterial cellulose ionogel for multisensory electronic skin, Research, 2022 DOI:10.34133/2022/9814767.
- F. Garavand, M. Nooshkam, D. Khodaei, S. Yousefi, I. Cacciotti and M. Ghasemlou, Recent advances in qualitative and quantitative characterization of nanocellulose-reinforced nanocomposites: A review, Adv. Colloid Interface Sci., 2023, 102961 CrossRef CAS PubMed.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Cellulose: fascinating biopolymer and sustainable raw material, Angew. Chem., Int. Ed., 2005, 44(22), 3358–3393 CrossRef CAS PubMed.
- K. Nagarajan, et al., A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization, Polym. Compos., 2021, 42(4), 1588–1630 CrossRef CAS.
- J. George and S. Sabapathi, Cellulose nanocrystals: synthesis, functional properties, and applications, Nanotechnol., Sci. Appl., 2015, 8, 45 CrossRef CAS PubMed.
- D. Dey, et al., Physical, antifungal, and biodegradable properties of cellulose nanocrystals and chitosan nanoparticles for food packaging application, Mater. Today: Proc., 2021, 38, 860–869 CAS.
- N. Nepomuceno, A. Seixas, E. Medeiros and T. Mélo, Evaluation of conductivity of nanostructured polyaniline/cellulose nanocrystals (PANI/CNC) obtained via in situ polymerization, J. Solid State Chem., 2021, 302, 122372 CrossRef CAS.
- J. C. Jackson, C. H. Camargos, V. T. Noronha, A. J. Paula, C. A. Rezende and A. F. Faria, Sustainable cellulose nanocrystals for improved antimicrobial properties of thin film composite membranes, ACS Sustainable Chem. Eng., 2021, 9(19), 6534–6540 CrossRef CAS.
- L. Qin, H. Gao, S. Xiong, Y. Jia and L. Ren, Preparation of collagen/cellulose nanocrystals composite films and their potential applications in corneal repair, J. Mater. Sci.: Mater. Med., 2020, 31, 1–11 CrossRef PubMed.
- U. Mamudu, M. R. Hussin, J. H. Santos and R. C. Lim, Synthesis and characterisation of sulfated-nanocrystalline cellulose in epoxy coatings for corrosion protection of mild steel from sodium chloride solution, Carbohydr. Polym. Technol. Appl., 2023, 5, 100306 CAS.
- N. F. S. M. Azani, M. M. Haafiz, A. Zahari, S. Poinsignon, N. Brosse and M. H. Hussin, Preparation and characterizations of oil palm fronds cellulose nanocrystal (OPF-CNC) as reinforcing filler in epoxy-Zn rich coating for mild steel corrosion protection, Int. J. Biol. Macromol., 2020, 153, 385–398 CrossRef CAS PubMed.
- N. F. S. M. Azani and M. H. Hussin, Comparison of cellulose nanocrystal (CNC) filler on chemical, mechanical, and corrosion properties of epoxy-Zn protective coatings for mild steel in 3.5% NaCl solution, Cellulose, 2021, 28(10), 6523–6543 CrossRef CAS.
- X. Wang, K. Gao, E. B. Caldona, M. R. R. Ali, X. Zhang and Z. Zhang, Cellulose nanocrystals-reinforced waterborne epoxy coatings with enhanced corrosion resistance for steel, Int. J. Biol. Macromol., 2024, 257, 128755 CrossRef CAS PubMed.
- Y. He, Y. Boluk, J. Pan, A. Ahniyaz, T. Deltin and P. M. Claesson, Corrosion protective properties of cellulose nanocrystals reinforced waterborne acrylate-based composite coating, Corros. Sci., 2019, 155, 186–194 CrossRef CAS.
- S. Tamantini, S. Bergamasco, F. Zikeli, M. Humar, M. Cavalera and M. Romagnoli, Cellulose nano crystals (CNC) as additive for a bio-based waterborne acrylic wood coating: decay, artificial weathering, physical and chemical tests, Nanomaterials, 2023, 13(3), 442 CrossRef CAS PubMed.
- M. N. Khan, C. M. Clarkson, M. Nuruddin, A. Sharif, E. Ahmad and J. P. Youngblood, Performance of advanced waterborne wood coatings reinforced with cellulose nanocrystals, ACS Appl. Bio Mater., 2022, 5(9), 4179–4190 CrossRef CAS PubMed.
- Z. Wang, B. Hu and G. Z. Chen, The role of 1-octyl-3-methylimidazolium hexafluorophosphate in anticorrosion coating formula development, J. Saudi Chem. Soc., 2022, 26(3), 101446 CrossRef CAS.
- Z. Wang, B. Hu, H. Yu and G. Z. Chen, Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings, Polymers, 2023, 15(4), 810 CrossRef CAS PubMed.
- Y. Kong, Encapsulation of Room Temperature Ionic Liquids by Miniemulsion Polymerisation for Application in Low-Emitting Latex Coating, University of Nottingham, 2016 Search PubMed.
- T. Sakai, K. Kamogawa, F. Harusawa, N. Momozawa, H. Sakai and M. Abe, Direct observation of flocculation/coalescence of metastable oil droplets in surfactant-free oil/water emulsion by freeze-fracture electron microscopy, Langmuir, 2001, 17(2), 255–259 CrossRef CAS.
- T. M. Ho, A. Razzaghi, A. Ramachandran and K. S. Mikkonen, Emulsion characterization via microfluidic devices: A review on interfacial tension and stability to coalescence, Adv. Colloid Interface Sci., 2022, 299, 102541 CrossRef CAS PubMed.
- Z. Hu, S. Ballinger, R. Pelton and E. D. Cranston, Surfactant-enhanced cellulose nanocrystal Pickering emulsions, J. Colloid Interface Sci., 2015, 439, 139–148 CrossRef CAS PubMed.
- K. Reinheimer, M. Grosso and M. Wilhelm, Fourier transform rheology as a universal non-linear mechanical characterization of droplet size and interfacial tension of dilute monodisperse emulsions, J. Colloid Interface Sci., 2011, 360(2), 818–825 CrossRef CAS PubMed.
- K. von Nessen, M. Karg and T. Hellweg, Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: Tailoring particle size by interfacial tension control, Polymer, 2013, 54(21), 5499–5510 CrossRef CAS.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, New Pickering emulsions stabilized by bacterial cellulose nanocrystals, Langmuir, 2011, 27(12), 7471–7479 CrossRef CAS PubMed.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface, Biomacromolecules, 2011, 13(1), 267–275 CrossRef PubMed.
- I. Kalashnikova, H. Bizot, P. Bertoncini, B. Cathala and I. Capron, Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions, Soft Matter, 2013, 9(3), 952–959 RSC.
- I. Capron and B. Cathala, Surfactant-free high internal phase emulsions stabilized by cellulose nanocrystals, Biomacromolecules, 2013, 14(2), 291–296 CrossRef CAS PubMed.
- S. Tasset, B. Cathala, H. Bizot and I. Capron, Versatile cellular foams derived from CNC-stabilized Pickering emulsions, RSC Adv., 2014, 4(2), 893–898 RSC.
- B. P. Binks and T. S. Horozov, Colloidal Particles at Liquid Interfaces, Cambridge University Press, 2006 Search PubMed.
- R. I. Dekker, et al., Is there a difference between surfactant-stabilised and Pickering emulsions?, Soft Matter, 2023, 19(10), 1941–1951 RSC.
- T. Okubo, Surface tension of structured colloidal suspensions of polystyrene and silica spheres at the air-water interface, J. Colloid Interface Sci., 1995, 171(1), 55–62 CrossRef CAS.
- N. Saleh, T. Sarbu, K. Sirk, G. V. Lowry, K. Matyjaszewski and R. D. Tilton, Oil-in-water emulsions stabilized by highly charged polyelectrolyte-grafted silica nanoparticles, Langmuir, 2005, 21(22), 9873–9878 CrossRef CAS PubMed.
- N. Glaser, D. J. Adams, A. Böker and G. Krausch, Janus particles at liquid− liquid interfaces, Langmuir, 2006, 22(12), 5227–5229 CrossRef CAS PubMed.
- S. Kutuzov, J. He, R. Tangirala, T. Emrick, T. Russell and A. Böker, On the kinetics of nanoparticle self-assembly at liquid/liquid interfaces, Phys. Chem. Chem. Phys., 2007, 9(48), 6351–6358 RSC.
- J. Forth, P. Y. Kim, G. Xie, X. Liu, B. A. Helms and T. P. Russell, Building reconfigurable devices using complex liquid–fluid interfaces, Adv. Mater., 2019, 31(18), 1806370 CrossRef PubMed.
- D. Sun, S. Kang, C. Liu, Q. Lu, L. Cui and B. Hu, Effect of zeta potential and particle size on the stability of SiO2 nanospheres as carrier for ultrasound imaging contrast agents, Int. J. Electrochem. Sci., 2016, 11(10), 8520–8529 CrossRef CAS.
- D. J. Pochapski, C. Carvalho dos Santos, G. W. Leite, S. H. Pulcinelli and C. V. Santilli, Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results, Langmuir, 2021, 37(45), 13379–13389 CrossRef CAS PubMed.
- P. A. Lovell and F. J. Schork, Fundamentals of emulsion polymerization, Biomacromolecules, 2020, 21(11), 4396–4441 CrossRef CAS PubMed.
- P. Jenkins and M. Snowden, Depletion flocculation in colloidal dispersions, Adv. Colloid Interface Sci., 1996, 68, 57–96 CrossRef CAS.
- A. Wittmar, D. Ruiz-Abad and M. Ulbricht, Dispersions of silica nanoparticles in ionic liquids investigated with advanced rheology, J. Nanopart. Res., 2012, 14(2), 651 CrossRef.
- N. Bechthold and K. Landfester, Kinetics of miniemulsion polymerization as revealed by calorimetry, Macromolecules, 2000, 33(13), 4682–4689 CrossRef CAS.
- A. Einstein, Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen, Ann. Phys., 1905, 322(8), 549–560 CrossRef.
- S. Eve and J. Mohr, Effects of UV-irradiation on the thermo-mechanical properties of optical grade poly (methyl methacrylate), Appl. Surf. Sci., 2010, 256(9), 2927–2933 CrossRef CAS.
- B. Schneider, J. Štokr, P. Schmidt, M. Mihailov, S. Dirlikov and N. Peeva, Stretching and deformation vibrations of CH2, C (CH3) and O (CH3) groups of poly (methyl methacrylate), Polymer, 1979, 20(6), 705–712 CrossRef CAS.
- G. Duan, C. Zhang, A. Li, X. Yang, L. Lu and X. Wang, Preparation and characterization of mesoporous zirconia made by using a poly (methyl methacrylate) template, Nanoscale Res. Lett., 2008, 3(3), 118 CrossRef CAS PubMed.
- S. Ramesh, K. H. Leen, K. Kumutha and A. Arof, FTIR studies of PVC/PMMA blend based polymer electrolytes, Spectrochim. Acta, Part A, 2007, 66(4–5), 1237–1242 CrossRef CAS PubMed.
- M. Schwanninger, J. Rodrigues, H. Pereira and B. Hinterstoisser, Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose, Vib. Spectrosc., 2004, 36(1), 23–40 CrossRef CAS.
- J. Lamaming, R. Hashim, O. Sulaiman, C. P. Leh, T. Sugimoto and N. A. Nordin, Cellulose nanocrystals isolated from oil palm trunk, Carbohydr. Polym., 2015, 127, 202–208 CrossRef CAS PubMed.
- A. Mandal and D. Chakrabarty, Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization, Carbohydr. Polym., 2011, 86(3), 1291–1299 CrossRef CAS.
- W. Wulandari, A. Rochliadi and I. Arcana, Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2016, vol. 107, supp. 1, p. 012045 Search PubMed.
- A. Alemdar and M. Sain, Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls, Bioresour. Technol., 2008, 99(6), 1664–1671 CrossRef CAS PubMed.
- Y. Liu, et al., Modified ammonium persulfate oxidations for efficient preparation of carboxylated cellulose nanocrystals, Carbohydr. Polym., 2020, 229, 115572 CrossRef CAS PubMed.
- B. Abderrahim, E. Abderrahman, A. Mohamed, T. Fatima, T. Abdesselam and O. Krim, Kinetic thermal degradation of cellulose, polybutylene succinate and a green composite: comparative study, World J. Environ. Eng., 2015, 3(4), 95–110 Search PubMed.
- C. Campano, P. Lopez-Exposito, L. Gonzalez-Aguilera, Á. Blanco and C. Negro, In-depth characterization of the aggregation state of cellulose nanocrystals through analysis of transmission electron microscopy images, Carbohydr. Polym., 2021, 254, 117271 CrossRef CAS PubMed.
- N. Anton and T. F. Vandamme, Nano-emulsions and micro-emulsions: clarifications of the critical differences, Pharm. Res., 2011, 28(5), 978–985 CrossRef CAS PubMed.
- Q. Yuan and R. A. Williams, CO-stabilisation mechanisms of nanoparticles and surfactants in Pickering Emulsions produced by membrane emulsification, J. Membr. Sci., 2016, 497, 221–228 CrossRef CAS.
- K. Guo, P. Wei, Y. Xie and X. Huang, Smart ultra-stable foams stabilized using cellulose nanocrystal (CNC) gels via noncovalent bonding, Chem. Commun., 2022, 58(30), 4723–4726 RSC.
- J. Hu, et al., Carboxylation of cellulose nanocrystals for reinforcing and toughing rubber through dual cross-linking networks, ACS Appl. Polym. Mater., 2021, 3(12), 6120–6129 CrossRef CAS.
- T. Cao and M. Elimelech, Colloidal stability of cellulose nanocrystals in aqueous solutions containing monovalent, divalent, and trivalent inorganic salts, J. Colloid Interface Sci., 2021, 584, 456–463 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |










