Open Access Article
Open Access ArticleImmunocytochemistry response of a hybrid (chitosan-g-glycidyl methacrylate)-xanthan scaffold for cell proliferation
Argelia Rosillo-de la Torrea,
Tonantzi Pérez-Morenob,
Iraís A. Quintero-Ortegaa,
Padmavati Saharec,
Diego A. Bravo-Alfaro c,
Héctor E. Martínez-Floresd,
Friné López-Medinae,
Ramón Román-Doval
c,
Héctor E. Martínez-Floresd,
Friné López-Medinae,
Ramón Román-Doval f,
Alejandro Gómez-Sánchezf,
Sandra Herrera-Perezg,
Julie E. Goughh,
Zaira Yunuen García Carvajal*i,
Cristina Velasquillo*j and
Gabriel Luna-Barcenas*c
f,
Alejandro Gómez-Sánchezf,
Sandra Herrera-Perezg,
Julie E. Goughh,
Zaira Yunuen García Carvajal*i,
Cristina Velasquillo*j and
Gabriel Luna-Barcenas*c
aDivisión de Ciencias e Ingenierías, Universidad de Guanajuato, Guanajuato, Leon, GTO 37150 Mexico. E-mail: rosillo.a@ugto.mx; iraisq@fisica.ugto.mx
bDivisión de Investigación y Posgrado, Facultad de Ingeniería, Universidad Autónoma de Querétaro, Santiago de Querétaro, QRO 76010, Mexico. E-mail: tonantzi.perez@uaq.mx
cTecnologico de Monterrey, Institute of Advanced Materials for Sustainable Manufacturing, Santiago de Queretaro 76130, Mexico. E-mail: padma.sahare@tec.mx; diego.bravo@tec.mx; gabriel.luna@tec.mx
dFaculty of Chemical Pharmacology, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, MICH 58240, Mexico. E-mail: hedu65@hotmail.com
eUniversidad Autónoma de Tlaxcala, Apizaco, TLAX 90401, Mexico. E-mail: frine.lopez@uatx.mx
fTecnológico Nacional de México, Instituto Tecnológico del Valle de Etla, Santiago Suchilquitongo, OAX 68230, Mexico. E-mail: rrdoval.11@gmail.com; drek26@hotmail.com
gTecnológico Nacional de México en Celaya, Celaya, Gto. 38010, Mexico. E-mail: sandra.herrera@itcelaya.edu.mx
hDepartment of Materials and Henry Royce Institute, The University of Manchester, Manchester M13 9PL, UK. E-mail: j.gough@manchester.ac.uk
iBiotecnología Médica y Farmacéutica, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. (CIATEJ), Guadalajara 44270, Jalisco, Mexico. E-mail: zgarcia@ciatej.mx
jUnidad de Ingeniería de Tejidos, Terapia Celular y Medicina Regenerativa, Instituto Nacional de Rehabilitación Luis Guillermo Ibarra Ibarra, Ciudad de México 14389, CDMX, Mexico. E-mail: mvelasquillo@inr.gob.mx
First published on 10th March 2025
Abstract
In the present work, chitosan-glycidyl methacrylate-xanthan ((CTS-g-GMA)-X) hydrogel was successfully synthesized by the chemical reaction of a co-polymer of chitosan-g-glycidyl methacrylate (CTS-g-GMA), under different stoichiometric molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and xanthan (X). (CTS-g-GMA)-X was synthesized by two methods to obtain a hydrogel: an aqueous acid media followed by neutralization and a neutral aqueous media, with an extended reaction time. Human epidermal keratinocytes (HEK) and nerve cells (neuroblastoma × glioma hybrid cell NG108-15) were sown over the hydrogels, and their vitality was determined using a calcein stain. The viability for keratinocytes and nerve cells was quantified using a DNA (proliferation) assay at several intervals. Additionally, immunocytochemistry, detecting E-cadherin, fibronectin, and laminin proteins in HEK, was performed to discard possible diseases caused by (CTS-g-GMA)-X hydrogels. The (CTS-GMA) shows greater results compared with the positive control (glass) and the pure chitosan; all polymers show similar satisfactory behaviors between them.
4, and xanthan (X). (CTS-g-GMA)-X was synthesized by two methods to obtain a hydrogel: an aqueous acid media followed by neutralization and a neutral aqueous media, with an extended reaction time. Human epidermal keratinocytes (HEK) and nerve cells (neuroblastoma × glioma hybrid cell NG108-15) were sown over the hydrogels, and their vitality was determined using a calcein stain. The viability for keratinocytes and nerve cells was quantified using a DNA (proliferation) assay at several intervals. Additionally, immunocytochemistry, detecting E-cadherin, fibronectin, and laminin proteins in HEK, was performed to discard possible diseases caused by (CTS-g-GMA)-X hydrogels. The (CTS-GMA) shows greater results compared with the positive control (glass) and the pure chitosan; all polymers show similar satisfactory behaviors between them.
1. Introduction
Due to their abundant availability and unique biological properties, natural polymers have attracted significant interest in regenerative medicine, particularly for applications such as cell scaffolding, wound dressing, tissue regeneration, and wound healing. These materials exhibit vital attributes, including biocompatibility and structures similar to the extracellular matrix (ECM) components. As a result, they have facilitated the development and application of new natural and semi-synthetic materials that mimic the structure and functionality of tissues while also providing favorable conditions for cell growth.1 For example, designing a cellular scaffold involves tailoring the biomaterial's properties to encourage proper interaction between cells and the ECM, which is crucial for effective tissue formation.2 Therefore, selecting the appropriate components for the biomaterial is essential. Materials under investigation for these applications include collagen, cellulose, alginate, chitin, hyaluronic acid, starch, and chitosan.Chitin, the second most abundant polysaccharide in nature after cellulose, is converted into chitosan (CTS), a natural polymer, through deacetylation.3 Due to its structural similarity to natural glycosaminoglycans and its biodegradability by human enzymes such as lysozyme, CTS has been extensively explored for various tissue engineering applications.4–6 Furthermore, CTS possesses several attractive properties, including low toxicity, a cationic nature, and versatile preparation methods that allow for producing a wide range of biomaterials (e.g., scaffolds, films, hydrogels, and foams). Its functional groups also enable the functionalization or conjugation of biomolecules, making it highly suitable for tissue engineering.7 CTS-based hydrogels are widely investigated in skin tissue engineering, with numerous studies demonstrating the in vitro proliferation of various cell types, such as fibroblasts and keratinocytes, to form skin layers.8–12 Additionally, neuronal cell growth has been observed in vivo and in vitro.13,14 Moreover, there is strong evidence supporting the acceleration of the wound-healing process when wounds are in direct contact with CTS-based hydrogels, as shown in various in vivo models15–18 and clinical studies.19
Xanthan gum (X), an exopolysaccharide produced by the bacterium Xanthomonas, is an intriguing biomaterial for various biomedical applications, including tissue engineering and drug delivery, due to its desirable properties such as excellent biocompatibility, biodegradability, non-toxicity, and immunological activity.20,21 Several studies have focused on evaluating X-based biomaterials to promote the growth and proliferation of specialized cells for skin regeneration.22,23 However, X-based biomaterials for nerve cell tissue engineering24–26 have not been as extensively investigated as their chitosan (CTS) counterparts. This study evaluates the in vitro biocompatibility of (CTS-g-GMA)-X hydrogels as extracellular matrix (ECM) supports (scaffolds) for fibroblasts, keratinocytes, and nerve cells, using assays such as calcein viability, DNA quantification for cell number, peroxide levels, interleukin-1β, and immunocytochemistry.
2. Materials and methods
2.1. Materials
The commercial reagents were from Aldrich Chemical and were used without additional purification. The chitosan had a degree of deacetylation of about 87%, determined by a titration method, and an average molecular weight of 300 kDa, determined by viscometry in a 0.1 M acetic acid and 0.2 M sodium chloride solution at 25 °C. The Mark–Houwink constants, α, and K, were taken from Milas et al.27 for the xanthan (X) and from Kasaai et al.28 for chitosan (CTS). The liquid glycidyl methacrylate (GMA) had 97% purity and was used without additional purification.2.2. Cells culture
2.3. (CTS-g-GMA)-X hydrogel synthesis
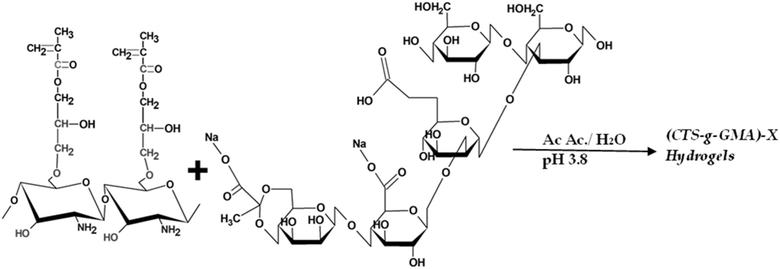
The (CTS-g-GMA)-X hydrogels were synthesized by modifying the method proposed by Elizalde-Peña et al. 2008.33 The (CTS-g-GMA)-X hydrogels were labeled Z11E, Z12E, Z13E, and Z14E, respectively. The numbers 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 correspond to stoichiometric molar ratios of CTS
4 correspond to stoichiometric molar ratios of CTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMA. E = A for CTS-g-GMA materials dissolved in acid solution and E = B for neutral dissolution (Table 1).33,34
GMA. E = A for CTS-g-GMA materials dissolved in acid solution and E = B for neutral dissolution (Table 1).33,34
| Material | General conditions | |||
|---|---|---|---|---|
Stoichiometric ratio (CTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMA) GMA) |
CTS-g-GMA solvent | Xanthan solvent | Gravimetric ratio (CTS-g-GMA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X X |
|
| Z11A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Acetic acid 0.4 M | Distilled water | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Z12A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|||
| Z13A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
|||
| Z14A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|||
| Z11B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Distilled water | ||
| Z12B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|||
| Z13B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
|||
| Z14B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|||
To obtain a pH similar to physiological, we used a neutral and acid aqueous media to synthesize CTS-g-GMA-X hydrogels. In an Erlenmeyer flask, a known quantity, in dry weight, of CTS-g-GMA was placed, and then an acetic acid solution of 0.4 M (pH mixture 3.1) was added for series A or distilled water for series B. When the CTS-g-GMA was dissolved, the xanthan solution was added slowly. The vial was closed, then a nitrogen flow was introduced; the temperature was 50 ± 1 °C, and constant magnetic agitation was used; the reaction scheme is shown in Fig. 1. The gas flow, heating, and agitation were stopped after one hour (for series A) or four hours (for series B). The product was cooled down in an ice bath for 15 minutes to complete the reaction.
NaOH 0.2 M solution was added for the polymer's series A until a pH of ca. 6.8 was reached. Under these conditions, white pearls remained formed, immersed in a colorless solution, and they were recovered by decantation. The pearls were rinsed several times in distilled water to eliminate the residual reactants. The material was recovered by decantation and washed several times in distilled water for the series B polymers to eliminate the residual reactants. Both products were dried in an oven for one hour at 45 °C to obtain films for the subsequent characterization.
All the items used for the tests were conditioned to avoid introducing microbes that could have been picked up while handling the materials in non-sterile places. First, material discs of 10 mm diameter were placed in 48-well plates and were immersed twice in 1 mL of ethanol (70%) for 15 minutes. Then, the ethanol was removed, washed twice with sterile PBS, and maintained overnight in a sterile environment.33
2.4. Infrared characterization
Infrared spectroscopy determined the structural differences between both series of materials. The FTIR spectra were recorded on a PerkinElmer spectrometer (model Spectrum One) in the 4000 to 450 cm−1 range at a resolution of 4 cm−1 in transmission mode.2.5. Live/dead assay
A LIVE–DEAD® Viability/Cytotoxicity Kit (L-3224, Molecular Probes, Invitrogen) was used to check the cell viability. The cells were seeded at a density of 4 × 104 cells per mL in corresponding media onto each disc of polymer; additionally, glasses were used as a positive control. Each well received 500 mL of calcein AM in PBS containing 2 mM of EthD1, which was added for 20 minutes of incubation. The times for this assay were 1 and 5 days, after which media was removed, and the samples were rinsed three times with PBS. Following removing the media, each sample was placed on a glass microscope slide and observed under a fluorescence microscope; healthy cells fluoresce green, while damaged cells fluoresce red. All assays were made in triplicate.2.6. DNA assay for cell number
H413 keratinocytes were seeded at a density of 4 × 104 cells per mL in DMEM:F12 onto each polymer; additionally, glass coverslips were used as a positive control. The keratinocyte's behaviors were observed quantitatively on the materials mediated by a DNA assay for cell number. The times for this assay were 1, 3, and 7 days. After this time, the media was removed, and samples were rinsed with PBS. Each sample was transferred to a separate replicate multiwell plate. Distilled water was added to each new well, and samples were freeze-thawed three times. Aliquots of 100 mL of the specimens, standards, and blank (distilled water) were placed into a 96-well plate, and 100 mL of Hoechst stain was added to each well. The plate was shaken for 10 seconds, and fluorescence measurements at 355 nm excitation and 460 nm emission were carried out using a fluorescence plate reader (Fluostar Optima).35,36 All assays were made in triplicate.2.7. Interleukin 1b assay (IL-1β)
This assay quantitatively determines the IL-1β in cell culture supernatant released for the macrophages when seeded onto the materials. Cells were cultured for 2 and 48 hours on the material samples.We used 50 μg mL−1 of lipopolysaccharide (LPS) as a positive control for the Il-1β release at various concentrations at both times. The assay was performed, and the optical density of each well was determined using a microplate reader at 450 nm (measurement) and 540 nm (reference) wavelengths.32 All assays were made in triplicate.
2.8. Immunocytochemistry (ICC)
This test has been made only for the HEKa. Fibronectin, laminin, and E-cadherin antibodies were detected in the cells by ICC protocol, using rabbit IgG as a secondary antibody for fibronectin and laminin; for E-cadherin, mouse IgG and rabbit IgG were used. This assay was performed for 1 and 5 days, respectively, after which media was removed, and the samples were rinsed twice with PBS. The cells were fixed in 3–4% paraformaldehyde in PBS for 15 minutes at room temperature and washed twice using ice-cold PBS. The samples were incubated for 10 minutes with PBS containing 0.25% Triton X-100. Samples were washed in PBS three times for 5 minutes.Cells were incubated with 1% Bovine Serum Albumin (BSA) in PBS for 30 minutes to block the unspecific binding of the antibodies. Next, the solution was removed, and cells were incubated in the diluted antibody in 1% BSA in PBS in a humidified chamber for 1 hour at room temperature. After that, the solution was removed, and the cells were washed thrice in PBS, 5 minutes for each wash. Next, the HEKa cells were incubated with the secondary antibody corresponding to 1% BSA for 1 hour at room temperature in the dark, and the solution was removed. After that, the samples were washed thrice with PBS for 5 minutes each in the dark. Finally, the cells were mounted in a coverslip with a drop of mounting medium and stored in the dark at 4 °C overnight to be analyzed using a fluorescence microscope. All assays were made in triplicate.
3. Results
3.1. (CTS-g-GMA)-X hydrogel synthesis and characterization
Our research group was the first to report synthesizing and characterizing a functionalized CTS-based biomaterial using the synthetic polymer glycidyl methacrylate (GMA). The resulting hybrid hydrogel (CTS/GMA) exhibited superior mechanical properties compared to CTS hydrogels.34 Subsequently, we provided evidence for synthesizing a (CTS-g-GMA)-xanthan (X) hydrogel. Rheological analysis demonstrated that the composite biomaterial exhibited physical hydrogel behavior, while in vitro qualitative studies revealed a decrease in cell viability associated with the GMA content.37 However, in vivo studies highlighted the significant potential of this composite biomaterial for treating spinal cord injuries.25In this study, the films obtained after drying had an optimal thickness for infrared characterization. Fig. 2a shows the infrared spectra of the hydrogels (CTS-g-GMA)-X (Z) series B, where all spectra display the same bands with minimal changes. For example, it can be observed that in the region corresponding to the OH groups, the Z11B and Z13B samples show wider bands. The band's widening in this region is commonly associated with hydrogen bonding interactions. The OH groups can form hydrogen bonds either with each other or with neighboring water molecules, which causes the band to expand in the FTIR spectrum. This hydrogen bonding is a characteristic phenomenon of materials that contain water or have a high capacity to form hydrogen bonds, such as gels.
Nevertheless, these spectra maintain the characteristic bands compared to the material obtained in an acidic medium without neutralization.33 However, some differences are evident when comparing series B with series A.
Fig. 2b shows the infrared spectra of series Z A compared to series B. The most notable difference is observed in the bands around 1695 and 1560 cm−1, which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the GMA group attached to chitosan. This result suggests that the chemical treatment used for neutralization slightly degraded the chemical structure of the CTS-g-GMA fraction, as reported in previous studies, where it was indicated that the affected groups in CTS-g-GMA are the terminal methyl-vinyl groups, which are primarily in contact with the basic medium.34 In an acid environment, the protonated groups can interact with the epoxy group, decreasing the signals between 1070 and 1035 cm−1, which is associated with the C–O–C group of GMA. This phenomenon correlates with the shift to the left of NH2 and OH signal compared to Fig. 2a. However, as in series B, the rest of the bands for series A show the same position as the un-neutralized biomaterials described in previous work.33–36,38,39
C stretching vibrations of the GMA group attached to chitosan. This result suggests that the chemical treatment used for neutralization slightly degraded the chemical structure of the CTS-g-GMA fraction, as reported in previous studies, where it was indicated that the affected groups in CTS-g-GMA are the terminal methyl-vinyl groups, which are primarily in contact with the basic medium.34 In an acid environment, the protonated groups can interact with the epoxy group, decreasing the signals between 1070 and 1035 cm−1, which is associated with the C–O–C group of GMA. This phenomenon correlates with the shift to the left of NH2 and OH signal compared to Fig. 2a. However, as in series B, the rest of the bands for series A show the same position as the un-neutralized biomaterials described in previous work.33–36,38,39
In summary, although no significant differences were found between the spectra of series A and B, the slight degradation observed in the functional groups of GMA suggests that the neutralization treatment influences the chemical structure of the material, especially the groups that react with the basic medium. Finally, the composed biomaterial showed no apparent immune rejection or severe tissue damage; it is necessary to verify the biocompatibility at a cellular level; the scaffold is an exogenous agent that will be interacting with a living host, which could cause different cellular responses, such as inflammatory responses (acute and chronic), foreign body reactions, fibrous capsule development and wound healing responses.40
3.2. Epithelial cells
The biocompatibility of a material means that it does not harm cells, allowing them to survive, proliferate, and support tissue formation. To assess the biocompatibility of the (CTS-g-GMA)-X hydrogel, we employed calcein, live/dead, and DNA assays with keratinocytes H413 and human epidermal keratinocytes.41,42 All assays were performed in triplicate, and no significant differences were observed, with a p-value > 0.05.In Fig. 3(a–d), the positive control shows a high number of live cells (green). Meanwhile, all materials exhibit numerous live cells organized into clusters, although some dead cells (red) are also present. In comparison, chitosan shows a more significant proportion of dead cells.
Fig. 3(e–h) presents the live/dead assay results after 5 days of cell inoculation. The glass surface displays a high number of nuclei (dead cells in red) embedded within a layer of cytoplasm (live cells). These nuclei could be due to the high pressure applied to the coverslip. In the CTS group, only remnants of cytoplasm are visible, with no live cells present. The tendency for cells to organize into clusters persists across the biomaterials, though dead cells are evident within these clusters.
 | ||
| Fig. 4 Cell growth of keratinocytes lines H413 onto polymers and glass (positive control). All assays were made in triplicate; there are no significant differences, with a p > 0.05. | ||
For materials in series A, it is noteworthy that there is a peak in cell growth on day 3, followed by a decrease in cell numbers on subsequent days. This growth pattern could be attributed to the chemical structure of the biomaterials, which may create an unfavorable environment for keratinocyte attachment and growth on the material surface. In contrast, the series B polymers support keratinocyte growth at all time points across the biopolymers. This observation could be due to the neutral nature of the reaction, which likely results in a different three-dimensional arrangement in this material, promoting better cell adhesion and proliferation.
The viability of HEKa cells was quantified using the DNA (proliferation) assay. Fig. 5 shows cell growth on the biomaterials. The results indicate that most samples, except for Z13B and Z14B, exhibit better performance than the positive control (glass with a treated surface), with an increase in cell numbers over time. However, while the cell count in Z13B and Z14B is low on day 1, these samples show a significant increase in cell numbers by day 7, with cells doubling in that period.
Likewise in Fig. 5, the samples Z13A and Z14A, containing higher concentrations of GMA, demonstrated increased interactions with CTS through hydroxyl groups. These interactions reduced the availability of amino groups in CTS, which would otherwise interact with X, leading to a notable decrease in the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signals compared to samples with lower GMA concentrations.37,43 This effect not only limits the availability of positively charged groups but also enhances the exposure and prevalence of negatively charged groups, such as –COOH and –OH, on the surface. The increased density of negative surface charges provides potential chelation sites for positively charged ions or enzymes, thereby modifying the local biochemical environment.44,45 These surface modifications can negatively affect cell viability, as observed in primary keratocytes HEKa.46,47
O signals compared to samples with lower GMA concentrations.37,43 This effect not only limits the availability of positively charged groups but also enhances the exposure and prevalence of negatively charged groups, such as –COOH and –OH, on the surface. The increased density of negative surface charges provides potential chelation sites for positively charged ions or enzymes, thereby modifying the local biochemical environment.44,45 These surface modifications can negatively affect cell viability, as observed in primary keratocytes HEKa.46,47
Polymers from series A generally perform better than those from series B, with Z11A showing the most significant growth after seven days. These findings contrast with results obtained for H413 keratinocytes in previous work,33 where series B demonstrated better attachment and growth. This difference may be attributed to the distinct nature of the two cell types. The results show no significant differences between most polymers, though notable differences were observed between specific samples on days 1 and 7.
 | ||
| Fig. 6 Interleukin-1β production by mouse macrophages J774A.1 after 2 and 48 hours. All assays were made in triplicate; there are no significant differences, with a p > 0.05. | ||
In contrast, the remaining samples had values below 200 pg mL−1, with chitosan showing low activity and yielding the lowest value compared to the positive control.
All samples responded similarly when in contact with macrophages, although their values were generally higher than those reported by other studies. Our samples were used at 1 mg mL−1, whereas other studies employed concentrations of 50 or 500 mg mL−1 for incubation periods of 18 or 24 hours (Table 2).48–50 The observed increase in IL-1β production may be attributed to the expected behavior of macrophages in the presence of foreign materials, triggering a non-specific inflammatory response that could promote accelerated biodegradation.
3.3. Immunocytochemistry (ICC)
Cell adhesion is a critical cell proliferation process involving several multi-protein complexes. E-Cadherin (epithelial) is a crucial cell–cell adhesion molecule in epithelial tissues, playing an essential role in forming and maintaining these tissues' typical architecture and function.51Fibronectin is one of the most studied matrix glycoproteins, owing to its crucial role in the adhesion of various cell types. It is vital for regulating cell behavior through communication with intracellular and extracellular environments and is essential in wound healing.52,53 Laminin, the most abundant structural multidomain protein in basement membranes, regulates cell attachment, proliferation, differentiation, and motility.
Additionally, it promotes neurite regeneration, highlighting its multifunctionality.54–56 Cellular responses such as adhesion, spreading, proliferation, and differentiation are influenced by proteins at the cell–biomaterial interface. These proteins' proper expression and function—such as E-cadherin, fibronectin, and laminin—are essential for promoting favorable cellular responses. In contrast, dysfunctional or improperly expressed proteins have been linked to immune reactions, carcinogenic phenotypes, and dystrophies.57–61 Therefore, evaluating the behavior of these proteins when a biomaterial is exposed to the host is crucial.
3.4. Nerve cells (NG108-15)
While no significant differences were observed between most of the polymers, notable differences were found between some samples on days 1 and 7, particularly in the positive control. Although cell growth was lower than in the control specimens, there is sufficient evidence to support the viability of this type of cell for growth on the biomaterials.
The trend continues to organize the cells in straight clusters with a random orientation on the biomaterials. However, in all the polymers, including chitosan film, it is evident that dead cells are absent in these clusters. This observation could be attributed to the lack of attachment of dead cells and their possible discard in some of the washes, especially when DNA assay shows a low number of cells onto biomaterials compared with the glass.
In both cases, it is possible to observe the attachment to the surface of the polymers, and the cells present a habitual behavior and a web-like morphology. These images confirm the random orientation and the straight cluster formation observed in the live/dead assay. In addition, the morphology shown by the cells in the polymers demonstrates that, although the number of cells is low according to DNA assay, the cells are in a suitable environment for their growth and proliferation.
4. Discussion
Due to the ionic nature of the polymers, the reaction mechanism suggests an interaction between the carboxyl group of xanthan and the amino group of chitosan. Adding glycidyl methacrylate to chitosan does not appear to interact with xanthan, as infrared signals show no significant shifts in form or intensity. Based on the FTIR spectra, no significant difference was observed between the two methods, indicating that the materials can be used in cell culture without affecting the pH of the culture media.The results from the peroxide assay show that these materials do not provoke a substantial reaction in macrophages over short periods (6 hours), unlike the negative control (copper), which exhibited higher values than the polymers. These findings support that the material does not induce an immune response, allowing cell protection and growth on the biomaterial surface. However, the IL-1β assay reveals that the A series polymers promote an increase in the inflammatory response after 48 hours, while the B series materials do not. In the context of biomedical applications, this low inflammatory response enhances the potential for using these materials.
Calcein staining demonstrates an increase in the viability of human fibroblasts. The DNA assay results indicate that the materials have potential as scaffolds for H413 keratinocytes, as cell viability was maintained for 7 days in culture, with a high cell density (many cells per mL). These findings support using these materials in the biomedical field as scaffolds, given their ability to maintain cell viability in connective, skin, and nerve tissue.
The DNA assay for human epidermal keratinocytes shows strong cell preservation and growth in most samples, with results comparable to the blank (glass with a treated surface). Immunocytochemistry staining revealed good labeling for all antibodies tested in this study with human epidermal keratinocytes, and the results were comparable to the positive control (glass). These polymers do not negatively affect the growth and proliferation of this cell type. Additionally, live/dead, SEM, and DNA assays on NG108-15 nerve cells showed good viability, with a significant proportion of cells remaining active after 7 days.
Given the importance of laminin in promoting neurite regeneration, this result is crucial, as this study aims to regenerate spinal cord channels. If the laminin antibody staining is accurate for this cell type on our biomaterials, we can expect similar behavior in nerve cells, as reported in previous studies.62 Furthermore, the viability of dermal fibroblasts and keratinocytes aligns with the findings of López-Muñoz et al., who demonstrated that CTS-g-GMA materials with gold nanoparticles and type I collagen can promote effective skin wound healing.63
One of the main limitations of the present study is that, due to the use of membranes and the extended duration of the tests, we were unable to assess the viability of other cell types, which could expand the potential applications of our polymer. For future work, we propose differentiating NG108 cells to evaluate their application in model organisms, such as rats, to scale up to more complex organisms with advanced DNA structures.
In the present work, we have developed a simple yet effective synthesis of a material with good mechanical properties and cell viability for skin and nerve cells, complementing previous studies.62,63 This type of CTS-g-GMA composite supports the proper growth of various cell types and aids in protecting damaged areas. Our material offers a more straightforward and versatile synthesis than other materials, such as fiber matrices. However, its properties are currently more suited to wound care and surface treatments.64–67 That said, the material we developed demonstrates considerable versatility, as it can be modified to suit specific applications depending on the molecules with which it is functionalized, thus significantly expanding its potential applications.25,33,34,37,62,63
5. Conclusions
The present study aimed to synthesize and evaluate the biopolymer CTS-g-GMA-X, which maintains a physiological pH close to 7. No significant differences were observed between the two synthesis methods based on FTIR analysis, and neither method negatively affected cell viability, suggesting that these materials may be suitable for biomedical applications. However, whether they are biocompatible in vivo and effective for specific medical applications (e.g., skin graft devices) remains to be determined. Nevertheless, the results obtained in this study are promising and strongly support the potential use of these hydrogels as scaffolds for tissue engineering and the repair of spinal cord injuries.Data availability
The data presented in this article, including the descriptions and information related to , Fig. 4–6 and 9, as well as the ethics committee approval letter for the conducted studies, are available in the Science Data Bank repository at https://www.scidb.cn/en/anonymous/ZkVGejZq, with DOI: https://doi.org/10.57760/sciencedb.19980.Author contributions
A. R. T.: investigation, methodology, writing – original draft. T. P. M.: investigation, writing – review & editing. I. A. Q. O.: investigation, methodology, software. P. S.: data curation, writing – original draft, formal analysis. D. A. B. A.: writing – original draft, data curation, software. H. M. F.: conceptualization, methodology. F. L. M.: investigation, software. R. R. D.: formal analysis, visualization, writing – review & editing. A. G. S.: methodology, software, writing – review & editing. S. H. P.: funding acquisition, visualization, investigation. J. E. G.: methodology, validation, supervision. Z. Y. G. C.: conceptualization, formal analysis, resources, supervision. C. V.: validation, funding acquisition, formal analysis, writing – original draft. G. L. B.: conceptualization, funding acquisition, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate access to LIDTRA facilities through its projects: LN295261, LN254119, and LN299082. We also thank M. C. Reyna Araceli Mauricio Sanchez for technical assistance in the FTIR measurements.Notes and references
- S. Stratton, N. B. Shelke, K. Hoshino, S. Rudraiah and S. G. Kumbar, Bioactive Polymeric Scaffolds for Tissue Engineering, Bioact. Mater., 2016, 1, 93–108 Search PubMed.
- M. Jafari, Z. Paknejad, M. R. Rad, S. R. Motamedian, M. J. Eghbal, N. Nadjmi and A. Khojasteh, Polymeric Scaffolds in Tissue Engineering: A Literature Review, J. Biomed. Mater. Res., Part B, 2017, 105, 431–459 CrossRef CAS PubMed.
- G. A. F. Roberts, Chitin Chemistry, Macmillan Education UK, 1992 Search PubMed.
- M. M. Islam, M. Shahruzzaman, S. Biswas, M. Nurus Sakib and T. U. Rashid, Chitosan Based Bioactive Materials in Tissue Engineering Applications-A Review, Bioact. Mater., 2020, 5, 164–183 Search PubMed.
- S. Kim, Z. K. Cui, B. Koo, J. Zheng, T. Aghaloo and M. Lee, Chitosan-Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications, ACS Appl. Mater. Interfaces, 2018, 10, 41138–41145 CrossRef CAS PubMed.
- A. Lončarević, M. Ivanković and A. Rogina, Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds, Journal of Tissue Repair and Regeneration, 2017, 1, 12–22 Search PubMed.
- S. M. Ahsan, M. Thomas, K. K. Reddy, S. G. Sooraparaju, A. Asthana and I. Bhatnagar, Chitosan as Biomaterial in Drug Delivery and Tissue Engineering, Int. J. Biol. Macromol., 2018, 110, 97–109 CrossRef CAS PubMed.
- G. I. Howling, P. W. Dettmar, P. A. Goddard, F. C. Hampson, M. Dornish and E. J. Wood, The Effect of Chitin and Chitosan on the Proliferation of Human Skin Fibroblasts and Keratinocytes in Vitro, Biomaterials, 2001, 22, 2959–2966 CrossRef CAS PubMed.
- V. Vivcharenko, M. Wojcik and A. Przekora, Cellular Response to Vitamin C-Enriched Chitosan/Agarose Film with Potential Application as Artificial Skin Substitute for Chronic Wound Treatment, Cells, 2020, 9, 1185 CrossRef CAS PubMed.
- P. Makvandi, G. W. Ali, F. della Sala, W. I. Abdel-Fattah and A. Borzacchiello, Biosynthesis and Characterization of Antibac-terial Thermosensitive Hydrogels Based on Corn Silk Extract, Hyaluronic Acid and Nanosilver for Potential Wound Healing, Carbohydr. Polym., 2019, 223, 115023 CrossRef CAS PubMed.
- H. Xu, S. Huang, J. Wang, Y. Lan, L. Feng, M. Zhu, Y. Xiao, B. Cheng, W. Xue and R. Guo, Enhanced Cutaneous Wound Healing by Functional Injectable Thermo-Sensitive Chitosan-Based Hydrogel Encapsulated Human Umbilical Cord-Mesenchymal Stem Cells, Int. J. Biol. Macromol., 2019, 137, 433–441 CrossRef CAS PubMed.
- A. M. Heimbuck, T. R. Priddy-Arrington, M. L. Padgett, C. B. Llamas, H. H. Barnett, B. A. Bunnell and M. E. Caldorera-Moore, Development of Responsive Chitosan-Genipin Hydrogels for the Treatment of Wounds, ACS Appl. Bio Mater., 2019, 2, 2879–2888 CrossRef CAS PubMed.
- F. Yan, M. Li, H. Q. Zhang, G. L. Li, Y. Hua, Y. Shen, X. M. Ji, C. J. Wu, H. An and M. Ren, Collagen-Chitosan Scaffold Impregnated with Bone Marrow Mesenchymal Stem Cells for Treatment of Traumatic Brain Injury, Neural Regener. Res., 2019, 14, 1780–1786 CrossRef CAS PubMed.
- M. Mattotti, Z. Alvarez, L. Delgado, M. A. Mateos-Timoneda, C. Aparicio, J. A. Planell, S. Alcántara and E. Engel, Differential Neuronal and Glial Behavior on Flat and Micro Patterned Chitosan Films, Colloids Surf., B, 2017, 158, 569–577 CrossRef CAS PubMed.
- C. Intini, L. Elviri, J. Cabral, S. Mros, C. Bergonzi, A. Bianchera, L. Flammini, P. Govoni, E. Barocelli and R. Bettini, 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an in Vivo Wound Healing Evaluation in Experimental Diabetes in Rats, Carbohydr. Polym., 2018, 199, 593–602 CrossRef CAS PubMed.
- V. Lopes Rocha Correa, J. Assis Martins, T. Ribeiro de Souza, G. de Castro Nunes Rincon, M. Pacheco Miguel, L. Borges de Menezes and A. Correa Amaral, Melatonin Loaded Lecithin–Chitosan Nanoparticles Improved the Wound Healing in Diabetic Rats, Int. J. Biol. Macromol., 2020, 162, 1465–1475 CrossRef CAS PubMed.
- Z. Aliakbar Ahovan, S. Khosravimelal, B. S. Eftekhari, S. Mehrabi, A. Hashemi, S. Eftekhari, P. Brouki Milan, M. Mobaraki, A. M. Seifalian and M. Gholipourmalekabadi, Thermo-Responsive Chitosan Hydrogel for Healing of Full-Thickness Wounds Infected with XDR Bacteria Isolated from Burn Patients: In Vitro and In Vivo Animal Model, Int. J. Biol. Macromol., 2020, 164, 4475–4486 CrossRef CAS PubMed.
- N. Masood, R. Ahmed, M. Tariq, Z. Ahmed, M. S. Masoud, I. Ali, R. Asghar, A. Andleeb and A. Hasan, Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits, Int. J. Pharm., 2019, 559, 23–36 CrossRef CAS PubMed.
- G. Kratz, M. Back, C. Arnander and O. Larm, Immobilised Heparin Accelerates the Healing of Human Wounds in Vivo, Scand. J. Plast. ReConstr. Surg. Hand Surg., 1998, 32, 381–386 CrossRef CAS PubMed.
- G. Singhvi, N. Hans, N. Shiva and S. Kumar Dubey, Xanthan Gum in Drug Delivery Applications, In Natural Polysaccharides in Drug Delivery and Biomedical Applications, 2019, pp. 121–144 Search PubMed.
- A. Kumar, K. M. Rao and S. S. Han, Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review, Carbohydr. Polym., 2018, 180, 128–144 CrossRef CAS PubMed.
- M. Z. Bellini, A. L. R. Pires, M. O. Vasconcelos and A. M. Moraes, Comparison of the Properties of Compacted and Porous La-mellar Chitosan-Xanthan Membranes as Dressings and Scaffolds for the Treatment of Skin Lesions, J. Appl. Polym. Sci., 2012, 125, E421–E431 CrossRef CAS.
- A. Alves, S. P. Miguel, A. R. T. S. Araujo, M. J. de Jesus Valle, A. S. Navarro, I. J. Correia, M. P. Ribeiro and P. Coutinho, Xanthan Gum-Konjac Glucomannan Blend Hydrogel for Wound Healing, Polymers, 2020, 12, 99 CrossRef CAS PubMed.
- T. Glaser, V. B. Bueno, D. R. Cornejo, D. F. S. Petri and H. Ulrich, Neuronal Adhesion, Proliferation and Differentiation of Embryonic Stem Cells on Hybrid Scaffolds Made of Xanthan and Magnetite Nanoparticles, Biomed. Mater., 2015, 10, 045002 CrossRef PubMed.
- D. G. Zarate-Triviño, H. Pool, H. Vergara-Castañeda, E. A. Elizalde-Peña, V. Vallejo-Becerra, F. Villaseñor, E. Prokhorov, J. Gough, B. Garcia-Gaitan and G. Luna-Barcenas, (Chitosan-g-Glycidyl Methacrylate)-Collagen II Scaffold for Cartilage Re-generation, Int. J. Polym. Mater., 2020, 69, 1043–1053 CrossRef.
- E. Martín-López, M. Darder, E. Ruiz-Hitzky and M. Nieto Sampedro, Agar-Based Bridges as Biocompatible Candidates to Provide Guide Cues in Spinal Cord Injury Repair, Biomed. Eng. Mater., 2013, 23, 405–421 Search PubMed.
- M. Milas, M. Rinaudo and B. Tinland, Polymer Bulletin The Viscosity Dependence on Concentration, Molecular Weight and Shear Rate of Xanthan Solutions, Polym. Bull., 1985, 14, 157–164 CrossRef CAS.
- M. R. Kasaai, J. Arul and G. R. Charlet, Intrinsic Viscosity-Molecular Weight Relationship for Chitosan, J. Polym. Sci., Part B:Polym. Phys., 2000, 38, 2591–2598 CrossRef CAS.
- G. Biagini, A. Bertani, R. Muzzarelli, A. Damadei, G. Dibenedetto, A. Belligolli, G. Riccotti, C. Zucchini and C. Rizzoli, Wound Management with N-carboxybutyl Chitosan, Biomaterials, 1991, 12, 281–286 CrossRef CAS PubMed.
- T. Chandy and C. P. Sharma, Chitosan – as a Biomaterial, Biomater., Artif. Cells, Artif. Organs, 1990, 18, 1–24 CrossRef CAS PubMed.
- J. E. Gough, P. Christian, C. A. Scotchford and I. A. Jones, Craniofacial Osteoblast Responses to Polycaprolactone Produced Using a Novel Boron Polymerisation Technique and Potassium Fluoride Post-Treatment, Biomaterials, 2003, 24, 4905–4912 CrossRef CAS PubMed.
- J. E. Gough, P. Christian, J. Unsworth, M. P. Evans, C. A. Scotchford and I. A. Jones, Controlled Degradation and Macrophage Responses of a Fluoride-Treated Polycaprolactone, J. Biomed. Mater. Res., Part A, 2004, 69, 17–25 CrossRef CAS PubMed.
- E. A. Elizalde-Peña, N. Flores-Ramirez, G. Luna-Barcenas, S. R. Vásquez-García, G. Arámbula-Villa, B. García-Gaitán, J. G. Rutiaga-Quiñones and J. González-Hernández, Synthesis and Characterization of Chitosan-g-Glycidyl Methacrylate with Methyl Methacrylate, Eur. Polym. J., 2007, 43, 3963–3969 CrossRef.
- N. Flores-Ramírez, E. A. Elizalde-Peña, S. R. Vásquez-García, J. González-Hernández, A. Martinez-Ruvalcaba, I. C. Sanchez, G. Luna-Bárcenas and R. B. Gupta, Characterization and Degradation of Functionalized Chitosan with Glycidyl Methacrylate, J. Biomater. Sci., Polym. Ed., 2005, 16, 473–488 CrossRef PubMed.
- A. Pawlak and M. Mucha, Thermogravimetric and FTIR Studies of Chitosan Blends, Thermochim. Acta, 2003, 396, 153–166 CrossRef CAS.
- A. Pawlak and M. Mucha, Erratum: Thermogravimetric and FTIR Studies of Chitosan Blends [Thermochimica Acta, 2003, 396, p. 153–166], Thermochim. Acta, 2004, 409, 95–97 CrossRef CAS.
- E. A. Elizalde-Peña, D. G. Zarate-Triviño, S. M. Nuño-Donlucas, L. Medina-Torres, J. E. Gough, I. C. Sanchez, F. Villaseñor and G. Luna-Barcenas, Synthesis and Characterization of a Hybrid (Chitosan-g-Glycidyl Methacrylate)-Xanthan Hydrogel, J. Biomater. Sci., Polym. Ed., 2013, 24, 1426–1442 CrossRef PubMed.
- D. Lien-Vien, N. B. Colthup, W. G. Fateley and J. G. Grasselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, 1991 Search PubMed.
- M. Badertscher, P. Bühlmann and E. Pretsch, Structure Determination of Organic Compounds, Tables of Spectral Data, 2009 Search PubMed.
- Z. Sheikh, P. J. Brooks, O. Barzilay, N. Fine and M. Glogauer, Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials, Materials, 2015, 8, 5671–5701 CrossRef CAS PubMed.
- B. M. Gumbiner, Cell Adhesion: Review The Molecular Basis of Tissue Architecture and Morphogenesis, Cell, 1996, 84, 345–357 CrossRef CAS PubMed.
- A. Przekora, The Summary of the Most Important Cell-Biomaterial Interactions That Need to Be Considered during in Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications, Mater. Sci. Eng., C, 2019, 97, 1036–1051 CrossRef CAS PubMed.
- A. E. Aguiar, M. de O. Silva, A. C. D. Rodas and C. A. Bertran, Mineralized layered films of xanthan and chitosan stabilized by polysaccharide interactions: a promising material for bone tissue repair, Carbohydr. Polym., 2019, 207, 480–491 CrossRef CAS PubMed.
- T. L. Hwang, I. A. Aljuffali, C. F. Lin, Y. T. Chang and J. Y. Fang, Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles, Int. J. Nanomed., 2015, 10, 371–385 Search PubMed.
- A. M. Rios, A. S. Medina, J. M. Irache, A. L. M. López, I. A. R. Espejel and J. M. C. Bravo, Toxicity evaluation of thermosensitive nanogels in an in vivo model, Revista de Ciencias Tecnológicas, 2022, 5, e236 Search PubMed.
- K. Trescher, N. Scharnagl, K. Kratz, T. Roch, A. Lendlein and F. Jung, Adherence and viability of primary human keratinocytes and primary human dermal fibroblasts on acrylonitrile-based copolymers with different concentrations of positively charged functional groups, Clin. Hemorheol. Microcirc., 2012, 52, 391–401 Search PubMed.
- K. A. Bush, P. F. Driscoll, E. R. Soto, C. R. Lambert, W. G. McGimpsey and G. D. Pins, Designing tailored biomaterial surfaces to direct keratinocyte morphology, attachment, and differentiation, J. Biomed. Mater. Res., Part A, 2009, 90, 999–1009 CrossRef CAS PubMed.
- J. Feng, L. Zhao and Q. Yu, Receptor-Mediated Stimulatory Effect of Oligochitosan in Macrophages, Biochem. Biophys. Res. Commun., 2004, 317, 414–420 CrossRef CAS PubMed.
- T. Mori, Y. Irie, S. Nishimura, S. Tokura, M. Matsuura, M. Okumura, T. Kadosawa and T. Fujinaga, Endothelial Cell Re-sponses to Chitin and Its Derivatives, J. Biomed. Mater. Res., 1998, 43, 469–472 CrossRef CAS PubMed.
- F. Chellat, A. Grandjean-Laquerriere, R. le Naour, J. Fernandes, L. Yahia, M. Guenounou and D. Laurent-Maquin, Metallo-proteinase, and Cytokine Production by THP-1 Macrophages Following Exposure to Chitosan-DNA Nanoparticles, Biomaterials, 2005, 26, 961–970 CrossRef CAS PubMed.
- K. H. Biswas, Molecular Mobility-Mediated Regulation of E-Cadherin Adhesion, Trends Biochem. Sci., 2020, 45, 163–173 CrossRef CAS PubMed.
- E. Ruoslahti, Fibronectin in Cell Adhesion and Invasion, Cancer Metastasis Rev., 1984, 3, 43–51 CrossRef CAS PubMed.
- L. Parisi, A. Toffoli, B. Ghezzi, B. Mozzoni, S. Lumetti and G. M. Macaluso, A Glance on the Role of Fibronectin in Controlling Cell Response at Biomaterial Interface, Jpn. Dent. Sci. Rev., 2020, 56, 50–55 CrossRef PubMed.
- B. L. Patton, J. H. Miner, A. Y. Chiu and J. R. Sanes, Distribution and Function of Laminins in the Neuromuscular System of Developing, Adult, and Mutant Mice, J. Cell Biol., 1997, 139, 1507–1521 CrossRef CAS PubMed.
- M. Paulsson, The Role of Laminin in Attachment, Growth, and Differentiation of Cultured Cells: A Brief Review, Cytotechnology, 1992, 9, 99–106 CrossRef CAS PubMed.
- A. E. Haggerty, M. R. Bening, G. Pherribo, E. A. Dauer and M. Oudega, Laminin Polymer Treatment Accelerates Repair of the Crushed Peripheral Nerve in Adult Rats, Acta Biomater., 2019, 86, 185–193 CrossRef CAS PubMed.
- A. B. Reynolds and R. H. Carnahan, Regulation of Cadherin Stability and Turnover by P120ctn: Implications in Disease and Cancer, Semin. Cell Dev. Biol., 2004, 15, 657–663 CrossRef CAS PubMed.
- J. W. Rick, A. Chandra, C. Dalle Ore, A. T. Nguyen, G. Yagnik and M. K. Aghi, Fibronectin in Malignancy: Cancer-Specific Alterations, Protumoral Effects, and Therapeutic Implications, Semin. Oncol., 2019, 46, 284–290 CrossRef CAS PubMed.
- T. C. Lin, C. H. Yang, L. H. Cheng, W. T. Chang, Y. R. Lin and H. C. Cheng, Fibronectin in Cancer: Friend or Foe, Cells, 2020, 9, 27 CrossRef CAS PubMed.
- P. Barraza-Flores, C. R. Bates, A. Oliveira-Santos and D. J. Burkin, Laminin and Integrin in LAMA2-Related Congenital Muscular Dystrophy: From Disease to Therapeutics, Front. Mol. Neurosci., 2020, 13, 1 CrossRef CAS PubMed.
- P. D. Yurchenco, K. K. McKee, J. R. Reinhard and M. A. Rüegg, Laminin-Deficient Muscular Dystrophy: Molecular Pathogenesis and Structural Repair Strategies, Matrix Biol., 2018, 71–72, 174–187 CrossRef CAS PubMed.
- E. A. Elizalde-Peña, I. A. Quintero-Ortega, D. G. Zárate-Triviño, A. Nuño-Licona, J. Gough, I. C. Sanchez, D. I. Medina and G. Luna-Barcenas, (Chitosan-g-Glycidyl Methacrylate)-Xanthan Hydrogel Implant in Wistar Rats for Spinal Cord Regeneration, Mater. Sci. Eng., C, 2017, 78, 892–900 Search PubMed.
- H. A. López-Muñoz, M. Lopez-Romero, M. A. Franco-Molina, A. Manzano-Ramirez, C. Velasquillo, B. L. España-Sanchez, A. L. Martinez-Hernandez, H. Vergara-Castañeda, A. Giraldo-Betancur and S. Favela, Chitosan-G-Glycidyl Methacry-late/Au Nanocomposites Promote Accelerated Skin Wound Healing, Pharmaceutics, 2022, 14, 1855 Search PubMed.
- W. Xue, W. Shi, Y. Kong, M. Kuss and B. Duan, Anisotropic Scaffolds for Peripheral Nerve and Spinal Cord Regeneration, Bioact. Mater., 2021, 6, 4141–4160 CAS.
- S. Wu, T. Dong, Y. Li, M. Sun, Y. Qi, J. Liu, M. A. Kuss, S. Chen and B. Duan, State-of-the-Art Review of Advanced Electrospun Nanofiber Yarn-Based Textiles for Biomedical Applications, Appl. Mater. Today, 2022, 27, 101473 CrossRef PubMed.
- J. Liu, T. Li, H. Zhang, W. Zhao, L. Qu, S. Chen and S. Wu, Electrospun Strong, Bioactive, and Bioabsorbable Silk Fibroin/Poly(L-Lactic-Acid) Nanoyarns for Constructing Advanced Nanotextile Tissue Scaffolds, Mater. Today Bio, 2022, 14, 100243 CrossRef CAS PubMed.
- Y. Li, T. Dong, Z. Li, S. Ni, F. Zhou, O. A. Alimi, S. Chen, B. Duan, M. Kuss and S. Wu, Review of Advances in Electro-spinning-Based Strategies for Spinal Cord Regeneration, Mater. Today Chem., 2022, 24, 100944 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |