Open Access Article
Open Access ArticlePolycyclic aromatic hydrocarbons in freshwater organisms from Hubei, Central China: health risk assessment and source identification†
Ting Liu ab,
Zhiyu Liub,
Zhuozhen Qianb,
Li Hea,
Jie Penga,
Lang Zhanga,
Yali Yua and
Jinhua Gan*a
ab,
Zhiyu Liub,
Zhuozhen Qianb,
Li Hea,
Jie Penga,
Lang Zhanga,
Yali Yua and
Jinhua Gan*a
aYangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan, 430223, China. E-mail: gjh@yfi.ac.cn
bKey Laboratory of Cultivation and High-Value Utilization of Marine Organisms, Fisheries Research Institute Fujian, 361013, China
First published on 7th April 2025
Abstract
Polycyclic aromatic hydrocarbons (PAHs) can be absorbed and accumulated in aquatic organisms, posing a threat to human health through the food chain. However, the concentration, composition, potential source and health risks of PAHs in freshwater organisms remain largely unknown. This study aimed to investigate the presence of PAHs in freshwater organisms in Hubei, Central China. The levels of 16 PAHs in the studied freshwater organisms ranged from 4.31 to 49.60 ng g−1, with an average value of 38.40 ng g−1. Comprising 75–81% of the total, 3–4 ring PAHs were the primary components in freshwater organisms, with an average ratio of 77.3%. Molecular diagnostic ratios (MDR) and principal component analysis (PCA) successfully identified combustion-related contamination as the major source of PAHs in these organisms. The incremental lifetime cancer risk (ILCR) values associated with PAH exposure were higher for crabs and fishes and were greater for children than for adults, suggesting potential carcinogenic risks to humans via the consumption of freshwater organisms. A comparative analysis of PAH concentrations in marine and freshwater organisms showed that the levels of 16 PAHs in marine organisms were more than twice as high as those in freshwater organisms, indicating differing behaviors of PAHs in marine and freshwater environments. Overall, this study significantly enhances the understanding on PAH accumulation in freshwater organisms and provides valuable insights for preventing and controlling PAH pollution in freshwater aquatic products.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a class of persistent organic pollutants (POPs) primarily found in estuaries,1,2 harbors3 and coastal waters.4 They are composed of least two fused benzene rings. Unlike most POPs, many PAHs occur in nature.5 Natural sources of PAHs include volcanic eruptions,6 natural fires7 and geological diagenesis,8 while anthropogenic sources include biomass combustion, fossil fuel burning, vehicle exhaust, oil spills, waste incineration and various industrial emissions.9 Identifying PAH sources has received extensive attention in aquatic organism protection and is essential for pollution control of their growing environments. Several tools and techniques are used to identify PAH sources, such as diagnostic ratios, principal component analysis (PCA), positive matrix factorization (PMF) and isotopic analysis.10–12 Previous studies have applied these methods to source apportionment of PAHs in marine organisms. Petroleum pollution, coal and biomass combustion, and marine transport emissions were identified as the main anthropogenic sources of PAHs in marine organisms.13 However, current research on the source identification for freshwater organisms is still somewhat limited. Therefore, the application of these techniques is required to offer comprehensive insights into PAH sources in freshwater organisms.To date, studies on PAH contamination have focused on the marine environment and marine organisms. PAHs have been determined in different species of marine organisms14–16 and the marine environment, such as coastal sediments and water.17–21 Apart from oceans, freshwater resources, such as lakes and rivers, are also closely linked to human activity. Terrestrial life depends on freshwater, which is the cornerstone of human survival and development. Previous studies have reported PAH contamination in the freshwater bodies of rivers and lakes,22–26 but as far as we know, only a literature review has summarized the effects of anthropogenic pollutants, including petroleum hydrocarbons and PAHs, on freshwater organisms.27 There is a lack of studies on the occurrence of PAHs in freshwater organisms. Moreover, PAHs can easily accumulate in freshwater organisms through the direct intake of contaminated water and food or through exposure to polluted sediments.27 The effects of PAHs on freshwater organisms are both significant and multifaceted. PAHs can cause acute or chronic toxicity, DNA damage, reproductive impairment, developmental abnormalities, behavioral changes and immune system suppression in freshwater organisms,27 leading to increased susceptibility to diseases and risks of mortality for freshwater organisms. Owing to their hydrophobic properties, PAHs tend to biomagnify in organisms at higher trophic levels via the food chain28 and cause adverse health effects on the human reproductive system and immune system,29,30 posing increasing risks to public health. Hence, it is urgent to advance the understanding of PAH contamination in freshwater organisms.
The global population intake of freshwater fish has grown impressively over the past 30 years, and the growth has increased from 2.86 kg per capital per year in 1990 to 8.18 kg per capital per year in 2020, which is becoming the chief contribution of high-quality protein intake, accounting for nearly 41% of total protein intake.31 China, as a major freshwater aquaculture country in the world, has the largest production of freshwater fish in the world with total output stabilized at more than 26 million metric tons, accounting for about 42.2% of the total global production.31 The food supply quantity of freshwater fish consumed per person in China has also increased considerably over the past 30 years, reaching the top level of 17.83 kg per capital per year in 2017.31 Hubei province, Central China, has a tremendous industry of freshwater aquaculture, ranking first in the production of freshwater fish and crustaceans. The surface area of freshwater lakes, ponds or reservoirs reaches up to 5258.66 km2, ranking second in terms of the freshwater aquaculture area in China.32 Sufficient freshwater resources are now in use for culturing both indigenous and exotic species such as fish, crayfish and crab. Consequently, it is necessary to determine whether freshwater organisms from Hubei province are contaminated with PAHs. In this study, we investigated the occurrence of 16 PAHs in freshwater organisms, including fish, crayfish and crab in the typical freshwater aquaculture area of Hubei, Central China. The content, composition and possible sources of PAHs in freshwater organisms were analyzed, and the incremental lifetime cancer risks (ILCRs) in humans by consuming contaminated freshwater organisms were assessed. Although molecular diagnostic ratios for PAH source identification and the ILCR model for human exposure to PAHs have been well applied, there are still some issues that remain to be solved. First, ratios of different aromatic ring numbers can also offer a similar distinction because pyrogenic PAHs are enriched in high ring members (5–6 rings), while petrogenic PAHs contain lower ring number homologues (1–4 rings). Second, there is a need to refine the cancer risks for different life stages, especially for early-life exposure, because children are regarded as vulnerable groups. Based on this, we combined diagnostic ratios with principal component analysis to improve the accuracy of source identification and adopted an age-dependent adjustment factor to assess cancer risks for distinct population groups. The main purposes of this study are to (1) explore the presence of PAHs in freshwater organisms from Hubei and their pollution characteristics in different freshwater species from Hubei, (2) identify possible sources of PAHs in freshwater organisms from Hubei, (3) evaluate the incremental lifetime cancer risks by consuming freshwater organisms, and (4) compare the content of these PAHs between marine and freshwater organisms.
Materials and methods
Chemicals and reagents
The mixed standard solution of 16 priority PAHs (naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorine (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flo), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-c,d]pyrene (IcdP), diben-zo[a,h]anthracene (DahA), and benzo[g,h,i]perylene (BghiP) (2000 μg mL−1)) was obtained from Dr Ehrenstorfer (Augsburg, Germany). HPLC grade acetonitrile (ACN, 99.8%) was purchased from Merck (Darmstadt, Germany). Anhydrous magnesium sulfate (MgSO4), neutral alumina sorbents and anhydrous sodium sulfate (NaSO4) with analytical grade used in this study were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Flash C18 was supplied by Bonna-Agela Technologies Inc. (Tianjin, China).Study area and sample collection
Hubei is situated in the middle part of the Yangtze River and is located at 29°05′–33°20′N and 108°30′–116°10′E. Rich in freshwater resources, Hubei cultivated more than ten kinds of precious freshwater species. Three characteristic and important economic freshwater species in Hubei, red swamp crayfish (Procambarus clarkii), Chinese mitten crab (Eriocheir sinensis) and ricefield eel (Monopterus albus) were collected at 56 sampling sites from April 2021 to October 2022, involving Jianli, Tianmen, Xiantao, Honghu, Qianjiang city, with a collection of triplicate samples from each site. The detailed locations of sampling sites and details of species are shown in Fig. 1 and Table S1.† Samples of these freshwater species were rapidly preserved at a temperature of 4 °C and transferred to the laboratory in an ice box; after that, they were washed three times with distilled water. Samples of three species were first narcotized, and the shells and gills of red swamp crayfish (Procambarus clarkii) and Chinese mitten crab (Eriocheir sinensis) were incised with scissors. The skin of a ricefield eel (Monopterus albus) was removed, and then the edible portions of the three species were dissected from the whole body.Sample pretreatment and analysis
Two grams of homogeneous samples from freshwater organisms were accurately measured in a 50 mL centrifuge tube, and 15 mL of acetonitrile was added. After thorough mixing, 3 g MgSO4 and 1.5 g NaSO4 were added and then bath-sonicated for 10 min. After ultrasonic extraction, the extracts were centrifuged at 6000g for 5 min. The 8 mL of supernatants were purified using 0.5 g MgSO4, 0.5 g C18 and 0.2 g neutral alumina sorbents. The 5 mL of purified supernatants were evaporated under a mild nitrogen stream, and the residue was redissolved into 1.0 mL of acetonitrile for GC-MS/MS analysis. GC-MS/MS analysis was performed using a Thermo Scientific TSQ9000 triple quadrupole mass spectrometer equipped with a Trace1300 gas chromatograph and an AS1310 autosampler (Thermo Fisher Scientific, USA). A TG-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Thermo Fisher Scientific, USA) was used as a separation column. The oven temperature was programmed to increase from 90 °C to 220 °C at 20 °C min−1 and finally increased to 300 °C at a 5 °C min−1 rate for 5 min. The injection, MS transfer line and ion source temperatures were set at 280 °C. The selected reaction monitoring (SRM) method was employed to analyze all samples. The qualification of the 16 PAHs was based on the characteristic ions and retention time, and quantification was based on the calculation of the peak areas. The characteristic ion pairs used for qualification and quantification are shown in Table S2.†Quality assurance/quality control (QA/QC)
To monitor the performance of quantitative analysis, a solvent blank, a procedure blank and a matrix blank were run duplicately with every 10 samples to check for any interference or background contamination. The relative standard deviation (RSD) among replicates was below 15%, and the procedural blank samples contained no detectable amounts of PAHs. The matrix-matched calibrations were prepared at five levels of 5.0, 10.0, 20.0, 50.0 and 100.0 ng mL−1 for each PAH by spiking a series of PAH dilutions in blank samples. The linear regression coefficients for calibration curves were >0.992. To estimate the efficiency of pretreatment procedures towards the target analytes, samples spiked with 16 PAH standards at three concentrations (5.0, 10.0 and 50.0 ng g−1) were measured six times. The mean recoveries of 16 PAHs were 50.8–148.5% (Tables S3–S5†).Risk assessment
To evaluate the health risk for the consumer through the consumption of freshwater organisms contaminated by PAHs, the incremental lifetime cancer risk (ILCR) value was used for the risk assessment. The PAH estimated daily intake (EDI) was evaluated as follows:| EDI (ng per kg bw per day) = CR × CF/BW, |
| ILCR = ED × EF × EDI × OF × ADAF × CF/AL, |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 days.
550 days.
Statistical analysis
The statistical data were analyzed using Microsoft Excel 2016 (Microsoft Co., Redmond, WA, USA) and Origin Pro 2019b software (OriginLab Corporation, Northampton, MA, USA). A paired t-test measure was used to compare the PAH pollution characteristics between different species of freshwater organisms, and principal coordinates analysis (PCA) was performed to identify potential sources. Briefly, the data of PAH concentrations in freshwater samples were Z-score normalized; then, the correlation matrix of the standardized data was computed. After performing PCA on the correlation matrix using Origin Pro 2019b software, factors with eigenvalues greater than 1 were retained and rotated using the varimax method. The rotated factors can be directly linked to potential pollution sources, and the factor loadings can help to identify the source categories.Results and discussion
Concentrations and composition of PAHs in freshwater organisms
In the studied freshwater organisms, ∑16 PAH levels ranged from 4.31 to 49.60 ng g−1. The average of ∑16PAH concentration in the three species of freshwater organisms was 38.40 ng g−1, as shown in Table 1. The highest ∑16PAH concentrations were determined in ricefield eel (Monopterus albus), followed by Chinese mitten crab (Eriocheir sinensis) and red swamp crayfish (Procambarus clarkii). The concentration of ∑16PAH was significantly different among the three species of freshwater organisms. For the sixteen PAH compounds, only Acy, Phe and BghiP were found in all freshwater species. Nap was detected only in crayfish. BaA, BbF, BaP, Ant, Chr and DahA were only found in ricefield eel. Flu appeared as the most abundant PAH compound, followed by Phe and Ace in crab and fish, except for crayfish, in which Phe was the most abundant compound. The PAH distribution in different species is influenced by multiple factors, including exposure, lipid content, nutrition and metabolism of PAHs in fish.36,37 Significant correlations have been found between lipid content in the tissue of species and many organic pollutants.38,39 Higher lipid content than red swamp crayfish (Procambarus clarkii) can be a reason for the higher PAH levels in mitten crab and eel. It can also be found that the culture modes among the three species are quite different. The pond cage culture is a typical mode for ricefield eel (Monopterus albus), while the rice-fish culture is the main culture mode for mitten crab and crayfish. PAH levels in eels are much higher than in the other two species. Different life habits, feeding habits and exposure conditions of the three species can be the main reason.| PAHs | Total sample | Crayfish sample | Crab sample | Fish sample | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Median | Min. | Max. | Average | SD | Average | SD | Average | SD | |
| a 138 samples of crayfish, crab and eel were carried out. n.d.: not detected. SD: standard deviations. ∑PAHs: the total concentrations of 16 PAHs. | |||||||||||
| Nap | 0.41 | 0.0480 | 0.39 | 0.31 | 0.62 | 0.41 | 0.0480 | n.d. | — | n.d. | — |
| Acy | 1.28 | 0.0630 | 1.30 | 0.08 | 2.85 | 0.18 | 0.0850 | 2.39 | 0.0600 | 1.27 | 0.0440 |
| Ace | 4.83 | 0.0052 | 4.83 | 2.41 | 6.84 | n.d. | — | 3.13 | 0.0580 | 6.54 | 0.0460 |
| Flu | 6.18 | 0.0104 | 6.06 | 0.36 | 15.10 | 0.59 | 0.1380 | 9.76 | 0.0710 | 8.20 | 0.1020 |
| Phe | 5.96 | 0.0760 | 5.90 | 1.25 | 15.30 | 2.14 | 0.0650 | 2.63 | 0.0800 | 13.10 | 0.0820 |
| Flo | 0.80 | 0.0710 | 0.81 | 0.26 | 1.33 | 0.42 | 0.0280 | n.d. | — | 1.17 | 0.1140 |
| Pyr | 0.60 | 0.0710 | 0.61 | 0.07 | 1.25 | 0.17 | 0.0560 | n.d. | — | 1.03 | 0.0860 |
| BaA | 0.30 | 0.0440 | 0.29 | 0.19 | 0.48 | n.d. | — | n.d. | — | 0.30 | 0.0440 |
| BbF | 2.66 | 0.0910 | 2.69 | 2.21 | 2.96 | n.d. | — | n.d. | — | 2.66 | 0.0910 |
| BaP | 1.37 | 0.1080 | 1.37 | 1.08 | 1.69 | n.d. | — | n.d. | — | 1.37 | 0.1080 |
| Ant | 3.23 | 0.0470 | 3.24 | 2.24 | 4.34 | n.d. | — | n.d. | — | 3.23 | 0.0470 |
| Chr | 2.55 | 0.0720 | 2.41 | 2.30 | 3.56 | n.d. | — | n.d. | — | 2.55 | 0.0720 |
| BkF | 2.02 | 0.0780 | 2.03 | 1.39 | 3.09 | n.d. | — | 2.19 | 0.0950 | 1.85 | 0.0610 |
| IcdP | 1.27 | 0.0590 | 1.26 | 0.72 | 1.72 | n.d. | — | 1.45 | 0.0970 | 1.09 | 0.0210 |
| DahA | 3.51 | 0.0990 | 3.55 | 2.75 | 4.11 | n.d. | — | n.d. | — | 3.51 | 0.0990 |
| BghiP | 1.48 | 0.0660 | 1.40 | 0.15 | 4.11 | 0.41 | 0.0740 | 2.30 | 0.0420 | 1.72 | 0.0830 |
| ∑PAHs | 38.40 | 0.0720 | 38.10 | 17.80 | 69.40 | 4.32 | 0.0710 | 23.90 | 0.0720 | 49.60 | 0.0730 |
| 2–3 rings | 21.90 | 0.0650 | 21.70 | 6.65 | 45.10 | 3.32 | 0.0900 | 17.90 | 0.0630 | 32.30 | 0.0640 |
| 4–6 rings | 16.50 | 0.0760 | 16.40 | 11.10 | 24.30 | 1.00 | 0.0530 | 6.00 | 0.0780 | 17.30 | 0.0780 |
PAHs can be categorized as low-molecular weight PAHs (LMW PAHs; having less than four rings) and high-molecular-weight PAHs (HMW PAHs; having 4–6 rings) by their structure and volatility properties, which decline with the number of benzene-fused rings and are also influenced by the connected way of benzene rings.40,41 The composition profiles of the 16 PAH mixtures are shown in Fig. 2. The results showed that 3- and 4-ring PAHs were the most abundant compounds in these freshwater organisms, comprising 75% to 81% of the total, with an average ratio of 77.3%. In all species, the composition of LMW PAHs and HMW PAHs accounted for 28.9% and 71.1% of the total, respectively. In crayfish, no 5 ring PAHs were found. In crab and fish, no 2 ring PAH was detected, and LMW PAHs were comparable to HMW PAHs only in crab. The composition of PAHs in the environment depends on their sources. Pyrogenic PAHs (e.g., from combustion) are often associated with high-molecular-weight (HMW) PAHs.40,41 Petrogenic PAHs (e.g., oil spills) are dominated by low-molecular-weight (LMW) PAHs.40,41 LMW PAHs are more water-soluble and bioavailable to pelagic organisms, while HMW PAHs are fatter-soluble and tend to bind strongly to organic carbon in sediments, influencing their bioavailability to benthic organisms. Although mitten crabs share benthic characteristics with Monopterus albus and Procambarus clarkii, their unique migratory behavior sets them apart. Their catadromous migration (freshwater to brackish water) may expose them to varying PAH levels in different environments. This might be the main reason for the varying PAH composition patterns in freshwater organisms.
The concentrations of PAHs in freshwater organisms from Hubei were compared with those from a few other regions around the world. The concentrations of PAHs observed in the present study were very similar to those of marine organisms from Mischief Reef in the South China Sea (12.8 to 81.3 ng g−1 in 44 marine organisms)42 and higher than those from the northern coast of the Campania region (1.7 ng g−1 in the muscle of warty crabs)43 and Korea (2.13 ng g−1 in fishery products).44 Nevertheless, the contents of PAHs determined in the present study were obviously lower than those from Poyang Lake in China (53.9 to 513 ng g−1 in different tissues of fish),45 South Yellow Sea, China (86.37 to 350.53 ng g−1 in marine organisms)14 and those from northeastern Brazil in liver samples of green turtles.46 Taken together, the crayfish and crab samples tested in this study were mildly contaminated, and fish samples were moderately contaminated by PAHs compared with previous reports on aquatic organisms throughout the world.
Source identification of PAHs
In this study, possible sources of PAHs in freshwater organisms were identified by applying molecular diagnostic ratios and principle component analysis.As illustrated in Fig. 3, the results of Ant/(Ant + Phe) were all higher than the value of 0.1, suggesting a petrogenic combustion source. The ratios of Flo/(Flo + Pyr) ranged from 0.22 to 0.88, and the majority of values were higher than 0.5, implying that PAHs mainly came from biomass and bioenergy combustion (e.g. agricultural residues, grass and wood combustion). From the molecular indices, 4 sampling sites in Tianmen are contaminated by petroleum combustion, and 10 sampling sites in Xiantao are contaminated by biomass combustion. These results demonstrate that the sources of PAHs in ricefield eels from 14 sampling sites could arise from a mixture of petrogenic combustion and biomass combustion. Combustion-related contamination is the dominant source. The differences in the sources of PAHs in Tianmen and Xiantao may be related to the influence of environmental factors.
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| a Principal coordinate analysis loading value higher than 0.7 is marked in bold. | |||
| Nap | 0.000 | 0.000 | 0.000 |
| Acy | −0.147 | 0.366 | 0.257 |
| Ace | −0.118 | 0.289 | 0.921 |
| Flu | −0.184 | 0.804 | 0.036 |
| Phe | −0.209 | 0.766 | −0.307 |
| Flo | −0.188 | 0.180 | −0.524 |
| Pyr | −0.164 | 0.324 | 0.332 |
| BaA | 0.724 | 0.181 | −0.092 |
| BbF | 0.284 | 0.005 | 0.233 |
| BaP | 0.735 | 0.181 | −0.092 |
| Ant | −0.216 | 0.293 | −0.183 |
| Chr | 0.258 | 0.319 | −0.132 |
| BkF | 0.316 | 0.049 | 0.202 |
| IcdP | 0.720 | 0.180 | −0.091 |
| DahA | 0.755 | 0.181 | −0.092 |
| BghiP | 0.731 | 0.167 | −0.055 |
| Variance | 54.5% | 23.6% | 7.9% |
Human health risk assessment
Freshwater organisms are considered an excellent source of high-quality protein for people to improve their nutritional status and health.59 It is essential to assess the risk to people through the consumption of freshwater organisms contaminated by PAHs. A widely accepted guidance on risk criteria for carcinogens, which is defined at an acceptable risk level of 1.0 × 10−6 and a priority risk level of 1.0 × 10−4, was applied for evaluation. As shown in Table 3, the mean EDI values of total PAH for adults were 0.0052 ng per kg bw per day for crayfish, 0.28 ng per kg bw per day for crab and 3.82 ng per kg bw per day for fish, while those for children were 0.019 ng per kg bw per day for crayfish, 0.97 ng per kg bw per day for crab and 13.37 ng per kg bw per day for fish. High intake and long-term exposure to PAHs could increase the cancer risk.60,61 Consequently, incremental lifetime cancer risk was essentially calculated. The mean ILCR values calculated from human exposure to 16 PAHs through consumption of freshwater organisms for adults were 2.45 × 10−8 for crayfish samples, 1.24 × 10−6 for crab samples and 1.71 × 10−5 for fish samples, while those for children were 1.19 × 10−7 for crayfish samples, 1.73 × 10−6 for crab samples, and 8.36 × 10−5 for fish. Therefore, for all tested freshwater species, the mean ILCR values were slightly over the minimum risk level (1.0 × 10−6) for crabs and remarkably higher for fish than the minimum risk level (1.0 × 10−6), but all ILCR values were below the maximum risk level (1.0 × 10−4). These results suggested that the contamination levels of the 16 PAH in the tested freshwater organism showed a noticeable carcinogenic risk for consumers. Only the mean ILCR of total PAHs in crayfish samples was excluded in the range from 10−6 to 10−4. Based on this, both the mitten crab and ricefield eel within the study region were beyond the acceptable threshold. Moreover, children are more vulnerable to PAH exposure than adults owing to their lower body weight and less developing bodies. Bioaccumulation also increases PAH concentration in freshwater organisms compared to that in sediment owing to the uptake through all the routes of exposure, including dietary intake, dermal absorption, and biological heritage.36,37 Furthermore, our study is based on raw uncooked aquatic products. A previous study on the comparison of PAH content between fresh raw fish and processed fish proved that processed fish tend to have a higher content of PAHs than raw fish because PAHs are easily formed at high temperatures during the cooking process.62 The cooking process might greatly increase the chance of exposure to PAHs and increase the risk of cancer in consumers.| Species | B[a]P equivalent concentration of total 16-PAHs (ng g−1) | Estimated daily intake (EDI) of a total of 16 PAHs for adults (ng per kg bw per day) | Incremental lifetime cancer risk of total 16-PAHs for adults | Estimated daily intake (EDI) of a total of 16 PAHs for children (ng per kg bw per day) | Incremental lifetime cancer risk of total 16-PAHs for children |
|---|---|---|---|---|---|
| Red swamp crayfish (Procambarus clarkii) | 0.0080 | 0.0052 | 2.45 × 10−8 | 0.0191 | 1.19 × 10−7 |
| Chinese mitten crab (Eriocheir sinensis) | 0.41 | 0.28 | 1.24 × 10−6 | 0.97 | 1.73 × 10−6 |
| Ricefield eel (Monopterus albus) | 5.58 | 3.82 | 1.71 × 10−5 | 13.37 | 8.36 × 10−5 |
Comparison of PAH concentrations between marine organisms and freshwater organisms
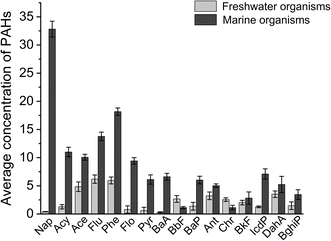
Compared with PAH studies on marine organisms, researchers are far less concerned about the pollution level of PAHs in edible freshwater organisms. Previous studies on PAHs accumulated in edible marine organisms worldwide indicated higher levels of PAHs than in freshwater organisms in our study. The levels of 16 PAHs were within the 29.73 to 87.02 ng g−1 concentration range in marine organisms from the coastal area of the East China Sea,63 94.88 to 557.87 ng g−1 in marine wild organisms from the South China Sea,64 81.499 to 5895.608 ng g−1 in some commercially important marine fish from the north western Gulf of Suez, Egypt65 and were 326–451 ng g−1 in edible marine fish from Kharg Coral Island, Persian Gulf.66 Coastal marine areas are heavily monitored, while studies on freshwater areas might be more scattered. This could introduce bias in the estimation of PAHs in marine organisms and freshwater organisms, making it seem that marine organisms have higher PAH levels because they have been studied extensively in polluted regions. Therefore, 16 PAHs in marine shellfish samples collected from Zhejiang, China were determined in this study. As shown in Fig. 4, the average values of PAH concentrations in marine shellfish samples were all higher than those in freshwater organisms, except for BbF. Nap, Acy, Ace, Flu, Phe, Flo, Pyr, BaA, BaP, IcdP, and BghiP were significantly higher than those in freshwater organisms. The results could be attributed mainly to environmental factors. The behaviors of marine PAHs can be influenced by oceanographic processes.67,68 Oceanographic processes, such as ocean currents, fronts and eddies, and air–sea interactions can impact the distribution of PAHs and marine dynamics by changing the surrounding temperature, nutrient levels, or productivity.69 The different patterns in the vertical distributions of PAHs are related to the ocean primary productivity and stratification index. Intensified stratification could prompt the accumulation of PAHs.70 In the freshwater environment, the behaviors of PAHs depend more on human activity, such as industrial wastewater discharge, traffic-related fuel combustion, garbage dumping and domestic heating. Rapid urbanization and industrialization for humanity produced large amounts of PAHs. They can be emitted into urban rivers and lakes, greatly affecting the quality of aquaculture water. PAHs were detected in 44 lakes in China, with a median concentration of 55.88 ng L−1.24 Among them, East Lake, located in Hubei, is the largest lake within a city in China, with 16 PAH concentrations of 36.95 ± 13.76 ng L−1 and 897.08 ± 232.34 ng g−1 in water and sediment, respectively.23 The occurrence of PAHs in aquatic organisms could also be strongly influenced by processing methods, such as boiling, frying and smoking.71,72 Smoked fish is one of the favorite foods throughout history, and it is tasty and can be stored for a long time. The smoking procedure involves treating pre-salted, whole, eviscerated, or filleted fish with wood smoke. The major components of wood are broken down in the burning process to form smoke and unique flavor substances, PAHs. It was reported that the amounts of BaP and PAH4 in Cambodian smoked freshwater fish exceeded the maximal limit set by the European Commission.62 Similarly, the mean concentrations of both BaP and PAHs in smoked catfish in Italy exceeded the EU maximum limits.73 There is no certainty about whether marine organisms or freshwater organisms are safer. By understanding only the characteristics and risks of marine organisms and freshwater organisms and adopting appropriate processing methods, we can fully ensure the safety and health of our diet.Conclusions
In the current study, we provided important data on the pollution characteristics, possible sources and health risks of PAHs in freshwater organisms from Hubei, Central China. The overall average concentration of ∑16PAH in the studied freshwater organisms was 38.40 ng g−1, which was a moderately contaminated level. 3–4 ring PAHs were dominant in freshwater organisms. HMW PAHs accounted for approximately three quarters of all samples. PAHs in freshwater organisms are derived mainly from vehicular exhaust emissions, a coke source and biomass and bioenergy combustion. The incremental lifetime cancer risk assessment indicated that the risk value of PAHs in fish was one magnitude higher than 10−6, with potential carcinogenic risks to human beings. Compared with marine organisms, the average PAH levels were lower in freshwater organisms, coinciding with other related reports around the world. According to the study, emissions of vehicular exhaust and coal tars should be reduced to prevent and control PAH pollution in freshwater aquatic products. In the future, more attention should be paid to the environmental situation in freshwater regions and study the environmental behavior of persistent toxic substances. More samples of freshwater organisms at different locations are needed for further investigation. More experimental studies are necessary to reveal the specific mechanism of PAH migration in the freshwater body.Consent to participate
All authors have given consent to their contribution.Consent to publish
All the authors are in agreement with the publication.Ethical statement
All experiments involving freshwater organisms in this study were performed in accordance with the experimental basic principles. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Yangtze River Fisheries Research Institute and approved by the Animal Ethics Committee of Yangtze River Fisheries Research Institute.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Ting Liu: methodology, investigation, writing original draft. Zhiyu Liu: methodology, formal analysis. Zhuozhen Qian: writing – review & editing. Li He: methodology, funding acquisition. Jie Peng: validation, data curation & editing. Lang Zhang: data curation. Yali Yu: data curation. Jinhua Gan: writing – review & editing, supervision.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The work is supported by the National Key Research and Development Program of China (No. 2022YFF0608203), the Open Program of Key Laboratory of Cultivation and High-value Utilization of Marine Organisms in Fujian Province (No. 2021fjscq14) and the Central Public-interest Scientific Institution Basal Research Fund (YFI20240405).References
- F. Liu, S. Hu, X. Guo, L. Niu, H. Cai and Q. Yang, Impacts of estuarine mixing on vertical dispersion of polycyclic aromatic hydrocarbons (PAHs) in a tide-dominated estuary, Mar. Pollut. Bull., 2018, 131, 276–283 CAS.
- A. Ramzi, H. K. Rahman, T. R. Gireeshkumar, K. K. Balachandran, J. Chacko and N. Chandramohanakumar, Dynamics of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of Cochin estuary, India, Mar. Pollut. Bull., 2017, 114(2), 1081–1087 CAS.
- P. P. O. Pinheiro, C. G. Massone and R. S. Carreira, Distribution, sources and toxicity potential of hydrocarbons in harbor sediments: a regional assessment in SE Brazil, Mar. Pollut. Bull., 2017, 120(1–2), 6–17 Search PubMed.
- H. H. Soclo, H. Budzinski, P. Garrigues and S. Matsuzawa, Biota accumulation of polycyclic aromatic hydrocarbons in benin coastal waters, Polycyclic Aromat. Compd., 2008, 28(2), 112–127 CAS.
- L. Wolska, A. Mechlinska, J. Rogowska and J. Namiesnik, Sources and Fate of PAHs and PCBs in the Marine Environment, Crit. Rev. Environ. Sci. Technol., 2012, 42(11), 1172–1189 CrossRef CAS.
- K. Ravindra, R. Sokhi and R. Van Grieken, Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation, Atmos. Environ., 2008, 42(13), 2895–2921 CrossRef CAS.
- K. A. Kieta, P. N. Owens, E. L. Petticrew, T. D. French, A. J. Koiter and P. M. Rutherford, Polycyclic aromatic hydrocarbons in terrestrial and aquatic environments following wildfire: a review, Environ. Rev., 2023, 31(1), 141–167 CrossRef CAS.
- W. Wilcke, Global patterns of polycyclic aromatic hydrocarbons (PAHs) in soil, Geoderma, 2007, 141(3–4), 157–166 Search PubMed.
- J. Zhang, C. Qu, S. Qi, J. Cao, C. Zhan and X. Xing, et al., Polycyclic aromatic hydrocarbons (PAHs) in atmospheric dustfall from the industrial corridor in Hubei Province, Central China, Environ. Geochem. Health, 2015, 37(5), 891–903 CrossRef CAS PubMed.
- J. Wu, G. Yang, H. Chen, Y. Zhai, Y. Teng and J. Li, et al., Source apportionment and source specific health risk assessment of HMs and PAHs in soils with an integrated framework in a typical cold agricultural region in China, Sci. Total Environ., 2023, 904, 167337 CrossRef CAS PubMed.
- M. Janneh, C. Qu, Y. Zhang, X. Xing, O. Nkwazema and F. Nyihirani, et al., Distribution, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in agricultural and dumpsite soils in Sierra Leone, RSC Adv., 2023, 13(11), 7102–7116 RSC.
- M. Mil-Homens, A. Cortes, S. Goncalves, B. L. van Drooge, H. de Stigter and J. O. Grimalt, et al., Sources and distribution of organic matter and polycyclic aromatic hydrocarbons in sediments of the southwestern Portuguese shelf, Mar. Pollut. Bull., 2025, 210, 117303 CrossRef CAS.
- H. Wang, X. Huang, Z. Kuang, X. Zheng, M. Zhao and J. Yang, et al., Source apportionment and human health risk of PAHs accumulated in edible marine organisms: a perspective of “source-organism-human”, J. Hazard. Mater., 2023, 453, 131372 CrossRef CAS PubMed.
- C. Zhang, Y. Li, C. Wang, Z. Feng, Z. Hao and W. Yu, et al., Polycyclic aromatic hydrocarbons (PAHs) in marine organisms from two fishing grounds, South Yellow Sea, China: bioaccumulation and human health risk assessment, Mar. Pollut. Bull., 2020, 153, 110995 Search PubMed.
- H. Zhang, Y. Chen, D. Li, C. Yang, Y. Zhou and X. Wang, et al., PAH residue and consumption risk assessment in four commonly consumed wild marine fishes from Zhoushan Archipelago, East China Sea, Mar. Pollut. Bull., 2021, 170, 112670 CrossRef CAS.
- D. Shi, F. Wu, J. He, Y. Sun, N. Qin and F. Sun, et al., Spatiotemporal distributions and ecological risk of polycyclic aromatic hydrocarbons in the surface seawater of Laizhou Bay, China, Environ. Sci. Pollut. Res., 2024, 31(8), 12131–12143 Search PubMed.
- S. Y. Pang, S. Suratman, M. T. Latif, M. F. Khan, B. R. T. Simoneit and N. M. Tahir, Polycyclic aromatic hydrocarbons in coastal sediments of Southern Terengganu, South China Sea, Malaysia: source assessment using diagnostic ratios and multivariate statistic, Environ. Sci. Pollut. Res., 2022, 29(11), 15849–15862 Search PubMed.
- M. Mora, T. R. Walker and R. Willis, Multiple contaminant ecological risk evaluation in small craft harbour sediments in Nova Scotia, Canada, Sci. Total Environ., 2022, 834, 155266 CAS.
- Z. Qian, Y. Mao, S. Xiong, B. Peng, W. Liu and H. Liu, et al., Historical residues of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in a flood sediment profile from the Longwang Cave in Yichang, China, Ecotoxicol. Environ. Saf., 2020, 196, 110542 CAS.
- R. X. S. Tulcan, L. Liu, X. Lu, Z. Ge, D. Y. F. Rojas and D. M. Silva, PAHs contamination in ports: status, sources and risks, J. Hazard. Mater., 2024, 475, 134937 Search PubMed.
- Y. Cao, J. Wang, M. Xin, B. Wang and C. Lin, Spatial distribution and partition of polycyclic aromatic hydrocarbons (PAHs) in the water and sediment of the southern Bohai Sea: Yellow River and PAH property influences, Water Res., 2024, 248, 120873 Search PubMed.
- R. A. Grmasha, C. Stenger-Kovacs, B. A. H. Bedewy, O. J. Al-sareji, R. A. Al-Juboori and M. Meiczinger, et al., Ecological and human health risk assessment of polycyclic aromatic hydrocarbons (PAH) in Tigris river near the oil refineries in Iraq, Environ. Res., 2023, 227, 115791 CAS.
- M. Shi, J. Zhu, T. Hu, A. Xu, Y. Mao and L. Liu, et al., Occurrence, distribution and risk assessment of microplastics and polycyclic aromatic hydrocarbons in East lake, Hubei, China, Chemosphere, 2023, 316, 137864 Search PubMed.
- Y. He, C. Yang, W. He and F. Xu, Nationwide health risk assessment of juvenile exposure to polycyclic aromatic hydrocarbons (PAHs) in the water body of Chinese lakes, Sci. Total Environ., 2020, 723, 138099 CAS.
- H. Wu, J. Wang, J. Guo, X. Hu and J. Chen, Sedimentary records of polycyclic aromatic hydrocarbons from three enclosed lakes in China: response to energy structure and economic development, Environ. Pollut., 2023, 318, 120929 Search PubMed.
- N. Y. Kim, B. G. Loganathan and G. B. Kim, Determination and comparison of freely dissolved PAHs using different types of passive samplers in freshwater, Sci. Total Environ., 2023, 892, 164802 Search PubMed.
- P. Amoatey and M. S. Baawain, Effects of pollution on freshwater aquatic organisms, Water Environ. Res., 2019, 91(10), 1272–1287 Search PubMed.
- N. J. Diepens and A. A. Koelmans, Accumulation of Plastic Debris and Associated Contaminants in Aquatic Food Webs, Environ. Sci. Technol., 2018, 52(15), 8510–8520 Search PubMed.
- M. V. Pena-Garcia, M. J. Moyano-Gallego, S. Gomez-Melero, R. Molero-Payan, F. Rodriguez-Cantalejo and J. Caballero-Villarraso, One-Year Impact of Occupational Exposure to Polycyclic Aromatic Hydrocarbons on Sperm Quality, Antioxidants, 2024, 13(10), 1181 Search PubMed.
- K. K. Ferguson, T. F. McElrath, G. G. Pace, D. Weller, L. Zeng and S. Pennathur, et al., Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women, Environ. Sci. Technol., 2017, 51(8), 4652–4660 CAS.
- Food and Agriculture Organization of the United Nations, FAOSTAT. Calculated from food balance sheets, https://faostat.fao.org/site/368/DesktopDefault.aspx?PageID5368#ancor, 2020, accessed 18 March 2025 Search PubMed.
- China Fishery Statistics Yearbook, Bureau of Fisheries, Ministry of Agriculture, Beijing, China, 2021, pp. 21–36 Search PubMed.
- USEPA, Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Volume 1: Fish Sampling and Analysis (EPA 823-B-00-007), Washington DC, 3rd edn, 2000, pp. 1–485 Search PubMed.
- USEPA, Guidance for Assessing Chemical Contaminant Data for Advisories Use in Fish, Volume 2: Risk Assessment and Fish Consumption Limits (EPA 823-B-00-008), Washington DC, 3rd edn, 2000, pp. 1–383 Search PubMed.
- USEPA, Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens, EPA/630/R-03/003F, Risk Assessment Forum, United States Environmental Protection Agency, Washington DC, USA, 2005, https://www3.epa.gov/airtoxics/childrens_supplement_final.pdf Search PubMed.
- J. Zhang, X. Zhang, T. Hu, X. Xu, D. Zhao and X. Wang, et al., Polycyclic aromatic hydrocarbons (PAHs) and antibiotics in oil-contaminated aquaculture areas: bioaccumulation, influencing factors, and human health risks, J. Hazard. Mater., 2022, 437, 129365 Search PubMed.
- H. Li, X. Wang, Y. Mai, Z. Lai and Y. Zeng, Potential of microplastics participate in selective bioaccumulation of low-ring polycyclic aromatic hydrocarbons depending on the biological habits of fishes, Sci. Total Environ., 2023, 858, 159939 Search PubMed.
- Y. Zhai, H. Wang, X. Lin and X. Xia, Bioconcentration of polycyclic aromatic hydrocarbons in different tissues of zebrafish (Danio rerio) investigated with PBTK model, Environ. Sci. Pollut. Res., 2023, 30(54), 116313–116324 CAS.
- J. Xiong and Z. Li, Predicting PFAS fate in fish: assessing the roles of dietary, respiratory, and dermal uptake in bioaccumulation modeling, Environ. Res., 2024, 252, 119036 CrossRef CAS.
- P. Devi and A. K. Dalai, Occurrence, distribution, and toxicity assessment of polycyclic aromatic hydrocarbons in biochar, biocrude, and biogas obtained from pyrolysis of agricultural residues, Bioresour. Technol., 2023, 384, 129293 CrossRef CAS PubMed.
- S. K. Singh and R. K. Singh, An overview on remediation technologies for polycyclic aromatic hydrocarbons in contaminated lands: a critical approach, Environ. Dev. Sustain., 2025, 27(2), 2753–2787 CrossRef.
- R.-X. Sun, X. Yang, Q. X. Li, Y.-T. Wu, H.-Y. Shao and M.-H. Wu, et al., Polycyclic aromatic hydrocarbons in marine organisms from Mischief Reef in the South China sea: implications for sources and human exposure, Mar. Pollut. Bull., 2019, 149, 110623 CrossRef CAS.
- S. Lambiase, A. Ariano, F. P. Serpe, M. Scivicco, S. Velotto and M. Esposito, et al., Polycyclic aromatic hydrocarbons (PAHs), arsenic, chromium and lead in warty crab (Eriphia verrucosa): occurrence and risk assessment, Environ. Sci. Pollut. Res., 2021, 28(26), 35305–35315 CrossRef CAS PubMed.
- Y.-Y. Kim, J.-K. Patra and H.-S. Shin, Evaluation of analytical method and risk assessment of polycyclic aromatic hydrocarbons for fishery products in Korea, Food Control, 2022, 131, 108421 CrossRef CAS.
- Z. Zhao, L. Zhang, Y. Cai and Y. Chen, Distribution of polycyclic aromatic hydrocarbon (PAH) residues in several tissues of edible fishes from the largest freshwater lake in China, Poyang Lake, and associated human health risk assessment, Ecotoxicol. Environ. Saf., 2014, 104, 323–331 CAS.
- S. Rossi, D. S. D. de Farias, A. d. C. Bomfim, R. S. Carreira, J. H. H. Grisi-Filho and C. G. Massone, et al., Concentrations of polycyclic aromatic hydrocarbons (PAHs) in liver samples of green turtles Chelonia mydas stranded in the Potiguar Basin, northeastern Brazil, Mar. Pollut. Bull., 2023, 193, 115264 Search PubMed.
- G. Sanli, S. Celik, V. Joubi and Y. Tasdemir, Concentrations, phase exchanges and source apportionment of polycyclic aromatic hydrocarbons (PAHs) In Bursa-Turkey, Environ. Res., 2023, 232, 116344 CAS.
- J.-N. Li, Y. Zhang, J.-X. Wang, H. Xiao, A. Nikolaev and Y.-F. Li, et al., Occurrence, Sources, and Health Risks of Polycyclic Aromatic Hydrocarbons in Road Environments from Harbin, a Megacity of China, Toxics, 2023, 11(8), 695 CAS.
- A. Sidhu, J. Bazely, E. Peeters and J. Cami, A principal component analysis of polycyclic aromatic hydrocarbon emission in NGC 7023, Mon. Not. R. Astron. Soc., 2022, 511(2), 2186–2200 Search PubMed.
- U. Edet, A. Joseph, G. Bebia, E. Mbim, B. Ubi and C. Archibong, et al., Health risk of heavy metals and PAHs contaminants in goat meat de-haired with waste tyres and plastic in Calabar, Nigeria, J. Food Compos. Anal., 2024, 131, 106216 CAS.
- R. K. Larsen and J. E. Baker, Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods, Environ. Sci. Technol., 2003, 37(9), 1873–1881 CAS.
- H. Guo, S. C. Lee, K. F. Ho, X. M. Wang and S. C. Zou, Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong, Atmos. Environ., 2003, 37(38), 5307–5317 CAS.
- R. de Abrantes, J. V. de Assunçao and U. R. Pesquero, Emission of polycyclic aromatic hydrocarbons from light-duty diesel vehicles exhaust, Atmos. Environ., 2004, 38(11), 1631–1640 CAS.
- Z. Wang, J. Chen, F. Tian, P. Yang, X. Qiao and Z. Yao, Application of Factor Analysis with Nonnegative Constraints for Source Apportionment of Soil Polycyclic Aromatic Hydrocarbons (PAHs) in Liaoning, China, Environ. Forensics, 2010, 11(1–2), 161–167 CAS.
- J. R. Sobus, S. Waidyanatha, M. D. McClean, R. F. Herrick, T. J. Smith and E. Garshick, et al., Urinary naphthalene and phenanthrene as biomarkers of occupational exposure to polycyclic aromatic hydrocarbons, Occup. Environ. Med., 2009, 66(2), 99–104 CAS.
- H. Huo, Y. Lei, Q. Zhang, L. Zhao and K. He, China's coke industry: recent policies, technology shift, and implication for energy and the environment, Energy Policy, 2012, 51, 397–404 CAS.
- A. M. Mastral, T. García, M. S. Callén, J. M. López, M. V. Navarro and R. Murillo, et al., Three-ring PAH removal from waste hot gas by sorbents: influence of the sorbent characteristics, Environ. Sci. Technol., 2002, 36(8), 1821–1826 CAS.
- Q. Shi, Y. Yan, X. Wu, S. Li, K. H. Chung and S. Zhao, et al., Identification of Dihydroxy Aromatic Compounds in a Low-Temperature Pyrolysis Coal Tar by Gas Chromatography-Mass Spectrometry (GC-MS) and Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS), Energy Fuels, 2010, 24(10), 5533–5538 Search PubMed.
- S. Sroy, E. Arnaud, A. Servent, S. In and S. Avallone, Nutritional benefits and heavy metal contents of freshwater fish species from Tonle Sap Lake with SAIN and LIM nutritional score, J. Food Compos. Anal., 2021, 96, 103731 CAS.
- S. Chen, S. Li, H. Li, M. Du, S. Ben and R. Zheng, et al., Effect of polycyclic aromatic hydrocarbons on cancer risk causally mediated via vitamin D levels, Environ. Toxicol., 2023, 38(9), 2111–2120 CAS.
- A. Olsson, N. Guha, L. Bouaoun, H. Kromhout, S. Peters and J. Siemiatycki, et al., Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Lung Cancer Risk: Results from a Pooled Analysis of Case-Control Studies (SYNERGY), Cancer Epidemiol. Biomark. Prev., 2022, 31(7), 1433–1441 CrossRef CAS PubMed.
- C. Douny, H. Mith, A. Igout and M. L. Scippo, Fatty acid intake, biogenic amines and polycyclic aromatic hydrocarbons exposure through the consumption of nine species of smoked freshwater fish from Cambodia, Food Control, 2021, 130, 108219 CrossRef CAS.
- S. Ji, F. Yin, W. Zhang, Z. Song, B. Qin and P. Su, et al., Occurrences, Sources, and Human Health Risk Assessments of Polycyclic Aromatic Hydrocarbons in Marine Organisms From Temperate Coastal Area, Front. Ecol. Evol., 2022, 10, 850247 CrossRef.
- C.-L. Ke, Y.-G. Gu, Q. Liu, L.-D. Li, H.-H. Huang and N. Cai, et al., Polycyclic aromatic hydrocarbons (PAHs) in wild marine organisms from South China Sea: occurrence, sources, and human health implications, Mar. Pollut. Bull., 2017, 117(1–2), 507–511 Search PubMed.
- O. E. Ahmed, A. M. Eldesoky and M. M. El Nady, Evaluation of petroleum hydrocarbons and its impact on organic matters of living organisms in the northwestern Gulf of Suez, Egypt, Pet. Sci. Technol., 2019, 37(24), 2441–2449 Search PubMed.
- A. R. Jafarabadi, A. R. Bakhtiari, Z. Yaghoobi, C. K. Yap, M. Maisano and T. Cappello, Distributions and compositional patterns of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in three edible fishes from Kharg coral Island, Persian Gulf, Iran, Chemosphere, 2019, 215, 835–845 Search PubMed.
- L. Zhang, Y. Ma, S. Vojta, M. Morales-McDevitt, M. Hoppmann and T. Soltwedel, et al., Presence, Sources and Transport of Polycyclic Aromatic Hydrocarbons in the Arctic Ocean, Geophys. Res. Lett., 2023, 50(1), e2022GL101496 CAS.
- M. Liu, M. Cai, M. Duan, M. Chen, R. Lohmann and Y. Lin, et al., PAHs in the North Atlantic Ocean and the Arctic Ocean: Spatial Distribution and Water Mass Transport, J. Geophys. Res.: Oceans, 2022, 127(6), e2021JC018389 CrossRef.
- M. Liu, H. Zheng, M. Chen, J. Liang, M. Duan and H. Du, et al., Dissolved PAHs impacted by air-sea interactions: net volatilization and strong surface current transport in the Eastern Indian Ocean, J. Hazard. Mater., 2022, 431, 128603 CAS.
- M. Liu, H. Zheng, M. Cai, K. M. Y. Leung, Y. Li and M. Yan, et al., Ocean Stratification Impacts on Dissolved Polycyclic Aromatic Hydrocarbons (PAHs): From Global Observation to Deep Learning, Environ. Sci. Technol., 2023, 57(46), 18339–18349 CAS.
- S. Giandomenico, M. Nigro, I. Parlapiano, L. Spada, A. Grattagliano and E. Prato, et al., Effect of in-house cooking in Mytilus galloprovincialis and Trachurustrachurus: lipid and fatty acids quality and polycyclic aromatic hydrocarbons formation, Food Chem. Toxicol., 2023, 173, 113606 CAS.
- K. Dutta, S. Shityakov, W. Zhu and I. Khalifa, High-risk meat and fish cooking methods of polycyclic aromatic hydrocarbons formation and its avoidance strategies, Food Control, 2022, 142, 109253 CAS.
- S. Squadrone, P. Brizio, M. C. Abete, F. Busico and B. Neri, Polycyclic Aromatic Hydrocarbons and Mercury in Smoked Catfish: A Case Study, Water, Air, Soil Pollut., 2021, 232(7) DOI:10.1007/s11270-021-05204-1.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05983b |
| This journal is © The Royal Society of Chemistry 2025 |