Open Access Article
Open Access ArticleSolvent-free synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones using a magnetic nickel–zinc ferrite nanocatalyst†
Sreelakshmi Sreekandana,
Anjitha Thadathilb,
Bindu Mavilac,
Kannan Vellayan*a and
Pradeepan Periyat *c
*c
aDepartment of Chemistry, Government College, Kattappana, Kerala, India. E-mail: kannanpvl@gmail.com
bDepartment of Chemistry, University of Calicut, Kerala, India
cDepartment of Environmental Studies, Kannur University, Kerala, India. E-mail: pperiyat@kannuruniv.ac.in
First published on 10th February 2025
Abstract
A high quality magnetic nanocatalyst Ni0.5 Zn0.5Fe2O4 (NZF) was synthesized via a sol–gel auto combustion method. The structure and morphology of the synthesized Ni0.5Zn0.5Fe2O4 (NZF) nanocatalyst were examined using various physico-chemical methods. The catalytic performance of the catalyst with varying transition metal composition was investigated for the solvent-free synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones, and it was found that the yield of the product was 87%. The NZF nanocatalyst showed excellent catalytic performance for a wide range of substrates with a very low catalyst loading. The super paramagnetic nature of the NZF nanocatalyst enabled the separation of the catalyst from the reaction mixture by magnetic recovery. Recyclability of the NZF nanocatalyst was also investigated, and it was found that the catalyst exhibited a recyclability of three cycles, without any significant loss in its activity.
1 Introduction
The new era of chemistry focuses on the use of inexpensive catalysts, with an eco-friendly aspect, which allow aqueous or solvent-free synthesis without affecting the yield and quality of the product.1 Nanocatalysis has become an important field to achieve such goals. Nanostructured materials have emerged as powerful heterogeneous catalysts for various organic transformations, in terms of selectivity, reactivity, and improved yields of products. The high surface-to-volume ratio of nanoparticles provides a larger number of active sites per unit area.2 Nano-catalysts combine the advantages of both homogeneous and heterogeneous catalytic systems, exhibiting high catalytic activity (turnover frequency) and selectivity similar to homogeneous catalytic systems while allowing the recycling and separation of the catalyst after completion of the reaction as shown by heterogeneous catalytic systems.3Magnetically recyclable nano-catalysts (MRNCs) have been recognized for their role in organic synthesis.4 Such catalytic systems reduce the number of reaction steps, lower the cost of the process and minimize waste generation.5 Recently, multimetallic systems with magnetic properties have been used as catalysts for conducting one-pot multistep reactions.6 Multimetallic nanoparticles, which contain more than two distinct metals, exhibit synergistic effects that enhance the catalytic activity.7 These multimetallic nanoparticles combine the properties of their individual constituents, resulting in characteristics that are superior to those of their constituents, thereby achieving improved activity, selectivity and stability.8 The difference in electronegativity between the individual metal nanoparticles can induce electron transfer, leading to the generation of electron-poor and -rich regions on the multi metallic surface. This electronic effect promotes high electron uptake and increases the electron transfer between the target molecules, resulting in a high catalytic activity of multi metallic nanocatalysts.9
According to previous researches, trimetallic NPs exhibit unique characteristics and demonstrate higher reactivity than bimetallic and monometallic nanomaterials. They can be employed as efficient nanocatalysts, antimicrobials and anticancer agents.10 Trimetallic nanocatalysts display enhanced catalytic performance and higher stability, making them promising photocatalysts and adsorbents for the elimination of hazardous pollutants and contaminants.11
Ferrite nanoparticles provide increased surface area for organic groups to anchor, which enhances the product yield and reduces the reaction time, providing a cost-effective and efficient method for organic transformations.12 Various organic reactions are reported, such as the photocatalytic decomposition of different dyes, alkylation, dehydrogenation, oxidation and C–C coupling, with nano-ferrites as the catalyst.13 Multimetallic ferrites such as Ni–Mg–Zn ferrite, Ni–Co–Zn ferrite, Ni–Zn–Al ferrite, Pd–Bi ferrite, Co–Sm ferrite, Co–Ce ferrite, Mg–Ce ferrite, and Co–Zn–Gd ferrite have been proven to be promising materials for waste water treatment, computer memory, electromagnetic interference filtration, transesterification and gas sensing applications.14–22 The pure zinc ferrite (ZnFe2O4) and ZnNd2O4 have demonstrated high activity for the coupling reaction.23 Ferrite nanomaterials have been employed as a catalyst for condensation and cyclization reactions, oxidation of various alkenes, alkylation, C–C coupling, dehydrogenation reactions and synthesis of organic compounds such as arylamines and acetylferrocene chalcones.11,24 CuO nanoparticles are potential candidates, showing antibacterial effect against both Gram-negative and Gram-positive bacteria.25,26 Magnetic nanodendrimers have shown promising catalytic activity for a wide variety of organic transformations such as coupling, reduction, cycloaddition and oxidation.27 Green-synthesized Zn0.5Ni0.5FeCrO4 magnetic nanoparticles (MNPs), which are magnetically recoverable, exhibit significant photocatalytic activity due to their unique composition, which enhances the generation of reactive species when exposed to light. Their synthesis through environmentally friendly methods often involves the use of plant extracts, which not only reduces the use of harmful chemicals but can also enhance the properties of nanocatalysts.28
The recovery of catalysts from the system is a crucial characteristic for the catalyst to be acceptable in green chemical manufacturing processes in industry. The ferrite nanocatalysts can be separated easily from reaction systems using an external magnet and can be reused with minimal loss of catalytic activity. Magnetic separation reduces secondary waste generation and energy consumption.29 Multicomponent reactions are gaining increasing interest in the synthesis of biologically important compounds, as they simplify the possess of building up complex molecules.30
Heterocyclic compounds (HC) with five and six membered rings play a vital role in medicinal chemistry, thus making the synthesis of heterocycles with reusable nanocatalysts increasingly important in the pharmaceutical and organic chemistry fields. Oxazinone, benzoxazinone and their derivatives are important classes of heterocyclic compounds that exhibit biological activities, such as HIV-1 reverse transcriptase inhibitors.31
Various heterocyclic compounds have demonstrated antibacterial, antifungal, antiviral and anti-inflammatory properties.32 Heterocycles constitute a common structural unit of most marketed drugs, with 60% of unique small-molecule drugs containing nitrogen.33 Recent advancements have utilized nanoparticles as effective catalysts in the synthesis of nitrogen-containing heterocyclic compounds; for example, MgO nanocatalysts have been employed in the preparation of dihydropyrano [2,3-c]pyrazole derivatives.34 Additionally a Schiff base complex of copper immobilized on core–shell magnetic nanoparticles Cu(II)-SB/GPTMS@SiO2@Fe3O4 has been reported to be efficient in the synthesis of biologically active polyhydroquinoline derivatives.35,36
2-Naphthol and naphthoxazinones and their derivatives were reported to possess bactericidal, antioxidant and chelating properties in the metal-catalysed asymmetric synthesis and they have cytotoxic and antifungal activities.37,38 Chiral Mannich bases of β-naphthol are commonly used as metal-mediated and ligand-accelerated catalysts in enantioselective carbon–carbon bond formation.39,40
Various strategies employing harsh reaction conditions have been reported for the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones by the condensation of aldehyde with β-naphthol and urea in the presence of FeCl3 under microwave assistance,41 thiamine hydrochloride catalyst in alcohol42 and Sr(OTf)2 as the catalyst in chloroform.43 It is desirable to develop a more efficient, simple, less expensive and less toxic one-pot method for the synthesis of 1,2-dihydro-1-aryl naphtho[1,2-e] [1,3] oxazine-3-ones. In this work, we demonstrate a method to extend the scope of the three-component condensation of β-naphthol, aryl aldehydes and urea, using efficient, readily available, simply synthesized and magnetically retrievable nickel zinc ferrite (NZF) nanoparticles as the catalyst under solvent free conditions.
2 Experimental
2.1 Synthesis of Ni0.5Zn0.5Fe2O4 (NZF) nanocatalysts
The nanocatalyst Ni0.5Zn0.5Fe2O4 (NZF) was synthesized by a sol–gel auto combustion method (Scheme 1).43–45 Solutions of iron nitrate (Fe (NO3)3·9H2O) at a concentration of 2.0 M, nickel nitrate (Ni (NO3)2·6H2O) at a concentration of 0.5 M and zinc nitrate (Zn (NO3)2·6H2O) at a concentration of 0.5 M were used as the precursors for the nanoparticle synthesis. The iron nitrate, nickel nitrate, and zinc nitrate solutions were mixed thoroughly with citric acid (C6H8O7) at a concentration of 2.2 M to form a clear solution. The pH of the solution was adjusted to 7 by the addition of ammonia solution. This step was important to create optimal conditions for the gel formation. The solution was stirred continuously and kept at a temperature of 90 °C until a gel-like substance formed. The gelling process helped to form the desired nanoparticle structure. The gel was then heated to 150 °C to initiate a self-propagating combustion process. This combustion helped to further transform the gel into the desired NZF nanoparticle structure. The resultant loose powder from the combustion process was crushed well to break down any large particles. This step ensured uniform particle size distribution. The crushed powder was then calcined at a temperature of 550 °C for 4 h. It helped further solidification of particles and formation of the spinal phase. The ratio of nitrates to citric acid used in the synthesis is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.77. This ratio had been optimized to obtain the desired properties of the NZF nanoparticles.
2.77. This ratio had been optimized to obtain the desired properties of the NZF nanoparticles.
2.2 Characterisation of Ni0.5Zn0.5Fe2O4 (NZF) nanocatalysts
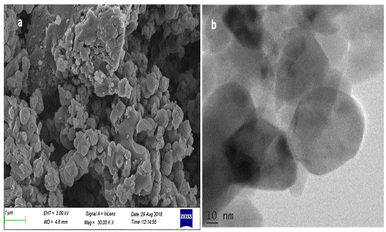
The as synthesized Ni0.5Zn0.5Fe2O4 (NZF) nanocatalysts were characterized by powder X-ray diffraction (XRD) (X'Pert3 Powder with CuKα (λ = 1.5406 Å) radiation) and FTIR spectroscopy (JASCO FTIR-model 4100, in the range of 350–4000 cm−1 by the KBr pellet method).The morphology was investigated by scanning electron microscopy (Hitachi S-3000H) and transmission electron microscopy (JEOL/JEM2100 with a 200 kV accelerating voltage and a lattice resolution of 0.14 nm) analysis.
The XRD pattern of the catalyst shows an average size of 10 nm, calculated using the Scherrer formula in eqn (1). The broadening of diffraction peaks can be attributed to the bulk to nanoscale conversion of crystallite sizes.
D = κλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
These well characterized Ni0.5Zn0.5Fe2O4 (NZF) nanocatalysts were used for the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones.
2.3 Synthesis of 1,2-dihydro-1-arylnaphtho [1,2-e] [1,3] oxazine-3-ones using Ni0.5Zn0.5Fe2O4 (NZF)
A mixture of aryl aldehyde (1 mmol), β-naphthol (1 mmol) and urea in the presence of the Ni0.5Zn0.5Fe2O4 catalyst (80 mg) was stirred under solvent free conditions at a temperature of 120 °C. The progress and completion of the reaction were monitored by thin layer chromatography. The insoluble catalyst was magnetically removed and the solvent was evaporated from the reaction mixture followed by column chromatography over silica gel using the solvent mixture of chloroform-ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to get the pure product. The product was then characterised by 1H NMR (nuclear magnetic resonance) spectroscopy to determine the molecular structure.
3) to get the pure product. The product was then characterised by 1H NMR (nuclear magnetic resonance) spectroscopy to determine the molecular structure.
3 Results and discussion
3.1 Catalyst characterization
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. The characteristic diffraction peaks were in good agreement with JCPDS-00-008-0234. Each peak can be evaluated independently and should produce a consistent crystalline domain size as long as the sample can be roughly approximated as uniform, spherical particles.
m space group. The characteristic diffraction peaks were in good agreement with JCPDS-00-008-0234. Each peak can be evaluated independently and should produce a consistent crystalline domain size as long as the sample can be roughly approximated as uniform, spherical particles.
The average crystallite size (D) of the NZF nanoparticles (10 nm) was calculated from the broadening of the most intense peak (311) of the sample. The crystallite size increases when more Ni2+ ions are replaced by Zn2+ ions (0.83 Å) with a large size compared to Ni2+ ions (0.78 Å).
| Nanoparticles | TB (K) | Ms (emu g−1) | Mr (emu g−1) | Hc (Oe) |
|---|---|---|---|---|
| a TB (K): blocking temperature, Ms (emu g−1): saturation magnetization, Mr (emu g−1): remanent magnetization and Hc (Oe): coercivity. | ||||
| NZF | 56.2 | 45.38 | 4.85 | 28.8 |
3.2 Synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones
After the synthesis and complete characterisation of the NZF nanocatalyst, the nanocatalyst has been scrutinized in the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones. Various parameters such as reaction conditions, type of solvent used, reaction time, temperature and the amount of catalyst loading have been optimised.Solvent free condition has been tested at various temperatures starting from 30 °C. The maximum yield has been obtained at 120 °C, and no further increase in yield was obtained when increasing the temperature. In order to study the effect of the catalyst, the reaction has been carried out in the absence of the catalyst under the same reaction condition. A trace amount of product was obtained, clearly indicating the role of the catalyst.
| Solvent | Time (min) | Yield (%) |
|---|---|---|
| Water | 120 | 45 |
| Ethanol | 120 | 72 |
| Toluene | 120 | 55 |
| DCM | 120 | 65 |
| Solvent free | 120 | 87 |
| Amount of catalyst (g) | Time (min) | Yield (%) |
|---|---|---|
| 0.020 | 120 | 64 |
| 0.030 | 120 | 69 |
| 0.040 | 120 | 71 |
| 0.050 | 120 | 76 |
| 0.060 | 120 | 78 |
| 0.070 | 120 | 82 |
| 0.080 | 120 | 87 |
| 0.090 | 120 | 87 |
A comparison of catalytic activity of Ni0.5Zn0.5Fe2O4 (NZF) nanocatalysts with that of other catalysts in the solvent free synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones is given in Table 4.
In summary, the optimization studies revealed that the Ni0.5Zn0.5Fe2O4 (NZF) nanocatalyst showed good yield in the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones with a reaction time of 120 minutes at 120 °C under solvent free conditions (Scheme 2).
 | ||
| Scheme 2 Synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones under optimized conditions. | ||
The proposed mechanism for the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones is shown as Scheme 3. In the initial step, reactants get adsorbed on the surface of the catalyst. The nanocatalyst bears high surface area, which increases the adsorption of reactant molecules. The unique surface chemistry associated with the nanocatalyst and higher zeta potential value help in the stabilization of the nanocrystal system.54,55 In NZF, Zn2+ is the main active component having Lewis acid properties and superior catalytic properties.56,57 The NZF catalyst activates the carbonyl group of the aldehyde via co-ordination of the vacant orbital present on Ni2+ with carbonyl oxygen. The carbonyl group is then polarized because of the difference in electronegativities between carbon and oxygen. After this step, the unshared pair of electrons in the nitrogen atom of amine is attracted to the partially positive carbon of the carbonyl group. This facilitates the reaction of aryl aldehydes with beta-naphthol, generating an intermediate.58 Again, the catalyst activates the intermediate and thus accelerates intramolecular cyclization to give the desired product. It was reported previously that the use of smaller catalyst particles (10 nm in the present work) can enhance the catalytic performance by reducing the diffusion path, providing more active sites, enhancing stability and workability under reactive environments.59–61
 | ||
| Scheme 3 Proposed mechanism for the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones using NZF nanoparticles. | ||
Semi-empirical calculations provide a reliable method for the study of organic reaction mechanisms, balancing computational efficiency in modelling chemical processes, structures and properties to give evidence for the proposed mechanism.62 Theoretical calculations were performed to predict the transition state using the semiempirical PM6 method. The proposed mechanism is in agreement with the theoretically obtained transition state. The transition state obtained and IRC pathway details are included in the ESI.†
After optimizing the reaction conditions, in order to study the electronic effect of substituted aryl aldehydes, the reactions have been repeated with a variety of substituted aryl aldehydes with electron releasing and electron withdrawing groups while keeping 1 and 3 as fixed substrates. The results are summarized in Table 5. The yield of the product depends on both the electronic effect of reactants and the polarity of the carbonyl group in aldehydes. Aromatic aldehydes with both electron-donating and electron-withdrawing groups reacted and gave the products in good yields. Aldehydes with electron withdrawing groups gave higher yields (Table 5). Poor yield of product was obtained when using veratraldehyde, which may be due to the steric hindrance.
| Sl. no. | β-Naphthol | Aldehyde | Urea | Product | Yield % | M.P. (°C) |
|---|---|---|---|---|---|---|
| a Reaction condition: aryl aldehyde (1 mmol), β-naphthol (1 mmol) and urea in presence of catalyst Ni0.5Zn0.5Fe2O4 (80 mg), at a temperature of 120 °C under solvent free condition. | ||||||
| 1 |  |
 |
 |
 |
87 | 216 |
| 2 |  |
 |
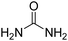 |
 |
89 | 188 |
| 3 | 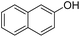 |
 |
 |
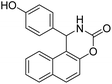 |
85 | 182 |
| 4 |  |
 |
 |
 |
86 | 183 |
| 5 |  |
 |
 |
 |
82 | 203 |
| 6 |  |
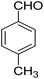 |
 |
 |
87 | 168 |
| 7 |  |
 |
 |
 |
85 | 225 |
| 8 |  |
 |
 |
 |
Trace | |
3.3 Recyclability studies
Recyclability is crucial in terms of economy and sustainability. The catalytic process was repeated over three consecutive reaction cycles to study the recyclability of the catalyst. Magnetic separation is an important property for the separation and reuse of nano-catalysts. The super paramagnetic nature of the NZF nanocatalyst enables the separation of the catalyst from the reaction mixture using a permanent magnet by magnetic recovery. The separation of magnetic solid catalysts was found to be fast, simple, convenient, and efficient by using an external magnet. After each cycle, the catalyst has been washed with ethanol at 60 °C, dried and used again. It was found out that the NZF catalyst can be reused three times without any significant loss of catalytic activity towards the synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e] [1,3] oxazine-3-ones. The variation of percentage yield with the number of cycles is depicted as Fig. 4.The H1-NMR and C13-NMR spectral data of the products are incorporated in the ESI file.†
4 Conclusions
The work presented here demonstrated a novel approach to the synthesis of 1,2-dihydro-1-aryl naphtho [1,2-e] [1,3] oxazine-3-ones using the nanocatalyst Ni0.5Zn0.5Fe2O4. The NZF nanocatalyst was prepared by using a sol–gel auto combustion method, involving the reaction between Fe (NO3)3·9H2O, Ni (NO3)2·6H2O and Zn (NO3)2·6H2O. The prepared NZF catalysts were found to be magnetically separable and demonstrated promising catalytic efficiency for organic conversions. The catalyst proved to be an effective heterogeneous catalyst for the one-pot synthesis of naphthoxazinones, which are of significant medicinal importance. The reaction involved three-component coupling of β-naphthol, aromatic aldehydes and urea using the NZF nanocatalyst, which had several advantages like high yield, environmental suitability and simple work-up procedure. A mechanism was proposed for the reaction, which was further supported by computational studies. The catalyst was recovered and reused without significant loss in the catalytic activity. The structures of the newly synthesized products were confirmed through spectroscopic data. The study demonstrated that the NZF nanocatalyst provided a promising route for the synthesis of naphthoxazinones and similar compounds in organic synthesis.Data availability
Data will be made available on request.Author contributions
Sreelakshmi Sreekandan: data curation, formal analysis, investigation, validation, methodology, writing – original draft; Anjitha Thadathil: data curation; Bindu Mavila: data curation; Kannan Vellayan: conceptualization, project administration, supervision, writing – review and editing; Pradeepan Periyat: conceptualization, supervision, writing – review and editing.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors are grateful to Government College for Women, Thiruvananthapuram, for NMR analysis.References
- K. Hemalatha, G. Madhumitha, A. Kajbafvala, N. Anupama, R. Sompalle and S. M. Roopan, J. Nanomater., 2013, 2013, 4 CrossRef.
- S. Khaturia, M. Chander, H. Sachdeva, Sangeeta and C. B. Mahto, J. Nanomed. Nanotechnol., 2020, 11, 1 Search PubMed.
- S. G. Babu and R. Karvembu, Catal. Surv. Asia, 2013, 17, 156 CrossRef CAS.
- M. B. Gawande, A. K. Rathi, P. S. Branco and R. S. Varma, Appl. Sci., 2013, 3, 656 CrossRef CAS.
- P. Singh, S. Misra, A. Sahoo and S. Patra, Sci. Rep., 2011, 11, 9305 CrossRef PubMed.
- J. A Mata, F. E Hahn and E. Peris, Chem. Sci., 2014, 5, 1723 RSC.
- H. Lv, A. Lopes, d. Xu and B. Liu, ACS Cent. Sci., 2018, 4, 1412 CrossRef CAS PubMed.
- S. Cai, D. Wang, Z. Niu and Y. Li, Chin. J. Catal., 2013, 34, 1964 CrossRef CAS.
- L. Qina, Z. Zengb, G. Zenga, C. Laia, A. Duana, R. Xiaob and D. Huanga, Appl. Catal., 2019, 259, 1180 Search PubMed.
- X. Jia, J. Wang and E. Wang, Adv. Mater., 2024, 36, 2309261 CrossRef CAS PubMed.
- M. Sajjadi, S. Iravani, R. S. Varma and N. M. Nasrollahzadeh, Nanomaterials, 2020, 10, 1784 CrossRef PubMed.
- N. Maji and H. S. Dosanjh, Magnetochemistry, 2023, 9, 156 CrossRef CAS.
- M. Amiri, K. Eskandari and M. S. Niasari, Adv. Colloid Interface Sci., 2019, 271, 101982 CrossRef CAS PubMed.
- W. Huang, S. Pan, Q. Yu, X. Liu, Y. Liu and R. Liu, J. Inorg. Organomet. Polym. Mater., 2019, 29, 1755 CrossRef CAS.
- E. Leal, S. T. Basílio, J. Dantas, P. Richa, R. da C. Lima, R. H. G. A. Kiminami and A. C. F. de M. Costa, Arabian J. Chem., 2020, 13, 8100 CrossRef CAS.
- S. S. Hossain and P. K. Roy, J. Mater. Sci.:Mater. Electron., 2017, 28, 18136 CrossRef CAS.
- S. T. Fardood, A. Ramazani, Z. Golfar and S. W. Joo, Appl. Organomet. Chem., 2017, 31, 3823 CrossRef.
- P. Saha, S. Das and S. Sutradhar, J. Appl. Phys., 2018, 124, 045303 CrossRef.
- M. A. Dar, V. Verma, S. P. Gairola, W. A. Siddiqui, R. K. Singh and R. K. Kotnala, Appl. Surf. Sci., 2012, 258, 5342 CrossRef.
- J. Dantas, J. R. D. Santos, R. B. L. Cunha, R. H. G. A. Kiminami and A. C. F. M. Costa, Mater. Res., 2013, 16, 625 CrossRef CAS.
- L. Ma, L. Chen and S. Chen, Mater. Chem. Phys., 2009, 114, 692 CrossRef CAS.
- S. B. Madake, J. B. Thorat and K. Y. Rajpure, Sens. Actuators, A, 2021, 331, 112919 CrossRef CAS.
- B. I. Kharisov, H. V. R. Dias and O. V. Kharissova, Arabian J. Chem., 2019, 12, 1234 CrossRef CAS.
- S. B. Singh and P. K. Tandon, J. Energy Chem., 2014, 2, 106 Search PubMed.
- B. P. Rickard, M. Overchuk, G. Obaid, M. K. Ruhi, U. Demirci, S. E. Fenton, J. H. Santos, D. Kessel and I. Rizvi, J. Photochem. Photobiol., A, 2023, 99, 448 CrossRef CAS PubMed.
- S. T. Fardood, F. Moradnia, S. Heidarzadeh and A. Naghipour, Nanochem. Res., 2023, 8, 134 CAS.
- F. Alantari, S. Rezayati, A. Ramazani and M. R. Poor Heravi, Appl. Organomet. Chem., 2023, 37, e7064 CrossRef.
- S. T. Fardood, F. Moradnia and T. M. Aminabhavi, Environ. Pollut., 2024, 358, 124534 CrossRef PubMed.
- L. M. Nainwal, S. Tasneem, W. Akhtar, G. Verma, M. F. Khan, S. Parvez, M. Shaquiquzzaman, M. Akhter and M. M. Alam, Eur. J. Med. Chem., 2019, 164, 121 CrossRef CAS PubMed.
- X. Xu, Z. Guo, D. Zhu, J. Pan, C. Yang and S. Li, Process Saf. Environ. Prot., 2024, 183, 59 CrossRef CAS.
- A. Hajra, D. Kundu and A. Majee, J. Heterocycl. Chem., 2009, 46, 1019 CrossRef CAS.
- E. Kabir and M. Uzzaman, Results Chem., 2022, 4, 100606 CrossRef CAS.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- A. Chanderiya, R. Das, G. Naikoo, I. U. Hassan, S. K. Kashaw and S. Pandey, Chem.–Asian J., 2021, 33, 949 CAS.
- S. Rezayati, M. M. Moghadam, Z. Naserifar and A. Ramazani, Inorg. Chem., 2024, 63, 1652 CrossRef CAS PubMed.
- M. Kumar, A. K. Singh, V. K. Singh, R. K. Yadav, A. P. Singh and S. Singh, Coord. Chem. Rev., 2024, 505, 215635 CrossRef.
- W. Su, W. Tang and J. Li, J. Chem. Res., 2008, 3, 123 CrossRef.
- A. Chaskar, V. Vyavhare1, V. Padalkar1, K. Phatangare and H. Deokar, J. Serb. Chem. Soc., 2011, 76, 21 CrossRef CAS.
- J. X. Ji, L. Q. Qiu, C. W. Yip and A. S. C. Chan, J. Org. Chem., 2003, 68, 1589 CrossRef CAS PubMed.
- A. S. Dzunuzovic, N. I. Ilic, M. V Petrovic, J. D. Bobic, B. Stojadinovic, M. Z. Dohcevic and B. D. Stojanovic, J. Magn. Magn. Mater., 2015, 374, 245 CrossRef CAS.
- S. T. Fardood, A. Ramazani, M. Ayubi, M. F Moradnia, S. Abdpoura and R. Forootana, Chem. Methodol., 2019, 3, 519 Search PubMed.
- M. Lei, L. Ma and L. Hu1, Tetrahedron Lett., 2009, 50, 6393 CrossRef CAS.
- J. H. Forsberg, V. T. Spaziano, T. M. Balasubramanian, G. K. Liu, S. A. Kinsley, C. A. Duckworth, J. J. Poteruca, P. S. Brown and J. L. Miller, J. Org. Chem., 1987, 52, 1017 CrossRef CAS.
- O. Yavuz, M. K. Ram, M. Aldissi, P. Poddar and S. Hariharan, J. Mater. Chem., 2005, 15, 810 RSC.
- A. Rostamnejadi, H. Salamati and P. Kameli, J. Supercond. Novel Magn., 2012, 25, 1123 CrossRef CAS.
- K. Praveena and K. Sadhana, Int. J. Sci. Res. Publ., 2015, 5, 1 Search PubMed.
- A. B. Mapossa, J. Dantas, M. R. Silva, R. H. Kiminami, A. C. F. Costa and M. O. Daramola, Arabian J. Chem., 2020, 13, 4462 CrossRef CAS.
- R. Thejas, T. L. Saundariya, G. Nagaraju, K. Swaroop, S. C. Prashantha, M. Veena, E. Melagiriyappa and C. S. Naveen, Mater. Lett.:X, 2022, 15, 10015 Search PubMed.
- C. S. M. Havan, M. K Babrekar, S. S. More and K. M. Jadhav, J. Alloys Compd., 2010, 507, 21 CrossRef.
- J. Massoudi, M. Smari, K. Nouri, E. Dhahri, K. Khirouni, S. Bertaina, L. Bessais and E. K. Hill, RSC Adv., 2020, 10, 34556 RSC.
- S. Toh, C. B. Mcauly, K. Tschulik, M. Uhlemann, A. Crossley and R. C. Compton, Nanoscale, 2013, 5, 4884 RSC.
- R. Sharmal, D. P. Bisen, U. Shukla and B. G. Sharma, Recent Res. Sci. Technol., 2012, 4, 77 Search PubMed.
- A. Kocjan, M. Logar and Z. Shen, Sci. Rep., 2017, 7, 2541 CrossRef PubMed.
- H. Yin, P. S. Casey, M. J. McCall and M. Fenech, Langmuir, 2010, 26, 15399 CrossRef CAS PubMed.
- R. K. Singh, R. Bala and S. Kumar, Indian J. Chem., 2016, 55, 381 Search PubMed.
- H. Moghanian, A. Mobinikhaledi, A. G. Blackman and E. S. Farahani, RSC Adv., 2014, 5, 2816 Search PubMed.
- J. W. M. Crawley, I. E. Gow, N. Lawes, I. Kowalec, L. Kabalan, C. R. A. Catlow, A. J Logsdail, S. H Taylor, N. F. Dummer and G. J. Hutchings, Chem. Rev., 2022, 122, 6795 CrossRef CAS PubMed.
- A. R. Liandi, A. H. Cahyana, A. J. F. Kusumah, A. Lupitasari, D. N. Alfariza, R. Nuraini, R. W. Sari and F. C. Kusumasari, Case Stud. Chem. Environ. Eng., 2023, 7, 100303 CrossRef CAS.
- P. Li and H. C. Zeng, ACS Appl. Mater. Interfaces, 2016, 8, 29551 CrossRef CAS PubMed.
- S. Chaturvedi, P. N. Dave and N. K. Shah, J. Saudi Chem. Soc., 2012, 16, 307 CrossRef CAS.
- A. Chaudhary, Mol. Diversity, 2021, 25, 1211 CrossRef CAS PubMed.
- F. Passamani, I. A. Guerra, P. R. Filgueiras, B. A. M. C. Santos and A. de Silva Goncalves, Orbital:Electron. J. Chem., 2022, 14, 168 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05486e |
| This journal is © The Royal Society of Chemistry 2025 |