Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
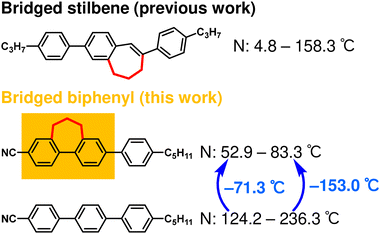
Innovative molecular design of bridged biphenyls for calamitic nematic liquid crystals with extensive π-conjugated mesogens†
Yoshimichi
Shimomura
a,
Yuuto
Iida
a,
Eiji
Tsurumaki
 b and
Gen-ichi
Konishi
b and
Gen-ichi
Konishi
 *a
*a
aDepartment of Chemical Science and Engineering, Institute of Science Tokyo, Ookayama, Meguro-ku, Tokyo 152-8552, Japan. E-mail: konishi.g.aa@m.titech.ac.jp
bDepartment of Chemistry, Institute of Science Tokyo, Ookayama, Meguro-ku, Tokyo 152-8552, Japan
First published on 14th February 2025
Abstract
To develop advanced materials based on calamitic nematic liquid crystals, it is essential to design functional optoelectronic mesogens that can form nematic phases at low temperatures. This study proposes a new molecular design strategy for low-temperature nematic liquid crystals using large π-conjugated mesogens with optical/electrical functions. Bridged biphenyls were synthesized by bridging the two phenyl rings with propylene. This bridging structure reduced the molecular planarity and prevented the molecules from aligning neatly in one direction, resulting in lowering the temperature range of the nematic phases. Terphenyl and phenyltolane derivatives exhibited supercooled nematic phases at room temperature, while quarterphenyl and bis(phenylethynyl)-biphenyl derivates exhibited nematic phases below 100 °C. The proposed design is more effective for rigid mesogens compared to conventional calamitic nematic liquid crystal design.
Introduction
Since their discovery, calamitic nematic liquid crystals (NLCs) have been intensively investigated owing to their unique properties, including electrical, magnetic, and optical anisotropy, as well as high fluidity.1 In recent years, new nematic phases as the chiral nematic (cholesteric),2–6 twist-bend,7–10 biaxial,11 blue,12–14 splay nematic phases,15 and ferroelectric structures16 have been investigated. In addition, there has been the development of unique liquid crystal molecules which have a nematic phase.17–28 From a materials viewpoint, calamitic NLCs are used in liquid crystal displays29,30 and other functional materials, such as optical films31–34 and distributed-feedback lasers.35–39 These applications exploit the anisotropy and fluidity of liquid crystals. Additionally, molecules with optical/electrical functions within the liquid crystals are also gaining significant attention, leading to the development of calamitic NLCs with large π-conjugated systems.40–45 More sophisticated materials and devices, such as dye-doped high-speed fluorescent switching devices46–51 and liquid-crystalline organic semiconductors,52–55 are currently being studied. In general, the nematic phase temperature range of π-conjugated liquid crystals with optical/electrical functions is often too high owing to the high melting point of the mesogen itself and strong intermolecular interactions resulting from their rigid skeletons and functional groups.56–58 Extending the hydrocarbon chains in the tail to lower this temperature range tends to promote the formation of smectic phases.59,60The most common approach to addressing this issue is to introduce fluorine into the lateral position of mesogens.61 This method has been effective, as some terphenyl and phenyltolane molecules form a nematic phase below 100 °C due to fluorine incorporation.23,40 However, introducing fluorine can sometimes alter the electronic states of the original molecules, significantly changing their optical and electrical properties.24,61–63 Other molecular design strategies include introducing a substituent larger than fluorine and using branch chains in the tail.64–67 Nevertheless, a new molecular design strategy that can be applied to various mesogens with optical and electrical functions is required for further development of calamitic NLCs.
Very recently, we reported π-extended bridged stilbenes that exhibited nematic phases at near room temperature with luminescence properties.68 Bridged stilbenes have the 7-membered ring structure that connects the vinyl group and the phenyl ring of stilbenes with a propylene group, referred to as the ‘bridging structure.’ The flexible bridging structure promoted the intramolecular rotation and the bending motion of the π-conjugated skeletons, enabling the formation of various conformations. This increased the phase-transition entropies and contributed to the lowering of the temperature range of the nematic phase.
In this study, we synthesized bridged biphenyls by introducing a bridging structure to biphenyls, which are more common mesogens compared to stilbenes (Fig. 1).
Additionally, we developed biphenyl, terphenyl, quarterphenyl, phenyltolane, and bis(phenylethynyl)-biphenyl-type molecules. Terphenyl- and phenyltolane-type molecules that formed a nematic phase, even at room temperature, were obtained. Additionally, we achieved nematic phases below 100 °C with quarterphenyl and bis(phenylethynyl)-biphenyl molecules. The effect of the bridging structure in the bridged biphenyl also investigated and found to differ from that in the bridged stilbene. Despite this difference, the molecular design strategy of introducing a bridging structure proved superior to previous strategies in terms of lowering the nematic phase temperature range and applicability to large π-conjugated molecules.
Results and discussion
Synthesis and characterization
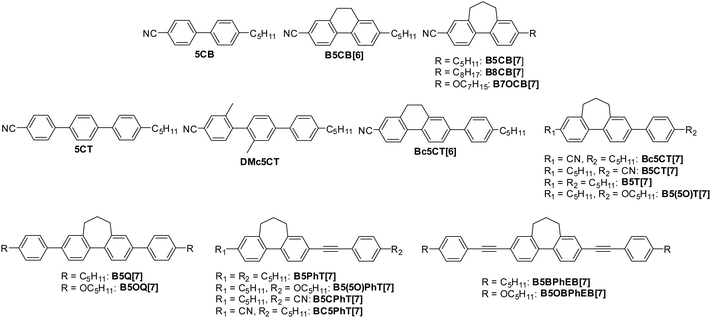
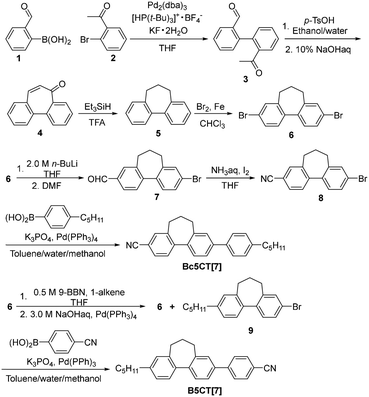
We synthesized biphenyl, terphenyl, quarterphenyl, phenyltolane, and bis(phenylethynyl)-biphenyl derivatives containing bridged biphenyl by introducing alkyl cyclic structure into biphenyl. For comparison, we synthesized a terphenyl derivative with two methyl groups introduced into the mesogen. The chemical structures of the molecules used in this study are shown in Fig. 2. The synthesis procedures for seven-membered bridged biphenyl, Bc5CT[7], and B5CT[7] are illustrated in Scheme 1, and those for the other final products are shown in Schemes S3–S5 (ESI†).Seven-membered bridged biphenyl was synthesized from 2-formylphenylboronic acid (1) and 2′-bromoacetophenone (2) using Suzuki–Miyaura cross-coupling reaction, intermolecular aldol condensation and reduction by triethylsilane in a trifluoro acetate solution.69 The cyano group was introduced through the formylation of the bromo group, followed by treatment with iodine and ammonia.70 The alkoxy group was formed by first converting the bromo group to a boronate, then reducing it with m-chloroperoxybenzoic acid, and finally performing a Williamson ether reaction. Alkylation of the bromo group was achieved using a B-alkyl Suzuki–Miyaura cross-coupling reaction with 9-BBN.71 Terphenyl and quarterphenyl derivatives were synthesized from the corresponding bridged biphenyl derivatives and arylboronic acids via Suzuki–Miyaura cross-coupling.72–74 Phenyltolane and bis(phenylethynyl)biphenyl derivatives were synthesized from the corresponding bridged biphenyl derivatives and 4-ethynyl aryl via Sonogashira cross-coupling.24
The synthesized compounds were identified using 1H-NMR, 13C-NMR, and HR-EI spectroscopies. FT-IR spectroscopy was used for molecules with cyano groups.
Phase transition behaviours
The phase transition behaviors of the synthesized compounds were evaluated using DSC and POM. Using POM, we observed the phase transition of the compounds during cooling and heating at a rate of 10 °C min−1 for a molten sample sandwiched between untreated glass plates. The phase transition temperatures and enthalpies of synthesized molecules, 5CB, and 5CT during the second heating/cooling process are listed in Table 1. The bar graphs of the phase transition temperatures and ranges are shown in Fig. 3. The DSC thermograms are provided in Fig. 4a, 5a, and ESI.†| Entry | Phase transition temperature [°C] (enthalpy [kJ mol−1]) | |
|---|---|---|
| Heating | Cooling | |
| a There were no peak in DSC thermogram and no phase transition in POM observation at a rate of 10 °C min−1, but B8CB[7] and B7OCB[7] showed a smectic C and a crystalline phase at room temperature after a few days in POM observation, respectively. On the other hand, B5CT[7] exhibited an isotropic–nematic phase transition after a few minutes and a gradual nematic–crystalline phase transition after a few more days at room temperature. b Phase transition temperatures were determined by POM. | ||
| 5CB | N 35.8 (0.6) Iso | Iso 33.8 (−0.6) N |
| B5CB[6] | SmC 5.6 (0.4) Iso 31.5 (—) Cry 43.4 (—) Iso | Iso 3.7 (−0.4) SmC |
| B5CB[7] | — | — |
| B8CB[7] | — | —a |
| B7OCB[7] | — | —a |
| 5CT | Cry1 105.6 (0.8) Cry2 130.9 (10.6) N 238.2 (1.2) Iso | Iso 236.3 (−1.1) N 126.8, 124.2 (−10.0) Cry1 |
| DMc5CT | Cry1 −5.9 (−0.5) Cry2 84.0 (23.7) Iso | Iso 9.3 (−15.4) Cry1 |
| Bc5CT[6] | N 43.6, 59.0 (−18.6) Cry 113.5 (28.3) N 208.4 (1.0) Iso | Iso 206.6 (−0.9) N |
| Bc5CT[7] | Cry1 36.0 (−7.7) Cry2 67.6 (−3.2) Cry3 120.7 (28.7) Iso | Iso 83.3 (−1.4) N 52.9 (−3.8) Cry1 |
| B5CT[7] | — | —a |
| B5T[7] | N 10.9 (0.25) Iso | Iso 8.9 (−0.2) N |
| B5(5O)T[7] | N 18.1 (−15.8) Cry 50.2 (21.5) N 59.7, 61.6 (2.2) Iso | Iso 57.6 (−0.5) N |
| B5Q[7] | Cry 142.6 (32.2) N 178.4 (1.0) Iso | Iso 176.3 (−1.1) N 82.4 (−26.3) Cry |
| B5OQ[7] | Cry 164.6 (40.9) N 229.2 (1.3) Iso | Iso 227.3 (−1.3) N 136.4 (−41.1) Cry |
| B5PhT[7] | N 49.2 (0.9) Iso | Iso 47.0 (−1.0) N |
| B5(5O)PhT[7] | N 36.2 (−23.2) Cry 78.9 (31.7) N 94.6 (1.4) Iso | Iso 92.5 (−2.0) N |
| BC5PhT[7] | Cry 132.2 (25.9) Iso | Iso 130.7 (−1.2) N 115.6 (−22.9) Cry |
| B5CPhT[7] | N 101.1 (0.3), 104.9 (0.6) Iso | Iso 102.4 (0.9) N |
| B5BPhEB[7] | Cry 120 N 240 Iso | Iso 237 N 52 Cry |
| B5OBPhEB[7] | Cry 150 N 260 Iso | Iso 254 N113 Cry |
 | ||
| Fig. 3 Schematic diagram showing the phase transition temperatures and ranges of the compounds, except for B5CB[7], B8CB[7], B7OCB[7], and B5CT[7]; upper bar: upon heating, lower bar: upon cooling. | ||
 | ||
| Fig. 4 (a) DSC thermograms of DMc5CT at a rate of 10 °C min−1 upon 2nd heating and cooling, and POM images of DMc5CT at (b) 0 °C during cooling and (c) room temperature left for a few days. | ||
None of the seven-membered bridged biphenyl derivatives exhibited phase transitions in the DSC thermograms (Fig. S4, S5, and S7, ESI†). B5CB[7] maintained a dark field, B8CB[7] formed a schlieren texture (Fig. S6, ESI†), and B7OCB[7] crystallized when each prepared slide was left at room temperature for a few days (Fig. S8, ESI†). The schlieren texture of B8CB[7] exhibited no fluidity; thus, this phase is smectic C.
Bridged biphenyl derivatives did not show a nematic phase, unlike 5CB. These results were attributed to multiple factors, such as the steric hindrance of the bridging moiety, decrease in the planarity of the biphenyl, and absence of a biaryl rotational axis.
Bc5CT[6] formed an enantiotropic nematic phase. The DSC thermograms exhibited two exothermic peaks at 43.6 and 59.0 °C and two endothermic peaks at 113.5 and 208.4 °C during heating, while only one exothermic peak was observed at 206.6 °C during cooling (Fig. S10, ESI†). POM observations revealed that Bc5CT[6] exists in a nematic phase (Fig. S11b, ESI†). A gradual nematic-to-crystalline phase transition was observed at 40 °C (Fig. S11c, ESI†). The crystallization rate of Bc5CT[6] was lower than that of 5CT. In particular, the lower temperature limit of the nematic phase during cooling was significantly reduced.
In contrast, Bc5CT[7] formed a monotropic nematic phase, showing two exothermic peaks and one endothermic peak during heating and two exothermic peaks during cooling in the DSC thermograms (Fig. 5a). In POM, a nematic phase was observed between the two exothermic peaks during cooling (Fig. 5d). Moreover, unlike Bc5CT[6], Bc5CT[7] exists in at least three different crystalline phases (Fig. 5b, c and e). The isotropic-to-nematic and the nematic-to-crystalline phase transition temperatures of Bc5CT[7] are below at 153.0 °C and 71.5 °C compared to those of 5CT, respectively.
B5CT[7], with a different bridge position from Bc5CT[7], exhibited no phase transition peak during heating at a rate of 10 °C min−1 (Fig. S12, ESI†). When the prepared slide of the once-melted B5CT[7] was left at room temperature for a few minutes, the isotropic–nematic phase transition proceeded, and the nematic–crystalline phase transition developed gradually after a few more days (Fig. S13, ESI†). This indicates that steric hindrance of the bridging moiety inhibit crystallization. The crystallized B5CT[7] transitioned to an isotropic phase at 86.7 °C upon heating at a rate of 10 °C min−1 in POM.
The diversity of crystalline systems due to the bridging structure and the associated significant reduction in the phase transition temperatures occurred, which is also common to the stilbene and distyrylbenzene dyes we have previously reported.68,75–78 This result suggests that the molecular design strategy of the seven-membered bridging is effective for the development of calamitic liquid crystals with a nematic phase near room temperature. The effect of the bridging structure in the bridged biphenyl is discussed later.
B5(5O)T[7] also exhibited an enantiotropic nematic phase, with one exothermic peak and three endothermic peaks during heating. Moreover, it exhibited one exothermic peak during cooling in the DSC thermograms (Fig. S16, ESI†) and a schlieren texture (Fig. S17b and c, ESI†). B5(5O)T[7] maintained the nematic phase after cooling to 0 °C at a rate of 10 °C min−1 and underwent cold crystallization upon heating to 20 °C, as indicated by a broad peak, indicating the remarkable effect of the seven-membered bridging structure. Prepared slide of B5(5O)T[7] transitioned from the nematic to the crystalline phase when left at room temperature for a few minutes (Fig. S17a, ESI†). There were two exothermic peaks near the nematic to isotropic phase transition during heating, indicating that a nematic–nematic phase transition would occur. However, we could not observe any event in POM and could not conduct the WAXD measurement in this temperature range as it was only 1.9 °C. Therefore, this is not discussed in detail.
Regardless of the substituents, the seven-membered bridging structure contributed significantly to the reduction in the temperature range of the nematic phase. The results of applying this molecular design strategy to even larger π-conjugated molecules are presented below.
B5(5O)PhT[7] exhibited an enantiotropic nematic phase with phase transition behavior similar to B5(5O)T[7] (Fig. S23, ESI†). The phase transition temperatures of B5(5O)PhT[7] increased by 18–35 °C compared to those of B5(5O)T[7]. In the POM observations, a schlieren texture was observed, and the nematic-to-isotropic phase transition of B5(5O)PhT[7] occurred over a period of 15 minutes at room temperature (Fig. S24, ESI†).
BC5PhT[7] formed a monotropic nematic phase, and its DSC thermograms exhibited an endothermic peak at 133 °C during heating. Additionally, two exothermic peaks at 131 and 116 °C during cooling were observed (Fig. S25, ESI†). Under POM, BC5PhT[7] exhibited a schlieren texture between the two exothermic peak temperatures (Fig. S26, ESI†).
On the other hand, B5CPhT[7] exhibited a nematic phase; the nematic ⇄ isotropic phase transition temperatures during heating and cooling were 105 and 103 °C, respectively (Fig. S27, ESI†). Moreover, the nematic-to-nematic phase transition was also observed on heating in the DSC chart. However, this is not discussed in detail for the same reason as for B5(5O)T[7]. While B5CPhT[7] did not show any crystallization peak in the DSC measurement despite cooling to 0 °C, the prepared B5CPhT[7] crystallized when left at room temperature for a few minutes (Fig. S28, ESI†). Although the crystallization temperature was not determined, this crystalline phase melted at 100.0 °C and transitioned to the nematic phase.
Unlike B5CPhT[7], where the bridge is located near the pentyl group, BC5PhT[7], where the bridge is situated near the cyano group, crystallized on a time scale of 10 °C min−1. This trend was also observed for terphenyl derivatives. This can be attributed to the significantly increased flexibility of the molecules due to the proximity of the pentyl chains and bridging structures. The cause of this phenomenon remains unclear, and the molecular alignments were not determined. This will be explored in future investigations.
The bis(phenylethynyl)-biphenyl derivatives transitioned from colorless to brown upon heating to their isotropic phases. In particular, B5BPhEB[7] exhibited an enantiotropic nematic phase, displaying a schlieren texture at 120–240 °C and 237–52 °C during heating and cooling, respectively (Fig. 6c and Fig. S29, ESI†). B5OBPhEB[7] also exhibited an enantiotropic nematic phase at 150–260 °C and 254–113 °C during heating and cooling, respectively (Fig. S30, ESI†).
The use of the seven-membered bridging molecular design strategy has enabled these large π-conjugated molecules to successfully form nematic phases at temperatures below 100 °C, and some terphenyl and phenyltolane derivatives exhibited nematic phases at room temperature. Even in very rigid skeletons such as quaterphenyl and bis(phenylethynyl)-biphenyl, this strategy was effective in lowering the nematic phase temperatures.
Birefringence
To investigate the effect of the bridge structure on the birefringence, we measured the temperature dependence of birefringence (Δn) for 5CT and Bc5CT[7] in the nematic phases, using the method described in our previous report.79–81 However, Bc5CT[7] crystallized during the measurement process, thus we conducted measurements for quarterphenyl and bis(phenylethynyl)-biphenyl derivatives, whose mesogens consist of large π-conjugated skeletons.This study followed the general practice of indexing the temperature dependence properties in nematic phases to the reduced temperature (TIN − T), where TIN is the isotropic-to-nematic phase transition temperature, and T is the measurement temperature. Fig. 7a compares the dependence of the Δn at 550 nm on TIN − T for 5CT, B5Q[7], B5OQ[7], B5BPhEB[7], and B5OBPhEB[7]. Quarterphenyl and bis(phenylethynyl)-biphenyl derivatives with the bridging structure exhibited larger Δn than that of 5CT at the same TIN − T value, despite 5CT having a cyano group at the end. Among the same mesogens, B5OQ[7] and B5OBPhEB[7] exhibited larger Δn than that of B5Q[7] and B5BPhEB[7], respectively, owing to the polarizability of terminal groups in the following order: alkoxy group > alkyl group. In addition, these results were applied to the empirical Haller's equation82 for further detailed analysis in nematic phases. The empirical Haller's equation is as follows;
| S = Δn/Δn0 |
| Δn = Δn0 (1 − T/TIN)β |
 | ||
| Fig. 7 (a) Δn and (b) ΔS of 5CT (●), B5Q[7] (▲), B5OQ[7] (△), B5BPhEB[7] (■), and B5OBPhEB[7] (□) at each TIN − T. | ||
Photophysical properties
The photophysical properties of B5PhT[7] and B5(5O)PhT[7], which exhibit nematic phases at room temperature, were measured in dilute solution, their solid states, and nematic phases (Table 3). Their absorption and fluorescence spectra are depicted in Fig. 8.| Entry | State | ε [M−1 cm−1] | λ abs [nm] | λ fl [nm] |
|---|---|---|---|---|
| a Excited at 304 nm for B5PhT[7]. b Excited at 309 nm for B5(5O)PhT[7]. | ||||
| B5PhT[7] | THF | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
304 (max), 321 | 340, 354 (max) |
| Solid | — | — | 365 (max), 408 | |
| Nematic phase | — | — | 353, 406 (max) | |
| B5(5O)PhT[7] | THF | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
309 (max), 326 | 346 (shoulder), 359 |
| Solid | 380, 394 (shoulder) | |||
| Nematic phase | 375 | |||
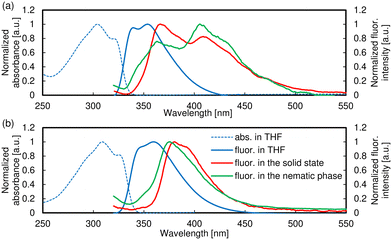 | ||
| Fig. 8 Absorption (dashed line) and fluorescence (solid line) spectra of (a) B5PhT[7] and (b) B5(5O)PhT[7]. | ||
The absorption spectrum of B5PhT[7] in THF solution exhibits a vibrational structure with peaks (the absorption wavelength, λabs) at 304 nm (max) and 321 nm (Fig. 8a). As indicated in a previous report,83 the peaks are derived from the S0 → S1 transition. The molar absorption coefficient (ε) was 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. The fluorescence spectrum of B5PhT[7] in THF also shows a vibrational structure, and the fluorescence wavelengths (λfl) are 346 nm and 359 nm (max). The λfl of B5PhT[7] in the solid state are 365 nm and 408 nm, and the longer λfl is red-shifted by 50 nm compared to that in THF. The λfl values in the nematic phase are almost identical to those in the solid state, 363 nm and 406 nm. On the other hand, the maximum λfl values in these states are different, which are 365 nm and 406 nm in the solid and nematic phases, respectively.
000 M−1 cm−1. The fluorescence spectrum of B5PhT[7] in THF also shows a vibrational structure, and the fluorescence wavelengths (λfl) are 346 nm and 359 nm (max). The λfl of B5PhT[7] in the solid state are 365 nm and 408 nm, and the longer λfl is red-shifted by 50 nm compared to that in THF. The λfl values in the nematic phase are almost identical to those in the solid state, 363 nm and 406 nm. On the other hand, the maximum λfl values in these states are different, which are 365 nm and 406 nm in the solid and nematic phases, respectively.
Both of the absorption/fluorescence spectra of B5(5O)PhT[7] in THF solution exhibit a vibrational structure similar to those of B5PhT[7] (Fig. 8b). The λabs values are 304 nm and 321 nm (max), and λfl values are 346 nm (shoulder) and 359 nm, all of which are red-shifted by 5 nm compared to those of B5PhT[7]. This can be attributed to the narrowing of the HOMO–LUMO gap by the electro-donating alkoxy group. The fluorescence spectrum in the solid state has a maximum peak at 380 nm and shoulder at 394 nm and that in the nematic phase only shows one peak at 375 nm, which is different from that of B5PhT[7]. The increase in the electronic molecular interaction owing to the alkoxy group is likely to have contributed to this result.
B5PhT[7] exhibits different fluorescence behavior in THF solution, in both the solid and nematic phases. To conduct detailed investigations, we first measured the absorption/fluorescence spectrum of the polymethacrylate (PMMA, Mn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) cast film doped with 0.1 wt% B5PhT[7]; however, they are almost the same as those in THF solution (Fig. S36, ESI†). Subsequently, a B5PhT[7] agglomeration experiment in a THF/water system was performed, and the aggregation of B5PhT[7] could be observed in ≤30% THF suspension. The absorption spectra in ≤30% THF suspension exhibit the decrease in ε and the slight red-shifted longer-wavelength peak (Fig. 9a). The fluorescence spectra in 20% and 30% THF solution also changed slightly, and in THF 10% solution, a fluorescence peak appears at 394 nm, with a spectrum similar to a superposition of those in the THF solution and nematic phase (Fig. 9b). These results suggest that the fluorescence peak near 394 nm was caused by intermolecular interactions. Finally, the fluorescence lifetimes of B5PhT[7] in the THF solution, solid state, and nematic phase were measured. Unfortunately, the lifetime in the THF solution could not be measured because it was very short (<1.0 ns) and we did not have the proper wavelength cutting filter or LED laser. However, one of the components in the solid and nematic phase exhibited long lifetimes (solid: 6.8 ns, nematic phase: 12.8 ns), complementing the presence of the fluorescence peak resulting from intermolecular interactions (Table S1 and Fig. S37, ESI†). A single-crystal X-ray structure analysis is effective for further investigations of the fluorescence behavior in the solid and nematic phases, but the single crystal of B5PhT[7] was not obtained.
000) cast film doped with 0.1 wt% B5PhT[7]; however, they are almost the same as those in THF solution (Fig. S36, ESI†). Subsequently, a B5PhT[7] agglomeration experiment in a THF/water system was performed, and the aggregation of B5PhT[7] could be observed in ≤30% THF suspension. The absorption spectra in ≤30% THF suspension exhibit the decrease in ε and the slight red-shifted longer-wavelength peak (Fig. 9a). The fluorescence spectra in 20% and 30% THF solution also changed slightly, and in THF 10% solution, a fluorescence peak appears at 394 nm, with a spectrum similar to a superposition of those in the THF solution and nematic phase (Fig. 9b). These results suggest that the fluorescence peak near 394 nm was caused by intermolecular interactions. Finally, the fluorescence lifetimes of B5PhT[7] in the THF solution, solid state, and nematic phase were measured. Unfortunately, the lifetime in the THF solution could not be measured because it was very short (<1.0 ns) and we did not have the proper wavelength cutting filter or LED laser. However, one of the components in the solid and nematic phase exhibited long lifetimes (solid: 6.8 ns, nematic phase: 12.8 ns), complementing the presence of the fluorescence peak resulting from intermolecular interactions (Table S1 and Fig. S37, ESI†). A single-crystal X-ray structure analysis is effective for further investigations of the fluorescence behavior in the solid and nematic phases, but the single crystal of B5PhT[7] was not obtained.
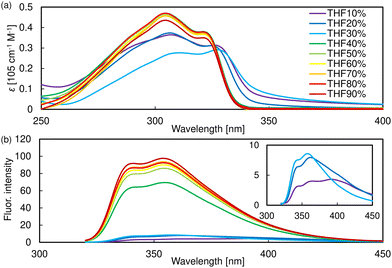 | ||
| Fig. 9 (a) Absorption and (b) fluorescence spectra of B5CT[7] in THF/water mixed solvent at 1.0 × 10−5 M. | ||
B5PhT[7] and B5(5O)PhT[7] show blue luminescence in THF, in their solid and nematic phases. They can be used as liquid crystalline and even optical materials. Furthermore, fluorescent liquid crystals show the potential to be applied for more advanced optoelectronics, such as electric-field-responsive fluorescent switch devices.68,84,85
Structural analysis
A single-crystal X-ray analysis of Bc5CT[7] was conducted (Fig. 10). Bc5CT[7] had two atropisomers, and it formed a γ structure. The torsional angle of the biaryl axis at the bridging part in both conformations was 46.8°, and the intermolecular aromatic carbon distance was 3.53 Å. In this single crystal, the molecular long axis was oriented in two different directions, forming an angle of about 60°. The single-crystal X-ray structure of 5CT was previously reported.86 In the single crystal, the molecule was determined to have only one conformation, and the molecular long axis was aligned in one direction (Fig. S38, ESI†). The torsional angle of the biaryl axis and intermolecular aromatic carbon distance of 5CT were 40.9° and 3.63 Å, respectively. | ||
| Fig. 10 (a) Molecular conformations and (b) the crystal structure of Bc5CT[7] (ESI†). | ||
To investigate the conformation of the bridged biphenyl in more detail, the optimized structure of BT[7] was calculated using density functional theory (DFT) at the B3LYP/6-311G(d) level87 (Fig. 11).
 | ||
| Fig. 11 (a) The chemical structure of BT[7], the conformations of (b) Opt-BT[7]-I and (c) Opt-BT[c]-II, and Ebar of (d) φ1 and (e) φ2. | ||
BT[7] showed two optimized structures (Fig. 11b and c). The torsional angle of φ1 of Opt-BT[7]-I and Opt-BT[7]-II were 47.8° and 47.3°, respectively, which were 6.6° and 6.1°, respectively, larger than that of biphenyl (φ1 = 41.2°). These structures were in an atropoisomeric relationship, in which the positions of Cα and Cβ relative to the paper plane were reversed, consistent with the results of single-crystal Bc5CT[7].
Calculations of Opt-BT[7]-I with φ1 varying in 5°-steps showed that the rotational energy barrier (Ebar) significantly changed around φ1 = 30° (Fig. 11d). Calculations performed for every 1° φ1 between 25–35° showed a sudden change at 28–29° (Fig. S43, ESI†). The maximum Ebar was 117.8 kJ mol−1, which was much larger than that of biphenyl (9.7 kJ mol−1). The scan calculation had two local minimum values at −50° and 45°, where the structures were almost identical to those of Opt-Bt[7]-I and Opt-BT[7]-II, respectively (Fig. S41, ESI†). The results of a set of calculations for varying φ2 are shown in Fig. 11e, and they exhibit a significant change of Ebar and two local minimum values, like φ1. The maximum Ebar of φ2 was about half that of φ1. Moreover, Ebar did not change much around φ2 = −20–30°. In this angular range, the change of φ1 was also suppressed (Fig. S45, ESI†). The bridging structure caused this behavior, resulting in the Ebar remaining almost constant. Conversely, the structures at each local minimum value were close to those of Opt-BT[7]-I and Opt-BT[7]-II (Fig. S42, ESI†). In these calculations, there were only two stable conformations of the seven-membered bridged biphenyl. This was different from the case of the bridged stilbenes, which had various conformations.68 The biaryl axis of the bridged biphenyl was more twisted compared to that of biphenyl. Considering the result of the single-crystal X-ray analysis (ESI†), the decrease in the intermolecular interaction due to the low molecular planarity and difficulty in aligning the molecular long axis in one direction contribute to the lower phase transition temperatures of bridged biphenyl derivatives. This mechanism is different from that of bridged stilbene derivatives, where the increase in the entropy from forming various conformations contributes significantly.
Conclusions
We synthesized biphenyl, terphenyl, quarterphenyl, phenyltolane, and bis(phenylethynyl)-biphenyl derivatives, incorporating a seven-membered bridged biphenyl structure in the mesogens. Except for the biphenyl-type compounds, all compounds formed nematic phases. The nematic phase temperature range of Bc5CT[7] was approximately 100 °C lower than that of 5CT, and it exhibited a nematic phase even at room temperature. In contrast, DMc5CT exhibited no liquid crystalline phase. Moreover, some terphenyl/phenyltolane skeleton molecules exhibited nematic phases at or below room temperature. B5Q[7] and B5BPhEB[7], whose mesogens are quarterphenyl and bis(phenylethynyl)-biphenyl, respectively, exhibited nematic phases even below 100 °C. These results indicate that the molecular design strategy of seven-membered bridging can potentially solve a long-standing challenge in the field of calamitic NLC research, which continues from Vorländer's research.88 In this study, we have focused on the phase transition behavior of calamitic molecules with only one bridging introduced. On the other hand, those of calamitic molecules with multiple bridging introduced and the effect of introducing the bridging structure into biphenyl on the nematic phases remain unexplored. We will investigate these unknowns in the future. In addition, functional materials, such as liquid crystalline organic semiconductors and advanced materials,89–100 will be developed using this molecular design strategy.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for Bc5CT[7] has been deposited at the CCDC under 2410034 and can be obtained from https://www.ccdc.cam.ac.uk/.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Yuki Sawatari (Department of Chemical Science and Engineering, Institute of Science Tokyo) for the birefringence measurements. We also thank Masato Koizumi (Materials Analysis Division, Institute of Science Tokyo) for the HRMS measurements. This division is independent of our laboratory to ensure fairness. YS thanks JSPS Research Fellowships for Young Scientists. This project was supported in part by MEXT/JSPS KAKENHI grants 24KJ1084 (YS), 23H02036 (GK), Iketani Science and Technology Foundation (YS), Murata Science and Education Foundation (GK). Tokyo Institute of Technology merged with Tokyo Medical and Dental University to form Institute of Science Tokyo (Science Tokyo) on October 1, 2024.Notes and references
- D. Demus, J. Goodby, G. W. Gray, H. W. Spiess and V. Vill, Physical Properties of Liquid Crystals, Wiley-VCH, Weinheim, 1999 Search PubMed.
- M. Mitov, Cholesteric Liquid Crystals with a Broad Light Reflection Band, Adv. Mater., 2012, 24, 6206–6276 CrossRef PubMed.
- G. Friedel, Les états mesomorphs de la matière, Ann. Phys., 1922, 9, 273–474 Search PubMed.
- M. Ozaki, Y. Matsuhisa, H. Yoshida, R. Ozaki and A. Fuji, Photonic crystals based on chiral liquid crystal, Phys. Status Solidi A, 2007, 204, 3777–3789 CrossRef CAS.
- Y. He, S. Lin, J. Guo and Q. Li, Circularly polarized luminescent self-organized helical superstructures: From materials and stimulus-responsiveness to applications, Aggregate, 2022, 3, e141 Search PubMed.
- J. Xiang, Y. Li, Q. Li, D. A. Paterson, J. M. D. Storey, C. T. Imrie and O. D. Lavrentovich, Electrically Tunable Selective Reflection of Light from Ultraviolet to Visible and Infrared by Heliconical Cholesterics, Adv. Mater., 2015, 27, 3014–3018 CrossRef CAS PubMed.
- M. Cestari, S. Diez-Berart, D. A. Dunmur, A. Ferrarinim, M. R. de la Fuente, D. J. B. Jackson, D. O. Lopez, G. R. Luckhurst, M. A. Perez-Jubindo, R. M. Richardson, J. Salud, B. A. Timimi and H. Zimmermann, Phase behavior and properties of the liquid-crystal dimer 1′′,7′′-bis(4-cyanobiphenyl-4′-yl) heptane: A twist-bend nematic liquid crystal, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 031704 CrossRef CAS PubMed.
- P. A. Henderson and C. T. Imrie, Methylene-linked liquid crystal dimers and the twist-bend nematic phase, Liq. Cryst., 2011, 38, 1407–1414 CrossRef CAS.
- R. J. Mandle, The dependency of twist-bend nematic liquid crystals on molecular structure: a progression from dimers to trimers, oligomers and polymers, Soft Matter, 2016, 12, 7883–7901 RSC.
- Y. Arakawa, K. Komatsu and H. Tsuji, Twist-bend nematic liquid crystals based on thioether linkage, New J. Chem., 2019, 43, 6786–6793 RSC.
- R. J. Mandle, N. Sebastián, J. Martinez-Perdiguero and A. Mertelj, On the molecular origins of the ferroelectric splay nematic phase, Nat. Commun., 2021, 12, 4962 CrossRef CAS PubMed.
- H. Coles and M. Pivnenko, Liquid crystal ‘blue phases’ with a wide temperature range, Nature, 2005, 436, 997–1000 CrossRef CAS PubMed.
- H. Kikuchi, M. Yokota, Y. Hisakado, H. Yang and T. Kajiyama, Polymer-stabilized liquid crystal blue phases, Nat. Mater., 2002, 1, 64–68 CrossRef CAS PubMed.
- J. Yang, W. Zaho, W. He, Z. Yang, D. Wang and H. Cao, Liquid crystalline blue phase materials with three-dimensional nanostructures, J. Mater. Chem. C, 2019, 7, 13352–13366 RSC.
- A. Mertelj, L. Cmok, N. Sebastián, R. J. Mandle, R. R. parker, A. C. Whitwood, J. W. Goodby and M. Čopič, Splay Nematic Phase, Phys. Rev. X, 2018, 8, 041025 CAS.
- R. J. Mandle, N. Sebastián, J. Martinez-Perdiguero and A. Mertelj, On the molecular origins of the ferroelectric splay nematic phase, Nat. Commun., 2021, 12, 4962 CrossRef CAS PubMed.
- S. Kumar and A. N. Gowda, The chemistry of bent-core molecules forming nematic liquid crystals, Liq. Cryst. Rev., 2015, 3, 99–145 CrossRef CAS.
- N. Sebastián, M. Čopič and A. Mertelj, Ferroelectric nematic liquid-crystalline phases, Phys. Rev. E, 2022, 106, 021001 CrossRef PubMed.
- T. Ghosh and M. Lehmann, Recent advances in heterocycle-based metal-free calamitics, J. Mater. Chem. C, 2017, 5, 12308–12337 RSC.
- R. Cai and E. T. Sanyksju, New thermotropic liquid crystals derived from thiophenes, Liq. Cryst., 1991, 9, 616–674 CrossRef.
- P. Kirsch and M. Bremer, Nematic Liquid Crystals for Active Matrix Displays: Molecular Design and Synthesis, Angew. Chem., Int. Ed., 2000, 39, 4216–4235 CrossRef CAS PubMed.
- S. A. Hudson and P. M. Maitlies, Calamitic Metallomesogens: Metal-Containing Liquid Crystals with Rodlike Shapse, Chem. Rev., 1993, 93, 861–885 CrossRef CAS.
- R. Dabrowski, P. Kula and J. Herman, High Birefringence Liquid Crystals, Crystals, 2013, 3, 443–482 CrossRef CAS.
- Y. Iida, Y. Shimomura, M. Tokita and G. Konishi, Push-Pull biphenyl and tolane derivatives as novel luminescent liquid crystals: synthesis and properties, Liq. Cryst., 2024, 51, 2032–2045 CrossRef CAS.
- Y. Arakawa, Q. Ning, S. Karthick and S. Aya, Sulfur-based ferroelectric nematic liquid crystals, J. Mater. Chem. C, 2024, 12, 16206–16217 RSC.
- Y. Arakawa, S. Kang, J. Watanabe and G. Konishi, Assembly of thioether-containing rod-like liquid-crystalline materials assisted by hydrogen-bonding terminal carboxyl groups, RSC Adv., 2015, 5, 8056–8062 RSC.
- S. Brown, E. Cruickshank, J. M. D. Storey, C. T. Imrie, D. Pociecha, M. Majewska, A. Makal and E. Gorecka, Multiple Polar and Non-polar Nematic Phases, ChemPhysChem, 2021, 22, 2506–2510 CrossRef CAS PubMed.
- V. Borshch, Y.-K. Kim, J. Ziang, M. Gao, A. Jákli, V. P. Panov, J. K. Vij, C. T. Imrie, M.-G. Tambe, G. H. Mehl and O. D. Lavrentovich, Nematic twist-bend phase with nanoscale modulation of molecular orientation, Nat. Commun., 2013, 4, 2635 CrossRef CAS PubMed.
- G. H. Heilmeier, L. A. Zanoni and L. A. Barton, Gest-host interactions in nematic liquid crystals. A new electro-optic effect, Appl. Phys. Lett., 1968, 13, 91–92 CrossRef CAS.
- G. H. Heilmeier, Liquid crystal displays: An experiment in interdisciplinary research that worked, IEEE Trans. Electron Devices, 1976, 23, 780–785 Search PubMed.
- R. G. Horn, J. N. Israelachvili and E. Perez, Forces due to structure in a thin liquid crystal film, J. Phys., 1981, 42, 39–52 CrossRef CAS.
- D. K. Sahu, S. Kol, S. Ramaswamy and S. Dhara, Omnidirectional transport and navigation of Janus particles through a nematic liquid crystal film, Phys. Rev. Res., 2020, 2, 032009 CrossRef CAS.
- A. Nych, J. Fukuda, U. Ognysta, S. Žumer and I. Muševič, Spontaneous formation and dynamics of half-skyrmions in a chiral liquid-crystal film, Nat. Phys., 2017, 13, 1215–1220 Search PubMed.
- I. Gharb, V. Palacio-Betancur, H. Ayeb, D. Demaile, J. J. de Pable, R. D. Kamien and E. Lacaze, Liquid Crystal Films as Active Substrates for Nanoparticle Control, ACS Appl. Nano Mater., 2021, 4, 6700–6708 Search PubMed.
- H. Coles and S. Morris, Liquid-crystal lasers, Nat. Photonics, 2010, 4, 676–685 CrossRef CAS.
- M. Uchimura, Y. Watanabe, F. Araoka, J. Watanabe, H. Takezoe and G. Konishi, Development of laser dyes to realize low threshold in dye-doped cholesteric liquid crystal lasers, Adv. Mater., 2010, 22, 4473–4478 CrossRef CAS PubMed.
- X. Zhan, F.-F. Xu, Z. Zhou, Z. Zhou, Y. Yan, J. Yao and Y. S. Zhao, 3D Laser Displays Based on Circularly Polarized Lasing from Cholesteric Liquid Crystal Arrays, Adv. Mater., 2021, 33, 2104418 CrossRef CAS PubMed.
- T. M. Sarukhanyan, H. Gharagulyan, M. L. Sargsan, H. Grigoryan, R. S. Hakobyan, A. H. Gevorgyan and R. B. Alaverdyan, Lasing peculiarities in cholesteric multilayer structure with dye-doped polymer film depending on the concentration of laser dye and pumping energy, Mol. Cryst. Liq. Cryst., 2020, 713, 15–25 CrossRef CAS.
- S. Y. Cho, H. Yoshida and M. Ozaki, Emission Direction-Tunable Liquid Crystal Laser, Adv. Opt. Mater., 2020, 8, 200375 Search PubMed.
- J. Dziaduszek, R. Dabrowski, S. Urban, K. Garbat, A. Glushchenko and K. Czupryński, Selected fluorosubstituted phenyltolanes with a terminal group: NCS, CN, F, OCF3 and their mesogenic and dielectric properties and se for the formulation of high birefringence nematic mixtures to GHz and THz applications, Liq. Cryst., 2017, 44, 1277–1292 CrossRef CAS.
- M. Echeverri, I. Martín, A. Concellón, C. Ruize, M. S. Anselmo, E. Gutiérrez-Puebla, J. L. Sarrano and B. Gómez-Lor, Fluorescent and Electroactive Monoalkyl BTD-Based Liquid Crystals with Tunable Self-Assembling and Electronic Properties, ACS Omega, 2018, 3, 11857–11864 CrossRef CAS PubMed.
- G. Liu, L. Ren, M. Zhang, S. Du, P. Chen, A. Gao, X. Chen and Z. An, Synthesis and properties of benzoxazole-based liquid crystals containing ethynyl group, Liq. Cryst., 2020, 47, 1719–1728 CrossRef CAS.
- S. K. Saha, G. Mohiuddin, M. K. Paul, S. P. Gupta, R. K. Khan, S. Chosh and S. K. Pal, Polar Switching and Cybotactic Nematic Ordering in 1,3,4-Thiadiazole-Based Short-Core Hockey Stick-Shaped Fluorescent Liquid Crystals, ACS Omega, 2019, 4, 7711–7722 CrossRef CAS PubMed.
- J. Buchs, A. Geßner, B. Heyne, D. Janietz and H. Sawade, Fluorescent liquid crystals with rod-shaped π-conjugated hydrocarbon core, Liq. Cryst., 2019, 46, 281–298 CrossRef CAS.
- F. N. da Silva, A. S. da Silva, I. H. Bechtold, E. Zapp and A. A. Vieira, Luminescent liquid crystals based on 2,1,3-benzoxadiazole: conducive heterocycle or poor cousin of benzothiadiazole?, Liq. Cryst., 2019, 46, 1707–1717 CrossRef CAS.
- B. A. S. Jose, J. Yan and K. Akagi, Dynamic Switching of the Circularly Polarized Luminescence of Disubstituted Polyacetylene by Selective Transmission through a Thermotropic Chiral Nematic Liquid Crystal, Angew. Chem., Int. Ed., 2014, 53, 10641–10644 CrossRef PubMed.
- J. Li, H. K. Bisoyi, J. Tian, J. Guo and Q. Li, Optically Rewritable Transparent Liquid Crystal Displays Enabled by Light-Driven Chiral Fluorescent Molecular Switches, Adv. Mater., 2019, 31, 1807751 CrossRef PubMed.
- Y. Tsutsui, W. Zhang, S. Chosh, T. Sakurai, H. Yoshida, M. Ozaki, T. Akutagawa and S. Seki, Electrically Switchable Amplified Spontaneous Emission from Liquid Crystalline Phase of an AIEE-Active SEIPT Molecule, Adv. Opt. Mater., 2020, 8, 1902158 CrossRef CAS.
- W. Zhang, T. Sakurai, M. Aotani, G. Watanabe, H. Yoshida, V. S. Padalkar, Y. Tsutsui, D. Sakamaki, M. Ozaki and S. Seki, Highly Fluorescent Liquid Crystals from Excited-State Intramolecular Proton Transfer Molecules, Adv. Opt. Mater., 2019, 7b, 1801349 CrossRef.
- Y. Tsutsui, T. Sakurai and S. Seki, Amplified spontaneous emission from a liquid crystalline phase: anisotropic property and active modulation, Faraday Discuss., 2024, 250, 271–280 RSC.
- H. Chen, H. Yang, H. Kuo, W. Ko, K. Uchida and H. Yoshida, Photo-switching behaviour in liquid crystalline materials incorporating a non-planar dithienylcyclopentene core and their birefringence properties, Liq. Cryst., 2022, 49, 1475–1487 CrossRef CAS.
- M. Funahashi, Development of liquid-crystalline semiconductors with high carrier mobilities and their application to thin-film transistors, Polym. J., 2009, 41, 459–469 CrossRef CAS.
- H. Iino, T. Usui and J. Hanna, Liquid crystals for organic thin-film transistors, Nat. Commun., 2015, 6, 6828 CrossRef CAS PubMed.
- K. Sun, Z. Xiao, S. Lu, W. Zajaczkowski, W. Pisula, E. Hanssen, J. M. White, R. M. Williamson, J. Subbiah, J. Ouyang, A. B. Holmes, W. W. H. Wong and D. J. Jones, A molecular nematic liquid crystalline material for high-performance organic photovoltaics, Nat. Commun., 2015, 6, 6013 CrossRef CAS PubMed.
- G. Hu, M. R. Billa, S. P. Kitney and S. M. Kelly, Symmetrical carbazole-fluorene-carbazole nematic liquid crystals as electroluminescent organic semiconductors, Liq. Cryst., 2018, 45, 965–979 CrossRef CAS.
- D. Demus, One Century Liquid Crystal Chemistry: From Vorländer's Rods to Disks, Stars, and Dendrites, Mol. Cryst. Liq. Cryst., 2006, 364, 25–91 CrossRef.
- Y.-M. Liao, N. Janarthanan, C.-S. Hsu, S. Gauza and S.-T. Wu, Synthesis and mesomorphic properties of fluoro and isothiocyanato biphenyl tolane liquid crystals, Liq. Cryst., 2006, 33, 1199–1206 CrossRef CAS.
- S. Kang, S. Nakajima, Y. Arakawa, G. Konishi and J. Watanabe, Large extraordinary refractive index in highly birefringent nematic liquid crystals of dinaphthyldiacetylene-based materials, J. Mater. Chem. C, 2013, 1, 4222–4226 RSC.
- J. P. Schroeder, Liquid Crystals: VII. Smectic-Nematic Transition Temperature as a Function of Alkyl End Group Length in p-Phenylene Di-p-n-alkoxynbenzoates, Mol. Cryst. Liq. Cryst., 1980, 61, 229–240 CrossRef CAS.
- E. Cruickshank, G. J. Strachan, J. M. D. Storey and C. T. Imrie, Chalcogen bonding and liquid crystallinity: Understanding the anomalous behaviour of the 4′-(alkylthio)[1,1′-biphenyl]-4-carbonitriles (nSCB), J. Mol. Liq., 2022, 346, 117094 CrossRef CAS.
- M. Hird, Fluorinated liquid crystals – properties and applications, Chem. Soc. Rev., 2007, 36, 2070–2095 RSC.
- H. A. Ahmed, M. Hagar and O. A. Alhaddad, Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems, Liq. Cryst., 2020, 47, 404–413 CrossRef CAS.
- D. G. McDonnell, E. P. Raynes and R. A. Smith, Dipole moments and dielectric properties of fluorine substituted nematic liquid crystals, Liq. Cryst., 2006, 6, 515–523 CrossRef.
- A. Chakraborty, B. Das, M. K. Das, S. Findeisen-Tandel, M.-G. Tamba, U. Baumeister, H. Kresse and W. Weissflog, New hockey stick compounds with a lateral methyl group showing nematic, synclinic and anticlinic smectic C phases, Liq. Cryst., 2011, 38, 1085–1097 CrossRef CAS.
- M. Hagar, H. A. Ahmed and O. A. Alhaddad, Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of aterally substituted azopyridines, Liq. Cryst., 2019, 46, 1440–1451 CrossRef CAS.
- Y. Matsunaga and N. Miyajima, Effects of Branching of the Ester Alkyl Chain on the Liquid Crystalline Properties of Alkyl 4-(4-Alkoxybenzylideneamino)-benzoates, Mol. Cryst. Liq. Cryst., 1985, 116(3–4), 207–216 CrossRef CAS.
- Y. Shimomura, M. Tokita, A. Kawamura, J. Watanabe and G. Konishi, Fluorinated Poly(pentylene 4,4′-bibenzoate)e with Low Isotropization Temperatures and Unique Phase Transition Behavior, Macromolecules, 2023, 56, 5152–5161 CrossRef CAS.
- R. Iwai, H. Yoshida, Y. Arakawa, S. Sasaki, Y. Iida, K. Igawa, T. Sakurai, S. Suzuki, M. Tokita, J. Watanabe and G. Konishi, Near-room temperature π-conjugated nematic liquid crystals in molecules with a flrxible seven-membered ring structure, Aggregate, 2025, 6, e660 CrossRef CAS.
- S. Lou and G. C. Fu, Palladium/Tris(tert-butyl)phosphine-Catalyzed Suzuki Cross-Couplings in the Presence of Water, Adv. Synth. Catal., 2010, 352, 2081–2084 CrossRef CAS PubMed.
- J.-J. Shie and J.-M. Fang, Direct Conversion of Aldehydes to Amide, Tetrazoles, and Triazines in Aqueous Media by One-Pot Tandem Reactions, J. Org. Chem., 2003, 68, 1158–1160 CrossRef CAS PubMed.
- N. Miyaura, T. Ishiyama, H. Sasaki, M. Ishikawa, M. Sato and A. Suzuki, Palladium-catalyzed inter- and intramolecular cross-coupling reactions of B-alkyl-9-borabicyclo[3.3.1]nonane derivatives with 1-halo-1-alkenes of haloarenes. Synthesis of functionalized alkenes, arenes, and cycloalkenes via a hydroboration-coupling sequence, J. Am. Chem. Soc., 1989, 111, 312–321 CrossRef.
- N. Miyaura and A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev., 1994, 94, 2457–2483 Search PubMed.
- A. Sen and Y. M. A. Yamada, Latest Developments on Palladium- and Nickel-Catalyzed Cross-Couplings Using Aryl Chlorides: Suzuki–Miyaura and Buchwald–Hartwig Reactions, Synthesis, 2024, 3555–3574 CAS.
- T. Tanaka, A. Matsumoto, A. S. Klymchenko, E. Tsurumaki, J. Ikenouchi and G. Konishi, Fluorescent Solvatochromic Probe for Long-Term Imaging of Lipid Order in Living Cells, Adv. Sci., 2004, 11, 2309721 CrossRef PubMed.
- R. Iwai, S. Suzuki, S. Sasaki, A. S. Sairi, K. Igawa, T. Suenobu, K. Morokuma and G. Konishi, Bridged Stilbenes: AIEgens Designed via a Simple Strategy to Control the Non-radiative Decay Pathway, Angew. Chem., Int. Ed., 2020, 59, 10566–10573 CrossRef CAS PubMed.
- Y. Shimomura, K. Igawa, S. Sasaki, N. Sakakibara, R. Goseki and G. Konishi, Flexible Alkylene Bridges as a Tool to Engineer Crystal Distyrylbenzene Structures Enabling Highly Fluorescent Monomeric Emission, Chem. – Eur. J., 2022, 28, e202201884 CrossRef CAS PubMed.
- Y. Shimomura and G. Konishi, Push-Pull Bridged Distyrylbenzene with Highly Bright Solid-State Red-Orange Aggregation-Induced Emission, Chem. – Eur. J., 2023, 29, e202301191 CrossRef CAS PubMed.
- G. Konishi, Y. Sawatari, R. Iwai, T. Tanaka, Y. Shimomura and M. Tokita, Synthesis of Side-Chain Liquid Crystalline Polyacrylates with Bridged Stilbene Mesogens, Molecules, 2024, 29, 5220 CrossRef CAS PubMed.
- Y. Arakawa, S. Nakajima, R. Ishige, M. Uchimura, S. Kang, G. Konishi and J. Watanabe, Synthesis of diphenyl-diacetylene-based nematic liquid crystals and their high birefringence properties, J. Mater. Chem., 2012, 22, 8394–8398 RSC.
- Y. Arakawa, S. Kang, H. Tsuji, J. Watanabe and G. Konishi, The design of liquid crystalline bistolane-based materials with extremely high birefringence, RSC Adv., 2016, 6, 92845–92851 RSC.
- Y. Arakawa, S. Kang, H. Tsuji, J. Watanabe and G. Konishi, Development of novel bistolane-based liquid crystalline molecules with an alkylsulfanyl group for highly birefringent materials, RSC Adv., 2016, 6, 16568–16574 RSC.
- I. Haller, Thermodynamic and static properties of liquid crystals, Prog. Solid State Chem., 1975, 10, 103–118 CrossRef.
- M. Krämer, U. H. F. Bunz and A. Dreuw, Comprehensive Look at the Photochemistry of Tolane, J. Phys. Chem. A, 2017, 121, 946–953 CrossRef PubMed.
- J. Kobayashi, H. Yoshida and M. Ozaki, Planar optics with patterned chiral liquid crystals, Nat. Photonics, 2016, 10, 389–392 CrossRef.
- S. Y. Cho, M. Takahashi, J. Fukuda, H. Yoshida and M. Ozaki, Directed self-assembly of soft 3D photonic crystals for holograms with omnidirectional circular-polarization selectivity, Commun. Mater., 2021, 2, 39 CrossRef CAS.
- W. Haase, H. Paulus, Z. X. Fan, I. H. Ibrahim and M. Mokhles, The Crystal and Molecular Structure of the mesogenic 4-Cyano-4′-n-pentyl-p-terphenyl (T15) and its Solid State Polymorphism, Mol. Cryst. Liq. Cryst., Lett., 1988, 6, 113–121 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- D. W. Bruce, K. Heyns and V. Vill, Vorlander's wheel, Liq. Cryst., 1997, 23, 813–819 CrossRef CAS.
- S. Kapuściński, J. Szczytko, D. Pociecha and P. Kaszyński, Mesogenic Behavior of a 6-Oxoverdazyl Diradical: Towards Organic High-Spin Liquid Crystals, Mater. Chem. Front., 2024, 8, 1112–1119 RSC.
- S. Cao, C. Liu and M. Yoshio, Mater. Chem. Front., 2023, 7, 2828–2838 RSC.
- K. Sambe, T. Takeda, N. Hoshino, W. Matsuda, K. Shimada, K. Tsujita, S. Maruyama, S. Yamamoto, S. Seki, Y. Matsumoto and T. Akutagawa, Carrier Transport Switching of Ferroelectric BTBT Derivative, J. Am. Chem. Soc., 2024, 146, 8557–8566 CrossRef CAS PubMed.
- S. Ishikawa, K. Yamasumi, S. Sugiura, S. Sato, G. Watanabe, Y. H. Koo, S. Seki, Y. Bando, Y. Haketa, H. Shinokubo and H. Maeda, Norcorroles as antiaromatic π-electronic systems that form dimension-controlled assemblies, Chem. Sci., 2024, 15, 7603–7609 RSC.
- Y. Kobayashi, A. Muranaka, K. Kato, A. Saeki, T. Tanaka, M. Uchiyama, A. Osuka, T. Aida and T. Sakurai, A structural parameter to link molecular geometry to macroscopic orientation in discotic liquid crystals: study of metalloporphyrin tapes, Chem. Commun., 2021, 57, 1206–1209 RSC.
- K. Igeta, A. Higuchi, J. Kobashi, Y. Tomioka, S. Oka and H. Yoshida, ACS Appl. Opt. Mater., 2024, 2, 1314–1320 CrossRef CAS.
- G. Washio, T. Kajitani, S. Nishimura and A. Shishido, Design of ionic liquid crystals enabled by [2]rotaxane structure formation, Mol. Syst. Des. Eng., 2024, 9, 826–831 RSC.
- Y. Arakawa, S. Sasaki, K. Igawa, M. Tokita, G. Konishi and H. Tsuji, Birefringence and photoluminescence properties of diphenylacetylene-based liquid crystal dimers, New J. Chem., 2020, 44, 17531–17541 RSC.
- C. Anders, T. Tan, V.-M. Fischer, R. Wang, M. Alaasar, R. Waldecker, Y. Cao, F. Liu and C. Tschierske, Engineering “Meso-Atom” Bonding: Honeycomb-Network Transitions in Reticular Liquid Crystals, Aggregate, 2025, e728 Search PubMed (Early View).
- Z. Deng, X. Chen, X. Deng, J. Yang, S. Zhou, J. Chen, P. Wang, H. Yang and R. Lan, Programmable light-driven soft actuator enabled by structurally anisotropic liquid crystalline network, Aggregate, 2025, 6, e633 CrossRef CAS.
- Y. He, J. Zhang, C. Ma, J. Liu, J. Guo, T. Han, R. Hu, B. S. Li and B. Z. Tang, Multifaceted regulation of chiroptical properties and self-assembly behaviors of chiral fluorescent polymers, Aggregate, 2024, 5, e642 CrossRef CAS.
- Y. Sawatari, Y. Shimomura, M. Takeuchi, R. Iwai, T. Tanaka, E. Tsurumaki, M. Tokita, J. Watanabe and G. Konishi, Supramolecular liquid crystals from the dimer of L-shaped molecules with tertiary amide end groups, Aggregate, 2024, 5, e507 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: DSC charts, POM figures, absorption and fluorescence spectra, synthetic procedures, NMR and mass charts, crystallographic analysis. CCDC 2410034. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qm01116c |
| This journal is © the Partner Organisations 2025 |