Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Feed supplementation with molybdenum complexes improves honey bee health†
Arcadie
Fuior
 ab,
Loïc
Colin-Duchevet
ab,
Loïc
Colin-Duchevet
 c,
Valentina
Cebotari
c,
Valentina
Cebotari
 d,
Amélie
Noël
d,
Amélie
Noël
 c,
Isabelle
Ribaud
e,
Isabelle
Gérard
c,
Isabelle
Ribaud
e,
Isabelle
Gérard
 a,
Olga
Garbuz
a,
Olga
Garbuz
 d,
Mathieu
Fregnaux
d,
Mathieu
Fregnaux
 a,
Xavier
López
a,
Xavier
López
 f,
Virginie
Larcher
c,
Michael A.
Shestopalov
f,
Virginie
Larcher
c,
Michael A.
Shestopalov
 g,
Anastasiya O.
Solovieva
g,
Anastasiya O.
Solovieva
 h,
Tatiana N.
Pozmogova
h,
Tatiana N.
Pozmogova
 gi,
Olesea
Gliga
gi,
Olesea
Gliga
 d,
Nadejda
Railean
d,
Nadejda
Railean
 d,
Précillia
Cochard
d,
Précillia
Cochard
 j,
Benjamin
Poirot
j,
Benjamin
Poirot
 j,
Leonidas
Charistos
j,
Leonidas
Charistos
 k,
Fani
Hatjina
k,
Fani
Hatjina
 k,
Andrea
Somogyi
k,
Andrea
Somogyi
 l,
Kadda
Medjoubi
l,
Kadda
Medjoubi
 l,
Sébastien
Gaumer
l,
Sébastien
Gaumer
 m,
Aurelian
Gulea
m,
Aurelian
Gulea
 b,
Ion
Toderas‡
b,
Ion
Toderas‡
 d,
Jean-Christophe
Sandoz‡
d,
Jean-Christophe
Sandoz‡
 c and
Sébastien
Floquet‡
c and
Sébastien
Floquet‡
 a
a
aInstitut Lavoisier de Versailles, Université Paris-Saclay, UVSQ, CNRS, UMR 8180, 78000 Versailles, France. E-mail: sebastien.floquet@uvsq.fr
bState University of Moldova, MD-2009 Chisinau, Republic of Moldova
cEvolution Genomes Behaviour & Ecology, University Paris-Saclay, CNRS, IRD, 91198 Gif Sur Yvette, France. E-mail: jean-christophe.sandoz@cnrs.fr
dInstitute of Zoology, MD-2028 Chisinau, Republic of Moldova
eUniversité Paris Saclay, CNRS, IN2P3, IJCLab, 91403 Orsay, France
fUniversitat Rovira i Virgili, Departament de Química Física i Inorgànica, Marcel·lí Domingo 1, 43007 Tarragona, Spain
gNikolaev Institute of Inorganic Chemistry SB RAS, 630090 Novosibirsk, Russia
hResearch Institute of Clinical and Experimental Lymphology – Branch of the ICG SB RAS, 630090 Novosibirsk, Russia
iNovosibirsk State University, 630090 Novosibirsk, Russia
jApinov S.A.S, 17140 Lagord, La Rochelle, France
kDepartment of Apiculture, Institute of Animal Science, ELGO ‘DIMITRA’, Nea Moudania, 63200, Greece
lNanoscopium beamline, Synchrotron SOLEIL, L'Orme des Merisiers, Saint-Aubin, 91192 Gif-sur-Yvette, France
mGenetics and Cell Biology Laboratory, Université Paris-Saclay, UVSQ, UR 4589, 78180 Montigny-le-Bretonneux, France
First published on 30th June 2025
Abstract
This study investigates the effects of dietary supplementation with molybdenum-based compounds on honey bee health. Among a series of dinuclear Mo(V) complexes, the most stable and non-toxic complexes were selected and tested over an extensive eight-year field campaign involving more than 700 beehives across diverse environmental conditions in Moldova, France, Greece and the United States. In a first part, we established that the administration of a few milligrams of the compounds Na-Mo2O4-EDTA or Li-Mo2O4-EDTA in spring or autumn enhanced colony performance: queen fecundity, hygienic behaviour, and honey production increased, while Varroa destructor infestation rates and winter losses were substantially reduced. A second part of the work focused on understanding these effects in beehives. Hive monitoring showed that the Mo-containing syrup can be consumed over 1.5 months and is well assimilated by larvae and workers. In particular, Mo levels increased significantly in the head of the bees. X-ray fluorescence measurements demonstrated that Na-Mo2O4-EDTA increases Mo levels in the brain, neurolemma and hypopharyngeal glands, which play a crucial role in honey bee health. The metabolism of Mo complexes was addressed using X-ray photoelectron spectroscopy (XPS) on bee fæces, which revealed that the complexes are oxidized into Mo(VI), suggesting that Mo complexes may function as antioxidant agents in bees. These findings offer promising solutions for the beekeeping industry, struggling with weakening honey bee colonies.
1. Introduction
About 80% of all cultivated plant species and 40% of our diet directly depend on pollination.1 The economic impact of insect pollinators, especially bees, has thus been estimated at 150 billion euros in 2005.2 While the honey bee Apis mellifera plays a major role in insect-mediated pollination, it is gravely endangered by colony losses,3 which have systematically increased in the last decades through a multifactorial process involving biological agents (Varroa destructor, Vairimorpha, etc.), the use of pesticides, and a lack of resources among others.4 In this context, the development of new solutions to protect bees from these external aggressions and strengthen their health and resilience is therefore of great interest.One way to improve honey bees’ health and resilience to stress is to optimize their nutrition.5–7 This is a highly complex issue as it requires a perfect balance between macronutrients (carbohydrates, proteins, and lipids) and micronutrients (vitamins, minerals) to meet all the needs of honey bees. Floral sources are honey bees’ major source of macronutrients. Nectar provides them with carbohydrates, while pollen supplies bees with lipids, proteins, and minerals. Pollen quality strongly depends on the plant species, the soil, and the time of year in which it is produced8 and honey bee health is directly correlated with the quality of pollen.9–11 These sources dynamically change in the landscape in both space and time, which can cause periods of deficiencies for honey bee colonies5,6 and this issue can be exacerbated by climate changes, which can potentially create mismatches between plants and honeybees.6,12
To overcome nutritional limitations, beekeepers rely on food supplements usually given in spring at the begining of the beekeeping season, during dearth periods, and in autumn to prepare the colonies for the wintering.5 This supplementation is usually in the form of sugar syrup or fondants, which primarily deliver carbohydrates to the colony but do not cover the other needs. Other additives are therefore needed to fill these gaps and prevent bees from feeding on risky sources containing toxic, inedible substances as much as possible.13 Interestingly of lot of products are used by beekeepers but very few of them are proven by scientific studies. In this field some researchers explored formulations containing vitamins,14,15 or proteins to boost colony strength or reduce mortality16–19 even if some other studies have reported that colonies fed protein supplements do not perform as well when compared with colonies receiving real pollen.20 In contrast, a very recent study form Bogaert et al. reported impressive results on hives for the development of the colonies from May to October by supplementation with a mixture of sterols, especially isofucosterol, which appears as an essential nutrient for honeybees.21 More recently, the supplementation with pre and post-biotics emerged with the idea that the gut microbiome plays important roles in honey bees, including a role in honey bee colony health (pathogen defense) and nutrition.22
Metals, are also important micronutrients for animals’ health as they play an essential role in numerous physiological processes. However, surprisingly, they have been poorly studied in honey bees.23 Honey bees likely obtain minerals from two main sources: pollen and “dirty” or turbid water. Yet, very little is known about the metals that are needed for honey bees’ health.23 Several studies have focused on the physiological role and/or toxicity of elements like Fe, Mn, Cu and Zn, as well as the deleterious effects of pollution by heavy elements such as As, Cd, and Pb.24–28 However, the role and the importance of trace elements for honey bees remains scarce,29–31 leaving us with limited knowledge of their potential role or the consequence of a deficiency in these elements.
Among trace elements, molybdenum (Mo) is of essential importance for life in almost all living organisms from bacteria to humans.32 Mo is present in about fifty enzymes, namely molybdoenzymes, which are essential constituents of the global carbon, sulfur and nitrogen metabolism in plants and animals. In these enzymes, Mo atoms play a key role in the catalysis of diverse redox reactions thanks to their oxidation state which can vary from Mo(+VI) to Mo(+IV).32,33 Molybdoenzymes are generally classified into different families. In eukaryotes, these enzymes mainly belong to the Sulfite Oxidase (SO) and Xanthine Oxidase (XO) families.33 The role of SO is important for sulfite detoxification. XO molybdoenzymes are very useful for the degradation of xenobiotics and possess a broad substrate spectrum. As we demonstrate here, Mo is also present in honey bee foragers, with an average content of about 0.39 ppm (see Part I, ESI†) but to our knowledge its role in this insect is utterly unknown.
The objectives of this study are to fill this gap and thus to evidence that supplementing food for honey bees with stable, non-toxic, easy to handle, and bio-available simple molybdenum-based coordination complexes can induce positive effects on the global health of the colonies. A first part of this study is thus dedicated to the evaluation of the impact of supplementing bees feed with Mo-based compounds through an extensive eight-year campaign of field trials -conducted in Moldova, in France, in Greece and in the USA to identify the best complexes among a dozen of candidates, their effects in hives for the key parameters interesting beekeeping industry (development of the colony, productivity, mortality, action against pathogens), their optimal dose and their side effects, if so, in case of an overdosage.
For this, we chose coordination complexes of general formula (cation)x[Mo2O2E2(L)y] (x = 0, 2, 4; y = 1 or 2, E = O or S), hereafter referred to as Cation-Core-Ligand combining the [Mo2O4]2+ or [Mo2O2S2]2+ clusters with organic ligands, such as EDTA or cysteine (L-Cys), and counter cations which can be alkali or organic cations. The organic ligand should facilitate their uptake by bees and rapidly involve redox processes due to the reduced oxidation state of the Mo core. These complexes have been described in the literature as biomimetic models of molybdoenzymes since the 1970s34 and more recently, it has been demonstrated that they have little to no toxicity35,36 with some showing potential for cyanide detoxification,37 cell penetration capabilities,38 and stimulation of biomass production, or acting as potent anti-oxidants.36 Among a dozen previously published potential candidates,36 three were selected for their high chemical stability (see Part II, ESI†) and their lack of toxicity (Part III, ESI†), especially when used as feed supplement for honey bees: [Mo2O4(EDTA)]2−, [Mo2O2S2(EDTA)]2−, and [Mo2O2S2(L-Cys)2]2− (see Fig. 1). [Mo2O4(EDTA)]2− was used as PPh4+, Na+ and Li+ salts (PPh4-Mo2O4-EDTA, Na-Mo2O4-EDTA and Li-Mo2O4-EDTA), the sulphurated analogue [Mo2O2S2(EDTA)]2− was used as the PPh4+ salt (PPh4-Mo2O2S2-EDTA) and [Mo2O2S2(LCys)2]2− was used as the K+ salt (K-Mo2O2S2-LCys).
 | ||
| Fig. 1 Structures of the three complexes tested in beehives. Color code: Mo in green, S in yellow, O in red, N in blue, C in black, H in white. | ||
A second part of this study aims to bring element to understand the mode of action of the most interesting complexes in beehives: cycle of Mo in the colonies, bio-assimilation, organs targeted by our molecules, metabolism and anti-oxidative properties.
2. Results
2.1 Tests in beehives
Field experiments aimed to assess the effects of these complexes on critical indicators of colony health, such as queen reproductive performance, honey production, levels of Varroa destructor infestation, and winter survival rates. The first field campaigns aimed to identify the most interesting complexes. Subsequent campaigns focused on the most promising results, particularly with regard to Varroa infestation, the maximum doses that can be administered without risk to the colony, the extension of tests under real operating conditions, and the study of the impact of these complexes on winter mortality in a geographical area particularly affected by these losses (California, USA).The treatment had a significant effect on the worker bee infestation rate by Varroa (Kruskal Wallis test, p = 0.0024). With an overall dose of 2 mg of Li-Mo2O4-EDTA per beehive, infestation of worker bees by Varroa fell by −47% compared to the control group (multiple comparison Dunn's test, p = 0.080), similarly to the previous campaign (−42.9%). This effect increased to −61.3% with 6 mg per beehive (Dunn's test, p = 0.002). Despite a lithium concentration 3.7 times higher, the effect of lithium acetate was weaker and non-significant (−30.4%, p = 1). It could suggest that the Mo-complex in Li-Mo2O4-EDTA has a protective action against Varroa, in synergy with Li+ cations. Interestingly, the hygienic behaviour of hives fed with 2 and 6 mg of Li-Mo2O4-EDTA concomitantly increased by +9.3% (p = 0.007) and +12% (p < 0.001), respectively. It is known that honey bee colonies with higher hygienic behaviour tend to keep Varroa infestation at lower levels.41
We also observed a significant effect of the treatment on the brood infestation rate by Varroa (Kruskal Wallis test, p < 0.001). Interestingly, Varroa infestation was very high in control hives, i.e. 28.2%. After feeding with the reference compound LiOAc (2 mg per hive), the rate was lowered by −35.1% in this group (Dunn's test, p = 1). Again, the Li-Mo2O4-EDTA complex produced stronger effects, reducing brood infestation by −57.4% compared to the control group with 2 mg (Dunn's test, t, p = 0.067), and up to −81.6% with 6 mg per beehive (Dunn's test, p = <0.001). Beyond this result, it also suggests that the complex is consumed not only by adult workers, but is also transferred to the brood.
A new test campaign was thus carried out in France, with the feeding protocol adapted to commercial beekeeping practices. The test was performed on 22 beehives of Apis mellifera “Buckfast”, widely used in beekeeping, in Gif-sur-Yvette (France), split into two groups: 11 control and 11 test colonies. For the test colonies, 2 mg of Li-Mo2O4-EDTA complex were introduced on 1st April in one 0.5 L syrup feeding. We monitored colony weight and the quantity of honey produced on 15th July (Table SIV.5, Part IV, ESI†). The results depicted in Fig. 2C show that a single dose of Li-Mo2O4-EDTA complex introduced at the beginning of spring resulted in a +23.6% increase in colony weight compared to the control group (U-test, p = 0.033), and in a +58.7% increase in honey production (30.3 kg on average for the test group vs. 19.1 kg for the control group, U-test, p = 0.014). This result is similar to the one from the previous campaign with 2 mg of the Li-Mo2O4-EDTA (+47%, U-test compared to control group, p = 0.03) in Moldova on Apis mellifera carpatica after multiple feedings, which validates the efficacy of the complex when applied in a single feeding, under professional beekeeper practices in a second geographical location and on another honey bee subspecies.
Two test campaigns were conducted during the winters of 2019 and 2020 in apiaries in the San Francisco Bay area (USA), a region consistently reporting high levels of winter colony losses.45–48 In the first campaign, 151 beehives were distributed across 6 different apiaries. In each apiary, the colonies were randomly divided into a control group (76 hives), receiving 1 US Gallon (3.78 Liters) of sugar syrup, and a test group (75 hives) receiving syrup containing 4 mg of Li-Mo2O4-EDTA complex. The feeding took place at the end of October 2019, and the surviving colonies were counted on 7th January 2020. In the control group, 47 colonies were lost out of 76, i.e. 61.8% winter mortality, while in the test group only 26 colonies were lost, i.e. 34.7% winter mortality. This 43.8% mortality rate decrease was significant (chi2 test, p = 0.0008).
A second campaign (winter 2020) involved 220 beehives, distributed across 11 apiaries. The beehives were divided into 4 groups of 55 colonies. Each group was fed twice, in September and in October, with 1 US gallon of syrup each time for a total of 2 US gallons for each beehive. The control group received only syrup, the second group received 8 mg Li-Mo2O4-EDTA in September only, the third one received the same dosage in October only, and the fourth group received 4 mg of the complex in both feedings. In summary, the three test groups of colonies received 8 mg of the complex and only differed in the supplementation schedule. Lost colonies were counted on 31st December, 2020. The period of supplementation strongly and significantly impacted the results. The mortality rate in the control group was 15/55, i.e. 27.3% loss. The supplementation in October reduced mortality by 33% compared to the control group (10/55, i.e. 18.2% loss), which was not significant (Chi2 test, p = 0.25). The supplementation in September resulted in no winter losses (0/55, 0%, Chi2 test, p = 3.1 10−5). And finally, supplementation in both September and October resulted in 3 lost colonies out of 55 (5.5% loss, Chi2 test, p = 0.002), corresponding to 80% reduction in winter mortality compared to the control group.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mg in total) and then in syrup in March (4 liters at 10 mg L−1, 40 mg in total, “MoLi-A” batch). The size of the colonies and the number of dead bees at the entrance of the colonies (using Gary traps) were monitored throughout the whole period (see Part IV, ESI†). During the first period (December to March), we found that feeding the bee colonies with high Li-Mo2O4-EDTA dosage (colonies from both MoLi-A and MoLi-B groups) had deleterious effects. On average, about 185 dead bees were found in front of the colonies in these groups, compared to about 130 for the control group (t-test, p = 0.0001). This effect was stronger during the second period (March to April) in the “MoLi-A” group (160 dead bees per hive vs. 97 for the control group, p = 0.0001), while the increased mortality stopped for the MoLi-B group (57 dead bees per hive during the second period). While feeding 2–8 mg of complex Li-Mo2O4-EDTA appears to have very positive effects in hives, overdosing with 40 and 80 mg per hive increases bee mortality.
40 mg in total) and then in syrup in March (4 liters at 10 mg L−1, 40 mg in total, “MoLi-A” batch). The size of the colonies and the number of dead bees at the entrance of the colonies (using Gary traps) were monitored throughout the whole period (see Part IV, ESI†). During the first period (December to March), we found that feeding the bee colonies with high Li-Mo2O4-EDTA dosage (colonies from both MoLi-A and MoLi-B groups) had deleterious effects. On average, about 185 dead bees were found in front of the colonies in these groups, compared to about 130 for the control group (t-test, p = 0.0001). This effect was stronger during the second period (March to April) in the “MoLi-A” group (160 dead bees per hive vs. 97 for the control group, p = 0.0001), while the increased mortality stopped for the MoLi-B group (57 dead bees per hive during the second period). While feeding 2–8 mg of complex Li-Mo2O4-EDTA appears to have very positive effects in hives, overdosing with 40 and 80 mg per hive increases bee mortality.
For the sake of comparison, the impact of a high Na-Mo2O4-EDTA dosage on mortality was evaluated in a field campaign performed in the west of France (see Part III.5 of the ESI† for more details) from May to the end of June 2022. 40 colonies divided into five groups of 8 beehives were each fed with 4 L of sugar syrup (2 times 2 L with 1 week between the two feedings), and received 0, 4, 8, 16, and 80 mg of Na-Mo2O4-EDTA per beehive, respectively. Mortality was monitored in all groups over 2 months with Gary traps positioned in front of the hives. No significant difference in mortality was measured between hives supplemented with Na-Mo2O4-EDTA and control hives, even at the highest dose (80 mg, p = 0.57, see Fig. SIII.24, Part III, ESI†). Conversely to Li-Mo2O4-EDTA, Na-Mo2O4-EDTA demonstrated a superior safety profile, even at high concentrations, supporting its potential for safe, large-scale apicultural use.
2.2 Tracking molybdenum in beehives
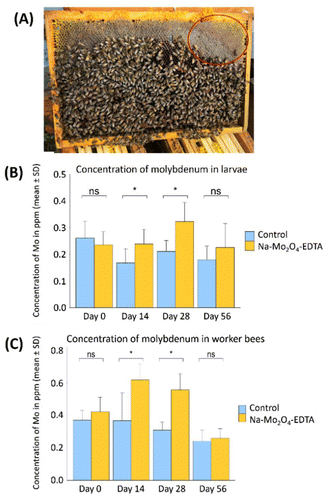
Since supplementation with the complexes showed positive effects several months after administration, it was important to monitor the molybdenum cycle within the beehives to understand the underlying reasons. To this end, the control and 8 mg Na-Mo2O4-EDTA groups from the prior experiment were employed.At Day 0 (D0), the level of Mo in the colonies’ food stores was below 0.1 ppm in all groups (Table SV.1, Part V, ESI†). At D28 and D42 the Mo content matched the levels of Mo from the syrups used for the feeding, showing that the syrup was not yet consumed. At D56, the Mo level was again below 0.1 ppm in almost all groups. The 1H NMR studies were additionally conducted on these samples, revealing the absence of disaccharides and confirming it is pure and unadulterated honey. This study demonstrates that the syrup is stored and consumed over several weeks by the colonies (over 1.5 months in our case). This gradual consumption enables several generations of bees to be fed with Na-Mo2O4-EDTA. Once the feeding is consumed, we confirmed that the supplementation does not impact the honey produced by the colony.
Fig. 3B and C show the variations in Mo-contents in bee larvae and in worker bees between D0 and D56 for control and 8 mg per hive batches. In bee larvae, at D0, Mo levels were similar in the control and 8 mg per beehive groups: 0.24 vs. 0.26 ppm. After two weeks (D14) a difference of +42% in Mo levels was measured in the 8 mg per hive group (U-test, p = 0.02). This difference increased again at D28, reaching +53% Mo (U-test, p = 0.004). At D56, the difference between the two groups decreased and was not significant (p = 0.36).
In the worker bees, Mo levels were also similar at D0 in the control and 8 mg per beehive groups: around 0.4 ppm. At D14, the Mo level increased by +68% in the 8 mg per hive group (0.62 ppm, U-test, p = 0.01), reaching +80% at D28 (p = 0.001). The workers consumed the syrup and assimilated the complex of molybdenum. At D56, Mo levels were again similar in both groups (p = 0.46). This experiment demonstrates the assimilation of Mo by larvae and worker bees over at least 1 month after the supplementation.
2.3 Effect of chronic feeding of bees in laboratory conditions
To further confirm the safety of Mo-complexes for honey bees, a chronic feeding experiment was conducted in laboratory conditions.Three different concentrations were used in addition to a control group: 2, 20 and 400 mg L−1 for both Na-Mo2O4-EDTA and Li-Mo2O4-EDTA complexes. For each group, 3 cages of 50 workers collected at emergence were fed ad libitum with water and with Mo-enriched sugar solution. The survival was monitored throughout their lifetime (Fig. 4).
The survival curves are depicted in Fig. 4B and C (see also section III.4, Part III, ESI†). We found that the average curves obtained for control batches and for bees fed with 2, 20 and 400 mg L−1 solutions of both complexes are similar. The statistical analysis confirms that no difference in mortality appeared between the control group and the groups fed with both Na-Mo2O4-EDTA and Li-Mo2O4-EDTA during the first 10 days (Table SIII.10, Part III, ESI†), and for the whole period for all concentrations of Li-Mo2O4-EDTA (Table SIII.11, Part III, ESI†). In contrast, the Cox model indicates a slight negative impact of Na-Mo2O4-EDTA at 2 and 20 mg L−1 but a positive impact at 400 mg L−1. In summary, no notable positive or negative effect of feeding with Mo-complexes was observed on bees’ longevity in laboratory conditions, even at a very high concentration.
2.4 Assimilation of Mo by the honey bees in laboratory
Dead bees collected in the previous experiment were used to evaluate the assimilation of Mo complexes within the body of the bees. The dead bees were divided into 3 batches (periods I, II, III, see Fig. 4B and C) depending on feeding duration. The Mo content was determined in heads, thoraxes, and abdomens by ICP-MS (see Table 1 and Tables SVI.2, SVI.3, Part VI, ESI†). First, we found low Mo levels in the control bees, with values respectively around 0.2 ppm for Na-Mo2O4-EDTA groups and 0.5–0.7 ppm for Li-Mo2O4-EDTA groups in the head and in the abdomen, and lower levels in the thorax (0.12 and 0.25 ppm respectively). An increase in Mo levels was found in the head, thorax and abdomen when the concentration of Na-Mo2O4-EDTA or Li-Mo2O4-EDTA increased in the feeding syrup. For each concentration, the Mo level reached a different plateau which did not depend on the duration of feeding. More precisely, Mo levels in the head increased from ∼0.19 ppm in the control group, to 0.58, 1.83 and 27 ppm, respectively for the groups fed with syrups at 2, 20 and 400 mg L−1 of Na-Mo2O4-EDTA, i.e. up to a 140-fold increase. At the same time, Mo-level variations were higher in the thorax, from 0.12 ppm to 0.73, 2.09 and 58 ppm with the same syrups. Similar observations were made with Li-Mo2O4-EDTA, with levels reaching 34.8 ppm in the head and 75.2 ppm on average in the thorax. With both complexes, as bees defecate very little in cages, strong accumulation was observed in the bees’ abdomen, in particular in the faeces (up to 3600 ppm), and so these parts were further excluded from the experiment. In the case of Li-Mo2O4-EDTA, an accumulation of Lithium was also observed, in particular in the head, in which we estimate that 5.6 Li+ are assimilated for each Mo atom (see Table 1 and Fig. SVI.5, Part VI, ESI†).| Complex | Modality | Periods | Head | Thorax | ||
|---|---|---|---|---|---|---|
| Mo (ppm) | Na (ppm) | Mo (ppm) | Na (ppm) | |||
| Na-Mo2O4-EDTA | Control | I | 0.20 ± 0.01 | 720 ± 40 | 0.12 ± 0.01 | 350 ± 5 |
| II | 0.20 ± 0.01 | 650 ± 50 | 0.13 ± 0.01 | 375 ± 3 | ||
| III | 0.17 ± 0.01 | 680 ± 30 | 0.11 ± 0.01 | 330 ± 10 | ||
| Na-Mo2O4-EDTA 2 mg L−1 | I | 0.70 ± 0.01 | 728 ± 9 | 0.6 ± 0.1 | 380 ± 10 | |
| II | 0.63 ± 0.01 | 710 ± 10 | 0.9 ± 0.3 | 400 ± 60 | ||
| III | 0.42 ± 0.01 | 710 ± 6 | 0.7 ± 0.1 | 370 ± 30 | ||
| Na-Mo2O4-EDTA 20 mg L−1 | I | 2.02 ± 0.01 | 761 ± 8 | 2.06 ± 0.01 | 340 ± 20 | |
| II | 1.33 ± 0.01 | 661 ± 3 | 2.12 ± 0.04 | 430 ± 20 | ||
| III | 2.15 ± 0.75 | 760 ± 10 | 2.08 ± 0.08 | 409 ± 8 | ||
| Na-Mo2O4-EDTA 400 mg L−1 | I | 30 ± 1 | 830 ± 5 | 51 ± 5 | 500 ± 60 | |
| II | 28 ± 1 | 834 ± 4 | 80 ± 25 | 530 ± 20 | ||
| III | 23 ± 1 | 1003 ± 4 | 43 ± 15 | 540 ± 6 | ||
| Complex | Modality | Periods | Head | Thorax | ||
|---|---|---|---|---|---|---|
| Mo (ppm) | Li (ppm) | Mo (ppm) | Li (ppm) | |||
| Li-Mo2O4-EDTA | Control | I | 0.66 ± 0.07 | 0.06 ± 0.01 | 0.27 ± 0.01 | 0.03 ± 0.01 |
| II | 0.46 ± 0.02 | 0.04 ± 0.01 | 0.26 ± 0.01 | 0.03 ± 0.01 | ||
| III | 0.40 ± 0.01 | 0.04 ± 0.01 | 0.23 ± 0.01 | 0.03 ± 0.01 | ||
| Li-Mo2O4-EDTA 2 mg L−1 | I | 1.86 ± 0.01 | 1.06 ± 0.01 | 3.07 ± 0.02 | 1.31 ± 0.02 | |
| II | 1.48 ± 0.01 | 0.84 ± 0.02 | 2.31 ± 0.04 | 0.93 ± 0.01 | ||
| III | 1.62 ± 0.04 | 1.11 ± 0.02 | 2.00 ± 0.04 | 1.23 ± 0.01 | ||
| Li-Mo2O4-EDTA 20 mg L−1 | I | 4.36 ± 0.04 | 1.77 ± 0.01 | 7.81 ± 0.04 | 2.27 ± 0.02 | |
| II | 3.72 ± 0.01 | 2.35 ± 0.01 | 5.50 ± 0.07 | 2.53 ± 0.02 | ||
| III | 3.39 ± 0.02 | 1.58 ± 0.01 | 3.45 ± 0.07 | 1.80 ± 0.02 | ||
| Li-Mo2O4-EDTA 400 mg L−1 | I | 31.9 ± 0.1 | 10.89 ± 0.08 | 63.1 ± 0.4 | 16.3 ± 0.1 | |
| II | 37.6 ± 0.2 | 14.0 ± 0.1 | 58.6 ± 0.2 | 18.87 ± 0.05 | ||
| III | 35.03 ± 0.03 | 16.5 ± 0.2 | 104.0 ± 0.3 | 24.9 ± 0.1 | ||
2.5 X-Ray fluorescence studies
As it was confirmed that Mo levels are increased in bees after the supplementation, another study was conducted to understand in which organs it is assimilated. X-ray fluorescence spectra were recorded on the Nanoscopium beamline of Synchrotron Soleil (Gif-sur-Yvette, France) on 20–50 μm-thick slices of head, thorax, and abdomen, and on hypopharyngeal glands extracted from the heads of worker bees. The study was conducted on bees fed over 14 days after their emergence with a sugar syrup containing Na-Mo2O4-EDTA at 400 mg L−1 or pure syrup for the control group. The analysis of the slices and glands of bees not supplemented with Na-Mo2O4-EDTA (see Fig. 5A–C) qualitatively revealed that Mo-content (i) is negligible in the brain, (ii) it is low in the neurolemma, hypopharyngeal glands and muscles (see Fig. 6A, 7A, and Fig. SVII.9, SVII.13, SVII.16, Part VII, ESI†) as evidenced by a shoulder on the X-ray fluorescence spectra at the energy expected for the element Mo (16.9 to 17.9 eV) and (iii) it is moderate but at least two times higher in the cuticle parts, as shown in the Fig. SVII.18 (Part VII, ESI†).Fig. 5D–H show the distribution of Mo on a 20 μm thick slice of the head of a bee fed with the complex Na-Mo2O4-EDTA. This slice contains elements of the brain, neurolemma, cuticle and oesophagus. No difference was observed in the intensity of the fluorescence of Mo in the cuticle (external cuticle and tentorial arms) and in the muscles in comparison with control bees (see Fig. SVII.18–SVII.20, Part VII, ESI†). More interestingly, the fluorescence of Mo is clearly seen in the brain and found especially in the neurolemma around the brain (Fig. 5F and G), in contrast with control bees (Fig. 5B). The Mo level appears slightly enhanced within the brain after feeding from an intensity of ca. 70–80 counts in control bees (Fig. 5C), comparable to the baseline, to ca. 130–140 counts in fed bees (Fig. 5H).
Concomitantly, the increase of Mo in the neurolemma is much more pronounced. From several X-ray spectra recorded in the same experimental conditions for several individuals from both groups (Fig. 6 and Fig. SVII13 and SVII14, Part VII, ESI†), Mo-content in bees fed with Na-Mo2O4-EDTA appeared around 10-fold higher than in the control group, showing an accumulation of Mo in the neurolemma.
In control bees (Fig. 7A), the fluorescence spectrum reveals a low presence of Mo in the glands. Feeding with a sugar syrup containing the Na-Mo2O4-EDTA complex induced a remarkable fluorescence peak, as shown in Fig. 7B. The results obtained for 7 acini of hypopharyngeal glands from 2 individuals are similar (see Part VII, ESI†). A 14× increase in Mo content was estimated in bees’ hypopharyngeal glands after feeding bees with Na-Mo2O4-EDTA at 400 mg L−1. These results show that the hypopharyngeal glands are a primary target for the Mo complex, which increases the Mo level naturally present in these glands and, due to the importance of these glands, could explain its impact on the health of the bees.
2.6 Metabolism and antioxidant properties of Mo-complexes within the bee organism
The last part of this study aimed to provide elements for understanding the metabolism of the Mo complexes in the honey bee organism.X-ray photoelectron spectroscopy (XPS) experiments were performed on the fæces of individuals fed with Na-Mo2O4-EDTA at 400 mg L−1. The Mo 3d spectral region of Na-Mo2O4-EDTA and of bee fæces, as well as their associated reconstruction, are presented in Fig. 8.
The Na-Mo2O4-EDTA spectrum can be resolved into three doublets, with the most intense corresponding, as expected, to the Mo+V oxidation state (Mo 3d5/2 = 231.2 eV, full width at half maximum FWHM = 1.4 eV).52 For the bee fæces, the Mo 3d core level spectrum displays only one broad doublet (Mo 3d5/2 at BE 232.7 eV, FWHM = 3.5 eV). The energy position is fully consistent with a Mo+VI phase.52 The presence of Mo in +V or +IV oxidation states was not identified. This experiment thus unambiguously shows that the complex Na-Mo2O4-EDTA was oxidized in the bees, probably into the molybdate anion MoO42−. It constitutes the first element suggesting that the Na-Mo2O4-EDTA complex may act as an antioxidant by oxidation of the Mo(V) atoms into the Mo(VI) oxidation state.
The antioxidant activity (AOA) of the complexes Na-Mo2O4-EDTA and Li-Mo2O4-EDTA was evaluated through field tests performed on Apis mellifera carnica. Experimental beehives were fed at the beginning of spring for 14 days with 50% sugar syrup enriched or not with Na-Mo2O4-EDTA and Li-Mo2O4-EDTA at 0.2 mg L−1. Sodium molybdate Na2MoO4·2H2O was also used, for comparison, with a Mo content equivalent to that of the Na-Mo2O4-EDTA and Li-Mo2O4-EDTA compounds. The AOA was evaluated by the ABTS method53 on hemolymph from worker bees and larvae, wax, honey, propolis, royal jelly, and bee bread. The results are expressed as IC50 values, the half-maximal Inhibitory Concentration, defined as the concentration that causes the loss of 50% of the activity of ABTS radicals. The lower the IC50 values, the higher the antioxidative activity. Some selected results are depicted in Fig. 9 while the other results are presented in the ESI (Fig. SVIII-6–SVIII-12, Part VIII, ESI†).
As shown in Fig. 9, the IC50 values obtained for the hemolymph of worker bees are low, indicating a naturally good antioxidant activity (Fig. 9A). The AOA of hemolymph in worker bees increased for both Na-Mo2O4-EDTA and Li-Mo2O4-EDTA complexes compared to the control group, suggesting that these complexes act on the organisms of bees as antioxidants or induce AOA. The AOA of both complexes appears similar, slightly better with Na-Mo2O4-EDTA. Interestingly, even though sodium molybdate Na2MoO4 cannot chemically act as a direct antioxidant, the AOA of the hemolymph of bees treated with this compound increased, suggesting a more complex mechanism.
In the case of larval hemolymph (Fig. 9B), the findings are similar for the control group and sodium molybdate, showing no increase in AOA. Both Na-Mo2O4-EDTA and Li-Mo2O4-EDTA complexes showed an important increase in AOA in larvae with the ABTS method.
Regarding hive products (see Part VIII, ESI†), the AOA of honey samples was similar for control, sodium molybdate and Na-Mo2O4-EDTA groups but strongly enhanced with Li-Mo2O4-EDTA. This result is surprising and could be either due to traces of the feeding syrup in the honey for this group or to an action of this complex on the enzymes produced in the bees’ crop to produce honey. The AOA of royal jelly and bee wax taken from the 4 groups were similar in all cases. Finally, both complexes significantly amplified the antioxidant activity of bee bread (Fig. 9C), showcasing remarkable efficacy in enhancing its overall antioxidant potential54 for the benefit of the entire colony.
3. Discussion
This study provides the first large-scale demonstration of the beneficial impact of molybdenum complexes dietary supplementation on the health of honey bee colonies (Apis mellifera). Conducted over eight years in Moldova, in France, in Greece, and in the USA, field trials, encompassing over 700 hives across diverse ecological settings in Europe and the United States, revealed that administering only a few milligrams of Na-Mo2O4-EDTA or Li-Mo2O4-EDTA complexes in spring or autumn produces consistent improvements in colony development, hygienic behaviour, honey and wax production for both complexes, whereas a strong reduction in Varroa destructor infestation and winter losses have been observed with the lithium salt.All the field-tests were achieved with low doses of Mo-complexes. Such a low dosage (2–8 mg of complex, which corresponds to 0.6–2.4 mg of the Mo element) is in fact of the same order as the needs of Mo we can globally estimate for a colony across one beekeeping season. Indeed, considering an average level of about 0.4 μg g−1 (see Part I, ESI†), an average weight of bees of about 80 mg, a lifespan of 40–45 days for the workers bees and a population ranging from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 50
000 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 individuals, we can estimate a need around 30 ng of Mo/bee and around 4 mg of Mo per colony per season. These needs must be met by pollen, but due to a growing lack of resources, the depletion of these resources, and a temporal mismatch between pollen availability and honey bees’ activity,5,6,12 it is likely that the needs are not being totally met and that appropriate supplementation with Mo could cover the shortfall. Besides, as we demonstrated that these complexes are consumed over a long period of time, they have a prolonged action in the beehives. Therefore, they can feed several generations of bees and fill their deficiency in the Mo trace element, if so. This is a first hypothesis to explain the benefits of the colonies with our complexes, but this environmental deficiency would need to be proven in future studies and the role of Mo must be elucidated.
000 individuals, we can estimate a need around 30 ng of Mo/bee and around 4 mg of Mo per colony per season. These needs must be met by pollen, but due to a growing lack of resources, the depletion of these resources, and a temporal mismatch between pollen availability and honey bees’ activity,5,6,12 it is likely that the needs are not being totally met and that appropriate supplementation with Mo could cover the shortfall. Besides, as we demonstrated that these complexes are consumed over a long period of time, they have a prolonged action in the beehives. Therefore, they can feed several generations of bees and fill their deficiency in the Mo trace element, if so. This is a first hypothesis to explain the benefits of the colonies with our complexes, but this environmental deficiency would need to be proven in future studies and the role of Mo must be elucidated.
To date, the main part of the in-field experiments in the literature are focused on protein-based diets and very little is known about the mineral supplements and especially how transition metals cations can modulate the defence mechanisms of the bees against biotic and abiotic sources of stress and then reinforce the health of the colonies. In particular, to date, there are no studies in the literature that examine the effects of molybdenum on bees. After absorption, mineral elements enter the haemolymph and cells and then activate a large number of enzymes involved in the detoxification of xenobiotics.55 Among transition metals, zinc has received particular attention. Zinc used as simple salt, has been shown to stimulate hypopharyngeal glands development and upregulate major royal jelly proteins.27c De Almeida Longuini et al. evidenced an increase in the level of proteins involved in defence systems by feeding with Zinc sulfate at 50–75 ppm concentration, thus affecting nutrition and maintenance of colonies.27d However, excessive zinc supplementation can result in colony-level disturbances and even abandonment.27b More recently, Ghasemi et al. reported the effect of a mixture of transition metal complexes containing Ca, Mg, Fe, Mn, Cu, Zn, Cr and Co at various dosages.31 When low concentration of the mixture is used the authors observed the promotion of HPG growth, together with an increase in the level of nutrient in the bees and a better resistance to the pesticide dimethoate at a sublethal level in laboratory conditions. In contrast, the mixture proved to be toxic when the concentration increases. The use of molybdenum complexes offers distinct advantages compared to these studies since no toxicity was evidenced even at high concentration for Na-Mo2O4-EDTA both in laboratory and in field conditions. Besides, in-depth studies in the field, in the laboratory and at the synchrotron facility have confirmed the bioavailability and assimilation of Mo in both worker bees and larvae by feeding with our complexes. In particular, we demonstrated that Mo accumulation was especially prominent in the neurolemma and hypopharyngeal glands—two critical tissues involved in nutrition, immunity, and neural function49–51 which already naturally contain traces of molybdenum (see Fig. 6A and 7A). These findings suggest that Mo may play a previously unrecognized physiological role in honey bee health, particularly through its incorporation into key organs. In particular, the HPGs are involved in the production of royal jelly which is the primary food of the queen bee, whereas the drone, worker bees and larvae feed upon royal jelly, honey, and pollen. An effect on the quality of the royal jelly should impact the health and the behaviour of the queen bee, what we observe in hives with an increase fecundity of 11–13%.
Honey bee gut microbiome research is an emerging field.5 A recent review of Smriti et al. evidences that many authors demonstrated that the use of probiotic-supplemented diet can promote honeybee gut health, enhance immunity, and overall well-being.55 One of the major reasons for the loss of bee colonies is infections caused by different pathogens like Paenibacillus larvae, Varroa mites and Nosema cerana. Probiotics can increase the gut health of honeybees and a better gut health favours the development of gut microbiota, which plays a crucial role to induce immune response56 and serve various immunomodulatory functions so that they can fight bacterial infections. In particular, beneficial bacteria in the gut of bees help to reduce the impact of nosemosis and varroosis infections.57 Thus, probiotics help to increase the survival rate of bees by preventing pathogenic infections and increase the colony strength. It also leads to more honey and royal jelly production by increasing the expression of several genes. In another study, Tejerina et al. demonstrated the effectiveness of feed supplementation with probiotics derived from lactic acid bacteria against mites such as Varroa.58 All these elements lead to lower colony loss due to infections and increase the survival rate of bees. For instance, Kaznowski et al. revealed that probiotics promoted bee survival, as bee losses were 30–50% lower in colonies fed probiotics compared to control hives.59 However, Tlak Gajger et al. showed that if a 5% probiotic supplementation to sugar syrup increased colony strength, a 10% concentration leads to honeybee mortality.60
The effects observed by sugar syrup supplemented with probiotics are similar to those we observed in our field trials with Mo-complexes. It thus appears appealing to hypothesize that our Mo-complexes could have a positive impact on the gut microbiota of honey bees to explain the variety of effect observed in hives. In a previous study, we evidenced that these complexes have no antibacterial effects and can promote the development of biomass,36 which is compatible with this hypothesis.
Finally, X-ray photoelectron spectroscopy (XPS) analyses on bee's faeces revealed the oxidation of Mo(V) to Mo(VI) within bee organisms, indicating a potential antioxidant mechanism, which was confirmed by field test experiments on hemolymph of larvae and worker bees. Given the metabolic demands and oxidative stress encountered by bees, especially during periods of environmental or pathogenic stress, the antioxidant properties of these complexes could underlie many of their observed benefits in hives. Such antioxidant activity (AOA) in both bees and larvae can hold particular significance for insects with high metabolic rates, inherently generating substantial volumes of free radicals.61 Therefore, implementing proactive measures to mitigate oxidative stress effects can substantially boost the resilience of honey bees62 and improve their health and their productivity.
Taken together, molybdenum-based supplementation appears to offer a multifunctional mode of action: improving colony productivity, modulating physiological and neurological tissues, and providing redox-related protection—all without compromising honey safety. The impact on the hypopharyngeal glands and on the intestinal microbiota, in addition to compensating for molybdenum deficiency and providing an antioxidant effect, are plausible hypotheses to explain the varied effects we have observed in hives.
Of course, this does not rule out other hypotheses and paves the way towards many further investigations to elucidate the mode of action of our complexes. In particular, further studies are needed (i) to evidence an environmental defiencies in Mo, (ii) to explore the expression and activity of Mo-dependent enzymes in bees, and to assess how these complexes might protect against combined stressors such as pesticides, pathogens, and poor nutrition, (iii) to investigate more deeply the impact on HPG glands (size, vitellogenin production), the production and quality of royal jelly and its impact on queen bees, (iv) to study the impacts of Mo-complexes on the longevity of bees in hives, on the gut microbiota, or on social behaviour, and (v) to extend this work on other pollinators.
4. Conclusion
Based on a large set of field-test campaigns unprecedented in the literature and lab experiments, this study establishes the importance of the molybdenum trace element for honey bee health. These results are promising for the beekeeping industry, which is currently suffering from numerous challenges weakening honey bee colonies, to improve winter survival and colony productivity.In this study, we demonstrated that feeding with only a few milligrams of the coordination complexes Li-Mo2O4-EDTA or Na-Mo2O4-EDTA during spring or autumn improves key parameters of colony performance: it enhances in particular colony development, and honey and wax production. Furthermore, it also reduces infestation by the mite Varroa destructor and decreases winter colony losses. Importantly, feeding with Mo-complexes had no effect on the quality of the produced honey.
In-depth studies in the field, in the laboratory and at the synchrotron facility have demonstrated that these complexes have a prolonged action in the beehives and that the Mo trace element is naturally present in bees’ cuticle, muscles, hypopharyngeal glands and in the neurolemma. Interestingly, Mo levels increased in the neurolemma and in the hypopharyngeal glands after supplementation with Mo-complexes. Lastly, our experiments suggest that the complexes Li-Mo2O4-EDTA and Na-Mo2O4-EDTA act as antioxidant agents in bees.
This work repositions molybdenum—until now largely neglected in apicultural science—as a key trace element for bee health and opens many avenues for future works not only for our molybdenum-based complexes but also for coordination complexes in general, which could play a significant role in helping to protect pollinator insects.
Author contributions
I. T., J.-C. S. and S. F. initiated and coordinated the project. A. F. synthesized and characterized the complexes. X. L. performed DFT studies. M. A. S, A. O. S., and T. N. P. performed the toxicity studies on mice. O. Ga. and A. G. coordinated and performed toxicity studies on Daphnia Magna and Anti oxidative Properties studies. L. C-D., A. N., V. L. performed the toxicity studies on bees in laboratory conditions. V. C., N. R. and I. T. performed and followed the field tests in Moldova. A. F., S. F., P. C., and B. P. performed and followed the field tests in France and in USA. L. Ch. and F. H. performed the field tests in Greece. O. Gl. collected and provided bee samples from Moldova. I. R and L. C-D. performed the slices of bees. L. C., I. G., J.-C. S., S. F., A. S. and K. M. managed Synchrotron studies with L. C-D., I. G., S. F., and J.-C. S., M. F. performed the XPS studies. P. C. and S. G. performed statistics.Conflicts of interest
The results of this study are partially patented to protect the complexes for their potential use in beekeeping industry. Some authors, namely Arcadie Fuior, Valentina Cebotari, Aurelian Gulea, Ion Toderas and Sébastien Floquet are co-inventors of this patent delivered in France (FR3112667B1 on 23 feb. 2024), In Europe (EP4185594B1 on 4 dec2024) and in progress in USA, in Canada, in Argentina and in China.Data availability
All the details of the synthesis, characterizations, properties measurements, test in hives, etc. are given in the ESI† with the raw data.To facilitate the reading, the ESI† is divided into 8 independent parts built as reports to cover each topic. Part I contains data about Mo level measured in honeybees by ICP-MS or ICP-OES; Part II contains all details on the synthesis of complexes, their characterization and the stability studies by DFT calculation, 1H NMR and UV-visible spectroscopies; Part III contains the toxicity studies on mice, Daphnia Magna, and bees both in laboratory and in hives; Part IV gathers all the field tests in beehives and analyses on honey with the raw data obtained for each test campaign; Part V is focused on the cycle of Mo in beehives after feeding by monitoring Mo content by ICP-MS in larvae, worker bees, honey/syrup, wax, and bee bread; Part VI contains the data about the assimilation of Mo by honey bees in laboratory conditions in head, thorax and abdomen. The Mo, Na/Li contents are measured by ICP-MS; Part VII gathered all data of X-ray fluorescence acquired at synchrotron SOLEIL; Part VIII focuses on the metabolism and the antioxidant properties of Mo-complexes consumed by honey bees thank to an XPS study of bee faeces and spectroscopic determination of anti-oxidant activity of hemolymph of bees and larvae as well as hive products by ABTS and DPPH methods.
Acknowledgements
University of Versailles, the “Institut Universitaire de France, IUF” and the CNRS are gratefully acknowledged for financial support. AF gratefully acknowledge Campus France for Excellence Eiffel grant as well as State University of Moldova for funding his PhD thesis. This work was funded by the Labex Charmmmat (project “COMPA”), the IDEX Paris-Saclay (project “COMPA2”), the “lune de miel” foundation, the UVSQ foundation (project “COMPA3”), the CNRS MITI (call Metallomix, project “MOLYBEE”), and the SATT Paris-Saclay (projects “COMPA4” and “APIMONA”). The financial support from National Agency for Research and Development (ANCD) of the Republic of Moldova (Project No. 20.80009.5007.10) is also acknowledged. Dr Clémence Riva is gratefully acknowledged for her help for the statistical analyses. The NIIC team thanks the Ministry of Science and Higher Education of the Russian Federation (No. 121031700321-3). AOS acknowledges the support of assays by state funding of RICEL—Branch of IC&G SB RAS. Mrs Aneta Ozieranska and Dr Karen Wright from Institut Lavoisier of Versailles are gratefully acknowledged for English correction.References
- A. M. Klein, B. E. Vaissiere, J. H. Cane, I. Steffan-Dewenter, S. A. Cunningham, C. Krement and T. Tscharntke, Importance of pollinators in changing landscapes for world crops, Proc. Biol. Sci., 2007, 274, 303–313 Search PubMed.
- N. Gallai, J. M. Salles, J. Settele and B. E. Vaissiere, Economic valuation of the vulnerability of world agriculture confronted with pollinator decline, Ecol. Econ., 2009, 68, 810–821 CrossRef.
- S. G. Potts, S. P. M. Roberts, R. Dean, G. Marris, M. A. Brown, R. Jones, P. Neumann and J. Settele, Declines of managed honey bees and beekeepers in Europe, J. Apic. Res., 2010, 49(1), 15–22 CrossRef.
- D. Goulson, E. Nicholls, C. Botias and E. L. Rotheray, Bee declines driven by combined stress from parasites, pesticides, and lack of flowers, Science, 2015, 347, 1255957 CrossRef PubMed.
- J. M. Tsuruda, P. Chakrabarti and R. R. Sagili, Honey Bee Nutrition, Vet. Clin. Food Anim., 2021, 37, 505–519 CrossRef.
- P. W. Lau, I. L. Esquivel, K. A. Parys, K.-L. J. Hung and P. Chakrabarti, The nutritional landscape in agroecosystems: a review on how resources and management practices can shape pollinator health in agricultural environments, Ann. Entomol. Soc. Am., 2023, 116(5), 261–275 CrossRef CAS.
- R. Brodschneider and K. Crailsheim, Nutrition and health in honey bees, Apidologie, 2010, 41, 278–294 CrossRef.
- E. Topal, N. Çakıcı, R. Mărgăoan, Ç. Takma, F. Güney, M. Kösoğlu, M. Cornea-Cipcigan and H. Atmaca, Annual Development Performance of Fixed Honeybee Colonies Linked with Chemical and Mineral Profile of Bee Collected Pollen, Chem. Biodivers., 2022, 19, e202200468 CrossRef CAS.
- C. Alaux, F. Ducloz, D. Crauser and Y. Le Conte, Diet effects on honeybee immunocompetence, Biol. Lett., 2010, 6, 562–565 CrossRef PubMed.
- A. G. Dolezal, J. Carrillo-Tripp, T. M. Judd, W. A. Miller, B. C. Bonning and A. L. Toth, Interacting stressors matter: diet quality and virus infection in honeybee health, R. Soc. Open Sci., 2019, 6, 181803 CrossRef CAS PubMed.
- D. Frizzera, A. M. Ray, E. Seffin, V. Zanni, D. Annoscia, C. M. Grozinger and F. Nazzi, The Beneficial Effect of Pollen on Varroa Infested Bees Depends on Its Influence on Behavioral Maturation Genes, Front. Insect Sci., 2022, 2, 864238 CrossRef PubMed.
- M. Gérard, M. Vanderplanck, T. Wood and D. Michez, Global warming and plant– pollinator mismatches, Emerging Top. Life Sci., 2020, 4(1), 77–86 CrossRef.
- L. Desmedt, L. Hotier, M. Giurfa, R. Velarde and M. G. de Brito Sanchez, Absence of food alternatives promotes risk-prone feeding of unpalatable substances in honey bees, Sci. Rep., 2016, 6, 31809 CrossRef CAS PubMed.
- A. Brown, V. Rodriguez, J. Pfister, V. Perreten, P. Neumann and G. Retschnig, The dose makes the poison: feeding of antibiotic-treated winter honey bees, Apis mellifera, with probiotics and b-vitamins, Apidologie, 2022, 53, 19 CrossRef CAS.
- N. M. Jovanovic, U. Glavinic, B. Delic, B. Vejnovic, N. Aleksic, V. Mladjan and Z. Stanimirovic, Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance, Prev. Vet. Med., 2021, 190, 105322 CrossRef PubMed.
- R. Pavlović, B. Dojnov, M.Š. Slavić, M. Ristović, M. Vujčić, S. Stojanović and Z. Vujčić, Differential Processing of Sucrose and Invert Syrup in Honey Bees, Arch. Insect Biochem. Physiol., 2025, 118, e70052 CrossRef PubMed.
- V. A. Ricigliano, S. T. Williams and R. Oliver, Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota, BMC Vet. Res., 2022, 18, 52 CrossRef CAS.
- A. Saffari, P. G. Kevan and J. L. Atkinson, Consumption of three dry pollen substitutes in commercial apiaries, J. Apic. Sci., 2010, 54(2), 13–20 Search PubMed.
- E. J. García-Vicente, M. Martín, I. Rey-Casero, A. Perez, J. Martín, A. García, J. M. Alonso and D. Risco, Effects of feeding with a protein liquid supplement on productivity, mortality and health of Apis mellifera hives in southwestern Spain, Res. Vet. Sci., 2024, 169, 105173 CrossRef PubMed.
- G. DeGrandi-Hoffman, Y. Chen, R. Rivera, M. Caroll, M. Chambers, G. Hidalgo and E. Watkins de Jong, Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements, Apidologie, 2016, 47, 186–196 CrossRef CAS.
- T. Bogaert, T. Reams, I. Maillet, K. Kulhanek, M. Duyck, F. Eertmans, A. M. Fauvel, B. Hopkins and J. Bogaert, A nutritionally complete pollen-replacing diet protects honeybee colonies during stressful commercial pollination —requirement for isofucosterol, Proc. R. Soc. B, 2025, 292, 20243078 CrossRef CAS PubMed.
- E. J. García-Vicente, M. Martín, I. Rey-Casero, A. Perez, R. Martínez, M. Bravo, J. M. Alonso and D. Risco, Effect of feed supplementation with probiotics and postbiotics on strength and health status of honey bee (Apis mellifera) hives during late spring, Res. Vet. Sci., 2023, 159, 237–243 CrossRef PubMed.
- (a) R. E. Bonoan, L. D. O'Connor and P. T. Starks, Seasonality of honey bee (Apis mellifera) micronutrient supplementation and environmental limitation, J. Insect Physiol., 2018, 107, 23–28 CrossRef CAS PubMed; (b) R. E. Bonoan, T. M. Tai, M. Tagle-Rogriguez, L. Feller, S. R. Daddario, R. Czaja, L. D. O'Connor, G. Buruss and P. T. Starks, Seasonality of salt foraging in honey bees (Apis mellifera), Ecol. Entomol., 2017, 42, 195–201 CrossRef.
- C. H. Liang, C. L. Chuang, J. A. Jiang and E. C. Yang, Magnetic Sensing through the Abdomen of the Honey bee, Sci. Rep., 2016, 6, 23657 CrossRef CAS PubMed.
- H. N. Koo, S. G. Lee, S. H. Yun, H. K. Kim, Y. S. Choi and G. H. Kim, Comparative Analyses of Cu-Zn Superoxide Dismutase (SOD1) and Thioredoxin Reductase (TrxR) at the mRNA Level between Apis mellifera L. and Apis cerana F. (Hymenoptera: Apidae) Under Stress Conditions, J. Insect Sci., 2016, 16(1), 4 CrossRef PubMed.
- (a) Y. Ben-Shahar, The Impact of Environmental Mn Exposure on Insect Biology, Front. Genet., 2018, 9, 70 CrossRef PubMed; (b) E. Sovik, C. J. Perry, A. LaMora, A. B. Barron and Y. Ben-Shahar, Negative impact of manganese on honey bee foraging, Biol. Lett., 2015, 11(3), 20140989 CrossRef PubMed.
- (a) M. P. Camilli, S. M. Kadri, M. V. N. Alvarez, P. E. M. Ribolla and R. O. Orsi, Zinc supplementation modifies brain tissue transcriptome of Apis mellifera honey bees, BMC Genomics, 2022, 23(1), 282 CrossRef CAS PubMed; (b) M. P. Carillo, A. Astolfi, E. J. D. Rodrigues, I. C. D. Lippi, L. G. R. Da Silva, M. G. Guimaraes, L. A. Justulin and R. D. Orsi, High doses of inorganic zinc modulate the hypopharyngeal glands of Apis mellifera but promote abandonment of colonies when offered over long periods, Bull. Insectology, 2022, 75(1), 27–31 Search PubMed; (c) G. D. Ribeiro, S. M. Kadri, L. A. Justulin, P. E. M. Ribolla and R. D. Orsi, Zinc methionine or zinc sulphate supplementation modulate the development of the hypopharyngeal gland and expression of major royal jelly protein genes in Apis mellifera L. bees, Physiol. Entomol., 2023, 48(2–3), 90–96 CrossRef; (d) L. A. de Almeida Longuini, G. Moreno Martineli, M. Polizel Camilli, D. Cavalcante Brambila de Barros, J. Cavalcante Souza Vieira, P. de Magalhães Padilha and R. de Oliveira Orsi, Supplementation with an Inorganic Zinc Source in the Metalloproteomic Profile of Royal Jelly in Apis mellifera, Biol. Trace Elem. Res., 2021, 199, 4308–4318 CrossRef PubMed.
- (a) T. V. Nikolic, D. Kojic, S. Orcic, D. Batinic, E. Vukasinovic, D. P. Blagojevic and J. Purac, The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions, Chemosphere, 2016, 164, 98–105 CrossRef CAS PubMed; (b) N. Di, K. R. Hladun, K. Zhang, T. X. Liu and J. T. Trumble, Laboratory bioassays on the impact of cadmium, copper and lead on the development and survival of honey bee (Apis mellifera L.) larvae and foragers, Chemosphere, 2016, 152, 530–538 CrossRef CAS; (c) C. Monchanin, E. Drujont, G. Le Roux, P. D. Lösel, A. B. Barron, J. M. Devaud, A. Elger and M. Lihoreau, Environmental exposure to metallic pollution impairs honey bee brain development and cognition, J. Hazard. Mater., 2024, 465, 133218 CrossRef CAS.
- E. W. Herbert, A New Ash Mixture for Honeybees Maintained on a Synthetic Diet, J. Apic. Res., 1979, 18, 144–147 CrossRef.
- G. Zhang, W. Zhang, X. Cui and B. Xu, Zinc nutrition increases the antioxidant defenses of honey bees, Entomol. Exp. Appl., 2015, 156, 201–210 CrossRef CAS.
- V. Ghasemi, G. N. Paghaleh, E. Torabi, Z. S. Arzanforoosh, R. Bideshki, S. Kalanaky, S. Fakharzadeh, M. Hafizi and M. H. Nazara, Effects of advanced chelate-based mineral supplement on adult worker honey bees’ (Apis mellifera L.) survival, nutritional indices, hypopharyngeal glands’ growth, energy reserve contents, and tolerance to dimethoate, J. Apic. Res., 2025 DOI:10.1080/00218839.2024.2446106.
- (a) R. Hille, Molybdenum and Tungsten in Biology, Trends Biochem. Sci., 2002, 27(7), 360–367 CrossRef CAS PubMed; (b) R. R. Mendel and T. Kruse, Cell biology of molybdenum in plants and humans, Biochim. Biophys. Acta, 2012, 1823, 1568–1579 CrossRef CAS PubMed.
- (a) T. Peng, Y. Xu and Y. Zhang, Comparative genomics of molybdenum utilization in prokaryotes and eukaryotes, BMC Genomics, 2018, 19, 691 CrossRef; (b) Y. Zhang, S. Rump and V. N. Gladyshev, Comparative genomics and evolution of molybdenum utilization, Coord. Chem. Rev., 2011, 255(9–10), 1206–1217 CrossRef CAS PubMed.
- V. R. Ott;, D. S. Swieter; and F. A. Schultz, Di-μ-Oxo, μ-Oxo- μ-Sulfido, and Di-μ-Sulfido Complexes of Molybdenum(V) with EDTA, Cysteine, and Cysteine Ester Ligands. Preparation and Electrochemical and Spectral Properties, Inorg. Chem., 1977, 16(10), 2538–2545 CrossRef.
- J. M. Gretarsdóttir;, S. Bobersky;, N. Metzler-Nolte and S. G. Suman, Cytotoxicity Studies of Water-Soluble Coordination Compounds with a [Mo2O2S2]2+ Core, J. Inorg. Biochem., 2016, 160, 166–171 CrossRef.
- A. Fuior, A. Hijazi, O. Garbuz, V. Bulimaga, L. Zosim, D. Cebotari, M. Haouas, I. Toderaş, A. Gulea and S. Floquet, Screening of biological properties of MoV2O2S2- and MoV2O4-based coordination complexes: Investigation of antibacterial, antifungal, antioxidative and antitumoral activities versus growing of Spirulina platensis biomass, J. Inorg. Biochem., 2022, 226, 111627 CrossRef CAS PubMed.
- J. M. Gretarsdottir;, S. Jonsdottir;, W. Lewis;, T. W. Hambley; and S. G. Suman, Water-Soluble Alpha-Amino Acid Complexes of Molybdenum as Potential Antidotes for Cyanide Poisoning: Synthesis and Catalytic Studies of Threonine, Methionine, Serine, and Leucine Complexes, Inorg. Chem., 2020, 59(24), 18190–18204 CrossRef.
- J. M. Gretarsdottir, I. H. Lambert, S. Sturup and S. G. Suman, In Vitro Characterization of a Threonine-Ligated Molybdenyl-Sulfide Cluster as a Putative Cyanide Poisoning Antidote; Intracellular Distribution, Effects on Organic Osmolyte Homeostasis, and Induction of Cell Death, ACS Pharmacol. Transl. Sci., 2022, 5(10), 907–918 CrossRef CAS PubMed.
- I. Toderas, A. Gulea, V. Cebotari, S. Floquet, E. Cadot and I. Buzu, A method for preparing a bio active food supplement for bees feed and process for feeding bee colonies Apis mellifera”, Patent No. 4438 MD–BOPI 4, 2016 p. 16.
- (a) B. Ziegelmann, E. Abele, S. Hannus, M. Beitzinger, S. Berg and P. Posenkranz, Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action, Sci. Rep., 2018, 8, 683 CrossRef PubMed; (b) S. Sevin, V. Bommuraj, Y. R. Chen, O. Afik, S. Zarchin, S. Barel, O. C. Arslan, B. Erdem, H. Tutun and J. A. Shimshoni, Lithium salts: assessment of their chronic and acute toxicities to honey bees and their anti-Varroa field efficacy, Pest Manage. Sci., 2022, 78(11), 4507–4516 CrossRef CAS PubMed.
- F. Mondet, S. Blanchard, N. Barthes, D. Beslay, C. Bordier, G. Costagliola, M. R. Hervé, B. Lapeyre, S. H. Kim, B. Basso, A. R. Mercer and Y. Le Conte, Chemical detection triggers honey bee defence against a destructive parasitic threat, Nat. Chem. Biol., 2021, 17, 524–530 CrossRef CAS.
- M.-P. Chauzat, M. Laurent, M.-P. Rivière, C. Saugeon, P. Hendricx and M. Ribiere-chabert, Report “A pan-European epidemiological study on honeybee colony losses 2012–2013”, Epilobee, 27th april 2024.
- A. Gray, N. Adjlane, A. Arab, A. Ballis, V. Brusbardis, A. Bugeja Douglas, L. Cadahía, J.-D. Charrière, R. Chlebo, M. F. Coffey, B. Cornelissen, C. A. D. Costa, E. Danneels, J. Danihlík, C. Dobrescu, G. Evans, M. Fedoriak, I. Forsythe, A. Gregorc, I. I. Arakelyan, J. Johannesen, L. Kauko, P. Kristiansen, M. Martikkala, R. Martín-Hernández, E. Mazur, C. A. Medina-Flores, F. Mutinelli, E. M. Omar, S. Patalano, A. Raudmets, G. San Martin, V. Soroker, P. Stahlmann-Brown, J. Stevanovic, A. Uzunov, F. Vejsnaes, A. Williams and R. Brodschneider, Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: the combined effects of operation size, migration and queen replacement, J. Apic. Res., 2023, 62, 204–210 CrossRef.
- F. Requier, K. Antúnez, C. L. Morales, P. Aldea Sánchez, D. Castilhos, P. M. Garrido, A. Giacobino, F. J. Reynaldi, J. M. Rosso Londoño, E. Santos and L. A. Garibaldi, Trends in beekeeping and honey bee colony losses in Latin America, J. Apic. Res., 2018, 57, 657–662 CrossRef.
- S. Bruckner, M. Wilson, D. Aurell, K. Rennich, D. van Engelsdorp, N. Steinhauer and G. R. Williams, A national survey of managed honey bee colony losses in the USA: results from the Bee Informed Partnership for 2017–18, 2018–19, and 2019–20, J. Apic. Res., 2023, 62, 429–443 CrossRef.
- D. van Engelsdorp and M. D. Meixner, A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them, J. Invertebr. Pathol., 2023, 103, S80–S95 CrossRef PubMed.
- D. van Engelsdorp, J. Hayes, R. M. Underwood and J. S. Pettis, A survey of honey bee colony losses in the United States, fall 2008 to spring 2009, J. Apic. Res., 2010, 49(1), 7–14 CrossRef.
- https://www.projectapism.org/colony-loss-information .
- S. Ahmad, S. A. Khan, K. A. Khan and J. K. Li, Novel Insight Into the Development and Function of Hypopharyngeal Glands in Honey Bees, Front. Physiol., 2021, 11, 615830 CrossRef.
- L. N. Standifer, A comparison of the protein quality of pollen for growth-stimulation of the hypopharyngeal glands and longevity of honey bees Apis Mellifera L. (Hymenoptera : Apidae), Insectes sociaux, Paris, 1967, 14(4), 415–426 CAS.
- G. M. Quinlan and C. M. Grozinger, Evaluating the role of social context and environmental factors in mediating overwintering physiology in honey bees (Apis meliffera), J. Exp. Biol., 2024, 227, jeb247314 CrossRef.
- (a) C. R. Clayton and Y. C. Lu, Electrochemical and XPS Evidence of the Aqueous Formation of Mo2O5, Surf. Interface Anal., 1989, 14(1–2), 66–70 CrossRef CAS; (b) P. A. Spevack and N. S. McIntyre, Thermal Reduction of Molybdenum Trioxide, J. Phys. Chem., 1992, 96(22), 9029–9035 CrossRef CAS; (c) X. Liao, A. R. Jeong, R. G. Wilks, S. Wiesner, M. Rusu and M. Bär, X-Ray, Irradiation Induced Effects on the Chemical and Electronic Properties of MoO3 Thin Films, J. Electron Spectrosc. Relat. Phenom., 2016, 212, 50–55 CrossRef CAS; (d) J. Baltrusaitis, B. Mendoza-Sanchez, V. Fernandez, R. Veenstra, N. Dukstiene, A. Roberts and N. Fairley, Generalized Molybdenum Oxide Surface Chemical State XPS Determination via Informed Amorphous Sample Model, Appl. Surf. Sci., 2015, 326, 151–161 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radicals Biol. Med., 1999, 26, 1231–1237 CrossRef CAS PubMed.
- J. Kocot, M. Kiełczykowska, D. Luchowska-Kocot, J. Kurzepa and I. Musik, Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application, Oxid. Med. Cell. Longevity, 2018, 7074209 CrossRef PubMed.
- Smriti, A. Rana, G. Singh and G. Gupta, Prospects of probiotics in beekeeping: a review for sustainable approach to boost honeybee health, Arch. Microbiol., 2024, 206, 205 CrossRef CAS PubMed.
- M. J. Molloy, N. Bouladoux and Y. Belkaid, Intestinal microbiota: shaping local and systemic immune responses, Semin. Immunol., 2012, 24(1), 58–66 CrossRef CAS.
- M. C. Audisio, Gram-positive bacteria with probiotic potential for the Apis mellifera L. honeybee: the experience in the northwest of Argentina, Probiotics Antimicrob. Proteins, 2017, 9, 22–31 CrossRef CAS PubMed.
- M. R. Tejerina, M. R. Benítez-Ahrendts and M. C. Audisio, Lactobacillus salivarius A3iob reduces the incidence of Varroa destructor and Nosema Spp. in commercial apiaries located in the northwest of Argentina, Probiotics Antimicrob. Proteins, 2020, 12, 1360–1369 CrossRef PubMed.
- A. Kaznowski, B. Szymas, E. Jazdzinska, M. Kazimierczak, H. Paetz and J. Mokracka, The effects of probiotic supplementation on the content of intestinal microflora and chemical composition of worker honeybees (Apis mellifera), J. Apic. Res., 2005, 44(1), 10–14 CrossRef.
- I. Tlak Gajger, J. Vlainić, P. Šoštarić, J. Prešern, J. Bubnič and M. I. Smodiš Škerl, Effects on some therapeutical, biochemical, and immunological parameters of honeybee (Apis mellifera) exposed to probiotic treatments, in field and laboratory conditions, Insects, 2020, 11(9), 638 CrossRef PubMed.
- D. J. Candy, A. Becker and B. Wegener, Coordination and integration of metabolism in insect flight, Comp. Biochem. Physiol., 1997, 117B, 497–512 CrossRef CAS.
- M. Farjan, M. Dmitryjuk, Z. Lipiński, E. Biernat-Łopieńska and K. Żółtowska, Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages, J. Apic. Res., 2012, 51(3), 263–270 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, stability studies, toxicity studies, details on test on beehives, monitoring of the Mo in beehives, assimilation of Mo by honey bees, synchrotron studies, XPS and anti-oxidative property studies. See DOI: https://doi.org/10.1039/d5qi00878f |
| ‡ These authors jointly supervised the study. |
| This journal is © the Partner Organisations 2025 |