Open Access Article
Open Access ArticleA DNA aptamer for trivalent lanthanide ions with low nanomolar affinity†
Jin
Wang
ab,
Xin
Wang
bc,
Xuesong
Li
bd,
Xiangmei
Li
 a,
Hongtao
Lei
a,
Hongtao
Lei
 a and
Juewen
Liu
a and
Juewen
Liu
 *b
*b
aGuangdong Provincial Key Laboratory of Food Quality and Safety, College of Food Science, South China Agricultural University, Guangzhou 510642, China
bDepartment of Chemistry, Waterloo Institute for Nanotechnology, University of Waterloo, Waterloo, Ontario N2L 3G1, Canada. E-mail: liujw@uwaterloo.ca
cBeijing Laboratory for Food Quality and Safety, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
dSchool of the Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, China
First published on 7th April 2025
Abstract
Lanthanides are extremely important for a variety of technological applications. In this work, DNA aptamers were selected using the library-immobilization method (capture-SELEX) with Tb3+ and Ce3+ as target metal ions. The Tb3+ selection yielded a new sequence named Tb-1 that has a Kd of 26.9 nM for La3+, 3.9 nM for Tb3+, and 2.3 nM for Lu3+ as determined by a DNA strand displacement assay. Non-lanthanide metal ions did not induce a fluorescence enhancement. Therefore, it is a general lanthanide binding aptamer. Another aptamer Tb-4 (Kd 290 nM) has some sequence similarity to a previously reported aptamer selected using Gd3+ (Kd 1.5 μM), as determined by a thioflavin T fluorescence assay. Compared to a previously reported aptamer named Sc-1, Tb-1 has faster exchange with EDTA for lanthanide binding, suggesting that Tb-1 is an outer-sphere ligand. By comparing different aptamers, we have gained fundamental insights into aptamer binding to lanthanide ions. Finally, using the strand displacement reaction, a detection limit of 0.5 nM Tb3+ was achieved in Lake Ontario water.
Introduction
Lanthanide ions comprise a group of 15 metal ions within the f-block of the periodic table. They are extremely important in modern technologies.1,2 With rich f-orbitals, lanthanide ions have unique optical and magnetic properties, allowing them to be used in magnetic imaging contrast agents, permanent magnets and upconverting nanoparticles.3,4 Yet, they all have similar chemical properties since electrons in the f-orbitals are shielded and do not usually participate in chemical bonding. Lanthanides are further categorized based on their atomic numbers to be light (from lanthanum to europium) and heavy (from gadolinium to lutetium).5Lanthanide ions can be accurately measured by inductively coupled plasma optical emission spectroscopy (ICP-OES),6–8 and ICP mass spectrometry (ICP-MS).9,10 However, these techniques require expensive instruments and can hardly be implemented for on-site and real-time detection. Many chemical probes have been developed for the detection of lanthanide ions,11,12 but most are less sensitive or selective. Lanthanide binding proteins have also been discovered with high binding affinity, although using them for detecting lanthanides could be challenging due to cost, stability and signal transduction considerations.13–15
DNA is composed of four types of nucleosides attached to a phosphate backbone, and both nucleobases and phosphate can bind to lanthanide ions.16–18 Attempts have been made to use DNA to detect lanthanides. Using a method called in vitro selection, our lab previously isolated five DNAzymes that can cleave an RNA substrate in the presence of trivalent lanthanide ions.2,19–22 These DNAzymes have different activity trends cross the lanthanide series, and in general they do not respond to non-lanthanide ions. Other groups also used lanthanide ions to catalyze reactions such as DNA cleavage.23 DNAzymes catalyze chemical reactions and they may not directly bind to lanthanides. It is important to isolate aptamers24–27 for lanthanide ions to achieve advanced applications such as separation, enrichment and detection.
A DNA aptamer named Ln-aptamer for Gd3+ was reported by Edogun and coworkers. To avoid confusion, in this work, we renamed it Gd-1.28 Their goal was to use this aptamer for the detection of free Gd3+ ions to ensure the safety of gadolinium-based contrast agents. The authors speculated that this aptamer can bind to all the lanthanide ions but they only measured binding using Gd3+. Our lab recently used the capture-SELEX method to isolate aptamers for Sc3+. Interestingly, this Sc3+ aptamer named Sc-1 can bind to all the trivalent lanthanide ions, and binding is stronger for heavier lanthanide ions. Based on the binding affinities and kinetics of Sc-1, rare earth elements were categorized into three groups.29
Since we already have an aptamer that binds to heavier lanthanides more strongly, in this work, we selected aptamers using terbium (Tb3+) and cerium (Ce3+). Tb is in the middle of the lanthanide series and is best known for its optical property with strong green emissions. Ce is a light lanthanide and it is often used in catalysis. In the end, we discovered an ultrahigh affinity aptamer that can bind to all the lanthanides with a similar affinity, so that the lanthanides can be detected as a group.
Materials and methods
Systematic evolution of ligands by the exponential enrichment (SELEX)
The SELEX experiments were conducted using the library immobilization following established procedures.30,31 The SELEX buffer contain 10 mM MES, pH 6.0, 100 mM NaCl, 2 mM MgCl2. TbCl3 hexahydrate and CeCl3 heptahydrate were dissolved in Milli-Q water at 100 mM. Then, they were diluted with SELEX buffer. The Tb3+ concentration was 10 μM for rounds 1 to round 7, 2 μM for rounds 8 and 9, 200 nM for round 10, and 100 nM for rounds 11 and 12. For the Ce3+ selection, the Ce3+ concentration was 10 μM for rounds 1 to 14 and 2 μM for rounds 15 and 16. The PCR products from the last rounds were deep sequenced at the facility in McMaster University.Thioflavin T (ThT) fluorescence-based binding assays
ThT fluorescence assays were conducted using a variant eclipse fluorescence spectrophotometer. A mixture containing 2.5 μL aptamer (100 μM), 5 μL ThT (100 μM) and 492.5 μL SELEX buffer was prepared. The mixture was then transferred to a quartz cuvette followed by titration with Tb3+ or other metal ions. The fluorescence intensity at 490 nm was recorded for analysis. The dissociation constant (Kd) was determined by fitting the equation: F = F0 + AKd/(Kd + x), where x denotes the concentration of the metal ion, and A denotes the maximal fluorescence variation upon full binding.30,32Fluorescent DNA strand-displacement sensor
The strand displacement sensor was tested in a 96-well microplate using a microplate reader (Tecan Spark F200Pro). 1 μL of FAM-labeled Tb-1 aptamer (100 μM) and 2 μL of quencher-labeled DNA (100 μM, Table S1†) were mixed in 97 μL SELEX buffer. This mixture was annealed at 85 °C and gradually cooled to room temperature, followed by incubation at 4 °C for 30 min and storage at −20 °C for an additional 30 min. The background fluorescence of the sensor was stabilized and recorded for 5 min before adding 2 μL Tb3+ or other metal ions. The kinetics of fluorescence signal change were then monitored for 10 min (Ex: 485 nm, Em: 520 nm). Each experiment was performed in triplicate.Results and discussion
Selection of aptamers for Tb3+
The capture-SELEX method was employed for aptamer selection,29–31 which started by the immobilization of a DNA library containing 30 random nucleotides via hybridization to a biotinylated capture strand (15 base pairs) bound to streptavidin agarose beads. After washing away unbound DNA, the immobilized library was then incubated with Tb3+. Some aptamers might be released from the capture strand upon binding to Tb3+, and the released DNA strands were amplified by PCR. We monitored the progress of the selection using real-time PCR by comparing the released DNA concentration eluted by buffer elution and by the same buffer containing Tb3+. The Tb3+ concentration was gradually decreased from 10 μM to 100 nM during the 12 rounds of selection. In the first 5 rounds, it appeared that more DNA was released by the buffer (Fig. S1†), suggesting that Tb3+ might have inhibited PCR. This phenomenon was also observed in our previous Sc3+ selection.29 Since round 7, the Tb3+ eluted DNA caught up and the PCR cycle difference reached 5, indicating the enrichment of Tb3+ binding aptamers. The PCR product of round 12 was deep sequenced.We ranked the sequences and the most abundant sequence named Tb-1 has only 218 copies (∼1% of the sequence obtained), whereas the rest of the sequences were all below 100 copies. Therefore, there were still a lot of diversity in the library, suggesting a large sequence variety of Tb3+ aptamers. To have an overall understanding, the top 10 sequences were aligned. We observed one family and some ungrouped sequences as shown in Fig. 1A. This family comprises 7 sequences, featuring conserved regions marked in red, and complementary base pairs highlighted in yellow. The first sequence in this family is named Tb-4. Tb-4 has a very clear secondary structure containing a large hairpin region and a conserved loop (Fig. 1C). Binding of Tb3+ likely takes place in the loop region. We also studied Tb-1, since it is the most abundant sequence, although it cannot be assigned to any family based on the top ten sequences. The mFold predicted secondary structure of Tb-1 is shown in Fig. 1B, which has two loops connected by two stem regions.
ThT fluorescence based binding assays
To evaluate the binding property of the aptamers, we first used thioflavin T (ThT) as a fluorescence probe. ThT is most well-known for its ability to bind G-quadruplex structures,33 but it can also bind to other DNA structures.27,34 Free ThT is almost non-fluorescent, and its fluorescence increases upon binding to DNA. As a DNA aptamer binds to its target, a fraction of the bound ThT molecules might be released, leading to decreased fluorescence (Fig. 2A).33 The ThT fluorescence assay has been widely applied to the study of binding to many aptamers.35,36 To evaluate whether the Tb-1 and Tb-4 aptamers can bind, we titrated Tb3+ ions into aptamer/ThT mixtures. The Tb-1 aptamer exhibited over 90% fluorescence drop with a fitted Kd of 106 nM, whereas Tb-4 showed only about 50% fluorescence drop with a Kd of 290 nM (Fig. 2B). The lower Kd indicates that Tb-1 has superior binding for Tb3+ compared to Tb-4.To confirm specific binding, we then performed some mutation studies on Tb-1. First, we mutated the “AT” highlighted by the red circle (Fig. 1B) by a “C” to form a “C–G” pair in the top stem region, and named this mutated aptamer Tb-1a. The ThT fluorescence assay showed that Tb-1a could still bind to Tb3+, whereas the Kd value increased slightly to 244 nM (Fig. 2B). Therefore, we believe that this aptamer has a simple structure with two loops connected by two base paired stems. Furthermore, we replaced the “GGTT” sequence with “TTGG” in the loop region (blue box at position b, Fig. 1B), and named the resulting mutant Tb-1b. This mutant showed significantly weakened binding to Tb3+, and the Kd increased to 1910 nM (Fig. 2B). Nevertheless, 90% fluorescence quenching was still observed upon the addition of 20 μM Tb3+. To test whether such a fluorescence drop was due to nonspecific fluorescence quenching by Tb3+, we tried two unrelated aptamers, OTC4337 and IBF-138 (see Table S1† for their sequences). For these two control aptamers, the fluorescence drop was less than 20% even with 500 μM Tb3+ (Fig. S2†). Therefore, Tb-1 and Tb-4 are true aptamers. We then studied the effect of salt and discovered that Mg2+ and Na+ ions had little effect on the binding property of the Tb-1 aptamer (Fig. S3†).
Tb-1 is selective for lanthanide ions as a group
We then assessed the selectivity of the Tb-1 aptamer. All trivalent rare earth metal ions (excluding radioactive Pm) were tested. Most of these metal ions, except for Sc3+, exhibited a similar degree of fluorescence drop of around 80%, indicating that they can all bind to Tb-1. The fluorescence decrease by Ce4+ was insignificant, suggesting this aptamer can only bind to trivalent lanthanides.Finally, other non-rare-earth metal ions, including Ca2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Ba2+, Hg2+, and Pb2+, were assessed at a higher concentration (5 μM). Among these, Cu2+ and Hg2+ exhibited a significant fluorescence decrease (purple bars, Fig. 2C) attributable to their strong fluorescence quenching properties. Since Cu2+ and Hg2+ are strongly thiophilic metals, for analytical applications, 2-mercaptoethanol can be used to chelate them, and this thiol compound should not affect the binding of Tb3+. Indeed, when Cu2+ was mixed with 2-mercaptoethanol, no fluorescence decrease was observed. However, upon the addition of Tb3+, fluorescence quenching occurred again (cyan-blue bars, Fig. S4A†). Similar results were obtained for Hg2+ (gray-blue bars, Fig. S4A†). Additionally, a slight fluorescence decrease was observed with Fe3+, Co2+, Ni2+, and Cd2+, which was also attributed to fluorescence quenching as confirmed through a strand-displacement assay (vide infra) (Fig. 2C). Therefore, Tb-1 is a general lanthanide binding aptamer.
A light-up DNA strand-displacement biosensor
The ThT fluorescence provides a simple and rapid binding assay, but it is in a signal turn-off mode. Certain metal ions such as Cu2+ and Hg2+ can strongly quench the fluorescence, leading to false-positive results. A turn-on design can overcome this problem. Therefore, we used a strand-displacement assay, which can also serve as a biosensor. Firstly, the 5′-end of aptamer Tb-1 was labeled with a carboxyfluorescein (FAM) fluorophore (Table S1†). The fluorescence of FAM was quenched upon hybridization with a short quencher-labeled complementary strand. In the presence of a lanthanide ion, binding of the aptamer led to the release of the quencher-labeled strand, resulting in an increase in fluorescence (Fig. 3A).29,39–41 We used 20 nM FAM-labeled strand and 40 nM quencher labeled strand to achieve a low background. When Tb3+ was added, the fluorescence immediately increased, indicating a rapid binding and subsequent release of the quencher-labeled strand. A series of concentrations of Tb3+ were tested (Fig. 3B), and a calibration curve with (F − F0)/F0 was fitted, and an apparent Kd of 72.4 nM was obtained (Fig. 3C). This is not the true Kd of the aptamer due to the presence of the quencher-labeled competing strand. Based on chemical equilibrium, we then calculated the true Kd for Tb-1 to be 3.9 nM, considering the quencher-labeled strand as a competitor (Fig. S6†).42 Similarly, titration experiments determined the true Kd values for Y3+, La3+, and Lu3+ to be 2.7 nM, 26.9 nM, and 2.3 nM, respectively (Fig. S5 and S6†).The selectivity of aptamer Tb-1 was further tested using the strand-displacement biosensor. Similar to the ThT assay, all rare earth metal ion (0.5 μM each), except for Sc3+, showed a similar fluorescence enhancement. In contrast, Sc3+ did not exhibit fluorescence enhancement, suggesting that Tb-1 does not bind to Sc3+, attributable to its much smaller size. Moreover, other metal ions were assessed at higher concentrations (5 μM), and none showed an increase in fluorescence. Notably, Cu2+ and Hg2+ continued to exhibit fluorescence quenching. Consistent with the ThT assay, 2-mercaptoethanol can mask Cu2+ and Hg2+ (Fig. S4B†).
Aptamer selection using Ce3+ as a target metal ion
So far, Tb3+, Gd3+ and Sc3+ have been used for aptamer selection. The resulting aptamers either have a similar affinity to all the lanthanide ions or have stronger affinities to heavier ones. Therefore, we wondered whether there are aptamers that can bind to light lanthanides more strongly. So, we chose to use a light lanthanide, Ce3+, for a new selection. Using 10 μM Ce3+ (later reduced to 2 μM), we performed 16 rounds of selection. We aligned the top 20 sequences and found that the top family in the Ce3+ selection belonged to family 1 in the Tb3+ selection (e.g. sequence Ce-1 is very similar to Tb-4, Fig. 1D and Fig. S7A†). Therefore, we did not study this family any further. We picked the first ungroup sequence (Ce-2, Fig. S7B†) for further studies. Using the ThT fluorescence assay, the Ce-2 aptamer showed comparable Kd for Ce3+ (169 nM) and Yb3+ (343 nM, Fig. S7C†). Thus, it is indeed difficult to find an aptamer that can bind to light lanthanides more strongly. In contrast, the Lu12 DNAzyme reacts faster in the presence of light lanthanide ions.21 This is an interesting comparison of lanthanides binding by DNAzymes and by aptamers. In DNAzyme catalysis, metal ions only need to serve as a cofactor and they mainly interact with the scissile phosphate group. In aptamers, a stable binding pocket involving nucleobases needs to form. It also needs to be pointed out that a faster DNAzyme cleavage rate does not mean tighter binding.Comparison of lanthanide binding aptamers: Tb-1, Tb-4, Gd-1 and Sc-1
In this work, we obtained two aptamers: Tb-1 and Tb-4. We then compared them with two previously reported aptamers obtained using Gd3+ and Sc3+ as target ions. We first used ThT fluorescence to measure the binding of Tb3+ by the Gd-1 aptamer and obtained a Kd of 1.5 μM (Fig. 4A). The secondary structure of Gd-1 (Fig. 4A) is quite similar to that of Tb-4. In both aptamers, two base paired stems are right next to each other, and they both contained CCGC in their loop regions. That being said, Tb-4 has a longer conserved loop (mostly purines), which is different from that in the Gd-1 aptamer (mostly pyrimidines). So, the Tb-4 and Gd-1 aptamers are related but different. Overall, the Tb-1 aptamer has higher affinity than both Tb-4 and Gd-1. From the base composition, Tb-1 is rich in guanine in the loops, Tb-4 has a balanced base composition, while Gd-1 is rich in pyrimidines. There are different ways for DNA to bind to lanthanide ions, and guanine-rich aptamers have higher affinities.43 | ||
| Fig. 4 (A) Titration curves of Tb3+ into the previously reported aptamer Gd-1.28 Inset: predicted secondary structures from mFold. (B) Kd values of the Tb-1 and Sc-1 aptamers for La3+, Tb3+, and Lu3+. Kinetics of fluorescence change of 0.5 μM (C) Tb-1 and (D) Sc-1 aptamer along with 1 μM ThT binding to 2 μM Lu3+, followed by the addition of 100 μM EDTA. Panel (D) adapted from ref. 27 with permission. Copyright 2025 American Chemical Society. | ||
Then, we compared Tb-1 with Sc-1. Sc-1 was previously obtained using Sc3+ as the target. Unlike Tb-1, Sc-1 binds to Sc3+ and exhibits a significant difference in Kd values between light and heavy lanthanide ions, with a Kd of 258.5 nM for La3+ and 0.6 nM for Lu3+ (a 475-fold difference). In contrast, Tb-1 does not bind Sc3+ and shows a much smaller difference in Kd values for light and heavy lanthanide ions, with Kd values of 26.9 nM for La3+ and 2.3 nM for Lu3+ (a 12-fold difference) (Fig. 4B).
Binding and dissociation kinetics indicating outer-sphere coordination
To gain insights into DNA coordination with lanthanide ions, ligand exchange kinetics is a simple experiment but it can provide key information. For example, inner-sphere coordinated water molecules exchange more slowly with bulk water than outer-sphere coordinated water, which is attributable to the stronger interactions and the need for a more complex mechanism for the inner-sphere exchange.44 Our previously reported Sc-1 aptamer is featured with slow binding kinetics to heavy lanthanide ions and resistant to dissociation in the presence of EDTA, even though EDTA has over 10 orders of magnitude higher affinity to the lanthanides than Sc-1 has.29 Such slow binding and dissociation is attributable to an inner-sphere coordination leading to a higher activation energy barrier. To further investigate the binding mechanism of Tb-1 to lanthanide ions, we performed a kinetic assay using ThT fluorescence. After the aptamer/ThT fluorescence stabilized, the addition of 2 μM Lu3+ caused a significant fluorescence decrease. Subsequent addition of 100 μM EDTA immediately restored the fluorescence of the Tb-1 sample to its initial level, whereas the Sc-1 fluorescence recovered much more slowly (Fig. 4C and D). Here, we used EDTA to exchange the aptamers for Lu3+ binding. This experiment suggests that Tb-1 binds lanthanides via outer-sphere interactions, while Sc-1 engages in inner-sphere binding. Further structural biology experiments and advanced spectroscopy studies are needed to confirm this hypothesis.Regardless of the aptamers, they all showed tighter binding to heavier lanthanide ions. The hydrated ionic radius of lanthanides decreases gradually from 1.16–1.22 Å for La3+ to 0.97–1.00 Å for Lu3+.45,46 This decrease size leads to a higher charge density for heavier lanthanides and thus stronger binding interactions with the aptamers.
Detection of lanthanide ions
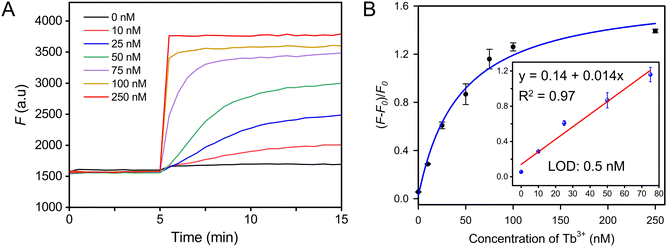
In this work, we have obtained an interesting aptamer, Tb-1, which has a high binding affinity to all the lanthanides. To evaluate the analytical performance of the Tb-1 aptamer, we further challenged our strand-displacement biosensor with Lake Ontario water samples. To remove potential interference from unknown substances in lake water, we filtered the samples by using a 0.2 μm membrane. Different concentrations of Tb3+ were then added to the filtered water, and each concentration was tested three times. As shown in Fig. 5A, higher Tb3+ concentrations resulted in a stronger final fluorescence. We then plotted the final fluorescence as a function of the Tb3+ concentration (Fig. 5B). The results demonstrated that the sensor developed based on aptamer Tb-1 enabled stable and highly sensitive detection of Tb3+ in lake waters, with a LOD of 0.5 nM (inset of Fig. 5B). Therefore, this sensor held significant potential for the detection of lanthanide ions.Conclusions
In this work, we used Tb3+ and Ce3+ as target metal ions for aptamer selection, and obtained a new high affinity aptamer named Tb-1 that has 3.9 nM Kd for Tb3+. It has slightly higher binding affinity for heavier lanthanides and the Kd difference between La3+ and Lu3+ is within 12-fold, which can be explained by the size difference of the lanthanides. Upon the addition of EDTA, the fast dissociation of lanthanides from Tb-1 is consistent with an outer-sphere coordination binding model. This is in sharp contrast to the Sc-1 aptamer, which showed a much larger affinity difference for different lanthanides and a very slow EDTA displacement kinetics for Lu3+. We attributed these properties of Sc-1 to inner-sphere binding to heavy lanthanides. Tb-1 is a general lanthanide binding aptamer motif, and we also showed its application for lanthanide detection using a fluorescence DNA strand displacement assay.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC). Jin Wang, Xin Wang, and Xuesong Li were supported by a China Scholarship Council grant (CSC) for their visit to the University of Waterloo.References
- K. Zheng and P. Ma, Recent advances in lanthanide-based POMs for photoluminescent applications, Dalton Trans., 2024, 53, 3949–3958 RSC.
- P.-J. J. Huang, M. Vazin, J. J. Lin, R. Pautler and J. Liu, Distinction of individual lanthanide ions with a DNAzyme beacon array, ACS Sens., 2016, 1, 732–738 CrossRef CAS.
- S. Kobayashi, M. Sugiura, H. Kitagawa and W. W.-L. Lam, Rare-earth metal triflates in organic synthesis, Chem. Rev., 2002, 102, 2227–2302 CrossRef CAS PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- Q. Yan, C. Liu, X. Zhang, L. Lei and C. Xiao, Selective dissolution and separation of rare earths using guanidine-based deep eutectic solvents, ACS Sustainable Chem. Eng., 2021, 9, 8507–8514 CrossRef CAS.
- E. Varbanova and V. Stefanova, A comparative study of inductively coupled plasma optical emission spectrometry and microwave plasma atomic emission spectrometry for the direct determination of lanthanides in water and environmental samples, Ecol. Saf., 2015, 9, 362–374 Search PubMed.
- M. He, B. Hu, B. Chen and Z. Jiang, Inductively coupled plasma optical emission spectrometry for rare earth elements analysis, Phys. Sci. Rev., 2017, 2, 20160059 Search PubMed.
- S. K. Pradhan and B. Ambade, Extractive separation of rare earth elements and their determination by inductively coupled plasma optical emission spectrometry in geological samples, J. Anal. At. Spectrom., 2020, 35, 1395–1404 RSC.
- N. Khumalo and M. Mathuthu, Determination of trace elements and lanthanide (REE) signatures in uranium mine products in South Africa by means of inductively coupled plasma mass spectrometry, J. Geochem. Explor., 2018, 186, 235–242 CrossRef CAS.
- Y. Zhu, K. Nakano, Y. Shikamori and A. Itoh, Direct determination of rare earth elements in natural water samples by inductively coupled plasma tandem quadrupole mass spectrometry with oxygen as the reaction gas for separating spectral interferences, Spectrochim. Acta, Part B, 2021, 179, 106100 CrossRef CAS.
- P. Das, A. Ghosh and A. Das, Unusual specificity of a receptor for Nd3+ among other lanthanide ions for selective colorimetric recognition, Inorg. Chem., 2010, 49, 6909–6916 CrossRef CAS PubMed.
- F. Zapata, A. Caballero, A. Espinosa, A. Tárraga and P. Molina, A Selective Chromogenic and Fluorescent Molecular Probe for YbIII Based on a Bichromophoric Azadiene, Eur. J. Inorg. Chem., 2010, 5, 697–703 CrossRef.
- J. A. Cotruvo Jr, E. R. Featherston, J. A. Mattocks, J. V. Ho and T. N. Laremore, Lanmodulin: a highly selective lanthanide-binding protein from a lanthanide-utilizing bacterium, J. Am. Chem. Soc., 2018, 140, 15056–15061 CrossRef PubMed.
- W. B. Larrinaga, J. J. Jung, C.-Y. Lin, A. K. Boal and J. A. Cotruvo Jr, Modulating metal-centered dimerization of a lanthanide chaperone protein for separation of light lanthanides, Proc. Georgian Natl. Acad. Sci., 2024, 121, e2410926121 CrossRef CAS PubMed.
- J. A. Mattocks, J. J. Jung, C.-Y. Lin, Z. Dong, N. H. Yennawar, E. R. Featherston, C. S. Kang-Yun, T. A. Hamilton, D. M. Park and A. K. Boal, Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer, Nature, 2023, 618, 87–93 CrossRef CAS PubMed.
- L. Xu, P. Zhang, Y. Liu, X. Fang, Z. Zhang, Y. Liu, L. Peng and J. Liu, Continuously tunable nucleotide/lanthanide coordination nanoparticles for DNA adsorption and sensing, ACS Omega, 2018, 3, 9043–9051 CrossRef CAS PubMed.
- W. Zhou, R. Saran and J. Liu, Metal sensing by DNA, Chem. Rev., 2017, 117, 8272–8325 CrossRef CAS PubMed.
- R. Nishiyabu, N. Hashimoto, T. Cho, K. Watanabe, T. Yasunaga, A. Endo, K. Kaneko, T. Niidome, M. Murata and C. Adachi, Nanoparticles of adaptive supramolecular networks self-assembled from nucleotides and lanthanide ions, J. Am. Chem. Soc., 2009, 131, 2151–2158 CrossRef CAS PubMed.
- P.-J. J. Huang, J. Lin, J. Cao, M. Vazin and J. Liu, Ultrasensitive DNAzyme beacon for lanthanides and metal speciation, Anal. Chem., 2014, 86, 1816–1821 Search PubMed.
- P.-J. J. Huang, M. Vazin, Ż Matuszek and J. Liu, A new heavy lanthanide-dependent DNAzyme displaying strong metal cooperativity and unrescuable phosphorothioate effect, Nucleic Acids Res., 2015, 43, 461–469 CrossRef CAS PubMed.
- P.-J. J. Huang, M. Vazin and J. Liu, In vitro selection of a new lanthanide-dependent DNAzyme for ratiometric sensing lanthanides, Anal. Chem., 2014, 86, 9993–9999 CrossRef CAS PubMed.
- P.-J. J. Huang, M. Vazin and J. Liu, In vitro selection of a DNAzyme cooperatively binding two lanthanide ions for RNA cleavage, Biochemistry, 2016, 55, 2518–2525 CrossRef CAS PubMed.
- V. Dokukin and S. K. Silverman, Lanthanide ions as required cofactors for DNA catalysts, Chem. Sci., 2012, 3, 1707–1714 Search PubMed.
- H. Yu, O. Alkhamis, J. Canoura, Y. Liu and Y. Xiao, Advances and challenges in small–molecule DNA aptamer isolation, characterization, and sensor development, Angew. Chem., Int. Ed., 2021, 60, 16800–16823 CrossRef CAS PubMed.
- A. Brown, J. Brill, R. Amini, C. Nurmi and Y. Li, Development of better aptamers: structured library approaches, selection methods, and chemical modifications, Angew. Chem., Int. Ed., 2024, 63, e202318665 CrossRef CAS PubMed.
- L. Gu, H. Zhang, Y. Ding, Y. Zhang, D. Wang and J. Liu, Capture-SELEX for a short aptamer for label–free detection of salicylic acid, Smart Mol., 2023, 1, e20230007 CrossRef.
- K. Yang, N. M. Mitchell, S. Banerjee, Z. Cheng, S. Taylor, A. M. Kostic, I. Wong, S. Sajjath, Y. Zhang and J. Stevens, A functional group–guided approach to aptamers for small molecules, Science, 2023, 380, 942–948 CrossRef CAS PubMed.
- O. Edogun, N. H. Nguyen and M. Halim, Fluorescent single-stranded DNA-based assay for detecting unchelated Gadolinium(III) ions in aqueous solution, Anal. Bioanal. Chem., 2016, 408, 4121–4131 CrossRef CAS PubMed.
- J. Wang, Y. A. Kaiyum, X. Li, H. Lei, P. E. Johnson and J. Liu, Kinetic and Affinity Profiling Rare Earth Metals Using a DNA Aptamer, J. Am. Chem. Soc., 2025, 147, 1831–1839 Search PubMed.
- X. Li, Z. Yang, M. Waniss, X. Liu, X. Wang, Z. Xu, H. Lei and J. Liu, Multiplexed SELEX for Sulfonamide Antibiotics Yielding a Group-Specific DNA Aptamer for Biosensors, Anal. Chem., 2023, 95, 16366–16373 Search PubMed.
- L. Gu, Y. Ding, Y. Zhou, Y. Zhang, D. Wang and J. Liu, Selective Hemin Binding by a Non-G-quadruplex Aptamer with Higher Affinity and Better Peroxidase-like Activity, Angew. Chem., Int. Ed., 2024, 63, e202314450 CrossRef CAS PubMed.
- L. Gu, Y. Zhang, D. Wang and J. Liu, Light–Up Sensing Citrate Using a Capture–Selected DNA Aptamer, Adv. Sens. Res., 2024, 2300167 CrossRef CAS.
- F. Y. Khusbu, X. Zhou, H. Chen, C. Ma and K. Wang, Thioflavin T as a fluorescence probe for biosensing applications, Trends Anal. Chem., 2018, 109, 1–18 CrossRef.
- Y. Ding, Y. Xie, A. Z. Li, P.-J. J. Huang and J. Liu, Cross-Binding of Four Adenosine/ATP Aptamers to Caffeine, Theophylline, and Other Methylxanthines, Biochemistry, 2023, 62, 2280–2288 CrossRef CAS PubMed.
- J. Wang, Y. Liu, X. Li, H. Lei and J. Liu, A high affinity and selective DNA aptamer for copper ions, Chem. Commun., 2024, 60, 14272–14275 RSC.
- Y. Liu and J. Liu, Selection of DNA aptamers for sensing uric acid in simulated tears, Analysis Sensing, 2022, 2, e202200010 CrossRef CAS.
- Y. Zhao, B. Gao, Y. Chen and J. Liu, An aptamer array for discriminating tetracycline antibiotics based on binding-enhanced intrinsic fluorescence, Analyst, 2023, 148, 1507–1513 RSC.
- J. Wang, X. Li, H. Lei and J. Liu, Selection of DNA aptamers for detecting metronidazole and ibuprofen: two common additives in soft drinks, Analyst, 2024, 149, 5482–5490 RSC.
- R. Nutiu and Y. Li, Structure-switching signaling aptamers, J. Am. Chem. Soc., 2003, 125, 4771–4778 CrossRef CAS PubMed.
- N. Nakatsuka, K.-A. Yang, J. M. Abendroth, K. M. Cheung, X. Xu, H. Yang, C. Zhao, B. Zhu, Y. S. Rim and Y. Yang, Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing, Science, 2018, 362, 319–324 CrossRef CAS PubMed.
- J. Canoura, T. Nguyen, C. Byrd, R. Hill, Y. Liu and Y. Xiao, Generation of High-Affinity Aptamers for Indazole Synthetic Cannabinoids, Anal. Chem., 2024, 96, 11488–11497 CrossRef CAS PubMed.
- J. Hu and C. J. Easley, A simple and rapid approach for measurement of dissociation constants of DNA aptamers against proteins and small molecules via automated microchip electrophoresis, Analyst, 2011, 136, 3461–3468 RSC.
- Z. Zhang, K. Morishita, W. T. D. Lin, P.-J. J. Huang and J. Liu, Nucleotide coordination with 14 lanthanides studied by isothermal titration calorimetry, Chin. Chem. Lett., 2018, 29, 151–156 CrossRef CAS.
- V. Jacques, S. Dumas, W.-C. Sun, J. S. Troughton, M. T. Greenfield and P. Caravan, High-Relaxivity Magnetic Resonance Imaging Contrast Agents Part 2: Optimization of Inner- and Second-Sphere Relaxivity, Invest. Radiol., 2010, 45, 613–624 Search PubMed.
- P. D'Angelo, A. Zitolo, V. Migliorati, G. Chillemi, M. Duvail, P. Vitorge, S. Abadie and R. Spezia, Revised ionic radii of lanthanoid (III) ions in aqueous solution, Inorg. Chem., 2011, 50, 4572–4579 Search PubMed.
- V. Solov'ev and A. Varnek, Thermodynamic radii of lanthanide ions derived from metal–ligand complexes stability constants, J. Inclusion Phenom. Macrocyclic Chem., 2020, 98, 69–78 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00391a |
| This journal is © the Partner Organisations 2025 |