Open Access Article
Open Access ArticleAmido/alkoxy–aryl–aryl–picolinate push–pull antennas for two-photon sensitization of Eu3+ luminescence†
Baptiste
Chartier
ab,
Alexei
Grichine
 d,
Lucile
Bridou
c,
Adam
Nhari
ab,
Guillaume
Micouin
c,
Akos
Banyasz
d,
Lucile
Bridou
c,
Adam
Nhari
ab,
Guillaume
Micouin
c,
Akos
Banyasz
 c,
Didier
Boturyn
c,
Didier
Boturyn
 b,
Jennifer K.
Molloy
b,
Jennifer K.
Molloy
 b,
Sule
Erbek
de,
Véronique
Martel-Frachet
b,
Sule
Erbek
de,
Véronique
Martel-Frachet
 de,
Olivier
Maury
de,
Olivier
Maury
 *c and
Olivier
Sénèque
*c and
Olivier
Sénèque
 *a
*a
aUniv. Grenoble Alpes, CNRS, CEA, IRIG, LCBM (UMR 5249), F-38000 Grenoble, France. E-mail: olivier.seneque@cea.fr
bUniv. Grenoble Alpes, CNRS, DCM (UMR 5250), F-38000 Grenoble, France
cUniv. Lyon, ENS de Lyon, CNRS UMR 5182, Laboratoire de Chimie, Lyon F-69342, France. E-mail: olivier.maury@ens-lyon.fr
dUniv. Grenoble Alpes, INSERM U1209, CNRS UMR 5309, Institute for Advanced Biosciences, F-38000 Grenoble, France
eEPHE, PSL Research University, 4-14 rue Ferrus, 75014 Paris, France
First published on 10th March 2025
Abstract
Luminescent two-photon (2P) absorbing lanthanide(III) complexes hold great promise for microsocpy imaging of biological samples. Conjugating such a complex to well-chosen cell penetrating peptides (CPP) allows its controlled delivery to the cytosol of live cells. However, alkoxy–phenyl–ethynyl–picolinate, one of the best antennae for 2P sensitization of Eu3+, undergoes side reactions at its ethynyl group during peptide synthesis or in biological media and thus cannot be used to create such a conjugate. In this article, we evaluate the effect of substituting the ethynyl group by a phenyl one. We describe the synthesis of conjugates of the TAT CPP with Eu3+ complexes featuring amido–phenyl–phenyl–picolinamide, alkoxy–phenyl–phenyl–picolinamide and amido–phenyl–phenyl–picolinate ter-aryl antennae and compare their spectroscopic properties to those of analogues with bi-aryl antennae, including the amido–phenyl–picolinamide already used for 2P live cell imaging. The absorption spectrum of the ter-aryl antennae is red-shifted and better covers the active spectral range for 2P excitation by a Ti-sapphire laser. Among compounds with ter-aryl antennae, those with an amido electron donating group are the most interesting, showing brightness ca. 4 times higher than their bi-aryl counterparts, and similar to the ethynyl-containing antenna. 2P microscopy imaging of live cells incubated with the TAT-Eu3+ conjugate and dFFLIPTAT, a non-luminescent CPP that promotes cytosolic delivery, showed diffuse cytosolic staining of the Eu3+ probe. The ter-aryl-based probes showed superior performances compared to bi-aryl, with ca. 80% of the cells showing Eu3+ staining of the cytosol.
Introduction
Lanthanide(III) (Ln3+) complexes have luminescent properties due to f–f transitions that are very interesting for biological applications, including fine emission bands (line widths of a few nanometers) at fixed wavelengths characteristic of each lanthanide, long luminescence lifetimes and high resistance to photobleaching.1–6 A major drawback to their use is the low efficiency of light absorption by Ln3+ with ε values in the order of 1–5 M−1 cm−1 for f–f transitions. This can be overcome by introducing a chromophore close to the lanthanide, capable of transferring to the lanthanide ion the energy it has acquired through light excitation, to bring it into its emissive excited state. This chromophore is called an antenna and the process of sensitizing the luminescence of the lanthanide is referred to as the antenna effect.7–10 Sensitization of Ln3+ that emit in the visible (Tb3+, Dy3+, Eu3+ and Sm3+) requires an antenna whose donor excited levels are above the Ln3+ excited emissive level (typically 2000–5000 cm−1 above). Consequently, for these lanthanides, it is mandatory to use antennae that are excited with UV light, which has the disadvantage of causing damage to biological samples. One solution to this problem is to use antennae that can be excited by two-photon (2P) absorption, a non-linear optical phenomenon that involves the simultaneous absorption of two photons instead of one, with half the energy required for excitation by a single photon.11–14 As a result, the excitation is shifted from the UV to the near infrared, causing less damage to cells, for instance.Over the last fifteen years, we have demonstrated that it is possible to perform 2P microscopy of fixed cells (i.e. cells treated with PFA or MeOH resulting in membrane permeabilization, but also causing cell death) with Ln3+ complexes based on a macrocyclic ligand TACN(pic)3 (Fig. 1) and equipped with 2P-absorbing push–pull antennae.13,15–17 More recently, we have used Ln3+ complexes based on a DO3Apic ligand equipped with a push–pull antenna and conjugated to a cell-penetrating peptide that enables the Ln complex to be delivered into the cytosol of living cells.18–20 2P microscopy imaging of live cells was performed using p-amido- or p-methoxy–phenyl–picolinamide antennae (A1 and A2 in Fig. 1, respectively) coordinated to the Ln3+. These antennae show an absorption band in the UV (Fig. 1) with a maximum at 315 nm (λmax) and that extends up ca. 360 nm (λcut-off). The Ti:sapphire lasers used for 2P excitation have an excitation wavelength between about 700 nm and 1050 nm, corresponding to the 350–525 nm range for 1P absorption. This means that only the tail of the absorption band of the Ln3+ complex is likely to be active for 2P excitation by the Ti:sapphire laser (Fig. 1). Additionally, the 2P cross-section of these antennae is small (35 GM at 700 nm for the TACN-based complex with three antennae, i.e. ca. 12 GM per antenna).
 | ||
| Fig. 1 Eu complexes with TACN-trispicolinate and DO3A-picolinate/picolimamide ligands, sensitizing push–pull antennas and their absorption spectra. | ||
A better antenna is the alkoxy–phenyl–ethynyl–picolinate antenna (A3 in Fig. 1), with λmax and λcut-off values around 325 nm and 370 nm, respectively, and a 2P cross-section of ca. 50 GM at 700 nm.21,22 However, its alkyne group is not completely stable and can react with nucleophiles (water, alcohols, thiols, phosphines, etc.) under certain conditions. For instance, under acidic conditions used during the preparation of Ln3+ complex-peptide conjugates (TFA treatment for protecting group removal), complete hydration of the triple bond or addition of other nucleophiles (e.g. the thioanisol scavenger) is observed.18 A solution to red-shift the absorption of the aryl-picolinate antenna was recently proposed, that consists in replacing the p-methoxy–phenyl–ethynyl (antenna A3 in Fig. 1) substituent of the picolinate with a p-thioanisolyl substituent (antenna A4 in Fig. 1).23 Such an antenna shows λmax and λcut-off values of ca. 330 nm and 375 nm, respectively, similar to the p-methoxy–aryl–ethynyl–picolinate antenna. A 2P cross-section of 35 GM at 700 nm was measured for the TACN-based complex that features three antennae. This accounts for ca. 12 GM per antenna. In this article, we explore an alternative solution: replacing the ethynyl linker with an aryl group. We describe the preparation and the luminescence properties of several Eu3+ complexes featuring p-amido- or p-methoxy–aryl–aryl–picolinamide antennae (A5 and A6 in Fig. 1, respectively), denoted here as ter-aryl antennae, in comparison with the corresponding bi-aryl p-amido- or p-methoxy–aryl–picolinamide antennae.
Results and discussion
Design and synthesis of the probes
In a previous article, we have described the conjugate mTAT[Eu·L-Ar-NHAc] (Fig. 2A). This compound comprises an Eu3+ ion bound to a cyclen-based macrocyclic ligand, DO3Apic, featuring a π-extended picolinamide antenna. The picolinamide moiety is substituted by a p-acetamido–phenyl group, in para position relative to the pyridine, the whole forming a bi-aryl push–pull antenna for 2P absorption. The Eu3+ complex is attached to a TAT peptide, which provides water solubility and cell penetration properties. Our first target probe was therefore the ter-aryl analogue mTAT[Eu·L-Ar-Ar-NHAc] (Fig. 2A). Conjugates mTAT[Eu·L-Ar-OMe] and mTAT[Eu·L-Ar-Ar-OMe] with a methoxy electron donating group instead of the acetamide group were also investigated (Fig. 2A).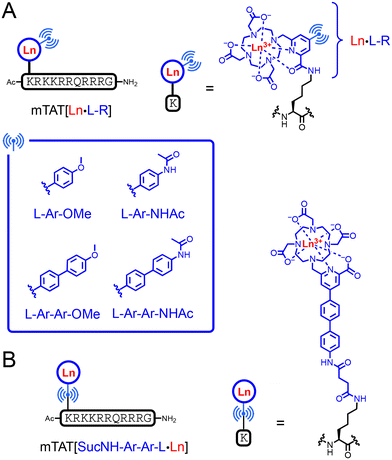 | ||
| Fig. 2 Chemical structures of (A) mTAT[Ln·L-R] conjugates (R = Ar-OMe, Ar-NHAc, Ar-Ar-OMe and Ar-Ar-NHAc; Ln = Eu3+ or Gd3+) and (B) the mTAT[SucNHArArL·Ln] conjugate. | ||
The three new probes were synthesized as already reported for mTAT[Eu·L-Ar-NHAc].19 The iodinated compound 1 (Fig. 3A) was engaged in a Miyaura–Suzuki coupling with borylated aromatics 3b–d to yield pro-ligands L-R(tBu)3 after hydrolysis of the picolinic methyl ester. All pro-ligands were obtained in similar yields (34–48%) after HPLC purification. Preparation of the conjugates was performed as already described.19 L-R(tBu)3 pro-ligands were then coupled to the selectively deprotected lysine side chain of the peptide, on resin using PyBOP/DIEA activation (Fig. S6 of ESI†). The peptide was cleaved from the resin and all protecting groups, including the macrocyclic ligand tBu ones, were removed by TFA/scavenger treatment for 4 h. The conjugates were purified by HPLC and metalation with Eu3+ was performed in water at pH 8 in the presence of excess EuCl3 to give mTAT[Eu·L-Ar-OMe], mTAT[Eu·L-Ar-Ar-NHAc] and mTAT[Eu·L-Ar-Ar-OMe] as pure compounds after HPLC purification as attested by LCMS analysis (Fig. S8 of ESI†).
 | ||
| Fig. 3 Synthetic pathways for pro-ligands (A) L-R(tBu)3 (R = Ar-NHAc,19 Ar-OMe, Ar-Ar-NHAc and Ar-Ar-OMe) and (B) L-Ar-Ar-NHSuc(Me)4. The carboxylic acid group used for peptide conjugation is highlighted in red. | ||
Due to the disappointing luminescence properties of mTAT[Eu·L-Ar-Ar-NHAc] and mTAT[Eu·L-Ar-Ar-OMe] (vide infra), we have also investigated the influence of the peptide anchoring position onto the complex. For this purpose, mTAT[SucNH-Ar-Ar-L·Eu] was prepared, in which the ter-aryl antenna is attached to the peptide through the electron donating group rather than through the picolinate moiety. This results in a negatively charged Eu3+ complex with a picolinate coordinating group rather than a neutral complex with a picolinamide group (Fig. 2). The synthesis of the pro-ligand L-Ar-Ar-NHSuc(Me)4 and its TAT conjugate were performed starting from the iodinated compound 2. It was coupled to boronic ester 3e to form L-Ar-Ar-NH2(Me)4, followed by treatment with succinic anhydride to give pro-ligand L-Ar-Ar-NHSuc(Me)4 (Fig. 3B), which was then coupled to the mTAT peptide on resin (Fig. S7 of ESI†). After resin cleavage in TFA and HPLC purification, the methyl protecting groups of the carboxylates were removed using a NaOH 2 M treatment for 15 minutes prior to metalation with EuCl3 in water, which gave mTAT[SucNH-Ar-Ar-L·Eu].
Photophysical properties
The photophysical properties of these probes were investigated in PBS buffer (pH 7.4). Spectroscopic data are summarized in Table 1.| Compound | λ max; λcut-off/nm | E(S1);bE(T1)c/cm−1 | ε at λmax/M−1 cm−1 | Φ Eu | Φ EuEu | η sens | σ 2P at 720 nm/GM | B 2P at 720 nm/GM | τ Eu (H2O, aerated); τEu (H2O, de-oxygenated); τEu (D2O, aerated)/ms | τ R/ms |
|---|---|---|---|---|---|---|---|---|---|---|
a Error is estimated ±5% on ε values and ±10% on ΦLn and σ2P. Error on τLn is estimated ± 0.03 ms.
b Energy of the excited singlet state is determined from the λcut-off.
c Energy of the excited triplet state is estimated from the wavelength at half-maximum on the onset of the time-gated phosphorescence spectrum of the Gd3+ analogue in PBS/glycerol 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v recorded at 77 K. 1 v/v recorded at 77 K.
|
||||||||||
| mTAT[Eu·L-Ar-NHAc] | 315; 366 | S1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300; T1: 22 300; T1: 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.15 | 0.22 | 0.67 | 5.3 | 0.8 | 1.05; 1.05; 1.63 | 4.68 |
| mTAT[Eu·L-Ar-OMe] | 318; 364 | S1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; T1: 22 500; T1: 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.15 | 0.22 | 0.67 | 3.5 | 0.5 | 1.05; 1.05; 1.65 | 4.56 |
| mTAT[Eu·L-Ar-Ar-NHAc] | 329; 384 | S1: 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; T1: 20 000; T1: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.075 | 0.16 | 0.48 | 33 | 2.5 | 0.71; 0.76; 0.99 | 4.56 |
| mTAT[Eu·L-Ar-Ar-OMe] | 333; 390 | S1: 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600; T1: 20 600; T1: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.035 | 0.086 | 0.41 | 41 | 1.4 | 0.39 (93%), 0.77 (7%); 0.40 (92%), 0.78 (8%); 0.46 (89%), 0.89 (11%) | 4.56 |
| mTAT[SucNH-Ar-Ar-L·Eu] | 326; 381 | S1: 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300; T1: 20 300; T1: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.15 | 0.22 | 0.67 | 24 | 3.6 | 1.02 1.03 1.51 | 4.54 |
In the case of Eu3+, the metal-centred emission quantum yield (ΦEuEu, also named intrinsic quantum yield) can be determined from the emission spectrum using eqn (1) and (2), where τR is the radiative lifetime of Eu3+, n is the refractive index of the medium, Itot and IMD are the total area of the corrected Eu3+ emission spectrum and the area of the 5D0 → 7F1 transition band, respectively, and AMD,0 is the spontaneous emission probability for the 5D0 → 7F1 transition.26AMD,0 was initially assumed to be constant and equal to 14.65 s−1,26 but significant deviations from this value were recently reported.27 Therefore, these equations have to be used with caution when comparing complexes. Nevertheless, (i) as all the complexes described here are based on the DO3Apic chelator, (ii) as they have nearly identical emission spectra and (iii) as they have the same hydration state (q = 0), the derived τR and ΦEuEu values can be compared. Their values are given in Table 1.
| ΦEuEu = τEu/τR | (1) |
| 1/τR = AMD,0 × n3 × (Itot/IMD) | (2) |
In agreement with their nearly identical emission spectra, all compounds have the same τR value, ca. 4.6 ms, and the differences in their intrinsic quantum yield values follow the differences in the τEu values.
The sensitizing efficiency, ηsens, which quantifies the efficiency of the electronic energy transfer from the antenna to the Eu3+, can be calculated from eqn (3).
| ΦEu = ηsens × ΦEuEu | (3) |
The conjugates with the bi-aryl antennae, mTAT[Eu·L-Ar-NHAc] and mTAT[Eu·L-Ar-OMe], show the same sensitization efficiency, 0.67, but their ter-aryl analogues, mTAT[Eu·L-Ar-Ar-NHAc] and mTAT[Eu·L-Ar-Ar-OMe], show significantly lower ηsens values, i.e. 0.48 and 0.41, respectively. With push–pull antennas, photoinduced electron transfer (PeT) from the excited antenna to the Eu3+ is likely to compete with the electronic energy transfer.20,28 The Gibbs energy of the PeT process can be evaluated using eqn (4).29–33
| ΔG0eT = (E0D − E0A) − E* + ΔGS+C | (4) |
Two-photon microscopy
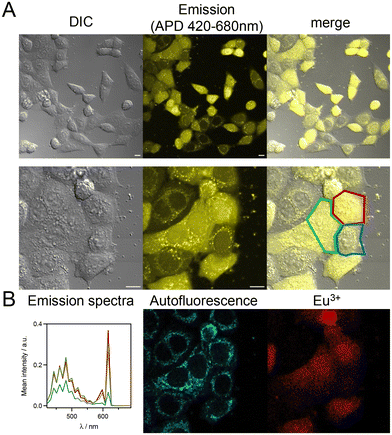
The mTAT conjugates with the ter-aryl antennae and an amido electron donating group showed better emission properties compared to the one with a methoxy group. Therefore, two-photon microscopy (2PM) on live HeLa cells was attempted with conjugates featuring complexes [Eu·L-Ar-NHAc], [Eu·L-Ar-Ar-NHAc] and [SucNH-Ar-Ar-L·Eu]. We were interested to know if cytosolic delivery of the Eu3+ probe could be achieved with these conjugates. 2P excitation was performed at 720 nm and emission was collected with an avalanche photodiode (APD) for better sensitivity or an array of photomultipliers (PMT) to achieve spectral detection. Under 720 nm 2P excitation, the main contributors to the emission are the Eu3+ probe and the cell autofluorescence arising from 2P-excited NAD(P)H and FAD.19,35–37 Cell autofluorescence mostly originates from mitochondria and has a perinuclear distribution. It is characterized by a broad emission with a maximum at ca. 480 nm extending up to 620 nm (Fig. 6B). The fine Eu3+ 5D0 → 7FJ emission bands at 595 nm (J = 1) and 615 nm can easily be discriminated from the cell autofluorescence by spectral detection. | ||
| Fig. 7 Chemical structures of (A) luminescent Eu3+ probes based on a dimeric TAT peptide and (B) non luminescent dimeric TAT derivative dFFLIPTAT. | ||
First, we examined their cytotoxicity on HeLa cells by the MTT proliferation assay in order to evaluate the concentration that could be used for 2PM experiments. While the previously described dTAT[Eu·L-Ar-NHAc] conjugate was not cytotoxic up to 20 μM (IC50 > 50 μM), both dTAT[Eu·L-Ar-Ar-NHAc] and dTAT[SucNH-Ar-Ar-L·Eu] were found to be more toxic (Fig. 8A), with an IC50 of 6 ± 1 and 12 ± 2 μM, respectively. This was not unexpected since we have observed that a higher antenna hydrophobicity in these compounds causes higher cytotoxicity.19,20 From the MTT assays, we found that the maximal nontoxic concentration that could be used for dTAT[Eu·L-Ar-Ar-NHAc] and dTAT[SucNH-Ar-Ar-L·Eu] is 2.5 μM. 2PM was performed on HeLa cells incubated 1 h with the probes at 2 μM. With dTAT[SucNH-Ar-Ar-L·Eu], an intense punctate emission was observed within the cell (Fig. 8B and Fig. S13 of ESI†), which was assigned to Eu3+ emission by spectral detection (Fig. 8C). dTAT-based probes were shown to accumulate in endosomes when incubated at a concentration ≤2.5 μM.19,38 Although co-localization with an endo/lysosome tracker was not attempted here, it is likely to be the case here again. A similar staining was obtained with dTAT[Eu·L-Ar-Ar-NHAc]. Therefore, the dTAT strategy is not pertinent for ter-aryl antennae in order to achieve cytosolic delivery.
 | ||
| Fig. 8 (A) MTT proliferation assays performed on HeLa cells with dTAT[Eu·L-Ar-NHAc] (green),19 dTAT[Eu·L-Ar-Ar-NHAc] (blue) and dTAT[SucNH-Ar-Ar-L·Eu] (red). (B) 2PM imaging (λex = 720 nm) of HeLa cells incubated 1 h with dTAT[SucNH-Ar-Ar-L·Eu] (2 μM) in RPMI medium, showing (top) the luminescence image recorded with 420–680 nm bp APD detection and (bottom) the superimposition of DIC and luminescence images. (C) Typical emission spectrum emanating from puncta. Scale bars correspond to 10 μm. | ||
Fig. 9A shows typical images obtained with mTAT[SucNH-Ar-Ar-L·Eu] in co-incubation with dFFLIPTAT. Contrarily to images obtained with the mTAT[SucNH-Ar-Ar-L·Eu] probe alone (Fig. 6), those obtained with dFFLIPTAT, show a majority of cells with dominant diffuse emission. In many cells, the perinuclear distribution characteristic of autofluorescence is hardly identified, hidden by the intense diffuse emission. Spectral detection (Fig. 9B) confirmed that the latter corresponds to Eu3+. Comparison of Fig. 6 and 9 clearly indicates that dFFLIPTAT boosts the internalization of the Eu3+ probe.
The three mTAT[Eu·L-Ar-NHAc], mTAT[Eu·L-Ar-Ar-NHAc] and mTAT[SucNH-Ar-Ar-L·Eu] probes in co-incubation with dFFLIPTAT gave similar results but the Eu3+ emission appeared lower by visual inspection in the case of mTAT[Eu·L-Ar-NHAc], with the perinuclear autofluorescence more easily discernible. Fig. 10A shows typical 2PM images obtained with each probe using APD detection and band-pass (bp) filtering and recorded in the same conditions (laser power, pixel dwell time …), the same day. Two detection channels were used: (i) 470–540 nm range (blue channel) that is specific for autofluorescence and (ii) 580–690 nm (red channel) that comprises the two Eu3+ 5D0 → 7FJ (J = 1 and 2) emission bands but also the red tail of the autofluorescence. A majority of cells show a diffuse emission within the cell in the red channel, with autofluorescence distribution hardly detectable, indicating that Eu3+ emission level is above the one of the red-edge of the autofluorescence emission. Fig. 10D quantifies the percentage of cells with diffuse staining as determined by visual inspection. For all conjugates it is above 60% and it increases in the order mTAT[Eu·L-Ar-NHAc] < mTAT[Eu·L-Ar-Ar-NHAc] < mTAT[SucNH-Ar-Ar-L·Eu]. With the latter ca. 90% of the cells show an unambiguous diffuse Eu3+ staining. The intensity of Eu3+ emission vary strongly from cell-to-cell. This is typical of CPP-based luminescent probes.38 In order to compare the three compounds, a quantitative assessment of Eu3+ staining was performed. For this purpose, the mean intensity (red channel) per pixel was measured using ImageJ in ca. 80 cells for cells treated with each compound and for cells not incubated with any of the compounds, as a control. It has been reported that CPP-based luminescent probes can adhere to the coverglass of the cell culture chamber providing a background signal outside cells, especially when 2P imaging is done close to the surface.19,40 Therefore, the background mean intensity per pixel was determined in several (>50) cell-free areas of the approximate size of a cell. Fig. 10B shows the mean intensity per pixel measured in cells and in background areas (Bg) for the three conjugates and in control experiments (Ctrl). First, for the three conjugates, this quantification shows a great dispersion in the staining level among cells, with the mean intensity spanning an order of magnitude. This confirms the visual inspection. For all three conjugates, the staining is significantly higher than the background (P value <0.0001), the staining of the two conjugates with a ter-aryl antenna is similar (P = 0.24) but significantly higher than that of mTAT[Eu·L-Ar-NHAc] with the bi-aryl antenna (P < 0.0001). Note also that the background is also higher in the case of the two ter-aryl antenna-based compounds (P < 0.0001). This suggests an identical behaviour for all conjugates but with an improved emission in the case of the ter-aryl compounds in agreement with their higher brightness. However, a higher cell penetration efficiency cannot be ruled out to explain this higher staining. Finally, the percentage of cells unambiguously stained with the Eu3+ probe (Fig. 10C) was determined by counting the cells with a mean intensity per pixel above the limit of detection (LoD) defined by eqn (5),
| LoD = <IBg> + IAF + 3 × SD(IBg) | (5) |
The quantitative data presented here allow comparison of two cell internalization strategies relying on (1) TAT dimers, with, on the one hand, dTAT[Eu·L-Ar-NHAc], and (2) TAT monomer + dFFLIPTAT, with, on the other hand, mTAT[Eu·L-Ar-NHAc], both conjugates having the same antenna. The former strategy, with the probe at 5 μM, yielded ca. 35% of cells with unambiguous diffuse cytosolic staining of Eu3+ (ref. 19) while the latter strategy gave ca. 60%, at the same probe concentration, i.e. with half the amount of Eu3+ complex. This means that superior cytosolic delivery is achieved by co-incubation of the mTAT probe with dFFLIPTAT compared to the dTAT probe. Combining the dFFLIPTAT strategy with a better ter-aryl antenna, enhances even more the number of cells with detectable diffuse staining.
Conclusions
Eu3+ based luminescent bioprobes with p-amido- or p-methoxy–aryl–picolinate antennae (i.e. bi-aryl antennae A1 and A2) that we have described previously suffer from a weak absorption above 350 nm. In this article, we have evaluated the benefit of inserting an additional aryl ring within these antennae to red shift the absorption and achieve superior 1P and 2P photophysical properties. Three compounds with ter-aryl antennae, mTAT[Eu·L-Ar-Ar-NHAc], mTAT[Eu·L-Ar-Ar-OMe] and mTAT[SucNH-Ar-Ar-L·Eu], were synthesized and studied, differing in the nature of the electron donating group and/or in the peptide grafting point. From our photophysical study, the best compromise between the red shift of the excitation wavelength and the 2P brightness is compound mTAT[SucNH-Ar-Ar-L·Eu]. The [SucNH-Ar-Ar-L·Eu] complex shows photophysical properties similar to those that can be obtained using the related p-alkoxy–aryl–ethynyl–picolinate (antenna A3), which cannot be incorporated into peptide conjugates due to side reactions arising with the alkyne group during peptide synthesis (especially, acidic deprotection treatments). Comparatively, the ter-aryl antenna is fully stable in peptide synthesis conditions. The increased 2P brightness at 720 nm gained by passing from a bi-aryl antenna to a ter-aryl antenna is likely to be responsible for the better staining and detection achieved with mTAT[SucNH-Ar-Ar-L·Eu] in 2PM experiments. This article demonstrates that the ter-aryl p-amido–aryl–aryl–picolinate antenna is an interesting alternative to p-alkoxy–aryl–ethynyl–picolinate for the 2P sensitization of Eu3+ luminescence.Author contributions
Conceptualization: O. S., O. M.; investigation: B. C., A. G., L. B., A. N., G. M., S. E., V. M.-F., O. S.; validation: O. S., A. G., A. B., O. M., V. M.-F., D. B., J. K. M.; writing (original draft): O. S.; writing (review & editing): all authors. Visualization: O. S.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors acknowledge the Agence Nationale de la Recherche (ANR-18-CE06-0022 and ANR-21-CE29-0018), the Labex ARCANE, CBH-EUR-GS (ANR-17-EURE-0003) and the CEA FOCUS Biomarqueurs program for financial support. Imaging experiments were done on Microcell core facility of the Institute for Advanced Biosciences (UGA – Inserm U1209 – CNRS 5309). This facility belongs to the IBISA-ISdV platform, member of the national infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04).References
- J.-C. G. Bünzli and S. V. Eliseeva, in Lanthanide Luminescence, ed. P. Hänninen and H. Härmä, Springer Berlin Heidelberg, 2011, pp. 1–45 Search PubMed.
- J.-C. G. Bünzli, Lanthanide light for biology and medical diagnosis, J. Lumin., 2016, 170, 866–878 CrossRef.
- M. Sy, A. Nonat, N. Hildebrandt and L. J. Charbonnière, Lanthanide-based luminescence biolabelling, Chem. Commun., 2016, 52, 5080–5095 RSC.
- E. Mathieu, A. Sipos, E. Demeyere, D. Phipps, D. Sakaveli and K. E. Borbas, Lanthanide-based tools for the investigation of cellular environments, Chem. Commun., 2018, 54, 10021–10035 RSC.
- G.-Q. Jin, Y. Ning, J.-X. Geng, Z.-F. Jiang, Y. Wang and J.-L. Zhang, Joining the journey to near infrared (NIR) imaging: the emerging role of lanthanides in the designing of molecular probes, Inorg. Chem. Front., 2020, 7, 289–299 RSC.
- I. Martinić, S. V. Eliseeva and S. Petoud, Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials, J. Lumin., 2017, 189, 19–43 CrossRef.
- S. I. Weissman, Intramolecular Energy Transfer: The Fluorescence of Complexes of Europium, J. Chem. Phys., 1942, 10, 214–217 CrossRef CAS.
- J.-C. G. Bünzli, On the design of highly luminescent lanthanide complexes, Coord. Chem. Rev., 2015, 293, 19–47 CrossRef.
- A. D'Aléo, F. Pointillart, L. Ouahab, C. Andraud and O. Maury, Charge transfer excited states sensitization of lanthanide emitting from the visible to the near-infra-red, Coord. Chem. Rev., 2012, 256, 1604–1620 CrossRef.
- W. Thor, H.-Y. Kai, Y.-H. Yeung, Y. Wu, T.-L. Cheung, L. K. B. Tam, Y. Zhang, L. J. Charbonnière, P. A. Tanner and K.-L. Wong, Unearthing the Real-Time Excited State Dynamics from Antenna to Rare Earth Ions Using Ultrafast Transient Absorption, JACS Au, 2024, 4, 3813–3822 CrossRef CAS PubMed.
- G. Piszczek, B. P. Maliwal, I. Gryczynski, J. Dattelbaum and J. R. Lakowicz, Multiphoton ligand-enhanced excitation of lanthanides, J. Fluoresc., 2001, 11, 101–107 CrossRef CAS PubMed.
- M. H. V. Werts, N. Nerambourg, D. Pélégry, Y. L. Grand and M. Blanchard-Desce, Action cross sections of two-photon excited luminescence of some Eu(III) and Tb(III) complexes, Photochem. Photobiol. Sci., 2005, 4, 531–538 CrossRef CAS PubMed.
- A. Picot, A. D'Aleo, P. L. Baldeck, A. Grichine, A. Duperray, C. Andraud and O. Maury, Long-lived two-photon excited luminescence of water-soluble europium complex: Applications in biological imaging using two-photon scanning microscopy, J. Am. Chem. Soc., 2008, 130, 1532–1533 CrossRef CAS PubMed.
- G.-L. Law, K.-L. Wong, C. W.-Y. Man, S.-W. Tsao and W.-T. Wong, A two-photon europium complex as specific endoplasmic reticulum probe, J. Biophotonics, 2009, 2, 718–724 CrossRef CAS PubMed.
- A. D'Aléo, A. Bourdolle, S. Brustlein, T. Fauquier, A. Grichine, A. Duperray, P. L. Baldeck, C. Andraud, S. Brasselet and O. Maury, Ytterbium-Based Bioprobes for Near-Infrared Two-Photon Scanning Laser Microscopy Imaging, Angew. Chem., Int. Ed., 2012, 51, 6622–6625 CrossRef PubMed.
- A. T. Bui, A. Roux, A. Grichine, A. Duperray, C. Andraud and O. Maury, Twisted Charge-Transfer Antennae for Ultra-Bright Terbium(III) and Dysprosium(III) Bioprobes, Chem. – Eur. J., 2018, 24, 3408–3412 CrossRef CAS PubMed.
- A. T. Bui, A. Grichine, S. Brasselet, A. Duperray, C. Andraud and O. Maury, Unexpected Efficiency of a Luminescent Samarium(III) Complex for Combined Visible and Near-Infrared Biphotonic Microscopy, Chem. – Eur. J., 2015, 21, 17757–17761 CrossRef CAS PubMed.
- J.-H. Choi, G. Fremy, T. Charnay, N. Fayad, J. Pécaut, S. Erbek, N. Hildebrandt, V. Martel-Frachet, A. Grichine and O. Sénèque, Luminescent Peptide/Lanthanide(III) Complex Conjugates with Push–Pull Antennas: Application to One- and Two-Photon Microscopy Imaging, Inorg. Chem., 2022, 61, 20674–20689 CrossRef CAS PubMed.
- K. P. Malikidogo, T. Charnay, D. Ndiaye, J.-H. Choi, L. Bridou, B. Chartier, S. Erbek, G. Micouin, A. Banyasz, O. Maury, V. Martel-Frachet, A. Grichine and O. Sénèque, Efficient cytosolic delivery of luminescent lanthanide bioprobes in live cells for two-photon microscopy, Chem. Sci., 2024, 15, 9694–9702 RSC.
- J.-H. Choi, A. Nhari, T. Charnay, B. Chartier, L. Bridou, G. Micouin, O. Maury, A. Banyasz, S. Erbek, A. Grichine, V. Martel-Frachet, F. Thomas, J. K. Molloy and O. Sénèque, Carbazole-Based Eu3+ Complexes for Two-Photon Microscopy Imaging of Live Cells, Inorg. Chem., 2025, 64, 2006–2019 CrossRef CAS PubMed.
- A. T. Bui, M. Beyler, Y.-Y. Liao, A. Grichine, A. Duperray, J.-C. Mulatier, B. L. Guennic, C. Andraud, O. Maury and R. Tripier, Cationic Two-Photon Lanthanide Bioprobes Able to Accumulate in Live Cells, Inorg. Chem., 2016, 55, 7020–7025 CrossRef CAS PubMed.
- S. Mizzoni, S. Ruggieri, A. Sickinger, F. Riobé, L. Guy, M. Roux, G. Micouin, A. Banyasz, O. Maury, B. Baguenard, A. Bensalah-Ledoux, S. Guy, A. Grichine, X.-N. Nguyen, A. Cimarelli, M. Sanadar, A. Melchior and F. Piccinelli, Circularly polarized activity from two photon excitable europium and samarium chiral bioprobes, J. Mater. Chem. C, 2023, 11, 4188–4202 RSC.
- D. Akl, L. Bridou, M. Hojorat, G. Micouin, S. R. Kiraev, F. Riobé, S. Denis-Quanquin, A. Banyasz and O. Maury, Comprehensive Photophysical and Nonlinear Spectroscopic Study of Thioanisolyl-Picolinate Triazacyclononane Lanthanide Complexes, Eur. J. Inorg. Chem., 2024, 27, e202300785 CrossRef CAS.
- K. Binnemans, Interpretation of europium(III) spectra, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. G. Williams and M. Woods, Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states, J. Chem. Soc., Perkin Trans. 2, 1999, 493–504 RSC.
- M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes, Phys. Chem. Chem. Phys., 2002, 4, 1542–1548 RSC.
- N. Kofod, L. G. Nielsen and T. J. Sørensen, Temperature Dependence of Fundamental Photophysical Properties of [Eu(MeOH-d4)9]3+ Solvates and [Eu·DOTA(MeOH-d4)]− Complexes, J. Phys. Chem. A, 2021, 125, 8347–8357 CrossRef CAS PubMed.
- D. Kovacs, E. Mathieu, S. R. Kiraev, J. A. L. Wells, E. Demeyere, A. Sipos and K. E. Borbas, Coordination Environment-Controlled Photoinduced Electron Transfer Quenching in Luminescent Europium Complexes, J. Am. Chem. Soc., 2020, 142, 13190–13200 CrossRef CAS PubMed.
- A. Weller, Electron-transfer and complex formation in the excited state, Pure Appl. Chem., 1968, 16, 115–124 CrossRef CAS.
- W. D. Horrocks, J. P. Bolender, W. D. Smith and R. M. Supkowski, Photosensitized near infrared luminescence of ytterbium(III) in proteins and complexes occurs via an internal redox process, J. Am. Chem. Soc., 1997, 119, 5972–5973 CrossRef CAS.
- A. Beeby, S. Faulkner and J. A. G. Williams, pH Dependence of the energy transfer mechanism in a phenanthridine-appended ytterbium complex, J. Chem. Soc., Dalton Trans., 2002, 1918–1922 RSC.
- D. Kocsi, D. Kovacs, J. A. L. Wells and K. E. Borbas, Reduced quenching effect of pyridine ligands in highly luminescent Ln(III) complexes: the role of tertiary amide linkers, Dalton Trans., 2021, 50, 16670–16677 RSC.
- D. Parker, J. D. Fradgley, M. Delbianco, M. Starck, J. W. Walton and J. M. Zwier, Comparative analysis of lanthanide excited state quenching by electronic energy and electron transfer processes, Faraday Discuss., 2022, 234, 159–174 RSC.
- P. Luo, E. C. Feinberg, G. Guirado, S. Farid and J. P. Dinnocenzo, Accurate Oxidation Potentials of 40 Benzene and Biphenyl Derivatives with Heteroatom Substituents, J. Org. Chem., 2014, 79, 9297–9304 CrossRef CAS PubMed.
- Y. Qin and Y. Xia, Simultaneous Two-Photon Fluorescence Microscopy of NADH and FAD Using Pixel-to-Pixel Wavelength-Switching, Front. Phys., 2021, 9, 642302 CrossRef.
- R. Cao, H. K. Wallrabe and A. Periasamy, Multiphoton FLIM imaging of NAD(P)H and FAD with one excitation wavelength, J. Biomed. Opt., 2020, 25, 014510 CAS.
- I. A. Gorbunova, M. K. Danilova, M. E. Sasin, V. P. Belik, D. P. Golyshev and O. S. Vasyutinskii, Determination of fluorescence quantum yields and decay times of NADH and FAD in water–alcohol mixtures: The analysis of radiative and nonradiative relaxation pathways, J. Photochem. Photobiol., A, 2023, 436, 114388 CrossRef CAS.
- A. Erazo-Oliveras, K. Najjar, L. Dayani, T.-Y. Wang, G. A. Johnson and J.-P. Pellois, Protein delivery into live cells by incubation with an endosomolytic agent, Nat. Methods, 2014, 11, 861–867 CrossRef CAS PubMed.
- J. Allen and J.-P. Pellois, Hydrophobicity is a key determinant in the activity of arginine-rich cell penetrating peptides, Sci. Rep., 2022, 12, 15981 CrossRef CAS PubMed.
- M. Serulla, P. Anees, A. Hallaj, E. Trofimenko, T. Kalia, Y. Krishnan and C. Widmann, Plasma membrane depolarization reveals endosomal escape incapacity of cell-penetrating peptides, Eur. J. Pharm. Biopharm., 2023, 184, 116–124 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00333d |
| This journal is © the Partner Organisations 2025 |