Open Access Article
Open Access Article‘Kick-in the head’: high-performance and air-stable mononuclear DyIII single-molecule magnets with pseudo-D6h symmetry from a [1 + 1] Schiff-base macrocycle approach†
Alexandros S.
Armenis
a,
Arpan
Mondal
b,
Sean R.
Giblin
c,
Dimitris I.
Alexandropoulos
 a,
Jinkui
Tang
a,
Jinkui
Tang
 d,
Richard A.
Layfield
d,
Richard A.
Layfield
 *b and
Theocharis C.
Stamatatos
*b and
Theocharis C.
Stamatatos
 *a
*a
aDepartment of Chemistry, University of Patras, Patras 26504, Greece. E-mail: thstama@upatras.gr; Tel: +30-2610996730
bDepartment of Chemistry, School of Life Sciences, University of Sussex, Brighton BN1 9QR, UK. E-mail: R.Layfield@sussex.ac.uk
cSchool of Physics and Astronomy, Cardiff University, Cardiff CF24 3AA, UK
dState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
First published on 23rd December 2024
Abstract
Using the [1 + 1] condensation approach for the preparation of new macrocyclic scaffolds (LN6phen and LN4O2phen) with the rigid phenanthroline-based ‘head’ unit produces the air-stable mononuclear compounds [Dy(LN6phen)(Ph3SiO)2](PF6) (1-LN6phen) and [Dy(LN4O2phen)(Ph3SiO)2](PF6) (1-LN4O2phen) through a stepwise metal-ion templated synthesis. Both compounds exhibit pseudo-D6h symmetry with different degrees of distortion from the ideal hexagonal bipyramidal geometry, depending on the planarity of the equatorial macrocycles and the intramolecular π–π stacking interactions between the phenyl groups of the apical siloxide ligand and the equatorial macrocycle. Both compounds are single-molecule magnets (SMMs) with large energy barriers for the magnetization reversal, exhibiting out-of-phase AC susceptibility signals up to 75 K or 90 K. The closer-to-ideal D6h complex 1-LN4O2phen possesses a Ueff of 1360 K, which is the highest reported barrier among all mononuclear DyIII SMMs synthesized using the [1 + 1] Schiff-base macrocycle approach. The experimental results are supported by ab initio calculations, which indicate relaxation of the magnetization via the first- or second-excited state for 1-LN6phen and 1-LN4O2phen, respectively. The results demonstrate the ability of Schiff-base macrocycles to facilitate the synthesis of high-performance and air-stable SMMs through a chemical modulation of the individual carbonyl ‘head’ and amine subunits, deciphering the factors which affect the magnetic dynamics of SMMs.
Introduction
Over the past decade, there has been a rapid increase in the number of studies on molecular compounds of f-elements that exhibit slow relaxation of their magnetic moment.1 The motivation behind this growing interest is the potential to use stable magnetic moments for storing information in molecules.2 Coordination compounds based on 4f-elements, particularly those containing dysprosium and terbium, are of great interest because their strong spin–orbit coupling and appreciable magnetic anisotropy can result in large and bistable magnetic moments separated by an energy barrier for the spin reversal (Ueff), thus leading to slow magnetization relaxation and single-molecule magnet (SMM) behavior.2 SMMs can retain their magnetization even in the absence of an external magnetic field, mimicking the behavior observed in bulk magnetic materials but at the molecular level.3 This ability to control and manipulate magnetic states at such small scales could revolutionize areas such as quantum computing,4 spintronics,5 and information storage,6 where traditional extended solid materials may face limitations.In lanthanide complexes, two critical factors influence their magnetic properties, namely the symmetry and strength of the ligand field that surrounds the 4f-metal ions.7 These factors contribute to the onset of significant crystal-field splitting, which in turn leads to large effective energy barriers for magnetization reorientation and high blocking temperatures (TB), both essential parameters for preserving magnetic information.8 One of the most promising approaches for developing high-performance and air-stable lanthanide SMMs is the synthesis of mononuclear DyIII complexes, where the metal center exhibits axial crystal-field (CF) symmetry, and adopts pentagonal bipyramidal (D5h) or hexagonal bipyramidal (D6h) coordination geometries.7,8 The primary intention of the CF symmetry is to eliminate, or at least significantly reduce, the transverse CF components that accelerate magnetic relaxation through quantum tunneling of the magnetization (QTM). Simultaneously, the strong axial CF is mandatory in the case of the oblate-shaped DyIII ion for achieving large energy separation of the CF-induced mJ states and high Ueff values.9
Although it is difficult to modulate the arrangement of the donor-atoms around a lanthanide ion and obtain perfect local symmetry, synthetic chemists have invented ways through ligand design and basic principles of molecular inorganic chemistry that result in the metal center adopting geometries with a near-ideal D5h or D6h symmetry. In this vein, there are three different synthetic strategies based on the preferences of the oblate-shaped electron density of DyIII, which requires strong metal–ligand interactions along the z-axis and weak interactions in the xy plane.9 The first strategy involves the coordination of five weakly bound monodentate ligands (e.g. pyridine, THF, H2O) in the equatorial plane in conjunction with two strong and bulky axial ligands (e.g. alkoxides, phenoxides or phosphine oxides) that reside along the z-axis. This approach prevents other ligands from coordinating with the metal, leading to almost perfect pentagonal bipyramidal compounds with remarkably high Ueff and TB values.10 The second approach is based on the rotating degrees of freedom and the twisting of the polydentate organic chelate (e.g. H2bbpen and its derivatives; H2bbpen = N,N′-bis(2-hydroxybenzyl)-N,N′-bis(2-methylpyridyl)ethylenediamine) that has the ability to coordinate both equatorially and axially to the metal center, providing the appropriate axial ligand field (RO− groups) around the oblate-shaped DyIII ions.11 The last route comprises the use of macrocyclic molecules with certain cavity sizes and numbers of donor-atoms, such as Schiff-bases and crown ethers. The latter encapsulate the lanthanide ion and promote a soft equatorial ligation, rendering the apical positions amenable to interact strongly with anionic O-donor ligands, such as phenoxides and siloxides.12 This synthetic strategy has yielded a variety of DyIII coordination compounds with D5h or D6h local symmetries,12 and record Ueff values for the magnetization reversal,13 comparable to the landmark dysprosium-metallocene SMMs.14
A common method to synthesize Schiff-base macrocyclic complexes involves a condensation reaction of a dicarbonyl (‘head’ unit) and diamine compounds in the presence of a template metal-ion that can foster the cyclization of the resulting product.15 Depending on the ratio of diamine to dicarbonyl, the amine chain length, the donor atoms, the ionic radius of the metal, and the cavity size, the reaction can yield [1 + 1], [2 + 2], or [3 + 3] macrocyclic products.15,16 Trivalent lanthanide ions (LnIII) have been employed as metal templates in the condensation reactions of diamines and dicarbonyl compounds to produce various [1 + 1] and [2 + 2] macrocyclic complexes, with a pyridine ‘head’ unit usually located at the macrocyclic scaffold.16 More specifically, the [2 + 2] products have led to a plethora of mononuclear DyIII complexes exhibiting D6h symmetry and enhanced SMM properties with extremely high Ueff values;17 this is mainly due to the ability of these large macrocycles to accommodate sizeable 4f-metal ions without suffering from significant bond strain within their combined carbonyl and amine units. In contrast, the [1 + 1] products can also yield DyIII complexes with D5h or D6h symmetries in a more designable manner, albeit the resulting coordination compounds are often more distorted than the [2 + 2] analogues, deviating from the ideal geometries.18 As a result, most of the high-performance hexagonal bipyramidal DyIII SMMs reported in the literature, have stemmed from the [2 + 2] approach, whereas only two examples have been reported that contain the [1 + 1] condensation reaction.18d,e
In this direction, we decided to utilize the metal-ion assisted [1 + 1] cyclocondensation approach by implementing the more robust phenanthroline-based ‘head’ unit to produce DyIII Schiff-base macrocyclic complexes with D6h symmetries and investigate their overall magnetic dynamics. To the best of our knowledge, phenanthroline-based dicarbonyl compounds have never been previously used in the coordination chemistry of lanthanide/Schiff-base macrocyclic complexes (Table S1†). Herein, we report the synthesis of two hexagonal bipyramidal DyIII complexes: [Dy(LN6phen)(Ph3SiO)2](PF6) (1-LN6phen) and [Dy(LN4O2phen)(Ph3SiO)2](PF6) (1-LN4O2phen), which bear the macrocycles LN6phen and LN4O2phen (Scheme 1), surrounding the equatorial plane of the metal centers, while two bulky triphenylsiloxide (Ph3SiO−) ligands occupy the apical positions. The two new, structurally similar, macrocycles have resulted from the [1 + 1] condensation reaction of 1,10-phenanthroline-2,9-dicarbaldehyde with either triethylenetetramine or 1,2-bis(2-aminoethoxy)ethane, yielding the all-N6 or the mixed-donor N4O2 scaffolds, respectively, with dissimilar equatorial environments, electronic properties, and degrees of distortion.
 | ||
| Scheme 1 Structural representation of the ‘head’ units (top) and the new [1 + 1] Schiff-base macrocyclic ligands LN6phen and LN4O2phen (bottom) discussed in this work. | ||
Interestingly, both DyIII complexes exhibit SMM behavior up to 75 K (1-LN6phen) and 90 K (1-LN4O2phen), albeit with distinctly different Ueff values depending on the deviation of the macrocyclic ligands from the perfect planar conformation and the overall distortion from the ideal D6h local symmetry. Apparently, the replacement of the –NH groups by the etheric O-donor atoms within the macrocycle scaffolds has yielded a substantial increase in the Ueff value, thus rendering 1-LN4O2phen as a record holder in energy barriers amongst all reported [1 + 1] macrocycle-based DyIII SMMs.
Results and discussion
Synthesis and molecular structures
We recently reported the mononuclear complexes [Dy(LN6py)(Ph3SiO)2](PF6) and [Dy(LN3O3py)(Ph3SiO)2](PF6), which feature pseudo-D6h symmetry and exhibit enhanced SMM properties with Ueff values up to 1300 K.18e The two air-stable compounds have resulted from the [1 + 1] Schiff-base macrocyclic approach, utilizing the macrocycles derived from the condensation of the well-known 2,6-diacetylpyridine ‘head’ unit (Table S1†) with either tetraethylenepentamine (LN6py) or 1,11-diamino-3,6,9-trioxaundecane (LN3O3py). In the present study, we decided to shift from pyridine to phenanthroline ‘head’ unit, thus exploiting several new opportunities, such as the offer of an additional N-donor atom from the ‘head’ unit for coordination to the metal center, as well as the rigidity, bulkiness and planarity of phenanthroline over pyridine groups. Further, we have retained the Ph3SiO− groups as strong axial ligands for directing the magnetic anisotropy along the Ising-type axis.The new phenanthroline-based compounds 1-LN6phen and 1-LN4O2phen were prepared by following a well-known synthetic protocol (Scheme 2), which was firstly introduced by Murrie and coworkers for the synthesis of [1 + 1] DyIII complexes with D5h symmetries.18c In particular, the stoichiometric reaction of 1,10-phenanthroline-2,9-dicarbaldehyde and the respective diamine was carried out in the presence of DyCl3·6H2O as a template agent to form the DyIII/Cl−/macrocycle precursor. Subsequently, the bulky triphenylsilanol pro-ligand (Ph3SiOH) was used, which upon deprotonation with Et3N, can occupy the axial sites of the DyIII center and replace the weakly bound Cl− ions and/or the coordinated solvate molecules (i.e., MeOH, H2O). Finally, an anion metathesis reaction with NH4PF6 or KPF6 was performed to aid crystallization, resulting in pale yellow plate-like crystals of the mononuclear complexes [Dy(LN6phen)(Ph3SiO)2](PF6) (1-LN6phen) and [Dy(LN4O2phen)(Ph3SiO)2](PF6) (1-LN4O2phen).
 | ||
| Scheme 2 General synthetic route for the preparation of complexes 1-LN6phen (X = NH and A+ = NH4+) and 1-LN4O2phen (X = O and A+ = K+). | ||
Both 1-LN6phen and 1-LN4O2phen are thermally robust, with a high decomposition temperature approaching 250 °C (Fig. S3†). The powder X-ray diffraction patterns of both compounds show good agreement with the simulated ones, confirming the phase purity of the samples (Fig. S4 and S5†).
Single-crystal X-ray diffraction revealed that complexes 1-LN6phen and 1-LN4O2phen crystallize in the orthorhombic P212121 and triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space groups, respectively (Fig. S6†). Crystallographic details for both complexes are presented in Table S2.† Selected interatomic distances and angles for complexes 1-LN6phen and 1-LN4O2phen are listed in Tables S3 and S4,† respectively. For the sake of brevity, only important metrical parameters will be discussed in the main text. The molecular structure of 1-LN6phen is composed of the mononuclear cationic complex [Dy(LN6phen)(Ph3SiO)2]+ (Fig. 1A) counterbalanced by a PF6− ion. The DyIII center is encapsulated in the cavity of the LN6phen Schiff-base macrocycle, which provides an equatorial N6 coordination environment, whereas the axial positions are occupied by two strongly bound Ph3SiO− ligands, leading to an overall N6O2 coordination environment. The eight-coordinate DyIII center adopts a significantly distorted hexagonal bipyramidal geometry according to the SHAPE program (Fig. 1B),19 as indicated by the large Continuous Shape Measure (CShM) value of 4.35 (Table S5†). Values of CShM greater than 3 correspond to a significant distortion from the ideal coordination geometry. The relatively large Dy–Nequatorial bond distances, which fall into the range of 2.558(8)–2.695(9) Å, in conjunction with the two short and strong Dy–Oaxial bond lengths of 2.159(5) and 2.163(5) Å, demonstrate the compressed D6h local symmetry favoring the axial crystal-field required for an oblate-shaped DyIII ion. Additionally, the O–Dy–O bond angle of 164.6(2)° significantly deviates from the ideal angle of 180° for a perfect hexagonal bipyramid, as well as the O–Dy–N bond angles, which diverge from the ideal angle of 90° (Table S3†), corroborating the distorted polyhedron of 1-LN6phen.
space groups, respectively (Fig. S6†). Crystallographic details for both complexes are presented in Table S2.† Selected interatomic distances and angles for complexes 1-LN6phen and 1-LN4O2phen are listed in Tables S3 and S4,† respectively. For the sake of brevity, only important metrical parameters will be discussed in the main text. The molecular structure of 1-LN6phen is composed of the mononuclear cationic complex [Dy(LN6phen)(Ph3SiO)2]+ (Fig. 1A) counterbalanced by a PF6− ion. The DyIII center is encapsulated in the cavity of the LN6phen Schiff-base macrocycle, which provides an equatorial N6 coordination environment, whereas the axial positions are occupied by two strongly bound Ph3SiO− ligands, leading to an overall N6O2 coordination environment. The eight-coordinate DyIII center adopts a significantly distorted hexagonal bipyramidal geometry according to the SHAPE program (Fig. 1B),19 as indicated by the large Continuous Shape Measure (CShM) value of 4.35 (Table S5†). Values of CShM greater than 3 correspond to a significant distortion from the ideal coordination geometry. The relatively large Dy–Nequatorial bond distances, which fall into the range of 2.558(8)–2.695(9) Å, in conjunction with the two short and strong Dy–Oaxial bond lengths of 2.159(5) and 2.163(5) Å, demonstrate the compressed D6h local symmetry favoring the axial crystal-field required for an oblate-shaped DyIII ion. Additionally, the O–Dy–O bond angle of 164.6(2)° significantly deviates from the ideal angle of 180° for a perfect hexagonal bipyramid, as well as the O–Dy–N bond angles, which diverge from the ideal angle of 90° (Table S3†), corroborating the distorted polyhedron of 1-LN6phen.
On the other hand, the DyIII center in 1-LN4O2phen (Fig. 1C) is equatorially coordinated by the LN4O2phen mixed-donor macrocycle via two pyridine N-atoms and two N-atoms of the secondary amine (–NH–) groups (same as in 1-LN6phen), as well as two ether-type O-donors in place of the secondary amine N-atoms in 1-LN6phen. Likewise, two axial triphenylsiloxide ligands, each located above and below the DyN4O2 plane, complete the overall N4O4 coordination environment. In contrast to 1-LN6phen, the Oax–Dy–Oax angle of 174.52(7) and the Oax–Dy–Neq/Oeq bond angles within 1-LN4O2phen are closer to the ideal 180° and 90°, respectively, for a perfect hexagonal bipyramid (Table S4†), resulting in a CShM value of 1.74 (Table S5†), implying a smaller distortion from ideal D6h symmetry (Fig. 1D). Moreover, the Dy–Oax bonds are 2.142(2) and 2.153(2) Å, noticeably shorter than the analogous bonds in 1-LN6phen, while the Dy–Neq/Oeq lie in the range of 2.570(2)–2.628(2) Å.
A closer inspection of the degree of planarity of the equatorial plane, according to the relative positions of the donor atoms with respect to the hexagonal plane, revealed that the macrocycle LN4O2phen exhibits a more planar conformation than LN6phen. This observation was validated by the strikingly different CShM values regarding the ideal hexagon between 1-LN6phen and 1-LN4O2phen (CShM = 4.13 vs. 1.32) (Fig. S7†). The packing diagram of neighboring monomeric complexes revealed that the shortest intermolecular Dy⋯Dy distance is 10.848(4) Å for 1-LN6phen and 8.015(5) Å for 1-LN4O2phen (Fig. S8 and S9†).
Attempting to rationalize the observed differences in the macrocycles’ planarity and the Oax–Dy–Oax angles, it is worth noting that the phenyl rings of the triphenylsilanolate ligands interact through π–π stacking and C–H⋯π intramolecular interactions with the phenanthroline moiety of the macrocyclic scaffold in both compounds (Fig. S10†). More specifically, the strength of the C–H⋯π interactions is of the same magnitude for both complexes, as indicated by the relatively long C52–H52⋯Ar1 centroid (1-LN6phen) and C21–H21⋯Ar1 centroid (1-LN4O2phen) distances of 2.936 Å and 2.935 Å, respectively. However, the π–π stacking interactions within 1-LN6phen are much stronger than 1-LN4O2phen, as demonstrated by the centroid⋯centroid distances of 3.538 Å (Ar2⋯Ar4) for the former and 3.719 Å (Ar1⋯Ar4) for the latter. This discrepancy may be responsible for the tilting of the axial triphenylsilanolate ligand towards the macrocycle, thus leading to a more bent hexagonal bipyramidal coordination geometry and less axial Oax–Dy–Oax angle in 1-LN6phen.
Magnetic properties
To probe the static magnetic properties of the two complexes, direct current (dc) magnetic susceptibility studies were conducted on analytically pure microcrystalline samples in the temperature range of 2–300 K under an applied field of 0.1 T (Fig. 2). The room temperature χMT values of 14.10 (1-LN6phen) and 14.06 (1-LN4O2phen) cm3 mol−1 K are very close to the theoretical value of 14.17 cm3 mol−1 K for an isolated DyIII ion (6H15/2, S = 5/2, L = 5, g = 4/3). For both complexes, the χMT product decreases smoothly upon cooling until ∼14 K, and then more sharply reaching values of 12.49 (1-LN6phen) and 10.00 (1-LN4O2phen) cm3 mol−1 K at 2 K. The abrupt decrease of the χMT products can be attributed to the depopulation of the low-lying crystal-field mJ states of the ground 6H15/2 state as the temperature is lowering, combined with the onset of magnetic blocking on approaching the low temperature limit of the experiment. | ||
| Fig. 2 Temperature dependence of the χMT product for complexes 1-LN6phen (black circles) and 1-LN4O2phen (red circles). | ||
The isothermal field (H) dependence of the magnetization (M) was measured at 2, 3, and 5 K for both complexes in the range of 0–7 T (Fig. S11 and S12†). The M(H) curves at 2 K are similar for both complexes, exhibiting an abrupt increase of magnetization at low fields while remaining almost constant at high fields. The magnetization values at the maximum applied field of 7 T and the lowest temperature of 2 K are 5.30 (1-LN6phen) and 5.07 NμB (1-LN4O2phen), much lower than the expected saturation value (MS) for one free DyIII ion (MS/NμB = 10NμB); this can be ascribed to the crystal field effects that induce significant magnetic anisotropy.
To assess the slow magnetization relaxation phenomena and the SMM behavior of 1-LN6phen and 1-LN4O2phen, alternating current (ac) magnetic susceptibility studies were performed under zero applied dc field and a weak ac field of 3.0 Oe, oscillating with frequencies ranging from 10 to 10000 Hz. Interestingly, both compounds exhibit frequency-dependent in-phase  (Fig. S13†) and out-of-phase
(Fig. S13†) and out-of-phase  (Fig. 3A and Fig. 4A) peaks of signals with well-defined maxima up to 75 and 90 K for 1-LN6phen and 1-LN4O2phen, respectively. The appearance of the out-of-phase magnetic susceptibility signals denotes the presence of slow magnetization relaxation due to an energy barrier for the spin-reversal consistent with SMM behavior. Furthermore, the detection of the frequency dependence of the out-of-phase susceptibility up to this high-temperature regime suggests the existence of significant magnetization reversal barriers for both complexes. The ac frequency at which the maximum occurs in the corresponding
(Fig. 3A and Fig. 4A) peaks of signals with well-defined maxima up to 75 and 90 K for 1-LN6phen and 1-LN4O2phen, respectively. The appearance of the out-of-phase magnetic susceptibility signals denotes the presence of slow magnetization relaxation due to an energy barrier for the spin-reversal consistent with SMM behavior. Furthermore, the detection of the frequency dependence of the out-of-phase susceptibility up to this high-temperature regime suggests the existence of significant magnetization reversal barriers for both complexes. The ac frequency at which the maximum occurs in the corresponding 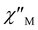 vs. v plots shows a slight variation below 10 K, suggesting the presence of quantum tunneling of magnetization (QTM) relaxation process. At elevated temperatures, this maximum becomes significantly dependent on temperature, shifting to higher frequencies as the temperature increases, indicating a relaxation process driven by thermal activation.20
vs. v plots shows a slight variation below 10 K, suggesting the presence of quantum tunneling of magnetization (QTM) relaxation process. At elevated temperatures, this maximum becomes significantly dependent on temperature, shifting to higher frequencies as the temperature increases, indicating a relaxation process driven by thermal activation.20
To obtain the temperature-dependence of relaxation times (τ) and derive the operating relaxation processes, we constructed the Arrhenius plot (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. 1/T) by fitting the data through the equation:
τ vs. 1/T) by fitting the data through the equation:
| τ−1 = τ0−1e−Ueff/kT + CTn + τQTM−1, | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. 1/T plots, while below 10 K the relaxation time becomes temperature-independent for both complexes.20,21 Accordingly, in the high-temperature regime, the thermally-activated Orbach process becomes the dominant relaxation pathway and the relaxation time shows an exponential dependence on temperature (linear region in ln
τ vs. 1/T plots, while below 10 K the relaxation time becomes temperature-independent for both complexes.20,21 Accordingly, in the high-temperature regime, the thermally-activated Orbach process becomes the dominant relaxation pathway and the relaxation time shows an exponential dependence on temperature (linear region in ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. 1/T plots).20,21 Therefore, the best-fit to the experimental data afforded the following parameters: Ueff = 779 (54) K, τ0 = 9.98 × 10−10 s, C = 0.5477 (0.045) s−1 K−n, n = 2.42 (0.03), τQTM = 1.57 × 10−2 (2.19 × 10−4) s for complex 1-LN6phen and Ueff = 1360 (21) K, τ0 = 5.88 × 10−12 s, C = 0.0777 (0.01) s−1 K−n, n = 2.45 (0.06), τQTM = 1.77 × 10−2 (6.94 × 10−4) s for complex 1-LN4O2phen. The values of the parameters τ0, C, and n are within the expected range for mononuclear DyIII-based SMMs,10,11,17,18 while the exponent n of the Raman process takes a significantly smaller value than the expected for a Kramers ion (n = 9). However, it has been reported that deviations in n values suggest the mixing of optical and acoustic phonons, most probably due to the presence of low energy optical phonons that contribute to the Raman demagnetization.17,18,21,22 The obtained Ueff for compound 1-LN4O2phen is the highest effective energy barrier yet reported among all mononuclear DyIII SMMs, which have derived from the [1 + 1] Schiff-base macrocycle approach (Table S1†). Li, Yin and coworkers have recently reported the complex [Dy(LN61)(Ph3SiO)2](BPh4) with a pseudo-D6h symmetry, bearing the neutral hexaaza macrocyclic Schiff base ligand derived from the [1 + 1] condensation of 6,6′-diformyl-2,2′-bipyridyl and triethylenetetramine.18d This complex behaves as an SMM with a Ueff of ∼584 K, much smaller than the corresponding values of 1-LN6phen and 1-LN4O2phen, which is rationalized in terms of the severe distortion from the ideal D6h symmetry (CShM = 3.91) and the significant deviation from the perfect axiality (O–Dy–O = 169.1°) and equatorial hexagonal plane (best mean angle of N–Dy–N = 50.8°).
τ vs. 1/T plots).20,21 Therefore, the best-fit to the experimental data afforded the following parameters: Ueff = 779 (54) K, τ0 = 9.98 × 10−10 s, C = 0.5477 (0.045) s−1 K−n, n = 2.42 (0.03), τQTM = 1.57 × 10−2 (2.19 × 10−4) s for complex 1-LN6phen and Ueff = 1360 (21) K, τ0 = 5.88 × 10−12 s, C = 0.0777 (0.01) s−1 K−n, n = 2.45 (0.06), τQTM = 1.77 × 10−2 (6.94 × 10−4) s for complex 1-LN4O2phen. The values of the parameters τ0, C, and n are within the expected range for mononuclear DyIII-based SMMs,10,11,17,18 while the exponent n of the Raman process takes a significantly smaller value than the expected for a Kramers ion (n = 9). However, it has been reported that deviations in n values suggest the mixing of optical and acoustic phonons, most probably due to the presence of low energy optical phonons that contribute to the Raman demagnetization.17,18,21,22 The obtained Ueff for compound 1-LN4O2phen is the highest effective energy barrier yet reported among all mononuclear DyIII SMMs, which have derived from the [1 + 1] Schiff-base macrocycle approach (Table S1†). Li, Yin and coworkers have recently reported the complex [Dy(LN61)(Ph3SiO)2](BPh4) with a pseudo-D6h symmetry, bearing the neutral hexaaza macrocyclic Schiff base ligand derived from the [1 + 1] condensation of 6,6′-diformyl-2,2′-bipyridyl and triethylenetetramine.18d This complex behaves as an SMM with a Ueff of ∼584 K, much smaller than the corresponding values of 1-LN6phen and 1-LN4O2phen, which is rationalized in terms of the severe distortion from the ideal D6h symmetry (CShM = 3.91) and the significant deviation from the perfect axiality (O–Dy–O = 169.1°) and equatorial hexagonal plane (best mean angle of N–Dy–N = 50.8°).
To further examine the distribution of relaxation times (α) across the entire temperature range, the Cole–Cole plots (Fig. S14†) of both complexes were fitted using a generalized Debye model (eqn (S1) and (S2), see ESI† for details). The non-semicircular shape of the plots indicates a wide distribution of relaxation times, most probably due to overlapping of different relaxation mechanisms. Indeed, the large α values span the range 0.35–0.18 for 1-LN6phen (Table S6†) and 0.33–0.11 for 1-LN4O2phen (Table S7†), corroborating the above hypothesis. In particular, the largest α values are observed in the low-T regime, denoting the coexistence of Raman and QTM relaxation processes as the temperature is lowered.
To verify the blocking temperature of both compounds, magnetization (M) vs. field (H) hysteresis studies were performed at an average field sweep rate of 40 Oe s−1 and different low temperatures by sweeping the field between −5 and 5 T (Fig. 5A and Fig. S15†). Both compounds showed butterfly-shaped loops, characteristics of the fast tunneling near zero field, which results in small coercivities.23 In particular, complexes 1-LN4O2phen and 1-LN6phen exhibit opening of the hysteresis loops up to 9 K (Fig. 5B) and 5 K (Fig. S15†), respectively, thus implying a smaller transverse anisotropy and a higher pseudo-D6h symmetry for the former compound, in agreement with the results obtained from the ab initio calculations (vide infra).
Ab initio calculations
Detailed insight into the magnetic properties of 1-LN6phen and 1-LN4O2phen was obtained through ab initio calculations, using the SINGLE_ANISO approach implemented in the ORCA 5.0.2 software package24a (see the ESI† for details), to further analyze the mechanism that governs magnetization relaxation in both complexes. As expected from the strong axial ligand field, the DyIII ions in both compounds exhibit large crystal-field splitting of 1854 K (1-LN6phen) and 2126 K (1-LN4O2phen) (Table S8†). The calculated energies of the eight lowest Kramer Doublets (KDs) of the 6H15/2 ground multiplet of the DyIII centers along with the principal components of the g-tensors and the angles between the ground and excited KDs of 1-LN6phen and 1-LN4O2phen are tabulated in Tables S9 and S10,† respectively. The ground KDs in both complexes possess strong axial magnetic anisotropy, which can be reflected in the wavefunction composition of nearly 100% of the mJ = 15/2, as well as the respective g-tensors approaching the Ising limit (gx = 3.3 × 10−4, gy = 5.7 × 10−4, gz = 19.85 for 1-LN6phen and gx = 6.8 × 10−4, gy = 8.0 × 10−4, gz = 19.87 for 1-LN4O2phen). In addition, the calculated orientations of the ground state anisotropic axes in both complexes point towards the axial Dy–O bonds of the triphenylsilanolate ligands (Fig. 6C/D). Moreover, the anisotropic axes of the ground doublet for both compounds are almost collinear with those of the first two excited doublets, exhibiting angles of 0.462° and 0.99° with the second KD and 1.413° and 3.07° with the third KD for 1-LN6phen and 1-LN4O2phen, respectively. Although the high purity of the first excited doublet (mJ = ±13/2) for 1-LN6phen can be validated by the relative wavefunction composition of 99.4%, the axial g-tensor is significantly lower (gz = 16.92) and the transversal components of the g-tensor are also small but non-negligible (gx = 0.1176, gy = 0.1195). In contrast, complex 1-LN4O2phen exhibits a purer and more anisotropic first excited doublet composed of 99.67% of the mJ = ±13/2, while the values of the g-tensors are: gz = 16.94, gx = 0.081, and gy = 0.089. However, the third KD in 1-LN4O2phen is an admixture of mJ = ±11/2 (96.47%) and mJ = ±1/2 (2.60%), while the low axiality within this KD is corroborated by the significant contribution of the transverse g-components (gx = 0.106, gy = 0.298) along with the decreased value of the axial component (gz = 13.77). These observations suggest that the gz-component is not sufficiently axial to suppress the through-barrier relaxation mechanisms within the second KD in 1-LN6phen and the third KD in 1-LN4O2phen. This is further confirmed by the experimentally determined Ueff values following the calculated energies of the second KD in 1-LN6phen and the third in KD 1-LN4O2phen which are located at 680 K and 1352 K, respectively. In detail, the experimental Ueff value of 1360 K for 1-LN4O2phen is in excellent agreement with the calculated energy of the third KD (1352 K), whereas the experimental Ueff value of 779 K for 1-LN6phen is slightly higher than the calculated energy state of the second KD (680 K). The small deviation between the energy of the second KD and the experimentally observed Ueff in 1-LN6phen most likely results from neglecting electron correlation outside the 4f orbital space in the CASSCF calculations.14a Therefore, we applied an alternative fit to the experimental data for 1-LN6phen in the temperature range of 10–75 K, discarding the QTM term from eqn (1). This was due to the relaxation time becoming temperature-independent below 10 K, and indeed the alternative fit gave a Ueff value of 653(27) K (Fig. S16;† see the caption for additional fit parameters), very close to the computed energy of the second KD in 1-LN6phen.Fig. 6 illustrates the probability of the magnetic relaxation pathways where the most intense red arrows show the most probable relaxation route. According to the above observations, combined with the magnitudes of the transition moment matrix elements (Tables S11 and S12†), which show transitions between different KDs, the crossing of the magnetization relaxation barrier is predicted to proceed through the second and third KDs in 1-LN6phen and 1-LN4O2phen, respectively. In the case of 1-LN6phen the predicted barrier of 473 cm−1 is somewhat lower than the experimental barrier of 541 cm−1, although, based on the appreciable transverse components of the g-tensors in the first-excited KD, relaxation via any higher-lying states seems unlikely. The discrepancy in the experimental and calculated barriers could be a consequence of electron correlation effects outside of the 4f orbital manifold not being captured by the calculations. For 1-LN4O2phen, the first-excited KD at 512 cm−1 has more pronounced axial character, increasing the probability of reaching the second-excited KD at 940 cm−1, where a barrier-crossing transition then occurs. If valid, this analysis provides a good match with the experimental barrier of 945 cm−1.
To further quantify the axiality of both compounds and the magnitude of the crystal-field induced energy separation between the ground and the low-lying excited states, we calculated the crystal-field parameters by implementing SINGLE_ANISO module (Table S13, see ESI† for details). To calculate the crystal-field parameters was used the following Hamiltonian:24b,c
 | (2) |
| ĤCF = B02Ô02+ B04Ô04+ B06Ô06+ B66 (Ô66 + Ô−66), | (3) |
Conclusions
In summary, we have synthesized two new air-stable DyIII macrocyclic complexes with hexagonal bipyramidal geometries by implementing for the first time a phenanthroline-based ‘head’ unit through the metal-ion assisted [1 + 1] Schiff-base cyclocondensation reaction. Both compounds 1-LN6phen and 1-LN4O2phen behave as zero-field SMMs with high energy barriers stemming from the strongly axial ligand fields in conjunction with the weak coordination from the all N-donor LN6phen macrocycle in complex [Dy(LN6phen)(Ph3SiO)2](PF6) and the mixed N/O-donor LN4O2phen macrocycle in complex [Dy(LN4O2phen)(Ph3SiO)2](PF6). The replacement of the secondary amine groups in LN6phen with etheric O-atoms in LN4O2phen seems to have a significant impact on the overall planarity of the equatorial plane, as well as improved magnetic axiality, thereby leading to a closer-to-ideal D6h symmetry for complex 1-LN4O2phen. Additionally, it appears that the strength of the intramolecular π–π stacking interactions between the aromatic groups of the apical and equatorial ligands has its own effect on the geometric and electronic structures of the two compounds via modulating not only the planarity of the Schiff-base macrocycle but also the axial O–Dy–O tilt. Theoretical studies have shed light on the magnetization relaxation mechanisms of both complexes, presaging a dominant relaxation pathway through the first excited doublet for 1-LN6phen and the second excited doublet for 1-LN4O2phen. Furthermore, the larger value of the axial CF parameter |B02|, when shifting from 1-LN6phen to 1-LN4O2phen, was in agreement with the experimental results, and particularly the 2-fold enhancement of the Ueff value from 779 K in compound 1-LN6phen to 1360 K in compound 1-LN4O2phen. The latter Ueff is a record value among all (D5h and D6h) previously reported mononuclear DyIII SMMs resulted from the [1 + 1] Schiff-base macrocyclic approach.Ongoing research involves the enrichment of the family of [1 + 1] Schiff-base macrocyclic DyIII complexes through the designed synthesis and use of macrocycles with reduced equatorial binding strength and improved planarity by modulating either the carbonyl ‘head’ or the amine(s) units, or both. In turn, the implementation of aliphatic instead of aromatic substituted O-donor axial ligands, as a means of eliminating the intramolecular π–π stacking interactions, can lead to more linear O–Dy–O bond angles and increase the axial magnetic anisotropy of the DyIII ions, and the energy barrier for the magnetization reorientation.
Author contributions
Th. C. S. conceived the research idea. A. S. A. designed the structures and performed their synthesis and characterization. A. M., S. R. G., D. I. A. and J. T. made contributions to the data acquisition and analysis (XRD, magnetism, ab initio calculations). R. A. L. and Th. C. S. wrote the paper, with contributions from all the co-authors.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 1-LN6phen and 1-LN4O2phen have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under 2393281 and 2393282, respectively.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
A. M. and R. A. L. thank the UK EPSRC (grants EP/V003089/1, EP/X036626/1) for financial support. J. T. acknowledges the National Natural Science Foundation of China (grant 92261103).References
- K. Bernot, Get under the Umbrella: A Comprehensive Gateway for Researchers on Lanthanide-Based Single-Molecule Magnets, Eur. J. Inorg. Chem., 2023, 26, e202300336 CrossRef CAS.
- (a) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Lanthanide Single-Molecule Magnets, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed; (b) L. Sorace, C. Benelli and D. Gatteschi, Lanthanides in molecular magnetism: old tools in a new field, Chem. Soc. Rev., 2011, 40, 3092–3104 RSC; (c) M. Atzori and R. Sessoli, The second quantum revolution: role and challenges of molecular chemistry, J. Am. Chem. Soc., 2019, 141, 11339–11352 CrossRef CAS PubMed.
- A. Zabala-Lekuona, J. M. Seco and E. Colacio, Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time, Coord. Chem. Rev., 2021, 441, 213984 CrossRef CAS.
- (a) S. Thiele, F. Balestro, R. Ballou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Electrically driven nuclear spin resonance in single-molecule magnets, Science, 2014, 344, 1135–1138 CrossRef CAS PubMed; (b) R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Electronic read-out of a single nuclear spin using a molecular spin transistor, Nature, 2012, 488, 357–360 CrossRef CAS PubMed; (c) E. Moreno-Pineda and W. Wernsdorfer, Measuring molecular magnets for quantum technologies, Nat. Rev. Phys., 2021, 3, 645–659 CrossRef CAS.
- (a) A. Candini, S. Klyatskaya, M. Ruben, W. Wernsdorfer and M. Affronte, Graphene Spintronic Devices with Molecular Nanomagnets, Nano Lett., 2011, 11, 2634–2639 CrossRef CAS PubMed; (b) I. S. Zlobin, Y. V. Nelyubina and V. V. Novikov, Molecular Compounds in Spintronic Devices: An Intricate Marriage of Chemistry and Physics, Inorg. Chem., 2022, 61, 12919–12930 CrossRef CAS PubMed; (c) M. Ganzhorn and W. Wernsdorfer, in Molecular Magnets, ed. J. Bartolomé, F. Luis and J. F. Fernández, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014, pp. 319–364 Search PubMed; (d) M. Urdampilleta, N.-V. Nguyen, J.-P. Cleuziou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Molecular Quantum Spintronics: Supramolecular Spin Valves Based on Single-Molecule Magnets and Carbon Nanotubes, Int. J. Mol. Sci., 2011, 12, 6656–6667 CrossRef CAS PubMed.
- (a) M. D. Korzyński, Z. J. Berkson, B. Le Guennic, O. Cador and C. Copéret, Leveraging Surface Siloxide Electronics to Enhance the Relaxation Properties of a Single-Molecule Magnet, J. Am. Chem. Soc., 2021, 143, 5438–5444 CrossRef PubMed; (b) G. Gabarró-Riera, G. Aromí and E. C. Sañudo, Magnetic molecules on surfaces: SMMs and beyond, Coord. Chem. Rev., 2023, 475, 214858 CrossRef; (c) E. Coronado, Molecular magnetism: from chemical design to spin control in molecules, materials and devices, Nat. Rev. Mater., 2019, 5, 87–104 CrossRef; (d) M. Mannini, F. Pineider, P. Sainctavit, C. Danieli, E. Otero, C. Sciancalepore, A. M. Talarico, M.-A. Arrio, A. Cornia, D. Gatteschi and R. Sessoli, Magnetic memory of a single-molecule quantum magnet wired to a gold surface, Nat. Mater., 2009, 8, 194–197 CrossRef CAS PubMed.
- (a) T. G. Ashebr, H. Li, X. Ying, X.-L. Li, C. Zhao, S. Liu and J. Tang, Emerging Trends on Designing High-Performance Dysprosium(III) Single-Molecule Magnets, ACS Mater. Lett., 2022, 4, 307–319 CrossRef CAS; (b) S. K. Gupta and R. Murugavel, Enriching lanthanide single-ion magnetism through symmetry and axiality, Chem. Commun., 2018, 54, 3685–3696 RSC.
- (a) V. Vieru, S. Gómez-Coca, E. Ruiz and L. F. Chibotaru, Increasing the Magnetic Blocking Temperature of Single–Molecule Magnets, Angew. Chem., Int. Ed., 2024, 63, e202303146 CrossRef CAS; (b) Z. Zhu and J. Tang, Lanthanide single-molecule magnets with high anisotropy barrier: where to from here?, Natl. Sci. Rev., 2022, 9, nwac194 CrossRef PubMed.
- (a) J.-L. Liu, Y.-C. Chen and M.-L. Tong, Symmetry strategies for high performance lanthanide-based single-molecule magnets, Chem. Soc. Rev., 2018, 47, 2431–2453 RSC; (b) J. D. Rinehart and J. R. Long, Exploiting single-ion anisotropy in the design of f-element single-molecule magnets, Chem. Sci., 2011, 2, 2078–2085 RSC.
- (a) L.-L. Li, H.-D. Su, S. Liu, Y.-C. Xu and W.-Z. Wang, A new air-and moisture-stable pentagonal-bipyramidal DyIII single-ion magnet based on the HMPA ligand, Dalton Trans., 2019, 48, 2213–2219 RSC; (b) Y. Ding, T. Han, Y. Zhai, D. Reta, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, A Study of Magnetic Relaxation in Dysprosium(III) Single–Molecule Magnets, Chem. – Eur. J., 2020, 26, 5893–5902 CrossRef CAS; (c) S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, An air-stable Dy(III) single-ion magnet with high anisotropy barrier and blocking temperature, Chem. Sci., 2016, 7, 5181–5191 RSC; (d) A. B. Canaj, M. K. Singh, C. Wilson, G. Rajaraman and M. Murrie, Chemical and in silico tuning of the magnetisation reversal barrier in pentagonal bipyramidal Dy(III) single-ion magnets, Chem. Commun., 2018, 54, 8273–8276 RSC; (e) S. K. Gupta, S. Dey, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Deciphering the Role of Anions and Secondary Coordination Sphere in Tuning Anisotropy in Dy(III) Air–Stable D5h SIMs, Chem. – Eur. J., 2022, 28, e202103585 CrossRef CAS PubMed; (f) Y. Chen, J. Liu, Y. Lan, Z. Zhong, A. Mansikkamäki, L. Ungur, Q. Li, J. Jia, L. F. Chibotaru, J. Han, W. Wernsdorfer, X. Chen and M.-L. Tong, Dynamic Magnetic and Optical Insight into a High Performance Pentagonal Bipyramidal DyIII Single–Ion Magnet, Chem. – Eur. J., 2017, 23, 5708–5715 CrossRef CAS PubMed; (g) K.-X. Yu, J. G. C. Kragskow, Y.-S. Ding, Y.-Q. Zhai, D. Reta, N. F. Chilton and Y.-Z. Zheng, Enhancing magnetic hysteresis in single-molecule magnets by ligand functionalization, Chem, 2020, 6, 1777–1793 CrossRef CAS; (h) Y. Ma, Y. Zhai, Q. Luo, Y. Ding and Y.-Z. Zheng, Ligand Fluorination to Mitigate the Raman Relaxation of DyIII Single–Molecule Magnets: A Combined Terahertz, Far–IR and Vibronic Barrier Model Study, Angew. Chem., Int. Ed., 2022, 61, e202206022 CrossRef CAS PubMed; (i) Y. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, On Approaching the Limit of Molecular Magnetic Anisotropy: A Near-Perfect Pentagonal Bipyramidal Dysprosium(III) Single-Molecule Magnet, Angew. Chem., Int. Ed., 2016, 55, 16071–16074 CrossRef CAS PubMed; (j) X. Ding, Q. Luo, Y. Zhai, X. Zhang, Y. Lv, X. Zhang, C. Ke, C. Wu and Y.-Z. Zheng, Rigid Dysprosium(III) Single–Molecule Magnets Exhibit Preserved Superparamagnetism in Solution, Chin. J. Chem., 2022, 40, 563–570 CrossRef CAS; (k) Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, Symmetry-Supported Magnetic Blocking at 20 K in Pentagonal Bipyramidal Dy(III) Single-Ion Magnets, J. Am. Chem. Soc., 2016, 138, 2829–2837 CrossRef CAS PubMed; (l) I. F. Díaz-Ortega, J. M. Herrera, S. Dey, H. Nojiri, G. Rajaraman and E. Colacio, The effect of the electronic structure and flexibility of the counteranions on magnetization relaxation in [Dy(L)2(H2O)5]3+ (L = phosphine oxide derivative) pentagonal bipyramidal SIMs, Inorg. Chem. Front., 2020, 7, 689–699 RSC.
- (a) L. Zhu, B. Yin, P. Ma and D. Li, Tuning the Equatorial Crystal-Field in Mononuclear DyIII Complexes to Improve Single-Molecule Magnetic Properties, Inorg. Chem., 2020, 59, 16117–16121 CrossRef CAS PubMed; (b) L. Zhu, Y. Dong, B. Yin, P. Ma and D. Li, Improving the single-molecule magnet properties of two pentagonal bipyramidal Dy3+ compounds by the introduction of both electron-withdrawing and -donating groups, Dalton Trans., 2021, 50, 12607–12618 RSC; (c) Z. Jiang, L. Sun, Q. Yang, B. Yin, H. Ke, J. Han, Q. Wei, G. Xie and S. Chen, Excess axial electrostatic repulsion as a criterion for pentagonal bipyramidal DyIII single-ion magnets with high Ueff and TB, J. Mater. Chem. C, 2018, 6, 4273–4280 RSC; (d) J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen and M.-L. Tong, A Stable Pentagonal Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K, J. Am. Chem. Soc., 2016, 138, 5441–5450 CrossRef CAS PubMed.
- Y. Gil, A. Castro-Alvarez, P. Fuentealba, E. Spodine and D. Aravena, Lanthanide SMMs Based on Belt Macrocycles: Recent Advances and General Trends, Chem. – Eur. J., 2022, 28, e202200336 CrossRef CAS PubMed.
- W.-J. Xu, Q.-C. Luo, Z.-H. Li, Y.-Q. Zhai and Y.-Z. Zheng, Bis-Alkoxide Dysprosium(III) Crown Ether Complexes Exhibit Tunable Air Stability and Record Energy Barrier, Adv. Sci., 2024, 11, 2308548 CrossRef CAS PubMed.
- (a) F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit, Angew. Chem., Int. Ed., 2017, 56, 11445–11449 CrossRef CAS PubMed; (b) F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet, Science, 2018, 362, 1400–1403 CrossRef CAS PubMed; (c) F.-S. Guo, M. He, G.-Z. Huang, S. R. Giblin, D. Billington, F. W. Heinemann, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Discovery of a Dysprosium Metallocene Single-Molecule Magnet with Two High-Temperature Orbach Processes, Inorg. Chem., 2022, 61, 6017–6025 CrossRef CAS PubMed.
- W. Radecka-Paryzek, V. Patroniak and J. Lisowski, Metal complexes of polyaza and polyoxaaza Schiff base macrocycles, Coord. Chem. Rev., 2005, 249, 2156–2175 CrossRef CAS.
- (a) V. Alexander, Design and Synthesis of Macrocyclic Ligands and Their Complexes of Lanthanides and Actinides, Chem. Rev., 1995, 95, 273–342 CrossRef CAS; (b) D. E. Fenton and P. A. Vigato, Macrocyclic Schiff base complexes of lanthanides and actinides, Chem. Soc. Rev., 1988, 17, 69–90 RSC.
- (a) M. S. Raju, K. Paillot, I. Breslavetz, G. Novitchi, G. L. J. A. Rikken, C. Train and M. Atzori, Optical Readout of Single-Molecule Magnets Magnetic Memories with Unpolarized Light, J. Am. Chem. Soc., 2024, 146, 23616–23624 CrossRef CAS PubMed; (b) A. B. Canaj, S. Dey, E. R. Martí, C. Wilson, G. Rajaraman and M. Murrie, Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets, Angew. Chem., 2019, 131, 14284–14289 CrossRef; (c) Z. Li, Y. Zhai, W. Chen, Y. Ding and Y.-Z. Zheng, Air-Stable Hexagonal Bipyramidal Dysprosium(III) Single-Ion Magnets with Nearly Perfect D6h Local Symmetry, Chem. – Eur. J., 2019, 25, 16219–16224 CrossRef CAS PubMed; (d) Z. Zhu, C. Zhao, Q. Zhou, S. Liu, X.-L. Li, A. Mansikkamäki and J. Tang, Air-Stable Dy(III)-Macrocycle Enantiomers: From Chiral to Polar Space Group, CCS Chem., 2022, 4, 3762–3771 CrossRef CAS; (e) Z. Zhu, C. Zhao, T. Feng, X. Liu, X. Ying, X.-L. Li, Y.-Q. Zhang and J. Tang, Air-Stable Chiral Single-Molecule Magnets with Record Anisotropy Barrier Exceeding 1800 K, J. Am. Chem. Soc., 2021, 143, 10077–10082 CrossRef CAS PubMed; (f) C. Zhao, Z. Zhu, X.-L. Li and J. Tang, Air-stable chiral mono- and dinuclear dysprosium single-molecule magnets: steric hindrance of hexaazamacrocycles, Inorg. Chem. Front., 2022, 9, 4049–4055 RSC; (g) S. Liu, Y. Gil, C. Zhao, J. Wu, Z. Zhu, X.-L. Li, D. Aravena and J. Tang, A conjugated Schiff-base macrocycle weakens the transverse crystal field of air-stable dysprosium single-molecule magnets, Inorg. Chem. Front., 2022, 9, 4982–4989 RSC.
- (a) B. Zhang, X. Guo, P. Tan, W. Lv, X. Bai, Y. Zhou, A. Yuan, L. Chen, D. Liu, H.-H. Cui, R. Wang and X.-T. Chen, Axial Ligand as a Critical Factor for High-Performance Pentagonal Bipyramidal Dy(III) Single-Ion Magnets, Inorg. Chem., 2022, 61, 19726–19734 CrossRef CAS PubMed; (b) J.-L. Wang, J.-T. Chen, H. Yan, T.-T. Wang, Y.-Q. Zhang and W.-B. Sun, Constructing high axiality mononuclear dysprosium molecular magnets via a regulation-of-co-ligands strategy, Dalton Trans., 2024, 53, 10982–10990 RSC; (c) A. B. Canaj, S. Dey, C. Wilson, O. Céspedes, G. Rajaraman and M. Murrie, Engineering macrocyclic high performance pentagonal bipyramidal Dy(III) single-ion magnets, Chem. Commun., 2020, 56, 12037–12040 RSC; (d) S. Jia, X. Zhu, B. Yin, Y. Dong, A. Sun and D. Li, Macrocyclic Hexagonal Bipyramidal Dy(III)-Based Single-Molecule Magnets with a D6h Symmetry, Cryst. Growth Des., 2023, 23, 6967–6973 CrossRef CAS; (e) A. S. Armenis, A. Mondal, S. R. Giblin, C. P. Raptopoulou, V. Psycharis, D. I. Alexandropoulos, J. Tang, R. A. Layfield and Th. C. Stamatatos, Unveiling new [1 + 1] Schiff-base macrocycles towards high energy-barrier hexagonal bipyramidal Dy(III) single-molecule magnets, Chem. Commun., 2024, 60, 12730–12733 RSC.
- M. Llunell, D. Casanova, J. Girera, P. Alemany and S. Alvarez, SHAPE, version 2.0, Universitat de Barcelona, Barcelona, Spain, 2010 Search PubMed.
- (a) J. Tang and P. Zhang, Lanthanide Single Molecule Magnets, Springer Berlin Heidelberg, Berlin, Heidelberg, 2015 CrossRef; (b) R. A. Layfield and M. Murugesu, Lanthanides and Actinides in Molecular Magnetism, John Wiley & Sons, Ltd, 2015 CrossRef.
- S. T. Liddle and J. Van Slageren, Improving f-element single molecule magnets, Chem. Soc. Rev., 2015, 44, 6655–6669 RSC.
- (a) E. Garlatti, A. Chiesa, P. Bonfà, E. Macaluso, I. J. Onuorah, V. S. Parmar, Y.-S. Ding, Y.-Z. Zheng, M. J. Giansiracusa, D. Reta, E. Pavarini, T. Guidi, D. P. Mills, N. F. Chilton, R. E. P. Winpenny, P. Santini and S. Carretta, A Cost-Effective Semi-Ab Initio Approach to Model Relaxation in Rare-Earth Single-Molecule Magnets, J. Phys. Chem. Lett., 2021, 12, 8826–8832 CrossRef CAS PubMed; (b) J. G. C. Kragskow, A. Mattioni, J. K. Staab, D. Reta, J. M. Skelton and N. F. Chilton, Spin–phonon coupling and magnetic relaxation in single-molecule magnets, Chem. Soc. Rev., 2023, 52, 4567–4585 RSC; (c) A. Lunghi, F. Totti, R. Sessoli and S. Sanvito, The role of anharmonic phonons in under-barrier spin relaxation of single molecule magnets, Nat. Commun., 2017, 8, 14620 CrossRef PubMed; (d) S. Dey, T. Sharma and G. Rajaraman, Unravelling the role of spin–vibrational coupling in designing high-performance pentagonal bipyramidal Dy(III) single ion magnets, Chem. Sci., 2024, 15, 6465–6477 RSC; S. Mondal and A. Lunghi, Unraveling the contributions to spin-lattice relaxation in Kramers single-molecule magnets, J. Am. Chem. Soc., 2022, 144, 22965–22975 Search PubMed.
- F. Ortu, D. Reta, Y.-S. Ding, C. A. P. Goodwin, M. P. Gregson, E. J. L. McInnes, R. E. P. Winpenny, Y.-Z. Zheng, S. T. Liddle, D. P. Mills and N. F. Chilton, Studies of hysteresis and quantum tunnelling of the magnetisation in dysprosium(III) single molecule magnets, Dalton Trans., 2019, 48, 8541–8545 RSC.
- (a) F. Neese, Software Update: The ORCA Program System—Version 5.0. WIREs Computational Molecular Science, 202212e1606; (b) K. W. H. Stevens, The theory of paramagnetic relaxation, Rep. Prog. Phys., 1967, 30, 189–226 CrossRef; (c) K. W. H. Stevens, Matrix elements and operator equivalents connected with the magnetic properties of rare earth ions, Proc. Phys. Soc. A, 1952, 65, 209–215 CrossRef.
- J. Li, S. Gómez-Coca, B. S. Dolinar, L. Yang, F. Yu, M. Kong, Y.-Q. Zhang, Y. Song and K. R. Dunbar, Hexagonal Bipyramidal Dy(III) Complexes as a Structural Archetype for Single-Molecule Magnets, Inorg. Chem., 2019, 58, 2610–2617 CrossRef CAS PubMed.
- H. Liu, J.-F. Li and B. Yin, The coexistence of long τQTM and high Ueff as a concise criterion for a good single-molecule magnet: a theoretical case study of square antiprism dysprosium single-ion magnets, Phys. Chem. Chem. Phys., 2022, 24, 11729–11742 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, single-crystal X-ray data, IR, p-XRD, TGA, structural and magnetism plots, and ab initio calculations. CCDC 2393281 and 2393282. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02756f |
| This journal is © the Partner Organisations 2025 |