Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Probing the influence of imidazolylidene- and triazolylidene-based carbenes on the catalytic potential of dioxomolybdenum and dioxotungsten complexes in deoxygenation catalysis†
Florian R.
Neururer
 a,
Florian
Heim
a,
Marc
Baltrun
a,
Florian
Heim
a,
Marc
Baltrun
 a,
Philipp
Boos
a,
Philipp
Boos
 b,
Julia
Beerhues
c,
Michael
Seidl
a and
Stephan
Hohloch
b,
Julia
Beerhues
c,
Michael
Seidl
a and
Stephan
Hohloch
 *a
*a
aUniversity of Innsbruck, Department of General, Inorganic and Theoretical Chemistry, Innrain 80–82, 6020 Innsbruck, Austria. E-mail: Stephan.Hohloch@uibk.ac.at
bUniversity of Paderborn, Department of Chemistry, Warburger Straße 100, 33098 Paderborn, Germany
cFreie Universität Berlin, Department of Inorganic Chemistry, Fabeckstraße 34–36, 14195 Berlin, Germany
First published on 24th December 2024
Abstract
We report the synthesis of dianionic OCO-supported NHC and MIC complexes of molybdenum and tungsten with the general formula (OCO)MO2 (OCO = bis-phenolate benzimidazolylidene M = Mo (1-Mo), bis-phenolate triazolylidene M = Mo (2-Mo), M = W (2-W) and bis-phenolate imidazolylidene, M = Mo (3-Mo), W (3-W)). These complexes are tested in the catalytic deoxygenation of nitroarenes using pinacol as a sacrificial oxygen atom acceptor/reducing agent to examine the influence of the carbene and the metal centre in this transformation. The results show that the molybdenum-based triazolylidene complex 2-Mo is by far the most active catalyst, and TOFs of up to 270 h−1 are observed, while the tungsten analogues are basically inactive. Mechanistic studies suggest that the superiority of the triazolylidene-based complex 2-Mo is a result of a highly stable metal carbene bond, strongly exceeding the stability of the other NHC complexes 1-Mo and 3-Mo. This is proven by the structural isolation of a triazolylidene pinacolate complex (5-Mo) that can be thermally converted to a μ-oxodimolybdenum(V) complex 7-Mo. The latter complex is very oxophilic and stoichiometrically deoxygenates nitro- and nitrosoarenes at room temperature. In contrast, azoarenes are not reductively cleaved by 7-Mo, suggesting direct deoxygenation of the nitroarenes to the corresponding anilines with nitrosoarenes as intermediates. In summary, this work showcases the superior influence of MIC donors on the catalytic properties of early transition metal complexes.
Introduction
The creation of sustainable and environmentally benign chemical processes is one of the most important tasks of our time.1,2 In this context, the development of resource-saving, environmentally friendly, and efficient catalytic reactions is a crucial task to realise this goal. Given the current transition from fossil fuels to environmentally favourable carbon sources, such as biomass,1,2 or more generally, oxidatively over-functionalized materials, the need for their efficient reduction to industrially compatible starting materials is of growing importance.Targeting this goal, a plethora of strategies have been introduced in the past decades,3–5 which include the use of either molecular hydrogen6–8 or hydrogen surrogates e.g., in transfer-hydrogenation processes.9–14 However, both strategies rely on expensive transition metal catalysts,5,10,11,15–17 are relatively unselective towards functional groups,18–22 and (for direct hydrogenations) pressurized reaction vessels (e.g. 50 bar H2 pressure) are needed.23 Furthermore, transfer hydrogenation requires a large excess of the hydrogen surrogate reagent (e.g., isopropanol, silanes or boranes) producing vast amounts of chemical waste and complicated waste remediation strategies.17,24–27
Overcoming many of these drawbacks, oxygen atom transfer reactions (OATR) have proven to be a useful alternative: they are very atom efficient,28–33 do not need pressurized reaction vessels, and most catalysts used in this transformation are based on early transition metals,34–36 primarily molybdenum37–39 and tungsten.40–45 Furthermore, they only “focus” on oxygen-containing groups, leaving other functional groups (alkenes, alkynes, etc.) unchanged.4,46,47 However, one major drawback in this reaction so far was the need for quite high catalyst loadings of 5–10 mol%48,49 for the catalytic deoxygenation of phosphine oxides, sulfoxides, or nitroarenes using simple MoO2Cl2L2 (L = DMF, bpy, etc.) catalysts.46,47,50–54 Studying the reduction of nitroarenes, we have found that the use of OCO-chelating N-heterocyclic carbenes55–67 with a benzimidazolylidene donor68,69 (1-Mo, Fig. 1) has a significant effect on the efficiency of the catalyst and we were able to lower the catalyst loading to 0.25 mol%.70,71
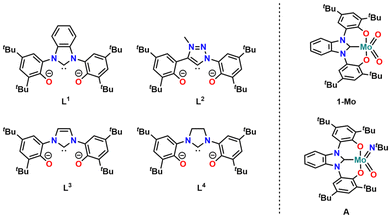 | ||
| Fig. 1 Overview of OCO chelating ligands L1–L4 used in this work (left) and previously reported NHC molybdenum deoxygenation catalysts 1-Mo and A reported by our group (right). | ||
Here, we expand this strategy to the use of other N-heterocyclic and mesoionic carbene donors, namely imidazolylidene,69 imidazolidinylidene55 and triazolylidene units.56 In the past decade, triazolylidene-based carbenes72 have found wide application in chemistry.73–78 The increased stability of their metal complexes, their modular synthesis via the copper,79,80 ruthenium,81 or base catalysed82 [3 + 2] azide-alkyne cycloaddition, and strong σ-donor properties73,83 have made them superior ligands in organometallic chemistry62,84–91 and chemical catalysis.11,12,92–103 The aim of this work is to investigate whether the carbene has an influence on the catalytic potential of the corresponding molybdenum complex in the deoxygenation of nitroarenes, using pinacol as a sacrificial reductant. Furthermore, we aimed at expanding this concept to tungsten-based catalysts supported by the NHC/MIC ligands L,1–4 as it was shown that tungsten can sometimes outperform the catalytic potential of molybdenum in OAT reactions.40
Results & discussion
Molybdenum & tungsten dioxo complexes
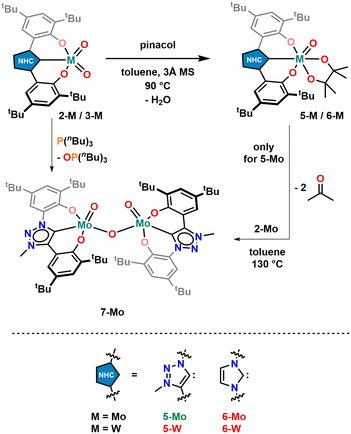
Triazolylidene supported dioxocomplexes 2-Mo and 2-W were prepared via an in situ deprotonation procedure as described for the benzimidazolylidene-supported complex 1-Mo (Scheme 1).71 The ligand precursor was first treated with an excess of triethylamine and then added to a solution/suspension containing the corresponding dioxo metal dichloride (MoO2Cl2(dme)104 or WO2Cl2) in THF. After stirring the reaction overnight, subsequent removal of triethylammonium chloride and precipitation using n-hexane, molybdenum complex 2-Mo was isolated as a bright yellow powder (91% yield), while its tungsten analogue 2-W was obtained as an off-white solid (70% yield). Notably, the molybdenum complex 2-Mo is soluble in THF, dichloromethane, and aromatic hydrocarbons, poorly soluble in diethyl ether, and almost insoluble in aliphatic hydrocarbons. In contrast, the tungsten complex 2-W is only soluble in THF, dichloromethane, and acetonitrile, sparingly soluble in aromatic hydrocarbons, and almost insoluble in diethyl ether, and aliphatic hydrocarbons. In the 1H NMR spectra of both compounds, the absence of the triazolium 5-H and the phenolic protons is indicative of the presence of MIC complexes (Fig. S1 and S6†). 13C-NMR resonances corresponding to a carbene carbon atom are found at 159.3 ppm for 2-Mo (in benzene-d6, Fig. S2†) and 168.1 ppm for 2-W (in dichloromethane-d2, Fig. S7†), which is in line with previously reported early transition metal triazolylidene complexes.56,61 The infrared-stretching frequencies at 904 cm−1 and 861 cm−1 corresponding to the terminal molybdenum-oxo frequencies in 2-Mo are well in line with the literature (Fig. S73†).71 For 2-W, these frequencies are observed at 904 cm−1 and 861 cm−1 (Fig. S74†).40 Furthermore, single crystals suitable for X-ray diffraction analysis could be obtained for both compounds (Fig. 2). Molybdenum compound 2-Mo was crystallised by slow diffusion of n-pentane into a benzene-d6 solution of the complex. It crystallises in the monoclinic space group P21/c with two complex units and one benzene-d6 molecule in the asymmetric unit. The molybdenum centre is five-coordinate, displaying a τ5 value of 0.53. With a carbon-metal bond (Mo1–C1) of 2.170(5) Å, the complex compares to other molybdenum complexes bearing a tridentate OCO ligand framework.56,71 The distances between the molybdenum centre and the terminal oxo ligands (Mo1–O10 and Mo1–O11) are 1.696(3) Å and 1.705(3) Å, respectively. Notably, 2-Mo can also be crystallised in the presence of triethylphosphine oxide (OPEt3) displaying no interaction between the phosphine oxide and the molybdenum centre (Fig. S86†), contrasting the complexation and coordination behaviour of 1-Mo in the presence of OPEt3.70 Interestingly, also in solution, no interactions between OPEt3 and 2-Mo could be observed (Fig. S62†). Single crystals of 2-W were grown from a concentrated acetonitrile solution at −40 °C. This compound crystallises in the monoclinic space group C2/c with four complex units and four solvent molecules in the asymmetric unit. Like in 2-Mo, the metal centre in 2-W is five-fold coordinated by the ligand and two terminal oxo ligands, displaying a τ5 value of 0.46. The bonding distances differ in each of the four complex units. For instance, the carbon-metal distances vary between 2.172(6) Å and 2.208(7) Å and are similar to previously reported tungsten(0) triazolylidene complexes.105,106 The slight variation in the C1–W1 bond distance is attributed to packing effects. The distance between the metal centre and the terminal oxo ligands (W1–O10 and W1–O11) are 1.714(5) Å and 1.714(5) Å, respectively. Confirming our previous results,71 both complexes are stable against air and moisture and dry samples or solutions may be kept under air at room temperature. | ||
| Scheme 1 (Attempted) synthesis of an isostructural series of Mo and W complexes supported by OCO-chelating bis-phenolate NHC/MIC donors. Green indicates successful, red unsuccessful synthesis. | ||
Having the triazolylidene complexes 2-M (M = Mo, W) and the molybdenum benzimidazolylidene complex 1-Mo previously reported by us70,71 in hand, we aimed for the isolation of the imidazolylidene based complexes 3-Mo and 3-W, as well as the tungsten benzimidazolylidene complex 1-W, to complete the series. 3-Mo was prepared following a procedure analogous to 1-Mo and 2-Mo (Scheme 1). The 1H-NMR spectrum shows the expected signature with absent phenolic OH and imidazolium 2-H protons (Fig. S11†). A resonance in the 13C{1H}-NMR spectrum at 171.9 ppm can be assigned to the carbene carbon atom (Fig. S12†). In comparison with 2-Mo (159.3 ppm), the carbene carbon resonance is significantly shifted downfield, but upfield from 1-Mo (183.1 ppm).71 This indicates that the imidazole-containing ligand is a weaker donor than the phenolate-tethered triazolylidene but stronger than the benzimidazolylidene ligand. X-ray quality single crystals were grown from tetrahydrofuran/hexane mixtures at low temperatures (Fig. 3). Apart from the Mo1–C1 distance, which was found at 2.225(3) Å in 3-Mo and is thus slightly longer compared to 2-Mo (2.170(5) Å) and 1-Mo (2.193(4) Å),71 the remaining structural parameters of 3-Mo strongly resemble those of complex 1-Mo and 2-Mo, wherefore a detailed discussion will be omitted here (see ESI for further information, Tables S1 and S2†). The synthesis of the tungsten analogues 1-W and 3-W, however, turned out to be more challenging. Unfortunately, neither the general procedure71 nor any other protocol (varying solvent, base, and temperature) yielded an analogous benzimidazolylidene complex 1-W, and only intractable mixtures could be obtained. In contrast, the imidazolylidene complex 3-W can be accessed following the general procedure, however, in poor yields only (18%, not optimized). Successful synthesis of 3-W is deduced from the 1H NMR spectrum, showing the presence of four resonances and the absence of the characteristic imidazole 2-H proton (Fig. S16†). Furthermore, the 13C NMR shows the distinctive downfield resonance at 181.1 ppm, which is characteristic for an NHC complex (vide supra and Fig. S17†). Finally, crystals suitable for X-ray diffraction analysis could be grown from a concentrated solution of 3-W in n-hexane at room temperature. Although these crystals were of poor quality and strongly twinned, the model confirms that 3-W is an NHC complex (Fig. S85†). Interestingly, the asymmetric unit contains two different conformers of 3-W: the expected monomeric form of 3-Wmono and a trimeric form 3-Wtrimer in which three tungsten atoms are bridged by μ-oxo units, depending on the ligand conformation (Fig. S85†). A comparable effect has already been found with 1-Mo, which was observed as a monomer and a dimer in the solid state.71 Overall, each tungsten atom is coordinated by two oxo ligands and one ligand L3, unambiguously confirming the assignment of the +VI oxidation state for each tungsten atom. Due to the low quality of the structure, no bond distances can be discussed here. Finally, all attempts to isolate an imidazolidine-2-yldidene complex (putative 4-Mo and 4-W, Scheme 1) have failed so far for both molybdenum and tungsten. We thus conclude that only electron-rich NHC ligands are compatible with the strongly Lewis-acidic MO2 (M = Mo, W) frameworks.
Catalytic deoxygenation of nitroarenes
Since dioxomolybdenum and dioxotungsten compounds are known to efficiently catalyse deoxygenation reactions,4,40,46,50,54e.g. the deoxygenation of sulfoxides, N-oxides, or the reduction of nitroaromatic compounds to the corresponding anilines,4,46,50–53 the catalytic potential of 2-Mo was investigated for nitroarene reductions. As a model reaction for initial studies, the conversion of o-nitrotoluene to o-toluidine using pinacol as a sacrificial reducing agent was chosen. To test the general activity of complex 2-Mo, the deoxygenation reaction was conducted in anhydrous toluene at 130 °C, and four equivalents of pinacol and 1.00 mol% of 2-Mo were applied. After 6 hours, an aliquot was removed from the reaction and analysed via gas chromatography, which confirmed that the reaction was complete. Given the fact that the analogous benzimidazolylidene complex 1-Mo allowed catalyst loadings as low as 0.25 mol%,70 we aimed at reducing the catalyst loading of 2-Mo as well. Catalyst loadings as low as 0.25 mol% resulted in complete conversion within 6 hours. (Table 1, entry 2) Furthermore, time-dependent experiments have shown that applying 0.25 mol% is almost as efficient as applying 1.00 mol%, indicating that the reaction does not benefit from increasing the catalyst loading (Fig. 4, bottom). Further decrease of the catalyst loading of 2-Mo resulted in lower conversions. However, at loadings of 0.1 mol%, still 94% conversion is achieved within 6 hours (Table 1, entry 3). Further reduction of the catalyst loading to 0.05 mol% shows 79% conversion after 6 hours (Table 1, entry 4). Translated into turnover numbers (TON) and turnover frequencies (TOF): at 0.05 mol%, a TON of 1600 and a TOF of approx. 270 h−1 is achieved with 2-Mo as a catalyst. To set this into relation, initial reports by Sanz et al. have used 5 mol% catalyst loadings, resulting in a TON of 20 and TOF of 9 h−1.54 Compared to 1-Mo, which has shown a TON of 280 and a TOF of 47 h−1 at 0.25 mol% after 6 hours, the triazolylidene complex 2-Mo exhibits increased activity. To set the activity of 2-Mo into further relation with other metals, Grieco and Blacque recently reported the use of (IMes)2ReVOBr3 using phenylsilane as a sacrificial reductant in a microwave-assisted reaction. This was performed at 0.5 mol% of catalyst and only nitrobenzene could be converted, while functionalized nitroarenes could not be reduced.107 Thus, we can confidently state, that the triazolylidene complex 2-Mo is by far the most active group VI NHC/MIC-based catalyst in the deoxygenation of nitroarenes reported so far.| Entry | Cat. | Loading mol% | Conv./% | TON/TOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d h−1 d h−1 |
|---|---|---|---|---|
| a Reactions were carried out in 10 ml J-Young tubes, using 1 mmol of substrate, 4 mmol of pinacol and the indicated amount of catalyst in 3 ml toluene. Conversions were determined by GC using mesitylene as an internal standard. Each reaction was performed three times to achieve redundancy of the values. b Conversion after 6 hours. c Conversion after 8 hours. d TOF values correspond to a reaction time of 6 h. e conversion is measured after 4 hours. f TON/TOF values are rounded to two significant digits. | ||||
| 1 | 2-Mo | 1.00 | >99b | 100/17 |
| 2 | 2-Mo | 0.25 | >99b | 400/100e |
| 3 | 2-Mo | 0.10 | 94b | 940/160f |
| 4 | 2-Mo | 0.05 | 79b/84c | 1600/270f |
| 5 | 1-Mo | 0.25 | 70b/93c | 280/47 |
| 6 | 3-Mo | 0.25 | 35b/49c | 140/35 |
| 7 | 2-W | 1.00 | 0c | — |
| 8 | 3-W | 1.00 | 0c | — |
To further study the influence of the NHC donor group, time-dependent catalysis was performed with 0.25 mol% catalyst loading, applying the molybdenum dioxo complex 1-Mo, 2-Mo, and 3-Mo (Fig. 4, top), respectively. As already indicated by the comparison between 1-Mo and 2-Mo (vide supra), the ligand has a major impact on the catalytic activity in the model reaction. Under these specified conditions (0.25 mol% catalyst loading), as expected, complex 2-Mo with the mesoionic triazolylidene donor was found to be the most active catalyst and full conversion was observed within 4 hours (TON 400, TOF 100 h−1). In contrast, the imidazolylidene complex 3-Mo was found to be the least active and only 35% conversion was observed over the course of 6 hours/49% over the course of 8 h (TON 140/200; TOF 35/25 h−1). The decrease of the TOF in 3-Mo after 6 compared to 8 hours of reaction time further indicates a limited stability of the active species in solution for 3-Mo (vide infra). The performance of the benzimidazolylidene complex 1-Mo, was found to lie in between 2-Mo and 3-Mo, showing conversion of 70% after 6 and 93% after 8 hours (TON 280/370 TOF 47/47 h−1). Finally, we were interested in how the tungsten complexes 2-W and 3-W would compete in the reaction. However, the tungsten analogues did not show any activity, even after prolonged reaction times or higher catalyst loadings of 1 mol% (Table 1, entries 7 and 8).
Since o-nitrotoluene is efficiently converted to o-toluidine, we were finally interested in the scope of the triazolylidene complex 2-Mo and whether easy-identifiable substrate limitations would exist (Scheme 2). To ensure full conversion, all further nitro-reduction reactions were conducted with 0.50 mol% of 2-Mo and four equivalents of pinacol in anhydrous toluene. All yields reported correspond to isolated yields. The scope shows that complex 2-Mo is a valuable catalyst, transforming protic (phenols), halogenated, nitriles, amides, alkyne and keto-functionalized nitroarenes efficiently to the corresponding anilines. We furthermore studied the scope of functionalized heterocycles. This shows, that complex 2-Mo efficiently deoxygenates nitropyridines and quinolines, but no product formation is observed if more strained substrates such as differently substituted furans and thiophenes are used. It is unclear, if the catalyst fails using these substrates, or if the substrates do not tolerate the conditions required. In the case of thiophenes fast precipitation of insoluble, black solids is observed, indicating potential polymerisation of the thiophene units. Notably, the low isolated yield of 68% of 4-fluoroaniline most likely results from mechanical losses during column chromatography, since crude NMR spectra recorded after the catalytic transformation indicate 92% product formation (Fig. S32†).
 | ||
| Scheme 2 Substrate scope. Yields given correspond to isolated yields (see ESI† for further information on workup). Reactions were carried out in 10 ml J-Young tubes, using 1 mmol of substrate, 4 mmol of pinacol and the indicated amount of catalyst in 3 ml toluene. | ||
Mechanistic investigations for the molybdenum mediated process
To examine the activity differences observed in the series 1-Mo, 2-Mo, 3-Mo, 2-W and 3-W, further mechanistic studies were executed. First, we examined the stability of the complexes towards pinacol, which serves as a sacrificial reducing agent. Previous results have shown that the benzimidazolylidene complex 1-Mo is not stable towards pinacol, resulting in the partial protonation of the benzimidazolylidene ligand.70 To examine whether an analogous, partially hydrolysed intermediate is possible with 2-Mo and 3-Mo both complexes were mixed with pinacol (2 equiv.) in benzene-d6 (Scheme 3). However, after a few hours at room temperature, no new resonances were observed in the 1H-NMR spectrum (Fig. S63 and S65†). When these mixtures are heated to 70 °C, they take on a blood red colour but turn bright yellow again when cooled to room temperature, indicating a strongly dynamic reaction/equilibrium. Notably, this “switching” can be done for 2-Mo several times without the indication of any complex decomposition (Fig. S64†), while for 3-Mo severe signs of multiple decomposition products are already observed within the first cycle (Fig. S66†). This indicates that similar to 1-Mo, the imidazolylidene complex 3-Mo is not stable under protic conditions, while the triazolylidene complex 2-Mo is unaffected by the presence of pinacol. This supports the observed catalytic potential of 3-Mo, and decreasing TOF values, which already indicated decomposition of the imidazolylidene complex during catalysis (vide supra). We propose that the red product present at elevated temperatures in the reaction between 2-Mo and pinacol is a molybdenum oxo pinacolate complex 5-Mo forming under the concomitant release of water (1 equiv.). The released water facilitates hydrolysis back to 2-Mo, which likely explains why complex 5-Mo is not readily isolable after heating. To verify this, the experiment was repeated in the presence of a water scavenger, i.e. molecular sieves, giving access to 5-Mo (Scheme 3). The 1H-NMR spectrum of 5-Mo shows a slight redistribution of the ligand resonances along with two singlets in the aliphatic region integrating to 12 protons, which are attributed to the pinacolate-CH3 groups (Fig. S21†). These can also be found in the 13C NMR spectrum of 5-Mo, which shows two distinct resonances at 25.6 and 25.8 ppm (Fig. S22†). Additionally, a quaternary carbon resonance at δC = 97.0 ppm in 5-Mo is observed, corresponding to the carbon atom adjacent to the pinacolate oxygen atoms. Full proof of the molecular structure was obtained via X-ray diffraction analysis (Fig. 5). Suitable single crystals were grown from a concentrated diethyl ether solution. Complex 5-Mo crystallises in the orthorhombic space group P212121 in a distorted octahedral geometry. The attachment of the pinacolate leads to a slight stretching of the ligand-metal bonds with respect to the parent dioxo complex 2-Mo. Bond lengths for the phenolates (Mo1–O1 and Mo1–O2) are 2.013(9) Å and 2.044(8) Å, respectively. The carbene bond is stretched to 2.187(12) Å and the terminal oxide bond length is found at 1.699(8) Å, comparable to the dioxo complex (see Tables S1 and S2† for more information). Focussing on tungsten, no reaction between 2-W/3-W and pinacol was observed, neither at room temperature nor at 130 °C in C6D6. This suggests that the oxo groups in the tungsten framework are less basic compared to those in the molybdenum complexes. This could also be one reason why the tungsten complexes are completely inactive in the catalytic deoxygenation of nitroarenes (Table 1, entries 7 and 8), as the initial reaction step (deprotonation of pinacol) appears to be thermodynamically hindered.To further demonstrate the participation of pinacolate complex 5-Mo in the deoxygenation reaction, stoichiometric reactions starting from o-nitrosotoluene or o-nitrotoluene were conducted. For each oxygen atom to be removed one equivalent of pinacolate complex was added (overall stoichiometry 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 5-Mo:nitrotoluene and 1
1 for 5-Mo:nitrotoluene and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 5-Mo:nitrosotoluene) and the reaction progress was monitored by 1H-NMR spectrometry (Fig. S67 and S68†). As expected, no reaction occurred at room temperature. But when heated to 130 °C, the blood red mixtures turned dark brown within one hour and light orange within four hours. After 12 hours, the 1H-NMR spectrum showed complete conversion of 5-Mo to 2-Mo.
1 for 5-Mo:nitrosotoluene) and the reaction progress was monitored by 1H-NMR spectrometry (Fig. S67 and S68†). As expected, no reaction occurred at room temperature. But when heated to 130 °C, the blood red mixtures turned dark brown within one hour and light orange within four hours. After 12 hours, the 1H-NMR spectrum showed complete conversion of 5-Mo to 2-Mo.
Although in many cases a MoIV species is proposed as the catalytically active species, it is also known that such MoIV complexes immediately comproportionate with remaining MoVI to form μ-oxo bridged dimeric MoV compounds.29,34,39 Thus, the thermolysis of 5-Mo was conducted in the presence of one equivalent of 2-Mo (Scheme 3).70 The mixture turned dark brown within 2 hours at 130 °C and the corresponding NMR spectrum suggests the presence of a paramagnetic compound 7-Mo (Scheme 3 and Fig. S26, S27†).70
To verify the assumption that this new compound arises from reduction and is indeed a μ-oxo bridged dimeric complex, 2-Mo was also reduced with tri-n-butylphosphine (P(nBu)3) at 130 °C (Scheme 3). After the work-up, a dark grey powder was obtained, and the NMR signature is in-line with the results from thermolysis of 5-Mo, indicating the formation of 7-Mo (Fig. S26†). An effective magnetic moment of 1.94 μB was observed, which is consistent with previously reported μ-oxo dimolybdenum(V) complexes.70 To unambiguously determine the molecular structure of this complex, single crystals were grown from a concentrated toluene solution of the complex at −40 °C (Fig. 5). Complex 7-Mo crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and each of the two molybdenum centres is pentacoordinate in a distorted trigonal pyramidal geometry (τ5 = 0.38 for Mo1 and 0.13 for Mo1A). The Mo1–O1–Mo1A bridge was found to be slightly deviating from linearity, showing an angle of 162.62(17)° with Mo–O distances of 1.890(3) Å (Mo1–O10) and 1.896(3) Å (Mo1A–O10). These Mo–O distances are substantially longer compared to the terminal Mo
and each of the two molybdenum centres is pentacoordinate in a distorted trigonal pyramidal geometry (τ5 = 0.38 for Mo1 and 0.13 for Mo1A). The Mo1–O1–Mo1A bridge was found to be slightly deviating from linearity, showing an angle of 162.62(17)° with Mo–O distances of 1.890(3) Å (Mo1–O10) and 1.896(3) Å (Mo1A–O10). These Mo–O distances are substantially longer compared to the terminal Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units at 1.656(3) Å (Mo1–O11) and 1.667(3) Å (Mo1A–O11A). The molybdenum carbene distance was found to be 2.123(4) Å and 2.124(4) Å for Mo1–C1 and Mo1A–C1A respectively, indicating some minor back-bonding effects from the d1 Mo centre. Similar observations have already been made in other d1 NHC/MIC complexes with vanadium60,61 and molybdenum.70,108
O units at 1.656(3) Å (Mo1–O11) and 1.667(3) Å (Mo1A–O11A). The molybdenum carbene distance was found to be 2.123(4) Å and 2.124(4) Å for Mo1–C1 and Mo1A–C1A respectively, indicating some minor back-bonding effects from the d1 Mo centre. Similar observations have already been made in other d1 NHC/MIC complexes with vanadium60,61 and molybdenum.70,108
To further check whether a MoIV species could also be isolated we attempted various trapping strategies. However, all attempts to accomplish this, i.e. conducting the deoxygenation in the presence of stabilizing ligands (e.g. PMe3), remained unsuccessful. A different strategy, that led to the successful isolation of ReV di-oxo compounds from ReVII tri-oxo compounds, was the addition of alkynes to generate a corresponding metallacyclopropene complex.2,109,110 Similar strategies have also been used to prepare “masked” low-valent MIV halide complexes of molybdenum111–115 and tungsten.111,116–118 In analogy to this, an NMR sample of complex 5-Mo was mixed with an excess of 3-hexyne and heated to 130 °C. The NMR spectrum however does not show a diamagnetic metallacyclopropene complex but the dimeric μ-oxo bridged complex 7-Mo was found instead (Fig. S71†). These findings suggest that no MoIV complex is formed during the catalytic process (Fig. 6, right part) and that the catalytic active complex is indeed the μ-oxo bridged dimeric complex 7-Mo. Further proof for this assumption was brought by stoichiometric reactions of the MoV compound 7-Mo and o-nitrotoluene or o-nitrosotoluene (Fig. S69 and S70†). Both reactions were conducted at room temperature and monitored via NMR spectroscopy. The reaction starting from nitrosotoluene is complete within a few minutes and complete deoxygenation of the nitro compound is achieved within 12 hours. In contrast, azobenzene does not react with 5-Mo and 7-Mo at 130 °C within 24 h, which is in accordance with previous experiments using 1-Mo as a catalyst.70 This further indicates, that despite changing the NHC donor, the general mechanism of nitroarene deoxygenation remains the same and follows a direct deoxygenation pathway (Route A, Fig. 6) deoxygenating nitroarenes via nitrosoarenes to the corresponding anilines, with the “azobenzene pathway” (Route B, Fig. 6) being unlikely.11,119 This has also been confirmed computationally in a recent publication by Nieto-Faza and Sanz elucidating the mechanism of molybdenum/pinacol-mediated nitroarene reduction.120 Finally, the mechanistic similarity between our previous investigations using 1-Mo as a catalysts,70 in combination with the enhanced catalytic potential of 2-Mo in the deoxygenation of nitroarenes, unambiguously confirms that the carbene has a major influence on the catalytic potential of early transition metal complexes.
Conclusions
We have synthesized and isolated a series of OCO-chelated NHC/MIC complexes of MoVI and WVI. We found that the complexation reaction requires electron-rich carbenes and allows only distinctive combinations of NHC/MIC and MO2Cl2L precursors (L = DME or none, M = Mo, W). In catalytic deoxygenations, such as the reduction of nitroarenes to anilines, we found that the tungsten complexes are inactive, but that mesoionic carbene based complex 2-Mo (with a triazolylidene donor) exhibits unprecedented catalytic activity, reaching a TOF of 270 h−1 at catalyst loadings of 0.05 mol%. Compared to previous systems,54 this states an increase of the catalytic activity of over one order of magnitude (30-fold increase). We were further able to show, that the NHC donor has a drastic influence on the catalytic activity, but also stability of the complexes. While nNHC based complexes tend to decompose under the conditions chosen (pinacol, 130 °C) the MIC metal carbon was found to be highly stable, and storage of stock solutions and synthesis of this unique triazolylidene complex can be even performed under air and moisture. Mechanistic experiments suggest that the reaction occurs via the stepwise formation of a molybdenum(VI) pinacolate complex, that is thermally converted to a μ-oxo dimolybdenum(V) complex, which is capable of directly deoxygenating nitroarenes, via nitrosoarenes, to the corresponding anilines, with no azobenzene playing a role in the reduction process.70This is the first report showcasing the drastic influence of NHC and MIC donors on the potential and stability of early transition metal catalysts, highlighting the importance and future of this research field and the necessity to further study the chemistry and potential of MIC ligands in early transition metal chemistry.
Author contributions
The project was designed by FRN and SH. Complex synthesis was carried out by FRN, FH and MB. Catalytic experiments were carried out by FRN, FH and PB. Mechanistic investigations were performed by FRN. FRN recorded all experimental data/spectra except for X-ray diffraction analysis, which was performed by SH, MS and JB. The manuscript was written by FRN and SH and final versions were proof read and acknowledged by all authors.Data availability
NMRs, IR and cyclic voltammograms have been included (plotted) into the ESI.† Raw data is stored on the university servers and can be accessed via us if necessary.Crystallographic data (CIF-files) have been uploaded to the CCDC and can be obtained via the CCDC homepage using the CCDC numbers assigned in the ESI.† Raw data and frames are stored on the university servers and can be accessed via us if necessary.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded in whole or in part by the Austrian Science Fund (FWF); Grant-DOI: https://doi.org/10.55776/P34626. For open access purposes, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. We are furthermore thankful to the University of Innsbruck as well as the University of Paderborn for partial funding of this work.References
- K. A. Rogers and Y. Zheng, Selective Deoxygenation of Biomass-Derived Bio-oils within Hydrogen-Modest Environments: A Review and New Insights, ChemSusChem, 2016, 9, 1750–1772 CrossRef CAS PubMed
.
- M. Shiramizu and F. D. Toste, Deoxygenation of biomass-derived feedstocks: oxorhenium-catalyzed deoxydehydration of sugars and sugar alcohols, Angew. Chem., Int. Ed., 2012, 51, 8082–8086 CrossRef CAS PubMed
.
- S. Kim, E. E. Kwon, Y. T. Kim, S. Jung, H. J. Kim, G. W. Huber and J. Lee, Recent advances in hydrodeoxygenation of biomass-derived oxygenates over heterogeneous catalysts, Green Chem., 2019, 21, 3715–3743 RSC
.
- R. Rubio-Presa, M. A. Fernández-Rodríguez, M. R. Pedrosa, F. J. Arnáiz and R. Sanz, Molybdenum–Catalyzed Deoxygenation of Heteroaromatic N-Oxides and Hydroxides using Pinacol as Reducing Agent, Adv. Synth. Catal., 2017, 359, 1752–1757 CrossRef CAS
.
- J. Yi, S. Liu and M. M. Abu-Omar, Rhenium-catalyzed transfer hydrogenation and deoxygenation of biomass-derived polyols to small and useful organics, ChemSusChem, 2012, 5, 1401–1404 CrossRef CAS PubMed
.
- R. V. Jagadeesh, A.-E. Surkus, H. Junge, M.-M. Pohl, J. Radnik, J. Rabeah, H. Huan, V. Schünemann, A. Brückner and M. Beller, Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines, Science, 2013, 342, 1073–1076 CrossRef CAS PubMed
.
- G. Wienhöfer, M. Baseda-Krüger, C. Ziebart, F. A. Westerhaus, W. Baumann, R. Jackstell, K. Junge and M. Beller, Hydrogenation of nitroarenes using defined iron-phosphine catalysts, Chem. Commun., 2013, 49, 9089–9091 RSC
.
- E. Balaraman, B. Gnanaprakasam, L. J. W. Shimon and D. Milstein, Direct hydrogenation of amides to alcohols and amines under mild conditions, J. Am. Chem. Soc., 2010, 132, 16756–16758 CrossRef CAS PubMed
.
- D. Wang and D. Astruc, The golden age of transfer hydrogenation, Chem. Rev., 2015, 115, 6621–6686 CrossRef CAS PubMed
.
- G. K. Ramollo, I. Strydom, M. A. Fernandes, A. Lemmerer, S. O. Ojwach, J. L. van Wyk and D. I. Bezuidenhout, Fischer Carbene Complexes of Iridium(I) for Application in Catalytic Transfer Hydrogenation, Inorg. Chem., 2020, 59, 4810–4815 CrossRef CAS PubMed
.
- S. Hohloch, L. Suntrup and B. Sarkar, Arene–Ruthenium(II) and −Iridium(III) Complexes with “Click”-Based Pyridyl-triazoles, Bis-triazoles, and Chelating Abnormal Carbenes: Applications in Catalytic Transfer Hydrogenation of Nitrobenzene, Organometallics, 2013, 32, 7376–7385 CrossRef CAS
.
- L. Suntrup, S. Hohloch and B. Sarkar, Expanding the Scope of Chelating Triazolylidenes: Mesoionic Carbenes from the 1,5-“Click”-Regioisomer and Catalytic Synthesis of Secondary Amines from Nitroarenes, Chem. – Eur. J., 2016, 22, 18009–18018 CrossRef CAS PubMed
.
- S. Sabater, H. Müller-Bunz and M. Albrecht, Carboxylate-Functionalized Mesoionic Carbene Precursors: Decarboxylation, Ruthenium Bonding, and Catalytic Activity in Hydrogen Transfer Reactions, Organometallics, 2016, 35, 2256–2266 CrossRef CAS
.
- I. Sorribes, G. Wienhöfer, C. Vicent, K. Junge, R. Llusar and M. Beller, Chemoselective transfer hydrogenation to nitroarenes mediated by cubane-type Mo3S4 cluster catalysts, Angew. Chem., Int. Ed., 2012, 51, 7794–7798 CrossRef CAS PubMed
.
- K. H. Hopmann and A. Bayer, Enantioselective imine hydrogenation with iridium-catalysts: Reactions, mechanisms and stereocontrol, Coord. Chem. Rev., 2014, 268, 59–82 CrossRef CAS
.
- T. Ikariya and A. J. Blacker, Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts, Acc. Chem. Res., 2007, 40, 1300–1308 CrossRef CAS PubMed
.
- R. Malacea, R. Poli and E. Manoury, Asymmetric hydrosilylation, transfer hydrogenation and hydrogenation of ketones catalyzed by iridium complexes, Coord. Chem. Rev., 2010, 254, 729–752 CrossRef CAS
.
- A. Bolje, S. Hohloch, J. Košmrlj and B. Sarkar, RuII, IrIII and OsII mesoionic carbene complexes: efficient catalysts for transfer hydrogenation of selected functionalities, Dalton Trans., 2016, 45, 15983–15993 RSC
.
- J. A. M. Brandts and P. H. Berben, Application of Immobilized Rhodium Catalyst Precursors in Enantio- and Chemoselective Hydrogenation Reactions, Org. Process Res. Dev., 2003, 7, 393–398 CrossRef CAS
.
- N. Gorgas, A. Ilic and K. Kirchner, Chemoselective transfer hydrogenation of aldehydes in aqueous media catalyzed by a well-defined iron(II) hydride complex, Chem. Mon., 2019, 150, 121–126 CrossRef CAS PubMed
.
- Y. Himeda, N. Onozawa-Komatsuzaki, S. Miyazawa, H. Sugihara, T. Hirose and K. Kasuga, pH-Dependent catalytic activity and chemoselectivity in transfer hydrogenation catalyzed by iridium complex with 4,4′-dihydroxy-2,2′-bipyridine, Chem. – Eur. J., 2008, 14, 11076–11081 CrossRef CAS PubMed
.
- B. S. Takale, X. Feng, Y. Lu, M. Bao, T. Jin, T. Minato and Y. Yamamoto, Unsupported Nanoporous Gold Catalyst for Chemoselective Hydrogenation Reactions under Low Pressure: Effect of Residual Silver on the Reaction, J. Am. Chem. Soc., 2016, 138, 10356–10364 CrossRef CAS PubMed
.
- P. M. Reis and B. Royo, Chemoselective hydrogenation of nitroarenes and deoxygenation of pyridine N-oxides with H2 catalyzed by MoO2Cl2, Tetrahedron Lett., 2009, 50, 949–952 CrossRef CAS
.
- C. Johnson and M. Albrecht, Triazolylidene Iron(II) Piano-Stool Complexes: Synthesis and Catalytic Hydrosilylation of Carbonyl Compounds, Organometallics, 2017, 36, 2902–2913 CrossRef CAS
.
- M. L. Shegavi and S. K. Bose, Recent advances in the catalytic hydroboration of carbonyl compounds, Catal. Sci. Technol., 2019, 9, 3307–3336 RSC
.
- Y. Wei, S.-X. Liu, H. Mueller-Bunz and M. Albrecht, Synthesis of Triazolylidene Nickel Complexes and Their Catalytic Application in Selective Aldehyde Hydrosilylation, ACS Catal., 2016, 6, 8192–8200 CrossRef CAS
.
- J. Zheng, L. C. Misal Castro, T. Roisnel, C. Darcel and J.-B. Sortais, Iron piano-stool phosphine complexes for catalytic hydrosilylation reaction, Inorg. Chim. Acta, 2012, 380, 301–307 CrossRef CAS
.
- T. Chantarojsiri, Y. Sun, J. R. Long and C. J. Chang, Water-Soluble Iron(IV)-Oxo Complexes Supported by Pentapyridine Ligands: Axial Ligand Effects on Hydrogen Atom and Oxygen Atom Transfer Reactivity, Inorg. Chem., 2015, 54, 5879–5887 CrossRef CAS PubMed
.
- R. H. Holm, Metal-centered oxygen atom transfer reactions, Chem. Rev., 1987, 87, 1401–1449 CrossRef CAS
.
- G. Licini, V. Conte, A. Coletti, M. Mba and C. Zonta, Recent advances in vanadium catalyzed oxygen transfer reactions, Coord. Chem. Rev., 2011, 255, 2345–2357 CrossRef CAS
.
- F. Weisser, H. Stevens, J. Klein, M. van der Meer, S. Hohloch and B. Sarkar, Tailoring Ru(II) pyridine/triazole oxygenation catalysts and using photoreactivity to probe their electronic properties, Chem. – Eur. J., 2015, 21, 8926–8938 CrossRef CAS PubMed
.
- J. Xiao and X. Li, Gold α-oxo carbenoids in catalysis: catalytic oxygen-atom transfer to alkynes, Angew. Chem., Int. Ed., 2011, 50, 7226–7236 CrossRef CAS PubMed
.
- F. Weisser, S. Hohloch, S. Plebst, D. Schweinfurth and B. Sarkar, Ruthenium complexes of tripodal ligands with pyridine and triazole arms: subtle tuning of thermal, electrochemical, and photochemical reactivity, Chem. – Eur. J., 2014, 20, 781–793 CrossRef CAS PubMed
.
- T. Schindler, A. Sauer, T. P. Spaniol and J. Okuda, Oxygen Atom Transfer Reactions with Molybdenum Cofactor Model Complexes That Contain a Tetradentate OSSO-Type Bis(phenolato) Ligand, Organometallics, 2018, 37, 4336–4340 CrossRef CAS
.
- I. A. Tonks, Organometallic mechanisms: Measuring up with the early metals, Nat. Chem., 2017, 9, 834–836 CrossRef CAS PubMed
.
- E. P. Beaumier, A. J. Pearce, X. Y. See and I. A. Tonks, Modern applications of low-valent early transition metals in synthesis and catalysis, Nat. Rev. Chem., 2019, 3, 15–34 CrossRef PubMed
.
- M. Schubert, J. Leppin, K. Wehming, D. Schollmeyer, K. Heinze and S. R. Waldvogel, Powerful fluoroalkoxy molybdenum(V) reagent for selective oxidative arene coupling reaction, Angew. Chem., Int. Ed., 2014, 53, 2494–2497 CrossRef CAS PubMed
.
- J. Leppin, C. Förster and K. Heinze, Molybdenum complex with bulky chelates as a functional model for molybdenum oxidases, Inorg. Chem., 2014, 53, 12416–12427 CrossRef CAS PubMed
.
- K. Heinze, Bioinspired functional analogs of the active site of molybdenum enzymes: Intermediates and mechanisms, Coord. Chem. Rev., 2015, 300, 121–141 CrossRef CAS
.
- M. Z. Ćorović, F. Wiedemaier, F. Belaj and N. C. Mösch-Zanetti, Replacement of Molybdenum by Tungsten in a Biomimetic Complex Leads to an Increase in Oxygen Atom Transfer Catalytic Activity, Inorg. Chem., 2022, 61, 12415–12424 CrossRef PubMed
.
- R. W. Gosselink, D. R. Stellwagen and J. H. Bitter, Tungsten-based catalysts for selective deoxygenation, Angew. Chem., Int. Ed., 2013, 52, 5089–5092 CrossRef CAS PubMed
.
- H. Ren, Y. Chen, Y. Huang, W. Deng, D. G. Vlachos and J. G. Chen, Tungsten carbides as selective deoxygenation catalysts: experimental and computational studies of converting C3 oxygenates to propene, Green Chem., 2014, 16, 761–769 RSC
.
- D. R. Stellwagen and J. H. Bitter, Structure–performance relations of molybdenum- and tungsten carbide catalysts for deoxygenation, Green Chem., 2015, 17, 582–593 RSC
.
- M. Z. Ćorović, F. Belaj and N. C. Mösch-Zanetti, Dioxygen Activation by a Bioinspired Tungsten(IV) Complex, Inorg. Chem., 2023, 62, 5669–5676 CrossRef PubMed
.
- S. B. Yu and R. H. Holm, Aspects of the oxygen atom transfer chemistry of tungsten, Inorg. Chem., 1989, 28, 4385–4391 CrossRef CAS
.
- R. Hernández-Ruiz and R. Sanz, Dichlorodioxomolybdenum(VI) Complexes: Useful and Readily Available Catalysts in Organic Synthesis, Synthesis, 2018, 4019–4036 Search PubMed
.
- S. Suárez-Pantiga, R. Hernández-Ruiz, C. Virumbrales, M. R. Pedrosa and R. Sanz, Reductive Molybdenum-Catalyzed Direct Amination of Boronic Acids with Nitro Compounds, Angew. Chem., Int. Ed., 2019, 58, 2129–2133 CrossRef PubMed
.
- R. Sanz and M. Pedrosa, Applications of Dioxomolybdenum(VI) Complexes to Organic Synthesis, Curr. Org. Synth., 2009, 6, 239–263 CrossRef CAS
.
-
R. Sanz and M. R. Pedrosa, in Advances in Organic Synthesis, ed. Atta-ur-Rahman, Bentham Science Publishers, pp. 182–266 Search PubMed
.
- R. Rubio-Presa, M. A. R. Pedrosa, M. A. Fernández-Rodríguez, F. J. Arnáiz and R. Sanz, Molybdenum-Catalyzed Synthesis of Nitrogenated Polyheterocycles from Nitroarenes and Glycols with Reuse of Waste Reduction Byproduct, Org. Lett., 2017, 19, 5470–5473 CrossRef CAS PubMed
.
- J. Robertson and R. S. Srivastava, Mo-catalyzed deoxygenation of epoxides to alkenes, Mol. Catal., 2017, 443, 175–178 CrossRef CAS
.
- J. R. Dethlefsen, D. Lupp, A. Teshome, L. B. Nielsen and P. Fristrup, Molybdenum-Catalyzed Conversion of Diols and Biomass-Derived Polyols to Alkenes Using Isopropyl Alcohol as Reductant and Solvent, ACS Catal., 2015, 5, 3638–3647 CrossRef CAS
.
- S. Asako, T. Sakae, M. Murai and K. Takai, Molybdenum–Catalyzed Stereospecific Deoxygenation of Epoxides to Alkenes, Adv. Synth. Catal., 2016, 358, 3966–3970 CrossRef CAS
.
- N. García, P. García-García, M. A. Fernández-Rodríguez, R. Rubio, M. R. Pedrosa, F. J. Arnáiz and R. Sanz, Pinacol as a New Green Reducing Agent: Molybdenum–Catalyzed Chemoselective Reduction of Sulfoxides and Nitroaromatics, Adv. Synth. Catal., 2012, 354, 321–327 CrossRef
.
- S. Bellemin-Laponnaz, R. Welter, L. Brelot and S. Dagorne, Synthesis and structure of V(V) and Mn(III) NHC complexes supported by a tridentate bis-aryloxide-N-heterocyclic carbene ligand, J. Organomet. Chem., 2009, 694, 604–606 CrossRef CAS
.
- M. Baltrun, F. A. Watt, R. Schoch, C. Wölper, A. G. Neuba and S. Hohloch, A new bis-phenolate mesoionic carbene ligand for early transition metal chemistry, Dalton Trans., 2019, 48, 14611–14625 RSC
.
- C. Romain, S. Bellemin-Laponnaz and S. Dagorne, Recent progress on NHC-stabilized early transition metal (group 3–7) complexes: Synthesis and applications, Coord. Chem. Rev., 2020, 422, 213411 CrossRef CAS
.
- R. Taakili and Y. Canac, NHC Core Pincer Ligands Exhibiting Two Anionic Coordinating Extremities, Molecules, 2020, 25(9), 2231 CrossRef CAS PubMed
.
- F. R. Neururer, K. Huter, M. Seidl and S. Hohloch, Reactivity and Structure of a Bis-phenolate Niobium NHC Complex, ACS Org. Inorg. Au, 2023, 3, 59–71 CrossRef CAS PubMed
.
- F. R. Neururer, D. Leitner, S. Liu, K. Wurst, H. Kopacka, M. Seidl and S. Hohloch, The Chemistry of Vanadium bis–Phenolate NHC Complexes in Three Oxidation States, Eur. J. Inorg. Chem., 2023, 26, e202300180 CrossRef CAS
.
- F. R. Neururer, S. Liu, D. Leitner, M. Baltrun, K. R. Fisher, H. Kopacka, K. Wurst, L. J. Daumann, D. Munz and S. Hohloch, Mesoionic Carbenes in Low- to High-Valent Vanadium Chemistry, Inorg. Chem., 2021, 60, 15421–15434 CrossRef CAS PubMed
.
- B. Wittwer, D. Leitner, F. R. Neururer, R. Schoch, M. Seidl, J. Pecak, M. Podewitz and S. Hohloch, Scrutinizing the redox chemistry of group 10 complexes supported by a redox-active bis-phenolate mesoionic carbene, Polyhedron, 2024, 250, 116786 CrossRef CAS
.
- C. Romain, L. Brelot, S. Bellemin-Laponnaz and S. Dagorne, Synthesis and Structural Characterization of a Novel Family of Titanium Complexes Bearing a Tridentate Bis-phenolate-N-heterocyclic Carbene Dianionic Ligand and Their Use in the Controlled ROP of rac -Lactide, Organometallics, 2010, 29, 1191–1198 CrossRef CAS
.
- C. Romain, S. Choua, J.-P. Collin, M. Heinrich, C. Bailly, L. Karmazin-Brelot, S. Bellemin-Laponnaz and S. Dagorne, Redox and luminescent properties of robust and air-stable N-heterocyclic carbene group 4 metal complexes, Inorg. Chem., 2014, 53, 7371–7376 CrossRef CAS PubMed
.
- C. Romain, C. Fliedel, S. Bellemin-Laponnaz and S. Dagorne, NHC Bis-Phenolate Aluminum Chelates: Synthesis, Structure, and Use in Lactide and Trimethylene Carbonate Polymerization, Organometallics, 2014, 33, 5730–5739 CrossRef CAS
.
- C. Romain, K. Miqueu, J.-M. Sotiropoulos, S. Bellemin-Laponnaz and S. Dagorne, Non-innocent behavior of a tridentate NHC chelating ligand coordinated onto a zirconium(IV) center, Angew. Chem., Int. Ed., 2010, 49, 2198–2201 CrossRef CAS PubMed
.
- S. Dagorne, S. Bellemin-Laponnaz and C. Romain, Neutral and Cationic N-Heterocyclic Carbene Zirconium and Hafnium Benzyl Complexes: Highly Regioselective Oligomerization of 1-Hexene with a Preference for Trimer Formation, Organometallics, 2013, 32, 2736–2743 CrossRef CAS
.
- E. Despagnet-Ayoub, L. M. Henling, J. A. Labinger and J. E. Bercaw, Addition of a phosphine ligand switches an N-heterocyclic carbene-zirconium catalyst from oligomerization to polymerization of 1-hexene, Dalton Trans., 2013, 42, 15544–15547 RSC
.
- E. Borré, G. Dahm, A. Aliprandi, M. Mauro, S. Dagorne and S. Bellemin-Laponnaz, Tridentate Complexes of Group 10 Bearing Bis-Aryloxide N-Heterocyclic Carbene Ligands: Synthesis, Structural, Spectroscopic, and Computational Characterization, Organometallics, 2014, 33, 4374–4384 CrossRef
.
- S. Liu, J. I. Amaro-Estrada, M. Baltrun, I. Douair, R. Schoch, L. Maron and S. Hohloch, Catalytic Deoxygenation of Nitroarenes Mediated by High-Valent Molybdenum(VI)–NHC Complexes, Organometallics, 2021, 40, 107–118 CrossRef CAS
.
- M. Baltrun, F. A. Watt, R. Schoch and S. Hohloch, Dioxo-, Oxo-imido-, and Bis-imido-Molybdenum(VI) Complexes with a Bis-phenolate-NHC Ligand, Organometallics, 2019, 38, 3719–3729 CrossRef CAS
.
- P. Mathew, A. Neels and M. Albrecht, 1,2,3-Triazolylidenes as versatile abnormal carbene ligands for late transition metals, J. Am. Chem. Soc., 2008, 130, 13534–13535 CrossRef CAS PubMed
.
- G. Guisado-Barrios, M. Soleilhavoup and G. Bertrand, 1 H-1,2,3-Triazol-5-ylidenes: Readily Available Mesoionic Carbenes, Acc. Chem. Res., 2018, 51, 3236–3244 CrossRef CAS PubMed
.
- R. Maity and B. Sarkar, Chemistry of Compounds Based on 1,2,3-Triazolylidene-Type Mesoionic Carbenes, JACS Au, 2022, 2, 22–57 CrossRef CAS PubMed
.
- D. Schweinfurth, L. Hettmanczyk, L. Suntrup and B. Sarkar, Metal Complexes of Click-Derived Triazoles and Mesoionic Carbenes: Electron Transfer, Photochemistry, Magnetic Bistability, and Catalysis, Z. Anorg. Allg. Chem., 2017, 643, 554–584 CrossRef CAS
.
- W. Stroek and M. Albrecht, Application of first-row transition metal complexes bearing 1,2,3-triazolylidene ligands in catalysis and beyond, Chem. Soc. Rev., 2024, 53, 6322–6344 RSC
.
- G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Crystalline 1H-1,2,3-triazol-5-ylidenes: new stable mesoionic carbenes (MICs), Angew. Chem., Int. Ed., 2010, 49, 4759–4762 CrossRef CAS PubMed
.
- K. F. Donnelly, A. Petronilho and M. Albrecht, Application of 1,2,3-triazolylidenes as versatile NHC-type ligands: synthesis, properties, and application in catalysis and beyond, Chem. Commun., 2013, 49, 1145–1159 RSC
.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes, Angew. Chem., 2002, 114, 2708–2711 CrossRef
.
- C. W. Tornøe, C. Christensen and M. Meldal, Peptidotriazoles on solid phase: 1,2,3-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed
.
- L. K. Rasmussen, B. C. Boren and V. V. Fokin, Ruthenium-catalyzed cycloaddition of aryl azides and alkynes, Org. Lett., 2007, 9, 5337–5339 CrossRef CAS PubMed
.
- S. W. Kwok, J. R. Fotsing, R. J. Fraser, V. O. Rodionov and V. V. Fokin, Transition-metal-free catalytic synthesis of 1,5-diaryl-1,2,3-triazoles, Org. Lett., 2010, 12, 4217–4219 CrossRef CAS PubMed
.
- D. Yuan and H. V. Huynh, 1,2,3-Triazolin-5-ylidenes: Synthesis of Hetero-bis(carbene) Pd(II) Complexes, Determination of Donor Strengths, and Catalysis, Organometallics, 2012, 31, 405–412 CrossRef CAS
.
- P. Dierks, A. Kruse, O. S. Bokareva, M. J. Al-Marri, J. Kalmbach, M. Baltrun, A. Neuba, R. Schoch, S. Hohloch, K. Heinze, M. Seitz, O. Kühn, S. Lochbrunner and M. Bauer, Distinct photodynamics of κ-N and κ-C pseudoisomeric iron(II) complexes, Chem. Commun., 2021, 57, 6640–6643 RSC
.
- L. Hettmanczyk, S. J. P. Spall, S. Klenk, M. van der Meer, S. Hohloch, J. A. Weinstein and B. Sarkar, Structural, Electrochemical, and Photochemical Properties of Mono– and Digold(I) Complexes Containing Mesoionic Carbenes, Eur. J. Inorg. Chem., 2017, 2017, 2112–2121 CrossRef CAS
.
- R. Maity, S. Hohloch, C.-Y. Su, M. van der Meer and B. Sarkar, Cyclometalated mono- and dinuclear Ir(III) complexes with “click”-derived triazoles and mesoionic carbenes, Chem. – Eur. J., 2014, 20, 9952–9961 CrossRef CAS PubMed
.
- A. Pavun, R. Niess, L. A. Scheibel, M. Seidl and S. Hohloch, A mesoionic carbene stabilized nickel(II) hydroxide complex: a facile precursor for C-H activation chemistry, Dalton Trans., 2024, 53, 2749–2761 RSC
.
- P. Pinter, C. M. Schüßlbauer, F. A. Watt, N. Dickmann, R. Herbst-Irmer, B. Morgenstern, A. Grünwald, T. Ullrich, M. Zimmer, S. Hohloch, D. M. Guldi and D. Munz, Bright luminescent lithium and magnesium carbene complexes, Chem. Sci., 2021, 12, 7401–7410 RSC
.
- B. Wittwer, N. Dickmann, S. Berg, D. Leitner, L. Tesi, D. Hunger, R. Gratzl, J. van Slageren, N. I. Neuman, D. Munz and S. Hohloch, A mesoionic carbene complex of manganese in five oxidation states, Chem. Commun., 2022, 58, 6096–6099 RSC
.
- K. Matsubara, K. Tomomatsu, A. Tajiri, A. Watanabe, Y. Koga, R. Ishikawa and Y. Yamada, Pincer-Type Mesoionic Carbene Nickel(II) Complexes: Synthesis, Properties, Reactions, and Catalytic Application to the Suzuki–Miyaura Coupling Reaction of Aryl Bromides, Eur. J. Inorg. Chem., 2022, e202100870 CrossRef CAS
.
- K. Matsubara, Y. Yamada, H. Iwasaki, H. Ikeda, Y. Kanetsugu, S. Kawata and Y. Koga, A 1,2,3-triazole-derived pincer-type mesoionic carbene complex of iron(II): carbonyl elimination and hydrosilylation of aromatic aldehydes via the concerted reaction with hydrosilane and a base, Dalton Trans., 2023, 52, 572–582 RSC
.
- M. Rigo, L. Hettmanczyk, F. J. L. Heutz, S. Hohloch, M. Lutz, B. Sarkar and C. Müller, Phosphinines versus mesoionic carbenes: a comparison of structurally related ligands in Au(I)-catalysis, Dalton Trans., 2016, 46, 86–95 RSC
.
- R. Maity, A. Verma, M. van der Meer, S. Hohloch and B. Sarkar, Palladium Complexes Bearing Mesoionic Carbene Ligands: Applications in α-Arylation, α-Methylation and Suzuki-Miyaura Coupling Reactions, Eur. J. Inorg. Chem., 2016, 111–117 CrossRef CAS
.
- S. Hohloch, L. Suntrup and B. Sarkar, Exploring potential cooperative effects in dicopper(I)-di-mesoionic carbene complexes: applications in click catalysis, Inorg. Chem. Front., 2016, 3, 67–77 RSC
.
- S. Hohloch, S. Kaiser, F. L. Duecker, A. Bolje, R. Maity, J. Košmrlj and B. Sarkar, Catalytic oxygenation of sp3 “C-H” bonds with Ir(III) complexes of chelating triazoles and mesoionic carbenes, Dalton Trans., 2015, 44, 686–693 RSC
.
- S. Hohloch, L. Hettmanczyk and B. Sarkar, Introducing Potential Hemilability into “Click” Triazoles and Triazolylidenes: Synthesis and Characterization of d 6 –Metal Complexes and Oxidation Catalysis, Eur. J. Inorg. Chem., 2014, 3164–3171 CrossRef CAS
.
- W. Stroek, M. Keilwerth, D. M. Pividori, K. Meyer and M. Albrecht, An Iron-Mesoionic Carbene Complex for Catalytic Intramolecular C-H Amination Utilizing Organic Azides, J. Am. Chem. Soc., 2021, 143, 20157–20165 CrossRef CAS PubMed
.
- M. van der Meer, E. Glais, I. Siewert and B. Sarkar, Electrocatalytic Dihydrogen Production with a Robust Mesoionic Pyridylcarbene Cobalt Catalyst, Angew. Chem., Int. Ed., 2015, 54, 13792–13795 CrossRef CAS PubMed
.
- F. A. Watt, B. Sieland, N. Dickmann, R. Schoch, R. Herbst-Irmer, H. Ott, J. Paradies, D. Kuckling and S. Hohloch, Coupling of CO2 and epoxides catalysed by novel N-fused mesoionic carbene complexes of nickel(II), Dalton Trans., 2021, 50, 17361–17371 RSC
.
- L. A. Hudson, W. Stroek and M. Albrecht, Tailoring C-H amination activity via modification of the triazole-derived carbene ligand, Dalton Trans., 2024, 53, 14795–14800 RSC
.
- W. Stroek, N. A. V. Rowlinson, L. A. Hudson and M. Albrecht, Heteroleptic Triazole-Bisoxazoline Iron Complexes Reveal Lability of the Iron-Carbene Bond Even Within a Chelating Scaffold, Inorg. Chem., 2024, 63(37), 17134–17140 CrossRef CAS PubMed
.
- M. Abdellaoui, K. Oppel, A. Vianna, M. Soleilhavoup, X. Yan, M. Melaimi and G. Bertrand, J. Am. Chem. Soc., 2024, 146, 2933–2938 CrossRef CAS PubMed
.
- C. Liu, Z. Zhang, L.-L. Zhao, G. Bertrand and X. Yan, Angew. Chem. Int. Ed., 2023, 62, e202303478 CrossRef CAS PubMed
.
- K. A. Rufanov, D. N. Zarubin, N. A. Ustynyuk, D. N. Gourevitch, J. Sundermeyer, A. V. Churakov and J. A. Howard, Synthesis and structure of a series of new haloaryl imido complexes of molybdenum, Polyhedron, 2001, 20, 379–385 CrossRef CAS
.
- P. Boden, P. Di Martino-Fumo, T. Bens, S. Steiger, U. Albold, G. Niedner-Schatteburg, M. Gerhards and B. Sarkar, NIR-Emissive Chromium(0), Molybdenum(0), and Tungsten(0) Complexes in the Solid State at Room Temperature, Chem. – Eur. J., 2021, 27, 12959–12964 CrossRef CAS PubMed
.
- T. Bens, D. Marhöfer, P. Boden, S. T. Steiger, L. Suntrup, G. Niedner-Schatteburg and B. Sarkar, A Different Perspective on Tuning the Photophysical and Photochemical Properties: The Influence of Constitutional Isomers in Group 6 Carbonyl Complexes with Pyridyl-Mesoionic Carbenes, Inorg. Chem., 2023, 62, 16182–16195 CrossRef CAS PubMed
.
- G. Grieco and O. Blacque, Microwave–assisted reduction of aromatic nitro compounds with novel oxo–rhenium complexes, Appl. Organomet. Chem., 2022, 36, e6452 CrossRef CAS
.
- D. Leitner, F. R. Neururer and S. Hohloch, Synthesis and Electrochemical Properties of Molybdenum Nitrido Complexes Supported by Redox-Active NHC and MIC Ligands, Dalton Trans., 2025, 54, 582–594 RSC
.
- M. A. Ehweiner, F. Belaj, K. Kirchner and N. C. Mösch-Zanetti, Synthesis and Reactivity of a Bioinspired Molybdenum(IV) Acetylene Complex, Organometallics, 2021, 40, 2576–2583 CrossRef CAS PubMed
.
- J.-H. Jung, D. M. Hoffman and T. Lee, Synthesis, X-ray crystallographic, and reactivity studies of rhenium(V) alkyne complexes, J. Organomet. Chem., 2000, 599, 112–122 CrossRef CAS
.
- A. Greco, F. Pirinoli and G. Dallasta, Reactions of molybdenum and tungsten halides with acetylenic hydrocarbons: an approach to the structure and pathways of formation of metathesis catalysts, J. Organomet. Chem., 1973, 60, 115–124 CrossRef CAS
.
- E. Hey, F. Weller and K. Dehnicke, Diphenylacetylen–Komplexe von Niob, Molybdän, Wolfram und Rhenium Die Kristallstruktur von [NbCl3(PhCCPh)]4, Z. Anorg. Allg. Chem., 1984, 514, 25–38 CrossRef CAS
.
- Y. Takashima, Y. Nakayama, H. Yasuda, A. Nakamura and A. Harada, Synthesis of cis-dichloride complexes of Group 6 transition metals bearing alkyne and chalcogen-bridged chelating bis(aryloxo) ligands as catalyst precursors for ring-opening metathesis polymerization, J. Organomet. Chem., 2002, 654, 74–82 CrossRef CAS
.
- E. C. Walborsky, D. E. Wigley, E. Roland, J. C. Dewan and R. R. Schrock, Preparation and characterization of molybdenum(IV)-alkyne complexes containing bulky aryloxide or arenethiolate ligands, Inorg. Chem., 1987, 26, 1615–1621 CrossRef CAS
.
- E. Roland, E. C. Walborsky, J. C. Dewan and R. R. Schrock, Preparation, structure, and coordination chemistry of molybdenum(IV) tetrathiolates, Mo[S-2,4,6-C6H2(CHMe2)3]4, J. Am. Chem. Soc., 1985, 107, 5795–5797 CrossRef CAS
.
- K. H. Theopold, S. J. Holmes and R. R. Schrock, Alkyne Complexes of Tungsten(IV), Angew. Chem., Int. Ed. Engl., 1983, 22, 1010 CrossRef
.
- K. H. Theopold, S. J. Holmes and R. R. Schrock, Some Chemistry of Tungsten(IV) Alkyne Complexes, Angew. Chem. Suppl., 1983, 1409–1423 CrossRef
.
- Y. Nakayama, H. Saito, N. Ueyama and A. Nakamura, Isomerization and α-H Elimination of Dialkyltungsten Complexes Stabilized by a Sulfur-Bridged Chelating Diaryloxo Ligand, Organometallics, 1999, 18, 3149–3158 CrossRef CAS
.
- S. P. Annen and H. Grützmacher, Nitrosobenzene as a hydrogen acceptor in rhodium catalysed dehydrogenation reactions of alcohols: synthesis of aldehydes and azoxybenzenes, Dalton Trans., 2012, 41, 14137–14145 RSC
.
- S. Kiriakidi, C. Silva López, R. Sanz and O. N. Faza, Deciphering Nitroaromatics Reduction: Theoretical Insights into Dioxomolybdenum Catalysis with Biomass–Derived Pinacol, ChemCatChem, 2024, 16, e202301575 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2383969–2383974. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02392g |
| This journal is © the Partner Organisations 2025 |