Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Geometry-directed cyclisation within the transfer-dominated branching radical telomerisation of dimethacrylates†
Corinna
Smith
ab,
Oliver B.
Penrhyn-Lowe
ab,
Samuel
Mckeating
ab,
Stephen
Wright
ab,
Andrew B.
Dwyer
ab and
Steve P.
Rannard
 *ab
*ab
aDepartment of Chemistry, University of Liverpool, Crown Street, L69 7ZD, UK. E-mail: srannard@liv.ac.uk
bMaterials Innovation Factory, University of Liverpool, Crown Street, L69 7ZD, UK
First published on 25th February 2025
Abstract
The use of Transfer-dominated Branching Radical Telomerisation (TBRT) in the homopolymerisation of neopentyl glycol dimethacrylate has shown the formation of highly cyclised structures even at relatively highly concentrated reaction conditions. This is contrary to previous reports of the TBRT of unconstrained multi-vinyl taxogens and is the first indication of geometry directed cyclisation within the formation of branched polyesters via TBRT methods. Surprisingly, there was limited impact of increased reaction temperature on recovered samples. Dilution led to an expected increase in cyclisation, however, the recovered polymer samples are unprecedented in the extent of cyclisation and the reduction in the use of telogen required to suppress gelation and form soluble branched polymers.
Introduction
The introduction of new polymer synthesis techniques has often led to a sudden growth of innovation driven by the novel opportunities achievable through a higher level of control,1,2 formation of new polymer architectures,3,4 and/or enhanced access to different functionality/chemistry.5,6 In many cases mechanistic studies highlight the novel nature of a previously unseen synthetic strategy, but identification of unexpected behaviour or reactivity allow the benefits or limitations to be understood.7,8The first reports of Transfer-dominated Branching Radical Telomerisation (TBRT) in 2020 showed that the manipulation of reaction conditions normally applied to free radical telomerisation9–11 (targeting number average degrees of polymerisation (DPn) of less than 5 monomer units) can prevent gelation and allow the homopolymerisation of multi-vinyl monomers to complete vinyl group consumption.12 Flory–Stockmayer (F–S) theory was initially created to describe gel formation within step-growth polymerisations of A2, B2 and An (where n > 2) monomer mixtures,13 and was rapidly extended to vinyl polymerisations under chain-growth conditions.14 The application of F–S theory to chain-growth polymerisations predicts that a copolymerisation of mono- and multi-vinyl monomers will lead to gel formation at low vinyl group consumption; experimental verification has been reported many times, with initial reports from as early as 1945.15
TBRT appears to contradict F–S theory as complete consumption of vinyl groups, and fully soluble high molecular weight branched polymers are readily achieved during the homopolymerisation of multi-vinyl substrates. F–S theory ignores intramolecular cyclisation and assumes all vinyl groups have equal reactivity.16 Essentially, TBRT uses chain transfer reactions to control the kinetic chain length within a free radical chain-growth polymerisation to <2 monomer units, Fig. 1; reaction temperature provides additional control.17
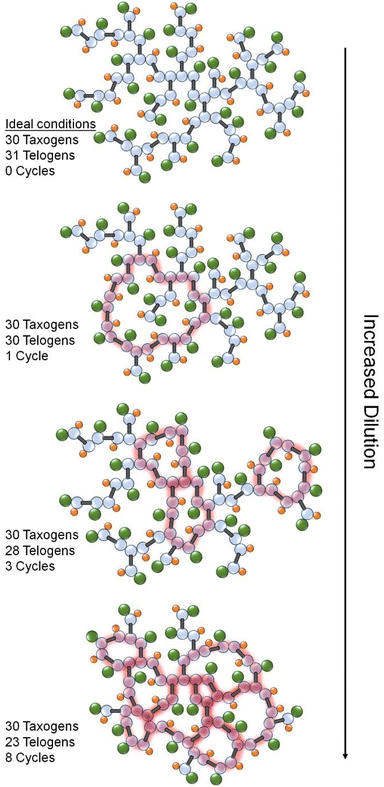
The DPn of the telomer subunit distribution within the branched polymer architecture is, therefore, extremely small. Under these conditions, a multi-vinyl monomer is known as a multi-vinyl taxogen (MVT) and a chain transfer agent is described as a telogen.9,18 To achieve a DPn < 2 monomer units, a large number of DP1 structures must be formed within an MVT homopolymerisation under TBRT conditions, Fig. 1.12 This is not considered within F–S theory and is key to the success of an ideal TBRT reaction forming soluble polymer products; such reactions are typically conducted at solids concentrations >40 wt% and are ideally characterised by an MVT/Telogen ratio approaching 1.00 within the final purified polymer composition.19 Under high solvent conditions (≤30 wt% solids) increasing intramolecular cyclisation with dilution has been observed, Fig. 2.19
Cyclisation can also be observed experimentally as the ability to form soluble highly branched polymers at significantly increased [MVT]0/[Telogen]0 ratios within the initial reaction mixture (i.e. less telogen required to control DPn to <2 monomer units). Analytically, this is also seen as the recovered polymers exhibit compositions, as measured by 1H nuclear magnetic resonance (NMR) spectroscopy, with MVT/Telogen ratios > 1 (i.e. cyclisation diminishes the need for a prevalence of DP1 structures to avoid gelation and requires one fewer telogen within the final structure per cycle formed), Fig. 2.19
During the early exploration of TBRT ethylene glycol dimethacrylate (EGDMA) was used as the MVT with 1-dodecanethiol (DDT) acting as the telogen.12 This has allowed direct comparisons of previous reports as the many variables available within TBRT have been studied. Here, we study the impact of MVT conformation by comparing EGDMA with neopentyl glycol dimethacrylate (NPGDMA) under TBRT conditions. The simple inclusion of a quaternary carbon at the centre of the otherwise simple aliphatic dimethacrylate MVT has modified the outcomes of the TBRT reaction considerably. These observations have been studied further through investigations into the impact of dilution and reaction temperature.
Results and discussion
Investigation of the conformations of ethylene glycol dimethacrylate and neopentyl glycol dimethacrylate
A study of the conformation of EGDMA and NPGDMA was conducted using Spartan (v24.110) software and modelling a polar solvent environment to mimic the reaction conditions utilised during the TBRT reactions described here (see ESI†). The energy minimised conformations for each MVT are shown in Fig. 3. | ||
| Fig. 3 Comparative evaluation of the proximity of vinyl functional groups within (A) ethylene glycol dimethacrylate (EGDMA), and (B) neopentyl glycol dimethacrylate (NPGDMA). | ||
Despite the longer alkyl chain length within the diol fragment of the dimethacrylate, NPGDMA displayed a 7.305 Å distance between vinyl groups, whereas the flexibility of the shorter EGDMA allowed a larger separation of 8.899 Å. Despite the absence of the extra atom in the central backbone of the MVT, the vinyl groups within EGDMA are, therefore, approximately 22% further away when comparing against NPGDMA. Our previous reports have shown that increased MVT dimensions can radically impact the outcomes of TBRT, with intermolecular branching reactions being more favoured as the dimethacrylate MVT chain length increases.20 Having an aliphatic, acyclic MVT with an inter-vinyl distance shorter than EGDMA was surprising and offered the opportunity to investigate this trend further.
TBRT synthesis of polymers via neopentyl glycol dimethacrylate homopolymerisation
The TBRT of NPGDMA was conducted using DDT as the telogen, utilising ethyl acetate (EtOAc; 70 °C) as the reaction solvent and 2,2′-azobis(isobutyronitrile) (1.5 mol% based on vinyl group concentration) as the radical source to allow a direct comparison with previous reports.At 50 wt% solids, and a [NPGDMA]0/[DDT]0 = 0.73, full consumption of vinyl groups was achieved, and a soluble polymer product was recovered, Table 1. This was to be expected as EGDMA also avoids gelation at this [MVT]0/[Telogen]0 ratio;12 as mentioned above, longer MVTs enhance intermolecular branching reactions and both 1,6-hexanediol dimethacrylate and 1,12-dodecanediol dimethacrylate fail to avoid gelation under these conditions.20
| Solids content (wt%) | 1H NMR (CDCl3) | TD-SEC (THF/TEA)c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| [NPGDMA]0/[DDT]0a | [NPGDMA]f/[DDT]f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
% Cyclisationd | DDT (wt%) | M w (g mol−1) | M n (g mol−1) | Đ | α | dn/dc | |
| a Calculated from 1H NMR analysis of the reaction mixture at t = 0 (ESI Fig. S1, S4 and S6†). b Determined by 1H NMR analysis of purified polymers (examples ESI Fig. S3, S5 and S7†) – note: purification is conducted to remove excess telogen and is expected to remove some low molecular weight species. c Determined by triple-detection size exclusion chromatography using a 0.5% v/v TEA/THF eluent system (examples ESI Fig. S14 and S15†). d See ref. 19 and ESI eqn (S4). e All polymerisations achieved >99% vinyl group consumption as determined by 1H NMR (CDCl3) analysis of crude samples of the reaction mixtures after 24 h (ESI Fig. S2, S4 and S7†). | |||||||||
| 50 | 1.10 | Gel formed | |||||||
| 50 | 1.01 | 1.43 | 30.1 | 37.1 | 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2200 | 59.87 | 0.22 | 0.088 |
| 50 | 0.96 | 1.48 | 32.4 | 36.3 | 133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7400 | 17.76 | 0.35 | 0.090 |
| 50 | 0.91 | 1.30 | 23.1 | 39.3 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1800 | 23.52 | 0.26 | 0.091 |
| 50 | 0.85 | 1.48 | 32.4 | 36.3 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
3100 | 10.72 | 0.32 | 0.090 |
| 50 | 0.79 | 1.40 | 28.6 | 37.6 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1200 | 19.38 | 0.25 | 0.089 |
| 50 | 0.73 | 1.38 | 27.5 | 37.9 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1500 | 8.38 | 0.26 | 0.088 |
| 30 | 1.90 | Gel formed | |||||||
| 30 | 1.73 | 1.98 | 49.5 | 29.8 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423 423![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
6500 | 372.50 | 0.41 | 0.098 |
| 30 | 1.45 | 2.33 | 57.1 | 26.6 | 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2800 | 52.86 | 0.36 | 0.095 |
| 30 | 1.29 | 1.98 | 49.5 | 29.8 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
3100 | 13.08 | 0.35 | 0.100 |
| 30 | 1.21 | 1.56 | 35.9 | 35.1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1300 | 17.54 | 0.30 | 0.095 |
| 30 | 1.12 | 1.52 | 34.2 | 35.7 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2000 | 9.74 | 0.29 | 0.095 |
| 30 | 1.01 | 1.56 | 35.9 | 35.1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2200 | 6.26 | 0.29 | 0.094 |
| 30 | 0.92 | 1.52 | 34.2 | 35.7 | 8300 | 1300 | 6.20 | 0.26 | 0.092 |
| 10 | 4.90 | 5.00 | 80.0 | 14.4 | 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
7200 | 28.17 | 0.31 | 0.106 |
| 10 | 2.70 | 4.78 | 79.1 | 15.0 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2800 | 7.74 | 0.30 | 0.102 |
| 10 | 2.50 | 4.56 | 78.1 | 15.6 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4100 | 5.18 | 0.33 | 0.104 |
| 10 | 2.00 | 3.69 | 72.9 | 18.6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2600 | 4.12 | 0.28 | 0.101 |
| 10 | 1.60 | 3.15 | 68.3 | 21.1 | 6120 | 1500 | 4.20 | 0.25 | 0.099 |
| 10 | 1.50 | 3.17 | 68.5 | 21.0 | 6600 | 2500 | 2.67 | 0.27 | 0.098 |
| 10 | 1.40 | 2.94 | 66.0 | 22.3 | 5700 | 2000 | 2.77 | 0.25 | 0.098 |
| 10 | 1.30 | 2.82 | 64.5 | 23.0 | 4800 | 1600 | 3.09 | 0.26 | 0.099 |
| 10 | 1.20 | 2.61 | 61.7 | 24.4 | 4200 | 1300 | 3.24 | 0.22 | 0.099 |
Interestingly, triple detection size exclusion chromatography (TD-SEC) analysis showed a weight average molecular weight (Mw) of the polymer derived from [NPGDMA]0/[DDT]0 = 0.73 that is remarkably low when compared with p(DDT-EGDMA) formed under these conditions (Mw ≈ 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1);12,19 at an Mw = 12
000 g mol−1);12,19 at an Mw = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1, this p(DDT-NPGDMA) has an Mw of approximately 6.5% of the equivalent p(DDT-EGDMA) sample. As mentioned previously, [MVT]F/[Telogen]F ratios > 1.00 within the purified polymer are indicative of cyclisation,19 and characterisation by 1H NMR (ESI eqn S4†) showed a very high value (1.38) which corresponds to 30.6% of NPGDMA residues being involved in cyclisation (cf. p(DDT-EGDMA) ≈ 1.02).19 Ideal p(DDT-NPGDMA) TBRT polymers ([MVT]F/[Telogen]F = 1.00) will have a composition containing NPGDMA and DDT residues comprising 54.5 wt% and 45.5 wt% respectively; DDT was calculated as only contributing 37.9 wt% of this recovered polymer.
900 g mol−1, this p(DDT-NPGDMA) has an Mw of approximately 6.5% of the equivalent p(DDT-EGDMA) sample. As mentioned previously, [MVT]F/[Telogen]F ratios > 1.00 within the purified polymer are indicative of cyclisation,19 and characterisation by 1H NMR (ESI eqn S4†) showed a very high value (1.38) which corresponds to 30.6% of NPGDMA residues being involved in cyclisation (cf. p(DDT-EGDMA) ≈ 1.02).19 Ideal p(DDT-NPGDMA) TBRT polymers ([MVT]F/[Telogen]F = 1.00) will have a composition containing NPGDMA and DDT residues comprising 54.5 wt% and 45.5 wt% respectively; DDT was calculated as only contributing 37.9 wt% of this recovered polymer.
As with other TBRT studies, the [NPGDMA]0/[DDT]0 ratio was carefully and systematically increased to identify conditions where the reducing telogen was able to maintain control of the polymerisation and avoid gelation. Soluble p(DDT-NPGDMA) polymers were able to be formed without gelation at [NPGDMA]0/[DDT]0 ratios > 1.00 with obvious network formation at 1.10 (cf. p(DDT-EGDMA) limiting gel point ratio ≈ 0.85).12 Under these conditions, the composition of the p(DDT-NPGDMA) polymers remained relatively consistent throughout, as did the amount of cyclisation.
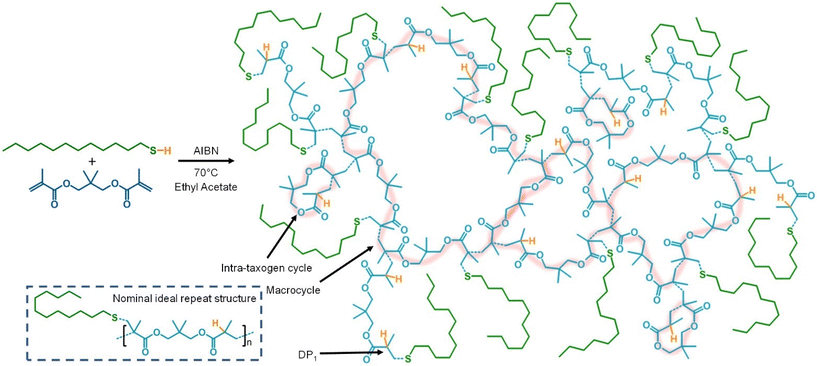
The TBRT synthesis of a branched p(DDT-NPGDMA) polyester is clearly subject to cyclisation, however, the 1H NMR analysis is not able to show the fine detail of the nature of the cycles present. It is possible that large macrocycles are formed at a concentration of 50 wt% solids, Fig. 4, but the degree of cyclisation at low [NPGDMA]0/[DDT]0 ratios is suggestive of the added role of the MVT in directing intra-taxogen cycle formation where the two vinyl groups react to form a 10-membered cyclic diester subunit within the structure, Fig. 4. This is not dissimilar to cycle formation during p(diallyl dimethylammonium chloride), p(DADMAC), synthesis which, although utilising a diallyl monomer, readily forms a linear polymer with a cyclic repeating structure under free radical conditions;21 diallyl dimethylammonium chloride is not dissimilar to NPGDMA in structure.
Investigation of reaction dilution for the TBRT of NPGDMA and DDT
TBRT polymers typically rely on the formation of DP1 structures to avoid gelation, Fig. 4, but clearly the propensity of NPGDMA to cyclise during TBRT opens the potential to work at significantly lower telogen (thiol) concentrations. Additionally, dilution is known to enable cycle formation in TBRT polymerisations. To study the effect of dilution, p(DDT-NPGDMA) was synthesised at 30 wt% and 10 wt% solids (70 wt% and 90 wt% ethyl acetate, respectively), Table 1.At 30 wt% solids, fully soluble branched polyesters were able to be achieved at [NPGDMA]0/[DDT]0 ratios ≤ 1.73 with Mw values ranging from 8300–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423
423![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Fig. 5A, (cf. limiting gel point ratio for EGDMA under these conditions ≈ 1.25).19 Comparing polymerisations conducted at 50 wt% and 30 wt% solids using an [NPGDMA]0/[DDT]0 ratio of 1.01, the decrease in solids concentration led to an approximate 10-fold decrease in Mw, a noticeable increase in [NPGDMA]f/[DDT]f ratio within the purified polymer, and a concomitant decrease in DDT residues that indicate a greater level of cyclisation. This is consistent with previous TBRT reports, however, further reductions in telogen within these polymerisations (higher [NPGDMA]0/[DDT]0 values) leads to more significant increases in cyclisation than those previously reported. At [NPGDMA]0/[DDT]0 values unobtainable when using EGDMA as the MVT, up to approximately 50% of NPGDMA residues are involved in cycle formation19 and the mass of the final polymer that is derived from DDT telogen residues decreased to <30 wt%.
000 g mol−1, Fig. 5A, (cf. limiting gel point ratio for EGDMA under these conditions ≈ 1.25).19 Comparing polymerisations conducted at 50 wt% and 30 wt% solids using an [NPGDMA]0/[DDT]0 ratio of 1.01, the decrease in solids concentration led to an approximate 10-fold decrease in Mw, a noticeable increase in [NPGDMA]f/[DDT]f ratio within the purified polymer, and a concomitant decrease in DDT residues that indicate a greater level of cyclisation. This is consistent with previous TBRT reports, however, further reductions in telogen within these polymerisations (higher [NPGDMA]0/[DDT]0 values) leads to more significant increases in cyclisation than those previously reported. At [NPGDMA]0/[DDT]0 values unobtainable when using EGDMA as the MVT, up to approximately 50% of NPGDMA residues are involved in cycle formation19 and the mass of the final polymer that is derived from DDT telogen residues decreased to <30 wt%.
Further dilution to 10 wt% solids led to highly unexpected results. For context, the formation of p(DDT-EGDMA) under these conditions allowed [MVT]0/[Telogen]0 values to exceed 2.00 (purified polymer maximum [EGDMA]f/[DDT]f = 1.83)21 with a limiting gel point value ≈ 2.20. When using NPGDMA, values of [NPGDMA]0/[DDT]0 up to 2.70 led to purified polymers with Mw < 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Table 1. Due to these TBRT polymerisations appearing to be far below the limiting gel point values, a further reaction was conducted at [NPGDMA]0/[DDT]0 = 4.90, leading to a purified polymer sample with an Mw = 202
000 g mol−1, Table 1. Due to these TBRT polymerisations appearing to be far below the limiting gel point values, a further reaction was conducted at [NPGDMA]0/[DDT]0 = 4.90, leading to a purified polymer sample with an Mw = 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, approximately 80% of NPGDMA residues involved in cyclisation, and <15 wt% of the composition of the final polymer being derived from the telogen DDT, Table 1 & Fig. 5B.
800 g mol−1, approximately 80% of NPGDMA residues involved in cyclisation, and <15 wt% of the composition of the final polymer being derived from the telogen DDT, Table 1 & Fig. 5B.
The ability to homopolymerise a dimethacrylate to complete vinyl group consumption without gelation, using a 5-fold excess of dimethacrylate MVT to telogen, and forming a fully soluble branched polymer, is remarkable. The degree of cyclisation within this complex soluble macromolecular architecture is unprecedented within the knowledge of the authors.
The impact of cyclisation can also be observed within the physical properties of p(DDT-NPGDMA) samples made under different reaction dilutions. At similar Mw and number average molecular weight (Mn) values, the polymers prepared at 50 wt% were highly viscous liquids, however those generated at 30 wt% and 10 wt% solids were solid powders, (Fig. 5Bi–iii).
To be clear, cyclisation would be expected to impact the rigidity of the branched polymer architecture, however, and as shown in Fig. 2, each cycle requires one fewer DDT telogen residue. The variation in the physical properties may therefore be influenced by both the increasing presence of cycles (30.1%, 57.1%, and 80.0% of NPGDMA residues involved in cyclisation in the polymer architectures formed at 50 wt% through to 10 wt% solids, respectively, (Fig. 5Bi–iii)) and the decreasing presence of telogen residues within the polymer sample (p(DDT-NPGDMA) composition contains 37.1 wt%, 26.6 wt%, and 14.4 wt% DDT as reaction conditions vary from 50 wt% to 30 wt% and 10 wt% solids, respectively).
The telogen residue within TBRT polymers is not a conventional chain-end, as would be expected when using thiols as chain-transfer agents at low concentration. Within the nominal repeat unit of an ideal TBRT polymer (no cyclisation), Fig. 1 & 2, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of MVT and telogen leads to the thiol residue acting as a pendant group.22 For DDT telogen residues in p(DDT-NPGDMA), the DDT pendant group is analogous to the lauryl chain within p(lauryl methacrylate). A significant reduction in DDT residues would, therefore, be expected to lead to a less mobile branched polyester backbone.
1 ratio of MVT and telogen leads to the thiol residue acting as a pendant group.22 For DDT telogen residues in p(DDT-NPGDMA), the DDT pendant group is analogous to the lauryl chain within p(lauryl methacrylate). A significant reduction in DDT residues would, therefore, be expected to lead to a less mobile branched polyester backbone.
Investigation of reaction temperature for the TBRT of NPGDMA utilising DDT as telogen
Our previous reports of conducting TBRT polymerisations of EGDMA in the presence of DDT at temperatures from 70 °C–100 °C showed a considerable impact on the formation of soluble branched polymer.17 Despite the dramatic impact on the half-life of AIBN degradation, as the reaction temperature increased, it was clear that the chain-transfer coefficient (CT) became increasingly dominated by chain-transfer, rather than propagation, leading to a shorter telomer distribution within the resulting branched polymers and reduced branching. A dominance of chain-transfer also allowed higher [MVT]0/[Telogen]0 ratios to be employed whilst avoiding gelation.A similar approach was studied for the TBRT of NPGDMA utilising DDT by varying the reaction temperature of the TBRT reactions conducted at 50 wt% solids at 90 °C and 100 °C and employing toluene as the reaction solvent, Table 2. At 90 °C the TBRT reactions were clearly able to form soluble branched polymer at higher [NPGDMA]0/[DDT]0 ratios than those conducted at 70 °C (limiting gel point ratios > 1.23), strongly suggesting an increase in CT, as would be expected. At near equivalent [NPGDMA]0/[DDT]0 ratios the Mw of the recovered polymers were also considerably reduced; as an example, at 70 °C (EtOAc): [NPGDMA]0/[DDT]0 = 1.01, Mw = 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 and at 90 °C (Toluene): [NPGDMA]0/[DDT]0 = 1.02, Mw = 31
200 g mol−1 and at 90 °C (Toluene): [NPGDMA]0/[DDT]0 = 1.02, Mw = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1. Interestingly, the number of NPGDMA residues contributing to cyclised structures within the polymers synthesised at different temperatures, but similar [NPGDMA]0/[DDT]0 ratios, were remarkably similar. Where Mw values were comparable (different [NPGDMA]0/[DDT]0 ratios) the samples generated at 90 °C appear to have a lower DDT content and higher cyclisation, however, this initial observation would require additional investigation.
100 g mol−1. Interestingly, the number of NPGDMA residues contributing to cyclised structures within the polymers synthesised at different temperatures, but similar [NPGDMA]0/[DDT]0 ratios, were remarkably similar. Where Mw values were comparable (different [NPGDMA]0/[DDT]0 ratios) the samples generated at 90 °C appear to have a lower DDT content and higher cyclisation, however, this initial observation would require additional investigation.
| Reaction temperature (°C) | 1H NMR (CDCl3) | TD-SEC (THF/TEA)c | |||||||
|---|---|---|---|---|---|---|---|---|---|
[NPGDMA]0/[DDT]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
[NPGDMA]f/[DDT]f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
% Cyclisationd | DDT (wt%) | M w (g mol−1) | M n (g mol−1) | Đ | α | dn/dc | |
| a Calculated from 1H NMR analysis of the reaction mixture at t = 0 (examples ESI Fig. S8 and S11†). b Determined by 1H NMR analysis of purified polymers (examples ESI Fig. S10 and S13†) note: purification is conducted to remove excess telogen and is expected to remove some low molecular weight species. c Determined by triple-detection size exclusion chromatography using a 0.5% v/v TEA/THF eluent system (example ESI Fig. S16 and17†). d See ref. 19 and ESI eqn (S4).† e All polymerisations achieved >99% vinyl group consumption as determined by 1H NMR (CDCl3) analysis of crude samples of the reaction mixtures after 24 h (examples ESI Fig. S9 and S12†). | |||||||||
| 90 | 1.31 | Gel formed | |||||||
| 90 | 1.23 | 1.60 | 37.5 | 37.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 086![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
5200 | 208.70 | 0.38 | 0.092 |
| 90 | 1.18 | 1.59 | 37.1 | 37.2 | 721![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
5600 | 129.00 | 0.37 | 0.094 |
| 90 | 1.16 | 1.48 | 32.4 | 32.7 | 276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2800 | 97.16 | 0.36 | 0.093 |
| 90 | 1.04 | 1.44 | 30.6 | 31.2 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
3700 | 24.75 | 0.35 | 0.091 |
| 90 | 1.02 | 1.46 | 31.5 | 33.0 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2800 | 10.99 | 0.34 | 0.089 |
| 90 | 0.92 | 1.28 | 21.9 | 24.2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2000 | 10.92 | 0.30 | 0.090 |
| 90 | 0.83 | 1.25 | 20.0 | 23.0 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2400 | 5.65 | 0.31 | 0.090 |
| 100 | 1.19 | Gel formed | |||||||
| 100 | 1.13 | 1.48 | 32.4 | 32.9 | 196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
3100 | 64.23 | 0.35 | 0.091 |
| 100 | 1.07 | 1.42 | 29.6 | 30.4 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3700 | 23.65 | 0.34 | 0.092 |
| 100 | 1.03 | 1.37 | 27.0 | 27.7 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2500 | 31.75 | 0.33 | 0.090 |
| 100 | 0.96 | 1.35 | 25.9 | 27.2 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3700 | 13.76 | 0.34 | 0.092 |
| 100 | 0.88 | 1.36 | 26.5 | 28.9 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3000 | 8.25 | 0.32 | 0.089 |
| 100 | 0.79 | 1.55 | 35.5 | 38.3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3000 | 5.10 | 0.28 | 0.088 |
Further increases in reaction temperature to 100 °C appear to not lead to any dramatic differences in the recovered and purified polymers although, where comparable, there may be an indication of a minor further impact on CT. For example, at [NPGDMA]0/[DDT]0 ratios of 1.02 (90 °C), 1.03 (100 °C), 1.04 (90 °C), and 1.07 (100 °C) the Mw values vary in a relatively systematic manner, namely 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 78
000 g mol−1, 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, 91
400 g mol−1, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, and 87
100 g mol−1, and 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, respectively. The decrease in Mw at the highest [NPGDMA]0/[DDT]0 ratio and higher temperature may be indicative of a further increase in CT, and the clear decrease in DDT wt% for the polymers synthesised at 100 °C within this series (27.7–30.4 wt%) compared to those at 90 °C (33.0–31.2 wt%) may also suggest a slight increase in cyclisation.
500 g mol−1, respectively. The decrease in Mw at the highest [NPGDMA]0/[DDT]0 ratio and higher temperature may be indicative of a further increase in CT, and the clear decrease in DDT wt% for the polymers synthesised at 100 °C within this series (27.7–30.4 wt%) compared to those at 90 °C (33.0–31.2 wt%) may also suggest a slight increase in cyclisation.
The inability to generate soluble polymer at [NPGDMA]0/[DDT]0 ratios ≈ 1.19, in reactions conducted at 100 °C, may suggest that the higher temperature has impacted the conformation of NPGDMA and allowed a fraction of the MVT population to extend to vinyl group distances that more closely align with EGDMA, although the authors have no direct evidence for this supposition. This is the first example of an increase in temperature leading to a decrease in limiting [MVT]0/[Telogen]0 gel point ratios. Overall, the impact of temperature is observable but not as dramatic as seen in our previous report of p(DDT-EGDMA) synthesis.17 This would, again, indicate a major influence on the outcomes of the TBRT reaction from the geometry of the MVT being used here.
Final note
It is important to state here that the physical properties of the polymer synthesised within this study have been highly difficult to characterise. The use of differential scanning calorimetry and dynamic mechanical analysis have yielded data that has proved challenging to interpret. Research is ongoing to establish appropriate analytical protocols and an understanding of these novel materials to allow clear and consistent data.Conclusions
With the considerable scope of multi-vinyl taxogens that may be utilised in TBRT polymerisations, it is important to understand the impact of chemical structure on polymerisation outcomes. Previously, aromatic and aliphatic dimethacrylates have been studied and the impact of rigidity and dimensions of the MVTs has been described.22 Directing the formation of cyclised TBRT polymers has been achieved by dilution but here the restricted geometry of NPGDMA has been shown to lead to significant cyclisation even at relatively high MVT/telogen concentrations. The precedent set by the cyclopolymerisation during p(DADMAC) synthesis is useful in interpreting the observations here. This offers the potential to use less telogen during a TBRT polymer synthesis, but also to create a range of polymer structures with the simple manipulation of reaction conditions, notably initial [MVT]/[Telogen] ratios, dilution, and temperature. The resulting polymers may also be tuned to contain varying amounts of thioether residues (or sulfur content). Although there are general trends in the measured dn/dc values of the recovered polymers (higher values at higher degrees of cyclisation) it is unclear whether this is due to the modified composition of the materials, as increasing cyclisation leads to a lower contribution of the telogen to the mass of the polymer relative to taxogen. This will require additional study.Whether the effects here are replicated when using a range of telogens, or within copolymerisation studies, is yet to be established. NPGDMA, however, represents an interesting case study for TBRT and the application of NPGDMA-containing polymers will be the subject of considerable further research.
Author contributions
C. S. was responsible for conceptualisation, methodology, experimentation, investigation, data curation, formal analysis, visualisation and editing of the original draft. O. B. P-L. contributed to supervision, methodology, experimentation, data curation and editing of the original and final drafts. S. M., S. W., and A. B. D. all contributed to validation. S. P. R. was responsible for funding acquisition, conceptualisation of the original research programme, methodology, validation, visualisation, supervision, project administration and manuscript review and editing.Data availability
All data generated during this study supporting its findings are available within the manuscript and the ESI.† All data is available from the corresponding author upon reasonable request.Conflicts of interest
S. W. and S. P. R. are co-inventors on patents that protect the TBRT chemistry; several of these patents have been licensed to Scott Bader and form the basis of Polymer Mimetics Ltd (Company number 12598928). No other co-authors have any competing interests.Acknowledgements
The Engineering & Physical Sciences Research Council (EPSRC) are grateful acknowledged for funding through grant EP/X010864/1. CS is grateful to the Centre of Excellence for Long-acting Therapeutics (CELT) for PhD funding. The authors would like to thank the Materials Innovation Factory (University of Liverpool) for analytical support.References
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- A. Skandalis, T. Sentoukas, D. Selianitis, A. Balafouti and S. Pispas, Materials, 2024, 17, 1947 CrossRef CAS PubMed.
- G. M. Dykes, J. Chem. Technol. Biotechnol., 2001, 76, 903–918 CrossRef CAS.
- T. Kaiser and H. Frey, Polymer, 2020, 211, 123113 CrossRef CAS.
- F. Hatton, Polym. Chem., 2020, 11, 220–229 RSC.
- J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249–1262 RSC.
- N. P. Truong, G. R. Jones, K. G. E. Bradford, D. Konkolewicz and A. Anastasaki, Nat. Rev. Chem., 2021, 5, 859–869 CrossRef CAS PubMed.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, Polym. Chem., 2020, 11, 2595–2604 RSC.
- B. Boutevin, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3235–3243 CrossRef CAS.
- B. Boutevin, J.-M. Lusinchi, Y. Pietrasanta and J.-J. Robin, Eur. Polym. J., 1994, 30, 615–619 CrossRef CAS.
- C. Loubat and B. Boutevin, Polym. Bull., 2000, 44, 569–576 CrossRef CAS.
- S. R. Cassin, P. Chambon and S. P. Rannard, Polym. Chem., 2020, 11, 7637–7649 RSC.
- P. J. Flory, J. Am. Chem. Soc., 1941, 63, 3083–3090 CrossRef CAS.
- W. H. Stockmayer, J. Chem. Phys., 1944, 12, 125–131 CrossRef CAS.
- C. Walling, J. Am. Chem. Soc., 1945, 67, 441–447 CrossRef.
- J. Lyu, Y. Gao, Z. Zhang, U. Greiser, H. Tai and W. Wang, Sci. China: Chem., 2018, 61, 319–327 CrossRef.
- S. Flynn, O. B. Penrhyn-Lowe, S. Mckeating, S. Wright, S. Lomas, S. R. Cassin, P. Chambon and S. P. Rannard, RSC Adv., 2022, 12, 31424–31431 RSC.
- A. Behr, M. Becker, T. Beckmann, L. Johnen, J. Leschinski and S. Reyer, Angew. Chem., Int. Ed., 2009, 48, 3598–3614 CrossRef CAS PubMed.
- S. R. Cassin, S. Wright, S. Mckeating, O. B. Penrhyn-Lowe, S. Flynn, S. Lomas, P. Chambon and S. P. Rannard, Polym. Chem., 2023, 14, 1905–1914 RSC.
- O. B. Penrhyn-Lowe, S. Flynn, S. R. Cassin, S. Mckeating, S. Lomas, S. Wright, P. Chambon and S. P. Rannard, Polym. Chem., 2021, 12, 6472–6483 RSC.
- A. R. Biery and D. M. Knauss, Mater. Today Chem., 2022, 26, 101251 CrossRef CAS.
- S. R. Cassin, S. Flynn, P. Chambon and S. P. Rannard, Polym. Chem., 2022, 13, 2295–2306 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, full experimental details and characterisation. See DOI: https://doi.org/10.1039/d4py01368a |
| This journal is © The Royal Society of Chemistry 2025 |