 Open Access Article
Open Access ArticleNanosensors in healthcare: transforming real-time monitoring and disease management with cutting-edge nanotechnology
Shikha
Gulati
 *a,
Rakshita
Yadav
b,
Varsha
Kumari
c,
Shivangi
Nair
*a,
Rakshita
Yadav
b,
Varsha
Kumari
c,
Shivangi
Nair
 c,
Chetna
Gupta
c,
Chetna
Gupta
 d and
Meenal
Aishwari
c
d and
Meenal
Aishwari
c
aDepartment of Chemistry, Sri Venkateswara College, University of Delhi, Delhi 110021, India. E-mail: shikha2gulati@gmail.com
bDepartment of Biochemistry, Sri Venkateswara College, University of Delhi, Delhi 110021, India
cDepartment of Life Sciences, Sri Venkateswara College, University of Delhi, Delhi 110021, India
dDepartment of Chemistry, Hansraj College, University of Delhi, Delhi, India
First published on 25th June 2025
Abstract
The field of healthcare monitoring continuously strives to find new and better ways of improving healthcare access and advancing the accuracy and precision of diagnostic and treatment approaches. To add to its challenges, the modern and fast-paced lifestyle now presents the need for even more sensitive, specific, and rapid methods of continuous healthcare monitoring technology that can generate real-time information. The integration of cutting-edge nanotechnology in health care with its unique and versatile properties has brought a technological revolution in the way disease detection, management, and treatment are approached, finding applications from early-stage disease detection to real-time physiological parameter monitoring. The unique physical and chemical properties of nanoparticles provide a basic structural framework on which successive chemical and biological detection systems can be built. This characteristic of nanoparticles provided healthcare researchers with opportunities to create nanoparticle-based nanosensors, nanomedicine, bioimaging, point-of-care, and other such devices. Here we provide a comprehensive review of the development and advancement of nanosensors in healthcare monitoring, its types, applications, and future prospects, and highlight the development and challenges faced in the field. The review also sheds light on the all-encompassing nature of nanotechnology, in terms of compatibility with different existing streams of applied sciences in healthcare.
1. Introduction
Nanosensors are devices that can identify and react to physical, chemical, or biological events on a nanoscale and they are transforming the healthcare industry with their remarkable accuracy and effectiveness. These sensors—comprising biosensors, chemical sensors, and physical sensors—trace their origins back to the early developments in nanotechnology and materials science around the early 2000s. The initial efforts concentrated on creating materials capable of engaging with molecular interactions, resulting in remarkably sensitive tools that can detect subtle fluctuations in biological indicators and environmental factors. The ongoing trend of miniaturization and improved functionality of these sensors has been fuelled by crucial research and significant technological advancements, allowing them to conduct intricate analyses that were not possible with traditional sensors. In the realm of healthcare, nanosensors are essential for the early detection of diseases, constant monitoring of health status, and tailored medical treatments, offering immediate data for prompt medical responses. Their uses span from tracking glucose levels in diabetes care and identifying cancer biomarkers to assessing cardiovascular health and monitoring infectious diseases. This adaptability and ability to provide swift and precise results position nanosensors as a fundamental element of contemporary medical technology, facilitating more agile and effective healthcare solutions.The rapid growth of the nanosensor field in healthcare is evidenced by the increasing number of publications over the years. The upward trend reflects the expanding applications and rising popularity of nanosensors, driven by their adaptable design and ability to be modified to meet the evolving needs of the scientific and healthcare industries. The key milestones in the development and deployment of nanosensors are increased biocompatibility, biodegradability, and exceptional adsorption properties, which make them highly effective in various health monitoring applications. As the field progresses, nanosensors continue to gain traction for their versatility, opening new avenues for advanced, real-time health monitoring and diagnostics.
Nanosensors, which are engineered at the scale of nanometers, have now emerged as one of the transformative tools in the healthcare sector due to their strong ability to detect biochemical, physiological, and molecular changes with exceptional specificity and sensitivity.1 These devices are supported by advanced nanomaterials like carbon nanotubes, graphene, quantum dots, and metal nanoparticles, which help in constructing compact platforms that are capable of performing real-time biomarker detection and environment monitoring. Because of their miniaturised nature, they integrate into point-of-care (POC) diagnostic devices, wearable systems, and implantable biosensors, which offer significant advantages in early disease detection and personalised treatment.
Recent advancements have helped in integrating nanosensors into healthcare solutions, including chronic and infectious conditions like cardiovascular diseases, tuberculosis, and diabetes. Moreover, their uses go beyond human health, which includes applications like agricultural pathogen monitoring, pharmaceutical research, and therapeutic drug delivery. These vast applications are driven by the demand for portable, non-invasive, and highly responsive technologies that can perform rapid diagnostics and patient-centric care. Their success also includes innovation in fabrication techniques, nanomaterials selection, sensor designing, wireless data transmission, etc., which are essential for seamless real-time monitoring in clinical and remote environments.
In this paper, we present a comprehensive review of nanosensor technologies and their implementation and extension in health monitoring, mainly emphasizing applications in POC devices, wearable systems, and disease diagnostics. We have explored some transformative tools like materials and methods used for sensor fabrication, miniaturization strategies, power supply innovations, and wireless communication protocols, which contribute to the performance of nanosensors. Furthermore, we have taken a glance at real-world applications in cardiovascular monitoring, tuberculosis diagnosis, glucose sensing, infection tracking in plants, and even development in the pharmaceutical sector through nano-medicines. Our aim is to provide a holistic perspective on how nanosensors can shape the future of healthcare technologies. Even after considerable progress and a wide range of uses for nanosensors in healthcare, several research gaps persist that indicate areas needing further investigation and enhancement. A key lies in the connection of nanosensors with user-friendly, non-invasive devices intended for continuous, real-time health monitoring, as most of the existing systems are either intrusive or require sophisticated equipment. Moreover, although there is extensive research on the sensitivity and specificity of nanosensors, further investigation is necessary to assess their long-term stability and reproducibility in various biological contexts. Another significant gap pertains to the standardization and regulation of nanosensor technology to guarantee consistent performance and safety across various applications and patient demographics. Additionally, many related studies underscore the necessity for improved data processing and analysis methods to manage the substantial amounts of data generated by nanosensors. This includes creating advanced machine learning and artificial intelligence techniques to accurately interpret sensor outputs and yield actionable insights. Lastly, ethical and privacy issues related to the widespread use of nanosensors in health monitoring warrant attention, as highlighted in numerous studies. These issues encompass the secure management of sensitive health information and the preservation of patient confidentiality in a progressively interconnected healthcare landscape. By addressing these research gaps, future investigations can improve the practicality, reliability, and acceptance of nanosensors in health monitoring, ultimately aiding in the development of more efficient and personalized healthcare solutions.
The numerous discoveries in nanotechnology initially sparked interest in integrating it with other fields, such as healthcare and environmental monitoring. This focus has since led to the miniaturization of nanotechnology, enhancing its adaptability and performance.
2. Methodology
In order to objectively assess the current status of nanosensor research and its applications in therapeutic monitoring, diagnostics, and healthcare, this study uses a systematic literature review methodology for a thorough coverage and analysis of existing academic literature and relevant applications of nanosensors in the field of healthcare.The main objective of this review is to summarize and report developments in nanosensor technology in the field of health care in context. The following research questions serve as the main objectives for the review:
1. Which nanosensor classes and processes are most frequently investigated for use in healthcare applications?
2. To explore how nanosensors have been applied to drug administration, physiological monitoring, and diagnosis.
3. To summarize the main drawbacks, obstacles to translation, and potential paths forward in healthcare enabled by nanosensors?
2.1. Method of search
A thorough search was carried out following the PRISMA criteria. We conducted searches across the databases such as PubMed, ScienceDirect, and independent journals. Only papers released between 2014 and 2024 were included in the searches. Combinations of the following keywords were used to identify 400 initial records in total:Key words: “biosensors”, “nanosensors”, “nanotechnology”, “biosensors”, “nanomaterial” and “healthcare”. Secondary words include “drug delivery”, “disease detection”, “point-of-care diagnostics”, “real-time monitoring”, “biomedical equipment”, and “diagnostics”.
Criteria for inclusion and exclusion: Peer-reviewed publications and thorough evaluations, English-language studies that explain or assess the use of nanosensors in biomedicine or clinical settings were the criteria for inclusion. Criteria for exclusion were publications in languages other than English, articles that addressed non-medical applications (such as food safety, environmental sensing etc.).
Titles and abstracts were used to filter the first 400 articles. A total of 147 papers were chosen for full-text review after eliminating duplicates and irrelevant research. Following that, 68 papers that satisfied all requirements and provided comprehensive and detailed literature of nanotechnology developed and applied in healthcare were used for the final draft.
The approach of classification of nanosensors used here is based on their signal transduction mechanisms (e.g., optical, electrochemical, mechanical, magnetic, and biological) and healthcare application areas (e.g., diagnostics, monitoring, and drug delivery). This structured framework facilitates:
• Identification of research gaps: by segmenting nanosensor types and their respective uses.
• Tracking technological trends: categorization allows for analysis of which nanosensor types are mostly used, revealing patterns in innovation and adoption over time across different diseases and clinical needs.
• Application-oriented design insight: grouping nanosensors by type aids researchers and developers in aligning device selection with the demands of specific applications.
• Facilitation of interdisciplinary collaboration: the classification helps bridge the gap between nanotechnologists, clinicians, and biomedical engineers by offering a common language and functional mapping of technology to medical utility.
To enhance analytical clarity, nanosensors here are categorized based on their signal transduction mechanisms (e.g., optical, electrochemical, magnetic, and biological) in the overview section and application domains (e.g., diagnostics, monitoring, and drug delivery) covering their applicability in healthcare. This structured classification allows for better comparisons between sensing mechanisms and helps identify which technologies are better suited for specific clinical contexts. It also supports systematic mapping of research trends and technological maturity across categories, ensuring a more comprehensive review.
3. Nanosensors: an overview
There is a plethora of grounds on which nanosensors are defined and distinguished based on the need and utility of the scenario; they can be defined and categorized by their constituent materials, their target sites, dimensionality, detection analytes, and the signals they use to transmit information. Each basis of categorization has its own specialties and domain of use. However, in the general and broad terms of use, nanosensors are categorized either based on signal production or by the different methods they employ for signal transduction.2 These include electrical, biological, magnetic, optical, and mechanical transduction properties of the sensor upon interaction with the target analyte. The primary categories of nanosensors based on signal production or by the different methods they employ for the signal transduction categorization approach are: -3.1. Optical nanosensors
Optical nanosensors essentially detect changes in optical properties, such as absorbance, fluorescence, and refractive index, of the interacting molecules in response to target element binding. The method by which an optical nanosensor identifies the specific substance or stimulus is dependent on the choice of the nanomaterial, its interaction with the target, and how these interactions result in detectable changes in the sensor's characteristics. Some of these common optical nanosensors’ working principles that are utilized in the field of healthcare monitoring are: -The versatility of optical nanosensors lies in their effectiveness and convenient optimization to increase their sensitivity and selectivity to several biological analytes, because of which optical nanosensors have found applications in various fields, including health and environmental monitoring. Traditional instrumental methods for disease detection and monitoring, though powerful, are often time-consuming, labour-intensive, and expensive. Optical nanosensors work as promising alternatives or complementary tools and offer rapid, convenient, effective, and less costly solutions. Revolutionizing the approach of disease detection and monitoring.
3.2. Electrochemical nanosensors
Electrochemical techniques are surface techniques that are advantageous for the recognition of the bio-analytes present due to their standout advantages,6e.g., profound selectivity, high sensitivity, rapid response, repeatability, low volume of sample requirement, cost-effectiveness, user-friendliness, portability, etc. Electrochemical nanosensors detect changes in electrical properties (current, voltage, and impedance) due to chemical reactions at the sensor surface.2In electrochemical sensing techniques, recognition of the biological metabolites followed by analysis of the targeted analytes is generally carried out via different electrochemical sensing modes, which show the variation in electrical signals at the working electrode, followed by transduction of the concentration of the targeted analyte into readable signals.6 Based on the mechanism of detection of analyte at the surface of the sensor, electrochemical nanosensors may be of two types.
To acquire more stability, sensitivity, selectivity, simplicity, etc., the electrodes of non-enzymatic sensors are chemically modified using various types of nanomaterials, as nanomaterials have the capability to highly enhance the electrocatalytic properties of sensors. Some examples are carbon-based nanomaterials (e.g., CNT, carbon quantum dots, graphene, etc.), conducting polymer nanostructures (polyaniline and polypyrrole), and noble metals (e.g., Au, Pt, etc.).3
Electrochemical nanosensors are currently an intensive topic of research and development for diverse fields of healthcare, including but not limited to medical diagnostics (e.g. glucose, lactate, and urea monitoring), detection of disease biomarkers and pathogens, drug monitoring and pharmacokinetics, and integration with wearable POC devices for continuous health monitoring.
3.3. Biological nanosensors (biosensors)
Principle: biological nanosensors are the integration of nanotechnology with biological molecules (DNA, enzymes, and antibodies) that specifically interact with target biological entities, resulting in the production of reaction-specific analytes, which are then converted into detectable signals.7 When a biological recognition component is immobilized on an electrochemical cell's electrode, it is called a biosensor.2,5 An electrical signal is produced when the biological sample interacts with the biological recognition site of the biosensor.8These nanosensors are essentially the hybridization of specific biological molecules with other types of nanosensors, such as optical, electrochemical, mechanical, etc., designed with a narrow and specific goal. They are categorized based on the type of biological recognition element used, the detection mechanism, and the specific applications they are designed for primary categories are:
These specialized sensors find applications in various domains of healthcare, such as medical diagnostics (pathogens, genetic mutations, biomarkers detection, and cancer therapy),13 environmental monitoring (microbial contamination), and food safety (contaminants and pathogens). Many of these find use in rapid testing of viruses and toxins and monitoring of metabolic diseases.12
3.4. Magnetic nanosensors
Principle: magnetic nanosensors operate based on the detection of changes in magnetic properties or the detection of magnetic fields caused by the interactions of magnetic nanoparticles with target analytes. These changes can be in the form of magnetic field strength, magnetic susceptibility, magnetoresistance, or other magnetic parameters. Magnetic nanosensors allow for the measurement of various physical characteristics. Common physical effects utilized by magnetic sensors include the Hall effect, giant magnetoresistance (GMR), tunnel magnetoresistance (TMR), anisotropic magnetoresistance (AMR), giant magnetoimpedance (GMI), and magnetic tunnel junctions (MTJs).14Magnetic nanosensors are mainly categorized based on their detection mechanisms and the type of magnetic nanomaterials used. The primary categories are: -
Magnetic nanosensors find various applications in bioimaging, such as MRI,15 data storage (in continuous health care monitoring), targeted drug delivery, and targeted disease monitoring and treatment.
3.5. Mechanical nanosensors
Mechanical nanoparticles detect changes in forces, mass, or motion through nanoscale structures that change mechanical properties (resonance frequency and deflection) in response to stimuli. Mechanical nanoparticles measure the changes in mechanical forces at the molecular level.16 The main advantage of these sensors is that they are sensitive to mass. This property to measure the mass makes them versatile since nearly anything has a mass. But mechanical nanosensors face a major obstacle in fluid phases due to viscous damping, which critically reduces the sensitivity of the sensor.The changes in the mechanical properties are transduced through a variety of methods, such as electrical, optical, piezoelectric, etc. Based on the methods of transduction, mechanical sensors are broadly categorised into the following categories: -
Mechanical nanosensors are comparatively less focused on in terms of healthcare monitoring and have even less impact and applications as compared to their alternatives, but does have the potential to for a larger impact on further development and optimisation for applied usage in the healthcare sector. Currently, it has uses in molecular interaction detection,16 precise mass measurements, and mechanical sensors in MEMS.
3.6. Applications of nanosensors in drug delivery, point-of-care (POC) devices and wearable systems
“A POC system is a portable device (Fig. 1) used for the analysis and detection of disease outside a traditional laboratory”.18 POC devices are essentially the integration of existing or newfound diagnostic techniques with microscale technologies to develop devices capable of producing accurate results with minimum human intervention or effort. The use of nanotechnology in these systems significantly reduces the duration of diagnosis and minimises human errors. The advancement in nanosensors has resulted in the creation of portable, flexible, and wearable devices for point-of-care diagnostics and continuous health monitoring.18 | ||
| Fig. 1 Schematic illustration of the POC-based data collection, analysis by AI & ML, and EHR shared through IOT for smart hospital application.20 Reproduced from an open-access article with permission from the Royal Society of Chemistry. Originally published under a CC BY license. | ||
Wearable sensor devices have become fairly common and appreciated among the current society for their portability and non-invasive nature, suitable for self-monitoring of certain health and physical parameters such as heart rate, blood pressure, physical activity, etc.18 Innovations in the field of artificial intelligence and enhanced machine learning algorithms have substantially reduced the diagnostic period of certain diseases and demonstrated a remarkable improvement in the prognosis and treatment approaches as seen in Fig. 1, leading to the evolution of a completely new field of “digital healthcare” which is the result of the amalgamation of nanosensors, software, and digital devices for efficient and sustainable health systems.
Point-of-care (POC) and wearable devices can play a crucial role in significantly reducing the mortality rates for various fatal diseases through early symptom detection and accurate diagnosis without the need for time-consuming testing processes and expensive lab setups.19
Innovations in nanosensor technology have now also made targeted drug delivery possible through nanomaterials.21 They play a crucial role in creating smart drug delivery systems that can enhance efficiency and specificity and minimise risks and side effects in various disease management, such as cancer22 and cardiovascular theranostics.23,24 The use of nanomaterials allows for stimulus-controlled and hyper-sensitive drug delivery. Nanosensors are already being used for targeted and controlled drug delivery in cancer, cardiovascular diseases, and diabetes management.
Each type of nanosensor mentioned here has its unique advantages and limitations, providing researchers with a range of options for developing personalised and targeted health care and biosensing25 solutions which have the capability of revolutionizing disease detection, treatment, management, and aftercare.26
4. Applications of nanosensors in health monitoring
4.1. Early cancer detection
According to WHO, cancer is one of the leading causes of death worldwide, accounting for almost one out of every six deaths.27 As of 2020, nearly 10 million people died primarily due to cancer. If detected at early stages, most types of cancer can be cured through adequate treatment procedures, and an individual may return to their normal life. To improve the quality of life and its expectancy, a more cost-effective, fast, and accurate routine blood screening method should be devised for early cancer detection and diagnosis.28 Also, the present technologies like PET, CT, and MRI may detect the disease only after the physiological changes begin in the body, which might be too late for the individual, along with high cost and poor spatial resolution. This is where the knowledge of nanosensors can be applied. Highly selective nanomaterials can be incorporated to create selective and sensitive nanosensors that can detect the presence of biomarkers, tumour cells circulating in the blood vessels, early release of exosomes or other tumour-derived vesicles by tumour cells in blood, and help in early diagnosis of cancer.Tumour biomarkers are entities ranging from genes and nucleic acids to proteins, sugars, or other metabolites which depict the person's biological or pathological state, in the biological fluids (i.e., saliva, urine, serum, etc.)29 Another method to gain information regarding the pathological state is the detection of CTCs. Some of the dividing cancer cells enter the blood or lymphatic systems, providing a valuable means of information on the presence of tumours. They are most often found in patients with metastatic cancer. The exosomes (40–100 nm in diameter) are present in both normal and affected cells. The exosomes derived from the tumour cells contain bioactive materials packed in vesicles on reaching distant tissues through blood, transferring the bioactive molecules, changing the properties of normal healthy cells, and providing perfect conditions for metastatic cancer cell growth.30 Low amounts of CTCs or exosomes are found in the bloodstream; hence, highly selective and sensitive sensors are required to detect their presence and help in the early detection of cancer.
There have been significant advancements in the development of sensors made of nanomaterials for highly sensitive detection of specific target molecules for early diagnosis of cancer. The unmatched, flexible physical and chemical properties of certain nanoparticles, such as large area-to-volume ratio, fluorescence and absorption, high electrical conductivity, etc., make them great tools for biosensing. As discussed earlier, the identification of tumour biomarkers and the detection of CTCs or exosomes derived from tumours can help in the early diagnosis of the disease as well as monitoring it. Nanosensors can help achieve significant amplification of various signals and can also be used to isolate and concentrate the target analyte, which in turn helps to estimate the analyte's quantity in the body. Hence, the unique properties of nanosensors should be exploited to improve the already existing technologies. The nanoparticles offer high selectivity, sensitivity, quicker response, and portability and are easy to use, hence making them a good potential material for designing real-time Point-of-Care (POC) devices. Table 1 lists some nanoparticles and their unique features, which can be incorporated into developing nanosensors for early cancer detection.
| Nanomaterial | Special features for biomedical application | Ref. |
|---|---|---|
| Colloidal fluorescent and plasmonic NPs | Strong response to incident light helps in the detection and quantification of the target analyte. | 31 |
| Gold nanoparticles (AuNPs) | Excellent signal transduction- the target analyte modulates the NP's optical properties, confirming its presence. | 32 |
| Carbon Quantum Dots (CQDs) instead of intrinsically toxic semiconductor QDs based on heavy metals such as CdS, CdSe, PbSe, Ag2S. | Biocompatibility, lower toxicity, high chemical stability, and luminescence properties offer great potential in biomedical applications. | 33 |
4.2. Monitoring cardiovascular diseases
The heart consists of a variety of cells – endothelial, connective, or smooth cells. Malfunctioning in any of these cells or tissues can lead to cardiovascular diseases (CVD). Tobacco and alcohol consumption, obesity, high blood glucose levels, unhealthy lifestyles, and genetic factors are the major reasons for CVDs. Depending on the risk and severity of the CVD, there are different treatment methods, but all aim to improve the blood supply or reduce pressure on cardiac walls and prevent rupture of capillaries.The current methods of diagnosis of CVDs include MRI, X-ray, computed tomography (CT), ECG, and echocardiography. The current treatment methods are not specific to diseases and may lead to organ toxicity. Along with that, conventional diagnosis techniques lack precision and are not as efficient in early diagnosis due to the heterogeneity of cardiovascular diseases. Here, nanosensors can prove to be very useful. Development in diagnosis and bioimaging using nanosensors has significantly improved the detection of biomarkers and proteins in CVDs. Disease-specific molecule like specific integrins expressed by atherosclerotic plaques, can be targeted by nanosensors for detection. Pressure biosensors utilise self-oriented nanocrystals to detect pressure changes in cardiovascular walls. Also, in vitro programmable bio-nanochips can be used as Point-of-Care (POC) testing methods.34
4.3. Diagnosing tuberculosis
Tuberculosis is one of the most widespread infectious diseases in the world. Primarily caused by Mycobacterium tuberculosis, it affects the pulmonary organs, i.e. lungs and central nervous system, leading to meningitis. Nanomaterials are now being extensively used to detect the presence of analytes such as glucose, biomarkers, and microorganisms for the diagnosis of several diseases, including tuberculosis. To ensure better selectivity and higher sensitivity for tuberculosis, nanomaterials have been incorporated into electrochemical and optical sensors. Ag nanoparticles (AgNPs), AuNPs, Cadmium Telluride Quantum Dots (CdTe QDs), and Nickel Oxide (NiO) NPs are some of the nanomaterials used in the optical sensing of tuberculosis. The strategy of sensing tuberculosis incorporates physical phenomena like SERS (Surface-enhanced Raman Spectroscopy), SPR (Surface Plasmon Resonance), and fluorescence emission.354.4. Glucose monitoring among diabetes patients
Diabetes is one of the most common diseases in the world, with approximately 537 million adults affected as of 2021, as suggested by the 2021 report by the IDF Diabetes Atlas.36 As diabetes is the most widespread disease worldwide after cancer, its diagnosis plays a vital role in deciding effective treatment methods for its cure.37 Serious cases of diabetes may also lead to cardiovascular diseases and blindness. The patients affected by the disease require continuous monitoring of their blood glucose levels and keeping them minimum to lead a healthy life.38Mainly two approaches are made for glucose level monitoring through nanotechnology- firstly, modification of the sensor designs by incorporating nanomaterials to improve their catalytic activity, and secondly, creating injectable or implantable nano-sized glucose sensors with longer lifetime and higher accuracy compared to traditional sensors. David et al. reported that sensors made of enzyme-loaded chitosan-poly (styrene sulfonate) in a self-assembled layer-by-layer structure can utilise acid–base functionalized carbon nanomaterials as electrical bridges and help in the detection of glucose.39 Also, silver nanoflower-reduced graphene oxide composites can potentially be used for developing micro disk electrodes for biosensors to detect the presence of insulin in serum samples of the patient.40
4.5. Monitoring infections and pathogens in plants
Not just humans and other animals, but plants also suffer from several diseases due to environmental stress or infection by pathogens. Such diseases can negatively impact the health of crops and decrease the yield, affecting the food supply. Even though there are remote sensing instruments meant to detect stress in plants, they are not as effective in preventing crop damage as they rely on external environmental conditions or measure stress only after the plant's health has started to deteriorate. Nanosensor-powered electronic health devices are emerging candidates for early, real-time stress detection and monitoring tools for plant health. Accumulation of H2O2 is a sign of stress in plants and has been reported in most plant stresses, like light stress, salinity, heat stress, and pathogenic infection. Single-walled carbon nanotubes (SWCNTs) are promising materials for making H2O2 detection nanosensors. Wu et al. designed near-infrared (NIR) fluorescent SWCNTs and interfaced them with Arabidopsis thaliana leaves to monitor H2O2 levels, as they are a key indicator in detecting plant stress. They developed the nIR fluorescent SWCNTs with a DNA aptamer that binds to hemin (HeAptDNA-SWCNT), allowing remote monitoring of plant health and stress caused by environmental factors or pathogens.414.6. Therapeutic drug monitoring
Therapeutic drug monitoring refers to the monitoring of the effects of a drug concentration over time for efficiently managing a safe drug dosage for an individual. Therapeutic drug monitoring usually takes blood samples to test the effect of the drug, though more non-invasive methods are being developed using other biological fluids like saliva and interstitial fluid. It is a necessary step following organ transplantation. During transplantation, the organ recipient's immune system may reject the organ, considering it a foreign object; therefore, a specific dosage of an immunosuppressant drug must be given to the patient to suppress the immune response and make the transplantation successful. In such cases, nanosensors are an essential tool.424.7. Pharmaceutical applications and nanomedicine
Nanomedicine is a multidisciplinary branch of medicine involving biology, chemistry, engineering, and biotechnology, which helps in the regeneration and repair of biological systems at the cellular level. Through nanomedicine, repairable cells are repaired one by one, while the cells that cannot be repaired undergo apoptosis. Gene therapy utilises nanoparticle systems to deliver a target mechanism to a specific cell. The nanoparticles are delivered to the intracellular target through cleavable shells. The biosensors control the amount of gene therapy in a cell by activating the control switches for the therapeutic gene sequence in the concerned cell. The gene expression is controlled by these biosensors. When an issue is detected by the biosensor in the cell, it triggers the gene delivery system, and when the issue is resolved, the biosensor halts the process. Green Fluorescent Protein (GFP) reporter sequences have been successful in gene delivery. The field of nanomedicine is still in its initial growth stages, but it can prove to be a new age of medicine with nano-biosensors playing a key role in the future.434.8. Current market landscape and comparison of nanosensors in healthcare
The market of nanosensors has grown significantly, both financially and economically around the entire world, with it's current estimate between $637 million and $700 million. Experts predict that by 2032, the estimated worth of the market will be around $2.37 billion to $3.1 billion.43 In recent years, flexible sensors have become more popular than traditional sensors which are naturally stiff and hard. When traditional sensors are developed to be flexible and stretchy, they acquire a more extensive range of applications.44 Application of nano-sensors in healthcare is continuously evolving, with new functions and increasing efficiency for disease detection and body function monitoring. Recent surveys of the market show that healthcare professionals and companies prefer electrochemical nanosensors above other choices. Electrochemical nanosensors thus account for over 25% of the market share.43 Nanosensors operate at the molecular level inside the body and are now actively integrated into various medical applications. By analyzing their use across different domains in medicine, nanosensors in medicine can be categorized based on their specific applications, such as electrochemical nanosensors for detecting biomarkers in blood, optical nanosensors for imaging and diagnostics, and magnetic nanosensors used in targeted drug delivery. This functional classification helps us in understanding the diverse roles these sensors play in enhancing precision, speed, and sensitivity in healthcare diagnostics and treatment in present times.44Advancements in improved and enhanced material for sensing and nano-fabrication, using the Internet of Things (IoT) to monitor real time changes, and using artificial intelligence and machine learning to analyze nanosensor data are important innovations that are making nanosensors better and more useful thereby expanding the nanosensor market.45 To develop these advancements North America has positioned itself as a significant investor particularly in the integration of nanosensors with IOT, closely followed by Europe.43
5. Technology and mechanisms behind nanosensors
5.1. Materials and methods used for nanosensor fabrication
Recent years have seen the rapid progress of nanotechnology leading to revolutionary breakthroughs in the design, manufacture, and applications of nanosensors. Nanomaterials can be classified by dimension as shown in Fig. 2, morphology as shown in Fig. 3 and the type of material used.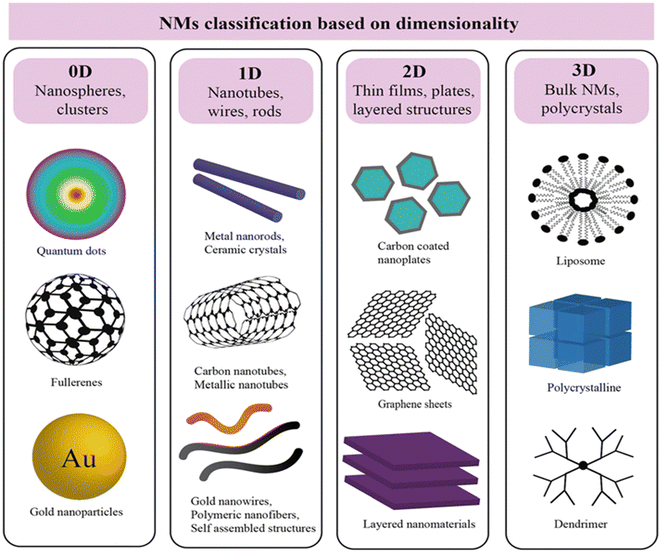 | ||
| Fig. 3 Schematic of nanoparticles by dimensionality with examples: 0D (nanoparticles), 1D (nanorods), 2D (films), and 3D nanocomposites, where synergistic properties emerge from distinct materials combined at the nanoscale. Reproduced from an open access article, ref. 44 with permission from the Royal Society of Chemistry, 2024. | ||
Nanomaterials like carbon nanotubes, graphene, nanowires, nanocomposites, and quantum dots are significant in the creation of nanosensors for wearable health monitoring.46
Metals like nickel and copper, metal oxides like zinc oxide and ferric oxide, and semiconductors like silicon and gallium nitride are commonly used materials in making nanowires used for the fabrication of nanosensors.47 To fabricate nanowire-based sensors, a variety of growth methods and design factors are used. A popular method for creating nanowires is the vapor–liquid–solid (VLS) method. Nanowires with the desired sizes, orientations, and crystal structures can be made using the VLS technique, as it allows controlled growth of nanowires. This mechanism uses a foreign element catalytic agent (FECA), which is a nanocluster made of metal atoms for the nucleation of nanowires.48 For nanowire fabrication, growth methods like electrodeposition and molecular beam epitaxy are also employed. Using these methods, one can precisely control the diameter, length, and doping concentration of nanowires.49
Graphene, a single atomic plane of graphite, is ideal for the fabrication of sensitive nanosensors and biosensors due to its advantageous physical and electrochemical properties like large surface area, electrical conductivity, high electron transfer rate etc.50 Graphene synthesis techniques can be roughly divided into two main categories: top-down (destruction) Fig. 4 and bottom-up (construction) as shown in Fig. 5.49 The top-down method involves exfoliating graphite or its derivatives to create nano-sized graphene sheets. Mechanical exfoliation, liquid phase exfoliation, arc discharge, and oxidative exfoliation-reduction are common exfoliating methods. In the bottom-up technique, graphene and its derivatives are formed using carbon precursors other than graphite. These nano-scale structures are formed by controlled deposition of materials using methods like chemical vapor deposition (CVD), substrate-free gas-phase synthesis, and epitaxial growth (Fig. 6).47
 | ||
| Fig. 4 Nanomaterials with different morphologies: (a) silica nanoparticles, (b) Au decorated ZnO rods, (c) MWCNTs, (d) 1D carbon nanofibers, (e) graphene oxide nanosheets, and (f) Ni crossed nanowires. Reproduced from open access article ref. 44 with permission from the Royal Society of Chemistry, 2024. | ||
 | ||
| Fig. 5 Graphene lines-based flexible transistor/hydroxypropyl cellulose (HPC) photonic thin film with a hexagonal nanopillar structure: (a) a schematic of the fabrication of the Cytop/Si mold; (b) simple procedures for transferring graphene lines from the Cytop/Si mold to the polyethylene terephthalate (PET) flexible substrate; (c) an image of flexible electrolyte-gated transistor (EGT) arrays, the magnified picture shows the composition of each transistor; (d and e) the transfer and output characteristics of the graphene electrodes. Reproduced from ref. 51with permission from ACS Nano, 2017. (f) Images of an HPC photonic crystal (top) and its mechanical flexibility with a free-standing property of the design (bottom); (g) schematics of two fabrication processes to make HPC photonic films-hot embossing and replica molding methods. The blue-coloured slide indicates the glass substrate, the green one for HPC, and the brown one for hard polydimethylsiloxane (h-PDMS); (h) special hexagonal nanopillar images obtained by scanning electron microscopy (SEM) in the lateral view. Paper substrates are used to imprint the predesigned nanopattern. Reproduced from ref. 51 with permission from Springer Nature, 2018. | ||
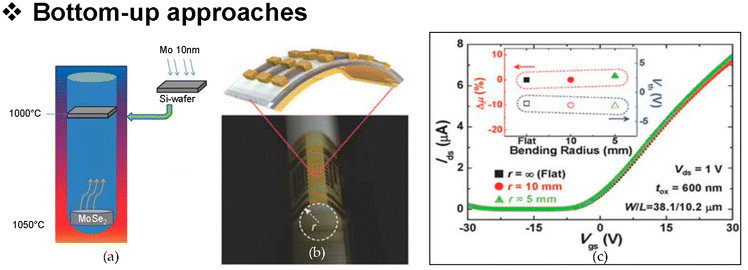 | ||
| Fig. 6 High-mobility transistors based on chemical vapor deposition (CVD)-grown MoSe2; (a) schematic of the synthesis of MoSe2 film with modified CVD method; (b) optical image of bended MoSe2 transistor; and (c) I–V characteristic of unbent and bent (up to 5 mm of radius) MoSe2 transistor. Reproduced from ref. 50 with permission from John Wiley and Sons, 2016. | ||
With their simple chemical makeup and atomic bonding configuration, carbon nanotubes (CNTs) are among the best examples of nanomaterials. Despite their simple composition, they might have the greatest structural and property diversity of all nanomaterials.52 CNTs are derived from rolled graphene planes and exist as single-walled carbon nanotube (SWCNT) or multi-walled carbon nanotube (MWCNT) structures, where the former is composed of a single cylindrical carbon layer, and the latter is composed of multiple co-axial cylindrical layers.53
In recent years, carbon nanotube fabrication has been done by various methods like arc discharge, laser ablation, chemical vapor deposition (CVD), and plasma-enhanced CVD (PECVD).54 Which of the two systems is better at this point is unclear: while MWCNTs have a larger surface that enables more effective internal encapsulation and external functionalization, SWCNTs have an extra photoluminescence feature that could be effectively employed in diagnostics.52 In the medical field, a great deal of research is being done to create novel CNT biomaterials that can be used for disease diagnosis and treatment. For instance, the use of CNTs in drug delivery systems (DDSs) for cancer treatment, hyperthermia management, and in vivo imaging is still being studied.53
Due to their optoelectronic properties—strong Stokes shifts, high fluorescence quantum yields, minimal photobleaching, and confined emission bands—quantum dots (QDs) as a nanomaterial have also attracted a great deal of attention.45 The striking variations in optical absorbance, exciton energies, and electron–hole pair recombination at varying particle sizes are the most intriguing features of QDs. Because these QDs’ characteristics’ intrinsic qualities depend on a variety of parameters, including size, shape, defect, impurities, and crystallinity, using them requires a high degree of control during their synthesis.55 The design of pH-sensitive QDs with organic ligands is made possible by QDs and has great promise for a range of analytical uses, particularly in the creation of luminous chemo-sensors and analyte-induced variations in quantum dot photoluminescence for ion sensing is also an active subject of study.56
5.2. Sensor design and characteristics
There is a vast array of nanosensors with different nanomaterials available, each with unique designs and characteristics. One such characteristic is the high surface-to-volume ratio that nanomaterials, especially graphene, display, which directly correlates with the increased sensitivity of sensors based on them. More binding sites are available due to the larger surface area, which improves the detection capabilities of the nano-sensors.49 Biochemical signals can be converted into detectable electrical signals through effective electron transfer made possible by the electrical conductivity of nanomaterials like nanotubes and quantum dots. Optical characteristics in quantum dot-based sensors identify changes in light absorption or emission and can be used to detect and quantify analytes using optical sensing techniques. All these properties of nanomaterials combine to enhance the sensitivity and performance of nanosensors. This increased sensitivity allows for the identification of minute and subtle changes in biomarker concentrations that may point to the existence of medical diseases, which shows great promise for early illness detection.57 A material property that changes with temperature is the basis for how temperature sensors work. These could be attributes of light, volume, or resistance. The property of interest fluctuates in response to variations in the surrounding temperature.58Although there is currently minimal review of the accuracy of the materials used in wearable health sensors, a variety of materials have been used for the detection of various health parameters. The statistical difference between recorded and actual data defines this accuracy. Furthermore, because the human body temperature fluctuates very little, wearable sensors need to be mechanically flexible and biocompatible in addition to having excellent precision, sensitivity, and resolution.59 Since the sensors must maintain sustained contact with the body for lengthy periods of time, it is crucial to select nanomaterials that exhibit great biocompatibility in order to guarantee that the sensors can interact with biological systems without producing adverse reactions or injury to the human body. Highly biocompatible nanomaterials are those with low immunological reactions and negligible cytotoxic consequences.57 The sensor's sustainable life is also determined by its durability. Flexibility qualities are now restricted to substrate materials and sensitive components. Adding external packaging to the flexible device can help increase longevity by limiting physical damage and exposure of the inner components.60 Multiplexing is another remarkable property of nanomaterial-based sensors, enabling them to detect several biomolecules at once. The functionalization of the sensors with receptors or ligands that bind to certain target biomolecules selectively allows for the achievement of this capability. Through the integration of these receptors or ligands, the sensors are able to concurrently record and measure multiple physiological indicators, offering all-encompassing health monitoring.57 Humidity sensing nano-devices can be easily attached under the nose of an individual and measure respiration-related signals such as temperature, humidity, and airflow rate, which can be correlated with respiratory diseases.58
The working of nanosensors is dependent on changes in physiological or mechanical conditions. The detection of carbon nanotube-based sensors is dependent on sensing processes such as humidity, temperature, and the piezoresistive effect.49 Flexible force-sensitive sensors may translate mechanical inputs, such as tension, pressure, torque, vibration, stress, and strain, among others, into electrical parameters using a number of representative sensing techniques.60
5.3. Miniaturization and integration with wearable devices
The light weight design and compact dimensions of wearable nano-sensors allow for easy integration with wearable devices. Furthermore, the high sensitivity, quick response, and multi-parameter detection capabilities of nanosensors enable thorough monitoring.4 These sensors can be manufactured at micro- or even nano-scale sizes, which makes them exceedingly small and flexible, allowing for easy integration into a variety of wearable gadgets, including fitness trackers, smartwatches, and biomedical patches.58 The wearable device's aesthetics are improved by this shrinking, which also guarantees that the sensors will not impede daily activities or create discomfort after extended use. Because of their flexibility, the nanomaterials utilized in these sensors can create a tight, conformal contact with the skin by adapting to its contours. For measurements to be precise and trustworthy, this conformal contact is necessary. The resistance and endurance of the sensor are other benefits of the flexibility of nanoparticles. The sensors’ long-term performance and dependability are ensured by the flexible nature of nanomaterials, which enables them to bear these mechanical forces without suffering damage.57 The indicators that need to be found in a health evaluation by wearable sensors attached to the human body fall into the following categories: vital signs: heart rate, pulse, blood pressure, breathing, etc.; bodily movements: hands, arms, knees, etc.61 The interior health of the human body have also been monitored via implantable sensors in a recent study. Implantable sensors are often smaller, more biocompatible, and biodegradable than wearable ones. Implantable electronics are more often used for adjuvant therapy and physiological parameter monitoring.605.4. Wireless communication and data transmission
In the realm of wearable health monitoring, the combination of wireless communication with sensors based on nanomaterials has proven to be a crucial breakthrough. The seamless transfer of data to phones and other portable devices is made possible by this connection. A flexible electronic board manages the raw data gathered from sensors and oversees directing sensor functioning and sending data in real-time.62 Monitoring of vital signs, biomarkers, and other physiological indicators can be done continuously due to the immediate data transmission capability. People can obtain quick insights into their health status due to this real-time feedback.57 An effective connection and communication infrastructure are necessary to gather and process data from the nano-sensors. For analysis and interpretation, the sensor data must be sent from the sensors to a central monitoring system. Data transmission can be accomplished by a variety of physical connections, including wired connections like Ethernet and fiber optic cables, or wireless technologies Table 2, including Bluetooth, Wi-Fi, and cellular networks.49 The development of wireless connectivity makes it possible for remote and individualized healthcare monitoring, giving people access to the most recent data and facilitating preventative actions for improved health management.57| Technology | Maximum coverage range | Power consumption | Data transmission rate | Frequency |
|---|---|---|---|---|
| IEEE 802.11 | 150 m | High | 54 Mbps | 2.4 GHz |
| ZigBee | 300 m | Low | Max. 250 Kbps | 868 MHz/902–928 MHz/2.4 GHz |
| ISA100.11a | 150 m | Low | Max. 250 Kbps | 2.4 GHz |
| Bluetooth | 300 m | Medium | Max. 2 Mbps | 2.4 GHz |
5.5. Real-time monitoring and data collection
Continuous and real-time data monitoring by nano-sensors allows preventive maintenance procedures, which maximize resource allocation and reduce potential health hazards. One trend in sensor functioning that is expanding quickly is the incorporation of nano-sensors into the Internet of Things (IoT) framework. It is possible to accomplish real-time data collection, transmission, and analysis by joining nano-sensors to a network infrastructure.49 In order to transform measured data into information, data analysis of data gathered from wired and wireless sensor networks is required. The features can be extracted and interpreted using a variety of techniques and algorithms. The benefits and drawbacks of various approaches vary widely and are largely dependent on the kind of data gathered from sensors like strain gauges, displacement, velocity, accelerometers, or ultrasonic transducers.635.6. Power sources and supply
For nanosensors to function properly, a steady and dependable power source is needed.The type of sensor, its placement, and the accessibility of power sources within the sensor infrastructure all influence the power supply issues. Batteries or energy-harvesting methods like solar cells, thermoelectric generators, vibrational energy harvesters, and piezoelectric vibration generators can be used to power embedded nano-sensors.64 Sensors that run on batteries provide mobility and independence from other power sources, but they also need to be replaced and maintained on a regular basis.49 Energy harvesting is one workable solution to the battery's low energy storage capacity. It involves gathering energy from the environment, such as vibration, radiofrequency, and renewable energy sources, among others, converting it into electricity, and putting it in rechargeable batteries for later use.63
One of the most widely used techniques for extending the battery life or eliminating the battery entirely from a wireless body area network is micro-scale energy scavenging. To assess if the associated scavenging technology is appropriate for a certain application, it is necessary to determine the sources of energy that energy scavenging circuits may access. Electrical energy can generally be produced by solar, thermal, mechanical motion or vibration, and ambient radiofrequency (RF) sources.65
6. Open issues
Even though remarkable advancements have been made in the field of nanosensor research and its successful application across diverse domains of healthcare, several critical issues and knowledge gaps remain, affecting the transition from lab innovations to widespread clinical integration.1. Regulatory approval and standardization: currently, there is no universally recognized regulatory framework for the quality assurance, standardization, and validation of nanosensor based medical devices. Consistent and reproducible clinical results are very few in number due to variations in composition, sensor calibration, and performance across various nanosenors.
2. Biocompatibility and long-term stability: although many nanosensors exhibit high levels of sensitivity and specificity in vitro, very little is known about their long-term biocompatibility, degradation, and reactions to biological systems in vivo. This raises concerns about long-term reliability and material toxicity when it comes to wearable technology or chronic implantation devices.
3. Data processing and integration with digital health ecosystems: nanosensors are increasingly integrated into medical devices, they generate very high-volumes and frequency of physiological data. However, the current healthcare infrastructure lacks proper techniques for handling such data with the existing healthcare system in a secure and interpretable format.
4. Scalability and cost-efficiency: fabrication of nanosensors often involves sophisticated procedures and require intensively trained personnel. Scaling up production while maintaining precision, functionality, and affordability remains a problem, particularly in limited healthcare resource settings or for global public health application.
5. Ethical, legal, and privacy concerns: the collection and transmission of sensitive physiological data via nanosensor-integrated systems introduce privacy and ethical concerns. They need to establish data governance models that ensure informed consent, secure transmission, and storage of patient data is paramount.
Addressing these open issues through interdisciplinary research, regulatory collaboration, and inclusive innovation models is critical for realizing the full potential of nanosensors in delivering equitable, scalable, and personalized healthcare solutions.
7. Conclusion and future outlook
The field of healthcare monitoring has entered a transformative era, driven by the integration of nanotechnology, particularly through the development of nanosensors. These advanced devices offer unprecedented sensitivity, specificity, and real-time monitoring capabilities, addressing the increasing demands of modern healthcare. Nanosensors have demonstrated their potential in various applications, from early-stage disease detection to continuous physiological monitoring, offering more accurate diagnostics and personalized treatment options. The unique properties of nanoparticles, such as their size, surface adaptability, and biocompatibility, have provided a solid foundation for creating cutting-edge solutions in diagnostics, bioimaging, and point-of-care devices.Despite the significant progress made, challenges remain in terms of scalability, long-term stability, and regulatory approval for widespread clinical use. The complexity of integrating nanosensors into existing healthcare systems, ensuring cost-effectiveness, and overcoming potential biocompatibility issues also presents hurdles that need to be addressed.
Looking forward, the future of nanosensors in healthcare monitoring appears promising. Continued advancements in nanomaterial synthesis, biocompatibility, and sensor miniaturization will likely drive further innovations. The convergence of nanotechnology with other fields, such as artificial intelligence, machine learning, and big data analytics, holds the potential to create intelligent healthcare systems capable of providing predictive diagnostics, automated monitoring, and even personalized treatment regimens. As research and development continue to push the boundaries of nanosensor technology, the future of healthcare will increasingly rely on these innovations to offer more efficient, accurate, and accessible medical solutions to meet the growing demands of a fast-paced, technologically driven environment, where early diagnosis, personalized treatment, and continuous health monitoring are essential.
Conflicts of interest
The authors declare no conflict of interest.Abbreviations
| AgNPs | Silver nanoparticles |
| AI | Artificial intelligence |
| AMR | Anisotropic magnetoresistance |
| AuNPs | Gold nanoparticles |
| BDA | Big data analytics |
| BioI | Bioimaging |
| Bios | Biosensors |
| CdTe QDs | Cadmium telluride quantum dots |
| ChemS | Chemical sensors |
| CNTs | Carbon nanotubes |
| CQDs | Carbon quantum dots |
| CT | Computed tomography |
| CTCs | Circulating tumour cells |
| CVD | Cardiovascular diseases |
| DDSs | Drug delivery systems |
| Dx | Diagnostics |
| ECG | Electrocardiography |
| EGT | Electrolyte-gated transistor |
| FECA | Foreign element catalytic agent |
| GFP | Green fluorescent protein |
| GMI | Giant magnetoimpedance |
| GMR | Giant magnetoresistance |
| HeAptDNA-SWCNT | Hemin aptamer DNA – single-walled carbon nanotube |
| HER | Electronic health record |
| HPC | Hydroxypropyl cellulose |
| h-PDMS | Hard polydimethylsiloxane |
| IoT | Internet of things |
| MEMS | Miniaturized mechanical and electro-mechanical elements |
| ML | Machine learning |
| MNPs | Magnetic nanoparticles |
| MRI | Magnetic resonance imaging |
| MSc | Materials science |
| MTJs | Magnetic tunnel junctions |
| MWCNTs | Multi walled carbon nanotube |
| NIR | Near Infrared |
| NPs | Nanoparticles |
| NSs | Nanosensors |
| NT | Nanotechnology |
| PECVD | Plasma enhanced CVD |
| PET | Polyethylene terephthalate |
| PhysS | Physical sensors |
| POC | Point-of-care |
| QDs | Quantum dots |
| R&D | Research and development |
| RF | Radiofrequency |
| SEM | Scanning electron microscopy |
| SERS | Surface-enhanced Raman spectroscopy |
| SPR | Surface plasmon resonance |
| SWCNTs | Single-walled carbon nanotubes |
| TMR | Tunnel magnetoresistance |
| VLS | Vapor–liquid–solid |
| WHO | World Health Organization |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.References
- A. Munawar, Y. Ong, R. Schirhagl, M. A. Tahir, W. S. Khan and S. Z. Bajwa, RSC Adv., 2019, 9, 6793–6803 RSC.
- M. A. Darwish, W. Abd-Elaziem, A. Elsheikh and A. A. Zayed, Nanoscale Adv., 2024, 6, 4015–4046 RSC.
- S. S. Agasti, S. Rana, M.-H. Park, C. K. Kim, C.-C. You and V. M. Rotello, Adv. Drug Delivery Rev., 2010, 62, 316–328 CrossRef CAS PubMed.
- J. B. Raval, V. N. Mehta, S. Jha, R. K. Singhal, H. Basu and S. K. Kailasa, Sens. Diagn., 2023, 2, 815–836 RSC.
- R. Das, S. Nag and P. Banerjee, Molecules, 2023, 28, 1259 CrossRef CAS.
- Y. Tian, G. Xu, K. Cai, X. Zhao, B. Zhang, L. Wang and T. Wang, Nanoscale, 2023, 15, 80–91 RSC.
- Y. Tian, G. Xu, K. Cai, X. Zhao, B. Zhang, L. Wang and T. Wang, Nanoscale, 2023, 15, 80–91 RSC.
- H. Meskher, H. C. Mustansar, A. K. Thakur, R. Sathyamurthy, I. Lynch, P. Singh, T. K. Han and R. Saidur, Nanoscale Adv., 2023, 5, 992–1010 RSC.
- S. Kumar, H. K. Sidhu, A. K. Paul, N. Bhardwaj, N. S. Thakur and A. Deep, Sens. Diagn., 2023, 2, 1390–1413 RSC.
- X. Liu, S. Cao, Y. Gao, S. Luo, Y. Zhu and L. Wang, Chem. Commun., 2023, 59, 3957–3967 RSC.
- P. Pandit, B. Crewther, C. Cook, C. Punyadeera and A. K. Pandey, Mater. Adv., 2024, 5, 5339–5350 RSC.
- D. Zhang, T. Luo, X. Cai, N. Zhao and C. Zhang, Chem. Commun., 2024, 60, 4745–4764 RSC.
- B. Jin, Z. Guo, Z. Chen, H. Chen, S. Li, Y. Deng, L. Jin, Y. Liu, Y. Zhang and N. He, J. Mater. Chem. B, 2023, 11, 1609–1627 RSC.
- T. Blachowicz, I. Kola, A. Ehrmann, K. Guenther and G. Ehrmann, Micro, 2024, 4, 206–228 CrossRef.
- E. Hoque Apu, M. Nafiujjaman, S. Sandeep, A. V. Makela, A. Khaleghi, S. Vainio, C. H. Contag, J. Li, I. Balasingham, T. Kim and N. Ashammakhi, Mater. Chem. Front., 2022, 6, 1368–1390 RSC.
- K. Y. Baek, S. Kim and H. R. Koh, Mol. Cells, 2022, 45, 26–32 CrossRef CAS.
- What is QCM?, https://www.biolinscientific.com/blog/what-is-qcm, (accessed 26 July 2024).
- K. R. Khondakar, M. S. Anwar, H. Mazumdar and A. Kaushik, Mater. Adv., 2023, 4, 4991–5002 RSC.
- A. Mishra, P. K. Singh, N. Chauhan, S. Roy, A. Tiwari, S. Gupta, A. Tiwari, S. Patra, T. R. Das, P. Mishra, A. S. Nejad, Y. K. Shukla, U. Jain and A. Tiwari, Sens. Diagn., 2024, 3, 718–744 RSC.
- K. R. Khondakar, M. S. Anwar, H. Mazumdar and A. Kaushik, Mater. Adv., 2023, 4, 4991–5002 RSC.
- S. Agrawal, Int. J. Pharm. Sci. Nanotechnol., 2012, 4, 1528–1535 Search PubMed.
- M. Khazaei, M. S. Hosseini, A. M. Haghighi and M. Misaghi, Sens. Bio-Sens. Res., 2023, 41, 100569 CrossRef.
- R. Sharma, S. J. Borah, Bhawna, S. Kumar, A. Gupta, V. Kumari, R. Kumar, K. K. Dubey and V. Kumar, Mater. Adv., 2023, 4, 3091–3113 RSC.
- Y. Liu, C. Li, X. Yang, B. Yang and Q. Fu, Biomater. Sci., 2024, 12, 3805–3825 RSC.
- L. Li, T. Wang, Y. Zhong, R. Li, W. Deng, X. Xiao, Y. Xu, J. Zhang, X. Hu and Y. Wang, J. Mater. Chem. B, 2024, 12, 1168–1193 RSC.
- M. C. Larkins and A. Thombare, in StatPearls, StatPearls Publishing, Treasure Island, FL, 2024 Search PubMed.
- WHO, Cancer, World Health Organization, Geneva, Switzerland, 2022 Search PubMed.
- E. Salvati, F. Stellacci and S. Krol, Nanomedicine, 2015, 10, 3495–3512 CrossRef CAS.
- A. N. Bhatt, R. Mathur, A. Farooque, A. Verma and B. S. Dwarakanath, Indian J. Med. Res., 2010, 132, 129 CAS.
- T. An, S. Qin, Y. Xu, Y. Tang, Y. Huang, B. Situ, J. M. Inal and L. Zheng, J. Extracell. Vesicles, 2015, 4, 27522 CrossRef.
- X. Liu, Y. Hu and F. Stellacci, Small, 2011, 7, 1961–1966 CrossRef CAS.
- P. D. Howes, R. Chandrawati and M. M. Stevens, Science, 2014, 346, 1247390 CrossRef.
- H. H. Jing, F. Bardakci, S. Akgöl, K. Kusat, M. Adnan, M. J. Alam, R. Gupta, S. Sahreen, Y. Chen, S. C. B. Gopinath and S. Sasidharan, J. Funct. Biomater., 2023, 14, 27 CrossRef CAS PubMed.
- F. Sabir, M. Barani, M. Mukhtar, A. Rahdar, M. Cucchiarini, M. N. Zafar, T. Behl and S. Bungau, Chemosensors, 2021, 9, 67 CrossRef CAS.
- R. M. El-Shabasy, M. Zahran, A. H. Ibrahim, Y. R. Maghraby and M. Nayel, Mater. Adv., 2024, 5, 1772–1782 RSC.
- International Diabetes Federation, IDF Diabetes Atlas, Brussels, Belgium, 10th edn, 2021 Search PubMed.
- J. Jeevanandam and M. K. Danquah, in Nanofabrication for Smart Nanosensor Applications, ed. K. Pal and F. Gomes, Elsevier, 2020, pp. 187–228 Search PubMed.
- K. J. Cash and H. A. Clark, Trends Mol. Med., 2010, 16, 584–593 CrossRef CAS.
- M. David, M. M. Barsan, M. Florescu and C. M. A. Brett, Electroanalysis, 2015, 27, 2139–2149 CrossRef CAS.
- A. K. Yagati, Y. Choi, J. Park, J.-W. Choi, H.-S. Jun and S. Cho, Biosens. Bioelectron., 2016, 80, 307–314 CrossRef CAS.
- H. Wu, R. Nißler, V. Morris, N. Herrmann, P. Hu, S.-J. Jeon, S. Kruss and J. P. Giraldo, Nano Lett., 2020, 20, 2432–2442 CrossRef CAS PubMed.
- A. Shafiee, E. Ghadiri, J. Kassis and A. Atala, Nanomedicine, 2019, 14, 2735–2747 CrossRef CAS.
- M. Rabbani, M. E. Hoque and Z. B. Mahbub, in Nanofabrication for Smart Nanosensor Applications, ed. K. Pal and F. Gomes, Elsevier, 2020, pp. 163–186 Search PubMed.
- M. A. Darwish, W. Abd-Elaziem, A. Elsheikh and A. A. Zayed, Nanoscale Adv., 2024, 6, 4015–4046 RSC.
- Y. Khalil and A. E. D. Mahmoud, Journal of Contemporary Healthcare Analytics, 2023, 7, 126–144 Search PubMed.
- A. A. Iqbal, N. Sakib, A. K. M. P. Iqbal and D. M. Nuruzzaman, Materialia, 2020, 12, 100815 CrossRef CAS.
- S. N. Mohammad, Nano Lett., 2008, 8, 1532–1538 CrossRef CAS.
- D. Han, H. Hosamo, C. Ying and R. Nie, Appl. Sci., 2023, 13, 11149 CrossRef CAS.
- C. I. L. Justino, A. R. Gomes, A. C. Freitas, A. C. Duarte and T. A. P. Rocha-Santos, TrAC, Trends Anal. Chem., 2017, 91, 53–66 CrossRef CAS.
- K. Kang, Y. Cho and K. Yu, Micromachines, 2018, 9, 263 CrossRef PubMed.
- H. Dai, Acc. Chem. Res., 2002, 35, 1035–1044 CrossRef CAS.
- N. Saito, H. Haniu, Y. Usui, K. Aoki, K. Hara, S. Takanashi, M. Shimizu, N. Narita, M. Okamoto, S. Kobayashi, H. Nomura, H. Kato, N. Nishimura, S. Taruta and M. Endo, Chem. Rev., 2014, 114, 6040–6079 CrossRef CAS PubMed.
- H. Dai, Acc. Chem. Res., 2002, 35, 1035–1044 CrossRef CAS PubMed.
- D. Bera, L. Qian, T.-K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260–2345 CrossRef CAS.
- M. F. Frasco and N. Chaniotakis, Sensors, 2009, 9, 7266–7286 CrossRef CAS.
- A. Nyabadza, M. Vázquez, S. Coyle, B. Fitzpatrick and D. Brabazon, Appl. Sci., 2021, 11, 8563 CrossRef CAS.
- S. Nasiri and M. R. Khosravani, Sens. Actuators, A, 2020, 312, 112105 CrossRef CAS.
- M. Cheng, G. Zhu, F. Zhang, W. Tang, S. Jianping, J. Yang and L. Zhu, J. Adv. Res., 2020, 26, 53–68 CrossRef CAS PubMed.
- Y. Liu, H. Wang, W. Zhao, M. Zhang, H. Qin and Y. Xie, Sensors, 2018, 18, 645 CrossRef.
- S. Yao, P. Swetha and Y. Zhu, Adv. Healthcare Mater., 2018, 7, 1700889 CrossRef CAS PubMed.
- U.-O. Dorj, M. Lee, J. Choi, Y.-K. Lee and G. Jeong, J. Sens., 2017, 2017, 1–9 CrossRef.
- S. Mustapha, Y. Lu, C.-T. Ng and P. Malinowski, Vibration, 2021, 4, 551–585 CrossRef.
- S. M. Demir, F. Al-Turjman and A. Muhtaroglu, IEEE Sens. J., 2018, 18, 6477–6488 Search PubMed.
- Z. L. Wang, Nano Res., 2008, 1, 1–8 CrossRef CAS.
- S. M. Demir, F. Al-Turjman and A. Muhtaroglu, IEEE Sens. J., 2018, 18, 6477–6488 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

