 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Asymmetric synthesis of C–N axially chiral carbazoles via axial-to-axial chirality transfer†
Sebastian
Myllek
 a,
Philip
Lencer
b,
Moritz K. T.
Klischan
a,
Philip
Lencer
b,
Moritz K. T.
Klischan
 a,
Birgit
Henssen
b,
Philipp
Neudecker
a,
Birgit
Henssen
b,
Philipp
Neudecker
 cd,
Martin
Breugst
cd,
Martin
Breugst
 *e and
Jörg
Pietruszka
*e and
Jörg
Pietruszka
 *ab
*ab
aInstitut für Bioorganische Chemie, Heinrich-Heine-Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, 52426 Jülich, Germany. E-mail: j.pietruszka@fz-juelich.de
bInstitut für Bio- und Geowissenschaften (IBG-1: Bioorganische Chemie), Forschungszentrum Jülich GmbH, Wilhelm-Johnen Straße, 52428 Jülich, Germany
cInstitut für Physikalische Biologie, Heinrich-Heine-Universität Düsseldorf, 40225 Düsseldorf, Germany
dIBI-7 – Strukturbiochemie, Forschungszentrum Jülich, 52425 Jülich, Germany
eInstitut für Chemie, Technische Universität Chemnitz, Straße der Nationen 62, 09111 Chemnitz, Germany. E-mail: martin.breugst@chemie.tu-chemnitz.de
First published on 16th April 2025
Abstract
C–N axially chiral carbazole derivatives were synthesised by an intramolecular Buchwald–Hartwig amination starting from C–C axially chiral biaryls utilising an axial-to-axial chirality transfer with enantiospecificities up to 91%. The mechanism of the chirality transfer was investigated via DFT calculations.
Axially chiral, N-arylated carbazoles are intriguing structural motifs that occur in natural products, chiral organocatalysts and phosphine ligands (Scheme 1a).1 So far, only a limited number of racemic and atroposelective syntheses have been reported in the literature, showcasing the relative scarcity of synthetic methods for this type of stereogenic C–N bond.2 Building on early reports on the synthesis of C–N axially chiral anilids via inter- or intramolecular Buchwald–Hartwig aminations, the development of atroposelective synthesis methods for C–N axially chiral biaryls has only recently gained momentum.3 Most of these methods follow one of three approaches: (I) de novo ring construction, (II) stereoselective functionalisation of C–N-linked biaryls or (III) direct formation of the stereogenic bond via aryl amination. Strategies following the first approach typically start from non-biaryl structures such as diarylamine precursors, which were successfully annulated via transition metal or Brønsted acid catalysis.4 The second approach focuses on establishing chirality via atroposelective ortho-functionalisation of achiral biaryls, while kinetic resolution and desymmetrisation were also reported.5 Other contributions include Rh-catalysed cycloadditions to alkynyl indoles and annulation of sulfoxonium ylides.6 The third concept consolidates C–N bond formation and establishment of axial chirality into a single step. Chiral Brønsted acids served as efficient catalysts for the asymmetric amination of azonaphthalenes and indolinone derivatives, while N-aryl-indolines and indolocarbazoles were accessed via transition metal catalysis.1a,2d,7 Recently, a C–C to C–N axial-to-axial chirality transfer was developed by Liu et al. in a Catellani-type reaction for the synthesis of C–N axially chiral phenanthridinones and later extended to other heterocycles.8 In this work, we have expanded the concept of axial-to-axial chirality transfer and developed an atroposelective synthesis of C–N axially chiral carbazoles starting from C–C axially chiral biaryls (Scheme 1b). Separating the formation of the C–C stereogenic bond and the chirality transfer into different reactions allowed us to elucidate the underlying principles and investigate the mechanism via DFT calculations.
 | ||
| Scheme 1 a) Axially chiral carbazoles; (b) axial-to-axial chirality transfer in carbazole synthesis. | ||
We started our investigations by computing the rotational barriers of different N-arylated carbazoles via DFT, identifying carbazoles 4, 5 and 8 as suitable chiral targets. With rotational barriers between 32 and 36 kcal mol−1, all carbazoles exceed the necessary barrier of 22.3 kcal mol−1 and have isomerisation half-lives of many years (Scheme 2).9 Interestingly, carbazole 8 showed a higher barrier than 1-methyl substituted derivatives 4 and 5. This can be attributed to a destabilised ground state due to repulsion between the C-1 methyl group and the N-aryl substituent, an effect previously described in the literature.8a,10
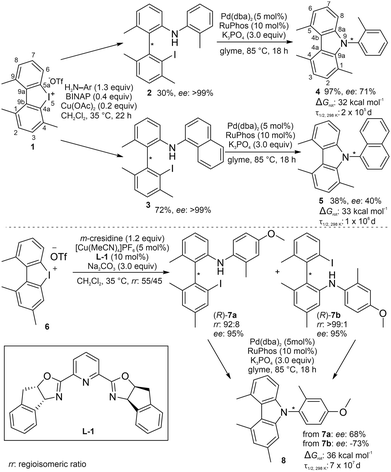 | ||
| Scheme 2 Synthesis of carbazoles 4, 5 and 8 starting from iodonium salts 1 and 6via C–C axially chiral biaryls 2, 3 and 7. ΔGrot: computed rotational barrier (ωB97M-V/def2-TZVPP//r2SCAN-3c);13τ1/2, 298 K: half-life at 298 K. | ||
Our synthetic strategy was based on a divergent approach utilising the copper-catalysed ring opening of strained iodonium salts as a branching point (Scheme 2).11 To utilise this route for the synthesis of C–N axially chiral carbazoles, the corresponding iodonium salt must not be C2-symmetric. Consequently, two regioisomers can result from the ring opening taking place at carbon C-4a or C-5a, respectively. Iodonium salt 1 was chosen as the first synthetic target, with the aim to sterically direct the ring opening towards carbon 5a via the C-4 substituent (Scheme 2). 1 was accessed in 59% total yield over 4 steps from commercial starting materials, as described by Zhao et al. (for full details, refer to the ESI†).11 Due to the sterically challenging nature of the ring opening of 1 using ortho-substituted anilines, a catalytic, enantioselective reaction was not successful.12 However, under high catalyst loadings of rac-BINAP-Cu(OAc)2, racemic biaryls 2 and 3 could be isolated in moderate to good yields and subsequently resolved using preparative chiral HPLC. Notably, in both cases, only one regioisomer was obtained. Subsequent intramolecular cyclisation under standard Buchwald–Hartwig conditions produced carbazoles 4 and 5 with 71% ee and 40% ee, respectively (Scheme 2).
Having demonstrated the viability of the chirality transfer, we directed our efforts towards the asymmetric construction of the key biaryls. To minimize steric hindrance in the ring opening reaction, iodonium salt 6 was chosen as an alternative branching point. [Cu(MeCN)4]PF6 in combination with PyBox-ligand L-1 was a suitable catalyst for the enantioselective ring opening, however, with low regioselectivity. Regioisomers 7a and 7b were obtained with 95% ee each, in a ratio of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45. m-Cresidine was used instead of o-toluidine to facilitate separation of the regioisomers using preparative HPLC. Cyclisation of 7a and 7b to carbazole 8 proceeded with similar efficiencies, but opposite selectivities in the chirality transfer. While 7a afforded (+)-8 with 68% ee, 7b yielded (−)-8 with 73% ee (Scheme 2). To assess whether the inverse enantioselectivity is a result of intrinsic enantiocomplementarity or if the regioisomers themselves are formed with opposite absolute configuration during the ring opening of 6, we assigned the absolute configuration of 7a and 7bvia CD spectroscopy. In both cases, the experimental CD spectrum showed good agreement with the computed spectra for (R)-7a and (R)-7b, which were obtained from TD-DFT calculations. As most biaryl precursors used in this study were not enantiopure (>99% ee), enantiospecificity (es) is used as a comparable measure to express the efficiency of the chirality transfer: es = (eeproduct/eestarting material) × 100%.14 For the synthesis of 8 starting from 7a and 7b, enantiospecificities of 72% and 77% are calculated. Correcting for the lower regioisomeric ratio of 7a compared to 7b (92
45. m-Cresidine was used instead of o-toluidine to facilitate separation of the regioisomers using preparative HPLC. Cyclisation of 7a and 7b to carbazole 8 proceeded with similar efficiencies, but opposite selectivities in the chirality transfer. While 7a afforded (+)-8 with 68% ee, 7b yielded (−)-8 with 73% ee (Scheme 2). To assess whether the inverse enantioselectivity is a result of intrinsic enantiocomplementarity or if the regioisomers themselves are formed with opposite absolute configuration during the ring opening of 6, we assigned the absolute configuration of 7a and 7bvia CD spectroscopy. In both cases, the experimental CD spectrum showed good agreement with the computed spectra for (R)-7a and (R)-7b, which were obtained from TD-DFT calculations. As most biaryl precursors used in this study were not enantiopure (>99% ee), enantiospecificity (es) is used as a comparable measure to express the efficiency of the chirality transfer: es = (eeproduct/eestarting material) × 100%.14 For the synthesis of 8 starting from 7a and 7b, enantiospecificities of 72% and 77% are calculated. Correcting for the lower regioisomeric ratio of 7a compared to 7b (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 vs. >99
8 vs. >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) an enantiospecificity of 81% is predicted for pure 7a, indicating small quantitative differences in the enantiospecificity, besides the previously discussed inverse selectivity.
1) an enantiospecificity of 81% is predicted for pure 7a, indicating small quantitative differences in the enantiospecificity, besides the previously discussed inverse selectivity.
We next proceeded with a screening of the intramolecular Buchwald–Hartwig amination. Due to the inverse enantioselectivity, facile separation of the biaryl regioisomers was required. Ring opening of chlorinated iodonium salt 9 yielded regioisomers 10a and 10b in a 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 ratio, which were separable via column chromatography (Scheme 3a) (for complete screening, see the ESI†). Starting with our initial catalyst system RuPhos-Pd(dba)2, a temperature screening from 65 °C to 85 °C showed only marginal effects on the es, while the yield deteriorated below 65 °C (Scheme 3b; entries 1–3). In contrast, the ligands had a much more pronounced effect on the es, and a positive correlation of enantiospecificity with steric bulk as well as an inverse correlation with electron richness was observed (entries 4–10). While electron-rich PCy3 produced only a marginal es of 9% (entry 4), P(4-CF3C6H4)3 was the best performing ligand with 88% es (entry 6). Buchwald-type and chelating ligands generally performed robustly in terms of yield and es. Interestingly, employing enantiopure chiral ligands had no effect on the es compared to the racemic ligand, and no matched or mismatched cases were observed (entries 7–9). The iPr-NHC ligand did not perform well in the reaction (entry 10). Switching the base to t-BuOK showed a small positive effect on yield and es (entry 11). In contrast, solvents had a pronounced influence, with toluene and 1,4-dioxane producing only 14% es and 54% es, respectively. DMF performed well, with 88% es (entries 12–14). Concluding the screening, we applied the optimized conditions to the syntheses of carbazoles 4, 5 and 8. While carbazoles 4 and 5 were synthesized with increased es of 84% (previously 71%) and 62% (previously 40%), respectively, 8 was formed with a lower es of 63% (previously 77%).
41 ratio, which were separable via column chromatography (Scheme 3a) (for complete screening, see the ESI†). Starting with our initial catalyst system RuPhos-Pd(dba)2, a temperature screening from 65 °C to 85 °C showed only marginal effects on the es, while the yield deteriorated below 65 °C (Scheme 3b; entries 1–3). In contrast, the ligands had a much more pronounced effect on the es, and a positive correlation of enantiospecificity with steric bulk as well as an inverse correlation with electron richness was observed (entries 4–10). While electron-rich PCy3 produced only a marginal es of 9% (entry 4), P(4-CF3C6H4)3 was the best performing ligand with 88% es (entry 6). Buchwald-type and chelating ligands generally performed robustly in terms of yield and es. Interestingly, employing enantiopure chiral ligands had no effect on the es compared to the racemic ligand, and no matched or mismatched cases were observed (entries 7–9). The iPr-NHC ligand did not perform well in the reaction (entry 10). Switching the base to t-BuOK showed a small positive effect on yield and es (entry 11). In contrast, solvents had a pronounced influence, with toluene and 1,4-dioxane producing only 14% es and 54% es, respectively. DMF performed well, with 88% es (entries 12–14). Concluding the screening, we applied the optimized conditions to the syntheses of carbazoles 4, 5 and 8. While carbazoles 4 and 5 were synthesized with increased es of 84% (previously 71%) and 62% (previously 40%), respectively, 8 was formed with a lower es of 63% (previously 77%).
 | ||
Scheme 3 a) Synthesis and resolution of substrates 10a and 10b; (b) screening conditions. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Conversion to product determined via1H-NMR. b Conversion to product determined via1H-NMR. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Enantiospecificity; for ee values, see the ESI.† Enantiospecificity; for ee values, see the ESI.† | ||
We next investigated the relationship between the structure of the biaryls and enantiospecificity of the cyclisation by synthesizing additional carbazole derivatives. As a logical extension to 4-chloro substituted 10a, the 4-fluoro derivative produced the corresponding carbazole 12 with a similar 86% es (Scheme 4). A fruitful approach was the introduction of a tosylate at position R1, which greatly facilitated the chromatographic separation of the corresponding biaryl regioisomers. Furthermore, cyclisation towards carbazole 13 showed the highest chirality transfer efficiency of 91%. Probing the effect of sterically demanding N-aryl substituents, 2′-ethyl and 2′-isopropyl substituted carbazoles 14 and 15 were accessed with reduced enantiospecificities of 44% es and 56% es, respectively. Concluding our synthetic studies, we explored the mechanism of the chirality transfer via DFT calculations. A plausible pathway is depicted in Scheme 5, following the generally accepted catalytic cycle of the Buchwald–Hartwig amination via oxidative addition, amine coordination, deprotonation, ligand exchange and reductive elimination.15 Our investigations were based on two hypotheses: (1) the reaction proceeds via two diastereomeric intermediates (Scheme 5: I-2a/I-2b), which form one or the other carbazole enantiomer. (2) These two intermediates interconvert via a C–N bond rotation (marked with °). If the interconversion of the intermediates (viaTSrot) is fast compared to carbazole formation, the chirality transfer proceeds under Curtin–Hammett conditions and the enantioselectivity is determined by the ΔΔG‡ of the corresponding transition states TSRE,1 and TSRE,2. Conformational analysis revealed intermediates I-2a and I-2b, with I-2a being slightly disfavoured by 0.3 kcal mol−1. TSrot was located at a dihedral angle of 3.3°, and a ΔG‡ of 11.7 kcal mol−1 was calculated with respect to I-2b. Two transition states were identified for reductive elimination. Starting from I-2a, TSRE,1 was located at a relative Gibbs energy of 14.7 kcal mol−1, while the corresponding TSRE,2 – starting from I-2b – had a higher energy of 16.7 kcal mol−1. From a ΔΔG‡1 of 3.0 kcal mol−1, it follows that the interconversion of I-2a and I-2b is more than 100-fold faster than the reductive elimination, indicating Curtin–Hammett conditions. The (S)-configuration is predicted for the main product, with an enatiomeric ratio (er) of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, matching the experimental absolute configuration of (S)-11 determined by CD spectroscopy (see the ESI†). The predicted er is in good agreement with the experimental distribution of 95
3, matching the experimental absolute configuration of (S)-11 determined by CD spectroscopy (see the ESI†). The predicted er is in good agreement with the experimental distribution of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of (S)-11 (assuming 90% es and enantiopure starting material).
5 of (S)-11 (assuming 90% es and enantiopure starting material).
 | ||
| Scheme 4 Scope of carbazole synthesis. es: enantiospecificity; for ee values, see the ESI.† | ||
 | ||
| Scheme 5 Plausible mechanistic pathway of the cyclisation and energy profile of the reductive elimination (ωB97M-V/def2-TZVPP/SMD(DMF)//TPSS-D4/def2-SVP).16 | ||
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Studienstiftung des deutschen Volkes e. V., the Heinrich Heine University Düsseldorf, the Forschungszentrum Jülich GmbH, the DFG (GRK2158 and BR 5154/4-1) and the Technische Universität Chemnitz for their continued support.References
- (a) W. Xia, Q.-J. An, S.-H. Xiang, S. Li, Y.-B. Wang and B. Tan, Angew. Chem., Int. Ed., 2020, 59, 6775–6779 CrossRef CAS PubMed; (b) K. B. Gan, R.-L. Zhong, Z.-W. Zhang and F. Y. Kwong, J. Am. Chem. Soc., 2022, 144, 14864–14873 CrossRef CAS PubMed; (c) C. Ito, Y. Thoyama, M. Omura, I. Kajiura and H. Furukawa, Chem. Pharm. Bull., 1993, 41, 2096–2100 CrossRef CAS; (d) Z. Xu, M. Baunach, L. Ding and C. Hertweck, Angew. Chem., Int. Ed., 2012, 51, 10293–10297 CrossRef CAS PubMed.
- (a) G. Bringmann, S. Tasler, H. Endress, J. Kraus, K. Messer, M. Wohlfarth and W. Lobin, J. Am. Chem. Soc., 2001, 123, 2703–2711 CrossRef CAS PubMed; (b) C. Börger, A. W. Schmidt and H.-J. Knölker, Synlett, 2014, 1381–1384 Search PubMed; (c) M. Zhang, P. Zhao, D. Wu, Z. Qiu, C. Zhao, W. Zhang, F. Li, J. Zhou and L. Liu, J. Org. Chem., 2023, 88, 2841–2850 Search PubMed; (d) Q. Ren, T. Cao, C. He, M. Yang, H. Liu and L. Wang, ACS Catal., 2021, 11, 6135–6140 CrossRef CAS.
- (a) O. Kitagawa, M. Takahashi, M. Yoshikawa and T. Taguchi, J. Am. Chem. Soc., 2005, 127, 3676–3677 Search PubMed; (b) O. Kitagawa, M. Yoshikawa, H. Tanabe, T. Morita, M. Takahashi, Y. Dobashi and T. Taguchi, J. Am. Chem. Soc., 2006, 128, 12923–12931 CrossRef CAS PubMed; (c) T. Hirata, I. Takahashi, Y. Suzuki, H. Yoshida, H. Hasegawa and O. Kitagawa, J. Org. Chem., 2016, 81, 318–323 CrossRef CAS PubMed.
- (a) A. Arunachalampillai, P. Chandrappa, A. Cherney, R. Crockett, J. Doerfler, G. Johnson, V. C. Kommuri, A. Kyad, J. McManus, J. Murray, T. Myren, N. Fine Nathel, I. Ndukwe, A. Ortiz, M. Reed, H. Rui, M. V. Silva Elipe, J. Tedrow, S. Wells, S. Yacoob and K. Yamamoto, Org. Lett., 2023, 25, 5856–5861 CrossRef CAS PubMed; (b) X. Wang, X.-J. Si, Y. Sun, Z. Wei, M. Xu, D. Yang, L. Shi, M.-P. Song and J.-L. Niu, Org. Lett., 2023, 25, 6240–6245 Search PubMed; (c) G. Zhang, B. Yang, J. Yang and J. Zhang, J. Am. Chem. Soc., 2024, 146, 5493–5501 Search PubMed; (d) P. Zhang, C.-Q. Guo, W. Yao, C.-J. Lu, Y. Li, R. S. Paton and R.-R. Liu, ACS Catal., 2023, 13, 7680–7690 CrossRef CAS; (e) P. Zhang, X.-M. Wang, Q. Xu, C.-Q. Guo, P. Wang, C.-J. Lu and R.-R. Liu, Angew. Chem., Int. Ed., 2021, 60, 21718–21722 CrossRef CAS PubMed.
- (a) M. E. Diener, A. J. Metrano, S. Kusano and S. J. Miller, J. Am. Chem. Soc., 2015, 137, 12369–12377 CrossRef CAS PubMed; (b) S. D. Vaidya, S. T. Toenjes, N. Yamamoto, S. M. Maddox and J. L. Gustafson, J. Am. Chem. Soc., 2020, 142, 2198–2203 CrossRef CAS PubMed; (c) H. M. Snodgrass, D. Mondal and J. C. Lewis, J. Am. Chem. Soc., 2022, 144, 16676–16682 CrossRef CAS PubMed; (d) Z.-J. Zhang, M. M. Simon, S. Yu, S.-W. Li, X. Chen, S. Cattani, X. Hong and L. Ackermann, J. Am. Chem. Soc., 2024, 146, 9172–9180 CrossRef CAS PubMed; (e) J. Zhang, Q. Xu, J. Wu, J. Fan and M. Xie, Org. Lett., 2019, 21, 6361–6365 CrossRef CAS PubMed; (f) U. Dhawa, T. Wdowik, X. Hou, B. Yuan, J. C. A. Oliveira and L. Ackermann, Chem. Sci., 2021, 12, 14182–14188 RSC; (g) L. Zhang, S.-H. Xiang, J. Wang, J. Xiao, J.-Q. Wang and B. Tan, Nat. Commun., 2019, 10, 566 CrossRef PubMed; (h) J. Wang, H. Chen, L. Kong, F. Wang, Y. Lan and X. Li, ACS Catal., 2021, 11, 9151–9158 CrossRef CAS.
- (a) P. Wang, H. Wu, X.-P. Zhang, G. Huang, R. H. Crabtree and X. Li, J. Am. Chem. Soc., 2023, 145, 8417–8429 CrossRef CAS PubMed; (b) P. Wang, Y. Huang, J. Jing, F. Wang and X. Li, Org. Lett., 2022, 24, 2531–2535 CrossRef CAS PubMed; (c) L. Zhou, Y. Li, S. Li, Z. Shi, X. Zhang, C.-H. Tung and Z. Xu, Chem. Sci., 2023, 14, 5182–5187 RSC; (d) X. Zhang, Q. Teng, Y. Ren, B. Wei, C.-H. Tung and Z. Xu, Org. Chem. Front., 2024, 11, 2857–2863 RSC.
- (a) J. Frey, A. Malekafzali, I. Delso, S. Choppin, F. Colobert and J. Wencel-Delord, Angew. Chem., Int. Ed., 2020, 59, 8844–8848 CrossRef CAS PubMed; (b) J. Rae, J. Frey, S. Jerhaoui, S. Choppin, J. Wencel-Delord and F. Colobert, ACS Catal., 2018, 8, 2805–2809 CrossRef CAS; (c) C. Qian, J. Huang, T. Huang, L. Song, J. Sun and P. Li, Chem. Sci., 2024, 15, 3893–3900 RSC.
- (a) Z.-S. Liu, P.-P. Xie, Y. Hua, C. Wu, Y. Ma, J. Chen, H.-G. Cheng, X. Hong and Q. Zhou, Chem, 2021, 7, 1917–1932 CrossRef CAS; (b) C. Wu, Z.-S. Liu, Y. Shang, C. Liu, S. Deng, H.-G. Cheng, H. Cong, Y. Jiao and Q. Zhou, Chin. J. Chem., 2024, 42, 699–704 CrossRef CAS.
- M. Ōki, in Topics in Stereochemistry, John Wiley & Sons Inc., 1983, pp. 1–81 Search PubMed.
- Y. Suzuki, I. Takahashi, Y. Dobashi, H. Hasegawa, C. Roussel and O. Kitagawa, Tetrahedron Lett., 2015, 56, 132–135 CrossRef CAS.
- K. Zhao, L. Duan, S. Xu, J. Jiang, Y. Fu and Z. Gu, Chem, 2018, 4, 599–612 CAS.
- X. Zhang, K. Zhao, N. Li, J. Yu, L.-Z. Gong and Z. Gu, Angew. Chem., Int. Ed., 2020, 59, 19899–19904 CrossRef CAS PubMed.
- (a) N. Mardirossian and M. Head-Gordon, J. Chem. Phys., 2016, 144, 214110 CrossRef PubMed; (b) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC; (c) S. Grimme, A. Hansen, S. Ehlert and J.-M. Mewes, J. Chem. Phys., 2021, 154, 064103 CrossRef CAS PubMed; (d) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS; (e) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- (a) D. Campolo, S. Gastaldi, C. Roussel, M. P. Bertrand and M. Nechab, Chem. Soc. Rev., 2013, 42, 8434–8466 RSC; (b) X.-L. Min, X.-L. Zhang, R. Shen, Q. Zhang and Y. He, Org. Chem. Front., 2022, 9, 2280–2292 RSC.
- (a) J. F. Hartwig, Synlett, 1997, 329–340 CrossRef CAS; (b) J. F. Hartwig, Synlett, 2006, 1283–1294 Search PubMed.
- (a) T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 Search PubMed; (b) J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 Search PubMed; (c) A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed; (d) E. Caldeweyher, C. Bannwarth and S. Grimme, J. Chem. Phys., 2017, 147, 034112 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00587f |
| This journal is © The Royal Society of Chemistry 2025 |
