 Open Access Article
Open Access ArticleSynthesis and biological evaluation of naphthoquinone-fused pyrrole and imidazopyridinedione heterocycles†
Rabindra Nath
Sana
ab,
Sudipta
Chakraborty
 c,
Vageesh
M.
c,
Vageesh
M.
 d,
Soumen
Adak
e,
Tanurima
Bhaumik
*b,
Raju
Dey
d,
Soumen
Adak
e,
Tanurima
Bhaumik
*b,
Raju
Dey
 *d and
Susanta Kumar
Manna
*d and
Susanta Kumar
Manna
 *a
*a
aDepartment of Chemistry, Bidhannagar College, Kolkata-700064, India. E-mail: Smanna19@gmail.com
bDepartment of Chemistry, Jadavpur University, Kolkata-700032, India. E-mail: tanurima.bhaumik@jadavpuruniversity.in
cDepartment of Microbiology, Government General Degree College, Narayangarh, West Bengal-721437, India
dDepartment of Chemistry, National Institution of Technology, Calicut-673601, India. E-mail: rajudey@nitc.ac.in
eDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, West, Bengal 741246, India
First published on 20th June 2025
Abstract
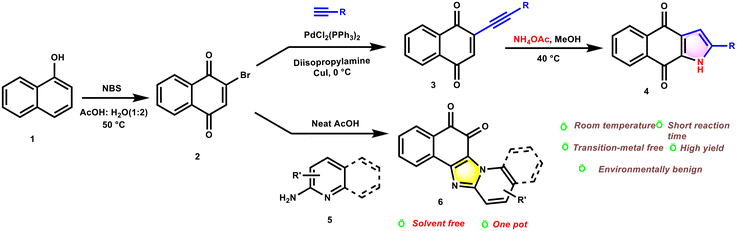
We have developed an efficient and straightforward synthetic route to obtain naphthoquinone-fused pyrrole derivatives (up to 93% yield) via an intramolecular cyclization of 2-(arylethynyl)naphthalene-1,4-dione derivatives with NH4OAc in methanol at ambient temperature. Furthermore, imidazopyridinedione derivatives were synthesized via coupling between 2-bromonaphthalene-1,4-dione and 2-amino pyridine through condensation and rearrangement under neat conditions in the presence of a catalytic amount of AcOH. A series of functionalized pyrrolonaphthoquinone and imidazopyridinedione derivatives were synthesized in high yields. Additionally, one of the synthesized pyrrolonaphthoquinones was selected as the model substrate for evaluating cytotoxicity and antimicrobial activity against both Gram-positive and Gram-negative biofilm bacteria.
Introduction
N-containing heterocycles fused with a quinone or naphthoquinone framework have received significant attention due to their wide range of biological and pharmacological activities, including anti-inflammatory,1 anticancer,2 anti-hyperproliferative, and antifungal activities.3a This motif is embedded in various natural and bioactive molecules.3b It also acts as a building block in the formation of many bioactive molecules. The pyrrole fused naphthoquinone utahmycin B, a natural product isolated from Streptomyces albus J1704, shows excellent antibiotic properties.4 3-Methyl-1H-benz[f] indole-4,9-dione (antiPAF) is a natural product obtained from the bark of G. tapis and G. uvaroides. This molecule has potential inhibitory effects against platelet-activating factor (PAF).5 Even recently, the synthesized molecule I (Fig. 1a) showed better cytotoxicity than the chemotherapy drug doxorubicin,6 while molecule II (Fig. 1a) showed specific cytotoxicity towards lung cancer cells, comparable to that shown by doxorubicin.7 Benzimidazole quinones exhibit cytotoxicity towards human skin fibroblast cells within the nanomolar range under hypoxic conditions (low pO2).8a Mitomycin C (Fig. 1b), a commercially available medicine, which has a pyrroloquinone moiety, has been effectively used as a chemotherapeutic drug.8b It prevents anal cancers, gastro-intestinal cancers (e.g. esophageal carcinoma) and breast cancers. Analogues of pyrrolo [1,2-a]benzimidazole (PBI) also act as antitumor agents.8c Thus, numerous synthetic methods have been developed to synthesize benzo[f]indole-4,9-dione derivatives.9 This inspired us to develop a convenient route to access naphthoquinone fused pyrrole and imidazopyridinedione derivatives (Scheme 1). Shvartsberg et al. developed a method for the synthesis of pyrrolonaphthoquinone, wherein a Sonogashira derivative bearing an acetylamine substituent undergoes intramolecular cyclization using K2CO3 in acetonitrile solvent at 80 °C (Scheme 2a).10 Annulation of naphthoquinones bearing acetylenic and amido substituents in the presence of ZnCl2 yielded bioactive 2-phenyl-1H-benzo[f]indole-4,9-dione (Scheme 2c).11 A mild strategy for constructing indolequinone motifs involved the Sonogashira reaction and a copper-catalyzed intramolecular cyclization (Scheme 2b).12 Maurya et al. documented the synthesis of highly functionalized naphthoquinones with 2,3-fused pyrrole through the coupling of α-azidochalcones with 2-hydroxy 1,4-naphthoquinone using Ru(bpy)3(PF6)2 as the catalyst under blue LED light irradiation (Scheme 2d).13 Here, the azide group serves as a source of pyrrole nitrogen. A novel, convenient, and economical protocol to prepare polyfunctionalized benzo[f]indole-4,9-diones catalyzed by Mn(II) acetate from vinyl azides and 2-hydroxynaphthoquinone has also been achieved (Scheme 2d).14 Very recently naphthoquinone with 3,4-fused pyrrole was synthesized by Deng et al., wherein they utilized naphthoquinone with aromatic aldehyde and ammonia as the nitrogen source.15 Starting from 2-substituted (attached to isoxazolones) 1,4-naphthoquinones, Broggini et al. synthesized pyrrolonaphthoquinones using [Ru(p-cymene)2Cl2]2 as the catalyst in DMSO at 100 °C.16 Here, the nitrogen of the pyrrole comes from the isoxazole ring. | ||
| Fig. 1 Representative examples of medicinally important naphthoquinone-fused pyrrole and benzimidazole fused quinone derivatives. | ||
 | ||
| Scheme 1 Outline of the synthesis of naphthoquinone-fused pyrrole and benzimidazole-fused quinone products. | ||
 | ||
| Scheme 2 State of the art in the synthesis of pyrrolonaphthoquinone and 1,2-naphthoquinone bearing fused-heterocycle rings. | ||
Choudhury et al. synthesized naphthoquinone-fused pyrroles attached to a pyrimidine moiety through a multicomponent reaction. The nitrogen from 2-amino-1,4-naphthoquinone is the source of the pyrrole nitrogen.17a Previous work also reports NaN3/NH4Cl mediated synthesis of pyrroles and isoquinolines through a transmolecular aza-exo-trig and aza-endo-dig cyclization of an aldehyde with an alkene or alkyne scaffold.17b Moreover, the synthesis of another interesting heterocycle benzimidazolequinone/imidazopyridinedione, was reported here by employing 2-bromo-1,4-naphthoquinone/2,3-dibromo-1,4-naphthoquinone and 2-aminopyridine under neat conditions with catalytic AcOH (Scheme 2i). Mosby and Boyle18a,b synthesized, for the first time, ortho-quinone fused benzimidazoles from 2,3-dichloro-1,4-naphthoquinone and 2-aminopyridine, and after that, several other groups18c–h have synthesized similar molecular frameworks by varying the reaction conditions. Anusevičius et al.18i,j and Castro et al.18k also developed this heterocyclic motif using various substituted o-aminopyridines and dichloronaphthoquinones/anthraquinones (Scheme 2f & g). Based on literature survey, this is the first documented synthesis of an o-quinone-fused imidazopyridine from 2-bromo naphthoquinone18f/2,3-dibromonaphthoquinone and o-aminopyridine (Scheme 1).
During our thorough literature survey, we did not come across any reports on the synthesis of naphthoquinone fused pyrroles in NH4OAc medium. Thus, in continuation of our synthetic efforts towards N-fused heterocycles,17b,19 herein, we report a NH4OAc promoted cyclization method for indoloquinone synthesis (Scheme 1, 2e).
Results and discussion
We prepared the starting materials from commercially available 1-naphthol 1. 1-Naphthol furnished bromonapthoquinone 2 by the treatment of NBS in an aqueous AcOH medium at 50 °C. The Sonogashira coupling of 2 with an aryl alkyne partner, in diisopropyl amine solvent at 0 °C, gave the desired 2-(arylethynyl) naphthalene-1,4-dione 3.20Furthermore, to optimize the reaction conditions, we carried out reactions using 2-(arylethynyl)naphthalene-1,4-dione 3a, varying the temperature, solvent, and reaction duration. Firstly, we carried out the reaction using NH4Cl as a nitrogen source and acetonitrile as the solvent under reflux conditions, obtaining the desired product in trace amounts (Table 1, entry 1). A similar result was observed when THF and DCM (Table 1, entries 2–4) were used as the reaction media. When methanol was used as a solvent along with an additive nitrogen source, the reaction proceeded to afford moderate to good product yields (Table 1, entries 5 and 6). Maximum yield was obtained when the reactions were carried out with NH4OAc as the nitrogen source in methanol at 40 °C for 30 minutes (Table 1, entry 7). However, alternative nitrogen sources like urea, (NH4)2SO4, and NH4SCN led to a decrease in the yield of the desired product (Table 1, entries 8–10). To establish the versatility of the protocol, the scope of the reaction was examined using different alkynyl substrates and the results are summarised in Table 2. The reaction proceeded smoothly with both electron-donating and -withdrawing substituents (Table 2). Even halogen-substituted and alkyl–alkynyl substrates furnished the corresponding desired products in moderate to good yields (Table 2compounds 4e, 4g and 4i).
| Entry | Medium | Additive | Temp. (°C) | Time | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 3a (1 mmol), additive (3 mmol), solvent (5 mL), stirred at the specified temperature for the required duration. b Yield refers to isolated yield. | |||||
| 1 | CH3CN | NH4Cl | Reflux | 8 h | Trace |
| 2 | THF | NH4PF6 | Reflux | 8 h | Trace |
| 3 | CH2Cl2 | (NH4)2CO3 | Reflux | 8 h | 21 |
| 4 | CH2Cl2 | NH4OAc | Reflux | 8 h | 25 |
| 5 | MeOH | (NH4)2CO3 | 40 °C | 4 h | 81 |
| 6 | MeOH | NH3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40 °C | 4 h | 80 |
| 7 | MeOH | NH 4 OAc | 40 °C | 30 min | 93 |
| 8 | MeOH | Urea | Reflux | 8 h | 25 |
| 9 | MeOH | (NH4)2SO4 | Reflux | 8 h | Trace |
| 10 | MeOH | NH4SCN | Reflux | 8 h | Trace |
The structures of the new molecules were characterized by 1H and 13C NMR and mass spectral analysis (see the ESI†). The formation of pyrrolonaphthoquinone derivatives could be explained by the nucleophilic addition of NH3 to the alkyne, followed by intramolecular Michael addition and subsequent oxidation to yield the desired molecule 4b (Scheme 3). Our designed molecule is analogous to the core structure of the natural product utahmycin B. In addition to NH4OAc-mediated synthesis towards pyrrolonaphthoquinones, we have also developed a method for the synthesis of benzimidazolequionones (Table 3). Herein, 2-aminopyridine 5b reacts with 2-bromo-1,4-naphthoquinone 2 under solvent-free conditions in the presence of catalytic amounts of AcOH to afford benzimidazolequionone 6b (Table 3, entry 10). Screening of other solvents such as acetonitrile, THF, and DCM gave trace amounts of the product under reflux conditions in the presence of CuI (Table 3, entries 1–3); however, polar solvents such as DMF, MeOH, n-butanol and ethoxyethanol furnished moderate product yields (Table 3, entries 4–7). Moreover, the use of AcOH in ethoxyethanol (entry 8) under neat conditions in the presence of CuI afforded 6b in moderate yield (entry 9). The reaction can be explained via the attack of the nucleophilic pyridyl nitrogen on the bromonaphthoquinone scaffold through a Michael addition followed by intramolecular cyclization of the carbonyl and amine, affording 6a′, which then undergoes hydrolysis followed by air oxidation to give the desired product 6a (Scheme 3).
| Entry | Medium | Catalyst | Temp. (°C) | Time (h) | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: 2 (1 mmol), 5 (1.1 mmol), AcOH (cat. amount), under neat conditions, heated in a preheated oil bath maintained at 100 °C for 8 h. b Yield refers to isolated yields of pure products characterized by 1H and 13C NMR and HRMS. | |||||
| 1 | CH3CN | CuI | Reflux | 12 | Trace |
| 2 | THF | CuI | Reflux | 12 | Trace |
| 3 | CH2Cl2 | CuI | Reflux | 12 | 21 |
| 4 | DMF | CuI | 90 | 12 | 40 |
| 5 | MeOH | CuI | Reflux | 8 | 43 |
| 6 | n-Butanol | CuI | 110 °C | 12 | 60 |
| 7 | Ethoxyethanol | CuI | Reflux | 12 | 53 |
| 8 | Ethoxyethanol | AcOH | Reflux | 12 | 62 |
| 9 | Neat | CuI | 100 °C | 12 | 62 |
| 10 | Neat | AcOH | 100 °C | 8 | 65 |
The applicability of this protocol was explored using different 2-aminopyridine derivatives and the results are summarized in Table 4 (compounds 6a–6h). A variety of tetracyclic and pentacyclic nitrogen-bridged imidazopyridine/imidazoquinoline fused o-quinones were furnished. The structures of the molecules were characterized by 1H and 13C NMR, 2D NMR and mass spectral analysis (see the ESI†). Single crystal X-ray analysis unambiguously confirmed the polycyclic framework of 6b (Fig. 2).
Biological studies
To investigate the biological properties of naphthoquinone fused pyrrole derivatives, we selected 4f, one of the synthesized compounds (Table 2), as the model compound, as 4f showed the lowest minimum inhibitory concentration (MIC) against the tested microorganisms S. aureus and E. coli (ESI Table S3†).Determination of minimum inhibitory concentration (MIC). We determined the antimicrobial activity of the compound by broth dilution assay. The compound in DMSO (70%, v/v %) at a concentration of 5 mg mL−1 was used as the working stock solution. Cultures of E. coli and S. aureus were incubated with different concentrations of the compound separately for 24 h, and then the optical density (OD) at 600 nm was recorded. The lowest concentration of the compound that can inhibit bacterial growth after a specified incubation period was determined as the MIC value of the compound against a specific bacterial species.21 ESI Fig. S1A and Table S3,† show the MIC values of the compound against S. aureus and E. coli. The results showed that the compound has potent antimicrobial activity against S. aureus with a MIC value of 120 μg mL−1 compared to E. coli (MIC = 200 μg mL−1) (see Fig. S1A†). Later, we determined the minimum bactericidal concentration (MBC) of the compound against S. aureus and E. coli.
The value of the MBC was found to be 180 μg mL−1 and 260 μg mL−1 for S. aureus and E. coli, respectively. We further intended to determine the MBC/MIC ratio of the compound against each tested microorganism to determine whether the compound is exhibiting bactericidal or bacteriostatic actions against them.19d Notably, the value of the MBC/MIC ratio was 1.5 and 1.3 for S. aureus and E. coli, respectively, which confirms that the compound exhibited bactericidal activity against the tested microorganisms; this activity is stronger against S. aureus than E. coli. The DMSO solvent concentration used in the experiments showed no antimicrobial activity.
Determination of zone of inhibition by agar diffusion assay. To further substantiate our results, we also tested the antimicrobial activity of the compound against S. aureus and E. coli separately in solid media using the agar diffusion assay. To determine this, different concentrations of the compound were added into wells on microbial lawns followed by incubation at 37 °C for 24 h. A clear zone appears due to the growth inhibition of sensitive microorganisms (see ESI Fig. S1B†). The diameter of the clear zone around the wells was measured and presented as a histogram (Fig. S1A†). It was found that the compound exhibits a strong antimicrobial response against the Gram-positive bacteria S. aureus at lower concentrations while a similar inhibitory effect at higher concentrations was observed both for Gram-positive (S. aureus) and negative (E. coli) bacteria (see ESI Fig. S1B† and Fig. 3A). Notably, where 60 μg mL−1 concentrations of the compound failed to produce any zone of inhibition in the E. coli plate, the same concentration produced a 10 mm zone in S. aureus, which must be considered as typical manifestation of selective antimicrobial effects (Fig. S1B† and Fig. 3A). From these two results, we can preliminarily conclude that the compound exhibited more promising antimicrobial action against Gram-positive bacteria than Gram-negative bacteria.
Compound 4f treated S. aureus cell show reduced growth: To further validate our results, we examined the effect of different sub-MIC doses of the compound on the growth kinetics of S. aureus and E. coli cells. For this, we compared the growth profile of the two bacterial species separately after treatment with different sub-MIC doses of the compound (1/8 MIC, 1/4 MIC, and 1/2 MIC). One set served as the untreated control. Aliquots of the microbial culture were collected aseptically from each set at regular intervals and the absorbance was measured at 600 nm.
The results demonstrated that there was no significant difference in the growth profiles of treated and untreated sets of E. coli cells (Panel A, Fig. 3B); in contrast, it was observed that S. aureus cells showed concentration-dependent growth inhibition (Panel B, Fig. 3B). Nearly 50% growth inhibition was observed after treatment with 1/2 MIC (60 μg mL−1) of the compound (compare Panel A with B, Fig. 3B). Collective data confirmed that the compound exhibited better antimicrobial efficacy towards S. aureus than E. coli. Thus, from these results, we can conclude the compound is more active towards Gram-positive bacteria compared to Gram-negative ones.
Conclusion
We have demonstrated a new straightforward methodology for the synthesis of various pyrrolonaphthoquinone derivatives via a unique cyclization between alkynylnaphthoquinone and ammonium acetate. We have also developed a synthetic route to obtain imidazopyridinedione derivatives via an unprecedented cyclization reaction. The compounds were characterized precisely by 1H-NMR, 13C-NMR, and HRMS. Additionally, the potential role of these types of molecules as antimicrobial agents against S. aureus and E. coli was established using 4f as the model compound. Surprisingly, this molecule exhibited low cytotoxicity against two mammalian cell lines: RAW 264.7 (murine macrophages) and human kidney epithelial cell (HEK 293). We anticipate that these molecules will exhibit significant medicinal properties due to the co-existence of both naphthoquinone and pyrrole pharmacophores.Experimental data
Procedure for the preparation of 2-bromonaphthalene-1,4-dione (2)
1.45 g (5 mmol) of 1-napthol was mixed with 7.12 g (20 mmol) of N-bromosuccinamide (NBS) in 50 mL glacial acetic acid and 100 mL water in a 500 mL round-bottom flask. The reaction mixture was then stirred for 45 minutes at 50 °C. The progress of the reaction was monitored by TLC, in comparison with the starting material. The product formed was extracted with ethyl acetate and water. It was purified by column chromatography with petroleum ether and ethyl acetate (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent.
1, v/v) as eluent.
Preparation of 2-(p-tolylethynyl)naphthalene-1,4-dione (3)
2-Bromo-1,4-naphthoquinone 2 (0.92 mmol, 220 mg) and phenylacetylene (0.143 g, 1.4 mmol, 1.5 equiv.) were mixed with CuI (19 mg, 0.1 mmol) and Pd(PPh3)2Cl2(35 mg, 0.05 mmol) catalyst in 10 mL THF. Diisopropylamine (0.281 g, 2.78 mmol, 3 eq.) was added gradually in portions to the reaction mixture at 0 °C and stirred for 45 minutes. The progress of the reaction was monitored by TLC. The product formed was extracted in diethyl ether/ethyl acetate and water with the addition of 10 mL 1N HCl. The crude product was purified by column chromatography with petroleum ether and ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to obtain the desired product.
1, v/v) as eluent to obtain the desired product.
General procedure for the preparation of pyrrole-fused naphthoquinone derivatives (4)
2-(p-Tolylethynyl)naphthalene-1,4-dione (1 mmol) 3 was stirred with NH4OAc (3 mmol) in 5 mL MeOH for 30 minutes at 40 °C. The reaction was monitored by TLC. The product formed was extracted with diethyl ether and the extract was evaporate under reduced pressure using a rotary evaporator to obtain the crude product, which was further purified by column chromatography with hexane and ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to obtain the desired product.
1, v/v) as eluent to obtain the desired product.
General procedure for the preparation of imidazopyridinedione derivatives (6)
2-Bromo-1,4-naphthoquinone 2 (0.63 mmol, 0.150 g) and 2-aminopyridine (0.071 g, 0.75 mmol) were taken in a round-bottom flask and were heated at 100 °C in the presence of a catalytic amount of AcOH (5 mol%) for 8 h. The progress of the reaction was monitored by thin-layer chromatography (TLC). The crude product was purified directly by column chromatography using silica gel as the stationary phase with ethyl acetate and chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) as eluent.
4, v/v) as eluent.
Author contributions
All authors have read and agreed to the published version of the manuscript. RNS and VM performed the experiments, and SA collected the data related to the crystal structure. SC collected the data related to the biological study. SKM and RD analyzed the data. SKM, SC, TB, and RD drafted the manuscript, and supervised, reviewed, and edited the text.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available within the article and the ESI.†Acknowledgements
Financial support from SERB (no. Sur/2022/001548) and Bidhannagar College is gratefully acknowledged. R. D. thanks the National Institute of Technology Calicut for funding. V. M. is thankful to NIT Calicut for his fellowship. The authors are grateful to DST-FIST for providing the HRMS facility at the Department of Chemistry, NIT Calicut and CMC-NIT Calicut for providing NMR facility.References
- (a) Y.-R. Chen, C.-H. Tseng, Y.-L. Chen, T.-L. Hwang and C.-C. Tzeng, Int. J. Mol. Sci., 2015, 16, 6532 CrossRef CAS PubMed; (b) M.-E. Suh, M.-J. Kang and S.-Y. Park, Bioorg. Med. Chem., 2001, 9, 2987 CrossRef CAS PubMed.
- M. Inman, A. Visconti, C. Yan, D. Siegel, D. Ross and C. J. Moody, Org. Biomol. Chem., 2014, 12, 4848 RSC.
- (a) S. Bannwitz, D. Krane, S. Vortherms, T. Kalin, C. Lindenschmidt, N. ZahediGolpayegani, J. Tentrop, H. Prinz and K. Müller, J. Med. Chem., 2014, 57, 6226 CrossRef CAS PubMed; (b) B. Huang, A. Desai, S. Tang, T. P. Thomas and J. R. Baker, Org. Lett., 2010, 12, 1384 CrossRef CAS PubMed.
- J. D. Bauer, R. W. King and S. F. Brady, J. Nat. Prod., 2010, 73, 976 CrossRef CAS PubMed.
- M. Efdi, S. Fujita, T. Inuzuka and M. Koketsu, Nat. Prod. Res., 2010, 24, 657 CrossRef CAS PubMed.
- H. J. Lee, M. E. Suh and C. O. Lee, Bioorg. Med. Chem., 2003, 11, 1511 CrossRef CAS PubMed.
- H. J. Park, H.-J. Lee, E.-J. Lee, H. J. Hwang, S.-H. Shin, M.-E. Suh, C. Kim, H. J. Kim, E.- K. Seo and S. K. Lee, Biosci. Biotechnol. Biochem., 2003, 67, 1944 CrossRef CAS PubMed.
- (a) M. Lynch, S. Hehir, P. Kavanagh, D. Leech, J. O'Shaughnessy, M. P. Carty and F. Aldabbagh, Chem. – Eur. J., 2007, 133218 Search PubMed; (b) A. Abdukader, Q. Xue, A. Lin, M. Zhang, Y. Cheng and C. Zhu, Tetrahedron Lett., 2013, 54, 5898 CrossRef CAS; (c) W. A. Craigo, B. W. LeSueur and E. B. Skibo, J. Med. Chem., 1999, 42, 3324 CrossRef CAS PubMed.
- (a) See review; S. K. Manna, S. Mondal, T. Ghosh and S. Bandyopadhyay, ChemistrySelect, 2024, 9, e202403426 CrossRef CAS and reference cited therein. (b) A. K. Panday, D. Ali, T. Parvin and L. H. Choudhury, ChemistrySelect, 2023, 8, e202300158 CrossRef CAS; (c) U. Chaurasia and T. Parvin, J. Chem. Sci., 2023, 135, 60 CrossRef; (d) F. R. F. Dias, F. S. Guerra, F. A. Lima, Y. K. C. de Castro, V. F. Ferreira, V. R. Campos, P. D. Fernandes and A. C. Cunha, J. Braz. Chem. Soc., 2021, 32, 476 CAS; (e) Y. Dong, T. Mei, Q.-Q. Luo, Q. Feng, B. Chang, F. Yang, H.-W. Zhou, Z.-C. Shi, J.-Y. Wang and B. He, RSC Adv., 2021, 11, 6776 RSC; (f) T. Q. Nguyen, T. G. L. Nhat, D. V. Ngoc, T. A. D. Thi, H. T. Nguyen, P. H. Thi, H. H. Nguyen, H. T. Cao, K. A. Tehraniand and T. V. Nguyen, Tetrahedron Lett., 2016, 57, 4352 CrossRef CAS; (g) S. Maity, S. K. Gupta and N. Panda, Asian J. Org. Chem., 2021, 10, 3355 CrossRef CAS.
- M. S. Shvartsberg, E. A. Kolodina, N. I. Lebedeva and L. G. Fedenok, Tetrahedron Lett., 2009, 50, 6769 CrossRef CAS.
- P. G. Dalai, S. Swain, S. Mohapatra and N. Panda, J. Org. Chem., 2023, 88, 13760 CrossRef CAS PubMed.
- M. Mau, K. Ueda, K. Sakaguchi and A. Iida, Tetrahedron Lett., 2011, 52, 4665 CrossRef.
- S. Borra, D. Chandrasekhar, U. D. Newar and R. A. Maurya, J. Org. Chem., 2019, 84, 1042 CrossRef CAS PubMed.
- S. Guo, B. Chen, X. Guo, G. Zhang and Y. Yu, Tetrahedron, 2015, 71, 9371 CrossRef CAS.
- C. Zhou, H. Zheng, Y. Chen, G. Mao and G. Deng, J. Org. Chem., 2023, 88, 1533 CrossRef CAS PubMed.
- M. S. Christodoulou, S. Giofrè, E. M. Beccalli, F. Foschi and G. Broggini, Org. Lett., 2020, 22(7), 2735 CrossRef CAS PubMed.
- (a) A. K. Panday, D. Ali and L. H. Choudhury, Org. Biomol. Chem., 2020, 18, 4997 RSC; (b) A. Jana, S. K. Manna, S. K. Mondal, A. Mandal, S. K. Manna, A. Jana, B. K. Senapati, M. Jana and S. Samanta, Tetrahedron Lett., 2016, 57, 3722 CrossRef CAS.
- (a) W. L. Mosby and R. J. Boyle, J. Org. Chem., 1959, 24, 374 CrossRef CAS; (b) P. Truitt, J. E. Cooper III and F. M. Wood Jr., J. Am. Chem. Soc., 1957, 79, 5708 CrossRef CAS; (c) Y. S. Kim, S. Y. Park, M. E. Suh, H. J. Lee and D. Schollmeyer, Bioorg. Med. Chem., 2003, 11, 1829 CrossRef CAS PubMed; (d) U. Balijapalli, M. D. Thiyagarajan, S. Manickam and K. I. Sathiyanarayanan, ChemistrySelect, 2016, 1, 2900 CrossRef CAS; (e) K. Podemska, R. Podsiadly, A. M. Szymczak and J. Sokolowska, Dyes Pigm., 2012, 94, 113 CrossRef CAS; (f) R. A. Tapia, L. Cantuarias, M. Cuéllar and J. Villena, J. Braz. Chem. Soc., 2009, 20, 999 CrossRef CAS; (g) P. Lareginie, V. Lokshin, A. Samat, R. Guglielmetti and G. Pepe, J. Chem. Soc., Perkin Trans. 1, 1996, I07, 2 Search PubMed; (h) D. Choudhari, D. N. Lande, D. Chakravarty, S. P. Gejji, P. Das, K. R. Pardesi, S. Satpute and S. Salunke-Gawali, J. Mol. Struct., 2019, 1176, 194 CrossRef CAS; (i) J. Šarlauskas, M. Pečiukaitytė-Alksnė, L. Misevičienė, A. Marozienė, E. Polmickaitė, Z. Staniulytė, N. Č. Ėnas and Ž. Anusevičius, Bioorg. Med. Chem. Lett., 2016, 26, 512 CrossRef PubMed; (j) M. Pečiukaitytė-Alksnė, J. Šarlauskas, L. Misevičienė, A. Marozienė, N. Čėnas, K. Krikštopaitis, Z. Staniulytė and Ž. Anusevičius, EXCLI J., 2017, 16, 663 Search PubMed; (k) M. Á. Castro, A. M. Gamito, V.-T. Castaño, V.-R. Linares, J. M. M. del Corral, A. C.-M. Arango, L.-B. Galvis, A. M. Francesch and A. S. Feliciano, RSC Adv., 2015, 5, 1244 RSC.
- (a) Sk. A. Azad, A. Bera, J. Samanta, N. Sepay, R. Jana, C. K. Pal, M. R. Molla, D. Maiti and S. Samanta, Chem. – Eur. J., 2023, e202303287 Search PubMed; (b) A. Jana, S. K. Manna, S. K. Mondal, A. Mandal, S. K. Manna, A. Jana, B. K. Senapati, M. Jana and S. Samanta, Tetrahedron Lett., 2016, 57, 3722–3726 CrossRef CAS; (c) Sk. A. Ali, S. Bhaumik, A. Jana, S. K. Manna, M. Ikbal, A. Mandal, A. Bera, A. Jana and S. Samanta, ChemistrySelect, 2018, 3, 11950 CrossRef CAS; (d) S. K. Manna, S. Chakraborty, A. K. Adak and S. Samanta, ChemistrySelect, 2022, 7, e20220072 CrossRef; (e) A. Mandal, S. K. Mondal, A. Jana, S. K. Manna, Sk. A. Ali and S. Samanta, J. Heterocycl. Chem., 2017, 54, 2529 CrossRef CAS.
- T. Yao, X. Zhang and R. C. Larock, J. Am. Soc., 2004, 126, 1164 Search PubMed.
- M. C. Das, S. Paul, P. Gupta, P. Tribedi, S. Sarkar, D. Manna and S. Bhattacharjee, J. Appl. Microbiol., 2016, 120, 842 CrossRef CAS PubMed.
- P. Biswas, S. Bose and S. Chakraborty, World J. Microbiol. Biotechnol., 2025, 13, 104 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2431475. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00564g |
| This journal is © The Royal Society of Chemistry 2025 |









