 Open Access Article
Open Access ArticleElectrophilic glycoluril-based reagents for atom-economic thiocyanation and selenocyanation of (hetero)arenes†
Paula
Casasús
 ,
Jordi
Mestre
,
Jordi
Mestre
 ,
Roksana
Radłowska
,
Roksana
Radłowska
 ,
Miguel
Bernús
,
Miguel
Bernús
 and
Omar
Boutureira
and
Omar
Boutureira
 *
*
Departament de Química Analítica i Química Orgànica, Universitat Rovira I Virgili, C/Marcel·lí Domingo 1, 43007 Tarragona, Spain. E-mail: omar.boutureira@urv.cat
First published on 9th April 2025
Abstract
Two electrophilic glycoluril-based N-XCN reagents (X = S, Se) were developed for introducing SCN/SeCN groups into aromatic substrates, including the late-stage modification of bioactive molecules. Their application produces minimal waste, enables simple purification, and offers potential for reagent regeneration. Additionally, their compatibility with green solvents and flow technology was demonstrated. The sustainability of the process was evaluated using green metrics and Ecoscale values, emphasizing the complementary roles of the reagents and solvent recovery in enhancing atom economy and reducing waste.
Introduction
Organic thiocyanates and their selenium analogues have attracted significant attention due to their presence in various natural products1a and pharmaceuticals,1b–h enhancing their antibacterial, antifungal, and anticancer properties (Fig. 1A). The thiocyanate group is a versatile yet underused functional group that can be converted into a range of sulfur-containing derivatives, such as thiols, disulfides, and thioethers.2 Likewise, selenocyanation serves as an efficient and straightforward method for incorporating selenium moieties into organic molecules that are often challenging to obtain.3 | ||
| Fig. 1 (A) Examples of bioactive molecules containing SCN/SeCN groups. (B) Selected electrophilic N-SCN and N-SeCN reagents. HDAC = histone deacetylase. | ||
Currently, several anionic reagents are employed for thiocyanation, often via the in situ formation of electrophilic agents,4 while selenocyanation remains less developed.5 Nucleophilic thio- and selenocyanate salts are commonly used not only in polar reactions but also in radical reactions (including those light-driven),6 particularly when combined with oxidants such as cerium ammonium nitrate (CAN),7tert-butyl hydroperoxide (TBHP),8 or potassium peroxodisulfate (K2S2O8).9 Additionally, these salts can also be used in cross-coupling reactions involving transition metals.10 In contrast, electrophilic sources of SCN and SeCN groups offer complementary reactivity.11 For example, Bacon and Angus first introduced thiocyanogen chloride (Cl-SCN) for the functionalization of alkenes and arenes; however, this reagent is inconvenient due to its poor stability and high toxicity.12 More recently, bench-stable thio- and selenocyanation reagents featuring N-chalcogen linkages have emerged as versatile alternatives for functionalizing various organic scaffolds (Fig. 1B).13–19 However, all of the reported reagents can only transfer one unit of SCN/SeCN per molecule, which decreases atom economy and contributes to significant waste generation. Furthermore, its application typically requires an additive/external catalyst, complicating purification and often necessitating flash-column chromatography for isolation. Our aim was to develop an electrophilic reagent capable of rapidly introducing thio- and selenocyanide groups into complex molecules with high selectivity, low waste, and simple purification. Given that N-SCN/SeCN motifs have demonstrated high efficiency in delivering thio- and selenocyanide groups, we turned our attention to glycoluril-based scaffolds, which feature four nitrogen centres per molecule. In fact, this core structure has been used for the electrophilic radioiodination of proteins20 and has also shown applications as a mild oxidizing agent.21 Surprisingly, no additional applications have been reported that utilized this glycoluril scaffold for installing other electrophiles. With four N-XCN (X = S, Se) bonds, the reagents can deliver four SCN or SeCN groups per equivalent, thereby maximizing the atom economy of the transformation.
Results and discussion
Synthesis of reagents
Their preparation can be achieved through a proposed 3-step synthesis for both reagents (Scheme 1). First, the condensation of urea 1 with benzyl 2 in the presence of trifluoroacetic acid affords the bicyclic diphenylglycoluril compound 3 in good yield (74%, 36 g).22 Next, chlorination with trichloroisocyanuric acid (TCCA) produces tetrachlorodiphenylglycoluril 4, also known as Iodo-Gen® (89%, 26 g),23 which can subsequently react with ammonium thiocyanate or potassium selenocyanate to yield 5a (95%, 4 g) or 5b (80%, 2.5 g). Importantly, all steps were carried out in multigram quantities without the use of column chromatography. Reagents 5a and 5b are free-flowing powders that remained stable without decomposition when stored at −20 °C under argon for several months. Moreover, differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) further demonstrated the stability of both reagents in the solid state at temperatures below 160 °C for 5a and 147 °C for 5b (Fig. S1 and S2, ESI†). | ||
| Scheme 1 Synthesis of 5a,b from commercially available urea 1 and benzyl 2. See the ESI† for details. TFA = trifluoroacetic acid, TCCA = trichloroisocyanuric acid. | ||
Scope and derivatization
With these reagents in hand, we performed a scope assessment using indoles as model substrates (Scheme 2A). Initially, a reaction was performed with 1 equivalent of 1H-indole and 0.33 equivalents of reagents (5a for thiocyanation or 5b for selenocyanation) in CH3CN (0.16 M) at room temperature. Pleasingly, 3-(thiocyano)indole 6a and its selenium analogue 6b (with antifungal activity)1g were obtained in 99% yield in just 10 min. Remarkably, the modified indoles 6a and 6b were obtained in high-purity through simple filtration followed by solvent removal. We then evaluated a series of halogen-substituted indoles, which afforded products 7–9a,b in good to excellent yields (up to 99%).1g Deactivated indoles with electron-withdrawing nitrile and nitro groups at the 5-position reacted smoothly, affording products 10–11a,b in >98% yield. The presence of a carboxylic acid group slightly reduced the yields, although 12a and 12b were still obtained in good to excellent yields (80% and 73%, respectively). Electron-donating 5-methoxy, N-methyl, and 2-methyl indoles were also well tolerated, delivering products 13–15a,b in excellent yields (91–99%). When the most reactive C3 position of the indole24 was substituted by a methyl group, functionalization selectively occurred at the C2-position, affording 16a and 16b in 64% and 79% yield, respectively. Moving to other heteroaromatics, the reaction of 1H-pyrrole afforded products 17a,b in good yields (up to 99%). However, using 2,5-dimethylpyrrole resulted in a mixture of products, with the monosubstituted product 18a obtained in 72% yield, and the di-functionalized product 19a isolated in 20% yield. For selenocyanation, a more complex mixture was obtained, although mono-functionalized 18b was isolated in 55% yield. Other electron-rich aromatic substrates, such as phenol, 3,5-dimethylphenol, and aniline, were functionalized in excellent yields, affording the corresponding para-substituted products 20–22a,b in up to 99% yield. However, the more deactivated N-acetyl aniline remained unreactive, even with trifluoromethanesulfonic acid (TfOH)15 as a catalyst. For less activated aromatic substrates, such as anisole, reactivity significantly diminished, resulting in conversions of only up to 50%. However, the addition of 1.5 equivalents of TfOH significantly increased reactivity, giving 23a,b in 75% and 69% yield, respectively. TfOH was also required for mesitylene 24a (86%) and 24b (70%) and anthracene 25a,b (73%). | ||
Scheme 2 (A) Scope and (B) derivatization. General conditions: (hetero)arene (0.3–0.5 mmol) and 5a,b (0.099–0.165 mmol) in CH3CN (0.16 M) unless otherwise indicated. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) With TfOH (0.45–0.75 mmol) in CH2Cl2 (0.16 M). b With TfOH (0.45–0.75 mmol) in CH2Cl2 (0.16 M). b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In CH2Cl2(0.16 M). c In CH2Cl2(0.16 M). c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a, 21a, or 21b (0.1–0.3 mmol), TMSCF3 (0.1–1 mmol), and KF (0.4–1 mmol) in DMF (0.2 M). See the ESI† for details. TMS = trimethylsilyl, DMF = N,N-dimethylformamide. NR = No Reaction. 6a, 21a, or 21b (0.1–0.3 mmol), TMSCF3 (0.1–1 mmol), and KF (0.4–1 mmol) in DMF (0.2 M). See the ESI† for details. TMS = trimethylsilyl, DMF = N,N-dimethylformamide. NR = No Reaction. | ||
We then aimed to functionalize more complex substrates by testing a series of natural products, pharmaceuticals, and agrochemicals (Scheme 2A). Preliminary attempts with dopamine and other neurotransmitters, hormones, and metabolites (such as melatonin, serotonin, L-adrenaline, tryptamine, and 5-hydroxytryptamine) using 5a resulted in a mixture of products. Similarly, a protected catechol dopamine derivative reacted with an electrophilic SCN reagent, revealing that certain aliphatic primary amines, such as dopamine, are initially converted to N-thiocyanoamines (RNHSCN). These relatively unstable intermediates subsequently evolve into quaternary salts (RNH3+SCN−), along with unidentified polymeric by-products.11b This suggests that protection of primary amines is necessary to achieve selective thiocyanation of the aromatic ring. For N-acetylated dopamine, reagent 5a delivered the corresponding product 26a in 58% yield, while no conversion was observed for 5b. The reaction of 5a and 5b with a precursor of the insecticide Fipronil25 afforded 27a,b in 40% and 35% yield, respectively.
Naproxen26 and ketorolac,27 anti-inflammatory agents, were functionalized to give 28a,b (80% and 42%, respectively), and 29a in 96% yield, while no conversion was observed for 29b. In the evaluation of Ivacaftor 30,28 a drug used to treat cystic fibrosis, we encountered an intriguing reaction previously reported with ortho-phenols and ortho-anilines.9b The thiocyanate group installed in the molecule reacted with the ortho-phenol moiety to form a stable oxathioimine 30a in 87% yield. This compound was confirmed by IR spectroscopy, which showed the absence of the characteristic C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching band (∼2100 cm−1).29 The analogous reaction with 5b resulted in no conversion of the starting material.
N stretching band (∼2100 cm−1).29 The analogous reaction with 5b resulted in no conversion of the starting material.
Next, to further complement our recently developed technologies focused on the incorporation and evaluation of polyfluoroalkylthio motifs (SRF),30 we carried out the derivatization of SCN and SeCN groups to demonstrate their utility as reactive handles for accessing trifluoromethylthio and selenoether derivatives (Scheme 2B).31 Indole and phenol derivatives 6a, 21a, and 21b were treated with TMSCF3 and potassium fluoride in DMF, converting the SCN and SeCN groups into the corresponding SCF3 and SeCF3 analogues in yields greater than 70%.
Reagent regeneration, compatibility with green solvents, and green metrics comparison
After assessing the reactivity of our reagents, we explored whether the sustainability of our protocol could be improved by regenerating them.15a,32 A key consideration was that, after the thio- or selenocyanation reaction, the precursor 3, generated from 5a and 5b, is poorly soluble in most organic solvents, allowing it to be easily separated by filtration and subjected to a two-step regeneration cycle (Scheme 3A). Thus, upon completion of the reaction between 5-fluoroindole 7 and 5a, the mixture was filtered to recover the solid precursor 3 (71%). Chlorination with TCCA, followed by reaction with ammonium thiocyanate enabled the synthesis of reagent 5a. The regenerated reagent 5a remained reactive toward 5-fluoroindole 7, achieving full conversion to 7a after 1 h. A further recycling-reactivity test revealed that, although 2 h were required to reach full conversion, the reagent maintained effectiveness. These experiments indicate a slight decrease in the reagent reactivity after each cycle, which can be compensated for by simply extending the reaction time.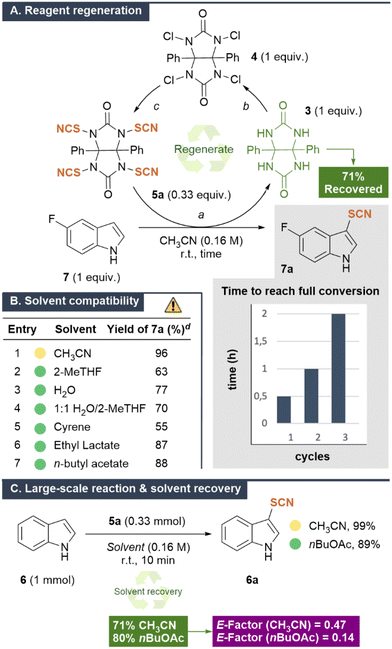 | ||
Scheme 3 (A) Reagent regeneration, (B) solvent compatibility, (C) large-scale reaction and solvent recovery. General conditions: a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (0.5 mmol) and 5a (0.19 mmol) in CH3CN (0.16 M). b 7 (0.5 mmol) and 5a (0.19 mmol) in CH3CN (0.16 M). b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (0.06 mmol), TCCA (0.1 mmol), and NaOAc (0.4 mmol) in H2O (0.08 M). c 3 (0.06 mmol), TCCA (0.1 mmol), and NaOAc (0.4 mmol) in H2O (0.08 M). c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (0.05 mmol) and NH4SCN (0.23 mmol) in CH3CN (0.04 M). d Determined by 19F NMR analysis of the crude reaction mixture, using 1,3-bis(trifluoromethyl)benzene as the internal standard. See the ESI† for details. Color code; volatile organic compound-VOC (yellow) and environmentally friendly solvent (green). 4 (0.05 mmol) and NH4SCN (0.23 mmol) in CH3CN (0.04 M). d Determined by 19F NMR analysis of the crude reaction mixture, using 1,3-bis(trifluoromethyl)benzene as the internal standard. See the ESI† for details. Color code; volatile organic compound-VOC (yellow) and environmentally friendly solvent (green). | ||
We then evaluated the compatibility of environmentally friendly, green solvents, including 2-methyltetrahydrofuran, water, Cyrene™, ethyl lactate, and n-butyl acetate, revealing that the reaction outcome was not significantly influenced by the solvent choice, except for the slight erosion observed with Cyrene™ (Scheme 3B).33
Additionally, the greenness of our process was assessed by evaluating several key green metrics (section 3, ESI†).31,34 Initially, the simple E-factor (sEF), which excludes solvents when calculating waste generation, was determined for the optimized reaction between 1H-indole 6 and reagent 5a, yielding a value of 0.67 – comparable to those in bulk chemical industries.35 However, when the comprehensive E-factor, which includes solvent contribution, was calculated, the value increased significantly to 28.1 for CH3CN and 31.4 for nBuOAc, highlighting the substantial impact of solvents on waste generation. To address this, two large-scale reactions were conducted using a waste-minimized protocol that enabled efficient solvent recovery by simple distillation (Scheme 3C).31 When CH3CN was used, 71% of the solvent was successfully recovered, reducing the E-factor to 0.47. Similarly, with nBuOAc as the solvent, an 80% recovery rate further minimized the E-factor to an impressive 0.14 – close to the limit observed in oil refining (E-factor < 0.1).35 These results underscore the critical role of solvent recovery in reducing waste generation and improving the sustainability of the process.
Further analysis focused on comparing the sustainability of our protocol with similar methods employing alternative thiocyanating reagents.15a,16a This was done by calculating Atom Economy (AE), Reaction Mass Efficiency (RME), and Ecoscale scores (sections 3 and 4, ESI†). Our protocol achieved an AE of 70% and an RME of 60%, outperforming alternative methods using reagents R1 and R2, which exhibited significantly lower AE values of 49% and 37%, respectively, as well as RME values of 40% and 30%, respectively (Table 1). Moreover, in these comparative protocols, solvent contribution was notably high, leading to E-factors of 35.6 and 25.5 for R1 and R2, respectively. Finally, an additional evaluation was conducted using Ecoscale analysis, highlighting the advantage of our protocol in eliminating the need for chromatographic purification. This aspect significantly improved the Ecoscale score, resulting in a value of 82 when using CH3CN. In contrast, the selected procedures were penalized for requiring purification, obtaining scores of 77 for R1 and 72 for R2, respectively. Notably, when nBuOAc was used as the solvent in our protocol, the highest Ecoscale value of 87 was achieved, as it avoided safety-related penalties while price and availability factors remaining consistent across all protocols. This highlights the superior sustainability of our method, which outperformed the alternatives in AE, RME, and Ecoscale value, despite a slight yield decrease with nBuOAc.
| Entry | Reagent | Solvent | AEa (%) | RMEa (%) | sEFb | E-Factorb | Solvent contribution (%) | Ecoscalea (%) | Yielda (%) 6a |
|---|---|---|---|---|---|---|---|---|---|
| AE = Atom Economy, RME = Reaction Mass Efficiency, sEF = simple E-factor.a 100 is the ideal value.b 0 is the ideal value.c Values considering solvent recovery. | |||||||||
| 1 | 5a | CH3CN | 70 | 60 | 0.67 | 0.47c | 97 | 82 | 99 |
| 2 | 5a | nBuOAc | 70 | 60 | 0.67 | 0.14c | 97 | 87 | 89 |
| 3 | R1 | THF | 49 | 40 | 1.52 | 35.6 | 95 | 77 | 98 |
| 4 | R2 | CH3CN | 37 | 30 | 2.38 | 25.5 | 90 | 72 | 98 |
Flow chemistry
Given the low solubility of reagents 5a,b (Table S1, ESI†), we considered immobilizing them in a packed bed reactor for compatibility with flow technology.36 This setup employs a syringe pump to push a solution of a nucleophile through a cartridge filled with thiocyanating reagent 5avia polytetrafluoroethylene (PTFE) tubing (section 5, ESI†). Because significant leaching of 5a was observed during initial attempts using CH3CN, the flow rate of the nucleophile was optimized using a 0.2 M chloroform solution of 5-fluoroindole 7 as a model substrate, as its reduced solubility suppressed the leaching. Flow rates greater than 0.67 mL min−1 resulted in a mixture of unreacted 5-fluoroindole 7 and product 7a (Table S2, entries 1–5, ESI†), while reducing the flow rate to 0.5 mL min−1 yielded the desired product with full conversion and 91% yield, with a residence time of approximately 8 min (Table S2, entry 6, ESI†). Once the optimal flow rate was established, a new cartridge containing 5a was prepared to evaluate consecutive reactions. A sequence of nucleophiles, interspersed with chloroform washes, was flowed through the same packed bed reactor without the need to load a new cartridge for each substrate, and the resulting crude products were collected.The thiocyanated products of 5-fluoroindole (7a, 91%), aniline (20a, 90%), and phenol (21a, >99%) were obtained in pure form after solvent evaporation (Fig. 2). At this point, we considered replacing chloroform with the greener alternative nBuOAc,33,37 which was used in our previous solvent screening assessment (Scheme 3B) and possesses a similar solubility profile compatible with our flow setup (Table S1, ESI†). Thus, the same procedure was repeated and, although a slight yield erosion was observed, the corresponding products, – 5-fluoroindole (7a, 82%), aniline (20a, 79%), and phenol (21a, 88%) – were still obtained in pure form. This demonstrates the compatibility of the thiocyanating reagent 5a with flow chemistry, highlighting that multiple consecutive reactions can be performed without manipulating the reagent. This results in clean product formation and facilitates easy purification, as the insoluble reagent's precursor 3 is effectively retained in the cartridge.
 | ||
| Fig. 2 Multiple consecutive experiments using flow chemistry. See the ESI† for details. Color code; volatile organic compound-VOC (yellow) and environmentally friendly solvent (green). | ||
Conclusions
In summary, two electrophilic reagents were developed for the direct introduction of SCN and SeCN groups via a three-step synthetic route. These readily available reagents enable late-stage thio- and selenocyanation across a broad substrate scope, including indoles, pyrroles, aniline, phenols, less-activated aromatics, natural products, pharmaceuticals, and agrochemicals. The reactions proceed under mild, metal-free conditions (catalyst-free for most substrates) with good to excellent yields. Their versatility was further demonstrated through trifluoromethylation of indole-SCN and phenol-SCN/SeCN derivatives. Additionally, reagent regeneration was achieved by leveraging the low-solubility of diphenylglycoluril precursor 3, formed during reactions with nucleophiles. Importantly, these reagents also proved compatible with environmentally friendly solvents. Sustainability assessments using green metrics and Ecoscale values highlighted significant improvements in atom economy (AE), reaction mass efficiency (RME), and waste reduction (E-factor) via efficient solvent recovery, outperforming similar protocols. Finally, the applicability of these reagents in flow chemistry was confirmed, enabling consecutive reactions without reagent manipulation. Overall, this study showcases the broad utility and potential of these reagents for efficient synthetic transformations, offering high atom economy and reduced waste generation.Author contributions
P. C., J. M, R. R., and M. B. performed all the experiments. O. B. supervised the project and was responsible for funding acquisition. All the authors contributed to the preparation of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish Government-MICIU, the national agency of investigation-AEI/10.13039/501100011033, the European Regional Development Fund-ERDF/EU, the European Social Fund-ESF/EU Investing in your future (projects; CTQ2017-90088-R, PID2020-120584RB-I00, PID2023-153360NB-I00 to O. B., FPU Fellowship; FPU19/01969 to M. B., and Ramón y Cajal Fellowship; RYC-2015-17705 to O. B.), and the Universitat Rovira i Virgili (Martí i Franquès Research Fellowship Programme; 2022PMF-PIPF-17 to P. C.). We thank Dr Miguel A. Rodríguez (COS, URV-Eurecat) for his assistance with 13C NMR experiments on insoluble samples, and Dr Adrian Moreno (URV) for the DSC and TGA measurements.References
- (a) C. Jiménez and P. Crews, Tetrahedron, 1991, 47, 2097–2102 CrossRef; (b) E. Elhalem, B. N. Bailey, R. Docampo, I. Ujváry, S. H. Szajnman and J. B. Rodriguez, J. Med. Chem., 2022, 45, 3984–3999 CrossRef PubMed; (c) D. Plano, D. N. Karelia, M. K. Pandey, J. E. Spallholz, S. Amin and A. K. Sharma, J. Med. Chem., 2016, 59, 1946–1959 CrossRef CAS PubMed; (d) W. Ali, M. A. Pérez, M. A. Marć, N. S. Jiménez, J. Handzlik and E. D. Álvarez, Curr. Pharmacol. Rep., 2018, 4, 468–481 CrossRef CAS; (e) Y. Huang, Y. Cheng, M. Wei, Z. Peng, W. Tian, Z. Liu, J. Li and J. Cui, Bioorg. Chem., 2024, 144, 107149 CrossRef CAS PubMed; (f) W. Hou and H. Xu, J. Med. Chem., 2022, 65, 4436–4456 CrossRef CAS PubMed; (g) P. M. Quatrin, D. F. Dalla Lana, L. C. G. Bazana, L. F. S. de Oliveira, M. L. Teixeira, E. E. Silva, W. Lopes, R. F. S. Canto, G. P. Silveira and A. M. Fuentefria, New J. Chem., 2019, 43, 926–933 RSC; (h) C. Morán-Serradilla, D. Plano, C. Sanmartín and A. K. Sharma, J. Med. Chem., 2024, 67, 7759–7787 CrossRef PubMed.
- (a) T. Castanheiro, J. Suffert, M. Donnard and M. Gulea, Chem. Soc. Rev., 2016, 45, 494–505 RSC; (b) A. W. Erian and S. M. Sherif, Tetrahedron, 1999, 55, 7957–8024 CrossRef CAS; (c) M. Gulea, F. Hammerschmidt, P. Marchand, S. Masson, V. Pisljagic and F. Wuggenig, Tetrahedron: Asymmetry, 2003, 14, 1829–1836 CrossRef CAS; (d) X. Lu, H. Wang, R. Gao, D. Sun and X. Bi, RSC Adv., 2014, 4, 28794–28797 RSC; (e) M. Pawliczek, L. K. B. Garve and D. B. Werz, Org. Lett., 2015, 17, 1716–1719 CrossRef CAS PubMed; (f) C. Matheis, M. Wang, T. Krause and L. J. Goossen, Synlett, 2015, 1628–1632 CAS.
- (a) J. M. Sonego, S. I. de Diego, S. H. Szajnman, C. G. Rodriguez and J. B. Rodriguez, Chem. – Eur. J., 2023, 29, e202300030 CrossRef CAS PubMed; (b) Y. A. Lin, O. Boutureira, L. Lercher, B. Bhushan, R. S. Paton and B. G. Davis, J. Am. Chem. Soc., 2013, 135, 12156–12159 CrossRef CAS PubMed; (c) J. C. Guillemin, G. Bajor, E. H. Riague, B. Khater and T. Veszprémi, Organometallics, 2007, 26, 2507–2518 CrossRef CAS; (d) A. Ghasseminn, X. V. Farrés, P. F. Alewood and T. Durek, Bioorg. Med. Chem., 2013, 21, 3473–3478 CrossRef PubMed.
- (a) H. Chen, X. Shi, X. Liu and L. Zhao, Org. Biomol. Chem., 2022, 20, 6508–6527 RSC; (b) D. He, J. Yao, B. Ma, J. Wei, G. Hao, X. Tuo, S. Guo, Z. Fu and H. Cai, Green Chem., 2020, 22, 1559–1564 RSC; (c) A. S. Kirillov, E. A. Semenov, O. V. Bityukov, M. A. Kuznetsova, V. N. Demidova, A. N. Rogozhin, A. P. Glinushkin, V. A. Vil’ and A. O. Terent'ev, Org. Biomol. Chem., 2023, 21, 3615–3622 RSC; (d) S. Saha, A. B. Pinheiro, A. Chatterjee, Z. T. Bhutia and M. Banerjee, Green Chem., 2024, 26, 5879–5889 RSC; (e) C. Wang, Z. Wang, L. Wang, Q. Chen and M. He, Chin. J. Chem., 2016, 34, 1081–1085 CrossRef CAS; (f) H. Zhang, Q. Wei, S. Wei, J. Qu and B. Wang, Eur. J. Org. Chem., 2016, 3373–3379 CrossRef CAS; (g) Q. Chen, Y. Lei, Y. Wang, C. Wang, Y. Wang, Z. Xu, H. Wang and R. Wang, Org. Chem. Front., 2017, 4, 369–372 RSC.
- (a) P. G. Karmaker and F. Huo, Asian J. Org. Chem., 2022, 11, e202200226 CrossRef CAS; (b) V. Nair, A. Augustine and T. G. George, Eur. J. Org. Chem., 2002, 2363–2366 CrossRef CAS; (c) A. Hassanpour, E. Ghavidelaghdam, A. G. Ebadi, M. R. Poor Heravi and E. Vessally, RSC Adv., 2021, 11, 22305–22316 RSC.
- (a) Q. Yan, S. Chen, J. Fan and Z. Li, Org. Biomol. Chem., 2023, 21, 9112–9122 RSC; (b) J. Vigier, M. Gao, P. Jubault, H. Lebel and T. Besset, Chem. Commun., 2024, 60, 196–199 RSC; (c) S. Chen, Q. Yan, J. Fan, Y. Gao, X. Yang, L. Li, Z. Liu and Z. Li, Green Chem., 2022, 24, 4742–4747 RSC; (d) Q. Xie, Y. Yang, W. Zhang, Z. Gao, X. Li, J. Tang, C. Pan and G. Yu, Chem. Sci., 2021, 12, 5631–5637 RSC; (e) F. Buttard, J. Vigier, H. Lebel and T. Besset, Eur. J. Org. Chem., 2024, e202301205 CrossRef CAS; (f) P. G. Karmaker, Md. A. Alam and F. Huo, RSC Adv., 2022, 12, 6214–6233 RSC.
- V. Nair, T. G. George, L. G. Nair and S. B. Panicker, Tetrahedron Lett., 1999, 40, 1195–1196 CrossRef CAS.
- N. Muniraj, J. Dhineshkumar and K. R. Prabhu, ChemistrySelect, 2016, 5, 1033–1038 CrossRef.
- (a) D. Yang, K. Yan, W. Wei, G. Li, S. Lu, C. Zhao, L. Tian and H. Wang, J. Org. Chem., 2015, 80, 11073–11079 CrossRef CAS PubMed; (b) T. B. Mete, T. M. Khopade and R. G. Bhat, Tetrahedron Lett., 2017, 58, 415–418 CrossRef CAS.
- (a) I. P. Beletskaya, A. S. Sigeev, A. S. Peregudov and P. V. Ptrovskii, Mendeleev Commun., 2006, 16, 250–251 CrossRef; (b) I. Yavari, T. Damghani and M. Nematpour, Helv. Chim. Acta, 2013, 96, 2214–2217 CrossRef CAS; (c) H. Jiang, W. Yu, X. Tang, J. Li and W. Wu, J. Org. Chem., 2017, 82, 9312–9320 CrossRef CAS PubMed; (d) S. Thanna, C. M. Goins, S. E. Knudson, R. A. Slayden, D. R. Ronning and S. J. Sucheck, J. Org. Chem., 2017, 82, 3844–3854 CrossRef CAS PubMed.
- (a) M. Gao, M. Vuagnat, M. Y. Chen, X. Pannecoucke, P. Jubault and T. Besset, Chem. – Eur. J., 2021, 27, 6145–6160 CrossRef PubMed; (b) A. Klásek and V. Mrkvička, J. Heterocycl. Chem., 2003, 40, 747–752 CrossRef; (c) F. Buttard and T. Besset, SynOpen, 2023, 7, 117–120 CrossRef CAS; (d) J.-A. Xiao, Y.-C. Li, X.-L. Cheng, W.-Q. Chen, J.-G. Cui, Y.-M. Huang, J. Huang, Q. Xiao, W. Su and H. Yang, Org. Chem. Front., 2019, 6, 1967–1971 RSC.
- A. B. Angus and R. G. R. Bacon, J. Chem. Soc., 1958, 774–778 RSC.
- (a) F. D. Toste, V. De Stefano and I. W. J. Still, Synth. Commun., 1995, 25, 1277–1286 CrossRef CAS; (b) J. Y. See and Y. Zhao, Org. Lett., 2018, 20, 7433–7436 CrossRef CAS PubMed; (c) M. Gao, M. Vuagnat, P. Jubault and T. Besset, Eur. J. Org. Chem., 2021, e202101255 Search PubMed.
- (a) J. Qiu, D. Wu, P. G. Karmaker, H. Yin and F. X. Chen, Org. Lett., 2018, 20, 1600–1603 CrossRef CAS PubMed; (b) J. Qiu, D. Wu, L. Yuan, P. Long, H. Yin and F.-X. Chen, J. Org. Chem., 2019, 84, 7917–7926 CrossRef CAS PubMed; (c) M. Gao, M. Chen, X. Pannecoucke, P. Jubault and T. Besset, Chem. – Eur. J., 2020, 26, 15497–15500 CrossRef CAS PubMed.
- (a) D. Wu, J. Qiu, P. G. Karmaker, H. Yi and F. X. Chen, J. Org. Chem., 2018, 83, 1576–1583 CrossRef CAS PubMed; (b) W. Wei, L. Liao, T. Qin and X. Zhao, Org. Lett., 2019, 21, 7846–7850 CrossRef CAS PubMed; (c) L. J. N. Waddell, M. R. Senkans and A. Sutherland, J. Org. Chem., 2023, 88, 7208–7218 CrossRef CAS PubMed.
- (a) C. Li, P. Long, H. Wu, H. Yi and F. X. Chen, Org. Biomol. Chem., 2019, 17, 7131–7134 RSC; (b) X.-F. Song, A.-H. Ye, Y.-Y. Xie, J.-W. Dong, C. Chen, Y. Zhang and Z.-M. Chen, Org. Lett., 2019, 21, 9550–9554 CrossRef CAS PubMed; (c) A.-H. Ye, Y. Zhang, Y.-Y. Xie, H.-Y. Luo, J.-W. Dong, X.-D. Liu, X.-F. Song, T. Ding and Z.-M. Chen, Org. Lett., 2019, 21, 5106–5110 CrossRef CAS PubMed; (d) H. Wu, C. Shao, D. Wu, L. Jiang, H. Yin and F.-X. Chen, J. Org. Chem., 2021, 86, 5327–5335 CrossRef CAS PubMed.
- J. A. Xiao, X. L. Cheng, R. F. Meng, X. S. Qin, H. Peng, J. W. Ren, Z. Z. Xie, J. G. Cui and Y. M. Huang, Synthesis, 2020, 954–960 Search PubMed.
- D. Wu, J. Qiu, C. Li, L. Yuan, H. Yin and F.-X. Chen, J. Org. Chem., 2020, 85, 934–941 CrossRef CAS PubMed.
- D. Zhu, A. H. Ye and Z. M. Chen, Synthesis, 2021, 3744–3750 CAS.
- P. J. Fraker and J. C. Speck, Biochem. Biophys. Res. Commun., 1978, 80, 849–857 CrossRef CAS PubMed.
- (a) T. Unak, Z. Akgun, Y. Yildirim, Y. Dumanb and G. Erenel, Appl. Radiat. Isot., 2001, 54, 749–752 CrossRef CAS PubMed; (b) H. Yuan, J. Luo, S. Field, R. Weissleder, L. Cantley and L. Josephson, Bioconjugate Chem., 2005, 16, 669–675 CrossRef CAS PubMed; (c) A. K. Zad, A. Shiri, M. A. Zolfigol and S. Mallakpour, Synthesis, 2009, 2729–2732 Search PubMed.
- P. J. Gilissen, A. Swartjes, B. Spierenburg, J. P. J. Bruekers, P. Tinnemans, P. B. White, F. P. J. T. Rutjes, R. J. M. Nolte and J. A. A. W. Elemans, Tetrahedron, 2019, 75, 4640–4647 CrossRef CAS.
- A. Shiri and A. K. Zad, Synthesis, 2009, 2797–2801 CAS.
- (a) M. Bandini and A. Eicholzer, Angew. Chem., Int. Ed., 2009, 48, 9608–9644 CrossRef CAS PubMed; (b) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449–4465 RSC.
- A. S. Moffat, Science, 1993, 261, 550–551 CrossRef CAS PubMed.
- N. M. Davies, A. G. Roseth, C. B. Appleyard, W. Mcknight, P. Del Soldato, A. Calignano, G. Cirino and J. L. Wallace, Aliment. Pharmacol. Ther., 1997, 11, 69–79 CrossRef CAS PubMed.
- B. H. Resman-Thrgoff, Ann. Pharmacother., 1990, 24, 1098–1104 Search PubMed.
- P. B. Davis, U. Yasothan and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2012, 5, 349–350 CrossRef PubMed.
- (a) P. O. Kinell and B. Strandberg, Acta Chem. Scand., 1959, 13, 1607–1622 CrossRef CAS; (b) A. Lex, P. Pacher, O. Werzer, A. Track, Q. Shen, R. Schennach, G. Koller, G. Hlawacek, E. Zojer, R. Resel, M. Ramsey, C. Teichert, W. Kern and G. Trimmel, Chem. Mater., 2008, 20, 2009–2015 CrossRef CAS.
- (a) M. Bernús, G. D. Núñez, W. C. Hartley, M. Guasch, J. Mestre, M. Besora, J. J. Carbó and O. Boutureira, J. Med. Chem., 2025, 68, 4787–4800 CrossRef PubMed; (b) M. Spennacchio, M. Bernús, J. Stanić, D. Mazzarella, M. Colella, J. J. Douglas, O. Boutureira and T. Noël, Science, 2024, 385, 991–996 CrossRef CAS PubMed; (c) P. Casasús, J. Mestre, M. Bernús, S. Castillón and O. Boutureira, Adv. Synth. Catal., 2023, 365, 3438–3443 CrossRef; (d) J. Mestre, M. Bernús, S. Castillón and O. Boutureira, J. Org. Chem., 2022, 87, 10791–10806 CrossRef CAS PubMed.
- C. Pooput, M. Medebielle and W. R. Dolbier, Org. Lett., 2004, 6, 301–303 CrossRef CAS PubMed.
- Y.-X. Cheng, X.-G. Yang, F.-H. Du and C. Zhang, Green Chem., 2024, 26, 5914–5920 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- (a) H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC; (b) D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- (a) R. A. Sheldon, Chem. Ind., 1992, 903–906 CAS; (b) R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC; (c) R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC; (d) R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- (a) V. Hessel, S. Mukherjee, S. Mitra, A. Goswami, N. N. Tran, F. Ferlin, L. Vaccaro, F. M. Galogahi, N.-T. Nguyen and M. Escribà-Gelonch, Green Chem., 2024, 26, 9503–9528 RSC; (b) L. Capaldo, Z. Wen and T. Noël, Chem. Sci., 2023, 14, 4230–4247 RSC; (c) A. A. Heredia, S. M. Soria-Castro, W. D. Castro-Godoy, I. D. Lemir, M. López-Vidal, F. R. Bisogno, J. E. Argüello and G. Oksdath-Mansilla, Org. Process Res. Dev., 2020, 24, 540–545 CrossRef CAS.
- F. Gao, F. Ferlin, R. Bai, M. Li, L. Vaccaro and Y. Gu, Green Chem., 2021, 23, 3588–3594 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00536a |
| This journal is © The Royal Society of Chemistry 2025 |

