Open Access Article
Open Access ArticleTriflic acid catalyzed intermolecular hydroamination of alkenes with Fmoc-NH2 as the amine source†
Aswathi C.
S.
,
Chinraj
Sivarajan
 and
Raja
Mitra
and
Raja
Mitra
 *
*
School of Chemical and Materials Sciences, Indian Institute of Technology Goa, Ponda, Goa–403401, India. E-mail: rajamitra@iitgoa.ac.in
First published on 8th May 2025
Abstract
Intermolecular hydroamination of alkenes is recognized as one of the most challenging synthetic pathways for directly obtaining primary amine derivatives from alkenes. While metal-catalyzed hydroamination is well established, metal-free hydroamination for synthesizing primary amines remains an attractive yet infrequent approach. In this study, we report the hydroamination of vinyl arenes using triflic acid as the catalyst and Fmoc-NH2 as the amine source. The optimized conditions proved effective for a range of vinyl arenes and some endocyclic alkenes, yielding moderate to excellent results (40–91%). Mechanistic investigations conducted through NMR, variable temperature NMR, kinetic studies, and control reactions indicated that the transient interaction between triflic acid and Fmoc-NH2 inhibited styrene polymerization. Primary amines were obtained by deprotecting the Fmoc group using KOH/MeOH.
Introduction
Primary amines are particularly interesting in the pharmaceutical and other industries.1–4 Synthesizing primary amines from feedstock materials is a challenging task. Hydroamination is a primal, atom-economical, and by-product-free synthetic route for the synthesis of alkyl amines from olefins and amines;2,3,5–8 however, additional efforts are required to acquire selectivity.5,9–13 Metal-catalyzed intra- and intermolecular hydroamination of various alkynes3,5,8,14–17 and alkenes2,12,18–28 with N-protected amines is an established synthetic strategy to obtain primary amines. In many metal-catalyzed hydroamination reactions, Brønsted acids are used as additives or co-catalysts.2,29–33 The emergence of organocatalysis34–37 has prompted many scientists to search for metal-free alternatives for hydroamination.30,38–41 However, an inherent problem of hydroamination using Brønsted acids is quenching of the catalyst by a basic nitrogen source. In 2002, Hartwig and co-workers addressed this problem using protected amines for intramolecular hydroamination (Fig. 1).42 Since then, intramolecular hydroamination has been reported using various Brønsted acids30,34,42,43 and enzymes44 using N-protected amine sources.Bergman and co-workers reported Brønsted acid-assisted intermolecular addition of anilines to alkenes (Fig. 1), highlighting the potential of Brønsted acids in assisting in hydroamination reactions, albeit with the concomitant alkylation reaction.45 Among the potential hydroaminations, reactions involving ammonia are especially valuable because they provide direct access to primary amines.46–49 Nevertheless, the challenging nature of this simplest amine has compelled researchers to look for ammonia surrogates; recently, Morandi and co-workers utilized ammonium carbamate as the “N” source for the oxidative amination of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.50 It is important to note that NH351–53 and various NH3 surrogates54–59 have been utilized mainly in C–N cross-coupling reactions. The main criterion for choosing these surrogates, along with their compatibility with hydroamination reactions, is their facile deprotection. To develop a Brønsted acid-catalyzed hydroamination, the nitrogen source must be acid-tolerant and sufficiently nucleophilic under acidic conditions. List and co-workers reported an organocatalyzed asymmetric three-component homoallylic amine synthesis with Fmoc-NH2 as the amine source, and facile single-step deprotection was performed to assign the configuration.60 Hydroamination of an alkene with Fmoc-NH2 as an ammonia surrogate is rare.49,61
C bonds.50 It is important to note that NH351–53 and various NH3 surrogates54–59 have been utilized mainly in C–N cross-coupling reactions. The main criterion for choosing these surrogates, along with their compatibility with hydroamination reactions, is their facile deprotection. To develop a Brønsted acid-catalyzed hydroamination, the nitrogen source must be acid-tolerant and sufficiently nucleophilic under acidic conditions. List and co-workers reported an organocatalyzed asymmetric three-component homoallylic amine synthesis with Fmoc-NH2 as the amine source, and facile single-step deprotection was performed to assign the configuration.60 Hydroamination of an alkene with Fmoc-NH2 as an ammonia surrogate is rare.49,61
Herein, we report a simple and efficient regioselective intermolecular hydroamination of primarily vinyl arenes with Fmoc-NH2 (2a), using triflic acid as a catalyst. Amine transfer from 2a in alkene hydroamination offers significant advantages over conventional hydroamination methods: (i) the product formed is an Fmoc-protected amine, (ii) Fmoc-NH2 is acid tolerant, and (iii) Fmoc groups can be readily removed under mild conditions (base-catalyzed) to access primary amines.62,63
Results and discussion
To determine the feasibility of the Brønsted acid-catalyzed intermolecular hydroamination reaction, we began our investigation using styrene (1a) and Fmoc-NH2 (2a) as the model substrates in the presence of triflic acid (5 mol%) in toluene at 60 °C (Table 1, entry 2). The expected hydroamination product 3a was detected in 35% yield64,65 along with ether 4a as a side product66 (4%), generated by the hydrolysis67 of Fmoc-NH2 (Table S1, ESI†). Despite the low yield in this initial run, we were encouraged by the exclusive formation of the desired Markovnikov selectivity. Increasing the temperature from 60 °C to 80 °C gave 47% yield of 3a within 4 h, and continuing the reaction for a longer time resulted in the formation of 4a (isolated yield 6%) (Table 1, entry 3). Furthermore, increasing the temperature to 100 °C accelerated the reaction, and in 30 min, 37% yield of 3a was formed along with 8% yield of 4a. However, continuing the reaction for 12 h led to simultaneous decomposition (3a), increasing the polymerization of styrene (Table S1, ESI†). Thus, the reaction temperature was identified as a key factor for controlling the yield of the desired product. The ether solvent suppressed 3a formation (Table 1, entry 4, and Table S2, ESI†). Halogenated solvents such as 1,2-dichloroethane (1,2-DCE) and DCM resulted in a lower yield compared to toluene (Table 1, entries 5 and 6), and chloroform gave the best yield of 3a (47%, Table 1, entry 7) at 60 °C. Other screened solvents did not improve the yield (Table S2, ESI†). Screening other Brønsted acids as catalysts revealed that triflic acid was the most efficient (Table 1, entries 7–12 and Table S3, ESI†). The well-explored triflimide gave 3a and 4a in isolated yields of 29% and 6%, respectively. As mentioned earlier, the competing formation of ether 4a and the tendency of styrene to undergo polymerization interfered with the hydroamination product yield. Increasing the equivalents of styrene with respect to Fmoc-NH2 is a viable solution to overcome both challenges; therefore, the styrene to 2a ratio was adjusted to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which resulted in a dramatic acceleration of the reaction with an improved product yield (63%) (Table 1, entry 13). However, a further increase in the styrene concentration decreased the yield from 63% to 56%. Increasing the equivalents of Fmoc-NH2 (2a) did not improve the yield (Table S4, ESI†). Because adventitious water is responsible for the competing side reaction leading to ether 4a,67 we performed the reaction in the presence of molecular sieves under argon. Nevertheless, similar yields were obtained, proving that the reaction was unaffected by the presence of water (Table S4, entry 5, ESI†). When the catalyst loading was changed from 5 mol% to 10 mol%, the product yield increased to 81% after 12 h (Table 1, entry 14 and Table S5, ESI†). At this point, we believe that continuing the reaction for a longer time may lead to the decomposition of the product as well as Fmoc-NH2. The reaction was carried out for 4 h and 5 h, resulting in 82% and 91% yield of 3a, respectively (Table 1, entries 15 and 16). Prolonging the reaction beyond 5 h led to a decrease in the yield (Table S6, ESI†). Thus, the optimized reaction conditions were: a 3
1, which resulted in a dramatic acceleration of the reaction with an improved product yield (63%) (Table 1, entry 13). However, a further increase in the styrene concentration decreased the yield from 63% to 56%. Increasing the equivalents of Fmoc-NH2 (2a) did not improve the yield (Table S4, ESI†). Because adventitious water is responsible for the competing side reaction leading to ether 4a,67 we performed the reaction in the presence of molecular sieves under argon. Nevertheless, similar yields were obtained, proving that the reaction was unaffected by the presence of water (Table S4, entry 5, ESI†). When the catalyst loading was changed from 5 mol% to 10 mol%, the product yield increased to 81% after 12 h (Table 1, entry 14 and Table S5, ESI†). At this point, we believe that continuing the reaction for a longer time may lead to the decomposition of the product as well as Fmoc-NH2. The reaction was carried out for 4 h and 5 h, resulting in 82% and 91% yield of 3a, respectively (Table 1, entries 15 and 16). Prolonging the reaction beyond 5 h led to a decrease in the yield (Table S6, ESI†). Thus, the optimized reaction conditions were: a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of styrene (1a) to Fmoc-NH2 (2a) and 10 mol% triflic acid at 60 °C for 5 h in chloroform as the solvent (Table 1, entry 16). Control reactions showed that no 3a was formed in the absence any one of these reactants (Table S7, ESI†).
1 ratio of styrene (1a) to Fmoc-NH2 (2a) and 10 mol% triflic acid at 60 °C for 5 h in chloroform as the solvent (Table 1, entry 16). Control reactions showed that no 3a was formed in the absence any one of these reactants (Table S7, ESI†).
| Entry | Acid | Solvent | Temperature |
1H NMR yield 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (%) e (%) |
|---|---|---|---|---|
a Reaction conditions: styrene (1a, 0.344 mmol, 1 equiv.), Fmoc-NH2 (2a, 0.344 mmol, 1 equiv.), TfOH (0.01 mmol, 5 mol%), solvent (0.5 M), temperature (°C), time 12 h.
b Reaction carried out at RT.
c Styrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fmoc-NH2 (3 Fmoc-NH2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio).
d Ether (4a) formation observed from Fmoc-NH2.
e
1H NMR yield was calculated using 1,3,5-trimethoxybenzene as an internal standard. TfOH = CF3SO3H (see the ESI for complete optimization details†). 1 ratio).
d Ether (4a) formation observed from Fmoc-NH2.
e
1H NMR yield was calculated using 1,3,5-trimethoxybenzene as an internal standard. TfOH = CF3SO3H (see the ESI for complete optimization details†).
|
||||
| 1 | TfOH | Toluene | 40 °C | 0 |
| 2 | TfOH | Toluene | 60 °C | 35d |
| 3 | TfOH | Toluene | 80 °C | 47d |
| 4 | TfOH | 1,4-Dioxane | 60 °C | 0 |
| 5 | TfOH | 1,2-DCE | 60 °C | 35 |
| 6b | TfOH | CH2Cl2 | RT | 25 |
| 7 | TfOH | CHCl3 | 60 °C | 47 |
| 8 | CH3SO3H | CHCl3 | 60 °C | 15 |
| 9 | H2SO4 | CHCl3 | 60 °C | 31 |
| 10 | p-TSA | CHCl3 | 60 °C | Trace |
| 11 | CF3COOH | CHCl3 | 60 °C | 0 |
| 12 | (CF3SO2)2NH | CHCl3 | 60 °C | 29d |
| 13c | TfOH | CHCl3 | 60 °C | 63 |
| 14c | TfOH (10 mol%) | CHCl3 | 60 °C | 81 |
| 15c | TfOH (10 mol%) | CHCl3 | 60 °C; 4 h | 82 |
| 16c | TfOH (10 mol%) | CHCl3 | 60 °C; 5 h | 91 |
Under the optimized reaction conditions, we investigated the substrate scope of different vinylarenes and Fmoc-NH2 (Table 2). A scaled-up reaction (4.1 mmol of 2a) resulted in 89% (1.26 g of 3a) yield. Hydroamination proceeded successfully with several para-substituted styrenes with various electronic and steric demands. Alkyl substitutions such as p-Me and p-tert-Bu resulted in good yields of 3b (73%) and 3c (81%), respectively. With respect to the aryl substituents at the para position, phenyl (3d), 2-naphthyl (3e), and 9-anthracenyl (3f) showed moderate yields of 63%, 57%, and 52%, respectively. The halogen substituents also performed well under the optimized conditions, affording 3g (73%) and 3h (68%). m-Phenyl and m-(2-naphthyl) substituents gave moderate yields of 3i (38%) and 3j (42%). However, o-chloro substitution resulted in a poor yield (3k, 14%), which might have been caused by steric hindrance near the reactive site, and decomposition of the product was also observed during purification. 1-Vinyl naphthalene exhibited moderate reactivity and afforded the desired product 3l in 58%yield.
| a Reaction conditions: vinylarenes (1.5 mmol, 3 equiv.), Fmoc-NH2 (0.5 mmol, 1 equiv.), TfOH (0.05 mmol, 10 mol%), CHCl3 (0.5 M), 60 °C, stipulated time (h). b Condition A: hydroamination product (0.524 mmol, 1 equiv.), KOH (1.05 mmol, 2 equiv.), MeOH (0.25 M), RT, 5 min, oxalic acid (0.786 mmol, 1.5 equiv.). c Large scale (4.1 mmol of Fmoc-NH2). |
|---|

|
We also examined the hydroamination of endocyclic olefins, namely, 3,4-dihydro-2H-pyran and bicyclo[2.2.1]hept-2-ene. Both compounds underwent hydroamination, affording 3m in 72% yield (74% yield in a large-scale reaction)68 and 3n in 62% yield. Interestingly, while cyclohexene remained inert, 3,4-dihydro-2H-pyran reacted within minutes, indicating that the reaction proceeds through a carbocation intermediate that is stabilized in the case of pyran through the oxocarbenium ion.69 To further expand the substrate scope, we examined the reactivity of styrene with EWGs, unactivated alkenes, alkynes, conjugated systems, and sterically demanding α and β-substituted vinyl arenes (section 2.6, ESI†). None of these substrates underwent hydroamination even under forced conditions; the by-product, ether 4a, was the only isolable product in most cases. This suggests that the initial protonation of alkenes to give a sterically unhindered yet stable carbocation intermediate is the primary requirement for successful hydroamination. Deprotection of the Fmoc group over the benzylic group was achieved under mild conditions using 2 equivalents of KOH to release free amine in an excellent yield. However, the amine was moderately sensitive; it was isolated as the corresponding oxalate salt (Table 2, 5a & 5b) in 58% and 80% yields from 3a and 3b, respectively.70
To understand the mechanism of the hydroamination reaction, we performed detailed 1H, 19F{1H} NMR studies. C6F6 (0.1 M in CDCl3) in a closed capillary was used as the reference (δ = –165 ppm) for 19F{1H} NMR. Independent 19F{1H} and 1H NMR analysis with triflic acid and reaction monitoring experiments indicated the interaction between 2a and 3a with triflic acid. In 19F{1H} NMR, triflic acid showed a peak at −79.1 ppm (−81.2 ppm due to moisture absorption)31,71 (Fig. 2a). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of triflic acid to Fmoc-NH2 (2a) showed a peak at −81.7 ppm (Fig. 2b and Fig. S2, ESI†), and triflic acid to product 3a showed a peak at −81.6 ppm (Fig. 2c and Fig. S3, ESI†). The reaction monitored by 19F{1H} NMR also shows an overlapping peak at −81.2 ppm, and no free triflic acid peak was observed at −79.1 ppm, which affirms the interaction (Fig. S4, ESI†).31,72,73
1 ratio of triflic acid to Fmoc-NH2 (2a) showed a peak at −81.7 ppm (Fig. 2b and Fig. S2, ESI†), and triflic acid to product 3a showed a peak at −81.6 ppm (Fig. 2c and Fig. S3, ESI†). The reaction monitored by 19F{1H} NMR also shows an overlapping peak at −81.2 ppm, and no free triflic acid peak was observed at −79.1 ppm, which affirms the interaction (Fig. S4, ESI†).31,72,73
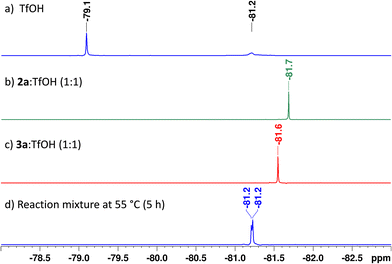 | ||
| Fig. 2 19F{1H} NMR analysis (the −78 to −83 ppm region was zoomed for clarity; C6F6 was used as a reference at −165 ppm) of TfOH along with Fmoc-NH2 (2a), the hydroamination product (3a) and the reaction mixture after 5 h. For detailed reaction conditions and analysis, see the ESI.† | ||
The 1H NMR study of styrene with 10 mol% triflic acid showed immediate decomposition, probably due to polymerization (Fig. S5, ESI†).74–76 The 1H NMR experiment of triflic acid and Fmoc-NH2 shows the disappearance of the NH peak at 4.71 ppm and a broad peak was observed with continuous drift (Fig. S6, ESI†). However, 1H NMR analysis of 3a with triflic acid showed substantial product decomposition in the presence of excess acid via benzyl group cleavage (Fig. S7, ESI†).77–79 This experimental evidence corroborates the interaction between triflic acid with Fmoc-NH2 and the product (3a). We believe that the interaction between triflic acid and 2a/3a is the major factor preventing styrene polymerization.
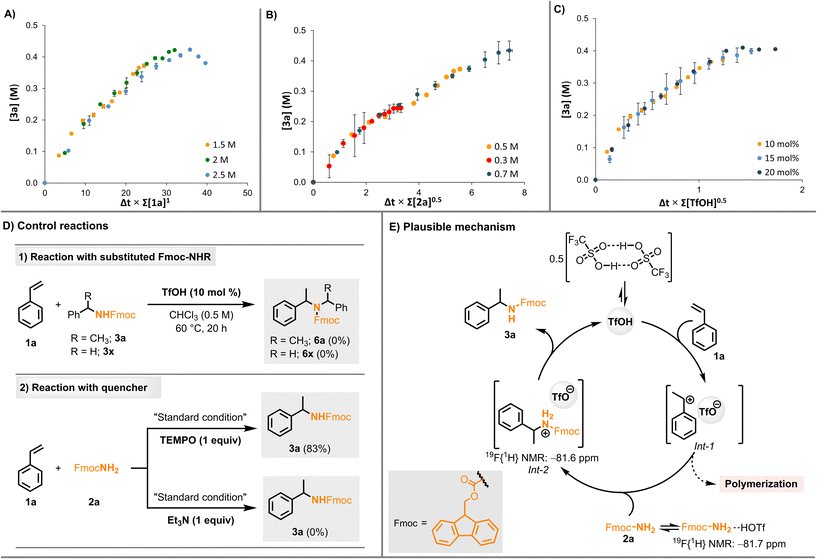
We envisioned that variable time normalisation analysis (VTNA) kinetic studies developed by Burés and co-workers and product inhibition studies80–84 could further confirm the interaction between triflic acid and 2a/3a. The experiments for VTNA analysis were conducted using HPLC (see section 2.4, ESI†). The different excess experiments resulted in a reaction order value of 1 for styrene, 0.5 for Fmoc-NH2, and 0.5 for triflic acid (Fig. 3A–C and Fig. S15–S17, ESI†). The fractional order for Fmoc-NH2 and triflic acid pointed toward either catalyst deactivation or product inhibition.82 To understand it further, a “same excess” kinetic experiment was conducted. A significant deviation from the standard reaction profile indicates product inhibition or catalyst deactivation (Fig. S14, ESI†). The deviation observed in the same excess experiments might be due to the interaction of the catalyst with Fmoc-NH2 (2a) or with the product (3a).
To understand the reason for the selectivity of 3a for over-alkylation, styrene (1a) was treated independently with 3a and 3x in the presence of triflic acid under optimized conditions as well as under forcing conditions, wherein 3a and 3x were recovered in near quantitative amounts (92% and 95%, respectively) (Fig. 3D1). We believe that the steric bulk of the Fmoc group may suppress over-alkylation. Next, we attempted to identify the nature of the intermediates involved in the hydroamination reaction. The potential of strong Brønsted acids to generate carbocations from styrene was previously reported by List and co-workers.85,86 In addition, the exclusive formation of the Markovnikov product provides reliable evidence for the involvement of benzylic carbocation intermediates. To rule out the participation of radical intermediates, we repeated the experiment in the presence of TEMPO. Only a slight decline in the yield (<10% decrease) was observed, which ruled out radical pathways. Adding a base, triethylamine, inhibited the hydroamination reaction completely, suggesting that the Brønsted acid acted as the catalyst (Fig. 3D2). The optimized reaction was carried out in CDCl3 instead of CHCl3, and no H/D exchange between triflic acid and the solvent was observed (section 2.5 in the ESI†). Based on these NMR studies, VTNA kinetic studies, and control reactions, a plausible mechanism for the hydroamination reaction is proposed, as shown in Fig. 3E. The triflic acid dimer releases the monomer triflic acid in CHCl3, as reported earlier.71,72,87 It protonates styrene to form a benzylic carbocation intermediate that is stabilized by the triflate counter anion (Int-1, Fig. 3E). Int-1 (styrene in the presence of triflic acid) may undergo cationic polymerization as observed in the control reaction without Fmoc-NH2 (Fig. S5, ESI†). The intermediate (Int-1) undergoes a nucleophilic attack by Fmoc-NH2, which results in the selective Markovnikov hydroamination product in the protonated form (Int-2, Fig. 3E), followed by deprotonation to yield the product and regenerate triflic acid. The free triflic acid and the protonated amines (Int-2 or Fmoc-NH2·TfOH) are likely in equilibrium. However, the equilibrium is largely shifted towards protonated amines, as we did not observe free TfOH in the 19F{1H} NMR of the reaction mixture. Hence, we speculate that a significantly low concentration of free TfOH prevents styrene polymerization.
Conclusions
In conclusion, we have demonstrated a scalable, metal-free intermolecular hydroamination reaction for the synthesis of Fmoc-protected 1-arylethylamines using triflic acid as the catalyst and Fmoc-NH2 as the amine source. Various vinylarene substrates afforded good yields (up to 91%). Activated endocyclic alkenes also exhibited good reactivity. Subsequently, deprotection allowed access to the primary amine oxalate salt in excellent yield. Mechanistic investigations were performed using 1H and 19F{1H} NMR techniques and showed the interaction between triflic acid and 2a/3a. VTNA kinetic studies and product inhibition experiments further supported the transient hydrogen bonding interactions. This interaction prevented styrene polymerization; however, it was sufficient for hydroamination. The control reactions further supported the proposed mechanistic cycle along with the selective mono-alkylation. We believe that mechanistic investigation and the developed methodology will allow a comprehensive understanding of the hydroamination reaction of alkenes.Author contributions
RM acquired the funding and supervised the project. ACS designed the project and performed the experiments. CS carried out mechanistic studies and reperformed a few substrate reactions. ACS prepared the first draft and wrote the manuscript with contributions from CS and RM. All the authors approved the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.† Materials and methods, detailed optimization table, experimental procedure, characterization data, NMR and VTNA analysis, and all NMR and HRMS spectra.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Indian Institute of Technology Goa (IIT Goa) for the infrastructure and Santu Sinha for assisting in recording the VT NMR spectra. The authors thank the Max Planck Society (MPG) and Max-Planck-Institut für Kohlenforschung (MPI Kofo) for generous funding through the “Max Planck India partner group” project. The authors are grateful to Prof. Benjamin List for his support throughout this project. The authors thank the CAIF of IISER Berhampur for HRMS sample analysis.References
- R. Y. Liu and S. L. Buchwald, Acc. Chem. Res., 2020, 53, 1229–1243 CrossRef CAS PubMed.
- S. Ma and J. F. Hartwig, Acc. Chem. Res., 2023, 56, 1565–1577 CrossRef CAS PubMed.
- T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed.
- I. P. Beletskaya, C. Naájera and M. Yus, Russ. Chem. Rev., 2020, 89, 1074 CrossRef CAS.
- J. Huo, G. He, W. Chen, X. Hu, Q. Deng and D. Chen, BMC Chem., 2019, 13, 89 CrossRef PubMed.
- K. C. Hultzsch, Org. Biomol. Chem., 2005, 3, 1819–1824 RSC.
- F. Pohlki and S. Doye, Chem. Soc. Rev., 2003, 32, 104–114 RSC.
- R. Severin and S. Doye, Chem. Soc. Rev., 2007, 36, 1407–1420 RSC.
- J. Escorihuela, A. Lledós and G. Ujaque, Chem. Rev., 2023, 123, 9139–9203 CrossRef CAS PubMed.
- J. F. Hartwig, Pure Appl. Chem., 2004, 76, 507–516 CrossRef CAS.
- A. M. Johns, M. Utsunomiya, C. D. Incarvito and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 1828–1839 CrossRef CAS PubMed.
- M. Nobis and B. Driessen-Hölscher, Angew. Chem., Int. Ed., 2001, 40, 3983–3985 CrossRef CAS PubMed.
- I. Bytschkov and S. Doye, Eur. J. Org. Chem., 2003, 935–946 CrossRef CAS.
- A. Reyes-Sánchez, I. García-Ventura and J. J. García, Dalton Trans., 2013, 43, 1762–1768 RSC.
- C. Lee, H.-J. Kang and S. Hong, Chem. Sci., 2024, 15, 442–457 RSC.
- Y. Li and T. J. Marks, J. Am. Chem. Soc., 1996, 118, 9295–9306 CrossRef.
- M. Patel, R. K. Saunthwal and A. K. Verma, Acc. Chem. Res., 2017, 50, 240–254 CrossRef CAS PubMed.
- A. N. Selikhov, A. V. Cherkasov, Y. V. Nelyubina and A. A. Trifonov, ACS Catal., 2023, 13, 12582–12590 CrossRef CAS.
- C. Lee, H.-J. Kang and S. Hong, Chem. Sci., 2024, 15, 442–457 RSC.
- P. Colonna, S. Bezzenine, R. Gil and J. Hannedouche, Adv. Synth. Catal., 2020, 362, 1550–1563 CrossRef CAS.
- J. S. Bandar, M. T. Pirnot and S. L. Buchwald, J. Am. Chem. Soc., 2015, 137, 14812–14818 CrossRef CAS PubMed.
- Y. Yang, S.-L. Shi, D. Niu, P. Liu and S. L. Buchwald, Science, 2015, 349, 62–66 CrossRef CAS PubMed.
- K. C. Hultzsch, Adv. Synth. Catal., 2005, 347, 367–391 CrossRef CAS.
- Y. Yang, N. I. Wong and P. Teo, Eur. J. Org. Chem., 2015, 1207–1210 CrossRef CAS.
- D. A. Patton and M. E. Cremeens, Rev. J. Chem., 2014, 4, 1–20 CrossRef.
- L. D. Julian and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 13813–13822 CrossRef CAS PubMed.
- C. Lepori and J. Hannedouche, Synthesis, 2017, 1158–1167 CAS.
- S. Bestgen and P. W. Roesky, in Early Main Group Metal Catalysis, John Wiley & Sons, Ltd, 2020, pp. 59–91 Search PubMed.
- J. G. Taylor, L. A. Adrio and K. K. (Mimi) Hii, Dalton Trans., 2010, 39, 1171–1175 RSC.
- J. Chen, S. K. Goforth, B. A. McKeown and T. B. Gunnoe, Dalton Trans., 2017, 46, 2884–2891 RSC.
- T. T. Dang, F. Boeck and L. Hintermann, J. Org. Chem., 2011, 76, 9353–9361 CrossRef CAS PubMed.
- S. Qin, Y. Liao, Q. Ni, R. Cao, A. S. C. Chan and L. Qiu, J. Org. Chem., 2025, 90, 1016–1023 CrossRef CAS PubMed.
- J. Penzien, R. Q. Su and T. E. Müller, J. Mol. Catal. A: Chem., 2002, 182–183, 489–498 CrossRef CAS.
- Y.-M. Wang, T.-T. Li, G.-Q. Liu, L. Zhang, L. Duan, L. Li and Y.-M. Li, RSC Adv., 2014, 4, 9517–9521 RSC.
- B. List, Chem. Rev., 2007, 107, 5413–5415 CrossRef CAS.
- T. Akiyama and K. Mori, Chem. Rev., 2015, 115, 9277–9306 CrossRef CAS PubMed.
- N. Frank, M. Leutzsch and B. List, J. Am. Chem. Soc., 2025, 147, 7932–7938 CrossRef CAS PubMed.
- E. Bernoud, C. Lepori, M. Mellah, E. Schulz and J. Hannedouche, Catal. Sci. Technol., 2015, 5, 2017–2037 RSC.
- Y.-D. Du, B.-H. Chen and W. Shu, Angew. Chem., Int. Ed., 2021, 60, 9875–9880 CrossRef CAS PubMed.
- P. Meena, A. M. Patel and A. K. Verma, Chem. Commun., 2022, 58, 8424–8427 RSC.
- X. Cheng, Y. Xia, H. Wei, B. Xu, C. Zhang, Y. Li, G. Qian, X. Zhang, K. Li and W. Li, Eur. J. Org. Chem., 2008, 1929–1936 CrossRef CAS.
- B. Schlummer and J. F. Hartwig, Org. Lett., 2002, 4, 1471–1474 CrossRef CAS PubMed.
- S. Guria, A. N. Volkov, R. Khudaverdyan, R. Van Lommel, R. Pan, C. G. Daniliuc, F. De Proft and U. Hennecke, J. Am. Chem. Soc., 2024, 146, 17180–17188 CrossRef CAS PubMed.
- F. C. Raps, A. Rivas-Souchet, C. M. Jones and T. K. Hyster, Nature, 2025, 637, 362–368 CrossRef CAS PubMed.
- L. L. Anderson, J. Arnold and R. G. Bergman, J. Am. Chem. Soc., 2005, 127, 14542–14543 CrossRef CAS PubMed.
- S. Streiff and F. Jérôme, Chem. Soc. Rev., 2021, 50, 1512–1521 RSC.
- J. I. van der Vlugt, Chem. Soc. Rev., 2010, 39, 2302–2322 RSC.
- S. Park, J. Jeong, K. Fujita, A. Yamamoto and H. Yoshida, J. Am. Chem. Soc., 2020, 142, 12708–12714 CrossRef CAS PubMed.
- J. Urbiña-Alvarez, S. Rincón-Carvajal and D. Gamba-Sánchez, Org. Biomol. Chem., 2023, 21, 7036–7051 RSC.
- Y. Brägger, A.-S. K. Paschke, N. Nasiri, B. B. Botlik, F. Felician and B. Morandi, Science, 2025, 387, 1108–1114 CrossRef PubMed.
- D. S. Surry and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 10354–10355 CrossRef CAS PubMed.
- P. G. Alsabeh, R. J. Lundgren, R. McDonald, C. C. C. Johansson Seechurn, T. J. Colacot and M. Stradiotto, Chem. – Eur. J., 2013, 19, 2131–2141 CrossRef CAS PubMed.
- A. Borzenko, N. L. Rotta-Loria, P. M. MacQueen, C. M. Lavoie, R. McDonald and M. Stradiotto, Angew. Chem., 2015, 127, 3844–3848 CrossRef.
- O. I. Afanasyev, E. A. Kuchuk, K. M. Muratov, G. L. Denisov and D. Chusov, Eur. J. Org. Chem., 2021, 543–586 CrossRef CAS.
- X. Huang and S. L. Buchwald, Org. Lett., 2001, 3, 3417–3419 Search PubMed.
- D.-Y. Lee and J. F. Hartwig, Org. Lett., 2005, 7, 1169–1172 Search PubMed.
- S. Lee, M. Jørgensen and J. F. Hartwig, Org. Lett., 2001, 3, 2729–2732 CrossRef CAS PubMed.
- C. Sivarajan, S. Saha, S. Mulla and R. Mitra, J. Org. Chem., 2024, 89, 17021–17030 CrossRef CAS PubMed.
- S. Ma, C. K. Hill, C. L. Olen and J. F. Hartwig, J. Am. Chem. Soc., 2021, 143, 359–368 CrossRef CAS PubMed.
- S. Gandhi and B. List, Angew. Chem., Int. Ed., 2013, 52, 2573–2576 CrossRef CAS PubMed.
- S. K. Alamsetti, A.KÅ. Persson and J.-E. Bäckvall, Org. Lett., 2014, 16, 1434–1437 CrossRef PubMed.
- R. Mansouri, Z. Aouf, S. Lakrout, M. Berredjem and N.-E. Aouf, J. Braz. Chem. Soc., 2016, 27, 546–550 CAS.
- G. B. Fields, in Peptide Synthesis Protocols, ed. M. W. Pennington and B. M. Dunn, Humana Press, Totowa, NJ, 1995, pp. 17–27 Search PubMed.
- K. D. Collins, A. Rühling, F. Lied and F. Glorius, Chem. – Eur. J., 2014, 20, 3800–3805 CrossRef CAS PubMed.
- A. Chakraborty, R. Purkait, U. C. De, D. K. Maiti and S. Majumdar, ChemistrySelect, 2016, 1, 2668–2672 CrossRef CAS.
- M. P. Doyle, A. B. Dyatkin and C. L. Autry, J. Chem. Soc., Perkin Trans. 1, 1995, 619–621 RSC.
- A. F. Hegarty and L. N. Frost, J. Chem. Soc., Perkin Trans. 2, 1973, 1719–1728 RSC.
- M. Sugiura, H. Hagio, R. Hirabayashi and S. Kobayashi, J. Am. Chem. Soc., 2001, 123, 12510–12517 CrossRef CAS PubMed.
- S. Lee, P. S. J. Kaib and B. List, J. Am. Chem. Soc., 2017, 139, 2156–2159 CrossRef CAS PubMed.
- H. R. Rajegowda, B. S. Chethan, R. U. R. Khan, N. K. Lokanath, P. A. Suchetan and P. R. Kumar, J. Mol. Struct., 2023, 1272, 134097 CrossRef CAS.
- M. P. Muñoz and G. C. Lloyd-Jones, Eur. J. Org. Chem., 2009, 516–524 CrossRef.
- E. S. Stoyanov, K.-C. Kim and C. A. Reed, J. Phys. Chem. A, 2004, 108, 9310–9315 CrossRef CAS.
- G. A. Olah, T. Heiner, G. Rasul and G. K. S. Prakash, J. Org. Chem., 1998, 63, 7993–7998 CrossRef CAS.
- A. D. Allen, M. Rosenbaum, N. O. L. Seto and T. T. Tidwell, J. Org. Chem., 1982, 47, 4234–4239 CrossRef CAS.
- M. Chmelir, N. Cardona and G. V. Schulz, Die Makromol. Chem., 1977, 178, 169–185 Search PubMed.
- T. Kunitake and K. Takarabe, Polym. J., 1978, 10, 105–110 CrossRef CAS.
- G. A. Olah and M. Calin, J. Am. Chem. Soc., 1968, 90, 401–404 CrossRef CAS PubMed.
- H. Kurouchi, K. Kawamoto, H. Sugimoto, S. Nakamura, Y. Otani and T. Ohwada, J. Org. Chem., 2012, 77, 9313–9328 CrossRef CAS PubMed.
- F. Rombouts, D. Franken, C. Martínez-Lamenca, M. Braeken, C. Zavattaro, J. Chen and A. A. Trabanco, Tetrahedron Lett., 2010, 51, 4815–4818 Search PubMed.
- C. D.-T. Nielsen and J. Burés, Chem. Sci., 2019, 10, 348–353 RSC.
- J. Burés, Angew. Chem., Int. Ed., 2016, 55, 16084–16087 CrossRef PubMed.
- J. Burés, Angew. Chem., Int. Ed., 2016, 55, 2028–2031 CrossRef PubMed.
- D. G. Blackmond, Angew. Chem., Int. Ed., 2005, 44, 4302–4320 CrossRef CAS PubMed.
- D. G. Blackmond, J. Am. Chem. Soc., 2015, 137, 10852–10866 CrossRef CAS PubMed.
- N. Tsuji, J. L. Kennemur, T. Buyck, S. Lee, S. Prévost, P. S. J. Kaib, D. Bykov, C. Farès and B. List, Science, 2018, 359, 1501–1505 CrossRef CAS PubMed.
- V. K. Singh, C. Zhu, C. K. De, M. Leutzsch, L. Baldinelli, R. Mitra, G. Bistoni and B. List, Science, 2023, 382, 325–329 Search PubMed.
- E. L. Varetti, Spectrochim. Acta, Part A, 1988, 44, 733–738 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00519a |
| This journal is © The Royal Society of Chemistry 2025 |