Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Domino synthesis of a new class of red-shifted and antimicrobial imidazole-based azo dyes from 5-aminoimidazole-4-carboxamidrazones†
Mariana P.
Silva
 a,
Bárbara
Silva
bc,
Inês
Costa
bc,
Paulo J. G.
Coutinho
a,
Bárbara
Silva
bc,
Inês
Costa
bc,
Paulo J. G.
Coutinho
 d,
Fátima
Cerqueira
efg,
Eugénia
Pinto
hi and
Alice M.
Dias
d,
Fátima
Cerqueira
efg,
Eugénia
Pinto
hi and
Alice M.
Dias
 *a
*a
aCentre of Chemistry of University of Minho (CQ-UM), Department of Chemistry, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: ad@quimica.uminho.pt
bAssociate Laboratory i4HB—Institute for Health and Bioeconomy, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal
cUCIBIO—Applied Molecular Biosciences Unit, Laboratory of Toxicology, Department of Biological Sciences, Faculty of Pharmacy, Porto University, 4050-313 Porto, Portugal
dPhysics Centre of Minho and Porto Universities (CF-UM-UP) and LaPMET (Laboratory of Physics for Materials and Emergent Technologies), Campus de Gualtar, 4710-057 Braga, Portugal
eFP-I3ID, FP-BHS, GIT-LoSa, University Fernando Pessoa, Praça 9 de Abril, 349, 4249-004 Porto, Portugal
fFaculty of Health Sciences, University Fernando Pessoa, Rua Carlos da Maia, 296, 4200-150 Porto, Portugal
gMolecular Oncology and Viral Pathology Group, Research Center of IPO Porto (CI-IPOP)/RISE@CI-IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto)/Porto Comprehensive Cancer Center (Porto.CCC), Porto, Portugal
hCIIMAR/CIMAR, Interdisciplinary Centre of Marine and Environmental Research, Terminal de Cruzeiros do Porto de Leixões, 4450-208 Matosinhos, Portugal
iLaboratory of Microbiology, Biological Sciences Department, Faculty of Pharmacy of University of Porto, 4050-313 Porto, Portugal
First published on 22nd April 2025
Abstract
Combining antimicrobial activity with halochromic properties offers an enticing opportunity to both combat and detect microbial infection in the medical field. Previous research has identified a class of compounds that demonstrate potent antimicrobial activity, particularly against pathogenic yeasts. Notably, these compounds also exhibit vibrant pink–blue colours and a halochromic behaviour within the therapeutic pH range. Aiming at developing a more sustainable synthetic method and to generate better red-shifted and pH-responsive imidazole-based azo dyes, a new domino reaction was discovered yielding a novel class of green-coloured 2-aminoimidazole azo-dyes. A strategy based on circular chemistry was achieved by introducing the accessible and environmentally benign O2/KI oxidant system, which is able not only to regenerate the oxidants but also minimize side reactions. The isolation of two key intermediates was also an important achievement in comprehending the underlying mechanisms. As expected, this new class of 2-aminoimidazole dyes also presented significant solvatochromism and pronounced halochromism with colours changing from green to purple as the pH decreases. Ab initio molecular quantum mechanics calculations supported the data obtained experimentally. A great number of compounds (12 in 18 compounds) showed potent fungicidal activity against C. neoformans and moderate activity against C. krusei.
1. Introduction
Infections caused by pathogenic fungi affect more than a billion people worldwide, and invasive fungal infections are responsible for higher mortality rates and result in higher death tolls than tuberculosis or malaria.1 The majority of these deaths are caused by pathogenic fungi of the genus Candida, Cryptococcus, Pneumocystis, and Aspergillus.2 As mycoses are one of the major causes of morbidity/mortality among immunocompromised individuals, the World Health Organization (WHO) defined a fungal pathogens priority list to guide research development, and public health action. The list includes Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus in the critical priority group while Pichia kudriavzevii (Candida krusei) is included in the medium priority group.3Nonetheless, the increased resistance to antifungal drugs of non-albicans Candida (NAC) species, including C. krusei, is changing the epidemiology of candidiasis. In addition, C. krusei can cause life-threatening infections in patients with hematologic malignancies and immune-compromised individuals, with a higher risk in cases of prolonged azole prophylaxis.2 Among the predominant fungi impacting human health, C. neoformans show alarming statistics with a global burden of cryptococcal meningitis of 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases/181
000 cases/181![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths annually, and involving a mortality rate of 100% for untreated infections.1,4 Moreover, treatment options for cryptococcosis are more limited, as Cryptococcus spp. has an inherent resistance to echinocandins.1
000 deaths annually, and involving a mortality rate of 100% for untreated infections.1,4 Moreover, treatment options for cryptococcosis are more limited, as Cryptococcus spp. has an inherent resistance to echinocandins.1
The few currently available antifungal drugs for invasive fungal infection treatments are limited to polyenes, azoles and echinocandins. However, their high host toxicity, the acquired fungal resistance, and the advent of other species with a broader resistance spectrum raise the need for new molecules that combine enhanced fungicidal activity with lower host toxicity while being capable of evading common resistance mechanisms.24,25
Chemical production faces major problems with the handling of waste, large expenses and reduction of resources, as well as inefficient processes.5 These problems require solutions that would not only be more environmentally friendly but also reduce resource consumption and cut production costs. Domino reactions (also known as cascade or Tandem reactions) are extremely beneficial to synthetic chemists as chemical conversions are sequential reactions occurring in the same reaction vessel, and products of one reaction are substrates for the next sequential reaction step. The benefits over the usual stepwise formation of the individual bonds include a high atom economy and a more streamlined process. In addition, the replacement of the expensive and toxic chemicals with environmentally benign reagents/catalysts and synthesis design closer to the principles of the circular economy aligns with sustainability principles while benefiting chemical production.6
Azobenzenes stand out concerning their colouring ability, which inspired the synthesis of a wide range of azo dyes with a myriad of applications including dyeing of natural and synthetic materials, medicine, inks, cosmetics, food and paints.7 The classical method to obtain azo dyes is a two-step pathway involving diazotisation and coupling reactions with electron-rich aromatic species.7,8 The azobenzene chromophore absorbs radiation in the visible region, around a wavelength of 450 nm (molar absorption coefficient (ε) ≈ 400 L mol−1 cm−1), due to a n–π* transition from the ground state (S0) to the first singlet excited state (S1), and in the ultraviolet (UV) region, around a wavelength of 300 nm (molar absorption coefficient (ε) ≈ 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1), due to a π–π* transition to the second excited state (S2).7,9–12 In the aminoazobenzenes, electron-donating amino groups at the 4-position, or further substitution with cyano or nitro groups, respectively, lead to a bathochromic shift that brings together the π–π* and n–π* bands.7 Aminoazobenzenes also display halochromic properties, meaning they are sensitive to pH and undergo visible colour change upon pH variation. If protonation occurs in the nitrogen of the azo-group, a bathochromic shift occurs, while a protonation at peripheral nitrogens causes a hypsochromic shift.13 Typically, an equilibrium between ammonium and azonium ions is established, where each of these two tautomeric forms originates a different absorption band and exhibits a different colour.14
000 L mol−1 cm−1), due to a π–π* transition to the second excited state (S2).7,9–12 In the aminoazobenzenes, electron-donating amino groups at the 4-position, or further substitution with cyano or nitro groups, respectively, lead to a bathochromic shift that brings together the π–π* and n–π* bands.7 Aminoazobenzenes also display halochromic properties, meaning they are sensitive to pH and undergo visible colour change upon pH variation. If protonation occurs in the nitrogen of the azo-group, a bathochromic shift occurs, while a protonation at peripheral nitrogens causes a hypsochromic shift.13 Typically, an equilibrium between ammonium and azonium ions is established, where each of these two tautomeric forms originates a different absorption band and exhibits a different colour.14
Red-shifted azobenzenes are promising candidates for use in photopharmacology as they exhibit photo-responsive signals within the spectrum of visible light,10 thereby avoiding the harmful side effects of UV radiation. These compounds can be obtained by three main strategies that involve push–pull systems,7,15 bridged azobenzenes,7 and ortho-substituted azobenzenes with two amino,16 two ortho-methoxy,17 two ortho-fluorine groups,18 or tetra-ortho-methoxy substituents.19–23
The addition of heterocyclic moieties to azobenzenes opens a new world of possibilities regarding their photophysical, chemical, and biological properties.24 Arylazoimidazoles, the second most utilized and studied heteroaryl azo-dyes after azopyridines,25,26 are a pivotal example of the hydrazone tautomerism mechanism, and may act as bases due to the basic nitrogen atom in the imidazole ring. Similarly to other azobenzenes, the classical synthetic route for azoimidazoles is diazotization of the aromatic or heterocyclic amine, followed by coupling of the diazonium salt with a nucleophile segment (Scheme 1A).27–29
Imidazoles are one of the most promising classes of contemporary antifungal agents. Showing broad spectrum and potent activity, excellent bioavailability, good tissue penetrability and permeability and a relatively low incidence of adverse and toxic effects, they have been found effective in the treatment of various infectious diseases.4 In addition, the 2-aminoimidazole moiety is an extremely useful building block for the design of small-molecule drugs as modulators of different targets.30 The methods for obtaining this moiety are the condensation of α-aminoketones or α-aminoaldehydes with cyanamides, or α-haloketones with guanidine derivatives; and the reaction between propargylic amines and carbodiimides,31 but expensive catalysts, harsh conditions and availability of reagents are still major limitations of these methods. The alternative functionalization of imidazole derivatives also requires multiple steps, protection/deprotection strategies, and activation of C-2 for the introduction of the amine.
Previous findings of our group led us to obtain a novel class of 2-aminoimidazole azo dyes (3) from Ag+-mediated oxidation of imidazole-based amidrazones 1, followed by the addition of secondary amines to the azo intermediates 2, generating the 2-aminoimidazole skeleton (Scheme 1B).32 These unique azo-dyes (3) exhibited unusual red-shifted absorption bands, and significant halochromism near the physiological pH with the vibrant magenta (neutral form) changing to a deep blue (protonated form) colour. They also demonstrated potent antimicrobial properties against infectious yeasts (minimum inhibitory concentration (MIC) ≈ 4 μg mL−1for C. krusei), and no cytotoxicity was detected for concentrations lower than 16 μg mL−1.32 The halochromism near the physiological pH, as well as the promising antifungal activity, allowed the development of (bio)medical and textile applications.33,34
Despite the previous method (B) being simple and fast at mild conditions, a large excess of the secondary amine is required to prevent degradation events and the formation of unwanted secondary products. Moreover, stoichiometric amounts of the expensive silver nitrate were required, and an additional purification step is mandatory to remove the metallic Ag0. On the search for greener catalysts and more sustainable conditions, we found a novel class of 2-aminoimidazole-based azo-dyes 5 (Scheme 1C) that showed an absorption band even more red-shifted than the previous azo-dyes 3, exhibiting a vibrant green colour that changes to purple upon protonation. These azo-dyes also showed moderate-strong activity against C. krusei and potent activity against C. neoformans.
2. Results and discussion
2.1. Synthesis
The first objective of our recent research was to replace silver nitrate with a more sustainable oxidant. Preliminary attempts to replace silver nitrate by atmospheric air evidenced a fast reaction with the production of hydrogen peroxide, but degradation and formation of unwanted secondary products were still major drawbacks. As hydrogen peroxide has been associated with the breakdown of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, leading to degradation of the azo chromophore,35 preliminary investigations of the stability of our azo dyes in the presence of hydrogen peroxide were conducted. Results evidenced a fast loss of colour, suggesting the reduction of the azo bond possibly followed by fragmentation reactions. These results led us to explore the use of potassium iodide (KI) to promote the cyclic decomposition of hydrogen peroxide (H2O2) and the in situ generation of O2, according to Scheme 2A.
N bonds, leading to degradation of the azo chromophore,35 preliminary investigations of the stability of our azo dyes in the presence of hydrogen peroxide were conducted. Results evidenced a fast loss of colour, suggesting the reduction of the azo bond possibly followed by fragmentation reactions. These results led us to explore the use of potassium iodide (KI) to promote the cyclic decomposition of hydrogen peroxide (H2O2) and the in situ generation of O2, according to Scheme 2A.
 | ||
| Scheme 2 Proposed reaction mechanism for the synthesis of azoimidazoles 5 from amidrazone precursors 1. | ||
Various amidrazones 1 were reacted with piperidine in the presence of KI, and it was found that amidrazones with aromatic substituents at the N1-position (R1 = aryl) enabled to isolate solid mixtures with a dark green colour (Table 1). NMR data of these mixtures was not sufficient to shed light on the structure of the products, but chemical shifts of the observed phenyl groups revealed the presence of two amidrazones and two azo compounds. One of the amidrazones was assigned to the starting reagent (1), but the other three compounds contained piperidine units, as three sets of methylenic N(CH2)2-protons were identified. These compounds were later identified as compounds 4I, 4II and 5.
| Starting reagent | Reagents | Solvents | Temp. (°C) | Products (1H NMR) | Yield (%) |
|---|---|---|---|---|---|
| a Yield was not calculated due to the presence of a complex mixture. | |||||
| 1e | 2 eq. Pip | H2O/ACN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 | 4i_I (36%) | |
| 3 eq. KI | 4i_II (14%) | ||||
| 5i (50%) | |||||
| 1e | 2 eq. Pip | ACN | 25 | 5i (100%) | 34 |
| 3 eq. KI | |||||
| 1e | 3 eq. Et3N | H2O/ACN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 5i (100%) | 77 |
| 2 eq. Pip | |||||
| 0.2 KI | |||||
| 1e | 2 eq. Pip | H2O/ACN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 5i (90%) | |
| 3 eq. KI | Unwanted products (10%) | ||||
| 1f | 2 eq. Pip | H2O/ACN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0 | 4j_I (100%) | 67 |
| 3 KI | |||||
| 4j | 3 eq. Et3N | ACN | 40 | 5j (100%) | 47 |
| 4j | 3 eq. Et3N | ACN | 40 | 5j (100%) | 46 |
Once the fast formation of azo-dyes in the sustainable O2/KI oxidation system was demonstrated, finding the ideal mixture of solvents that led to complete the consumption of amidrazone 1 was crucial, and it was found that the mixture of acetonitrile/water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in superior yields. Then, several attempts were performed to find the appropriate concentrations of KI and piperidine. It was concluded that only catalytic quantities of KI were needed and two equivalents of piperidine were sufficient to guarantee the success of the reaction, unlike the ten equivalents required in the synthesis of the azo-dyes 3. Temperature also played an essential role in reaction rate and selectivity, and it was possible to successfully isolate three intermediates 4 in the pure form by lowering the reaction temperature to 0 °C. Full characterization of these intermediates was not possible due to their unstable nature, as they promptly evolve to the final azo-dyes 5 when maintained in solution. However, structures of tautomer 4I could be assigned with significant confidence based on the data obtained from 1H, 13C NMR and 2D (HSQC and HMBC) spectroscopy. For tautomer 4d_II, although the isolation was also possible, its evolution in DMSO-d6 was so fast that it only allowed to infer its structure by comparison of the 1H NMR spectra of the two tautomers (4I and 4II). Both compounds present a phenyl group, but major noticeable differences exist. 4II shows an azo-like structure as the ortho protons of the aza phenyl group (N
1) resulted in superior yields. Then, several attempts were performed to find the appropriate concentrations of KI and piperidine. It was concluded that only catalytic quantities of KI were needed and two equivalents of piperidine were sufficient to guarantee the success of the reaction, unlike the ten equivalents required in the synthesis of the azo-dyes 3. Temperature also played an essential role in reaction rate and selectivity, and it was possible to successfully isolate three intermediates 4 in the pure form by lowering the reaction temperature to 0 °C. Full characterization of these intermediates was not possible due to their unstable nature, as they promptly evolve to the final azo-dyes 5 when maintained in solution. However, structures of tautomer 4I could be assigned with significant confidence based on the data obtained from 1H, 13C NMR and 2D (HSQC and HMBC) spectroscopy. For tautomer 4d_II, although the isolation was also possible, its evolution in DMSO-d6 was so fast that it only allowed to infer its structure by comparison of the 1H NMR spectra of the two tautomers (4I and 4II). Both compounds present a phenyl group, but major noticeable differences exist. 4II shows an azo-like structure as the ortho protons of the aza phenyl group (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh) appear at δ 7.25–7.26 ppm (doublet), while 4I presents the corresponding ortho protons of the phenylhydrazone group (C
NPh) appear at δ 7.25–7.26 ppm (doublet), while 4I presents the corresponding ortho protons of the phenylhydrazone group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NHPh) at δ 6.80–6.82 ppm, revealing an amidrazone-like structure. This was also deduced based on the presence and relative position of the NH and NH2 groups. For instance, 4II presents only one NH2 signal (δ 6.09–6.10 ppm) and two NH signals (the imino group at δ 9.11–9.12 ppm, and the imidazole NH at δ 9.26–9.37 ppm). In contrast with this, 4I shows two NH2 signals (δ 5.21–5.39 ppm and δ 5.60–5.65 ppm) and only one NH signal belonging to the phenylhydrazone unit (δ 7.84–7.89 ppm). Another significant and easily identifiable difference is the presence of a CH signal in 4II, whose proton appears as a singlet at δ 6.39–6.42 ppm, which is typical of a proton bonded to a sp3 carbon with two nitrogen neighbours. The presence of this tetrahedral carbon was also confirmed by 13C NMR spectroscopy as it appeared at δ 95.9 ppm and showed a direct correlation with the proton at δ 6.39–6.42 ppm, in the HSQC spectrum. Moreover, the two forms can be distinguished by the relative position of piperidine, which presents a chemical shift of δ 2.55–2.56 ppm in 4II and δ 2.77–2.78 ppm in 4I.
N–NHPh) at δ 6.80–6.82 ppm, revealing an amidrazone-like structure. This was also deduced based on the presence and relative position of the NH and NH2 groups. For instance, 4II presents only one NH2 signal (δ 6.09–6.10 ppm) and two NH signals (the imino group at δ 9.11–9.12 ppm, and the imidazole NH at δ 9.26–9.37 ppm). In contrast with this, 4I shows two NH2 signals (δ 5.21–5.39 ppm and δ 5.60–5.65 ppm) and only one NH signal belonging to the phenylhydrazone unit (δ 7.84–7.89 ppm). Another significant and easily identifiable difference is the presence of a CH signal in 4II, whose proton appears as a singlet at δ 6.39–6.42 ppm, which is typical of a proton bonded to a sp3 carbon with two nitrogen neighbours. The presence of this tetrahedral carbon was also confirmed by 13C NMR spectroscopy as it appeared at δ 95.9 ppm and showed a direct correlation with the proton at δ 6.39–6.42 ppm, in the HSQC spectrum. Moreover, the two forms can be distinguished by the relative position of piperidine, which presents a chemical shift of δ 2.55–2.56 ppm in 4II and δ 2.77–2.78 ppm in 4I.
When intermediates 4i reacted under atmospheric oxygen, the evolution to coloured products was fast, but contamination of azo-dyes 5 with traces of unwanted products was a limitation of the method (Table 1). These drawbacks led to optimising conditions by investigating the effects of increasing reaction temperature, as well as the presence of acid or base catalysts. When triethylamine was introduced in the reaction mixtures and the temperature was increased to 40 °C, products 5 were isolated pure. However, the additional step of isolating the intermediates 4 led to lower yields. As such, to prevent this issue, the sequential synthesis of 5i from amidrazones 1 and piperidine in a one-pot two-step method was attempted with success. These new conditions proved to be reproducible, including when different secondary amines were used (dimethylamine and pyrrolidine), allowing the isolation of eighteen azo-imidazoles 5 (Table 2) and demonstrating the versatility of the method.
This new class of azoimidazoles was characterized by 1H and 13C NMR spectroscopy. As azobenzene derivatives, these compounds contain a phenyl group, which was identified due to the presence of the aromatic protons at δ 7.40–7.72 ppm and the corresponding carbon atoms appearing at δ 152.8–121.4 ppm, identified by HMBC NMR spectroscopy. The core imidazole of this structure exhibits C-2, C-4 and C-5 signals that compare with the previous series of azo-dyes 3. However, the presence of the second sp2 nitrogen atom in the imidazole ring must be responsible for the observed shift of ca. 10 ppm to the lower field of C-2 signal (δ 169.0–171.9 ppm), as demonstrated by the three-bond HMBC correlation with the methylenic/methylic protons of the secondary amine moiety. Proton signals of this 2-amino substituent were also shifted to higher chemical shift values, as confirmed by the broad singlets (4H) at δ 3.88–3.89 ppm for piperidino, and δ 3.72–3.75 ppm for pyrrolidino, as well as the singlet (6 H) at δ 3.32–3.33 ppm for dimethylamino groups. C-4 signal is also shifted in the same direction (δ 136.7–137.5 ppm). The C-5 signal appears at δ 164.1–164.7 ppm and was assigned through correlations with the 5-NH proton (two-bond correlation) and the protons of Co of the R1 group's aromatic ring (four-bond correlations).
As proposed in Scheme 2, this reaction begins with the oxidation of amidrazone 1 to afford azoimidazole 2 in the presence of an O2/KI system (Scheme 2A). Once formed, this azoimidazole suffers a fast nucleophilic attack of the secondary amine to the C-2 carbon atom of the imidazole core (Scheme 2B). This attack weakens the structure of the imidazole core and allows a ring-opening reaction, which is favoured by the presence of the aromatic substituent because the negative charge developed will be particularly delocalized over the benzene ring. A rotation of the C4–C5 bond occurs, enabling the ring-closing by a nucleophilic attack of the NH group to the activated amidine group through a 5-exo-trig cyclization. This is the key step of this rearrangement that essentially causes the NR1 and NH groups to shift their positions when the imidazole ring is rebuilt. In the next steps, the tautomeric equilibria established must be shifted towards a more stable structure, restoring the aromaticity to the imidazole core of the target compound. Finally, an oxidation step results in the final azoimidazoles 5.
3. Photophysical characterization
The UV-Vis spectra of compounds 5 were obtained from 350 nm to 700 nm in ethanol, acetonitrile and 1,4-dioxane. The maximum absorption wavelength and molar absorption coefficients were also determined. Azoimidazoles 5 present two distinct bands in the visible region of the spectrum: one band around 413–441 nm, corresponding to an absorption in the purple region, and another band around 604–633 nm, corresponding to an absorption in the yellow-orange region of the spectrum. The complementary colours of purple and orange are, respectively, yellow and blue, which, when combined, explain the green colour presented by compounds 5. These compounds present molar absorption coefficients of 1.15–5.12 × 104 M−1 cm−1, regarding their main absorption band.In ethanol, all compounds present a similar position of the absorption bands, except 5j and 5m (R1 = C6H4(p)CH3 and R1 = C6H4(p)OCH3), which show a red-shift in both bands (Fig. 1). This could be related to the presence of an electron-donating group in the aromatic ring. When it comes to the intensity of the main absorption band, the presence of halogens in the substituent seems to be directly linked to a hyperchromic effect, which is more pronounced in 5g (R1 = C6H4(p)Br) There is also a significant hypochromic effect presented by 5m (R1 = C6H4(p)OCH3).
 | ||
| Fig. 1 UV-Vis spectra of compounds 5 with R2 = Pip in ethanol with varying R1 substituents, at 3.33 × 10−5 M. | ||
Regarding R2-substituents, there is no discernible pattern, as the relative absorbance of the different compounds varies depending on the R1-substituent. For instance, when R1 = C6H4(p)CH3, the compound containing a pyrrolidine unit shows higher absorbance throughout all wavelengths, followed by the piperidino-substituted compound. However, when R1 = C6H4(p)OCH3, the compound presenting the piperidine moiety presents a significantly lower absorbance than its counterparts.
When it comes to solvatochromism, hypsochromic shifts are observed when increasing solvent polarity in the sequence 1,4-dioxane; acetonitrile; ethanol. The difference between λmax values in ethanol and acetonitrile is 5 to 9 nm, while between acetonitrile and dioxane, it is only 1 to 5 nm. Fig. 2 presents the spectra of 5a (R1 = C6H4(p)F) in each solvent to illustrate the shift caused by these solvents, which is similar in all compounds. When it comes to the band around 430 nm, it is difficult to identify a pattern in the effects of the R1-substituents, but there is solvatochromism, particularly between compounds in acetonitrile and 1,4-dioxane solutions.
To demonstrate the halochromic properties of these molecules, the UV-Vis spectra of compound 5j were obtained from 350 nm to 700 nm, before and after adding 1 molar equivalent of sulfuric acid to a solution with a concentration of 4 × 10−5 M in ethanol (Fig. 3).
 | ||
| Fig. 3 UV-Vis spectra of azoimidazole 5j in ethanol at 4 × 10−5 M, before and after adding 1 molar equivalent of sulfuric acid, including photographs of the two forms. | ||
In its neutral form, 5j presents two distinct bands at 432 nm and 597 nm that result in the transmittance of the green colour. The acid form of this compound also presents two bands. The first, at 427 nm, shows a slight bathochromic shift. The second band, at 552 nm, suffers the most significant difference in both position and intensity. More specifically, there is a 45 nm hypsochromic shift into the yellow region that almost halves the absorbance value, explaining why this compound exhibits a colour in the purple region, as these two colours are complementary.
A more comprehensive study was also conducted, in which UV-Vis spectra of compound 5j were traced between 350 and 700 nm at different pH values, measured with a pH electrode (Fig. 4). This allowed the determination of pKa, using the graphical method reported by Salgado et al. The obtained pKa value was 4.89.
 | ||
Fig. 4 UV-Vis spectra of azoimidazole 5j at different pH values in water/ethanol (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40), and at 10−5 M. A pH electrode was used to determine pH values. 40), and at 10−5 M. A pH electrode was used to determine pH values. | ||
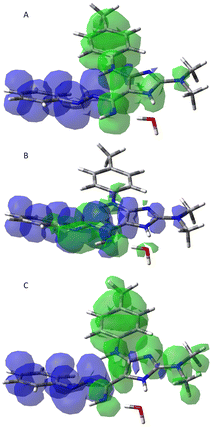
In order to further understand the photophysical behaviour of this compound, molecular quantum mechanical calculations were performed for compound 5k in ethanol. As various tautomers are possible, ground state geometry optimization and vibrational analysis were obtained for various possible tautomers, ensuring that no imaginary frequency modes were present. Within an energy difference of 15 kcal mol−1, five structures were found (ESI-Table 3†). Using TDDFT with the b3lyp functional, vertical electronic excitation energies were then obtained for the first eight excited states. Comparing with the experimental maximum wavelengths in ESI-Table 1† the tautomer with lowest energy (I) is significantly to the blue. This shift is not explainable by the used functional as it normally originates smaller excited state transition energies. Although being 14.6 kcal mol−1 more energetic, tautomer II shows bands at 436 nm and 701 nm with significant intensity, as seen from the oscillator strength values (fosc), that are more compatible with the observed experimental spectrum. Looking for possible mechanisms to explain the possible prominence of tautomer II, calculations were performed in the presence of one explicit water molecule (ESI-Table 4†). Hydrogen-bond type interaction was found and the excess energy of tautomer II decreased to 10 kcal mol−1. Additionally, the electronic transition energies and relative oscillator strengths became more compatible with the experimental spectrum. From these parameters an absorption spectrum can be calculated assuming Gaussian bands with 0.2 eV standard deviation. Transforming it in transmitted light and using the CIE colour matching functions it is possible to predict a corresponding perceived human colour. ESI-Fig. 10† shows a green colour for tautomer II confirming its importance. The nature of the electronic excitations can be inferred from the variations of electron density upon excitation. These are represented in Fig. 5. In the first and third electronic transitions, variations of electron density occur mainly at the π electron cloud perpendicular to the molecular plane, indicating them to be of type π–π*. On the contrary, in the second electronic transition electron density decreases in the nitrogen atoms along the molecular plane and increases in the π electron cloud. This behaviour is typical of an n–π* type transition and justifies its lower oscillator strength.
Calculations were also performed for protonated forms of both tautomer 5k_I and 5k_II (ESI-Table 5†). Protonation free energies were calculated assuming 0.4 kcal mol−1 (1050.2 kJ mol−1) for the free energy of the proton in ethanol.36 From the protonated form with lowest protonation free energy (II_A) a pKa value of 4.95 can be calculated, which is in very good accordance with determined value for compound 5j. Colours for the protonated forms of tautomer 5k_II are also shown in Fig. ESI-10† and again a very good match is obtained for protonated form 5k_II_A.
4. Antimicrobial activity
The antifungal and antibacterial activity of the newly synthesised compounds were evaluated (Table 3 and ESI-Table 6†). From the eighteen compounds tested, twelve derivatives showed potent activity against C. neoformans (MIC between 2–20 μg·mL−1), with compound 5j showing the best results. For Candida spp., seven compounds showed significant antifungal activity against C. krusei (MIC between 4–20 μg·mL−1) and nine compounds showed moderate activity (MIC between 20–80 μg·mL−1). The compounds showed greater selectivity for C. krusei, considering that the MIC values for C. albicans were higher than 256 μg·mL−1 for the majority of the compounds and the best was 89.6 μg·mL−1 albeit being fungistatic. Minimum lethal concentration (MLC) values for C. neoformans and C. krusei were identical to the MIC values (equal or one dilution higher), which represents an interesting fungicidal activity for these compounds against these species. Concerning filamentous fungi, some fungicidal activity was observed for some compounds on the dermatophyte T. rubrum, but for A. fumigatus all the compounds showed a MIC value ≥256 μg·mL−1, the maximum concentration tested (ESI Tables 3 and 4†). Concerning the antibacterial activity, only compound 5j showed good activity against Staphylococcus aureus with a MIC value of 10.7 μg·mL−1. However, the effect was bacteriostatic and not bactericidal as with C. neoformans and C. krusei. Some selectivity against Gram-positive bacteria was revealed, as no compound showed any activity against Escherichia coli.| MIC (MLC) μg mL−1 | |||
|---|---|---|---|
| 5j | 5b | 5k | |
| a Full results in ESI;† MIC/MLC, minimum inhibitory/lethal concentration. | |||
| C. albicans ATCC 10231 | 89.6 (≥256) | 192± (213.3) | 256 (256) |
| C. krusei ATCC 6258 | 4.0 (5.3) | 13.3 (13.3) | 16 (16) |
| C. neoformans CECT 1078 | 2.0 (3.3) | 8 (13.3) | 7.3 (13.3) |
| T. rubrum FF5 | 44.0 (48.0) | 128 (128) | 128 (128) |
| S. aureus ATCC 25923 | 10.7 (≥256) | 64 (>256) | >256 (>256) |
5. Conclusions
A sustainable and efficient method for the synthesis of a new class of 2-aminoimidazole azo-dyes with remarkable colorimetric, halochromic and antimicrobial properties was developed. The introduction of the O2/KI oxidation system in the synthesis pipeline as an alternative to silver nitrate was an innovative strategy that allowed the efficient and sustainable synthesis of various azoimidazoles 5. This is an entirely different class of azoimidazoles, when compared with classical azoimidazoles. However, they show some structural similarities with the 2-aminoimizole azo-dyes series previously reported by our group 3. The study of the mechanism involved in the synthesis of these unexpected green solids led to the isolation of the intermediate compounds 4I and 4II. A total of eighteen green solids were isolated and characterized by 1H, 13C and 2D NMR spectroscopy, infrared spectroscopy, and elemental analysis.The original 2-aminoimizole azo-dyes 3 presented unique properties, such as halochromism and antimicrobial activity, and these properties were also studied for this new azo-dyes (5). Interesting solvatochromism was found in azoimidazoles 5, with the highest shift in maximum wavelength of absorbance being found for 5 with R2 = Pyr. All 2-aminoimidazoles 5 presented a molar absorption coefficient around 104 M−1 cm−1. Protonation studies indicated the presence of halochromism in compounds 5, with their colours changing from green to purple when the pH decreases. ab initio molecular quantum mechanics calculations support the data obtained experimentally.
Data from the conducted antimicrobial activity assays evidences good antimicrobial activity of the newly synthesised 2-aminoimidazoles 5 against pathogenic yeasts. The majority of the tested derivatives showed potent activity against C. neoformans, and moderate activity against C. krusei. Compound 5j was the best hit compound for both C. neoformans (MIC = 2 μg·mL−1) and C. krusei (MIC = 4 μg·mL−1). The data also suggest that the antifungal activity was fungicidal and selective for these two species. These results are interesting since C. neoformans is responsible for a high mortality rate among infected individuals and drug resistance is an actual problem, while C. krusei is intrinsically resistant to fluconazole but is also able to develop acquired resistance to other azoles.
Altogether, the new azo-dyes 5 stand as promising candidates for the development of new compounds for (bio)medical applications.
6. Experimental
6.1. Materials and instruments
All new compounds were fully characterized by NMR (1H, 13C) on a Bruker Avance 3400 (1H: 400 MHz, 13C: 100 MHz), including the 1H–13C correlation spectra (HMQC and HMBC) using DMSO-d6 as solvent at room temperature or 50 °C. The coupling constants (J) are reported in Hertz (Hz) and chemical shifts (δ) were expressed in parts per million (ppm). IR spectra were recorded on a FT-IR Spectrum Two – PerkinElmer, at room temperature in the range of 4000–450 cm−1. Melting points were determined on a Stuart cat. SMP3 and are uncorrected. The reactions were monitored by TLC analysis, performed in Merck-Kieselgel 60 F254 silica plates and observed in a CN-6 ultraviolet light (ν = 50 Hz) chamber. UV-Vis spectra were obtained with UV-2501PC by Shimadzu Corporation, using 1 cm wide quartz cells. Elemental analysis data were obtained with the analyser Leco TruSpec CHNS Micro.6.2. Ab initio molecular quantum mechanics calculations
The electronic and structural properties of compound 5k were studied by ab initio molecular quantum chemistry calculations through orca software version 6.0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 with a def2-TZVP38 basis set using the DFT method and RIJCOSX approximation39 using def2/J40 as auxiliary basis. The B3LYP functional was used together with atom-pairwise dispersion correction based on EEQ partial charges (D4)41 and a conductor-like polarizable continuum model (CPCM) corresponding to ethanol. Excited state calculations were done through time-dependent DFT using the Tamm-Dancoff approximation (TDDFT-TDA).37 For the case of interaction with an explicit water molecule geometrical counter-poise correction (gCP) was used to correct artificial overbinding effects that can arise from basis set superposition error (BSSE).42
37 with a def2-TZVP38 basis set using the DFT method and RIJCOSX approximation39 using def2/J40 as auxiliary basis. The B3LYP functional was used together with atom-pairwise dispersion correction based on EEQ partial charges (D4)41 and a conductor-like polarizable continuum model (CPCM) corresponding to ethanol. Excited state calculations were done through time-dependent DFT using the Tamm-Dancoff approximation (TDDFT-TDA).37 For the case of interaction with an explicit water molecule geometrical counter-poise correction (gCP) was used to correct artificial overbinding effects that can arise from basis set superposition error (BSSE).42
6.3. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), presenting Rf values ranging from 0.41 to 0.73. At this point a filtration was performed with ethanol and diethyl ether and the pure amidrazones 1 were obtained.
1), presenting Rf values ranging from 0.41 to 0.73. At this point a filtration was performed with ethanol and diethyl ether and the pure amidrazones 1 were obtained.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), presenting Rf values ranging from 0.49 to 0.84. At this point, a filtration was performed with water, acetonitrile and diethyl ether and the pure azoimidazoles 4 were obtained.
1), presenting Rf values ranging from 0.49 to 0.84. At this point, a filtration was performed with water, acetonitrile and diethyl ether and the pure azoimidazoles 4 were obtained.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), presenting Rf values ranging from 0.62 to 0.88. At this point a filtration was performed with water, acetonitrile and diethyl ether and the pure azoimidazoles 5 were obtained.
3), presenting Rf values ranging from 0.62 to 0.88. At this point a filtration was performed with water, acetonitrile and diethyl ether and the pure azoimidazoles 5 were obtained.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding: Centro de Química da Universidade do Minho (CQ-UM) - UID/00686; Centro de Física das Universidades do Minho e do Porto (CF-UM-UP) - UID/04650; Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR) - UIDB/04423 and UIDP/04423; Portuguese Oncology Institute of Porto (project no. PI86-CI-IPOP-66-2019); and National NMR Network (RNRMN) - PINFRA/22161. Access to Cluster SeARCH of UMinho is acknowledged.References
- K. R. Iyer, N. M. Revie, C. Fu, N. Robbins and L. E. Cowen, Treatment strategies for cryptococcal infection: challenges, advances and future outlook, Nat. Rev. Microbiol., 2021, 19, 454–466, DOI:10.1038/s41579-021-00511-0.
- A. T. Jamiu, J. Albertyn, O. M. Sebolai and C. H. Pohl, Update on Candida krusei, a potential multidrug-resistant pathogen, Med. Mycol., 2021, 59, 14–30, DOI:10.1093/mmy/myaa031.
- WHO, WHO fungal priority pathogens list to guide research, development and public health action, 2022, vol. 1 Search PubMed.
- N. Rani, A. Sharma, G. Gupta and R. Singh, Imidazoles as Potential Antifungal Agents: A Review, Mini-Rev. Med. Chem., 2013, 13, 1626–1655, DOI:10.2174/13895575113139990069.
- L. F. Tietze, Domino Reactions in Organic Synthesis, Chem. Rev., 1996, 96, 115–136, DOI:10.1021/cr950027e.
- T. Keijer, V. Bakker and J. C. Slootweg, Circular chemistry to enable a circular economy, Nat. Chem., 2019, 11, 190–195, DOI:10.1038/s41557-019-0226-9.
- F. A. Jerca, V. V. Jerca and R. Hoogenboom, Advances and opportunities in the exciting world of azobenzenes, Nat. Rev. Chem., 2022, 6, 51–69, DOI:10.1038/s41570-021-00334-w.
- P. Griefs, Vorläufige Notiz über die Einwirkung von salpetriger Säure auf Amidinitro- und Aminitrophenylsäure, Ann. Chem. Pharm., 1858, 106, 123–125, DOI:10.1002/jlac.18581060114.
- H. M. D. Bandara and S. C. Burdette, Photoisomerization in different classes of azobenzene, Chem. Soc. Rev., 2012, 41, 1809–1825, 10.1039/C1CS15179G.
- S. Crespi, N. A. Simeth and B. König, Heteroaryl azo dyes as molecular photoswitches, Nat. Rev. Chem., 2019, 3, 133–146, DOI:10.1038/s41570-019-0074-6.
- G. Hartley, The Cis-form of Azobenzene, Nature, 1937, 140, 281 Search PubMed.
- V. Ladányi, P. Dvořák, J. Al Anshori, Ľ. Vetráková, J. Wirz and D. Heger, Azobenzene photoisomerization quantum yields in methanol redetermined, Photochem. Photobiol. Sci., 2017, 16, 1757–1761, 10.1039/c7pp00315c.
- A. Steinegger, O. S. Wolfbeis and S. M. Borisov, Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications, Chem. Rev., 2020, 120, 12357–12489, DOI:10.1021/acs.chemrev.0c00451.
- T. Stoyanova, S. Stoyanov, L. Antonov and V. Petrova, Ammonium-Azonium Tautomerism in Some N,N-Dialkylaminoazo Dyes. Part 1: General Considerations, 1996, vol. 31 Search PubMed.
- M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry and G. A. Woolley, Red-Shifting Azobenzene Photoswitches for in Vivo Use, Acc. Chem. Res., 2015, 48, 2662–2670, DOI:10.1021/acs.accounts.5b00270.
- O. Sadovski, A. A. Beharry, F. Zhang and G. A. Woolley, Spectral tuning of azobenzene photoswitches for biological applications, Angew. Chem., Int. Ed., 2009, 48, 1484–1486, DOI:10.1002/anie.200805013.
- A. A. Beharry, O. Sadovski and G. A. Woolley, Azobenzene photoswitching without ultraviolet light, J. Am. Chem. Soc., 2011, 133, 19684–19687, DOI:10.1021/ja209239m.
- C. Knie, M. Utecht, F. Zhao, H. Kulla, S. Kovalenko and A. M. Brouwer, et al., Ortho-Fluoroazobenzenes: Visible Light Switches with Very Long-Lived Z Isomers, Chem. – Eur. J., 2014, 20, 16492–16501, DOI:10.1002/chem.201404649.
- M. Dong, A. Babalhavaeji, C. V. Collins, K. Jarrah, O. Sadovski and Q. Dai, et al., Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions, J. Am. Chem. Soc., 2017, 139, 13483–13486, DOI:10.1021/jacs.7b06471.
- S. Samanta, A. Babalhavaeji, M. X. Dong and G. A. Woolley, Photoswitching of ortho-substituted azonium ions by red light in whole blood, Angew. Chem., Int. Ed., 2013, 52, 14127–14130, DOI:10.1002/anie.201306352.
- M. Dong, A. Babalhavaeji, M. J. Hansen, L. Kálmán and G. A. Woolley, Red, far-red, and near infrared photoswitches based on azonium ions, Chem. Commun., 2015, 51, 12981–12984, 10.1039/c5cc02804c.
- D. B. Konrad, J. A. Frank and D. Trauner, Synthesis of Redshifted Azobenzene Photoswitches by Late-Stage Functionalization, Chem. – Eur. J., 2016, 22, 4364–4368, DOI:10.1002/chem.201505061.
- M. J. Hansen, M. M. Lerch, W. Szymanski and B. L. Feringa, Direct and Versatile Synthesis of Red-Shifted Azobenzenes, Angew. Chem.,– Int. Ed., 2016, 55, 13514–13518, DOI:10.1002/anie.201607529.
- K. Mezgebe and E. Mulugeta, Synthesis and pharmacological activities of azo dye derivatives incorporating heterocyclic scaffolds: a review, RSC Adv., 2022, 12, 25932–25946, 10.1039/d2ra04934a.
- J. Calbo, C. E. Weston, A. J. P. White, H. S. Rzepa, J. Contreras-García and M. J. Fuchter, Tuning azoheteroarene photoswitch performance through heteroaryl design, J. Am. Chem. Soc., 2017, 139, 1261–1274, DOI:10.1021/jacs.6b11626.
- J. N. Bull, M. S. Scholz, N. J. A. Coughlan, A. Kawai and E. J. Bieske, Monitoring Isomerization of Molecules in Solution Using Ion Mobility Mass Spectrometry, Anal. Chem., 2016, 88, 11978–11981, DOI:10.1021/acs.analchem.6b04000.
- N. O. Mahmoodi, S. Rahimi and M. Pasandideh Nadamani, Microwave-assisted synthesis and photochromic properties of new azo-imidazoles, Dyes Pigm., 2017, 143, 387–392, DOI:10.1016/j.dyepig.2017.04.053.
- T. A. Khattab and M. Rehan, A review on synthesis of nitrogen-containing heterocyclic dyes for textile fibers - Part 2: Fused heterocycles, Egypt. J. Chem., 2018, 61, 989–1018, DOI:10.21608/ejchem.2018.4131.1363.
- V. Jelínková, A. Dellai, M. Vachtlová, M. Fecková, J. Podlesný and M. Klikar, et al., Molecular azo–imidazole photoswitches: Property tuning by substitution, J. Photochem. Photobiol., A, 2024, 449, 115390, DOI:10.1016/j.jphotochem.2023.115390.
- A. Žula, D. Kikelj and J. Ilaš, 2-Aminoimidazoles in Medicinal Chemistry, Mini-Rev. Med. Chem., 2013, 13, 1921–1943, DOI:10.2174/1389557511313130007.
- E. Li, Y. Lin, X. Wu, X. Mao, H. Kang and Y. Wen, et al., Regioselective single-step synthesis of 2-aminoimidazole derivatives, Tetrahedron Lett., 2019, 60, 151122, DOI:10.1016/j.tetlet.2019.151122.
- D. Dantas, A. I. Ribeiro, F. Carvalho, E. Gil-Martins, R. Silva and F. Remião, et al., Red-shifted and pH-responsive imidazole-based azo dyes with potent antimicrobial activity, Chem. Commun., 2023, 59, 2791–2794, 10.1039/d3cc00372h.
- A. I. Ribeiro, B. Vieira, C. Alves, B. Silva, E. Pinto and F. Cerqueira, et al., Halochromic Silk Fabric as a Reversible pH-Sensor Based on a Novel 2-Aminoimidazole Azo Dye, Polymers, 2023, 15, 1730, DOI:10.3390/polym15071730.
- A. I. Ribeiro, B. Vieira, D. Dantas, B. Silva, E. Pinto and F. Cerqueira, et al., Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles, Pharmaceutics, 2023, 15, 926, DOI:10.3390/pharmaceutics15030926.
- A. C. Serra, C. Docal and A. M. d'A. Rocha Gonsalves, Efficient azo dye degradation by hydrogen peroxide oxidation with metalloporphyrins as catalysts, J. Mol. Catal. A: Chem., 2005, 238, 192–198, DOI:10.1016/j.molcata.2005.05.017.
- A. Malloum, J. J. Fifen and J. Conradie, Determination of the absolute solvation free energy and enthalpy of the proton in solutions, J. Mol. Liq., 2021, 322, 114919, DOI:10.1016/j.molliq.2020.114919.
- F. Neese, Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606, DOI:10.1002/wcms.1606.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297, 10.1039/b508541a.
- F. Neese, An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix, J. Comput. Chem., 2003, 24, 1740–1747, DOI:10.1002/jcc.10318.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057, 10.1039/b515623h.
- E. Caldeweyher, S. Ehlert, A. Hansen, H. Neugebauer, S. Spicher and C. Bannwarth, et al., A generally applicable atomic-charge dependent London dispersion correction, J. Chem. Phys., 2019, 150, 154122, DOI:10.1063/1.5090222.
- H. Kruse and S. Grimme, A geometrical correction for the inter- and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems, J. Chem. Phys., 2012, 136, 154101, DOI:10.1063/1.3700154.
- A. I. Ribeiro, C. Gabriel, F. Cerqueira, M. Maia, E. Pinto and J. C. Sousa, et al., Synthesis and antimicrobial activity of novel 5-aminoimidazole-4-carboxamidrazones, Bioorg. Med. Chem. Lett., 2014, 24, 4699–4702, DOI:10.1016/j.bmcl.2014.08.025.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00463b |
| This journal is © The Royal Society of Chemistry 2025 |