Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of α,α-difluoro-β-amino amides using aldimines and bromodifluoroacetamides via the Reformatsky reaction†
Ryota
Ozawa
and
Tetsuya
Yamamoto
 *
*
Department of Materials Science and Engineering, Graduate School of Engineering, Tokyo Denki University, 5 Senju Asahi-cho Adachi-ku, Tokyo 120-8551, Japan. E-mail: t-yamamoto@mail.dendai.ac.jp
First published on 7th April 2025
Abstract
α,α-Difluoro-β-amino amides are attractive building blocks of biologically active compounds such as fluorinated pharmaceutical mimics and oligopeptides. Herein, we describe the zinc-promoted Reformatsky reaction of aldimines using bromodifluoroacetamides which provides a direct synthetic approach to α,α-difluoro-β-amino amides. This method gave various N-PMP protected α,α-difluoro-β-amino-β-aryl amides in 64–95% yields. Furthermore, these amides were efficiently converted into 2,2-difluoropropane-1,3-diamines under reductive conditions using a combination of NaBH4 and BF3.
Introduction
Fluorine atoms are nearly the same size as hydrogen atoms and are often used as isosteres of hydrogen in medicinal chemistry.1 For example, C–F bond containing molecules are less likely to undergo oxidative metabolism than fluorine-free molecules, and in some cases exhibit superior metabolic stability.2 Since β-amino amides are attractive scaffolds for various bioactive molecules such as andrimid, sitagliptin and L-carnosine,3 fluorinated β-amino amides such as α,α-difluoro-β-amino amides are also expected to be a scaffold that can be used to design novel biologically active compounds.4 As fluorinated analogues of natural products and pharmaceuticals with a β-amino amide moiety, antifungal tetrapeptides, renin inhibitory peptides and selective TAF1(2) bromodomain inhibitors have already been reported (Fig. 1).5In general, α,α-difluoro-β-amino amides have been synthesized by a condensation reaction between an amine and α,α-difluoro-β-amino acids (Fig. 2a)6 or α,α-difluoro-β-lactams (Fig. 2b),7 but their synthesis requires several steps from readily available substrates such as imines and halodifluoroacetic acid esters. In recent years, the Mannich-type reaction of imines using α,α-difluoro-α-trimethylsilylacetoamide (Fig. 2c),8 the three-component reaction of (bromodifluoromethyl)trimethylsilane, imines, and isocyanides (Fig. 2d),9 and the Reformatsky-type reaction of amides and bromodifluoroacetamides using an iridium catalyst (Fig. 2e)10 have been developed as direct synthesis methods for α,α-difluoro-β-amino amides. Herein, we report the zinc-promoted Reformatsky reaction of bromodifluoroacetamides with aldimines as a simpler synthetic method to directly access α,α-difluoro-β-amino amides (Fig. 2f).
Results and discussion
At first, we examined and optimized a zinc-mediated Reformatsky reaction of N-(4-methoxybenzylidene)aniline 1a and bromodifluoroacetamide 2a (Table 1). The reaction was carried out using 0.8 equivalents of trimethylsilyl chloride11 as an activator of zinc powder in THF to give α,α-difluoro-β-amino amide 3a in 95% yield (entry 1). The yield decreased to 86% on reducing the amount of trimethylsilyl chloride to 0.4 equivalents, and the yield decreased drastically to 44% in the absence of trimethylsilyl chloride (entries 2 and 3). The use of other ether solvents such as 2-Me-THF and 1,4-dioxane reduced the yields to 63% and 38% respectively (entries 4 and 5). DMSO promoted the reaction moderately, but other highly polar solvents such as acetonitrile and DMF were not suitable for the reaction (entries 6–8). Less polar solvents such as toluene and hexane were also ineffective in this reaction (entries 9 and 10).Recently, Blum reported that trimethylsilyl chloride aids to solubilise organozinc intermediates from zinc(0) metal after oxidative addition, and that this solubilisation can be sufficiently maintained with a catalytic amount of trimethylsilyl chloride.12 Based on Blum's report, we propose a plausible mechanism for this type of imino-Reformatsky reaction in Scheme 1. As trimethylsilyl chloride facilitates the oxidative addition of bromodifluoroacetamide to zinc(0) and the solubilisation of zinc enolates on the metal zinc surface, the nucleophilic addition of enolates to imines proceeded smoothly.
Under the optimized conditions, we synthesized various functionalized α,α-difluoro-β-amino amides 3 and clarified the scope and limitations of aldimines 1 and bromodifluoroacetamides 2 (Table 2). Benzaldimines bearing sterically hindered o-methoxyphenyl and 2-naphthyl groups were converted into the corresponding aminoamides 3b and 3g in moderate yields of 70% and 63%, respectively. The substituents at the positions distant from the imino group of benzaldimines did not have a significant effect on the reaction, whether they were electron-donating groups such as the p-methoxy group or electron-withdrawing groups such as p-methoxycarbonyl, p-chloro and p-cyano groups, and the desired aminoamides 3c–f were obtained in high yields. An aliphatic imine such as 1-cyclohexyl-N-(4-methoxyphenyl)methanimine was not suitable for this reaction, and the product 3h was afforded in only 25% yield. Other aliphatic aldimines derived from acetaldehyde and pivalaldehyde were difficult to purify and handle, so we were unable to use them in this reaction. Heteroaryl imines from 2-thiophenecarboxaldehyde or furfural reacted smoothly and afforded the desired products 3i and 3j in good yields. Replacing the PMP group of aldimine 1a with a benzyl group dramatically reduced its reactivity and the corresponding amine 3k was obtained in a poor yield. The tertiary amides gave the desired products 3l and 3m regardless of whether the alkyl group on the nitrogen had a linear or cyclic structure, but when a secondary amide was used as the substrate, a complex mixture 3n was obtained.
In general, amide groups can be easily converted into aminomethylene groups using hydride reducing agents such as LiAlH4, BH3 and others.13 Hence, one application of fluorinated β-amino amides would be their conversion into fluorinated 1,3-diamines, which are used as building blocks for a wide range of biologically active compounds such as antisense agents,14 selective TAF1(2) bromodomain inhibitors,5c and adenosine monophosphate-activated kinase activators.15
Table 3 shows the results of the reduction of α,α-difluoro-β-amino amide 3a using conventional hydride reagents. As reported by Leclerc,8 the use of NaBH4 resulted in negligible formation of 1,3-diamine 4a and gave γ-amino alcohol 5 in a moderate yield (entry 1). LiAlH4 is a typical reductant for amides, but like NaBH4 it gave γ-amino alcohol 5 instead of 1,3-diamine 4a (entry 2). Staas et al. reported that BH3·Me2S was effective in the reduction of α,α-difluoro-β-amino amides, but under their conditions, the reaction time was long and the yield was moderate.6b BH3·Me2S was suitable for the reduction of amides, but it also produced γ-amino alcohol 5 along with 1,3-diamine 4a (entry 3). Interestingly, when BH3 produced by the combination of NaBH4 and BF3·Et2O was used for the reduction,16 the yield of 4a was improved to 69%, and the by-product γ-amino alcohol 5 was suppressed (entry 4). Furthermore, when the reaction using NaBH4/BF3 was carried out for 1 hour at room temperature and then for 2 hours at 75 °C, the yield increased to 94% (entry 5). The yields of the reduction gradually decreased as the equivalent amount of NaBH4 was reduced (entries 6 and 7).
| Entry | Reductant (equiv.) | Reaction temp. and time | 4a (%) | 5 (%) |
|---|---|---|---|---|
| a Isolated yield. b Determined by 19F NMR of a crude mixture. | ||||
| 1 | NaBH4 (6) | r.t., 2 h | Traceb | 49 |
| 2 | LiAlH4 (6) | r.t., 2 h | Traceb | 47 |
| 3 | BH3·Me2S (6) | r.t., 2 h | 51 | 16 |
| 4 | NaBH4 (6) and BF3·Et2O (4) | r.t., 2 h | 69 | Traceb |
| 5 | NaBH4 (6) and BF3·Et2O (4) | r.t., 1 h, then 75 °C, 2 h | 94 | Traceb |
| 6 | NaBH4 (4) and BF3·Et2O (4) | r.t., 1 h, then 75 °C, 2 h | 91 | Traceb |
| 7 | NaBH4 (2) and BF3·Et2O (2) | r.t., 1 h, then 75 °C, 2 h | 83 | Traceb |
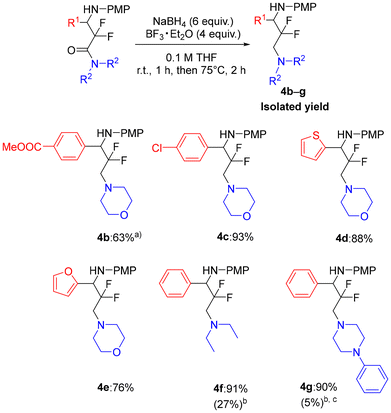
Next, we demonstrated the applicability of the NaBH4/BF3 reduction system for several α,α-difluoro-β-amino amides 3 (Table 4). Amide 3d with a methoxycarbonyl group, which is readily reduced by BH3, gave the corresponding amine 4b in a yield of 63% when reacted at room temperature. Additionally, a complex mixture of by-products was also formed under these conditions. The reduction of amides containing chlorine or heteroaromatic rings, such as thiophene and furan, proceeded smoothly and the desired amines 4c–e were obtained in excellent yields. Even when the morpholine moiety of the amide was replaced with diethylamine or 1-phenylpiperazine, the amines 4f and 4g were still provided in satisfactory yields of 91% and 90%, respectively. The reduction of amides 3l and 3m with LiAlH4 instead of NaBH4/BF3 gave a complex mixture containing unidentified products and the corresponding 1,3-diamines 4f and 4g were obtained in low yields. In addition, γ-amino alcohol 5 was not detected in either of these crude products by 19F NMR.
Conclusions
In conclusion, we have achieved a one-step synthesis of α,α-difluoro-β-amino amides from aldimines and bromodifluoroacetamides via the Reformatsky reaction using zinc and trimethylsilyl chloride as a zinc activator under mild conditions. The α,α-difluoro-β-amino amides were converted into 2,2-difluoropropane-1,3-diamines with high selectivity and excellent yields using the combination of NaBH4 and BF3 as reducing agents.Author contributions
T. Y. conceived and directed the project. R. O. performed the experiments and prepared the ESI.† T. Y. and R. O. wrote the manuscript, discussed the results, and commented on the manuscript.Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the TDU Analysis Center, Tokyo Denki University, for NMR spectroscopy. We thank Prof. Koguchi, Tokai University, for the HRMS measurements.References
- H. J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander and M. Stahl, ChemBioChem, 2004, 5, 637–643 CrossRef PubMed.
- J. W. Clader, J. Med. Chem., 2004, 47, 1–9 CrossRef CAS PubMed.
- (a) J. Needham, M. T. Kelly, M. Ishige and R. J. Andersen, J. Org. Chem., 1994, 59, 2058–2063 CrossRef CAS; (b) G. Xiang, K. Liu, T. Wang, X. Hu, J. Wang, Z. Gao, W. Lei, Y. Feng and T. H. Tao, Adv. Sci., 2021, 8, 2002328 CrossRef CAS PubMed; (c) T. Heck, V. S. Makam, J. Lutz, L. M. Blank, A. Schmid, D. Seebach, H. P. E. Kohler and B. Geueke, Adv. Synth. Catal., 2010, 352, 407–415 CrossRef CAS.
- T. L. March, M. R. Johnston, P. J. Duggan and J. Gardiner, Chem. Biodivers., 2012, 9, 2410–2441 CrossRef CAS PubMed.
- (a) S. Thaisrivongs, H. J. Schostarez, D. T. Pals and S. R. Turner, J. Med. Chem., 1987, 30, 1837–1842 CrossRef CAS PubMed; (b) K. Nakayama, H. C. Kawato, H. Inagaki, R. Nakajima, A. Kitamura, K. Someya and T. Ohta, Org. Lett., 2000, 2, 977–980 CrossRef CAS PubMed; (c) M. A. Clegg, N. H. Theodoulou, P. Bamborough, C. W. Chung, P. D. Craggs, E. H. Demont, L. J. Gordon, G. M. Liwicki, A. Phillipou, N. C. O. Tomkinson, R. K. Prinjha and P. G. Humphreys, ACS Med. Chem. Lett., 2021, 12, 1308–1317 CrossRef CAS PubMed.
- (a) M. Braun, Eur. J. Org. Chem., 2021, 1825–1836 CrossRef CAS; (b) D. D. Staas, K. L. Savage, C. F. Homnick, N. N. Tsou and R. G. Ball, J. Org. Chem., 2002, 67, 8276–8279 CrossRef CAS PubMed; (c) S. Thaisrivongs, H. J. Schostarez, D. T. Pals and S. R. Turner, J. Med. Chem., 1987, 30, 1837–1842 CrossRef CAS PubMed.
- A. Tarui, M. Ueo, M. Morikawa, M. Tsuta, S. Iwasaki, N. Morishita, Y. Karuo, K. Sato, K. Kawai and M. Omote, Synthesis, 2020, 3657–3666 CAS.
- A. Honraedt, A. Lee, J. Campagne and E. Leclerc, Adv. Synth. Catal., 2017, 359, 2815–2823 CrossRef CAS.
- R. Zhang, Z. Zhang, Q. Zhou, L. Yu and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 5744–5748 CrossRef CAS PubMed.
- P. Biallas, K. Yamazaki and D. Dixon, Org. Lett., 2022, 24, 2002–2007 CrossRef CAS PubMed.
- (a) J. K. Gawroński, Tetrahedron Lett., 1984, 25, 2605–2608 CrossRef; (b) R. J. Kloetzing, T. Thaler and P. Knochel, Org. Lett., 2006, 8, 1125–1128 CrossRef CAS PubMed.
- E. M. Hanada, P. J. McShea and S. A. Blum, Angew. Chem., Int. Ed., 2023, 62, e202307787 CrossRef CAS PubMed.
- For selected reviews, see: (a) H. C. Brown and S. Krishnamurthy, Tetrahedron, 1979, 35, 567–607 CrossRef CAS; (b) L. Pasumansky, C. T. Goralski and B. Singaram, Org. Process Res. Dev., 2006, 10, 959–970 CrossRef CAS; (c) A. F. D. Alcântara, H. dos Santos Barroso and D. Piló-Veloso, Quím. Nova, 2002, 25, 300–311 CrossRef.
- S. Ellipilli and K. N. Ganesh, J. Org. Chem., 2015, 80, 9185–9191 CrossRef CAS PubMed.
- S. J. Shaw, D. A. Goff, L. A. Boralsky, R. Singh, D. J. Sweeny, G. Park, T. Q. Sun, Y. Jenkins, V. Markovtsov, S. D. Issakani, D. G. Payan and Y. Hitoshi, J. Med. Chem., 2023, 66, 17086–17104 CrossRef CAS PubMed.
- S.-D. Cho, Y.-D. Park, J.-J. Kim, J. R. Falck and Y.-J. Yoon, Bull. Korean Chem. Soc., 2004, 25, 407–409 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00388a |
| This journal is © The Royal Society of Chemistry 2025 |