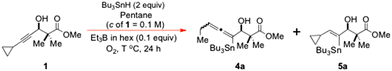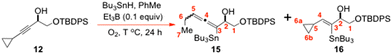Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rate constants and Arrhenius parameters for H-atom abstraction from Bu3SnH by the 2,2-dimethylvinyl radical in PhMe. Kinetic evidence for an entirely free radical mechanism for the O-directed hydrostannation of alkynols with stannanes and Et3B/O2†‡
Hamish A.
Watson
a,
K. Lawrence E.
Hale
a,
John M.
Marron
a,
Soraya
Manaviazar§
a,
Alistair J.
Fielding
 *b and
Karl J.
Hale§
*b and
Karl J.
Hale§
 *a
*a
aThe School of Chemistry and Chemical Engineering, Queen's University Belfast, Stranmillis Road, Belfast BT9 5AG, UK
bThe School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Byrom Street, Liverpool L3 3AF, UK. E-mail: A.J.Fielding@ljmu.ac.uk
First published on 14th April 2025
Abstract
Using the 2,2-dimethylvinyl radical 6 as a horological calibrant for the α-cyclopropyl-β-tributylstannylvinyl radicals 2a and 13 in PhMe, the k values and Arrhenius parameters for their cyclopropane ring-openings have been estimated by competition kinetics over a 293–353 K temperature range. The high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A values (14.95 and 14.55) for these reactions only satisfactorily align with a unimolecular, β-scissive, EH1 radical ring-opening being rate-determining, and the radicals 3a (R = Bu) and 14 undergoing H-atom abstraction from the stannane to give 4a and 15. The log
A values (14.95 and 14.55) for these reactions only satisfactorily align with a unimolecular, β-scissive, EH1 radical ring-opening being rate-determining, and the radicals 3a (R = Bu) and 14 undergoing H-atom abstraction from the stannane to give 4a and 15. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A data for these two reactions only endorse a totally free radical mechanism for the O-directed free radical hydrostannation of dialkyl acetylenes with stannanes and Et3B/O2. An estimated kH-atom abstraction Bu3SnH PhMe 293 K of 1.96 × 108 mol−1 s−1 is proposed for 6 in PhMe, along with an estimated kH-atom abstraction Ph3SnH PhMe 293 K of 1.36 × 109 mol−1 s−1.
A data for these two reactions only endorse a totally free radical mechanism for the O-directed free radical hydrostannation of dialkyl acetylenes with stannanes and Et3B/O2. An estimated kH-atom abstraction Bu3SnH PhMe 293 K of 1.96 × 108 mol−1 s−1 is proposed for 6 in PhMe, along with an estimated kH-atom abstraction Ph3SnH PhMe 293 K of 1.36 × 109 mol−1 s−1.
Introduction
Quantifying the rate constants and Arrhenius parameters for solution-phase free radical reactions of established synthetic worth is often a highly rewarding endeavour, since such information can frequently guide the design of efficient new synthetic pathways based upon those processes,1 while also providing important new mechanistic insights2 into the detailed inner workings of such reactions.In that very connection, we recently had cause to kinetically re-investigate the mechanism3 of the O-directed free radical hydrostannation reaction of dialkyl acetylenes4 using “radical clock” competition methods,5 due to a recent series of papers6 having postulated that O2-generated stannylvinyl cations are key synthetic intermediates in these reactions; these forming from stannylvinyl radical precursors by single electron transfer (SET) to O2, and subsequently undergoing facile ionic reduction by the stannane, to provide the allylically-oxygenated trisubstituted (Z)-vinylstannane products alongside regenerated O2 (see section 1.6 of the ESI‡ for more detail).
It was felt that if the O-directed free radical hydrostannation of alkynols 1 and 12 (Schemes 1 and 2) could be studied with Bu3SnH and cat. Et3B/O2 in PhMe, over a fairly wide temperature range, the product allenyltin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vinyltin ratios might yield rate constants and log
vinyltin ratios might yield rate constants and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A values for the ensuing cyclopropane ring-openings. The magnitude of that log
A values for the ensuing cyclopropane ring-openings. The magnitude of that log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A data might then give important clues as to the molecularity of the rate-determining step of these ring-openings, and reveal whether the mechanistic pathway to 4a and 15 was unimolecular, and exclusively free radical in its nature,3 or whether it proceeded via a putative α-cyclopropyl β-stannylvinyl cation and a cationic reduction, as would be advocated by the proponents6 of the stannylvinyl cation theory.
A data might then give important clues as to the molecularity of the rate-determining step of these ring-openings, and reveal whether the mechanistic pathway to 4a and 15 was unimolecular, and exclusively free radical in its nature,3 or whether it proceeded via a putative α-cyclopropyl β-stannylvinyl cation and a cationic reduction, as would be advocated by the proponents6 of the stannylvinyl cation theory.
 | ||
| Scheme 1 Use of the radical 6 as a calibrating free radical “clock” for α-cyclopropyl-β-stannylvinyl radical probe 2a (R = Bu). | ||
A key assumption in doing such work would be that the intermediary stannylvinyl radicals4d2a (Scheme 1) and 13 (Scheme 2) would be calibratable with the kH-atom abstraction value for a typical vinyl radical such as 6 from Bu3SnH in pentane and PhMe.
Although rate constants have long been known for the abstraction of a H-atom from Bu3SnH by several vinylic radicals,7,8 only one set of Arrhenius parameters has so far emerged from such studies.7 That work is due to Ingold et al.7 who measured the rate at which the 2,2-dimethylvinyl radical (6) abstracted a H-atom from Bu3SnH in pentane; a solvent rarely used in free radical chemistry.
Importantly, Ingold's study7 yielded a kH-atom abstraction 298 K value of 2.96 × 108 mol−1 s−1, an Ea of 1.624 ± 0.407 kcal mol−1, and a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 9.67 ± 0.33 (A = 4.67 × 109 mol−1 s−1) for this process7 (see Scheme 1 and the Ingold ESI‡7). Ingold generated his 2,2-dimethylvinyl radical 6 by laser flash photolysis (LFP) of 3-methyl-but-2-enoyl peroxide (8) at 308 nm;7 a process now widely accepted9–12 to produce the highly reactive 6 alongside the much more delocalised and less reactive 3,3-dimethylacryloyloxy radical (9). Both radicals are thought to emerge from a concerted two-bond homolytic cleavage reaction occurring within the photoexcited S1 form of peroxide 8 ([(Me)2C
A of 9.67 ± 0.33 (A = 4.67 × 109 mol−1 s−1) for this process7 (see Scheme 1 and the Ingold ESI‡7). Ingold generated his 2,2-dimethylvinyl radical 6 by laser flash photolysis (LFP) of 3-methyl-but-2-enoyl peroxide (8) at 308 nm;7 a process now widely accepted9–12 to produce the highly reactive 6 alongside the much more delocalised and less reactive 3,3-dimethylacryloyloxy radical (9). Both radicals are thought to emerge from a concerted two-bond homolytic cleavage reaction occurring within the photoexcited S1 form of peroxide 8 ([(Me)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–C(O)O]2), on a reaction timescale of 0.4 ps, given recent LFP and CIDNP-NMR studies of related acyl peroxides.9–12
C(H)–C(O)O]2), on a reaction timescale of 0.4 ps, given recent LFP and CIDNP-NMR studies of related acyl peroxides.9–12
Most importantly, Ingold's kH-atom abstraction Bu3SnH 298 K value7 for 6 aligned very well with Branchi, Galli and Gentili's8 independent k determination of 3.7 × 108 mol−1 s−1 for the encounter of a fluorenyl vinyl radical with Bu3SnH at 298 K in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (40
MeOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v); the latter radical itself having been generated from a vinylic bromide precursor by LFP. This means that Ingold's log
60 v/v); the latter radical itself having been generated from a vinylic bromide precursor by LFP. This means that Ingold's log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A, Ea and kH-atom abstraction Bu3SnH data7 for 6 can be relied upon for k calculations and radical probe calibrations (accepting a 25% level of error in the Ea).
A, Ea and kH-atom abstraction Bu3SnH data7 for 6 can be relied upon for k calculations and radical probe calibrations (accepting a 25% level of error in the Ea).
Given the dependability of Ingold's Arrhenius parameters for the 2,2-dimethylvinyl radical (6) in pentane,7 we set about using these to horologically calibrate the two stannylvinyl radical reporter probes 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b,c (R = Bu) and 13 as free radical “clocks”5 in PhMe, for a series of competition experiments aimed at establishing the relative rates of the two competing reactions shown in Schemes 1 and 2. Namely: (a) the EH1 stannylvinyl radical-induced cyclopropane ring-opening of radicals 2a and 13 and (b) the SH2 H-atom abstraction event involving Bu3SnH and radicals 2a and 13, to give the vinyltins 5a and 16.
3b,c (R = Bu) and 13 as free radical “clocks”5 in PhMe, for a series of competition experiments aimed at establishing the relative rates of the two competing reactions shown in Schemes 1 and 2. Namely: (a) the EH1 stannylvinyl radical-induced cyclopropane ring-opening of radicals 2a and 13 and (b) the SH2 H-atom abstraction event involving Bu3SnH and radicals 2a and 13, to give the vinyltins 5a and 16.
While conceptually analogous to the novel k determinations of Baines,13 Newcomb14 and Crich15 using other free radical “clocks”,5 the two reporter probes, (Z)-2a (R = Bu) (Scheme 1) and 13 (see Scheme 2) are themselves unique and conceptually new, having been purposely designed to allow an estimate of the k values for an event that has hitherto resisted k quantification by other means, namely, the radical ring-opening of α-cyclopropyl-β-tributylstannylvinyl radicals.
Results and discussion
Our precise experimental method is detailed here. It used the 2,2-dimethylvinyl radical (6) as a horological calibrant for the α-cyclopropyl-β-tri-n-butylstannylvinyl radical (Z)-2a (R = Bu) in pentane, with 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b,c itself being generated by an O-directed free radical hydrostannation4,5,16–19 of the alkynol 1
3b,c itself being generated by an O-directed free radical hydrostannation4,5,16–19 of the alkynol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b,c with Bu3SnH/cat. Et3B3b,4b,6 over a temperature range of 20–30 °C. Accordingly, at 298 K (25 °C), the radical 2a (R = Bu) was assigned Ingold's kH-atom abstraction value for the reaction of 6 with Bu3SnH in pentane,7 which is 2.96 × 108 mol−1 s−1. From Ingold's log
3b,c with Bu3SnH/cat. Et3B3b,4b,6 over a temperature range of 20–30 °C. Accordingly, at 298 K (25 °C), the radical 2a (R = Bu) was assigned Ingold's kH-atom abstraction value for the reaction of 6 with Bu3SnH in pentane,7 which is 2.96 × 108 mol−1 s−1. From Ingold's log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 9.67 and his Ea of +1.624 kcal mol−1 (6794.816 J mol−1) for 6,7 the corresponding Bu3SnH kH-atom abstraction values were calculated for 6/2a in pentane at 293 K and 303 K. These calculated values were then used alongside Ingold's experimentally-determined kH-atom abstraction value at 298 K, to allow a reasonably accurate experimental quantification of the kring-opening values (Scheme 1) for the α-cyclopropyl-β-tri-n-butylstannylvinyl radical 2a (R = Bu) in pentane at 293, 298 and 303 K using Baines’ proven method for α-cyclopropylvinyl radicals.13 The Baines formula of eqn (1) equates the ratio of the vinyltin
A of 9.67 and his Ea of +1.624 kcal mol−1 (6794.816 J mol−1) for 6,7 the corresponding Bu3SnH kH-atom abstraction values were calculated for 6/2a in pentane at 293 K and 303 K. These calculated values were then used alongside Ingold's experimentally-determined kH-atom abstraction value at 298 K, to allow a reasonably accurate experimental quantification of the kring-opening values (Scheme 1) for the α-cyclopropyl-β-tri-n-butylstannylvinyl radical 2a (R = Bu) in pentane at 293, 298 and 303 K using Baines’ proven method for α-cyclopropylvinyl radicals.13 The Baines formula of eqn (1) equates the ratio of the vinyltin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) allenyltin products in such radical “clock” experiments5 to the ratio of the k values for H-atom abstraction and cyclopropane ring-opening:
allenyltin products in such radical “clock” experiments5 to the ratio of the k values for H-atom abstraction and cyclopropane ring-opening: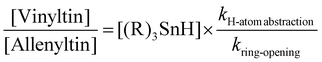 | (1) |
Of course, the latter expression rearranges to that in eqn (2):
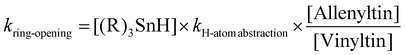 | (2) |
Following collation of the three experimentally-derived values (Scheme 1 entries 1–3) for the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kring-opening2ain pentanev 1/T in the form of an Arrhenius plot (see ESI‡), it was possible to deduce a log
kring-opening2ain pentanev 1/T in the form of an Arrhenius plot (see ESI‡), it was possible to deduce a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 13.274 (frequency factor A = 1.88 × 1013 s−1) for the ring-opening of 2a (R = Bu) in pentane, and a mean Ea of +7.18 kcal mol−1. The high magnitude of the log
A of 13.274 (frequency factor A = 1.88 × 1013 s−1) for the ring-opening of 2a (R = Bu) in pentane, and a mean Ea of +7.18 kcal mol−1. The high magnitude of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A for this ring-opening of 2a (R = Bu) unambiguously confirmed that it was a unimolecular EH1 free radical ring cleavage process that was leading to the radical 3a (R = Bu), which then H-atom abstracted from the Bu3SnH. Such a log
A for this ring-opening of 2a (R = Bu) unambiguously confirmed that it was a unimolecular EH1 free radical ring cleavage process that was leading to the radical 3a (R = Bu), which then H-atom abstracted from the Bu3SnH. Such a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A most definitely did not align with a stannylvinyl cation E1-ring-opening/reduction mechanism having led to 4a,6 nor a bimolecular SN2 stannylvinyl cation reduction, as would be invoked by advocates of the stannylvinyl cation mechanistic theory6 (see ESI‡).
A most definitely did not align with a stannylvinyl cation E1-ring-opening/reduction mechanism having led to 4a,6 nor a bimolecular SN2 stannylvinyl cation reduction, as would be invoked by advocates of the stannylvinyl cation mechanistic theory6 (see ESI‡).
Significantly, however, our experimentally-derived kring-opening value of 9.47 × 107 s−1 for 2a (R = Bu) in pentane at 298 K, and its accompanying log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 13.274, did align very satisfactorily with Newcomb's kring-opening value14a of 1.0 × 108 s−1 for the cyclopropylcarbinyl radical in THF at 298 K, and the log
A of 13.274, did align very satisfactorily with Newcomb's kring-opening value14a of 1.0 × 108 s−1 for the cyclopropylcarbinyl radical in THF at 298 K, and the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 13.15 that these workers reported for this process, which lends considerable confidence to the entirely free radical mechanistic proposal that is being advanced here (see Scheme 1).
A of 13.15 that these workers reported for this process, which lends considerable confidence to the entirely free radical mechanistic proposal that is being advanced here (see Scheme 1).
By comparing the experimentally-derived vinyltin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) allenyltin ratios 5a
allenyltin ratios 5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a (R = Bu) for the hydrostannation of 1 in pentane at 273, 298 and 303 K with the corresponding data gathered in PhMe, we were able to show that the rate of H-atom abstraction from Bu3SnH by the stannylvinyl radical 2a (R = Bu)/6 is approximately 1.47 times slower in PhMe than it is in pentane, which confirmed a noticeable solvent effect. Moreover, when the experimentally-determined rate constants obtained for 2a (R = Bu)/6 in PhMe were collated in the form of an Arrhenius plot (see ESI‡), this led to an Ea of +1.599 kcal mol−1 (i.e. 1.6 kcal mol−1) or 6693.84 J mol−1 being determined for the H-atom abstraction event involving 6/2a and Bu3SnH in PhMe. The resulting log
4a (R = Bu) for the hydrostannation of 1 in pentane at 273, 298 and 303 K with the corresponding data gathered in PhMe, we were able to show that the rate of H-atom abstraction from Bu3SnH by the stannylvinyl radical 2a (R = Bu)/6 is approximately 1.47 times slower in PhMe than it is in pentane, which confirmed a noticeable solvent effect. Moreover, when the experimentally-determined rate constants obtained for 2a (R = Bu)/6 in PhMe were collated in the form of an Arrhenius plot (see ESI‡), this led to an Ea of +1.599 kcal mol−1 (i.e. 1.6 kcal mol−1) or 6693.84 J mol−1 being determined for the H-atom abstraction event involving 6/2a and Bu3SnH in PhMe. The resulting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 9.4826 (A = 3.04 × 109 mol−1 s−1) also allowed a ΔS‡298 K of −17.148 e.u. or −71.75 J K−1 mol−1 to be deduced, which showed that the rate-determining step for this H-atom transfer was bimolecular and SH2.
A of 9.4826 (A = 3.04 × 109 mol−1 s−1) also allowed a ΔS‡298 K of −17.148 e.u. or −71.75 J K−1 mol−1 to be deduced, which showed that the rate-determining step for this H-atom transfer was bimolecular and SH2.
From the experimentally-derived log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A (9.4826 i.e. 9.48) and Ea (6693.84 J mol−1) data gathered on 2a (R = Bu) in PhMe, the theoretical kH-atom abstraction values could now be calculated for the reaction of the 2,2-dimethylvinyl radical 6/2a with Bu3SnH in PhMe at the higher temperatures of 313, 333 and 353 K (see Scheme 1). The availability of this log
A (9.4826 i.e. 9.48) and Ea (6693.84 J mol−1) data gathered on 2a (R = Bu) in PhMe, the theoretical kH-atom abstraction values could now be calculated for the reaction of the 2,2-dimethylvinyl radical 6/2a with Bu3SnH in PhMe at the higher temperatures of 313, 333 and 353 K (see Scheme 1). The availability of this log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A and these kH-atom abstraction values now allowed a complete experimental determination of the kring-opening values for the α-cyclopropyl-β-tri-n-butylstannylvinyl radical 2a (R = Bu) in PhMe over the temperature range 20–80 °C (293–353 K) at 0.2 M Bu3SnH concentration, and this k data is tabulated in Scheme 1.
A and these kH-atom abstraction values now allowed a complete experimental determination of the kring-opening values for the α-cyclopropyl-β-tri-n-butylstannylvinyl radical 2a (R = Bu) in PhMe over the temperature range 20–80 °C (293–353 K) at 0.2 M Bu3SnH concentration, and this k data is tabulated in Scheme 1.
An Arrhenius plot of the experimentally-derived log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kring-opening data for 2a (R = Bu) in PhMe vs. 1/T gave a straight line output (see Fig. 1 and ESI‡) from which a log
kring-opening data for 2a (R = Bu) in PhMe vs. 1/T gave a straight line output (see Fig. 1 and ESI‡) from which a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 14.951 (A = 8.93 × 1014 s−1) and an Ea of +9.47 kcal mol−1 (i.e. 9.5 kcal mol−1) could both be deduced for the ring-opening of 2a over the 293–353 K temperature range studied. The high mean log
A of 14.951 (A = 8.93 × 1014 s−1) and an Ea of +9.47 kcal mol−1 (i.e. 9.5 kcal mol−1) could both be deduced for the ring-opening of 2a over the 293–353 K temperature range studied. The high mean log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A for this cyclopropane ring-opening, and its substantially sized positive entropy of activation at 333 K (ΔS‡333 K = +32.09 J K−1 mol−1 or +7.67 e.u.) both immediately ruled out a stannylvinyl cation E1-ring-opening/reduction or a bimolecular ionic reduction mechanism6as having led to4a (see section 1.6 of the ESI‡ for an in depth discussion of these two invalid ionic mechanisms). Observations that were further supported by our previous unsuccessful cation-trappings with H2O in 4
A for this cyclopropane ring-opening, and its substantially sized positive entropy of activation at 333 K (ΔS‡333 K = +32.09 J K−1 mol−1 or +7.67 e.u.) both immediately ruled out a stannylvinyl cation E1-ring-opening/reduction or a bimolecular ionic reduction mechanism6as having led to4a (see section 1.6 of the ESI‡ for an in depth discussion of these two invalid ionic mechanisms). Observations that were further supported by our previous unsuccessful cation-trappings with H2O in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF
1 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O.3b,c
H2O.3b,c
 | ||
Fig. 1 Arrhenius plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kring-opening of 2avs. 1/T from the reaction of 1 with Bu3SnH/cat. Et3B/O2 in PhMe over 293–353 K. kring-opening of 2avs. 1/T from the reaction of 1 with Bu3SnH/cat. Et3B/O2 in PhMe over 293–353 K. | ||
Instead, our newly derived kinetic parameters only satisfactorily aligned with an entirely homolytic, unimolecular, EH1 fissive mechanism operating in the rate-determining step (Scheme 1), in which a very loose activated complex of the radical 2 was singularly transforming into the radical 3avia a product-like transition state in which cyclopropane bond-cleavage was already very advanced. The resulting stannylhomoallenyl radical 3a then H-atom abstracted from the Bu3SnH to ultimately yield 4a.
Critically, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A for this ring-opening of the α-cyclopropyl-β-stannylvinyl radical 2a (R = Bu) in PhMe aligned very well with the typical log
A for this ring-opening of the α-cyclopropyl-β-stannylvinyl radical 2a (R = Bu) in PhMe aligned very well with the typical log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A values (13.29–16.11) recorded by Frey20 for the unimolecular gas phase pyrolytic C–C bond homolyses of various cyclopropanes, which are always associated with large positive ΔS‡ values, due to the increased bond-loosening and much greater mobility that is experienced by such activated cyclopropane rings as they fissively transit into their initial biradical products.
A values (13.29–16.11) recorded by Frey20 for the unimolecular gas phase pyrolytic C–C bond homolyses of various cyclopropanes, which are always associated with large positive ΔS‡ values, due to the increased bond-loosening and much greater mobility that is experienced by such activated cyclopropane rings as they fissively transit into their initial biradical products.
We next elected to synthesize the sterically less encumbered chiral cyclopropylpropargylic alcohol 12 by the route shown in Scheme 2. This featured a catalytic Carreira alkynylation21 as a key step. The alkynol 12 was then subjected to an O-directed hydrostannation4,6,16–19 with Bu3SnH/cat. Et3B in PhMe, to generate 13, which now permitted an estimate of the kring-opening for its cyclopropane ring over a range of temperatures (Scheme 2).
Once again, it was assumed that the kH-atom abstraction values for 13 would very closely mirror those for 2a/6. If one is prepared to accept this key kinetic assumption, with the usual experimental caveats of course, then an Arrhenius plot of the resulting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kring-opening data v 1/T (see Fig. 2) reveals a log
kring-opening data v 1/T (see Fig. 2) reveals a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A of 14.549 (A = 3.54 × 1014 s−1), a ΔS‡333 K of +24.39 J K−1 mol−1 (+5.83 e.u.), and an Ea of +9.92 kcal mol−1 (i.e. +9.9 kcal mol−1).
A of 14.549 (A = 3.54 × 1014 s−1), a ΔS‡333 K of +24.39 J K−1 mol−1 (+5.83 e.u.), and an Ea of +9.92 kcal mol−1 (i.e. +9.9 kcal mol−1).
 | ||
Fig. 2 Arrhenius plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kring-opening of 13vs. 1/T from the reaction of 12 with Bu3SnH/cat. Et3B/O2 in PhMe over 313–353 K. kring-opening of 13vs. 1/T from the reaction of 12 with Bu3SnH/cat. Et3B/O2 in PhMe over 313–353 K. | ||
Critically, the above log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A and ΔS‡333 K data definitively ruled out a stannylvinyl cation reduction mechanism6 as having afforded 15 (see section 2.2 of the ESI‡ for a more detailed and in depth discussion of this point).
A and ΔS‡333 K data definitively ruled out a stannylvinyl cation reduction mechanism6 as having afforded 15 (see section 2.2 of the ESI‡ for a more detailed and in depth discussion of this point).
Significant also was the fact that our experimentally derived Ea of +9.9 kcal mol−1 was close in magnitude to the Ea of +10.7 kcal mol−1 calculated by Guo et al.22 for the closely related unimolecular radical-induced ring-opening22 of radical 17 (Scheme 3).
 | ||
| Scheme 3 Guo's calculations for the ring-opening of radical 17.22 | ||
While it is tempting to try to estimate the k values for the reaction of the β-triphenylstannylvinyl radical 2b (R = Ph) (Scheme 1) with Ph3SnH at different temperatures, by assuming that the kring-opening values for 2a and 2b would be identical, current EPR evidence suggests that β-triphenylstannylvinyl radicals are much more highly stabilised3dand potentially far less reactive than their β-trialkylstannylvinyl radical counterparts, which are generally unobservable by low temperature EPR spectroscopy.23
This is not the case with β-triphenylstannylvinyl radicals3d generated by the O-directed alkyne hydrostannation with Ph3SnH/cat. Et3B/O2.4 A process that has now allowed many such radicals to be routinely observed by EPR spectroscopy at low temperatures in PhMe and THF,3d due to the much greater lifetimes of β-triphenylstannylvinyl radicals in solution, even in the presence of excess Ph3SnH.
Possibly this enhanced longevity and much greater stability of β-triphenylstannylvinyl radicals is due to increased negative hyperconjugation (SOMO → σ*C–Sn) in such radicals (due to the electron-withdrawing Ph groups present on the Sn), as well as the reduced positive σC–Sn → SOMO hyperconjugation they experience.17e,f,g
Now even though it is not possible to reliably use the kring-opening values for 2a to directly calibrate 2b, a simple relative comparison of the 4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b product ratio of entry 10 in Scheme 1 with the ratio of 4a
5b product ratio of entry 10 in Scheme 1 with the ratio of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a obtained in entry 4, does suggest that the 2,2-dimethylvinyl radical 6 will likely react with Ph3SnH at a rate which is at least 6.95 times faster than the corresponding reaction with Bu3SnH in PhMe at 20 °C. This, in turn, points to a kH-atom abstraction value of no less than 1.36 × 109 mol−1 s−1 for 6 and 2b from Ph3SnH in PhMe (Scheme 1). While this relative kH-atom abstraction value for Ph3SnH can only ever be considered tentative, and a conservative minimal estimate at best, it does nevertheless confirm that such H-atom transfers do proceed at a very fast rate that is approximately an order of magnitude less than a diffusion-controlled reaction in PhMe (kdiffusion PhMe 293 K = 1.101 × 1010 mol−1 s−1). The availability of this kH-atom abstraction Ph3SnH value for 2b/6 has allowed a tentative estimate of the kring-opening value for 2b, which has clearly confirmed that the radical 2b has a lower level of reactivity with respect to its unimolecular ring-opening than 2a.
5a obtained in entry 4, does suggest that the 2,2-dimethylvinyl radical 6 will likely react with Ph3SnH at a rate which is at least 6.95 times faster than the corresponding reaction with Bu3SnH in PhMe at 20 °C. This, in turn, points to a kH-atom abstraction value of no less than 1.36 × 109 mol−1 s−1 for 6 and 2b from Ph3SnH in PhMe (Scheme 1). While this relative kH-atom abstraction value for Ph3SnH can only ever be considered tentative, and a conservative minimal estimate at best, it does nevertheless confirm that such H-atom transfers do proceed at a very fast rate that is approximately an order of magnitude less than a diffusion-controlled reaction in PhMe (kdiffusion PhMe 293 K = 1.101 × 1010 mol−1 s−1). The availability of this kH-atom abstraction Ph3SnH value for 2b/6 has allowed a tentative estimate of the kring-opening value for 2b, which has clearly confirmed that the radical 2b has a lower level of reactivity with respect to its unimolecular ring-opening than 2a.
Our collective findings to date do very strongly suggest that it is the fast rate of formation and trapping of β-triphenylstannylvinyl radicals, and their much lower tendency to β-scissively revert back into the starting propargyloxy O-coordinated tin radical, that is responsible for Ph3SnH generally outperforming Bu3SnH6,18 as a hydrostannylating reagent with most propargylically-oxygenated dialkylacetylene substrates under the rt Et3B-initiated reaction conditions.
It is also pertinent to point out that just because β-tributylstannylvinyl radicals are far less stable and more reactive than their β-triphenylstannylvinyl radical counterparts, this does not necessarily impose on them the requirement to preferentially engage in a fast bimolecular H-atom abstraction event with Bu3SnH. Such enhanced reactivity for β-tributylstannylvinyl radicals could manifest itself in other ways, such as through increased unimolecular β-scissive dissociation back into the starting alkyne in the form of its O-complexed Bu3Sn radical. This, in turn, might explain the generally lower levels of conversion4a,b,24 that one typically sees with Bu3SnH/cat. Et3B in most O-directed4 and non-directed24 alkyne free radical hydrostannations.
Although the latter may be synthetically detrimental to a significant number of intended applications,4 equally well, the enhanced reactivity of many β-tributylstannylvinyl radicals might sometimes be of direct benefit to certain tandem radical cyclisation processes.17 One case in point is Alabugin's brilliant O-directed hydrostannylative route to benzofluorenes from oligoalkynes,17a where Bu3SnH/AIBN was found to vastly outperform Ph3SnH/AIBN in PhMe in the tandem stannylvinyl radical cyclisation process conducted on a diyne model test substrate (86% yield vs. 40% yield). However, for most rt O-directed4 and non-directed24 dialkylacetylene hydrostannations with Et3B initiation, it is Ph3SnH4a,b that usually outperforms Bu3SnH, and this enhanced performance is almost certainly attributable to the higher stability of most β-triphenylstannylvinyl radical intermediates, which allows for their much more effective bimolecular trapping by the Ph3SnH at the fast, near diffusion-controlled, rates that we are seeing here.
Of further note in our current studies is the significant 5-fold rate acceleration seen for the ring-opening of 2a at 80 °C (kring-opening = 1.29 × 109 mol−1 s−1) relative to 13 (kring-opening = 2.66 × 108 mol−1 s−1). Such a marked increase in the rate of ring-opening of 2a possibly points to the potential constant recurrence of temporary transient internal MeO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) → Sn electron-donating events helping to accelerate the EH1 cyclopropane ring-opening event, by strongly reinforcing the σC–Sn → SOMO positive hyperconjugative interaction.17 Such Thorpe–Ingold-induced internal coordination in 2a might also be impeding the aforementioned reverse unimolecular β-scissive (R)3Sn˙ elimination back into the starting alkyne O-coordinated tin radical. Also, the much lower tendency of the stannylvinyl radical 2b (R = Ph) to engage in EH1 elimination to give the ring-cleaved 3b might simply be a reflection of the much higher stability of 2b, reduced conformational mobility induced by the Ph3Sn group, and the superior H-donor power of Ph3SnH. While our kring-opening and kH-atom abstraction data for 2a and 13 in PhMe are all based on Ingold's k and log
→ Sn electron-donating events helping to accelerate the EH1 cyclopropane ring-opening event, by strongly reinforcing the σC–Sn → SOMO positive hyperconjugative interaction.17 Such Thorpe–Ingold-induced internal coordination in 2a might also be impeding the aforementioned reverse unimolecular β-scissive (R)3Sn˙ elimination back into the starting alkyne O-coordinated tin radical. Also, the much lower tendency of the stannylvinyl radical 2b (R = Ph) to engage in EH1 elimination to give the ring-cleaved 3b might simply be a reflection of the much higher stability of 2b, reduced conformational mobility induced by the Ph3Sn group, and the superior H-donor power of Ph3SnH. While our kring-opening and kH-atom abstraction data for 2a and 13 in PhMe are all based on Ingold's k and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A data for 6 in pentane,7 clearly, our values will potentially be modifiable in the future, should improved k calibration data appear.
A data for 6 in pentane,7 clearly, our values will potentially be modifiable in the future, should improved k calibration data appear.
Conclusions
We expect that our new kH-atom abstraction data for the reaction of the 2,2-dimethylvinyl radical (6) with Bu3SnH and Ph3SnH in PhMe will aid much future synthetic planning with vinyl radicals in the commonly used solvent PhMe.Significantly, our new kinetic and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A work on the cyclopropane ring-openings of the β-stannylvinyl radicals derived from the probes 1 and 12 have further ruled out the hypothesised intermediacy of stannylvinyl cations6 in these Et3B/O2 radical-initiated alkyne hydrostannation reactions and, as such, the present work has confirmed an entirely free radical mechanism3 for the O-directed free radical hydrostannation of propargylically-oxygenated dialkylacetylenes (see sections 1.5 and 1.6 of the ESI‡ for more detailed discussion).4
A work on the cyclopropane ring-openings of the β-stannylvinyl radicals derived from the probes 1 and 12 have further ruled out the hypothesised intermediacy of stannylvinyl cations6 in these Et3B/O2 radical-initiated alkyne hydrostannation reactions and, as such, the present work has confirmed an entirely free radical mechanism3 for the O-directed free radical hydrostannation of propargylically-oxygenated dialkylacetylenes (see sections 1.5 and 1.6 of the ESI‡ for more detailed discussion).4
In the paper that accompanies this,25 other probe trapping studies will be described in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O that further invalidate the stannylvinyl cationic mechanistic theory6 of alkyne hydrostannation under the Et3B/O2-initiated reaction conditions. This work and the EPR studies that accompany it25 provide further new insights into the complex mechanistic events that proceed alongside these highly stereoselective, entirely free radical, O-directed hydrostannation reactions.3a,26
H2O that further invalidate the stannylvinyl cationic mechanistic theory6 of alkyne hydrostannation under the Et3B/O2-initiated reaction conditions. This work and the EPR studies that accompany it25 provide further new insights into the complex mechanistic events that proceed alongside these highly stereoselective, entirely free radical, O-directed hydrostannation reactions.3a,26
Experimental
General information
Unless stated otherwise, all reactions were run in dry solvents under an N2 atmosphere. Dry pentane was freshly distilled from CaH2 under an N2 atmosphere and dry PhMe was used as supplied by Sigma-Aldrich. Both anhydrous solvents were taken out by dry syringe under an N2 atmosphere. Ph3SnH was purchased from Sigma-Aldrich and used as supplied; it was always handled in a glove-bag under N2. Bu3SnH was purchased from Alfa and was used as supplied. It was also periodically tested on a known thiocarbonyl imidazolide substrate that typically deoxygenates in >95% yield; if a yield of this magnitude was obtained, then the Bu3SnH was used for the experiments reported. SiO2 flash chromatography was carried out using Fluorochem silica gel 60 Å, and petrol refers to the 40–60 °C b.p. fraction; it was distilled prior to use for chromatography. HPLC grade EtOAc was used for all chromatographic purifications. TLC analysis and preparative TLC were performed on Merck glass-backed TLC plates coated with silica gel 60 F254. NMR analyses were carried out using the QUB School of Chemistry Bruker Avance III HD Ascend 600 instrument operating at a frequency of 600.1337 MHz. Although the 600.13 MHz 1H spectra of 4a and 5a in CDCl3 (referenced upon tetramethylsilane (TMS) at δ 0.00 ppm, residual CHCl3 at δ 7.23 ppm) were previously published in ref. 3c (see: H. A. Watson, S. Manaviazar, H. G. Steeds and K. J. Hale, Tetrahedron, 2020, 76, 131061), we have included these spectra here in considerably abridged form, along with some of the previous spectra of 4b and 5b, in order to allow the readers of the present paper to conveniently gauge the new kinetic ratio determinations that we are presenting here for the very first time. Clearly, there are minor changes in the chemical shifts observed, in the new spectra, as one would expect.Experimental procedures for generating α-stannylvinyl radical 2a and stannyl homoallenyl radical 3aen route to 4a and 5a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5a ratio.
5a ratio.
To a round-bottomed flask containing a well-stirred solution of the cyclopropylacetylenic alcohol 1 (196.2 mg, 1 mmol) in dry pentane (10 mL) under N2 was added Bu3SnH (0.54 mL, 2 mmol) dropwise via syringe over 1 min. To this stirred mixture at the desired temperature (20, 25 and 30 °C) was successively added Et3B (0.1 mL, 1 M in hex, 0.1 mmol, 0.1 equiv.) dropwise via syringe followed by air (5 mL) from a syringe 5 min later. The reactants were stirred at the requisite temperature for 24 h, after which, the reaction flask was transferred to a rotary evaporator and the solvent removed in vacuo. A 1H NMR spectrum was recorded of a portion of the crude reaction mixture in CDCl3 to ascertain the crude ratio of products. Each reaction temperature was examined a minimum of three times and the average product ratio of 4a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a was taken to determine of the rate constant kring-opening for the (Z)-2a→3a (R = Bu) conversion at the designated temperature.
5a was taken to determine of the rate constant kring-opening for the (Z)-2a→3a (R = Bu) conversion at the designated temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5a ratio.
5a ratio.
To a small round-bottomed flask containing a well-stirred solution of the cyclopropylacetylenic alcohol 1 (196.2 mg, 1 mmol) in dry PhMe (10 mL) under N2 was added Bu3SnH (0.54 mL, 2 mmol) dropwise via syringe over 1 min. To this stirred mixture at the desired temperature (20, 25, 30, 40, 60 and 80 °C) was successively added Et3B (0.1 mL, 1 M in hex, 0.1 mmol) (0.1 equiv.) dropwise via syringe followed by air (5 mL, from a syringe) 5 min later. The reactants were then maintained at the desired temperature with stirring for 24 h, after which, the reaction flask was transferred to a rotary evaporator and solvent removed in vacuo. A 1H NMR spectrum was recorded of a portion of the crude reaction mixture in CDCl3 to ascertain the crude ratio of products. Each reaction temperature was examined a minimum of 2–4 times and the average product ratio of 4a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (R = Bu) was taken to determine of kring-opening for the (Z)-2a→3a conversion in PhMe at the designated temperature.
5a (R = Bu) was taken to determine of kring-opening for the (Z)-2a→3a conversion in PhMe at the designated temperature.
Synthetic route to the (R)-1-(tert-butyldiphenylsilyloxy)-4-cyclopropylbut-3-yn-2-ol (12)
To a round-bottomed flask containing ethylene glycol (20 mL, 357.7 mmol, 7 equiv.) in dry CH2Cl2 (200 mL) under N2 was added imidazole (6.69 g, 102.2 mmol, 2 equiv.) in one portion with vigorous stirring. THF (40 mL) was then added via syringe, and the reaction mixture was cooled to 0 °C using an ice bath. t-Butyldiphenylsilyl chloride (13.3 mL, 51.146 mmol, 1 equiv.) was then added dropwise over 30 min via syringe. When the addition was complete, the ice bath was removed and the reactants were allowed to stir at rt for 18 h before the reaction was diluted with CH2Cl2 (200 mL) and quenched with saturated aq. NaHCO3 solution (100 mL) and H2O (200 mL). The aqueous layer was washed with CH2Cl2 (50 mL × 3) and the combined organic layers were dried with MgSO4, filtered and concentrated in vacuo. The oily residue was purified by gradient elution SiO2 flash chromatography with petrol–EtOAc (50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 25
1 → 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 20
1 → 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 10
1 → 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 5
1 → 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the O–silyl ether 17 (10.67 g, 69%) as a slightly impure oil. This technical grade alcohol 17 was then used directly for the oxidation step.
1) to give the O–silyl ether 17 (10.67 g, 69%) as a slightly impure oil. This technical grade alcohol 17 was then used directly for the oxidation step.
To a stirred −78 °C solution of (COCl)2 (2.83 mL, 33.05 mmol, 1 equiv.) in dry CH2Cl2 (187 mL) under N2 was added DMSO (4.7 mL, 66.1 mmol, 2 equiv.) dropwise via syringe over 3 min. Stirring was continued at −78 °C for a further 30 min before a solution of the aforementioned alcohol 17 (9.93 g, 33.05 mmol, 1 equiv.) in dry CH2Cl2 (20 mL) was added dropwise via syringe over 15 min. After a further 7 min of stirring at −78 °C, Et3N (20.7 mL, 148.717 mmol, 4.5 equiv.) was added dropwise over 3 min and the reaction mixture then allowed to warm from −78 °C to rt, whereupon it was stirred for 2 h. The solvents were then removed in vacuo on the rotary evaporator. The crude residue of the aldehyde 11 was then suspended in petrol–EtOAc (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 500 mL), and the solid Et3NHCl filtered off under vacuum. The filtrate was concentrated in vacuo and the syrupy residue was purified by gradient elution SiO2 flash chromatography with petrol–EtOAc (20
1, 500 mL), and the solid Et3NHCl filtered off under vacuum. The filtrate was concentrated in vacuo and the syrupy residue was purified by gradient elution SiO2 flash chromatography with petrol–EtOAc (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 10
1 → 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the aldehyde 11 (6.73 g, 68%) as an oil.
1) to give the aldehyde 11 (6.73 g, 68%) as an oil.
To solid Zn(OTf)2 (2.05 g, 5.649 mmol, 0.3 equiv.) and (−)-N-methylephedrine (1.35 g, 7.532 mmol, 0.4 equiv.) in a small pear-shaped flask under N2 was successively added PhMe (20 mL) and Et3N (3.94 mL, 28.246 mmol, 1.5 equiv.) by syringe. Cyclopropylacetylene (5.62 mL, 66.284 mmol, 3.52 equiv.) was then added by syringe maintaining the N2 atmosphere throughout. The reactants were stirred vigorously at rt for 2 h whereafter a solution of aldehyde 11 (5.62 g, 18.831 mmol) (which had been pre-dried by coevaporation from PhMe × 2) in PhMe (7.1 mL) was added via syringe, along with a 1 mL rinse of the flask with more dry PhMe. The flask containing the reactants was next transferred to an oil bath and vigorously stirred at 40 °C for 22 h. The reaction mixture was then quenched by the addition of saturated aq. NH4Cl solution (50 mL) and diluted with EtOAc (50 mL). The organic extract was separated, and the aqueous layer was further extracted with more EtOAc (2 × 50 mL). The combined organic extracts were washed with H2O (50 mL), dried over MgSO4, filtered and concentrated in vacuo. The crude residue was purified by SiO2 flash chromatography with petrol–EtOAc (25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the alkynol 12 (4.33 g, 63%) as a thick oil. 1H NMR of 12 (600.13 MHz, CDCl3) δ: 7.73–7.64 (m, 4H, –Ph), 7.46–7.34 (m, 6H, Ph), 4.43 (m, 1H, H2), 3.765–3.75 (dd, J = 10.2, 3.6 Hz, 1H, H1a), 3.67 (dd, J = 10.2, 6.6 Hz, 1H, H1b), 2.58 (d, J = 5.4 Hz, 1H, –OH), 1.23 (m, 1H, H5), 1.07 (s, 9H, t-Bu), 0.74 (m, 1H, H6a), 0.66 (m, 1H, H6b). 13C NMR of 12 (150.9 MHz, CDCl3) δ: 135.6 (m-CH of Ph), 135.5 (m-CH of Ph), 133.0 (q of Ph), 132.9 (q of Ph), 129.9 (p-CH of Ph), 129.8 (p-CH of Ph), 127.8 (o-CH of Ph), 127.77 (o-CH of Ph), 89.4 (C3), 73.2 (C4), 67.9 (C2), 63.2 (C1), 26.8 (Me groups of t-Bu), 26.6 (C5), 19.2 (q carbon of t-Bu), 8.11 (C6a), 8.10 (C6b) ppm.
1) to give the alkynol 12 (4.33 g, 63%) as a thick oil. 1H NMR of 12 (600.13 MHz, CDCl3) δ: 7.73–7.64 (m, 4H, –Ph), 7.46–7.34 (m, 6H, Ph), 4.43 (m, 1H, H2), 3.765–3.75 (dd, J = 10.2, 3.6 Hz, 1H, H1a), 3.67 (dd, J = 10.2, 6.6 Hz, 1H, H1b), 2.58 (d, J = 5.4 Hz, 1H, –OH), 1.23 (m, 1H, H5), 1.07 (s, 9H, t-Bu), 0.74 (m, 1H, H6a), 0.66 (m, 1H, H6b). 13C NMR of 12 (150.9 MHz, CDCl3) δ: 135.6 (m-CH of Ph), 135.5 (m-CH of Ph), 133.0 (q of Ph), 132.9 (q of Ph), 129.9 (p-CH of Ph), 129.8 (p-CH of Ph), 127.8 (o-CH of Ph), 127.77 (o-CH of Ph), 89.4 (C3), 73.2 (C4), 67.9 (C2), 63.2 (C1), 26.8 (Me groups of t-Bu), 26.6 (C5), 19.2 (q carbon of t-Bu), 8.11 (C6a), 8.10 (C6b) ppm.
When run under identical conditions on 1 g (3.351 mmol) scale, with respect to aldehyde 11, the yield of 12 (0.87 g) was found to improve to 71%, possibly due to improved stirring.
General procedure for the O-directed hydrostannation of alkynol 12 with Bu3SnH in PhMe at various temperatures to obtain the 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 16 ratio
16 ratio
For each of these kinetic runs, a 1 M solution of Et3B in PhMe was freshly prepared by addition of Et3B (0.2 mL, 1 M solution in hexanes) to dry PhMe (2 mL) under N2; an aliquot of that solution was then taken and used as the reaction initiator, adhering to the general procedure set out below.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 in the crude concentrated reaction mixture was then determined by high field NMR spectroscopy in CDCl3 and this ratio was subsequently used alongside the theoretical or experimentally determined kH-atom abstraction values in Scheme 2, to determine the kring-opening values for the conversion of 13 into 14. Each reaction temperature was examined a minimum of 2–4 times and the average product ratio of 15
16 in the crude concentrated reaction mixture was then determined by high field NMR spectroscopy in CDCl3 and this ratio was subsequently used alongside the theoretical or experimentally determined kH-atom abstraction values in Scheme 2, to determine the kring-opening values for the conversion of 13 into 14. Each reaction temperature was examined a minimum of 2–4 times and the average product ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 was taken to determine of kring-opening for the 13 into 14 conversion in PhMe at the designated temperature.
16 was taken to determine of kring-opening for the 13 into 14 conversion in PhMe at the designated temperature.
In an attempt to obtain analytically pure samples of the two products 15 and 16, several of the aforementioned crude reaction mixtures were combined and partially purified by gradient-elution SiO2 flash chromatography using petrol–EtOAc (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 40
1 → 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 20
1 → 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 10
1 → 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent. A second flash chromatographic purification of this partially purified mixture (enriched in the stannylallene 15) was then performed with petrol–Et2O (150
1) as the eluent. A second flash chromatographic purification of this partially purified mixture (enriched in the stannylallene 15) was then performed with petrol–Et2O (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 100
1 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent, to isolate 15 in reasonably pure condition. A third analytical column with neat CH2Cl2 was then performed to allow isolation of the allene 15 as a 1
1) as the eluent, to isolate 15 in reasonably pure condition. A third analytical column with neat CH2Cl2 was then performed to allow isolation of the allene 15 as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture in near pure condition. The spectral data for this mixture of the two diastereosiomers of 15 is reported now in full: 1H NMR of 15 (600.13 MHz, CDCl3) δ: 7.71–7.63 (m, 4H, Ph), 7.46–7.34 (m, 6H, Ph), 4.77 (td, J = 6.6 and 3.0 Hz, 1H, H5 geometric isomer 1), 4.715 (td, J = 6.6 and 3.0 Hz, 1H, H5 geometric isomer 2), 4.383 (complex m, 1H, H2 both diastereomers), 3.68 (dd, J = 10.2, 3.0 Hz, 1H, H1a diastereomer 1) partially superimposed upon 3.66 (dd, J = 10.2, 3.6 Hz, 1H, H1a diastereomer 2), 3.49 (dd, J = 8.4, 4.8 Hz, 1H, H1b, diastereomer 1) partially superimposed upon 3.48 (dd, J = 8.4, 4.8 Hz, 1H, H1b diastereomer 2), 2.71 (d, J = 3.0 Hz, 1H, OH, diastereomer 1) superimposed upon 2.706 (d, J = 3.0 Hz, 1H, OH, diastereomer 2), 1.64 and 1.45 (m, 2H, H6a, H6b both diastereomers), 1.40–1.23 (complex m, 18 H, –CH2– regions of Bu3Sn, both diastereomers), 1.066 and 1.064 (2 × s, 9H, t-Bu, TBDPS, both diastereomers), 0.92 (t, J = 7.8 Hz, 9H, Me of Bu3Sn, superimposed upon m, 3H, H7, diastereomer 1), 0.86 (t, J = 7.2 Hz, 9H, Me of Bu3Sn, superimposed upon m, 3H, H7, diastereomer 2) ppm. 13C NMR of 15 (150.9 MHz, CDCl3) δ: 200.77 and 200.64 (1 × C4, both diastereomers), 135.56 and 135.54 (2 × m-CH of Ph, both diastereomers), 133.3 (1 × quaternary C of Ph, both diastereomers), 129.74 and 129.72 (1 × p-CH of Ph, both diastereomers), 127.71 (2 × o-CH of Ph carbons of both diastereomers) 96.28 and 96.22 (1 × C5, both diastereomers), 86.17 and 86.07 (1 × C3, both diastereomers), 72.94 and 72.73 (C2, both diastereomers), 68.74 and 68.43 (1 × C1, both diastereomers), 29.0, 27.84 and 27.28 (–CH2– groups of Bu3Sn, both diastereomers), 26.86 and 26.83 (t-Bu, both diastereomers), 21.65 and 21.60 (C6, of both diastereomers), 19.2 (quaternary C, t-Bu), 17.51 (–CH2– groups of Bu3Sn, 1J119Sn13C = 336.5 Hz, 1J117Sn13C = 321.4 Hz, –Sn
1 diastereomeric mixture in near pure condition. The spectral data for this mixture of the two diastereosiomers of 15 is reported now in full: 1H NMR of 15 (600.13 MHz, CDCl3) δ: 7.71–7.63 (m, 4H, Ph), 7.46–7.34 (m, 6H, Ph), 4.77 (td, J = 6.6 and 3.0 Hz, 1H, H5 geometric isomer 1), 4.715 (td, J = 6.6 and 3.0 Hz, 1H, H5 geometric isomer 2), 4.383 (complex m, 1H, H2 both diastereomers), 3.68 (dd, J = 10.2, 3.0 Hz, 1H, H1a diastereomer 1) partially superimposed upon 3.66 (dd, J = 10.2, 3.6 Hz, 1H, H1a diastereomer 2), 3.49 (dd, J = 8.4, 4.8 Hz, 1H, H1b, diastereomer 1) partially superimposed upon 3.48 (dd, J = 8.4, 4.8 Hz, 1H, H1b diastereomer 2), 2.71 (d, J = 3.0 Hz, 1H, OH, diastereomer 1) superimposed upon 2.706 (d, J = 3.0 Hz, 1H, OH, diastereomer 2), 1.64 and 1.45 (m, 2H, H6a, H6b both diastereomers), 1.40–1.23 (complex m, 18 H, –CH2– regions of Bu3Sn, both diastereomers), 1.066 and 1.064 (2 × s, 9H, t-Bu, TBDPS, both diastereomers), 0.92 (t, J = 7.8 Hz, 9H, Me of Bu3Sn, superimposed upon m, 3H, H7, diastereomer 1), 0.86 (t, J = 7.2 Hz, 9H, Me of Bu3Sn, superimposed upon m, 3H, H7, diastereomer 2) ppm. 13C NMR of 15 (150.9 MHz, CDCl3) δ: 200.77 and 200.64 (1 × C4, both diastereomers), 135.56 and 135.54 (2 × m-CH of Ph, both diastereomers), 133.3 (1 × quaternary C of Ph, both diastereomers), 129.74 and 129.72 (1 × p-CH of Ph, both diastereomers), 127.71 (2 × o-CH of Ph carbons of both diastereomers) 96.28 and 96.22 (1 × C5, both diastereomers), 86.17 and 86.07 (1 × C3, both diastereomers), 72.94 and 72.73 (C2, both diastereomers), 68.74 and 68.43 (1 × C1, both diastereomers), 29.0, 27.84 and 27.28 (–CH2– groups of Bu3Sn, both diastereomers), 26.86 and 26.83 (t-Bu, both diastereomers), 21.65 and 21.60 (C6, of both diastereomers), 19.2 (quaternary C, t-Bu), 17.51 (–CH2– groups of Bu3Sn, 1J119Sn13C = 336.5 Hz, 1J117Sn13C = 321.4 Hz, –Sn![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2– of Bu3Sn, both diastereomers), 14.0 and 13.9 (C7–Me of both diastereomers) 13.69, 13.65 and 13.59 (Me groups of Bu3Sn groups, both diastereomers), 10.88 and 10.83 (CH2– of Bu3Sn, both diastereomers) ppm.
H2– of Bu3Sn, both diastereomers), 14.0 and 13.9 (C7–Me of both diastereomers) 13.69, 13.65 and 13.59 (Me groups of Bu3Sn groups, both diastereomers), 10.88 and 10.83 (CH2– of Bu3Sn, both diastereomers) ppm.
Unfortunately, we were never able to obtain a satisfactory 1H NMR spectrum of the pure vinyltin product 16 of the hydrostannation of 12. Nonetheless, this did not prove especially problematical for the kinetic task at hand, since it was possible to readily determine the crude ratios of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 from the 1H NMR spectra run of the crude reaction mixtures. In this regard, the olefinic H4 peak of the vinyltin 16 clearly stood out, it resonating as a dd (J = 10.2 and 1.2 Hz) at δ 5.55 ppm in CDCl3. Its identity was readily confirmed by the small allylic coupling between H4 and H2 (4J = 1.2 H), and the large J coupling (3J = 10.2 Hz) with the cyclopropane CH (H5). The vinyltin geometry could be readily assigned from the large 119/117Sn–1H J couplings (119Sn–1H = 131.4 and 111.6 Hz) that accompanied this resonance.
16 from the 1H NMR spectra run of the crude reaction mixtures. In this regard, the olefinic H4 peak of the vinyltin 16 clearly stood out, it resonating as a dd (J = 10.2 and 1.2 Hz) at δ 5.55 ppm in CDCl3. Its identity was readily confirmed by the small allylic coupling between H4 and H2 (4J = 1.2 H), and the large J coupling (3J = 10.2 Hz) with the cyclopropane CH (H5). The vinyltin geometry could be readily assigned from the large 119/117Sn–1H J couplings (119Sn–1H = 131.4 and 111.6 Hz) that accompanied this resonance.
General procedure for the O-directed hydrostannation of 1 with Ph3SnH in PhMe at various temperatures to obtain the 4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 5b ratio
5b ratio
A 1 M solution of Ph3SnH in PhMe was prepared by accurately weighing out Ph3SnH, into an open-necked round-bottomed flask containing a magnetic stirring bar, inside a glove bag filled with dry N2. The reaction vessel was then capped with a closed 3-way tap possessing a Quickfit male joint, while still inside the glove bag. The sealed flask was then removed from the glove bag and connected to a vacuum line via a 3-way tap, which was also fitted with an N2-filled balloon. The reaction flask was then sequentially evacuated and purged with N2 from the balloon before it was clamped over a magnetic stirrer. Dry PhMe was then added to give a 1 M solution. An aliquot of that freshly prepared solution of Ph3SnH (2 mL, 1 M in PhMe, 2 mmol) was then added to the flask containing the acetylene 1 (196.2 mg, 1 mmol) and a magnetic stirring bar under N2. To this stirred mixture of the Ph3SnH and 1 at the desired temperature (20, 40, 60 and 80 °C) was then added Et3B (0.1 mL, 1 M in hex, 0.1 mmol) (0.1 equiv.) dropwise via syringe, followed by air (5 mL, from a syringe) 5 min later. The reactants were then stirred at the designated temperature for 24 h, after which, the reaction flask was transferred to a rotary evaporator and the solvent removed in vacuo. A 1H NMR spectrum was recorded of a tiny portion of the crude reaction mixture in CDCl3 to ascertain the crude ratio of products. The remaining crude concentrated residue was then purified by gradient-elution SiO2 flash chromatography using initially 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petrol
1 petrol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 to remove excess tin hydride, and then 30
CH2Cl2 to remove excess tin hydride, and then 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petrol
1 petrol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc to yield the allenylstannane product 4b as a clear oil. Finally the eluent was changed to 25
EtOAc to yield the allenylstannane product 4b as a clear oil. Finally the eluent was changed to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petrol
1 petrol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc to obtain the essentially pure vinylstannane product 5b as a white amorphous solid. Each reaction temperature was examined a minimum of two/four times and the average product ratio of 4b
EtOAc to obtain the essentially pure vinylstannane product 5b as a white amorphous solid. Each reaction temperature was examined a minimum of two/four times and the average product ratio of 4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b (R = Ph) was taken. This protocol allowed estimation of the kH-atom abstraction Ph3SnH (i.e.(Z)-2b→5b [R = Ph]) at 293 K (20 °C).
5b (R = Ph) was taken. This protocol allowed estimation of the kH-atom abstraction Ph3SnH (i.e.(Z)-2b→5b [R = Ph]) at 293 K (20 °C).
Data availability
The experimental data supporting this article can be found in the Experimental section of this paper and in the ESI.‡ The ESI‡ provides NMR spectra and product ratio determinations for 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a, 4b
5a, 4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b and 15
5b and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16. The ESI‡ also contains the theoretical rate constant calculations that were performed, and our experimental rate constant determinations, and the Arrhenius Plots that were associated with these studies in Excel format. The ESI‡ also provides details of how the log
16. The ESI‡ also contains the theoretical rate constant calculations that were performed, and our experimental rate constant determinations, and the Arrhenius Plots that were associated with these studies in Excel format. The ESI‡ also provides details of how the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A, Ea, ΔS‡ and ΔG‡ data were calculated from the experimentally-derived data gathered in these plots. Finally, the ESI‡ contains a detailed mechanistic interpretation of the new kinetic data gathered. Citations to references 26–43 can be found in the ESI.‡
A, Ea, ΔS‡ and ΔG‡ data were calculated from the experimentally-derived data gathered in these plots. Finally, the ESI‡ contains a detailed mechanistic interpretation of the new kinetic data gathered. Citations to references 26–43 can be found in the ESI.‡
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Leverhulme Trust (RPG-2015-438), QUB, LJMU, and the NI DfE for financial support (Ph.D Studentship to HAW), and Dr Joseph S. Vyle of QUB for generous gifts of chemicals.References
- S. Z. Zard, Radical Reactions in Organic Synthesis, Oxford University Press (OUP), 2003, ch. 1, p. 8 Search PubMed.
- (a) H. E. Avery, Theory of Reaction Rates, in Basic Reaction Kinetics and Mechanism, Macmillan, 1974, ch. 5, p. 59 CrossRef H. E. Avery, Reactions in Solution, in Basic Reaction Kinetics and Mechanism, Macmillan, 1974, ch. 8, p. 99 Search PubMed (b) R. A. Jackson, Kinetics, in Mechanism: An Introduction to the Study of Organic Reactions, OUP, 1985, ch. 3, p. 23 Search PubMed; (c) H. Maskill, The rates of simple chemical reactions, in The Physical Basis of Organic Chemistry, Oxford University Press, 1985, ch. 6, p. 216 Search PubMed.
- (a) P. Dimopoulos, J. George, D. A. Tocher, S. Manaviazar and K. J. Hale, Org. Lett., 2005, 7, 5377 CrossRef CAS PubMed; (b) H. A. Watson, S. Manaviazar, H. G. Steeds and K. J. Hale, Chem. Commun., 2019, 55, 14454 RSC; (c) H. A. Watson, S. Manaviazar, H. G. Steeds and K. J. Hale, Tetrahedron, 2020, 76, 131061 CrossRef CAS; (d) For the first solution phase EPR spectra of β-triphenyl-stannylvinyl radicals, see: H. A. Watson, A. J. Fielding and K. J. Hale, Chem. Commun., 2021, 57, 7449 RSC.
- (a) Review: K. J. Hale, S. Manaviazar and H. A. Watson, Chem. Rec., 2019, 19, 238 CrossRef CAS PubMed; (b) P. Dimopoulos, A. Athlan, S. Manaviazar, J. George, M. Walters, L. Lazarides, A. E. Aliev and K. J. Hale, Org. Lett., 2005, 7, 5369 CrossRef CAS PubMed; (c) S. Manaviazar, K. J. Hale and A. LeFranc, Tetrahedron Lett., 2011, 52, 2080 CrossRef CAS; (d) K. J. Hale, M. Grabski, S. Manaviazar and M. Maczka, Org. Lett., 2014, 16, 1164 CrossRef CAS PubMed; (e) K. J. Hale, M. Maczka, A. Kaur, S. Manaviazar, M. Ostovar and M. Grabski, Org. Lett., 2014, 16, 1168 CrossRef CAS PubMed; (f) K. Micoine, P. Persich, J. Llaveria, M.-H. Lam, A. Merderna, F. Loganzo and A. Furstner, Chem. – Eur. J., 2013, 19, 7370 CrossRef CAS PubMed.
- M. Newcomb, Radical Kinetics and Clocks, in Encyclopedia of Radicals in Chemistry, Biology and Materials, John Wiley, 2012. DOI:10.1002/9780470971253.rad007.
- (a) M. S. Oderinde, R. D. J. Froese and M. G. Organ, Angew. Chem., Int. Ed., 2013, 52, 11334 CrossRef CAS PubMed; (b) M. S. Oderinde, R. D. J. Froese and M. G. Organ, Chem. – Eur. J., 2014, 20, 8579 CrossRef CAS PubMed; (c) M. S. Oderinde and M. G. Organ, Chem. – Eur. J., 2013, 19, 2615 CrossRef CAS PubMed; (d) M. S. Oderinde, H. N. Hunter, R. D. J. Froese and M. G. Organ, Chem. – Eur. J., 2012, 18, 10821 CrossRef CAS PubMed; (e) M. Alami, A. Hamze and O. Provot, ACS Catal., 2019, 9, 3437 CrossRef CAS; (f) R. Hua, Functionalized Alkenes from Hydrofunctionalization of Alkynes, in Addition Reactions with Unsaturated Hydrocarbons, 1st edn, Wiley-VCH, 2022, ch. 3, p. 47 CrossRef.
- L. J. Johnston, J. Lusztyk, D. D. M. Wagner, A. N. Abeywickreyma, A. L. J. Beckwith, J. C. Scaiano and K. U. Ingold, J. Am. Chem. Soc., 1985, 107, 4594 CrossRef CAS.
- B. Branchi, C. Galli and P. Gentili, Eur. J. Org. Chem., 2002, 2844 CrossRef CAS.
- For Scaiano's observation of phenyl radicals by laser flash photolysis of benzoyl peroxide see: J. C. Scaiano and L. Stewart, J. Am. Chem. Soc., 1983, 105, 3609 CrossRef CAS.
- (a) C. Reichardt, J. Schroeder, P. Voghringer and D. Schwarzer, Phys. Chem. Chem. Phys., 2008, 10, 1662 RSC; (b) C. Reichardt, T. Schafer, J. Schroeder, P. Voghringer and D. Schwarzer, Ultrafast Phenomena XVI. Springer Series in Chemical Physics, Springer, Berlin, Heidelberg, 2009, vol. 92. DOI:10.1007/978-3-540-95946-5_159.
- (a) CIDNP-NMR work: A. Kitamura, H. Sakuragi, M. Yoshida and K. Tokumaru, Bull. Chem. Soc. Jpn., 1980, 53, 1393 CrossRef CAS; (b) Laser Flash Photolysis work: J. Wang, H. Itoh, M. Tsuchiya, K. Tokumaru and H. Sakuragi, Tetrahedron, 1995, 51, 11967 CrossRef CAS.
- P. Lebourgeois, R. Arnaud and J. Lemaire, J. Chim. Phys., 1972, 69, 1633 CrossRef.
- K. K. Milnes, S. E. Gottschling and K. M. Baines, Org. Biomol. Chem., 2004, 2, 3530 RSC.
- (a) M. Newcomb and A. G. Glenn, J. Am. Chem. Soc., 1989, 111, 275 CrossRef CAS; (b) J. Jin and M. Newcomb, J. Org. Chem., 2007, 72, 5098 CrossRef CAS PubMed.
- D. Crich and X.-S. Mo, J. Am. Chem. Soc., 1998, 120, 8298 CrossRef CAS.
- D. P. Curran and T. McFadden, J. Am. Chem. Soc., 2016, 138, 7741 CrossRef CAS PubMed.
- (a) K. Pati, G. dos Passos Gomes, T. Harris, A. Hughes, H. Phan, T. Banerjee, K. Hanson and I. V. Alabugin, J. Am. Chem. Soc., 2015, 137, 1165 CrossRef CAS; (b) N. P. Tsvetkov, E. Gonzales-Rodriguez, A. Hughes, G. dos Passos Gomes, F. D. White, F. Kuriakose and I. V. Alabugin, Angew. Chem., Int. Ed., 2018, 57, 3651 Search PubMed; (c) E. Gonzalez-Rodriguez, M. A. Abdo, G. dos Passos Gomes, S. Ayad, F. D. White, N. P. Tsvetkov, K. Hanson and I. V. Alabugin, J. Am. Chem. Soc., 2020, 142, 8352 CrossRef CAS PubMed; (d) C. Hu, L. Kuhn, F. D. Makurvet, E. S. Knorr, X. Lin, R. K. Kawade, F. Mentink-Vigier, K. Hamson and I. V. Alabugin, J. Am. Chem. Soc., 2024, 146, 4187 CrossRef CAS PubMed; (e) I. V. Alabugin, G. dos Passos Gomes and M. A. Abdo, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2019, 9, e1389 Search PubMed; (f) I. V. Alabugin, K. M. Gilmore and P. W. Peterson, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 109 CAS; (g) I. V. Alabugin, Stereoelectronic Effects: A Bridge Between Structure and Reactivity, John Wiley & Sons Ltd, Chichester, UK, 2016, ch. 3, p. 42 CrossRef.
- (a) C. Nativi and M. Taddei, J. Org. Chem., 1988, 58, 820 CrossRef; (b) H. E. Ensley, R. R. Buescher and K. Lee, J. Org. Chem., 1982, 47, 404 CrossRef CAS; (c) M. Lautens and A. H. Hudson, Tetrahedron Lett., 1990, 31, 3105 CrossRef CAS.
- R. Willem, A. Delmotte, I. De Borger, M. Biesemans, M. Gielen and F. Kayser, J. Organomet. Chem., 1994, 480, 255 CrossRef CAS.
- H. M. Frey and R. Walsh, Chem. Rev., 1969, 69, 103 CrossRef CAS.
- N. K. Anand and E. M. Carreira, J. Am. Chem. Soc., 2001, 123, 9687 CrossRef CAS PubMed.
- J. Shi, M. Zhang, Y. Fu, L. Liu and Q.-X. Guo, Tetrahedron, 2007, 63, 12681 CrossRef CAS.
- K. Suzuki, N. Sugihara, Y. Nishimoto and M. Yasuda, Angew. Chem., Int. Ed., 2022, 61, e202201883 CrossRef CAS PubMed.
- (a) K. Oshima, Bull. Chem. Soc. Jpn., 2008, 81, 1 CrossRef CAS; (b) K. Nozaki, K. Oshima and K. Utimoto, J. Am. Chem. Soc., 1987, 109, 2547 Search PubMed.
- K. L. E. Hale, A. J. Fielding and K. J. Hale, Org. Biomol. Chem., 2025, 23 10.1039/d4ob01847h.
- (The relevant citations to references 26–43 can be found in the mechanistic discussions in the accompanying ESI.‡) ref. 26 is: W. F. K. Wynne-Jones and H. Eyring, J. Chem. Phys., 1935, 3, 492 CrossRef CAS.
- E. V. Anslyn and D. A. Dougherty, Modern Physical Organic Chemistry, University Science, Sausalito, CA, 2006, p. 368 Search PubMed.
- (a) J. F. Bunnett and V.I Chapter, The Interpretation of Rate Data, in Technique of Organic Chemistry, Vol. VIII, Part I, Ed. A. Weissberger, Investigation of Rates and Mechanisms of Reactions, Part I, ed. S. L. Friess, E. S. Lewis and A. Weissberger, 2nd edn, 1963, p. 177 Search PubMed; (b) (See also Jerry March's book for the much more widespread publication of the Bunnett ΔS‡ equation, with due attribution to Bunnett, who made a major contribution to physical organic chemistry with his initial simplifying rearrangement of the original Eyring–Wynne–Jones–Polanyi equation to give the ΔS‡ equation shown). See: J. March, Advanced Organic Chemistry. Reactions, Mechanism and Structure, John Wiley & Sons, 4th edn, 1992, p. 225 Search PubMed.
- R. W. Alder, R. Baker and J. M. Brown, Mechanism and Reactivity, in Mechanism in Organic Chemistry, John Wiley & Sons, 1971, ch. 1, pp. 1–77 (see page 5 for their ΔS‡ equation) Search PubMed.
- (a) S. A. Sherrod and R. G. Bergman, J. Am. Chem. Soc., 1971, 93, 1925 CrossRef; (b) S. A. Sherrod and R. G. Bergman, J. Am. Chem. Soc., 1969, 91, 2115 CrossRef CAS.
- (a) M. Hanack and T. Bassler, J. Am. Chem. Soc., 1969, 91, 2117 CrossRef CAS; (b) For a detailed review by Hanack on cyclopropyl-stabilized vinyl cations, which discusses the special stability of α-cyclopropylvinyl cations, see: M. Hanack, Acc. Chem. Res., 1976, 9, 364 CrossRef CAS.
- M. M. Kreevoy, Chapter XXIII, Thermodynamics and Reaction Mechanism, in Technique of Organic Chemistry, Vol. VIII, Part II, Ed. A. Weissberger, Investigation of Rates and Mechanisms of Reactions, Part II, ed. S. L. Friess, E. S. Lewis and A. Weissberger, 2nd edn, 1963, p. 1361 Search PubMed.
- K. A. Dill, S. Bromberg and D. Stigter, Chapter 20, Coulomb's Law of Electrostatic Forces, in Molecular Driving Forces, ed. K. A. Dill and S. Bromberg, Garland Science, Taylor and Francis Group, New York, 2nd edn, 2010 Search PubMed.
- (a) N. Bjerrum, Kgl. Dan. Vidensk. Selsk. Mat.-Fys. Medd., 1926, 7(9), 1–48 CAS; (b) For an English Language version of the Introductory Survey of this paper, pages 1–17, see: Neils Bjerrum, Selected Papers, Chairmen of Editorial Committee, Neils Bohr, Einar Munksgaard, Copenhagen, 1949. See page 108 of the PDF of this book at: https://www.royalacademy.dk/Publications/Low/1686_Bjerrum,%20Niels.pdf. Accessed: 19 June, 2024.
- R. Moritz, G. Zardalidis, H.-J. Butt, M. Wagner, K. Mullen and G. Houdas, Macromolecules, 2014, 47, 191 CrossRef CAS.
- S. Winstein and A. H. Fainberg, J. Am. Chem. Soc., 1957, 79, 5937 CrossRef CAS.
- K. A. Cooper and E. D. Hughes, J. Chem. Soc., 1937, 1183 RSC.
- J. Biordi and E. A. Moelwyn-Hughes, J. Chem. Soc., 1962, 4291 RSC.
- G. R. Cowie, H. J. M. Fitches and G. Kohnstam, J. Chem. Soc., 1963, 1585 RSC.
- G. Velegraki and M. Stratakis, J. Org. Chem., 2013, 78, 8880 CrossRef CAS PubMed.
- P. G. Cookson, A. G. Davies and B. P. Roberts, J. Chem. Soc., Chem. Commun., 1976, 1022 RSC.
- (a) C. Hu, J. Mena and I. V. Alabugin, Nat. Rev. Chem., 2023, 7, 405 CrossRef PubMed; (b) A. M. Hughes, G. does Passos Gomes and I. V. Alabugin, J. Org. Chem., 2019, 84, 1853 CrossRef CAS PubMed.
- (a) For an excellent new 2024 review on alkyne hydrometallation with Group IV metal hydrides, see the following Book Chapter by: T. Wiesner and M. Haas, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2024. DOI:10.1016/B978-0-323-96025-0.00125-3 (b) For McLaughlan and Roberts’ recent highly regiocontrolled PtCl2/XPhos-catalysed hydrostannation of terminal aryl acetylenes and propargylic alcohols, see: D. D. Roberts and M. G. McLaughlin, Adv. Synth. Catal., 2023, 365, 1602 CrossRef CAS; (c) For McLaughlin's recent application of the PtCl2/XPhos/Et3SiH-catalyst system to the highly regiocontrolled hydroboration of terminal alkyl, aryl and heteroaryl acetylenes with HBPin, see: K. L. E. Hale, D. D. Roberts and M. G. McLaughlin, Eur. J. Org. Chem., 2025, e202401355 CrossRef CAS.
Footnotes |
| † Dedicated to the memory of Dr Clive W. Bird FRSC, former Reader in Chemistry at King's College London (University of London); a truly outstanding organic chemist of extraordinary chemical insight and teaching ability. Clive was a genuinely good human being who helped all around him. He was inventor of the now famous “Bird Aromaticity Index”. |
| ‡ Electronic supplementary information (ESI) available: Full experimental details, calculations and NMR data supporting the work. See DOI: https://doi.org/10.1039/d4ob01846j |
| § Current address: Halazar Pharma Ltd, Edgware, Middlesex, HA8 7RB, UK. E-mail: E-mail: k.hale120@btinternet.com |
| This journal is © The Royal Society of Chemistry 2025 |