Synthesis of benzosultams via Ag(I)-catalyzed alkylative cyclization of vinyl sulfonamides†
Raju
Dupud‡
ab,
Karthik Kumar
Merugu‡
a,
Remyachand
R
a and
Remya
Ramesh
 *ab
*ab
aDepartment of Organic Synthesis & Process Chemistry, CSIR-Indian Institute of Chemical Technology, Hyderabad 500007, India. E-mail: r.remya@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201 002, India
First published on 8th November 2024
Abstract
A convenient method to access benzo-fused-γ-sultams via alkyl radical induced cyclization of vinyl sulfonamides is presented. A wide range of carboxylic acids including sterically hindered adamantanes participated as alkyl donors in this Ag(I)-catalyzed decarboxylative alkylation. The reaction utilizes readily available starting materials and demonstrates a broad substrate scope.
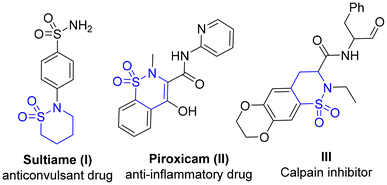
The sulfonamide functional group is present in over 100 drugs and biologically active compounds, dating back to the introduction of sulfa drugs in 1930s.1 Cyclic sulfonamides, commonly known as sultams, are considered lactam surrogates with superior metabolic stability and are therefore routinely used in medicinal chemistry optimizations.2 For instance, the sulfonamide peptide mimetic III is a potent and selective inhibitor of calpain I (Fig. 1).2b In addition to their interesting biological activities, sultams also find applications in synthetic organic chemistry, including their employment as chiral auxiliaries in asymmetric synthesis.3 In view of this importance, several synthetic methods have been developed for the efficient synthesis of sultams, involving cross-coupling, C–H activation, and cycloaddition.4 In addition, sulfur(VI) fluoride exchange chemistry (SuFEx), considered as a click reaction, has emerged as an efficient method for the rapid synthesis of sulphonamides.5
Radical-induced reactions are powerful methods to create multiple bonds and structurally complex molecules with higher atom-efficiency.6 Our group recently reported the radical cyclization of biphenyl acrylamides to generate SCF3-substituted dibenzolactams.7 Radical mediated difunctionalization of phenyl acrylamides to synthesize substituted oxindoles has been well studied.8 We hypothesized that vinyl sulfonamides could be efficient starting materials for radical cascade reactions and deliver synthetically useful products. Despite the potential, the radical cyclization of vinyl sulfonamides is not well explored. Zard and co-workers described a xanthate addition to vinyl sulfanilides, generating benzo-fused-γ-sultams.9 Vytla et al. reported a method for the trifluoromethylation of vinyl sulfonamides under blue LED irradiation.10 Considering their significance, the development of simple and cheap methods for ready access to sultams via radical cyclization would be appealing.
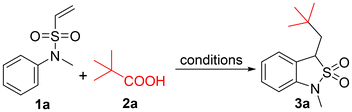
On the other hand, the generation of alkyl radicals from carboxylic acids via decarboxylation and subsequent synthetic transformations has garnered interest in recent times.11 Some of the early reactions such as the Kolbe electrolysis, Barton decarboxylation, and Hunsdiecker reaction use this strategy. However, many of these methods use high temperatures and harsh reaction conditions, which greatly limit their functional group tolerance and utility. Radical decarboxylation uses milder methods to generate alkyl radicals in the presence of external oxidants or under photo/electrochemical conditions. In continuation of our interest in radical-based reactions, we explored the decarboxylative alkylation/cyclization of vinyl sulfonamides for the synthesis of aryl fused γ-sultams (Scheme 1).
The decarboxylative alkylative cyclization of pivalic acid (2a) with N-methyl-N-phenylethenesulfonamide (1a) was chosen to analyze the feasibility and determine the optimal reaction conditions (Table 1). To begin with, the reaction was attempted with various oxidants (3 equiv.) in the presence of the catalyst Ag2CO3 (5 mol%) in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent at 85 °C (entries 1–5). In the presence of oxidants K2S2O8 and Na2S2O8, the desired product (3a) was obtained in moderate yields (58 and 65%, respectively) and confirmed by NMR and mass spectrometry. Increasing the water co-solvent ratio slightly improved the yield to 73%, with Na2S2O8 as the oxidant (entry 6). Replacing the co-solvent CH3CN with other solvents such as DMF, DMSO and THF resulted in diminished yields (entries 7–9). To our delight, increasing the equivalents of Na2S2O8, Ag2CO3, and pivalic acid enhanced the yield to 88% (entry 11), which was the best result we achieved in this study. Changing the catalyst to Ag2NO3 gave product 3a, albeit in a lower yield (entry 12; 63%). When the reaction was performed in the absence of the catalyst Ag2CO3, the starting materials remained unconsumed (entry 13).
1) solvent at 85 °C (entries 1–5). In the presence of oxidants K2S2O8 and Na2S2O8, the desired product (3a) was obtained in moderate yields (58 and 65%, respectively) and confirmed by NMR and mass spectrometry. Increasing the water co-solvent ratio slightly improved the yield to 73%, with Na2S2O8 as the oxidant (entry 6). Replacing the co-solvent CH3CN with other solvents such as DMF, DMSO and THF resulted in diminished yields (entries 7–9). To our delight, increasing the equivalents of Na2S2O8, Ag2CO3, and pivalic acid enhanced the yield to 88% (entry 11), which was the best result we achieved in this study. Changing the catalyst to Ag2NO3 gave product 3a, albeit in a lower yield (entry 12; 63%). When the reaction was performed in the absence of the catalyst Ag2CO3, the starting materials remained unconsumed (entry 13).
| Entry | Oxidant | Solvent | Yieldb |
|---|---|---|---|
| a All reactions were performed with 1a (1 equiv.), 2a (2 equiv.), oxidant and additive Ag2CO3 (5 mol%) in solvent (2 mL) at 85 °C for 2 h. b Isolated yield. c No reaction. d Ag2CO3 (10 mol%). e 3 equiv. of 2a. f Ag2NO3 (10 mol%). g No additive was used. | |||
| 1 | K2S2O8(3) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
58% |
| 2 | Oxone(3) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NRc |
| 3 | CAN(3) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 4 | (NH4)2S2O8(3) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Trace |
| 5 | Na2S2O8(3) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65% |
| 6 | Na2S2O8(3) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
73% |
| 7 | Na2S2O8(3) | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
30% |
| 8 | Na2S2O8(3) | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 9 | Na2S2O8(3) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
35% |
| 10 | Na2S2O8(5) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
81%d |
| 11 | Na 2 S 2 O 8 (5) |
CH
3
CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H
2
O (1 H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
88% , |
| 12 | Na2S2O8(5) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
63%f |
| 13 | Na2S2O8(5) | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NRg |
After identifying the optimal reaction conditions, we set out to explore the substrate scope of the reaction with various vinyl sulfonamides (Scheme 2). The required starting materials were synthesized through the reaction of the corresponding N-methyl anilines with 2-chloroethanesulfonyl chloride. Both electron donating (–CH3) and electron withdrawing (–COOMe) groups in the aromatic ring participated effectively in the reaction, affording sultams 3b and 3c, respectively. Halogens in the aromatic ring were also well tolerated, producing the corresponding fluoro (3d) and iodo (3e) derivatives in moderate yields. When subjected to the optimized conditions, the o-phenyl sulfonamide 1f delivered product 3f in 79% yield, indicating that the second phenyl ring did not participate in the cyclization reaction. Substrates with multiple substituents, both aromatic and cyano groups, also performed well, yielding sultams 3g and 3h in good yields. The structures of 3f and 3g were unambiguously confirmed with the help of X-ray crystal structure analysis (Fig. 2 and 3). To our delight, heterocyclic sulfonamides successfully underwent the cyclization reaction, resulting in the pyridine-fused sultam 3i and quinoline-fused sultam 3j. It is worth mentioning that in the case of the quinoline sulfonamide 1j, the desired product 3j was obtained with excellent regioselectivity. The structure was assigned based on the analysis of splitting patters in the 1H-NMR. Variations at the sulfonamide nitrogen were also explored and it was found that the N-ethyl derivative 3k could be obtained in good yields (85%).
 | ||
Scheme 2 Substrate scope of vinyl sulfonamides. Reaction conditions: 1 (0.2 mmol), 2a (0.6 mmol), Na2S2O8 (1 mmol), Ag2CO3 (0.02 mmol), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN 1 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 mL) at 85 °C. Isolated yields. H2O (3 mL) at 85 °C. Isolated yields. | ||
Next, the generality of the alkylative cyclization reaction was examined by employing a diverse array of carboxylic acids. The reaction proceeded well with the secondary carboxylic acid 2-methylbutanoic acid (2b) to deliver the desired sultam 3l in 86% yield as a mixture of diastereomers. Commendably, long chain primary carboxylic acids participated in the reaction to furnish the desired products 3m and 3n possessing dodecyl and chlorohexyl groups. Additionally, a variety of cyclic carboxylic acids including cyclobutyl, methylcyclohexyl, tetrahydropyranyl, and sterically hindered adamantyl groups were well tolerated to afford the corresponding products 3o–3t in good yields, showcasing the versatility of this strategy. Interestingly, adamantane groups are introduced in pharmaceuticals to increase lipophilicity and improve their pharmacokinetic properties.12 Unfortunately, the reaction failed in the case of benzoic acid and phenyl acetic acid. The desired product 3u could be obtained with phenyl butyric acid (Scheme 3).
 | ||
Scheme 3 Substrate scope of carboxylic acids. Reaction conditions: 1a (0.2 mmol), 2 (0.6 mmol), Na2S2O8 (1 mmol), Ag2CO3 (0.02 mmol), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN 1 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 mL) at 85 °C. Isolated yields. H2O (3 mL) at 85 °C. Isolated yields. | ||
To further expand the scope of this method, we attempted the reaction with 2-oxo acids, which can generate acyl radicals. However, we observed a decarboxylative–decarbonylative alkylation, leading to the formation of sultams 3a and 3v in good yields (Scheme 4).13
 | ||
Scheme 4 Alkylation using 2-oxo-acids. Reaction conditions: 1a (0.2 mmol), 2 (0.6 mmol), Na2S2O8 (1 mmol), Ag2CO3 (0.02 mmol), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN 1 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 mL) at 85 °C. Isolated yields. H2O (3 mL) at 85 °C. Isolated yields. | ||
A gram-scale reaction with 1.5 g of vinyl sulfonamide 1a produced the desired product 3a in 84% yield, indicating that scalability is feasible without significant challenges. Finally, we investigated the possible mechanistic pathways based on the available literature.14 The reaction, when conducted in the presence of the radical inhibitor TEMPO, failed to give the cyclized product, suggesting a radical pathway; the plausible mechanism is shown in Scheme 5.
In the presence of an oxidant, Ag(I) is converted to Ag(II), which then reacts with pivalic acid (2a), leading to decarboxylation and the generation of the tert-butyl radical A. This radical upon regioselective addition to the vinyl sulfonamide 1a produces radical B. Cyclization with the adjacent phenyl ring produces the delocalized radical intermediate C, which upon subsequent oxidation and elimination of H+ delivers the final product 3a.
Conclusions
In summary, a practical approach for the synthesis of benzo-fused-γ-sultams by a decarboxylative alkylation/cyclization of vinyl sulfonamides is presented. A variety of carboxylic acids possessing diverse electronic and steric features were employed as alkyl sources in this methodology. This study highlights the utility of vinyl sulfonamides as substrates in radical reactions, enabling the synthesis of structurally intriguing polycyclic sultams.Author contributions
R. D., K. K. M., and R. R. performed the reactions. R. D. and K. K. M. analysed the data and prepared the ESI.† R. R.* conceptualized the project and wrote the manuscript. All authors discussed and approved the manuscript.Data availability
The experimental data supporting this article have been included in the ESI.† Any other raw data including FIDS are available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Department of Science & Technology (DST), New Delhi, via SERB project SRG/2022/000157 is gratefully acknowledged. We thank the Analytical & Structural Chemistry Division at CSIR-IICT (Dr Sunil Kumar) for help with XRD data [CSIR-IICT Communication No. IICT/Pubs./2024/326].References
- S. Mondal and S. Malakar, Tetrahedron, 2020, 76, 131662 CrossRef CAS
.
-
(a) Y. K. Chong, Y. S. Ong and K. Y. Yeong, RSC Med. Chem., 2024, 15, 1798 RSC
; (b) G. J. Wells, M. Tao, K. A. Josef and R. Bihovsky, J. Med. Chem., 2001, 44, 3488 CrossRef CAS PubMed
.
-
(a)
O. Reiser, Oppolzer sultams in Organic Synthesis Set, ed. H. Waldman, 2003 Search PubMed
; (b) M. M. Heravi and V. Zadsirjan, Tetrahedron: Asymmetry, 2014, 25, 1061 CrossRef CAS
.
-
(a) K. C. Majumdar and S. Mondal, Chem. Rev., 2011, 111, 7749 CrossRef CAS PubMed
; (b) S. Debnath and S. Mondal, Eur. J. Org. Chem., 2018, 933 CrossRef CAS
; (c) X. Gong, G. Li and J. Wu, Org. Chem. Front., 2017, 4, 14 RSC
; (d) D. Zhong, D. Wu, Y. Zhang, Z. Lu, M. Usman, W. Liu, X. Lu and W.-B. Liu, Org. Lett., 2019, 21, 5808 CrossRef CAS
.
-
(a) J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430 CrossRef CAS
; (b) A. V. Bogolubsky, Y. S. Moroz, P. K. Mykhailiuk, S. E. Pipko, A. I. Konovets, I. V. Sadkova and A. Tolmachev, ACS Comb. Sci., 2014, 16, 192 CrossRef CAS
; (c) A. Dondoni and A. Marra, Org. Biomol. Chem., 2017, 15, 1549 RSC
.
-
(a) C. P. Jasperse, D. P. Curran and T. L. Fevig, Chem. Rev., 1991, 91, 1237 CrossRef CAS
; (b) M. Yan, J. C. Lo, J. T. Edwards and P. S. Baran, J. Am. Chem. Soc., 2016, 138, 12692 CrossRef CAS
; (c) M. P. Plesniak, H.-M. Huang and D. J. Procter, Nat. Rev. Chem., 2017, 1, 0077 CrossRef
; (d) K. J. Romero, M. S. Galliher, D. A. Pratt and C. R. J. Stephenson, Chem. Soc. Rev., 2018, 47, 7851 RSC
; (e) H.-M. Huang, M. H. Garduno-Castro, C. Morrill and D. J. Procter, Chem. Soc. Rev., 2019, 48, 4626 RSC
; (f) J. Liao, X. Yang, L. Ouyang, Y. Lai, J. Huang and R. Luo, Org. Chem. Front., 2021, 8, 1345 RSC
; (g) Q.-W. Gui, F. Teng, P. Yu, Y.-F. Wu, Z.-B. Nong, L.-X. Yang, X. Chen, T.-B. Yang and W.-M. He, Chin. J. Catal., 2023, 44, 111 CrossRef CAS
; (h) Y. Zhou, W.-H. Yang, N.-N. Dai, J.-Y. Feng, M.-Q. Yang, W. Gao, Q. Li, C. Deng, Z. Lu and W.-T. Wei, Org. Lett., 2024, 26, 5074 CrossRef CAS PubMed
; (i) N.-N. Dai, Y.-J. Lu, Z.-Q. Wu, Y. Zhou, Y. Tong, K. Tang, Q. Li, J.-Q. Zhang, Y. Liu and W.-T. Wei, Org. Lett., 2024, 26, 3014 CrossRef CAS
.
- R. Dupud, R. Thushara, K. K. Merugu, C. H. Suresh and R. Ramesh, Eur. J. Org. Chem., 2024, e202400450 CrossRef CAS
.
- Representative examples:
(a) K. Matcha, R. Narayan and A. P. Antonchick, Angew. Chem., Int. Ed., 2013, 52, 7985 CrossRef CAS
; (b) M. Zhang, X. Ding, A. Lu, J. Kang, Y. Gao, Z. Wang, H. Li and Q. Wang, Org. Chem. Front., 2021, 8, 961 RSC
; (c) X. Cheng, B. Hasimujiang, Z. Xu, H. Cai, G. Chen, G. Mo and Z. Ruan, J. Org. Chem., 2021, 86, 16045 CrossRef CAS
; (d) S. Ghosh, Z.-W. Qu, S. Pradhan, A. Ghosh, S. Grimme and I. Chatterjee, Angew. Chem., Int. Ed., 2022, 61, e202115272 CrossRef CAS
.
- C. Moutrille and S. Z. Zard, Tetrahedron Lett., 2004, 45, 4631 CrossRef CAS
.
- D. Vytla, K. Kaliyaperumal, R. Velayuthaperumal, P. Shaw, R. Gautam, A. Mathur and A. Roy, Synthesis, 2022, 667 CrossRef CAS
.
-
(a) L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886 CrossRef CAS PubMed
; (b) W.-P. Mai, J.-T. Wang, L.-R. Yang, J.-W. Yuan, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Lett., 2014, 16, 204 CrossRef CAS
; (c) R. Xia, M.-S. Xie, H.-Y. Niu, G.-R. Qu and H.-M. Guo, Org. Lett., 2014, 16, 444 CrossRef CAS PubMed
; (d) X. Sun and T. Ritter, Angew. Chem., Int. Ed., 2021, 60, 10557 CrossRef CAS
; (e) S. B. Beil, T. Q. Chen, N. E. Intermaggio and D. W. C. MacMillan, Acc. Chem. Res., 2022, 55, 3481 CrossRef CAS PubMed
; (f) L. Li, Y. Yao and N. Fu, Eur. J. Org. Chem., 2023, e202300166 CrossRef CAS
; (g) J. D. Tibbetts, H. E. Askey, Q. Cao, J. D. Grayson, S. L. Hobson, G. D. Johnson, J. C. Turner-Dore and A. J. Cresswell, Synthesis, 2023, 3239 CrossRef CAS
; (h) W. Wang, Y. Song, S. Xing, J. Li, W. Feng, X. Qu and S. Wang, ChemistrySelect, 2023, 8, e202300958 CrossRef CAS
; (i) D. L. Lipilin, M. O. Zubkov, M. D. Kosobokov and A. D. Dilman, Chem. Sci., 2024, 15, 644 RSC
; (j) C. R. Reddy, V. Edhara, A. Kumari, A. D. Patil and K. Thandavamurthy, Org. Biomol. Chem., 2024, 22, 6385 RSC
.
-
(a) L. Wanka, K. Iqbal and P. R. Schreiner, Chem. Rev., 2013, 113, 3516 CrossRef CAS PubMed
; (b) K. Spilovska, F. Zemek, J. Korabecny, E. Nepovimova, O. Soukup, M. Windisch and K. Kuca, Curr. Med. Chem., 2016, 23, 3245 CrossRef CAS PubMed
.
- J.-Q. Chen, R. Chang, Y.-L. Wei, J.-N. Mo, Z.-Y. Wang and P.-F. Xu, J. Org. Chem., 2018, 83, 253 CrossRef CAS PubMed
.
- C. R. Reddy, D. H. Kolgave, M. Subbarao, M. Aila and S. K. Prajapti, Org. Lett., 2020, 22, 5342 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectral data for all new compounds. CCDC 2387322 (3f) and 2387323 (3g). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob01583e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |