Transformation of CO2 and isocyanates mediated by N-borane-substituted cyclic phosphine imides (BCPIs) via λ5-oxazaphosphetanes†
Shun
Nagai
a,
Sensuke
Ogoshi
 a and
Yoichi
Hoshimoto
a and
Yoichi
Hoshimoto
 *ab
*ab
aDepartment of Applied Chemistry, Faculty of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: hoshimoto@chem.eng.osaka-u.ac.jp
bCenter for Future Innovation (CFi), Faculty of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
First published on 12th November 2024
Abstract
We herein report reliable evidence that λ5-oxazaphosphetane species are a key intermediate in the transformation of CO2 and isocyanates through their reaction with N-borane-substituted cyclic phosphine imides (BCPIs). We have isolated and fully characterized several λ5-oxazaphosphetane species prepared via formal [2 + 2] cycloaddition reactions between BCPIs and CO2 or isocyanates. The transformation of these λ5-oxazaphosphetanes via retro-ring opening reaction afforded an isocyanate and a carbodiimide from the CO2- and isocyanate-derived λ5-oxazaphosphetanes.
Introduction
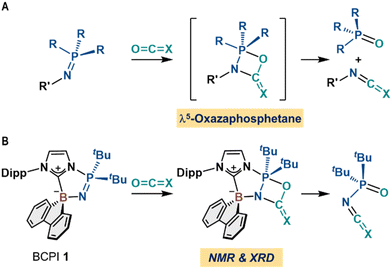
Phosphine imides (R′-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3), a nitrogen analogue of phosphine oxides, are widely used in the aza-Wittig reaction1 and the Staudinger reaction/ligation.2,3 The aza-Wittig reaction has been proposed to be initiated from a formal [2 + 2] cycloaddition between the phosphine imides and CO2 (or other heterocumulenes with an O
PR3), a nitrogen analogue of phosphine oxides, are widely used in the aza-Wittig reaction1 and the Staudinger reaction/ligation.2,3 The aza-Wittig reaction has been proposed to be initiated from a formal [2 + 2] cycloaddition between the phosphine imides and CO2 (or other heterocumulenes with an O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X moiety; X = O or NR′′) to yield phosphine oxides and R′-N
X moiety; X = O or NR′′) to yield phosphine oxides and R′-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X via λ5-oxazaphosphetane species as key intermediates (Fig. 1A).1b,4 However, the direct observation and isolation of λ5-oxazaphosphetane species from the reaction of a phosphine imide and CO2 have not yet been reported,5 even though related studies on their synthesis, characterization, and reactivity have been published by e.g., Röschenthaler et al.,6 Schmidpeter and von Criegern et al.,7 as well as Kawashima et al.8 Moreover, a plausible mechanism for the aza-Wittig reaction has been examined theoretically using DFT calculations, which supported the formation of λ5-oxazaphosphetane intermediates.9 Thus, direct evidence of these species in the transformations of heterocumulenes including CO2 would provide valuable fundamental insight into the reaction mechanism. Here, we report the isolation and characterization of λ5-oxazaphosphetane species via the direct reaction of CO2 or isocyanates with N-borane-substituted cyclic phosphine imides (BCPIs), which have recently been developed in our group (Fig. 1B).10 We also discuss the retro-ring opening reactions from the λ5-oxazaphosphetanes, which unambiguously confirm their intermediacy in aza-Wittig reactions.
X via λ5-oxazaphosphetane species as key intermediates (Fig. 1A).1b,4 However, the direct observation and isolation of λ5-oxazaphosphetane species from the reaction of a phosphine imide and CO2 have not yet been reported,5 even though related studies on their synthesis, characterization, and reactivity have been published by e.g., Röschenthaler et al.,6 Schmidpeter and von Criegern et al.,7 as well as Kawashima et al.8 Moreover, a plausible mechanism for the aza-Wittig reaction has been examined theoretically using DFT calculations, which supported the formation of λ5-oxazaphosphetane intermediates.9 Thus, direct evidence of these species in the transformations of heterocumulenes including CO2 would provide valuable fundamental insight into the reaction mechanism. Here, we report the isolation and characterization of λ5-oxazaphosphetane species via the direct reaction of CO2 or isocyanates with N-borane-substituted cyclic phosphine imides (BCPIs), which have recently been developed in our group (Fig. 1B).10 We also discuss the retro-ring opening reactions from the λ5-oxazaphosphetanes, which unambiguously confirm their intermediacy in aza-Wittig reactions.
 | ||
| Fig. 1 (A) Simplified scheme of the aza-Wittig reaction between phosphine imides and heterocumulenes such as CO2 (X = O) and isocynates (X = NR′′). (B) This work (X = O and NR′′). | ||
Results and discussion
We first explored the reaction between BCPI 1 and CO2 (Fig. 2A). Treatment of 1 with CO2 (5 atm) at room temperature (rt) in α,α,α-trifluorotoluene (TFT) resulted in the quantitative formation of λ5-oxazaphosphetane 2 (isolated yield: >99%) within 20 minutes.11 Compound 2 was characterized using multinuclear-NMR-spectroscopy techniques as well as single-crystal X-ray diffraction (SC-XRD) analysis. The 31P NMR spectrum of 2 shows a characteristic chemical shift (δP −18.5) that is consistent with the formation of a penta-coordinated phosphorus species. The molecular structure of 2 in the solid state, which was determined by SC-XRD analysis (Fig. 2B), confirmed the formation of the P–N3–C4–O1 ring. The phosphorus atom adopts a distorted trigonal bipyramidal geometry with the N2 and O1 atoms at the axial positions (N2–P–O1 = 158.3(1)°), while the N3 atom and two C atoms reside in the trigonal plane. The N3–P (1.673(3) Å) and N2–P (1.899(2) Å) bonds in 2 are notably elongated compared to those in 1, which suggests a decrease in their bond order. In contrast, the length of the B1–N3 bond remains almost unchanged (∼1.56 Å) through the transformation from 1 to 2. At rt, 2 gradually decomposed to yield N-phosphinoyl isocyanate 3 with concomitant formation of a dimer of an imidazole-substituted 9-borafluorene (4)2. These results constitute the direct observation of a λ5-oxazaphosphetane species as the intermediate in the aza-Wittig reaction between phosphine imides and CO2. Given that the thermolysis of 2 can be expected to be promoted by the formation of the thermodynamically favorable P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, we subsequently explored the cleavage of the P–O1 bond in 2. The reaction of 2 and HB(C6F5)2 afforded 5 in 97% yield through the coordination of the carboxylate moiety to the borane (O2–B2 = 1.518(4) Å; Fig. 2A and C).12,13 Thus, reconfiguration of the geometry around the phosphorus center to form a distorted tetrahedron occurs, together with a contraction of the N2–P bond (1.720(3) Å) and an elongation of the B1–N3 bond (1.608(5) Å). The length of the N3–P bond remained virtually unchanged (1.642(3) Å) compared to that of 2 and is thus significantly longer than that in 1. NMR analyses provided insight into the electronic state of the P atom in 5. The resonance at δP 59.9 indicates that the electron density on the P atom in 5 is nearly identical to that in 1, albeit that it should be slightly higher.14 Based on these experimental data, we conclude that the electron delocalization between the N3 atom and the adjacent carbonyl group partially weakens the negative hyperconjugation between the N3 and P atoms, while the increased π-donation from the imidazolium unit to the P atom compensates for the loss of electron density on the P atom.
O bond, we subsequently explored the cleavage of the P–O1 bond in 2. The reaction of 2 and HB(C6F5)2 afforded 5 in 97% yield through the coordination of the carboxylate moiety to the borane (O2–B2 = 1.518(4) Å; Fig. 2A and C).12,13 Thus, reconfiguration of the geometry around the phosphorus center to form a distorted tetrahedron occurs, together with a contraction of the N2–P bond (1.720(3) Å) and an elongation of the B1–N3 bond (1.608(5) Å). The length of the N3–P bond remained virtually unchanged (1.642(3) Å) compared to that of 2 and is thus significantly longer than that in 1. NMR analyses provided insight into the electronic state of the P atom in 5. The resonance at δP 59.9 indicates that the electron density on the P atom in 5 is nearly identical to that in 1, albeit that it should be slightly higher.14 Based on these experimental data, we conclude that the electron delocalization between the N3 atom and the adjacent carbonyl group partially weakens the negative hyperconjugation between the N3 and P atoms, while the increased π-donation from the imidazolium unit to the P atom compensates for the loss of electron density on the P atom.
Next, we investigated reactions between 1 and isocyanates. When p-toluenesulfonyl isocyanate (Ts–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) was employed, the expected λ5-oxazaphosphetane (6a) was isolated in >99% yield within 15 minutes at rt (Fig. 3). The molecular structure of 6a in the solid state was determined by SC-XRD analysis and also supports the formation of the P–N3–C2–O ring, in which the phosphorus atom adopts a distorted trigonal bipyramidal geometry, wherein the N1 and O atoms at located at the axial positions, whereas the N3 atom and two C atoms reside in the trigonal plane. In stark contrast to 2, 6a exhibits significant thermal stability, i.e., no transformation occurred even after 12 h at 100 °C. At 135 °C, 6a gradually decomposed to yield 1-(N-phosphinoyl)-3-tosylcarbodiimide 7a and (4)2. Again, these results clearly demonstrate the key intermediacy of λ5-oxazaphosphetane species in the aza-Wittig reaction between phosphine imides and isocyanates.
O) was employed, the expected λ5-oxazaphosphetane (6a) was isolated in >99% yield within 15 minutes at rt (Fig. 3). The molecular structure of 6a in the solid state was determined by SC-XRD analysis and also supports the formation of the P–N3–C2–O ring, in which the phosphorus atom adopts a distorted trigonal bipyramidal geometry, wherein the N1 and O atoms at located at the axial positions, whereas the N3 atom and two C atoms reside in the trigonal plane. In stark contrast to 2, 6a exhibits significant thermal stability, i.e., no transformation occurred even after 12 h at 100 °C. At 135 °C, 6a gradually decomposed to yield 1-(N-phosphinoyl)-3-tosylcarbodiimide 7a and (4)2. Again, these results clearly demonstrate the key intermediacy of λ5-oxazaphosphetane species in the aza-Wittig reaction between phosphine imides and isocyanates.
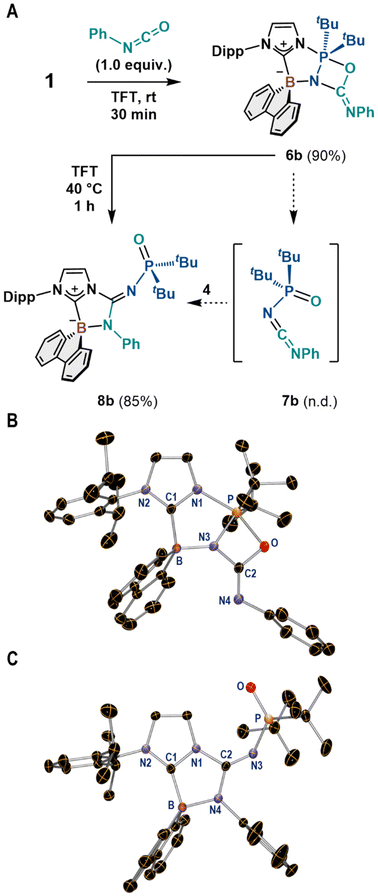
We also used phenyl isocyanate in the reaction with 1 and confirmed the formation of the corresponding λ5-oxazaphosphetane (6b), which was isolated in 90% yield (Fig. 4A). Thus, we expected to obtain carbodiimide 7b from 6b, in analogy to the case of 6a (Fig. 3); however, the thermolysis of 6b at 40 °C resulted in the quantitative formation of guanidine 8b, which was isolated in 85% yield (Fig. 4A). Compounds 6b and 8b were unambiguously characterized using multinuclear-NMR-spectroscopy techniques and SC-XRD analyses; the latter results are shown in Fig. 4B and C, respectively. The molecular structure of 8b includes an N-phosphinoylguanidine unit comprising the N1, N3, N4, and C2 atoms.
A plausible reaction mechanism affording 7a or 8b from 6 is shown in Fig. 5. First, the thermal decomposition of 6 occurs via the cleavage of the B–N, N–P, and C–O bonds, which results in the in situ formation of imidazole-substituted 9-borafluorene 4 and carbodiimides 7a or 7b. Subsequently, 4 dimerizes to form (4)2 when the Lewis basicity of the nitrogen atom in 7a is insufficient to form B–N adduct due to the electron-withdrawing nature of the N–Ts group. On the other hand, the N atom in 7b exhibits sufficient Lewis basicity to coordinate to the boron center in 4 before its dimerization, which eventually yields guanidine 8b.
Conclusions
In conclusion, we have gathered direct evidence and thus demonstrated for the first time that λ5-oxazaphosphetane species serve as key intermediates in aza-Wittig reactions involving CO2 and isocyanates. We used N-borane-substituted cyclic phosphine imides (BCPIs) for the isolation and characterization of several λ5-oxazaphosphetanes derived from CO2 and isocyanates via formal [2 + 2] cycloaddition reactions. Retro-ring opening reactions from the isolated λ5-oxazaphosphetanes afforded the expected isocyanates (from CO2) or carbodiimides (from TsNCO) with concomitant formation of a dimer of an imidazole-substituted 9-borafluorene. In their entirety, these results successfully provide evidence for a missing link in contemporary organic chemistry. Further research into the applications of BCPIs in the fields of organic and organometallic chemistry is currently in progress in our group.Data availability
The data supporting this article have been included as part of the ESI.†Metrical data for the solid-state structures are available from Cambridge Crystallographic Data Centre: CCDC 2240798 (2), 2240797 ((4)2), 2240796 (5), 2378677 (6a), 2378678 (6b), 2378679 (8b).†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by Grants-in-Aid for Transformative Research Area (A) Digitalization-driven Transformative Organic Synthesis (22H05363 to Y. H.), the JST FOREST Program (JPMJFR2222 to Y. H.), and a JSPS Research Fellowship (JSPS KAKENHI grant JP23KJ1443 to S. N.). The authors would like to thank Mr Takaya Hinogami (Department of Applied Chemistry, Faculty of Engineering, Osaka University) for his contribution to exploring the reactivity of BCPIs toward CO2.References
- (a) N. I. Gusar, Russ. Chem. Rev., 1991, 60, 146 CrossRef; (b) S. Eguchi, Y. Matsushita and K. Yamashita, Org. Prep. Proced. Int., 1992, 24, 209 CrossRef CAS; (c) P. Molina and M. J. Vilaplana, Synthesis, 1994, 1197 CrossRef CAS; (d) H. Wamhoff, G. Richardt and S. Stölben, Adv. Heterocycl. Chem., 1995, 64, 159 CrossRef CAS; (e) P. M. Fresneda and P. Molina, Synlett, 2004, 1 CrossRef CAS; (f) F. Palacios, C. Alonso, D. Aparicio, G. Rubiales and J. M. de los Santos, Tetrahedron, 2007, 63, 523 CrossRef CAS.
- (a) H. Staudinger and J. Meyer, Helv. Chim. Acta, 1919, 2, 635 CrossRef CAS; (b) H. Staudinger and E. Hauser, Helv. Chim. Acta, 1921, 4, 861 CrossRef CAS; (c) Y. G. Gololobov, I. N. Zhmurova and L. F. Kasukhin, Tetrahedron, 1981, 37, 437 CrossRef CAS; (d) E. W. Abel and S. A. Mucklejohn, Phosphorus, Sulfur Silicon Relat. Elem., 1981, 9, 235 CrossRef CAS; (e) Y. G. Gololobov and L. F. Kasukhin, Tetrahedron, 1992, 48, 1353 CrossRef CAS.
- (a) M. Köhn and R. Breinbauer, Angew. Chem., Int. Ed., 2004, 43, 3106 CrossRef PubMed; (b) E. Saxon and C. R. Bertozzi, Science, 2000, 287, 2007 CrossRef CAS PubMed; (c) S. S. van Berkel, M. B. van Eldijk and J. C. M. van Hest, Angew. Chem., Int. Ed., 2011, 50, 8806 CrossRef CAS PubMed; (d) Z.-P. A. Wang, C.-L. Tian and J.-S. Zheng, RSC Adv., 2015, 5, 107192 RSC; (e) C. Bednarek, I. Wehl, N. Jung, U. Schepers and S. Bräse, Chem. Rev., 2020, 120, 4301 CrossRef CAS.
- (a) L. Horner and H. Oediger, Chem. Ber., 1958, 91, 437 CrossRef CAS; (b) G. Aksnes and P. Frøyen, Acta Chem. Scand., 1969, 23, 2697 CrossRef CAS; (c) J. Barluenga and F. Palacios, Org. Prep. Proced. Int., 1991, 23, 1 CrossRef CAS; (d) L. Sarah, Doctoral thesis, Durham University, 1998.
- (a) T. Sasaki, S. Eguchi and T. Okano, J. Am. Chem. Soc., 1983, 105, 5912 CrossRef CAS; (b) F. A. Merckling and P. Rüedi, Tetrahedron Lett., 1996, 37, 2217 CrossRef CAS; (c) U. Seneviratne, L. C. Godoy, J. S. Wishnok, G. N. Wogan and S. R. Tannenbaum, J. Am. Chem. Soc., 2013, 135, 7693 CrossRef CAS PubMed; (d) H. B. Abed, O. Mammoliti, O. Bande, G. V. Lommen and P. Herdewijn, Org. Biomol. Chem., 2014, 12, 7159 RSC.
- (a) W. Storzer, G.-V. Röschenthaler, R. Schmutzler and W. S. Sheldrick, Chem. Ber., 1981, 114, 3609 CrossRef CAS; (b) R. Francke, D. Dakternieks, R. W. Gable, B. F. Hoskins and G.-V. Röschenthaler, Chem. Ber., 1985, 118, 922 CrossRef CAS; (c) D. V. Sevenard, E. Lork, K. I. Pashkevich and G.-V. Röschenthaler, Heteroat. Chem., 2002, 13, 97 CrossRef CAS.
- (a) A. Schmidpeter and T. von Criegern, Angew. Chem., Int. Ed. Engl., 1978, 17, 55 CrossRef; (b) W. S. Sheldrick, D. Schomburg, A. Schmidpeter and T. von Criegern, Chem. Ber., 1980, 113, 55 CrossRef CAS.
- (a) N. Kano, X. J. Hua, S. Kawa and T. Kawashima, Tetrahedron Lett., 2000, 41, 5237 CrossRef CAS; (b) N. Kano, J.-H. Xing, S. Kawa and T. Kawashima, Polyhedron, 2002, 21, 657 CrossRef CAS; (c) N. Kano, J.-H. Xing, A. Kikuchi, S. Kawa and T. Kawashima, Phosphorus, Sulfur Silicon Relat. Elem., 2002, 177, 1685 CrossRef CAS.
- (a) J. Koketsu, Y. Ninomiya, Y. Suzuki and N. Koga, Inorg. Chem., 1997, 36, 694 CrossRef CAS; (b) W. C. Lu, C. C. Sun, Q. J. Zang and C. B. Liu, Chem. Phys. Lett., 1999, 311, 491 CrossRef CAS; (c) W. C. Lu, C. B. Liu and C. C. Sun, J. Phys. Chem. A, 1999, 103, 1078 CrossRef CAS; (d) Y. Xue, D. Xie and G. Yan, J. Phys. Chem. A, 2002, 106, 9053 CrossRef CAS; (e) Y. Xue and C. K. Kim, J. Phys. Chem. A, 2003, 107, 7945 CrossRef CAS; (f) F. P. Cossío, C. Alonso, B. Lecea, M. Ayerbe, G. Rubiales and F. Palacios, J. Org. Chem., 2006, 71, 2839 CrossRef; (g) M. Fianchini and F. Maseras, Tetrahedron, 2019, 75, 1852 CrossRef CAS; (h) A. Adda and H. Sediki, Chem. Proc., 2021, 3, 47 Search PubMed.
- S. Nagai, T. Hinogami, S. Ogoshi and Y. Hoshimoto, Bull. Chem. Soc. Jpn., 2023, 96, 1346 CrossRef.
- For a related example using amidophosphoranes in CO2 fixation, see: L. J. Hounjet, C. B. Caputo and D. W. Stephan, Angew. Chem., Int. Ed., 2012, 51, 4714 CrossRef CAS PubMed.
- λ5-Oxazaphosphetanes could also be a key intermediate in the phosphine-imide-catalyzed conversion of CO2; for details, see: M.-A. Courtemanche, M.-A. Légaré, É. Rochette and F.-G. Fontaine, Chem. Commun., 2015, 51, 6858 RSC.
- We also carried out a reaction between 2 and HSiEt3, which unfortunately resulted merely in the thermal decomposition of 2 to 3 and (4)2.
- O. Kühl, Phosphorus-31 NMR Spectroscopy: A Concise Introduction for the Synthetic Organic and Organometallic Chemist, Springer-Verlag, Berlin, Heidelberg, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic, spectroscopic, and X-ray crystallographic details. CCDC 2240796–2240798 and 2378677–2378679. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob01565g |
| This journal is © The Royal Society of Chemistry 2025 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Based on the molar loading of 2. (B) Molecular structure of 2 with thermal ellipsoids at 30% probability; H atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: C1–B1 1.626(4), N3–B1 1.556(4), P–O1 1.831(2), O1–C4 1.334(5), C4–O2 1.212(4), C4–N3 1.371(3), N2–P–N3 84.6(1), N3–P–O1 73.7(1), P–O1–C4 89.2(2), O1–C4–N3 102.2(2), C4–N3–P 94.8(2). (C) Molecular structure of 5 with thermal ellipsoids at 30% probability; H atoms (except B2–H) are omitted for clarity. Selected bond lengths [Å] and angles [°]: C1–B1 1.624(5), N2–P–N3 92.7(2), P–N3–B1 119.8(2), N3–B1–C1 96.2(3), B1–N3–C4 122.8(3).
Based on the molar loading of 2. (B) Molecular structure of 2 with thermal ellipsoids at 30% probability; H atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: C1–B1 1.626(4), N3–B1 1.556(4), P–O1 1.831(2), O1–C4 1.334(5), C4–O2 1.212(4), C4–N3 1.371(3), N2–P–N3 84.6(1), N3–P–O1 73.7(1), P–O1–C4 89.2(2), O1–C4–N3 102.2(2), C4–N3–P 94.8(2). (C) Molecular structure of 5 with thermal ellipsoids at 30% probability; H atoms (except B2–H) are omitted for clarity. Selected bond lengths [Å] and angles [°]: C1–B1 1.624(5), N2–P–N3 92.7(2), P–N3–B1 119.8(2), N3–B1–C1 96.2(3), B1–N3–C4 122.8(3).