Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot hydroaminomethylation of an alkene under formation of primary amines by combining hydroformylation at elevated syngas pressure and biocatalytic transamination in water†
Jonas
Spang
a,
Hannah
Bork
 a,
Feodor
Belov
b,
Jan
von Langermann
a,
Feodor
Belov
b,
Jan
von Langermann
 b,
Andreas J.
Vorholt
b,
Andreas J.
Vorholt
 c and
Harald
Gröger
c and
Harald
Gröger
 *a
*a
aChair of Industrial Organic Chemistry and Biotechnology, Faculty of Chemistry, Bielefeld University, Universitätsstraße 25, 33615 Bielefeld, Germany. E-mail: harald.groeger@uni-bielefeld.de
bInstitute of Chemistry, Biocatalytic Synthesis Group, Otto von Guericke University Magdeburg, Building 28, Universitätsplatz 2, 39106 Magdeburg, Germany
cDepartment of Molecular Catalysis, Group Multiphase Catalysis, MPI for Chemical Energy Conversion, Stiftstrasse 34–36, 45470 Mülheim an der Ruhr, Germany
First published on 12th November 2024
Abstract
We report a novel one-pot chemoenzymatic synthesis of primary amines in water, combining rhodium-catalysed hydroformylation of styrene with a biocatalytic transamination. This process is starting from styrene at 50 mM substrate loading on a 10 mL preparative scale. Combined towards a one-pot process with both steps running concurrently, this chemoenzymatic synthesis involves a 6-DPPon/rhodium-catalysed hydroformylation of styrene at 20 bar of syngas, forming the iso- and n-aldehydes and an enzymatic transamination of the in situ-formed aldehydes to the corresponding primary amines catalysed by the amine transaminase from Chromobacterium violaceum, yielding the desired primary amines with 99% conversion.
Introduction
What can be considered as one of the most significant differences of today's way to carry out synthetic chemistry compared to the way nature prepares “chemicals” is the still often conducted isolation and purification of intermediates in multi-step transformations. Such multi-step syntheses are of importance for both nature and synthetic organic chemistry. In nature, biocatalytic cascades are conducted to get access to metabolites needed by the cell.1 In synthetic organic chemistry, multi-step syntheses enable the construction of structurally complex, often chiral chemicals mostly needed as fine chemicals and, in particular, pharmaceuticals.2 However, while in nature's approach these various reactions proceed in one cell and mostly in concurrent fashion, organic chemists typically conduct work-up of intermediates prior to using them for the next reaction step. Such a multi-step synthesis with intermediate isolation is also still the typical approach in industry when it comes to the production of industrial products, e.g., pharmaceuticals.2Although work-up of intermediates simplifies each individual reaction, the overall mass balance is disadvantageous resulting in a high overall need for, e.g., solvent and, thus, waste. Accordingly, in recent years an increasing tendency can be seen for combining chemical reaction steps towards one-pot processes without work-up.3–6 A particular challenge remained the combination of catalysts from different “worlds of catalysis” such as metal catalysts and enzymes.7–10 A key prerequisite to realize such resulting chemoenzymatic one-pot processes is to achieve compatibility. A particularly favoured reaction medium is water since it would, by definition, allow the use of any type of enzymes. Being still a young field of research, nonetheless various proof-of-concept works have demonstrated that chemo- and biocatalysts can be combined in a one-pot process in water.8,11–16 Very recently, we could show that even “antipodes” of such catalytic transformations such as high-pressure syngas chemistry with alcohol dehydrogenase-catalysed reductions can be combined.17
In continuation of this work,17 we became interested to make use of such a tolerance of hydroformylation and biocatalysis in water in order to expand the “synthetic space” achievable through combining chemo- and biocatalysis and in particular to realize a one-pot transformation, which remained difficult so far for primary amines when using only chemocatalysis, that is “hydroaminomethylation”.18,19 Hydroaminomethylation is a highly desired target reaction in chemocatalysis, but so far it mostly yields secondary or tertiary amines. To date, to best of our knowledge there is no report for realizing a chemocatalytic hydroaminomethylation leading with very high selectivity to a primary amino group (NH2), which, however, would be highly favoured in industry due to multiple applications of such compounds.20
We report that hydroformylation21–23 (as a reaction from the field of high-pressure syngas chemistry) can be cooperatively conducted with enzymatic transamination24 in a single reactor. This process, which will be described in the following, represents a one-pot hydroaminomethylation of a prochiral alkene that results in the formation of primary amines – a synthesis difficult to achieve through chemocatalytic steps alone (Fig. 1). This tandem-type one-pot process will be exemplified for the two-step transformation of styrene into 2-phenyl-1-propylamine via 2-phenylpropanal as intermediate, which has been conducted so far only with intermediate isolation and, thus, within two separated steps.25,26 It should be added that during the stage of the final preparation of this manuscript for submission and independent of our work, the Clark and Hartwig groups jointly reported a chemoenzymatic hydroaminomethylation of aliphatic alkenes for the synthesis of linear primary amines from olefins.27
Results and discussion
Hydroformylation: studies on compatibility with reaction conditions and components of enzymatic transamination
The first step consisted of studying the rhodium-catalysed hydroformylation under conditions needed for an enzymatic transamination in order to ensure the required compatibility of these two reaction steps for a tandem process. In accordance with our previous work,17 we utilised 6-(diphenylphosphino)-2(1H)-pyridinone (6-DPPon)23 as a phosphine ligand combined with [Rh(acac)(CO)2] as a precatalyst for the hydroformylation of styrene (1) in an aqueous reaction medium. Triton X-100 was employed as a surfactant to form micelles and, thus, facilitate mass transfer within the aqueous reaction medium. The 6-DPPon/rhodium catalyst was selected for its ability to enable the hydroformylation of styrene (1) under ambient conditions, in detail at 30 °C, and 20 bar H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) pressure. Additionally, the necessary components for enzymatic transamination were added in order to get an insight into the compatibility of the hydroformylation with those components of the biotransformation (Fig. 2).
1) pressure. Additionally, the necessary components for enzymatic transamination were added in order to get an insight into the compatibility of the hydroformylation with those components of the biotransformation (Fig. 2).
Conducting the hydroformylation of styrene (1) under these conditions in triplicate then yielded the desired iso- and n-aldehyde products rac-2-phenyl-propanal (2) and 3-phenyl-propanal (3) with an excellent overall conversion of 96 ± 3% and an iso/n ratio of 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (Fig. 2), thus being consistent with literature reports (for detailed reaction conditions, along with GC yield and 1H-NMR data, see ESI†).17,23 Thus, having been conducted in an aqueous reaction medium and in the presence of additives necessary for the enzymatic transamination, this hydroformylation experiment leading to such an excellent conversion indicates a high compatibility of the hydroformylation reaction with an enzymatic transamination reaction.
24 (Fig. 2), thus being consistent with literature reports (for detailed reaction conditions, along with GC yield and 1H-NMR data, see ESI†).17,23 Thus, having been conducted in an aqueous reaction medium and in the presence of additives necessary for the enzymatic transamination, this hydroformylation experiment leading to such an excellent conversion indicates a high compatibility of the hydroformylation reaction with an enzymatic transamination reaction.
Enzymatic transamination: bioprocess development
The next step consisted of developing a biotransformation of rac-2-phenylpropanal (2) as the main product received from hydroformylation of styrene (1). The enzymatic transamination was catalysed using the commonly applied and thermostable amine transaminase from Chromobacterium violaceum (Cv-ATA)28 and L-alanine as amino donor. In order to overcome the limitation of the non-favoured equilibrium of the (reversible) transamination, an enzymatic cascade was applied to shift the equilibrium of the transaminase-catalysed reactions towards the product side. This strategy involved the in situ-reduction of the by-product pyruvate, thereby driving the equilibrium towards the product side. For this purpose, lactate dehydrogenase from Bacillus subtilis (Bs-LDH) was employed in analogy to a previous protocol.29 Since Bs-LDH is NADH-dependent, NADH-recycling was accomplished using the thermostable glucose dehydrogenase mutant E170K Q252L from Bacillus subtilis (Bs-GDH).The biotransformation of rac-2-phenylpropanal (2) was conducted at a substrate concentration of 50 mM (corresponding to a substrate loading of 6.7 g L−1) and a 10 mL preparative scale (Fig. 3, details on the determination of e.r., GC yield, and 1H-NMR spectra are available in the ESI†). The effectiveness of the in situ-removal of pyruvate was demonstrated by significantly shifting the equilibrium towards the product side. The biotransformation carried out in triplicate constantly resulted in a high conversion of 86 ± 9%, showing the robustness of the bioprocess. Thus, this biotransformation represented a promising starting point for the next planned step consisting of combining this biocatalytic reaction with hydroformylation.
 | ||
| Fig. 3 Biotransformation of rac-2-phenylpropanal (2) to 2-phenyl-propan-1-amine (4) by means of a transaminase. | ||
Enzymatic transamination: studies on compatibility with reaction conditions and components of hydroformylation
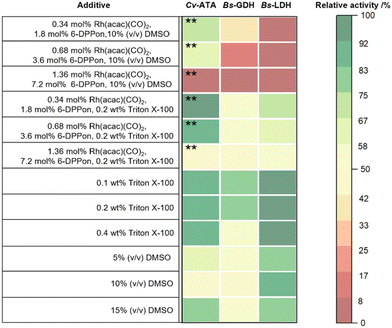
In parallel, we investigated the impact of components involved in hydroformylation on the enzymes. Therefore, we tested Cv-ATA, Bs-GDH, and Bs-LDH for compatibility with components from hydroformylation, in detail precatalyst [Rh(acac)(CO)2], phosphine ligand 6-DPPon, and surfactant Triton X-100 at different concentrations. Towards this end, standard spectrophotometric activity assays in PPB (100 mM, pH 7) containing the various additives ([Rh(acac)(CO)2], 6-DPPon, DMSO, or Triton X-100) were conducted to measure volumetric activities. The solvent DMSO was included as a co-solvent in order to dissolve [Rh(acac)(CO)2] and 6-DPPon in the aqueous medium. In contrast, the surfactant Triton X-100 served to form micelles in the aqueous system, thus embedding the 6-DPPon/rhodium catalyst in the micelles and “shielding” them from the components in the aqueous medium.The volumetric activity of the enzymes in the presence of each additive was referred to the volumetric activity of each enzyme without additives to determine the relative activity. The relative activities are summarised in Fig. 4 with red indicating low and green indicating high relative activity. The enzymes Cv-ATA, Bs-GDH, and Bs-LDH showed relative activities mainly in the 50–99% range in the presence of either Triton X-100 or DMSO at different concentrations. However, Cv-ATA, Bs-GDH and Bs-LDH exhibited relative activities predominantly below 25% in the presence of [Rh(acac)(CO)2], the 6-DPPon phosphine ligand, and the co-solvent DMSO (10%, v/v). In contrast, we were pleased to find that substantially higher relative activities of up to 75% were observed for all enzymes when [Rh(acac)(CO)2], 6-DPPon, and Triton X-100 (0.2 wt%) were used in combination as additives, most likely due to the formation of micelles that contain the chemocatalyst, thus avoiding contact with the enzymes being located in the aqueous medium.
Thus, all enzymes retained their activity in the aqueous reaction medium containing the components for hydroformylation, while interestingly the use of a co-solvent such as DMSO combined with [Rh(acac)(CO)2] and 6-DPPon resulted in a significant decrease in relative enzymatic activity. This finding highlights that the compartmentalization strategy applying Triton X-100 in order to form micelles for embedding the hydroformylation chemocatalyst enhances the compatibility of the biocatalysts with the 6-DPPon/rhodium catalyst (Fig. 4).
Combination of hydroformylation and enzymatic transamination towards a tandem-type one-pot process in water
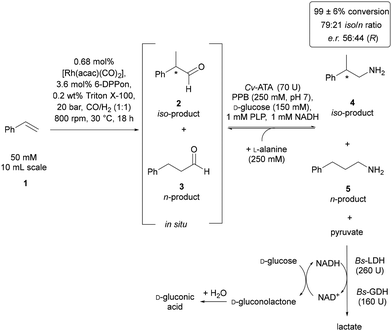
After developing the individual processes and achieving high conversions for both hydroformylation and biocatalytic transamination as well as demonstrating compatibility of these two reactions with each other, we next focused on combining these two reactions in a tandem-type one-pot process. In continuation of our previous work,17 we conducted the chemoenzymatic synthesis in an aqueous reaction medium. Thereby, the Rh/6-DPPon-catalysed hydroformylation of styrene (1) was combined with a subsequent enzymatic transamination of the in situ-formed aldehydes when using Cv-ATA along with in situ-removal of pyruvate facilitated by Bs-LDH in combination with a Bs-GDH-based recycling of the cofactor NADH. The chemoenzymatic synthesis was conducted in a five-fold determination and yielded constantly high conversions of >99 ± 6% for both of the resulting amines that is 2-phenyl-propan-1-amine (4) and 3-phenylpropan-1-amine (5) (Fig. 5). The iso/n-ratio of these amines (4 to 5) is 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21. Furthermore, the enantiomeric ratio (e.r.) of amine 4 was determined showing an e.r.-value of 56
21. Furthermore, the enantiomeric ratio (e.r.) of amine 4 was determined showing an e.r.-value of 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 with preference for the (R)-enantiomer. Work-up and product isolation of this chemoenzymatic synthesis has been done by means of preparative thin layer chromatography and resulted in an isolated yield of 52% for amine 4 and 8% for amine 5 (thus leading to a combined yield of 60% for amines 4 and 5). Details on work-up conditions for compound separation and purification as well as analytical characterisation are provided by the ESI.†
44 with preference for the (R)-enantiomer. Work-up and product isolation of this chemoenzymatic synthesis has been done by means of preparative thin layer chromatography and resulted in an isolated yield of 52% for amine 4 and 8% for amine 5 (thus leading to a combined yield of 60% for amines 4 and 5). Details on work-up conditions for compound separation and purification as well as analytical characterisation are provided by the ESI.†
It is noteworthy that this effective combination of 6-DPPon/Rh-catalysed hydroformylation of styrene (1) and enzymatic transamination of the in situ-formed aldehydes when using Cv-ATA has been achieved within a tandem-type process, in which both reactions proceed concurrently (Fig. 5). Most likely, the inclusion of Triton X-100 in this one-pot process facilitated the formation of an aqueous medium with micelles, which appears to be crucial for a high compatibility of the biocatalysts with the 6-DPPon/Rh catalyst and vice versa.
When comparing the various conversions achieved in this study with each other, hydroformylation of styrene (1) gave a conversion of 96 ± 3% while enzymatic transamination of rac-2-phenylpropanal (2) catalysed by Cv-ATA and combined with in situ-removal of pyruvate in the presence of Bs-LDH and Bs-GDH, led to a conversion of 86 ± 9%. Notably, the combination of both reactions (hydroformylation and biotransformation) towards a tandem-type one-pot process then resulted in an overall conversion of 99 ± 6%, yielding primary amines 4 and 5.
This indicates that the tandem-type one-pot process for the chemoenzymatic synthesis of primary amines can proceed without suffering from limited conversion compared to the isolated processes. The higher conversion in the transamination step when being conducted as a one-pot process might be attributed to the lower impact of an inhibition of Cv-ATA by the aldehyde substrates. These aldehydes 2 and 3 are formed in situ in the one-pot process by hydroformylation, being then directly converted by the Cv-ATA. Thus, in the one-pot process 2 and 3 are present at a lower concentration compared to their much higher initial concentration in case of an isolated transamination reaction. In addition, also the robustness of the chemoenzymatic synthesis should be highlighted since it was conducted in a five-fold determination consistently achieving excellent conversion of 99 ± 6% (Fig. 5).
As mentioned in the introduction, in parallel to our work a related study on chemoenzymatic synthesis of primary amines was conducted by Hartwig et al.,27 who focused on alternative substrates (e.g., 1-heptene). At different reaction conditions compared to our work, the simultaneous one-pot tandem process gave a maximum of 29% of 1-octylamine.27 One reason why in our work we achieved consistently high conversion (>99%) of styrene (1) is related to the use of the transaminase Cv-ATA that is highly suitable for the branched hydroformylation intermediate 2. In addition, the different applied amino donor system might represent a further relevant reaction parameter. At the same time, both achievements made by Hartwig et al.27 and us underline the suitability of chemoenzymatic synthesis for getting a one-pot access to primary amines from olefins by concurrently combining hydroformylation with enzymatic transamination.
Conclusions
In summary, the compatibility of a rhodium-catalysed hydro-formylation reaction with a biocatalytic transamination in the presence of Cv-ATA, Bs-LDH, and Bs-GDH, and vice versa, enabled a chemoenzymatic synthesis of 2-phenylpropan-1-amine (4) and 3-phenylpropan-1-amine (5) starting from styrene (1) in a tandem-type one-pot process running in an aqueous medium. This chemoenzymatic synthesis was carried out at a substrate loading of 50 mM (5.2 g L−1) and yielded an excellent conversion of 99 ± 6%. The process most likely involves the formation of micelles with the 6-DPPon/rhodium catalyst being included therein. In the aqueous phase, the biocatalysts, cofactors, and additives needed for the biotransformation are homogeneously dissolved. Thus, one can assume that through the presence of micelles chemocatalytic and biocatalytic reaction steps are effectively separated, preventing potential deactivation of the biocatalysts by, e.g., the rhodium catalyst and vice versa.The combination of hydroformylation and biocatalytic transamination in a one-pot process has the potential to significantly streamline the overall production of primary amines starting from olefins, e.g., by reducing the need for intermediate purification steps. In this study, we showcased such a chemoenzymatic one-pot synthesis of primary amines, exemplified for the one-pot transformation of styrene (1) into the chiral amine 2-phenylpropan-1-amine (4).
Among the tasks for future work is further process development aiming for an increased substrate loading as well as extension of the substrate scope.
Author contributions
Conceptualization: J. S., H. B., F. B., J. v. L., A. J. V., H. G.; data curation: J. S.; formal analysis: J. S.; funding acquisition: J. v. L., A. J. V., H. G.; investigation: J. S.; methodology: J. S., H. G.; project administration: H. G.; resources: A. J. V., H. G.; supervision: H. G.; validation: J. S.; visualization: J. S.; H. G.; writing – original draft: J. S., H. G.; writing – review & editing: J. S., H. B., F. B., J. v. L., A. J. V., H. G.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the German Federal Ministry for Economic Affairs and Energy (BMWi, Bundesministerium für Wirtschaft und Energie) within the funding project “Central Innovation Programme for small and medium-sized enterprises (SMEs)” (“Zentrales Innovationsprogramm Mittelstand (ZIM)”; grant number: ZF4832701CR9). J. V. L. acknowledges the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft) for financial support within the Heisenberg programme (grant number: 450014604). In addition, Bielefeld University, Otto von Guericke University Magdeburg and the Max-Planck Society are acknowledged for funding.References
- J. E. McMurry and T. P. Begley, The Organic Chemistry of Biological Pathways, Roberts and Company Publishers, Englewood, Colorado, 2005 Search PubMed.
- A. Kleemann, J. Engel, B. Kutscher and D. Reichert, Pharmaceutical substances: Syntheses, patents, applications, Thieme, Stuttgart, New York, 2001 Search PubMed.
- A. Behr, A. J. Vorholt, K. A. Ostrowski and T. Seidensticker, Green Chem., 2014, 16, 982 RSC.
- Cooperative Catalysis, ed. R. Peters, Wiley-VCH, Weinheim, 2015 Search PubMed.
- Domino Reactions – Concepts for Efficient Organic Synthesis, ed. L. F. Tietze, Wiley-VCH, Weinheim, 2018 Search PubMed.
- J. H. Schrittwieser, S. Velikogne, M. Hall and W. Kroutil, Chem. Rev., 2018, 118, 270–348 CrossRef CAS PubMed.
- H. Gröger and W. Hummel, Curr. Opin. Chem. Biol., 2014, 19, 171 CrossRef PubMed.
- H. Gröger, F. Gallou and B. H. Lipshutz, Chem. Rev., 2023, 123, 5262 CrossRef.
- S. González-Granda, L. Escot, I. Lavandera and V. Gotor-Fernández, Angew. Chem., Int. Ed., 2023, 62, e202217713 CrossRef.
- S. González-Granda, J. Albarrán-Velo, I. Lavandera and V. Gotor-Fernández, Chem. Rev., 2023, 123, 5297 CrossRef PubMed.
- M. Makkee, A. P. G. Kieboom, H. van Bekkum and J. A. Roels, J. C. S. Chem. Commun., 1980, 930 RSC.
- J. V. Allen and J. M. J. Williams, Tetrahedron Lett., 1996, 37, 1859 CrossRef CAS.
- E. Burda, W. Hummel and H. Gröger, Angew. Chem., Int. Ed., 2008, 47, 9551 CrossRef CAS PubMed.
- G. Rulli, N. Duangdee, K. Baer, W. Hummel, A. Berkessel and H. Gröger, Angew. Chem., Int. Ed., 2011, 50, 7944 CrossRef CAS PubMed.
- C. A. Denard, H. Huang, M. J. Bartlett, L. Lu, Y. Tan, H. Zhao and J. F. Hartwig, Angew. Chem., Int. Ed., 2014, 53, 465 CrossRef CAS PubMed.
- H. Sato, W. Hummel and H. Gröger, Angew. Chem., Int. Ed., 2015, 54, 4488 CrossRef CAS PubMed.
- H. Bork, K. Naße, A. J. Vorholt and H. Gröger, Angew. Chem., Int. Ed., 2024, 63, e202401989 CrossRef CAS PubMed.
- B. Zimmermann, J. Herwig and M. Beller, Angew. Chem., Int. Ed, 1999, 38, 2371 CrossRef.
- P. Kalck and M. Urrutigoïty, Chem. Rev., 2018, 118, 3833 CrossRef CAS PubMed.
- P. Roose, K. Eller, E. Henkes, R. Rossbacher and H. Höke, Amines, Aliphatic, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2015, pp. 1–55; DOI:10.1002/14356007.a02_001.pub2.
- A. Börner, D. Selent and R. Franke, Chem. Rev., 2012, 112, 5675 CrossRef PubMed.
- A. Börner and R. Franke, Hydroformylation – Fundamentals, Processes, and Applications in Organic Synthesis, Wiley-VCH, Weinheim, 2016 Search PubMed.
- A. T. Straub, M. Otto, I. Usui and B. Breit, Adv. Synth. Catal., 2013, 355, 2071 CrossRef CAS.
- M. Fuchs, J. E. Farnberger and W. Kroutil, Eur. J. Org. Chem., 2015, 6965 CrossRef CAS PubMed.
- C. S. Fuchs, M. Hollauf, M. Meissner, R. C. Simon, T. Besset, J. N. H. Reek, W. Riethorst, F. Zepeck and W. Kroutil, Adv. Synth. Catal., 2014, 356, 2257 CrossRef CAS.
- F. Belov, A. Gazizova, H. Bork, H. Gröger and J. von Langermann, ChemBioChem, 2024, 25, e202400203 CrossRef CAS PubMed.
- J. L. Manske, J. Nicolai, B. J. Bloomer, D. S. Clark and J. F. Hartwig, ACS Catal., 2024, 14, 14356 CrossRef CAS , published online on September 13, 2024.
- U. Kaulmann, K. Smithies, M. E. B. Smith, H. C. Hailes and J. M. Ward, Enzyme Microb. Technol., 2007, 41, 628 CrossRef CAS.
- F. Uthoff, H. Sato and H. Gröger, ChemCatChem, 2017, 9, 555 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01513d |
| This journal is © The Royal Society of Chemistry 2025 |