 Open Access Article
Open Access ArticleOne-pot synthesis of photonic microparticles doped with light-emitting quantum dots†
Simone
Bertucci
 ab,
Davide
Piccinotti
a,
Mauro
Garbarino
a,
Andrea
Escher
b,
Gianluca
Bravetti
c,
Christoph
Weder
ab,
Davide
Piccinotti
a,
Mauro
Garbarino
a,
Andrea
Escher
b,
Gianluca
Bravetti
c,
Christoph
Weder
 cd,
Paola
Lova
b,
Davide
Comoretto
cd,
Paola
Lova
b,
Davide
Comoretto
 b,
Ullrich
Steiner
b,
Ullrich
Steiner
 cd,
Francesco
Di Stasio
*a and
Andrea
Dodero
cd,
Francesco
Di Stasio
*a and
Andrea
Dodero
 *bcd
*bcd
aPhotonic Nanomaterials, Istituto Italiano di Tecnologia, Via Morego 30, 16163 Genoa, Italy. E-mail: francesco.distasio@iit.it
bDepartment of Chemistry and Industrial Chemistry, University of Genoa, Via Dodecaneso 31, 16146, Genoa, Italy
cAdolphe Merkle Institute, University of Fribourg, Chemin des Verdiers 4, 1700, Fribourg, Switzerland. E-mail: andrea.dodero@unifr.ch
dNational Center of Competence in Research Bio-Inspired Materials, Chemin des Verdiers 4, 1700, Fribourg, Switzerland
First published on 14th March 2025
Abstract
Colloidal quantum dots (QDs) exhibit size-dependent, tuneable optical properties that render them useful in a wide range of technological applications. However, integration of QDs into structured materials remains a significant challenge due to their susceptibility to degradation under chemical or physical perturbations. Here, we present a facile, scalable one-pot co-assembly strategy to embed commercially available CdSe/ZnS core–shell quantum dots into photonic microparticles via the confined self-assembly of a poly(styrene)-b-poly(2-vinylpyridine) block copolymer in emulsion droplets. The resulting hybrid particles exhibit a well-defined concentric lamellar structure and the quantum dots are selectively incorporated into the domains formed by the poly(2-vinylpyridine) blocks. This design enables two different optical responses, i.e., vivid, non-iridescent structural colouration from photonic bandgap effects and stable engineered photoluminescence from the embedded QDs. The use of swelling agents provides an effective means to tune the photonic bandgap spectral position, extending the optical range to the entire visible region. Optical experiments reveal a subtle interplay between the photonic structure and QD emission, and the emission properties remain intact despite variations in the structural periodicity and matrix refractive index. This work highlights a robust platform for the integration of functional nanomaterials into photonic architectures, offering significant potential for applications in advanced light sources, displays, and sensing technologies. The simplicity of the approach, combined with its scalability, sets the stage for future exploration into hybrid photonic materials with tailored optical properties.
1. Introduction
Controlling and manipulating the interaction of light with materials is critical to advancing a wide range of modern technologies.1 From optical communications to energy harvesting, photonic structures that consist of dielectric lattices in which at least two materials with different refractive indices are periodically alternated at the submicron scale have become indispensable.2–4 These structures inhibit the propagation of light in specific spectral regions and directions,5 a property that underpins their applications in fields as diverse as optical sensors, display technologies, solar cells, biomedical imaging, and anti-counterfeiting.6 Among the simplest yet most-studied structures, planar photonic crystals – also known as distributed Bragg reflectors (DBRs) – are optical structures consisting of alternating material layers with different refractive indices arranged periodically with sub-micron periodicity.7 These structures inhibit the propagation of specific wavelengths of light, a process driven by coherent diffraction, which can be described in a simple way according to the Bragg–Snell condition.8 The inhibited frequencies are known as photonic band gaps (PBGs) and can be easily tuned within the UV, visible and near-infrared spectral regions by controlling the periodicity and the refractive index of the alternating layers.9 Such precise control makes DBRs attractive for various devices in applications such as mirrors, filters, waveguides, and resonators in optical communications and sensing technologies.7Inorganic structures have shown outstanding performance due to the large dielectric contrast achievable among the building layers of DBRs,10–12 but their fabrication is generally complicated. Producing such structures with polymers greatly benefits material availability, cost, and ease of fabrication.13,14 Solution- or melt-based fabrication processes that apply to polymers – including spin/dip coating and co-extrusion – allow for large-area structures at costs compatible with packaging technologies.15 Polymer structures can redistribute the emission, enhance its intensity, and achieve low-threshold optically pumped lasing.16,17 In addition, recent developments in all-polymer DBRs have enabled effects such as emission rate control and strong coupling,18 offering significant potential for advanced optical applications.19–24 However, planar DBRs are inherently limited by their angular dispersion, i.e., the dependence of the PBG spectral response on the photon wave vector, which restricts their performance to a specific range of solid angles.24
One potential breakthrough is the fabrication of all-polymer photonic structures based on the self-assembly of block copolymers (BCPs) in confined environments.25–27 BCPs consist of two or more immiscible polymer chains covalently linked. While the covalent bond prevents macrophase segregation, favourable interactions between identical blocks allow the spontaneous formation of well-defined nanostructures through microphase separation.28 These nanostructures feature periodic domains with dimensions comparable to the wavelengths of visible light, making them well-suited for photonic applications when long-range order is achieved.29 The self-assembly behaviour of BCPs is primarily governed by three factors: (i) the Flory–Huggins interaction parameter χ, which quantifies the interactions between different polymer blocks, (ii) the molecular mass of the block polymer, and (iii) the volume fractions of each polymer block. The size and morphology of the resulting nanostructures can be precisely controlled by tuning these parameters.30–33 Confining the self-assembly of BCPs in emulsion droplets has recently emerged as a powerful strategy to fabricate photonic microparticles.34–41 These exhibit a concentric or stacked lamellar structure, which gives rise to vivid, tuneable structural colours due to their ordered internal arrangement without spectral dispersion because of their spherical symmetry. Such microparticles retain the advantageous light-manipulating properties of planar photonic crystals but offer greater versatility in applications, particularly due to their ability to be used as liquid dispersions or in solid casting. However, the incorporation of nanoscale objects with emissive properties into photonic structures with high spatial precision is very challenging due to the sensitivity of these materials to external agents.42 Interestingly, the co-assembly of BCPs and nanoparticles in emulsion droplets can lead to hybrid structures with precise spatial organization.43,44 This can be achieved by exploiting ligands on the surface of nanoparticles that allow fine-tuning of their enthalpic or entropic interactions with the BCPs during self-assembly. Depending on the surface chemistry of these nanoparticles, they can be selectively incorporated into specific polymer domains, confined to the interfaces between them, or embedded in the core of the particles.45,46 Despite these advantages, previous studies have only focused on low-molecular-weight BCPs and have not explored integrating light-emitting materials into self-assembled photonic microparticles. In particular, colloidal quantum dots (QDs) are attractive for this purpose due to their remarkable optical properties, including high fluorescence quantum yield,47 narrow emission linewidth,48 and tuneable emission spectra based on particle size49 and shape.50 Their surface chemistry can also be easily modified, making them compatible with a variety of polymer matrices and solution-processing techniques.47,51,52
Here, we present a one-pot preparation strategy for hybrid photonic microparticles composed of poly(styrene)-b-poly(2-vinylpyridine) (PS-P2VP) and CdSe/ZnS core–shell QDs. Using a simple evaporation-induced confined co-assembly process in emulsion droplets, we achieved well-defined concentric lamellar structures and the selective incorporation of QDs into one of the BCP domains (i.e., P2VP), as shown in Fig. 1. The fabrication starts with a biphasic mixture of chloroform, BCPs, and QDs in the dispersed phase and water and poly(vinyl alcohol) (PVA) in the continuous phase. The mixture was emulsified to form an oil-in-water (O/W) emulsion and as the chloroform slowly diffuses into the aqueous phase, the BCPs undergo spherical confinement within the droplets, which drives the self-assembly of the macromolecular chains into concentric lamellae. The resulting microparticles exhibit two different optical responses, i.e., vivid, non-iridescent structural colouration and bright and stable photoluminescence (PL).
2. Experimental details
2.1. Materials
Poly(styrene)-b-poly(2-vinyl pyridine) (PS-P2VP, Mn = 213-b-215 kg mol−1 and Mw/Mn = 1.29) was purchased from Polymer Source. 3-Pentadecylphenol (PDP, purity ≥ 88%) was obtained from Santa Cruz Biotechnology. Blue, green, and red-emitting CdSe/ZnS core–shell (oleic acid-functionalized) quantum dots, chloroform (stabilized with amylene), homopolymer poly(styrene) (hPS, Mw = 35 kg mol−1), poly(vinyl alcohol) (PVA, Mw = 13–23 kg mol−1, 87–89% hydrolysed), and aluminium oxide (Al2O3, activated, basic, Brockmann I) were purchased from Sigma-Aldrich. The chloroform was treated with Al2O3 to remove stabilizers and degradation products before use. Milli-Q water with conductivity equal to 0.055 μS cm−1 was used for all the experiments unless otherwise indicated.2.2. Methods
Transmission electron microscopy micrographs were acquired using a JEOL JEM-1011 instrument with accelerating voltage of 100 kV. Samples were prepared by drop-casting 10 μL of the diluted suspensions of QDs in toluene on the grids (Formvar/Carbon 200 mesh, Cu).
Optical microscopy of photonic microparticles was conducted using a custom-built microscope (ZEISS Axio Scope.A1) equipped with a diffusive CCD camera (Point Grey GS3-U3-28S5C-C) calibrated against a standard white diffuser and illuminated with a halogen lamp. Micrographs were captured in brightfield reflection mode configuration using a 50× objective (Zeiss LD EC Epiplan-Neofluar, NA = 0.8). Reflection spectra of the microparticles were acquired through micro-spectroscopy by coupling a microscope with a diode-array spectrometer (Ocean Optics QEPro) via an optical fibre positioned confocally to the microscope image plane (Avantes QP230-2-XSR, 230 μm core size) using an aluminium mirror as reference (Thorlabs PF10-03-G01). The photonic microparticles were characterized on optical glass slides covered with a glass coverslip.
Focused-ion-beam scanning electron microscopy (FIB-SEM) (Thermo Scientific Scios 2 DualBeam FIB-SEM, FEI, Eindhoven, the Netherlands) was used to investigate the internal structure of the photonic microparticles. Particle suspensions were first drop-cast on aluminium stubs covered with conductive carbon tape and oven-dried at 40 °C under vacuum before being coated with a 4 nm thick gold layer. Half the microparticles were milled away using a Ga+ ion beam set at an acceleration voltage of 30 kV and a current of up to 3 nA. The cut face was imaged using built-in SEM Everhart–Thornley (ETD, secondary electrons) and in-lens T1 (A + B composite mode, back-scattered electrons) detectors set to a voltage of 5 kV and a current of 0.4 nA. The image distortion induced by the acquisition at an angle of 52° was compensated with the built-in tilt correction feature. For each sample, the thicknesses of 20 lamellae of P2VP and 20 lamellae of PS were measured using ImageJ to obtain reliable statistical data. Confocal laser microscopy (CLM) was performed on single hybrid particles in suspension using a Leica LSM Stellaris 5 equipped with an HC PL Plan Apochromat 63×/1.40NA oil objective (brightfield, DIC). Three-dimensional images were reconstructed using Avizo software.
Ultra-small-angle X-ray scattering (USAXS) measurements were conducted on the ID02 beamline at the European Synchrotron Facility (ESRF) in Grenoble. Circular samples with an average thickness of 1 mm and a diameter of 4 mm were prepared by drop-casting particle suspensions in polytetrafluoroethylene (PTFE) disks closed on both sides using Kapton tape (DuPont).
Photoluminescence (PL) measurements were performed using a 405 nm diode laser at a repetition rate of 10 MHz and power of 13 nW through an optical microscope setup operated in reflection mode with a 100× NA = 1.45 objective lens. The PL emission from the microparticles was collected through the same objective and measured using a single photon avalanche diode (SPAD) detector (Micro Photon Devices, PDM series) connected to a time-correlated single photon counter (TCSPC, PicoHarp300), allowing lifetime measurements. PL spectra were recorded using a Gemini interferometer (Nireos), which works in combination with the described SPAD detector and the TCSPC instrument to record the spectrum of the input light based on a Fourier transform approach. Samples were scanned using a piezo stage (PI, Physik Instrumente) capable of nanometer spatial resolution, resulting in a 2D map where a time trace (or lifetime) was recorded for each pixel. The value of each pixel was obtained by summing all the counts of the corresponding time traces. The time traces of the resulting 2D map were fitted with a double exponential function, which allows a time constant to be extracted from the fit for each pixel, resulting in a fluorescence lifetime image (FLIM).
3. Results and discussion
Colloidal QDs were selected as prototype materials to demonstrate the feasibility of fabricating emissive photonic microparticles via a one-pot process. We used commercially available blue- (bQDs, λbQDsem = 470 nm), green- (gQDs, λgQDsem = 530 nm), and red (rQDs, λrQDsem = 650 nm)-emitting CdSe/ZnS core–shell QDs functionalized with oleic acid. Optical absorption, photoluminescence spectra, and transmission electron microscopy (TEM) micrographs of the QDs are shown in Fig. S1.† The absorption spectra (Fig. S1a–c,† black lines) of all three nanomaterials show a broad continuous background typical of colloidal QDs,53 where distinct exciton peaks are detected at λbQDsexc ∼ 465 nm, λgQDsexc ∼ 520 nm, and λrQDsexc ∼ 645 nm for the bQDs, gQDs, and rQDs, respectively. As expected, the intense fluorescence of the three samples (Fig. S1a–c,† coloured lines) is spectrally narrow with a slight Stokes shift (of about 5 nm) with peaks at 470 nm, 540 nm, and 650 nm for the different QDs. The TEM micrographs presented in Fig. S1a–c† show an almost spherical, homogeneous shape for all the QDs, whose average measured size increases from about 10 ± 1.2 nm for the blue QDs to 20 ± 2.0 nm for the red QDs. The correlation between the emission wavelength λem and the QD dimensions is related to quantum confinement effects.54 In smaller QDs, the confinement of charge carriers in a limited volume increases their energy gap, leading to higher energy emissions. As the QD size increases, the energy gap decreases, resulting in lower energy emissions.55QDs were then co-assembled with PS-P2VP in emulsion droplets without requiring specific, time-consuming surface modification. As mentioned, the co-assembly of BCPs and inorganic nanomaterials is challenging for several reasons. On the one hand, the QDs may prevent the BCP chains from self-assembly due to variations in enthalpy-driven interfacial interactions, structural strains, and entropy losses. On the other hand, the QDs themselves and their emissive properties are easily affected by changes in the external environment.56 The nature of the organic ligand on the surface of the QDs plays a crucial role in this context, as it can establish interactions between the BCPs and the QDs and reduce the conformational entropic penalties on the BCP chains.46 Additionally, specific interactions between the ligand and one of the blocks of the macromolecular chains can be exploited to facilitate control over the spatial distribution of the nanocrystals within the BCP structure. In this work, oleic acid-functionalized CdSe/ZnS quantum dots were selected on the expectation that the nitrogen atoms in the P2VP blocks would interact with the ZnS shell via electrostatic interactions, as previously reported in the literature.57–59 Additionally, oleic acid provides solubility of the BCPs and the QDs in the same solvent (i.e., chloroform), thus allowing for the one-pot synthesis of the desired self-assembled concentric structures. To further confirm the dispersion of the oleic acid-capped QDs into the PV2P domains, the fabrication procedure described in Fig. 1 was carried out with PS-P2VP, as well as the poly(methyl methacrylate)-b-poly(2-vinylpyridine) (PMMA-P2VP) and poly(styrene)-b-poly(methyl methacrylate) (PS-PMMA) block copolymers. Fig. S2† shows that while the QDs are not embedded in the PS-PMMA microparticles (Fig. S2a–c†), they are homogeneously dispersed in one of the layers of the PS-P2VP or PMMA-PV2P microparticles (Fig. S2d–f and S2g–i†), thus confirming the affinity of the capped nanocrystals for the PV2P domains. Fig. 2a–d shows the reflectance spectra obtained using microspectrophotometry and the optical microscope images of the resulting PS-PV2P microparticles containing the different QDs (around 5 wt%). The reflectance spectrum of the QD-free microparticles obtained from the neat BCP shows a sharp reflectance peak at 420 nm (Fig. 2a), which corresponds to the bright, structural colouration visible in the centre of the particle in the optical microscopy image. Note that macroscopically, the microparticle dispersions do not present structural colour due to the low microparticle concentration and scattering effects. Upon co-assembly with the blue-emitting QDs, the reflectance peak is slightly redshifted to 425 nm. In addition, a second peak at 473 nm is detected and assigned to the bQD emission (Fig. 2b) caused by the white-light illumination of the sample. Similar observations are made for the microparticles containing gQDs and rQDs, for which the reflectance peaks are shifted to 443 nm and 471 nm, respectively. Again, additional peaks at 547 nm and 650 nm are observed and related to the emission of the green and red QDs, respectively. This spectral behaviour is reflected in the optical microscopy images, where all particles show a bright blue colour in the centre and a different coloured hue at the periphery, which is assigned to the emission of the QDs. Notably, the presence of QDs in the BCP structure slightly affects the spectral position of the PBG, which mainly indicates low impact on the self-assembly process and the final structure. The observed redshift can be explained by considering two factors. First, the embedded QDs have a much higher refractive index – above 2 – than the polymer layers (i.e., nP2VP = 1.62 and nPS = 1.59).34,60,61 Consequently, selective loading of the QDs into the P2VP domains is responsible for an increase in the dielectric contrast between the lamellar phases, resulting in a broader and redshifted photonic band gap compared to the bare structure, as described by the Bragg–Snell law adapted to the case of the photonic particles:9
After investigating the optical response of the fabricated hybrid particles under white-light illumination, we further explored the emissive properties of the QDs embedded in the photonic microparticles. In particular, any potential changes in their optical properties due to the fabrication process of the hybrid structure and possible effects of the photonic structure were investigated.63 Although the fabrication process is robust and reproducible, the microparticles exhibit some variability in their size distribution (i.e., 20.7 ± 7.5 μm) and colour of the individual microparticles (Fig. S4†). Consequently, the detection of small changes in the photoluminescence of the QDs on a macroscopic scale becomes complicated and unreliable. For this reason, the emission of individual particles was measured using a microphotoluminescence setup and compared to the emission of the pure CdSe/ZnS QDs cast on a glass substrate. As previously highlighted, the spherical symmetry of the microparticles is responsible for the uniform colouration at their centre without any angle dependence (i.e., the observed colour does not change with the observation angle).34 In fact, the concentric multilayers can be approximated to be planar while aligning with the microparticle curvature so that they could be locally considered as a planar dielectric mirror (when the measured spot size is much smaller than the particle diameter).
Steady-state photoluminescence (PL) spectra and time-resolved photoluminescence (TRPL) measurements are shown in Fig. 3. The photoluminescence spectra of the hybrid particles (Fig. 3a–c) present only minor variations in the spectral shape of the emission and a redshift of the emission wavelength is detected when comparing the PL of pure QDs to that of QDs embedded in the microparticles, with 14, 20, and 8 meV redshifts for blue-, green- and red-emitting quantum dots, respectively. While this effect can be attributed to the close packing of the QDs and possible self-absorption, the PL spectra show no sign of broadening. Furthermore, no spectral redistribution of the emission is detected due to the limited (i.e., bQDs) or negligible (i.e., gQDs and rQDs) spectral superposition of the emission peaks with the PBG of the microparticles, as shown in Fig. 2b–d. Additionally, the variable redshift observed for the bQDs, gQDs, and rQDs is most likely associated with the varying degrees of organic ligand coverage on the surface of the nanocrystals, which leads to a different tendency for aggregation during the self-assembly processes.
TRPL measurements (Fig. 3d–f) reveal faster decays for the QDs embedded in the photonic microparticles than in their neat form. The decay is well fitted in each case with a double exponential function, as reported in the ESI.† The average PL lifetime ( ), reported in each panel for all decays, shows 2.9-, 5.3-, and 1.7-fold decreases for the blue, green, and red-emitting QDs, respectively, when embedded within the block copolymer particles. The PL lifetime reduction of the quantum dots could be due to several factors. First, an environment with a higher refractive index than air can lead to faster radiative recombination processes.64 Second, attractive interactions between oleic acid and P2VP may lead to the detachment of the ligands from the QD surface, resulting in the formation of defects that cause PL quenching, thus reducing the PL lifetime.65 However, although evident, the variations in the decay time are considered only qualitatively as a quantitative evaluation would require a more in-depth and sophisticated characterization, which is beyond the scope of the present study.
), reported in each panel for all decays, shows 2.9-, 5.3-, and 1.7-fold decreases for the blue, green, and red-emitting QDs, respectively, when embedded within the block copolymer particles. The PL lifetime reduction of the quantum dots could be due to several factors. First, an environment with a higher refractive index than air can lead to faster radiative recombination processes.64 Second, attractive interactions between oleic acid and P2VP may lead to the detachment of the ligands from the QD surface, resulting in the formation of defects that cause PL quenching, thus reducing the PL lifetime.65 However, although evident, the variations in the decay time are considered only qualitatively as a quantitative evaluation would require a more in-depth and sophisticated characterization, which is beyond the scope of the present study.
To demonstrate the possibility of tuning the photonic structure of the microparticles containing the emitting QDs, a single type of emitter (i.e., bQDs) was selected and the PBG of the microparticles was fine-tuned to ensure spectral overlap with the emission spectrum. To control the spectral position of the photonic bandgap, the periodicity of the lamellar structure was varied by adding swelling agents that increase the overall periodicity of the structure (i.e., both lamellar layers are swelled simultaneously to ensure structural symmetry). Therefore, adding increasing amounts of additives to the BCP(bQD) precursor mixture allows for the controlled swelling of the lamellar structure and, thereby, a spectral redshift of the photonic bandgap. The additive of choice for swelling the P2VP layers is 3-pentadecylphenol (PDP), which has previously been demonstrated to interact selectively with the pyridine blocks via hydrogen bonding.34,66 The samples thus made are classified according to the molar ratio (x-ratio) of PDP to pyridine residues. To maintain the balance between the volume ratio of the two blocks and to avoid a morphological transition away from the lamellar structure, counterbalancing amounts (the same volume as the added PDP) of a low-molecular-weight homopolymer poly(styrene) (hPS), which preferentially swells the PS domains, were added.67 By varying the content of the additives, the PBG can be tuned from λmax = 410 nm (i.e., x = 0.05) to λmax = 666 nm (i.e., x = 0.50), covering the entire visible spectrum, as shown in Fig. 4a–c. For all samples, the photoluminescence of the QDs is still clearly detectable at around λbQDsem = 475 nm in addition to the bandgap signal, even under white-light illumination, suggesting that the emissive properties of the QDs are not affected by the swelling process.
It should be noted that adding a small amount of PDP (i.e., x = 0.05) causes an initial blueshift of the PBG spectral position compared to the pristine block copolymer particles (Fig. 2b). This effect is attributed to the plasticizing behaviour of PDP, which allows the copolymer molecules to adopt different conformations.68 However, increasing the amount of swelling agents induces a linear proportional redshift of the PBG (Fig. 4b) due to the increased layer thickness of the lamellar structure. The variation of lamellar thickness upon additive addition, measured from the FIB-SEM cross-sections, is shown in Fig. 4d, while the corresponding micrographs are shown in Fig. 4e for x-ratios of 0.05 (i.e., lowest) and 0.50 (i.e., highest). Similar to what was observed for the PBG, a nearly linear increase with increasing amounts of additives is observed for both the P2VP and PS layers. In all cases, the presence of QDs and their selective loading into P2VP domains is confirmed by the FIB-SEM micrographs, indicating that the additives do not alter the interactions between the QDs and the block copolymer chains. At the same time, the embedded QDs preserve the possibility of fine-tuning the optical properties of the microparticles by adding small amounts of swelling agents.
After confirming the fine-tuning of the photonic bandgap, a systematic investigation of their emission properties was performed to elucidate any effects that arise from the photonic structure. Each sample was analysed using 2D photoluminescence intensity mapping of individual microparticles. First, an optical microscope equipped with a 100× oil objective was used to locate an isolated particle, where the emission intensity and lifetime were recorded over its surface area. Fig. 5a shows the photoluminescence map for the BCP(bQD) sample with an x-ratio of 0.15 (for other compositions, see Fig. S5a†) and the optical microscopy image of the particle is shown in Fig. 5b. The resulting map shows a uniform PL for most of the spherical microparticle surface, while lower intensities are observed at the edges where the amount of QDs is minimal, as shown in Fig. S6.† The fluorescence decay times (Fig. 5c) are consistent over the entire particle, indicating the uniformity of the effect on the exciton recombination process. Minor differences detected on the particle surfaces are assigned to a different dielectric environment of the exposed QDs. A comparison between the samples with different x-ratios (i.e., different spectral positions of the PBG) was performed by analysing the PL intensity and lifetime signals acquired at the centre of the microparticles, where the structure has a geometry that can be approximated to that of a planar DBR. No significant variations are observed when analysing possible differences in the shape and position of the photoluminescence (Fig. 5d, solid line). The emission is preserved when the microparticles are dispersed in water (Fig. 5d, dotted lines) and shows no difference compared to the single particles. This suggests that PDP does not significantly affect the oleic acid ligands on the quantum dot surface. If PDP interacts strongly with the ligands, we would expect aggregation phenomena, leading to a gradual redshift and broadening of the photoluminescence peak. Since no such effects are observed, we can conclude that PDP has minimal impact on the pristine ligand environment of the quantum dots. The lack of emission redistribution is attributed to the modest dielectric contrast (i.e., Δn ∼ 0.03 for PS vs. P2VP)34 between the two materials comprising the photonic structure and the relative inhomogeneity of the layer thickness compared to the standard planar structures. These effects will likely limit the redistribution of the local density of photonic states and, thus, the field enhancement in the respective layers.69 Therefore, it is realistic to consider that increasing the refractive index mismatch could lead to a more pronounced effect. Consistent with these data, the PL decay processes and their relative lifetimes remain unaffected by the addition of swelling agents (Fig. 5e). Indeed, Fig. 5f presents the two lifetimes of the particles (τ1 and τ2) and their weighted average ( ),revealing that no significant differences are present.
),revealing that no significant differences are present.
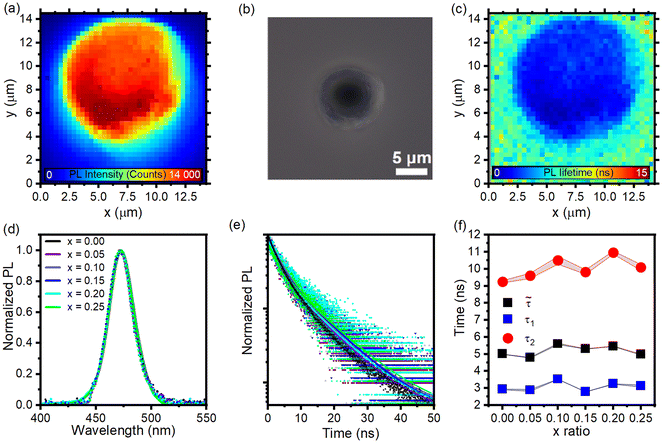 | ||
Fig. 5 (a) 2D photoluminescence map, (b) optical microscopy image, and (c) lifetime map showing the same emission behaviour throughout the photonic structure for a BCP(bQDs) microparticle with an x-ratio of 0.15. The complete dataset is shown in Fig. S5.† (d) PL spectra of individual BCP(bQDs) microparticles (solid lines) and water-dispersed microparticles (dotted lines) with different x-ratios. (e) Lifetime decays for individual water-dispersed microparticles with different additive contents. (f) Fitted emission lifetimes (τ1 and τ2) and their weighted average ( ). ). | ||
4. Conclusions
In summary, we report a scalable one-pot co-assembly method for fabricating hybrid photonic microparticles that contain colloidal quantum dots. By leveraging the self-assembly of PS-P2VP block copolymers in emulsion droplets, we successfully produce particles with highly organized concentric lamellar architectures that exhibit vivid, non-iridescent structural colouration due to the refractive index mismatch between the ordered domains. Fine-tuning the thermodynamic interactions between the organic and inorganic components allows the selective incorporation of CdSe/ZnS QDs into the P2VP domains, effectively addressing integration challenges while preserving their photoluminescence properties. Additionally, we show that the incorporation of swelling agents offers a straightforward means to finely tune the photonic bandgap across the entire visible spectral range. This capability, achieved without compromising the QD emission properties, positions the fabricated hybrid particles as promising candidates for diverse applications in optoelectronics, sensing, and light-emitting devices. The robustness and simplicity of this fabrication process, combined with its adaptability to various nanomaterials and potentially other polymer systems, underscore its versatility for tailoring the optical properties of hybrid photonic materials. The demonstrated retention of QD emission under different structural configurations validates the method's efficacy, offering a reliable platform for the development of advanced photonic architectures with customizable functionalities. This innovative approach lays the groundwork for next-generation photonic devices, unlocking new opportunities in technology-driven applications.Author contributions
SB contributed to the data curation, investigation, methodology and writing – original draft. DP contributed to the investigation. MG contributed to the investigation. AE contributed to the investigation. GB contributed to the investigation. CW contributed to the validation and writing – review & editing. PL contributed to the data curation and formal analysis. DC contributed to the supervision and validation. US contributed to the conceptualization, resources, validation and writing – review & editing. FDS contributed to the supervision, validation and writing – review & editing. AD contributed to the conceptualization, funding acquisition, methodology, project administration, supervision, validation, writing – original draft and writing – review & editing.Data availability
Data for this article are available from Zenodo at https://doi.org/10.5281/zenodo.14566782.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the Marie Sklodowska-Curie Action (MSCA) Postdoctoral Fellowship COLOUR (Grant No. 101062004) and the ELDOPP (Grant No. 101062372), the ERC Advanced Grant PrISMoID (Grant No. 833895), the ERC Starting Grant NANOLED (Grant No. 851794), the Adolphe Merkle Foundation, the NCCR Bio-Inspired Materials, and the Italian Ministry of University and Research (program project PRIN2022 WATERONIC, Grant No. 20227WZXJ3).The authors also acknowledge the European Synchrotron Radiation Facility (ESRF), Grenoble, France, for granting beamtime on beamline ID02 through the proposal SC-5454. The authors would like to thank Dr Theyencheri Narayanan and Dr Gouranga Manna for their assistance during the experiments.
References
- D. G. Baranov, M. Wersäll, J. Cuadra, T. J. Antosiewicz and T. Shegai, ACS Photonics, 2018, 5, 24–42 CrossRef CAS.
- V. V. Vogler-Neuling, M. Saba, I. Gunkel, J. O. Zoppe, U. Steiner, B. D. Wilts and A. Dodero, Adv. Funct. Mater., 2024, 34, 2306528 CAS.
- M. Bellingeri, A. Chiasera, I. Kriegel and F. Scotognella, Opt. Mater., 2017, 72, 403–421 CAS.
- V. Berger, Curr. Opin. Solid State Mater. Sci., 1999, 4, 209–216 CAS.
- J. B. Pendry, J. Mod. Opt., 1994, 41, 209–229 CAS.
- M. A. Butt, S. N. Khonina and N. L. Kazanskiy, Opt. Laser Technol., 2021, 142, 107265 CAS.
- E. Palo and K. S. Daskalakis, Adv. Mater. Interfaces, 2023, 10, 2202206 Search PubMed.
- H. Chen, J. Wei, F. Pan, T. Yuan, Y. Fang and Q. Wang, Adv. Mater. Technol., 2025, 10, 2400865 Search PubMed.
- P. Lova, G. Manfredi and D. Comoretto, Adv. Opt. Mater., 2018, 6, 800730 Search PubMed.
- H. Kajii, M. Yoshinaga, T. Karaki, M. Morifuji and M. Kondow, Org. Electron., 2021, 88, 106011 CAS.
- M. Portnoi, T. J. Macdonald, C. Sol, T. S. Robbins, T. Li, J. Schläfer, S. Guldin, I. P. Parkin and I. Papakonstantinou, Nano Energy, 2020, 70, 104507 CAS.
- J. Liu, D. Liu, Y. Shen, X. Yang, C. Zhao, R. Chen, Z. Yang, J. Liu, J. Ma and H. Xiao, Opt. Mater., 2020, 107, 110093 CAS.
- G. C. Righini, J. Krzak, A. Lukowiak, G. Macrelli, S. Varas and M. Ferrari, Opt. Mater., 2021, 115, 111011 CrossRef CAS.
- Z. Wang, C. L. C. Chan, R. M. Parker and S. Vignolini, Angew. Chem., Int. Ed., 2022, 61, e202117275 CrossRef CAS PubMed.
- L. Peponi, D. Puglia, L. Torre, L. Valentini and J. M. Kenny, Mater. Sci. Eng., R, 2014, 85, 1–46 CrossRef.
- D. Huang, M. Zeng, L. Wang, L. Zhang and Z. Cheng, RSC Adv., 2018, 8, 34839–34847 RSC.
- J. Xu, M. Hou, Y. Lu, L. Yang, J. Du, N. Li, H. Tan and L. Xu, React. Funct. Polym., 2022, 173, 105224 CrossRef CAS.
- A. Strang, V. Quirós-Cordero, P. Grégoire, S. Pla, F. Fernández-Lázaro, Á. Sastre-Santos, C. Silva-Acuña, P. N. Stavrinou and N. Stingelin, Adv. Mater., 2024, 36, 2212056 CrossRef CAS PubMed.
- H. Megahd, P. Lova, S. Sardar, C. D'Andrea, A. Lanfranchi, B. Koszarna, M. Patrini, D. T. Gryko and D. Comoretto, ACS Omega, 2022, 7, 15499–15506 CrossRef CAS PubMed.
- H. Megahd, M. Villarreal Brito, A. Lanfranchi, P. Stagnaro, P. Lova and D. Comoretto, Mater. Chem. Front., 2022, 6, 2413–2421 RSC.
- M. Li, X. Lai, C. Li and Y. Song, Mater. Today Nano, 2019, 6, 100039 CrossRef.
- G. Canazza, F. Scotognella, G. Lanzani, S. De Silvestri, M. Zavelani-Rossi and D. Comoretto, Laser Phys. Lett., 2014, 11, 035804 CrossRef CAS.
- L.-T. Shi, F. Jin, M.-L. Zheng, X.-Z. Dong, W.-Q. Chen, Z.-S. Zhao and X.-M. Duan, Phys. Chem. Chem. Phys., 2016, 18, 5306–5315 RSC.
- S. Wu, H. Xia, J. Xu, X. Sun and X. Liu, Adv. Mater., 2018, 30, 1803362 Search PubMed.
- J. J. Shin, E. J. Kim, K. H. Ku, Y. J. Lee, C. J. Hawker and B. J. Kim, ACS Macro Lett., 2020, 9, 306–317 CAS.
- N. Yan, Y. Zhu and W. Jiang, Chem. Commun., 2018, 54, 13183–13195 CAS.
- Y. Liu, F. Ke, Y. Li, Y. Shi, Z. Zhang and Y. Chen, Nano Res., 2023, 16, 564–582 Search PubMed.
- C. Cummins, R. Lundy, J. J. Walsh, V. Ponsinet, G. Fleury and M. A. Morris, Nano Today, 2020, 35, 100936 CAS.
- M. Stefik, S. Guldin, S. Vignolini, U. Wiesner and U. Steiner, Chem. Soc. Rev., 2015, 44, 5076–5091 CAS.
- J. H. Kim, H. M. Jin, G. G. Yang, K. H. Han, T. Yun, J. Y. Shin, S. Jeong and S. O. Kim, Adv. Funct. Mater., 2020, 30, 1902049 CAS.
- C. K. Wong, X. Qiang, A. H. E. Müller and A. H. Gröschel, Prog. Polym. Sci., 2020, 102, 101211 CAS.
- T. J. Giammaria, F. Ferrarese Lupi, G. Seguini, K. Sparnacci, D. Antonioli, V. Gianotti, M. Laus and M. Perego, ACS Appl. Mater. Interfaces, 2017, 9, 31215–31223 CAS.
- G. Seguini, F. Zanenga, M. Laus and M. Perego, Phys. Rev. Mater., 2018, 2, 055605 CAS.
- A. Dodero, K. Djeghdi, V. Bauernfeind, M. Airoldi, B. D. Wilts, C. Weder, U. Steiner and I. Gunkel, Small, 2023, 19, 2205438 CAS.
- U. Steiner and A. Dodero, Chimia, 2022, 76, 826 CAS.
- D.-P. Song, T. H. Zhao, G. Guidetti, S. Vignolini and R. M. Parker, ACS Nano, 2019, 13, 1764–1771 CAS.
- Z. Wang, C. L. C. Chan, T. H. Zhao, R. M. Parker and S. Vignolini, Adv. Opt. Mater., 2021, 9, 2100519 CAS.
- Y. Yang, Y. Chen, Z. Hou, F. Li, M. Xu, Y. Liu, D. Tian, L. Zhang, J. Xu and J. Zhu, ACS Nano, 2020, 14, 16057–16064 CAS.
- Y. Yang, H. Kim, J. Xu, M. Hwang, D. Tian, K. Wang, L. Zhang, Y. Liao, H. Park, G. Yi, X. Xie and J. Zhu, Adv. Mater., 2018, 30, 1707344 Search PubMed.
- Q. He, K. H. Ku, H. Vijayamohanan, B. J. Kim and T. M. Swager, J. Am. Chem. Soc., 2020, 142, 10424–10430 CAS.
- A. Escher, G. Bravetti, S. Bertucci, D. Comoretto, C. Weder, U. Steiner, P. Lova and A. Dodero, ACS Macro Lett., 2024, 1338–1344 CrossRef CAS PubMed.
- B. Sarkar and P. Alexandridis, Prog. Polym. Sci., 2015, 40, 33–62 CrossRef CAS.
- T. N. Hoheisel, K. Hur and U. B. Wiesner, Prog. Polym. Sci., 2015, 40, 3–32 CrossRef CAS.
- Z. Deng and S. Liu, Polymer, 2020, 207, 122914 CrossRef CAS.
- M. Xu, K. H. Ku, Y. J. Lee, J. J. Shin, E. J. Kim, S. G. Jang, H. Yun and B. J. Kim, Chem. Mater., 2020, 32, 7036–7043 CrossRef CAS.
- M. Xu, K. H. Ku, Y. J. Lee, T. Kim, J. J. Shin, E. J. Kim, S.-H. Choi, H. Yun and B. J. Kim, Macromolecules, 2021, 54, 3084–3092 CrossRef CAS.
- P. Rastogi, F. Palazon, M. Prato, F. Di Stasio and R. Krahne, ACS Appl. Mater. Interfaces, 2018, 10, 5665–5672 CrossRef CAS PubMed.
- O. Chen, J. Zhao, V. P. Chauhan, J. Cui, C. Wong, D. K. Harris, H. Wei, H.-S. Han, D. Fukumura, R. K. Jain and M. G. Bawendi, Nat. Mater., 2013, 12, 445–451 CrossRef CAS PubMed.
- J. Zhang, X. Zhang and J. Y. Zhang, J. Phys. Chem. C, 2009, 113, 9512–9515 CrossRef CAS.
- X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59–61 CrossRef CAS PubMed.
- Y. Barak, I. Meir, A. Shapiro, Y. Jang and E. Lifshitz, Adv. Mater., 2018, 30, 1801442 CrossRef PubMed.
- T. Pellegrino, L. Manna, S. Kudera, T. Liedl, D. Koktysh, A. L. Rogach, S. Keller, J. Rädler, G. Natile and W. J. Parak, Nano Lett., 2004, 4, 703–707 CrossRef CAS.
- A. P. Litvin, I. V. Martynenko, F. Purcell-Milton, A. V. Baranov, A. V. Fedorov and Y. K. Gun'ko, J. Mater. Chem. A, 2017, 5, 13252–13275 RSC.
- B. O. Dabbousi, J. Rodriguez-Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463–9475 CAS.
- A. R. C. Osypiw, S. Lee, S.-M. Jung, S. Leoni, P. M. Smowton, B. Hou, J. M. Kim and G. A. J. Amaratunga, Mater. Adv., 2022, 3, 6773–6790 CAS.
- J. L. Casas Espínola and X. A. Hernández Contreras, J. Mater. Sci.: Mater. Electron., 2017, 28, 7132–7138 CrossRef.
- M. Zhang, Y. Hu, Y. Hassan, H. Zhou, K. Moozeh, G. D. Scholes and M. A. Winnik, Soft Matter, 2013, 9, 8887 CAS.
- S. Singh, A. Horechyy, S. Yadav, P. Formanek, R. Hübner, R. K. Srivastava, S. Sapra, A. Fery and B. Nandan, Macromolecules, 2021, 54, 1216–1223 CAS.
- K. H. Ku, M. P. Kim, K. Paek, J. M. Shin, S. Chung, S. G. Jang, W. Chae, G. Yi and B. J. Kim, Small, 2013, 9, 2667–2672 CAS.
- A. Aubret, A. Pillonnet, J. Houel, C. Dujardin and F. Kulzer, Nanoscale, 2016, 8, 2317–2325 CAS.
- S. H. Mohamed and H. M. Ali, J. Appl. Phys., 2011, 109, 012108 Search PubMed.
- V. Bauernfeind, G. Bravetti, A. Dodero and V. V. Vogler-Neuling, Bio-Inspired Photonic Structures for Optical Materials, European Synchrotron Radiation Facility (ESRF), DOI:10.15151/ESRF-ES-1330737234.
- J. Kim, S. Song, Y.-H. Kim and S. K. Park, Small Struct., 2021, 2, 2000024 CrossRef CAS.
- M. Romero, J. R. Sánchez-Valencia, G. Lozano and H. Míguez, Nanoscale, 2023, 15, 15279–15287 RSC.
- C. Pu, X. Dai, Y. Shu, M. Zhu, Y. Deng, Y. Jin and X. Peng, Nat. Commun., 2020, 11, 937 CrossRef CAS PubMed.
- G. Moriceau, C. Kilchoer, K. Djeghdi, C. Weder, U. Steiner, B. D. Wilts and I. Gunkel, Macromol. Rapid Commun., 2021, 42, 2100522 CrossRef CAS PubMed.
- G. S. Doerk, R. Li, M. Fukuto and K. G. Yager, Macromolecules, 2020, 53, 1098–1113 CrossRef CAS.
- T. Ruotsalainen, J. Turku, P. Hiekkataipale, U. Vainio, R. Serimaa, G. ten Brinke, A. Harlin, J. Ruokolainen and O. Ikkala, Soft Matter, 2007, 3, 978 RSC.
- H. Megahd, C. Oldani, S. Radice, A. Lanfranchi, M. Patrini, P. Lova and D. Comoretto, Adv. Opt. Mater., 2021, 9, 2002006 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization data and fitting parameters. See DOI: https://doi.org/10.1039/d5nr00216h |
| This journal is © The Royal Society of Chemistry 2025 |





