Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold nanozymes for efficient degradation of organic dye pollutants: outperforming natural enzymes†
Giulia
Mirra‡
ab,
Lorenzo
Cursi‡
 a,
Marina
Veronesi
a,
Marina
Veronesi
 c,
Luca
Boselli
c,
Luca
Boselli
 a and
Pier Paolo
Pompa
a and
Pier Paolo
Pompa
 *a
*a
aNanobiointeractions&Nanodiagnostics, Istituto Italiano di Tecnologia (IIT), Via Morego 30, Genova 16163, Italy. E-mail: pierpaolo.pompa@iit.it
bDepartment of Chemistry and Industrial Chemistry, University of Genova, Via Dodecaneso 31, Genova 16146, Italy
cStructural Biophysics Facility, Istituto Italiano di Tecnologia (IIT), Via Morego 30, Genova 16163, Italy
First published on 4th February 2025
Abstract
Nanozymes (NZs) are raising increasing interest as effective tools for the degradation of organic pollutants dispersed in the environment. In particular, noble-metal NZs are extremely efficient and versatile, thanks to their multi-enzymatic activities, wide pH operational range, and thermal stability. However, whilst multifunctionality can be a key asset of NZs in some applications (e.g., by intrinsic self-cascade/tandem reactions), the “internal” competition between their different catalytic activities may strongly limit their specific efficiency towards some targets. In this scenario, a deep comprehension of their catalytic mechanisms and careful optimization of the operating conditions are crucial to disclose their full potential and maximize their performances. Here, we analyzed the ability of gold, palladium, and platinum NZs to degrade model organic pollutants of industrial relevance, i.e. rhodamine B, methylene blue, and methyl orange. Interestingly, we found that AuNZ is very efficient in degrading all three dyes via peroxidase-like activity, unlike the natural enzyme (horseradish peroxidase – HRP), which displayed weak degradative capabilities. On the other hand, Pd and PtNZs experience the internal competitive catalase-like reaction, strongly limiting their dye degradation performances. The mechanism underlying AuNZ's ability to degrade the synthetic dyes was investigated, revealing the preferential reactivity with the aromatic structures of the molecules. We also developed a proof-of-concept AuNZ-based dye-degrading filter system, showing excellent dye removal capability and good recyclability, even in real environmental samples.
Introduction
Synthetic dyes are widely employed in several industrial sectors, such as textile, painting, printing, pharmaceutics, cosmetics, and paper.1,2 However, they represent a serious threat when released in the environment, especially into water bodies: even at trace level, they can perturb light penetration, adversely affecting aquatic photosynthesis and consequently lowering oxygen availability for aquatic species.1,2 Furthermore, they are often classified as toxic or carcinogenic, both for humans and aquatic fauna.1,3 Notably, the complex aromatic and conjugated groups in their molecular structure, responsible for their characteristic color, are typically resistant to biodegradation, thus they persist for long times in the environment.4,5The treatment of synthetic dye contaminated waters mainly relies on “non-destructive” methods (adsorption, coagulation/flocculation, and filtration methods) that transfer the color from liquid to solid waste.3,4,6,7 However, these methods can hardly be considered a solution, since the solid waste can still represent a hazard for the environment and requires careful disposal. Advanced oxidizing processes8 such as photocatalysis and electrocatalysis are emerging as possible alternatives. These new technologies allow for mineralization of organic dyes,9–11 yet they are often energetically expensive, due to the necessity of power supply to produce UV light and current.12
Nanozymes (NZs), nanomaterials that mimic the activity of natural enzymes,13,14 have recently been proposed as an alternative tool for synthetic dye degradation.12,15–18 NZs offer wider pH and temperature operational range19,20 and are, in general, more robust than their natural counterparts,21,22 making them appealing in the field of pollutant remediation. Some drawbacks, however, include incomplete comprehension of their multifunctional catalytic behavior along with lack of substrate selectivity, which often limit their applicability. On the other hand, one NZ might potentially remove a plethora of contaminants. Au, Pd, and PtNZs are emerging nanotools that present multi-enzymatic activity,22–24 being able to mimic oxidase- (OX-), peroxidase- (POD-) and catalase-like (CAT-like) activities simultaneously, and they are therefore extremely versatile systems.
As previously reported, these NZs can catalytically oxidize a substrate (such as an organic dye) via OX-like or POD-like activity, thus using O2 or H2O2 as co-substrates, respectively. Alternatively, via CAT-like reaction, these NZs can lead to the disproportionation of H2O2. POD- and CAT-like activities are thus in competition. In both cases, the first step of the process is the NZ reaction with H2O2 forming highly reactive transient species (HRI*) at the surface (including hydroxyl radicals).21,24,25 During the second step, these reactive intermediates can, in one case, mainly react among them, or with another molecule of H2O2, eventually forming O2 and H2O, or instead react with a different substrate, such as a chromogenic one, oxidizing it. The highly oxidative reactions involved in the POD-like activity of NZs can also lead to multiple oxidations and eventually to oxidative degradation of a substrate,3,26 especially because, unlike their natural counterparts, NZs could exploit a remarkably high H2O2 concentration to boost the peroxidation processes.21 Furthermore, the possibility of immobilizing NZs on various supports facilitates their practical reuse, thus enhancing the relative economic feasibility.15,16,27
In this work we investigated the performances of Au, Pd, and PtNZs in the degradation of three widely employed synthetic dyes, i.e. Rhodamine B (RhB), Methylene Blue (MB), and Methyl Orange (MO). While other studies have started to explore the potential of NZs in this context,28–30 this work deepens the processes behind the degradation efficiency of different noble-metal NZs for specific organic dyes, analyzing their mechanisms and the differences with the activity of a natural peroxidase. We found that the multifunctionality and intrinsic competitive reactions of NZs are crucial to define their overall catalytic performance. Notably, the high CAT-like activity of Pt and PdNZs was found to strongly interfere with the process of dye degradation via POD-like activity. In contrast, despite AuNZs typically exhibit lower POD-like activity compared to Pt and PdNZs,24 they demonstrated superior efficiency in organic dye degradation, thanks to the lower interference of competitive CAT-like reaction. Interestingly, the degradation efficiency and versatility achieved by the AuNZ largely outperformed the activity of the natural horse radish peroxidase (HRP) enzyme, which was quite ineffective against the synthetic dyes analyzed herein.
Results and discussion
Au, Pd, and PtNZs are among the most promising NZs in several fields, thanks to their high catalytic efficiency and multiple enzyme-like activities.21,31 They have shown impressive POD-like activity21 that could be exploited in many applications,32–35 including environmental pollution remediation.36,37To best compare their performance in this field, we synthesized and characterized Au, Pd, and PtNZs with similar size (about 4 nm) and used citrate as capping agent to normalize their surface chemistry. The NZs were characterized by TEM and DLS, showing monodisperse and colloidally stable aqueous suspensions (Fig. S1–3†).
We chose RhB as our first target since it is one of the most employed synthetic dyes in industry, it is difficult to biodegrade, and it has been reported to be hazardous and carcinogenic.6 The reaction conditions (NZ and H2O2 concentrations) for RhB degradation via POD-like activity were optimized for each NZ (Fig. S4†). AuNZs resulted the most effective (Fig. 1A): a dispersion of 44 nM can degrade >90% of a concentrated RhB solution (40 μM) in the presence of H2O2.
In contrast, despite similar size and surface chemistry, Pd and PtNZs degraded only about 10% of RhB primarily within the first 10 minutes, after which their activity ceased. Notably, in the reaction solution of Pd and PtNZs, we observed intense and rapid bubble formation (oxygen development), while no bubbles were detectable in the reaction containing AuNZs. Monitoring the O2 production with a sensor in the same reaction conditions (Fig. 1B), we confirmed that oxygen was quickly and intensively produced in the presence of Pd and PtNZs, while almost no oxygen development was detected in the presence of AuNZs. These results clearly indicate that, in these experimental conditions, the strong competitive CAT-like activity rapidly consumed the H2O2 substrate necessary for POD functionality, thus completely inhibiting the POD-mediated degradation of RhB dye by Pd and PtNZs after a few minutes.
This outcome was not specific to RhB degradation. We tested the performance of the NZs against other two organic dyes commonly used in industrial applications, namely MB and MO (Fig. S5†). Similarly, AuNZ turned out to be very effective in the degradation of both dyes (Fig. 1C and D). In the case of MO, the difference in the degradation performance between Au and Pt/Pd was striking. With MB dye, Pt and Pd showed some POD-like activity at early time points (similar to Au), however after 10 min their degradation functionality underwent strong inhibition. Interestingly, the POD-mediated degradations of both MB and MO were consistent with the competitive CAT mechanism mentioned above: Pd and PtNZs were not able to degrade these synthetic dyes because they consumed most of the H2O2via CAT-like side reaction (Fig. S6†). Indeed, replenishing the H2O2 in the reaction solutions with a fresh aliquot, the degradation of the dyes resumed (Fig. S7†). Overall, this suggests that multifunctionality and internal competitive activities of nanozymes can be crucial in the design and development of applications targeting specific substrates.
Considering the superior performance exhibited by AuNZs with all the dyes tested, we focused on this functional material to get a deeper insight into the degradation mechanism, further optimize its performance, and make a direct comparison against a natural peroxidase enzyme (HRP), which can be used as a reference tool in this kind of environmental remediation applications.38–41 Since it is well established that the physical–chemical environment can significantly affect the activity of NZs,21 as a first point we investigated the performance of AuNZs in different pH, temperatures, and buffers. We observed that the process of dyes degradation via POD-like activity of AuNZs is favored under basic conditions (average 40% activity increase with all the 3 dyes with respect to neutral pH, see Fig. 2A), also due to the better colloidal stability of this nanomaterial in alkaline environment (Fig. S8†). Noteworthy, the performance of AuNZs is strongly enhanced (up to 8–9 times) by increasing the temperature from 20 to 70 °C, depending on the dye (Fig. 2B). This enhancement is attributed to the higher POD-like activity of AuNZ rather than to thermal degradation of the dye in these conditions (Fig. S9†). Such catalytic features are important in view of practical applications, since at the optimal pH and temperature it may be possible to achieve very efficient and fast degradation of the synthetic dyes, unlike biological enzymes that are typically unfolded/degraded at mid-high temperatures.21 In this respect, we analyzed the behavior of the natural enzyme (HRP), for direct comparison with the AuNZ in the degradation of the 3 synthetic dyes. Importantly, HRP was able to catalyze some degradative effects on MO, but was completely ineffective with the other two dyes, likely due to the lack of recognition of RhB and MB as enzymatic substrates. This was observed in very different conditions of H2O2 concentration (1–400 mM range); indeed, at the highest concentration, the natural HRP quickly lost its efficiency even with the MO dye (Fig. 2C), because of the harsh conditions, unlike the artificial AuNZ, which therefore exhibited unique performances in terms of versatility and efficiency.
MO solution treated with HRP was not completely decolorized after treatment, probably due to the formation of some colored byproducts, as suggested by the UV-vis spectra (Fig. S10†). Indeed, as analyzed in more detail in the following, the HRP enzyme did not elicit full degradation also in the case of MO, displaying rather a partial oxidation/decoloration of the dye.42–44
We also tested the activity of AuNZs in the presence of different buffers (Fig. S11†). The NZs were fairly stable in the presence of all the buffers, except TRIS (Fig. S12†), but their activity decreased significantly when TRIS, carbonate, or borate buffers were used. On the contrary, we observed no interference from HEPES buffer, which was thus selected as the buffer of choice for the following experiments mimicking real applications (see below).
We tried to investigate the mechanism underlying the AuNZ ability to degrade the synthetic dyes. First, we observed that, when the 3 dyes are simultaneously present in the solution, they are all degraded, though with different efficiency: first MB, then RhB, and MO (Fig. 3). To understand this pattern, we calculated the apparent Michaelis–Menten constants (Km) for the reaction with the different dyes and H2O2 (Fig. S13 and S14†).
The apparent Km represents the affinity of a substrate for the enzyme: the lower the Km the higher the affinity.45 Our results show that the sequence of dye degradation follows the order of the apparent Km, starting from MB (Km = 12 μM) then RhB (Km = 40 μM) and finally MO (Km = 90 μM). Thus, the dyes are degraded in succession, from the one with the highest affinity to the one with the lowest, in an almost selective way.
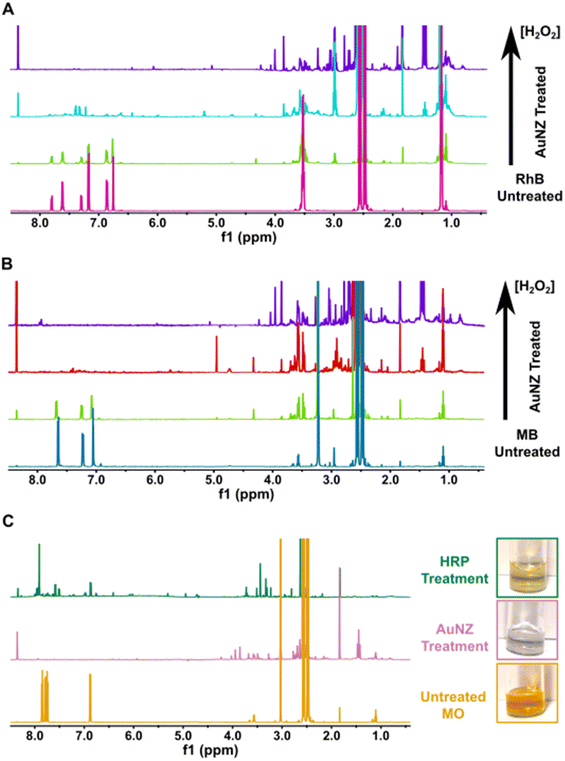
As a further characterization step, we analyzed the AuNZ-based degradation process of the organic dyes by NMR. It has been reported that dye degradation catalyzed by Fenton-like reactions can lead to complete oxidation of the organic molecules to H2O and CO2.4,16,27 We thus analyzed our dye solutions before and after the AuNZ treatment in the presence of increasing concentration of H2O2 (Fig. 4A, B and S15–17†). For all the dyes, the NMR spectra show that the signals of aromatic protons (∼7.0–8.0 ppm range) disappear with increasing H2O2 concentrations, while new signals appear in the aliphatic region (below 4.0 ppm). This suggests that the AuNZs degrade the aromatic system of the synthetic dyes, most likely via Fenton-like reaction (as already reported for other NZs),16,25,27 possibly leading to complete mineralization of the organic molecules. On the contrary, when treating MO with HRP (Fig. 4C and S16B†), the signals of aromatic protons do not disappear completely, although they decrease in intensity and shift. Accordingly, the MO solution does not appear completely clear upon HRP treatment, unlike the one treated by AuNZ (Fig. 4C, right). This confirms that, in these conditions, HRP is not capable of mineralizing the synthetic dye, but it only oxidizes the molecule, probably forming different byproducts. However, due to the complexity of the NMR spectra in the aliphatic region, further investigations are necessary beyond NMR to clearly identify the various degradation products. These results suggest that the AuNZ could be effective also on other emerging pollutants, in particular those with large aromatic groups, including some antibiotics as well as chemotherapeutic and anti-inflammatory drugs.
We finally designed a proof-of-concept filtering system to prove the suitability of the nanozymes to degrade the synthetic dyes in simulated real-world applications. The immobilization of the AuNZs onto a membrane provides an easy and effective way for their integration in a continuous flow system that simplifies both the reuse and the scale-up of the remediation process (Fig. 5A and Fig. S18†). Moreover, it also enables NZ stabilization and optimal activity even in complex media, e.g. at high ionic strength. As reported in Fig. 5B, using 1 mL solution of RhB (10 μM) in presence of H2O2, we observed >90% degradation of the dye by the AuNZs. In these conditions, the optimal degradation process required 3 filtrations steps, due to the short contact time between the sample and the NZs/membrane system (Fig. S19†) (see Methods for details). In view of future on-field applications, the presented “device” could be implemented by using filter chains (or immobilizing the NZ on columns) and/or inserting it in a continuous flow circulating system. Notably, this model system worked properly in terms of recyclability: the same AuNZ-based filter was tested with multiple cycles of dye degradation, and we observed no detectable loss of efficiency after 5 full RhB degradations (Fig. 5B). This is interesting as it confirms the high stability of the NZs together with their tolerance to harsh conditions (see also Fig. 2).
Finally, we tested the performance of the device with real samples. We prepared spiked solutions of RhB using tap, lake, river, and sea water and we compared the degradation performance of the AuNZ-filter versus pure laboratory water (milliQ). The results showed that tap, lake, and river waters do not cause strong interferences with the catalytic performance of the device (Fig. 5C). In the case of sea water, we observed a drop of efficiency with ca. 30% performance; however, the device proved its degradation capability even in such high ionic strength medium and using an unrealistically high dye concentration.
Conclusions
In this work, we explored the behavior of three noble-metal NZs, namely Au, Pd, and PtNZs, for the catalytic degradation of different synthetic dyes of industrial relevance. Our results highlight that a deep understanding of the NZs multi-enzymatic activity is essential to properly exploit their features. Indeed, AuNZ resulted the most efficient, despite showing lower POD-like activity, thanks to a negligible competitive interference from its CAT-like activity. On the contrary, Pt and PdNZs preferentially consumed H2O2 substrate via CAT-like activity, showing minor POD-like degradation activity towards the synthetic dyes. Interestingly, the natural HRP enzyme was found to be mostly ineffective against the organic pollutants. We also investigated the process of dye degradation by NMR, observing a preferential activity on the aromatic structure of the molecules. The reaction conditions were optimized to maximize the performance of the AuNZ, and a proof-of-concept dye-degrading filter system was implemented allowing for recovery and reuse of the nanocatalyst. The system showed excellent performance, being able to degrade more than 90% of the dye in contaminated solutions and excellent robustness with simulated real samples (contaminated tap, river, lake, and sea waters). These results may contribute to disclosing the potential of nanozymes for environmental remediation and sustainability applications and may encourage future studies with other classes of pollutants and multifunctional systems.Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is part of the “Technologies for Sustainability” Flagship program of the Italian Institute of Technology (IIT).References
- A. Tkaczyk, K. Mitrowska and A. Posyniak, Sci. Total Environ., 2020, 717, 137222 CrossRef CAS PubMed.
- A. K. D. Alsukaibi, Processes, 2022, 10, 1968 CrossRef CAS.
- C. Zaharia and D. Suteu, in Organic Pollutants Ten Years After the Stockholm Convention, ed. P. Tomasz and M.-S. Aleksandra, IntechOpen, Rijeka, 2012, ch. 3, DOI:10.5772/32373.
- M. C. Collivignarelli, A. Abbà, M. Carnevale Miino and S. Damiani, J. Environ. Manage., 2019, 236, 727–745 CrossRef CAS PubMed.
- S. M. Ghoreishi and R. Haghighi, Chem. Eng. J., 2003, 95, 163–169 CrossRef CAS.
- A. V. Mohod, M. Momotko, N. S. Shah, M. Marchel, M. Imran, L. Kong and G. Boczkaj, Water Resour. Ind., 2023, 30, 100220 CrossRef CAS.
- F. McYotto, Q. Wei, D. K. Macharia, M. Huang, C. Shen and C. W. K. Chow, Chem. Eng. J., 2021, 405, 126674 CrossRef CAS.
- J. Mukherjee, B. K. Lodh, R. Sharma, N. Mahata, M. P. Shah, S. Mandal, S. Ghanta and B. Bhunia, Chemosphere, 2023, 345, 140473 CrossRef CAS PubMed.
- F. Costantino, A. Armirotti, R. Carzino, L. Gavioli, A. Athanassiou and D. Fragouli, J. Photochem. Photobiol., A, 2020, 398, 112599 CrossRef CAS.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
- A. A. Özcan and A. Özcan, Chemosphere, 2018, 202, 618–625 CrossRef PubMed.
- Q. Feng, G. Wang, L. Xue, Y. Wang, M. Liu, J. Liu, S. Zhang and W. Hu, ACS Appl. Nano Mater., 2023, 6, 4844–4853 CrossRef CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- M. Liang and X. Yan, Acc. Chem. Res., 2019, 52, 2190–2200 CrossRef CAS PubMed.
- R. Dadigala, R. Bandi, M. Alle, C.-W. Park, S.-Y. Han, G.-J. Kwon and S.-H. Lee, J. Hazard. Mater., 2022, 436, 129165 CrossRef CAS PubMed.
- L. Wang, L. Zhao, D. Si, Z. Li, H. An, H. Ye, Q. Xin, H. Li and Y. Zhang, Sep. Purif. Technol., 2024, 331, 125571 CrossRef CAS.
- M. J. Jacinto, R. S. Souto, V. C. P. Silva, I. C. Prescilio, A. C. Kauffmann, M. A. Soares, J. R. de Souza, A. F. Bakuzis and L. C. Fontana, Water, Air, Soil Pollut., 2021, 232, 270 CrossRef CAS.
- Q. Diao, X. Chen, Z. Tang, S. Li, Q. Tian, Z. Bu, H. Liu, J. Liu and X. Niu, Environ. Sci.:Nano, 2024, 11, 766–796 RSC.
- A. Shamsabadi, T. Haghighi, S. Carvalho, L. C. Frenette and M. M. Stevens, Adv. Mater., 2024, 36, 2300184 CrossRef CAS PubMed.
- L. Gao, H. Wei, S. Dong and X. Yan, Adv. Mater., 2024, 36, 2305249 CrossRef CAS PubMed.
- L. Cursi, G. Mirra, L. Boselli and P. P. Pompa, Adv. Funct. Mater., 2024, 34, 2315587 CrossRef CAS.
- J. Sheng, Y. Wu, H. Ding, K. Feng, Y. Shen, Y. Zhang and N. Gu, Adv. Mater., 2024, 36, 2211210 CrossRef CAS PubMed.
- M. Zandieh and J. Liu, Adv. Mater., 2024, 36, 2211041 CrossRef CAS PubMed.
- G. Mirra, L. Cursi, L. Boselli and P. P. Pompa, Small Sci., 2024, 2400487 Search PubMed.
- N. Li, R. Li, Z. Li and X. Liu, New J. Chem., 2024, 48, 11407–11419 RSC.
- Y. Deng and R. Zhao, Curr. Pollut. Rep., 2015, 1, 167–176 CrossRef CAS.
- T. Yang, X. Liu, Z. Zeng, X. Wang, P. Zhang, B. Feng, K. Tian and T. Qing, Environ. Pollut., 2023, 316, 120643 CrossRef CAS PubMed.
- Z. Gao, H. Wu, Z. Liu, L. Liu, Z. Zeng, X. Yang, H. Wang, J. Du, B. Zheng and Y. Guo, ACS Appl. Nano Mater., 2023, 6, 11531–11540 CrossRef CAS.
- X. Zhao, T. Yang, D. Wang, N. Zhang, H. Yang, X. Jing, R. Niu, Z. Yang, Y. Xie and L. Meng, Anal. Chem., 2022, 94, 4484–4494 CrossRef CAS PubMed.
- Q. Li, D. Yu, C. Fan, Q. Huang, Y. Tang, R. Guo, Y. Huang, H. Wang, C. Lin and Y. Lin, ACS Appl. Nano Mater., 2022, 5, 94–100 CrossRef CAS.
- Y. Ai, Z.-N. Hu, X. Liang, H.-B. Sun, H. Xin and Q. Liang, Adv. Funct. Mater., 2022, 32, 2110432 CrossRef CAS.
- E. De Luca, D. Pedone, A. Scarsi, R. Marotta, F. Catalano, D. Debellis, L. Cursi, B. Grimaldi, M. Moglianetti and P. P. Pompa, Small Sci., 2024, 4, 2400085 CrossRef CAS.
- G. Tarricone, V. Castagnola, V. Mastronardi, L. Cursi, D. Debellis, D. Z. Ciobanu, A. Armirotti, F. Benfenati, L. Boselli and P. P. Pompa, Nano Lett., 2023, 23, 4660–4668 CrossRef CAS PubMed.
- A. Scarsi, D. Pedone and P. P. Pompa, Nanoscale Adv., 2023, 5, 2167–2174 RSC.
- T. Pomili, P. Donati and P. P. Pompa, Biosensors, 2021, 11, 2777–2809 CrossRef PubMed.
- G. Fang, R. Kang, S. Cai and C. Ge, Nano Today, 2023, 48, 101755 CrossRef.
- Y. Meng, W. Li, X. Pan and G. M. Gadd, Environ. Sci.:Nano, 2020, 7, 1305–1318 RSC.
- N. Elmerhi, K. Al-Maqdi, K. Athamneh, A. K. Mohammed, T. Skorjanc, F. Gándara, J. Raya, S. Pascal, O. Siri, A. Trabolsi, I. Shah, D. Shetty and S. S. Ashraf, J. Hazard. Mater., 2023, 459, 132261 CrossRef CAS PubMed.
- M. Bilal, H. M. N. Iqbal, H. Hu, W. Wang and X. Zhang, J. Environ. Manage., 2017, 188, 137–143 CrossRef CAS PubMed.
- S. M. A. Guelli Ulson de Souza, E. Forgiarini and A. A. Ulson de Souza, J. Hazard. Mater., 2007, 147, 1073–1078 CrossRef PubMed.
- S. V. Mohan, K. K. Prasad, N. C. Rao and P. N. Sarma, Chemosphere, 2005, 58, 1097–1105 CrossRef CAS PubMed.
- T. S. Shaffiqu, J. J. Roy, R. A. Nair and T. E. Abraham, Appl. Biochem. Biotechnol., 2002, 102, 315–326 CrossRef PubMed.
- M. Ambatkar and U. Mukundan, Appl. Water Sci., 2015, 5, 397–406 CrossRef CAS.
- V. S. Ferreira-Leitão, J. G. da Silva and E. P. S. Bon, Appl. Catal., B, 2003, 42, 213–221 CrossRef.
- P. K. Robinson, Essays Biochem., 2015, 59, 1–41 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr05137h |
| ‡ Equally contributing authors. |
| This journal is © The Royal Society of Chemistry 2025 |