Recent advances in flexible multifunctional electrochromic devices
Jiamin
Yu
a,
Shanjie
Wang
a,
Lin
Gao
b,
Guoqi
Qiao
a,
Meng-Fang
Lin
 c,
Cong
Wei
c,
Cong
Wei
 a,
Jingwei
Chen
a,
Jingwei
Chen
 *d and
Shaohui
Li
*d and
Shaohui
Li
 *a
*a
aSchool of Materials Science and Engineering, Zhengzhou University, Zhengzhou, 450001, P. R. China. E-mail: Shaohuili@zzu.edu.cn
bHubei Key Laboratory of Energy Storage and Power Battery, School of Mathematics, Physics and Optoelectronic Engineering, Hubei University of Automotive Technology, Shiyan 442002, P. R. China
cDepartment of Materials Engineering, Ming Chi University of Technology, New Taipei City 24301, Taiwan
dSchool of Materials Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China. E-mail: chenjingwei@ouc.edu.cn
First published on 26th November 2024
Abstract
Electrochromism refers to the phenomenon in which certain materials undergo a redox reaction under an applied voltage or current, resulting in reversible changes in their optical properties and color appearance. Electrochromic devices (ECDs) show great potential in smart windows, anti-glare rear-view mirrors and displays due to the advantages of low energy consumption and simple control mechanisms. However, traditional ECDs are unfavorable for wearable and deformable optoelectronics due to the structural rigidity and limited functions. Thus, flexible ECDs integrating visual information with other advanced technologies can realize multifunctionality and further expand their application fields. This review first introduces the structure and recent development of flexible ECDs, followed by comprehensively summarizing the recent development of flexible multifunctional ECDs, including energy harvesting, energy storage, multicolor displays, and smart windows. Finally, the challenges, development trends and future prospects in flexible multifunctional ECDs are proposed and discussed. We hope that this review can guide and accelerate the development of flexible multifunctional ECDs in the new era of smart optoelectronics.
1 Introduction
In nature, chameleons display different colors and patterns by controlling and regulating the activity of pigment cells on the skin.1 In the physical world, chromism is the result of alterations in the various structural parameters of the material in response to heat, applied potential, light, solvent/vapor, etc.2 To date, several types of chromism phenomenon have been discovered and studied, including thermochromism,3 electrochromism,4 photochromism5 and solvetochromism.2 Among these, electrochromism refers to the phenomenon of reversible changes in optical properties (absorbance/transmittance/reflectance) of materials/devices through redox reactions under small applied voltages or currents, which are then macroscopically manifested as a change in the color of the material.6–8In the early 1960s, Platt first proposed the concept of “electrochromism” and defined it as “a possible change of color producible in dyes by an electric field”.9 In the late 1960s, Deb first prepared ECDs using amorphous WO3 films and proposed the theory of “oxygen vacancies color centers”.10 Up to the 1980s, the concept of “smart windows” initiated a new era of research on the application of electrochromic technology.11 At this point, ECDs gradually began to form industrialized applications. In recent years, most of the ECDs are rigid due to the use of conductive glass substrates, which inevitably makes the devices rigid, bulky and heavy.12,13 Therefore, the limitations of rigid ECDs limit the realization of a multitude of potential applications. To further expand the practical applications of ECDs, researchers have improved the flexibility of the devices in terms of substrate, electrolyte, and device structure to realize applications in the emerging field of wearable and deformable electronics.14
In addition, to further achieve the wide adoption of EC technology in the new era of the internet of things (IoT), researchers’ interest has shifted in recent years to integrate EC technology with other advanced technologies to achieve multi-functionalities, which can promote and expand its potential applications.15–17 For example, in order to address the issue of an external power supply to the device, EC systems are integrated with energy harvesting devices, including solar cells, nanogenerators, and galvanic cells, to realize self-powered ECDs, which can greatly improve the independence and portability of the ECDs. Due to the analogous structural features and reaction mechanisms of EC and electrochemical materials, electrochromic energy storage devices (ECESDs) can also be designed to achieve both optical–thermal modulation and energy storage/release upon demand.18,19 In particular, by choosing a suitable flexible substrate, the ECDs can be constructed into fabric or fiber-shaped devices, which can minimize the size and provide suitability for wearable or deformable electronic applications. Although these interests in EC systems have gained considerable attention and the relevant publications and citations are rapidly increasing with time,18,20,21 a comprehensive and insightful summary on flexible multifunctional ECDs is still lacking. With the significant achievements made in transparent substrates, transparent conductors, EC materials, electrolytes, and device structures on flexible multifunctional ECDs, a timely and systematic summary is urgently needed.
Herein, in this review, we summarize and discuss the recent advances in flexible multifunctional ECDs, including flexible self-powered ECDs, flexible ESECDs, flexible multicolor displays, and flexible EC smart windows. In addition, the device configurations, design principles, integration mechanisms, material selection and performance optimization for flexible multifunctional ECDs are highlighted. Finally, the challenges and future development trends of flexible multifunctional ECDs are outlooked.
2 Configuration of flexible ECDs
As shown in Fig. 1, conventional ECDs have a sandwich symmetric structure, which includes a transparent substrate, transparent conductor, EC layer, electrolyte layer, ion storage layer, another transparent conductor, and a substrate layer.18,22,23 The configuration of flexible ECDs is similar to that of rigid ECDs. The only difference between the two is that flexible ECDs use flexible and mechanically robust substrates to replace the “rigid” substrates. By infusing ECDs with mechanical properties such as flexibility, stretchability and deformability, various new high-end applications, such as adaptive camouflage, biomimicry, wearable displays, and color-changing cloth, can be achieved.24 Although the structural differences between these two types of ECD are small, flexible ECDs still face numerous challenges that need to be overcome, including poor substrate flexibility, performance degradation, accompanying deformation, and electrolyte leakage during mechanical strain/stress processes.25 In addition to the conventional ECDs, which focus on ion intercalation/deintercalation as shown in Fig. 1, reversible metal electrodeposition-type electrochromic devices (RMEECDs) have also been widely explored in recent years.18 RMEECDs, which are based on reversible electrodeposition and dissolution of metals, have a simpler layer structure and a wider range of optical states (transparent, colored and mirrored states).26,272.1 Soft/flexible substrate
The substrate is the outermost layer of the ECDs and mainly plays the role of supporting the whole device to ensure a high mechanical strength. Compared with rigid substrates, flexible substrates offer the advantages of flexibility, light weight and low cost, ease of handling, and suitability for mass production, which allows flexible ECDs to maintain their EC performance under bending, stretching, or twisting, and expands the range of applications.28,29 Common flexible substrate materials include poly(ethylene) (PE), polyethyleneimine (PEI), polyimide (PI), polyethylene naphthalate (PEN), poly(ethylene terephthalate) (PET), and poly(dimethylsiloxane) (PDMS).30–35 Among these substrates, PET is the most widely reported substrate due to its colorlessness, chemical inertness, and excellent mechanical properties. Especially, PET also displays excellent thermal stability, which allows the facile preparation of PET-based transparent conductors under mild temperatures.25 Unfortunately, PET is non-biodegradable in nature, which easily causes environmental pollution problems. In recent years, researchers have identified and demonstrated the advantages of nanocellulose over commonly used plastic substrates due to its high mechanical strength, tunable optical transmittance, renewability, biodegradability and environmental friendliness.33,36,37 The fabricated ECDs also exhibit excellent flexibility, bendability and even foldability. However, to date, there is still a lack of an efficient way to fabricate transparent nanocellulose substrates on a grid-scale, which further limits the widespread application in EC systems.2.2 Transparent conductor
The transparent conductor as the connection between the device and the external power supply can reduce the electron transfer resistance and enhance the charge transfer, which is required to have the advantages of good light transmittance, low resistivity, and good electrochemical stability.23,24 Indium tin oxide (ITO) and fluorine-doped tin oxide (FTO) are the most commonly used transparent conductors in conventional ECD manufacturing due to their superior optical and electrical properties. The ITO or FTO layers are usually deposited on ultrathin silica-boron glass using vacuum evaporation, magnetron sputtering, chemical vapor deposition (CVD), or sol–gel methods.38 In order to satisfy application in flexible electronics, ITO can also be deposited on a PET or PEN substrate instead of rigid glass and this has been commercialized in the market.39 For example, Li et al.40 deposited W18O49 nanowires on a commercial ITO/PET transparent conductor as an EC electrode, and the fabricated ECDs display good mechanical flexibility. However, the inherent brittleness and poor adhesion of ITO makes it unfavorable in flexible and deformable ECDs, thus necessitating the development of more suitable flexible transparent conductors.41 In recent years, a variety of flexible transparent conductors have been investigated, including conducting polymers,42,43 carbon nanotubes (CNTs),44,45 graphene,46,47 MXene,48,49 metal nanowires,50 and metal grids,51 which can replace the traditional ITO/FTO and greatly promote the development of flexible ECDs. For example, Cai et al.52 prepared a stretchable transmission ECD by inkjet printing WO3 nanoparticles on an elastomeric transparent conductor based on silver nanowires (AgNWs). The device displayed excellent EC performance and can be stretched up to 50%, showing promise for wearable and deformable electronics.2.3 EC layer
The EC layer is the fundamental component for the flexible ECDs, and can change color under the action of an electric field. The EC layer is composed of EC materials and great efforts have been devoted to designing different EC materials during the past decades. Currently, the EC materials can be divided into inorganic materials (e.g., WO3,53,54 MoO3,55 TiO2,56,57 NiO,58,59 MnO2,60 V2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61,62), organic materials (e.g., polyaniline (PANI),63 polypyrrole (PPy),64 poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS),65 viologen66), and inorganic–organic hybrid materials20,21 (e.g., Ni(OH)2/PEDOT, PANI/WO2.7, MoO3−x@PANI, WO3/PEDOT:PSS).67–69 Inorganic materials usually have the advantages of good stability, high reliability and a wide operating temperature range, but suffer from monotonous colors, slow switching speed and high manufacturing costs.70,71 In contrast, organic materials can offer fast response times, rich colors, high processability, light weight, and natural flexibility, which makes them very suitable for fabricated flexible ECDs, but they usually have inferior heat resistance, durability, and chemical stability.72–74 Recently, coupling inorganic and organic materials together to construct flexible ECDs has attracted growing interest due to the synergistic effect. For instance, E. Eren et al.69 prepared a PANI and WO3 composite EC electrode and fabricated a flexible ECD, exhibiting a large optical modulation (38.7%), rapid coloration time (6.4 s), and excellent mechanical stability (only 7.5% optical contrast degradation after 100 bending cycles). The development of EC composite materials has shown great potential and may open new possibilities for flexible ECDs.
61,62), organic materials (e.g., polyaniline (PANI),63 polypyrrole (PPy),64 poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS),65 viologen66), and inorganic–organic hybrid materials20,21 (e.g., Ni(OH)2/PEDOT, PANI/WO2.7, MoO3−x@PANI, WO3/PEDOT:PSS).67–69 Inorganic materials usually have the advantages of good stability, high reliability and a wide operating temperature range, but suffer from monotonous colors, slow switching speed and high manufacturing costs.70,71 In contrast, organic materials can offer fast response times, rich colors, high processability, light weight, and natural flexibility, which makes them very suitable for fabricated flexible ECDs, but they usually have inferior heat resistance, durability, and chemical stability.72–74 Recently, coupling inorganic and organic materials together to construct flexible ECDs has attracted growing interest due to the synergistic effect. For instance, E. Eren et al.69 prepared a PANI and WO3 composite EC electrode and fabricated a flexible ECD, exhibiting a large optical modulation (38.7%), rapid coloration time (6.4 s), and excellent mechanical stability (only 7.5% optical contrast degradation after 100 bending cycles). The development of EC composite materials has shown great potential and may open new possibilities for flexible ECDs.
2.4 Electrolyte layer and ion storage layer
The electrolyte layer, also known as the ion conductive layer, supplies the compensation ions required by the EC material and prevents short circuits between the two electrodes.75 The ionic conductivity and temperature tolerance of the electrolyte can directly affect the performance of ECDs, including switching time, durability, and operating temperature range. The electrolyte can be classified into three categories based on its physical state: liquid, solid, and gel (semi-solid).76 In addition, the electrolyte must have suitable ion conductivity, good optical transmission, high thermal stability, and safety.77The ion storage layer, also known as the counter-electrode layer, serves the function of maintaining the charge balance and ion storage during electrochemical reactions, exemplified by NiO and TiO2.22 In addition, to enhance the optical modulation and coloration efficiency (CE) of ECDs, a complementary EC layer can also be used as an ion storage layer and exhibited a color change when the devices are colored. For the multivalent ion-based ECDs, the metal anodes can directly work as the ion storage layer via reversible metal plating/stripping kinetics.78 Taking the new type of zinc-based ECDs as an example, the zinc plays the role of a counter-electrode to balance the charge.
3 Recent progress of flexible multifunctional ECDs
3.1 Self-powered flexible multifunctional ECDs
Although ECDs have the advantages of a low driving voltage and small current, they still need an external energy supply to achieve the chromism function, which significantly affects the independence and portability of ECDs.79,80 To address these problems, the concept of self-powered ECDs is proposed based on the integration of ECDs and energy harvesting technology. This integrated technology enables the ECDs to obtain the necessary energy (e.g., solar, mechanical, chemical) from its operating environment and convert it into electrical energy, driving the ECDs to realize the color-changing function. | ||
| Fig. 2 (a) Schematic illustration of the PECD; (b–e) characterization of a self-powered EC system based on Ag@Au NWs. Reproduced with permission.80 Copyright 2022 Elsevier. | ||
 | ||
| Fig. 3 (a) Schematic illustration of the PENG-driven tactile display; (b) the color variance under different applied voltages. Reproduced with permission.82 Copyright 2022 Elsevier Ltd. (c and d) Schematic illustration and characterization of the self-powered flexible EC galvanic cell. Reproduced with permission.85 Copyright 2021 American Chemical Society. | ||
3.2 Flexible electrochromic energy storage
Energy storage devices (ESDs) mainly include supercapacitors and batteries.98 Importantly, ECDs share similar characteristics to ESDs in terms of material type, device configuration, and reaction mechanism. Therefore, it is possible to achieve a multifunctional integration of an EC and energy storage device in a single cell, which allows for both energy recovery in the process of color change and monitoring the level of stored energy in the device through the color change.20,99 In EC energy storage devices (ECESDs), advanced flexible devices possess better functionality than traditional rigid glass-based devices and can be integrated with a curved surface, showing potential applications in smart windows, smart wearables, low-energy electronics, and renewable energy sources.100 However, in flexible ECESDs, dissociation, degradation, and delamination occur at the electrodes/electrolytes interface, which seriously affect their performance, and become key issues that need to be solved before commercialization.21 In this section, we summarize the recent progress on flexible EC supercapacitors (ECSCs) and flexible EC batteries (ECBs).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles. Similarly, Zhang et al.108 coat the AgNW micromesh with a thin Au layer. Cai et al.109 protected the Ag grid/PET transparent conductor with a PEDOT:PSS layer. These strategies greatly improve the chemical stability of the Ag-based transparent conductor in acidic and alkaline conditions with robust mechanical stability. However, the poor electronic conductivity and limited specific capacity of WO3 hinder its further applications in ECSCs and in energy-saving fields. As an alternative, Hassan et al.110 prepared a multilayer flexible ECSC electrode by depositing W18O49 NW/Ti3C2Tx composite onto a pre-assembled Ag and W18O49 NW-based transparent conductive network. The obtained electrode can display a high areal capacitance of 125 mF cm−2 and remarkable optical modulation of 98.2%. The as-constructed bifunctional symmetric ECSC device delivered excellent SC properties (energy density of 10.26 μW h cm−2 and power density of 0.6 mW cm−2) and EC performance (fast switching time of 5/7 s (coloring/bleaching) and high CE of 116 cm2 C−1). Similarly, MoO3,111 V2O5,112 Nb2O5,113 Nb18W16O93,114 and NiO115etc. have also been investigated for the design of flexible ECSCs and great progress has been made, which significantly promotes the development of flexible ECSCs.
000 bending cycles. Similarly, Zhang et al.108 coat the AgNW micromesh with a thin Au layer. Cai et al.109 protected the Ag grid/PET transparent conductor with a PEDOT:PSS layer. These strategies greatly improve the chemical stability of the Ag-based transparent conductor in acidic and alkaline conditions with robust mechanical stability. However, the poor electronic conductivity and limited specific capacity of WO3 hinder its further applications in ECSCs and in energy-saving fields. As an alternative, Hassan et al.110 prepared a multilayer flexible ECSC electrode by depositing W18O49 NW/Ti3C2Tx composite onto a pre-assembled Ag and W18O49 NW-based transparent conductive network. The obtained electrode can display a high areal capacitance of 125 mF cm−2 and remarkable optical modulation of 98.2%. The as-constructed bifunctional symmetric ECSC device delivered excellent SC properties (energy density of 10.26 μW h cm−2 and power density of 0.6 mW cm−2) and EC performance (fast switching time of 5/7 s (coloring/bleaching) and high CE of 116 cm2 C−1). Similarly, MoO3,111 V2O5,112 Nb2O5,113 Nb18W16O93,114 and NiO115etc. have also been investigated for the design of flexible ECSCs and great progress has been made, which significantly promotes the development of flexible ECSCs.
 | ||
| Fig. 4 (a) Transmittance spectra of the Ag NW/WO3 electrode; (b) areal capacitance as a function of scan rate (the digital images of the flexible ECSC electrode); (c) optical density versus charge density of the Ag NW/WO3 electrode. Reproduced with permission.99 Copyright 2016 Royal Society of Chemistry. (d) The colored state of the flexible PANI film at the different potentials. Reproduced with permission.107 Copyright 2018 Elsevier B.V. (e) Schematic of flexible electrochromic-ZIBs; (f) the average CE of BCD-PI and DFD-PI; (g) cycling performance at a current density of 8 A g−1. Reproduced with permission.117 Copyright 2023 Wiley-VCH GmbH. (h) Schematic of the structure of a flexible ECB. (i) Photos of ECB in bleached and colored states. Reproduced with permission.118 Copyright 2020 Elsevier B.V. | ||
Due to the instinctive brittleness of metal oxides, the EC and energy storage performance of the fabricated flexible ECESDs were easily degraded by the cracks formed during the repeated bending process.116 In contrast, organic materials have the merits of flexibility, lightness, and compatibility, which find various applications in optoelectronics. For example, Zhang et al.117 prepared a flexible nanoporous 3D conjugated polymer network-based ECSC electrode by electropolymerizing triphenylamine (TPA) and 3,4-ethylenedioxythiophene (EDOT). The resulting electrode displayed a high specific capacitance (137 F g−1, 1 A g−1), excellent cycling stability (91.1% capacitance retention after 6000 cycles), and reliable EC behavior (optical modulation of 33% and coloration efficiency of 136 cm2 C−1 at 450 nm). The assembled flexible ECSC device also demonstrated excellent mechanical stability and EC performance with a multicolor change from blue-green to violet, manifesting the visual monitoring of energy storage. Similarly, Zhou et al.116 prepared a flexible PANI-based ECSC film on an ITO/PET substrate by galvanostatic and cyclic voltammetric electrodeposition. The obtained PANI electrode showed a reversible multicolor change between transparent, pale yellow, green, blue and blue-purple (Fig. 4d), a high coloration efficiency of 80.9 cm2 C−1 at 630 nm and highest specific capacitance of 473.3 F g−1 at a scan rate of 30 mV s−1, demonstrating its promising applications in flexible and multicolor electronics and optoelectronic devices.
3.3 Flexible multicolor displays
Multicolor ECDs (M-ECDs) are devices that are able to change between multiple colors based on the redox reaction of EC materials by adjusting their transmittance/reflectance in the visible light range.131 Compared with mature display technologies that have been commercialized, such as liquid crystal displays (LCD) and organic light-emitting diodes (OLED), M-ECDs have the advantages of high transparency, high energy efficiency, high contrast ratio, low energy consumption, fast response time, easy observation, optical memory effect, and eye-friendliness.74,80,132 Most of the current EC materials have good compatibility with a wide range of substrates, including glass, PET, fibers, and so on.80 These advantages enable applications in smart windows, displays, and flexible and wearable electronics.131 Generally, the M-ECDs can be classified into three categories: intrinsic chemical multicolored EC materials, color overlays from special architecture designs, and photonic crystal structure induced multicolors. | ||
| Fig. 5 (a) Reflectivity changes of the device in different states; (b) in situ reflectance change of the device; (c) device color modulation window. Reproduced with permission.126 Copyright 2022 American Chemical Society. (d) Illustration of a flexible ZIEB. Reproduced with permission.127 Copyright 2024 Wiley-VCH GmbH. (e) Photographs of poly(PEP) film with an applied voltage from −0.6 V to 0.5 V. Reproduced with permission.129 Copyright 2020 Elsevier B.V. (f) Structural images of the EC fibers; (g) digital photographs of the EC textile for large-area embroidery. Reproduced with permission.130 Copyright 2020 American Chemical Society. | ||
As well as transition metal oxides, organic EC materials have also been employed for M-ECDs due to their lack of expense, rich colors, faster response speed, and easy preparation. As a typical organic EC material, PANI can reversibly switch the color between green, yellow and blue. For example, Huang et al.139 reported PANI-based flexible and patterned ECDs by an inkjet-printing method. The ink was formulated with two-dimensional (2D) PANI in formic acid, which combines the nanoscale thickness and appropriate doping ratio, endowing the printed PANI electrodes with an excellent EC performance. The as-made EC electrode displays a high optical contrast (76% at 750 nm), fast coloration/bleaching response time (1.8/2.4 s), and superior CE (259.1 cm2 C−1). However, this color change still cannot meet the demand for a full spectral range of multicolor flexible ECDs. To solve this problem, Zhang et al.140 reported an EC polymer based on 5,7-bis(3,3-dimethyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepin-6-yl)-2,3-dihydrothieno[3,4-b][1,4]dioxine (PEP). PEP combines several distinct polymers, and can reversibly tune a multicolor range. When the applied potential was gradually increased from −0.6 V to 0.5 V, the polymer can change color between purple, red, orange, yellow-green, green and blue (Fig. 5e). Unfortunately, typical organic EC materials can only switch from the natural color to the oxidized transparent state. Therefore, it is extremely complex to realize a multicolor range by compositing several different organic EC materials.
Furthermore, apart from the planar flexible M-ECDs, fiber-shaped or fabric-shaped M-ECDs are also a research hotspot and have developed rapidly in recent years due to the potential applications in wearable, smart display, military camouflage, and anti-counterfeiting technologies. Recently, a continuous, hundreds of meters long EC fiber suitable for industrial weaving was developed by Fan et al.141 By introducing different EC materials and structural designs, multicolor changes can be achieved, including blue, magenta, green, and dull red. After introducing an outer polymer protective layer and electrochemical anticorrosive layer, the electrochemical, mechanical, washing, and thermal stabilities of as-produced fiber-shaped M-ECDs were greatly improved, making them wearable over large areas and implantable into smart color-changing textiles with complex patterns (Fig. 5f and g). Gao et al.142 constructed M-EC fabrics by using PET fabric, PEDOT:PSS/dimethyl sulfoxide (DMSO), and ionic liquid as the substrate, conductive EC layer and electrolyte, respectively. The M-EC fabrics display excellent EC behavior with reversible color variations between light blue and dark blue. After coating with commercial hydrophobic agents, the M-EC fabrics can exhibit impressive hydrophobicity and self-cleaning properties, showing potential applications in adaptive camouflage and wearable displays.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cyclic voltammetry cycles at a wide working voltage from −2 to 2.5 V (Fig. 6c–e). Xue et al.145 proposed a flexible M-ECD by sandwiching an ionic gel electrolyte between two cathodic nickel hexacyanoferrate (NiHCF) and PB EC layers. Owing to the color overlay effect by two EC electrodes, the device displays four colors of green, blue, yellow and colorless. When introducing an Al wire as the anode, the device can be formed into a self-powered flexible M-ECD system, which can change color without an external power supply. Similarly, Zhang et al.146 also demonstrated a self-powered M-ECD system by sandwiching a zinc anode between sodium vanadium oxide (SVO) and WO3 electrodes, which exhibited a full color tunability and displayed 16 different colors, showing great practical application of Zn anode-based M-ECDs.
000 cyclic voltammetry cycles at a wide working voltage from −2 to 2.5 V (Fig. 6c–e). Xue et al.145 proposed a flexible M-ECD by sandwiching an ionic gel electrolyte between two cathodic nickel hexacyanoferrate (NiHCF) and PB EC layers. Owing to the color overlay effect by two EC electrodes, the device displays four colors of green, blue, yellow and colorless. When introducing an Al wire as the anode, the device can be formed into a self-powered flexible M-ECD system, which can change color without an external power supply. Similarly, Zhang et al.146 also demonstrated a self-powered M-ECD system by sandwiching a zinc anode between sodium vanadium oxide (SVO) and WO3 electrodes, which exhibited a full color tunability and displayed 16 different colors, showing great practical application of Zn anode-based M-ECDs.
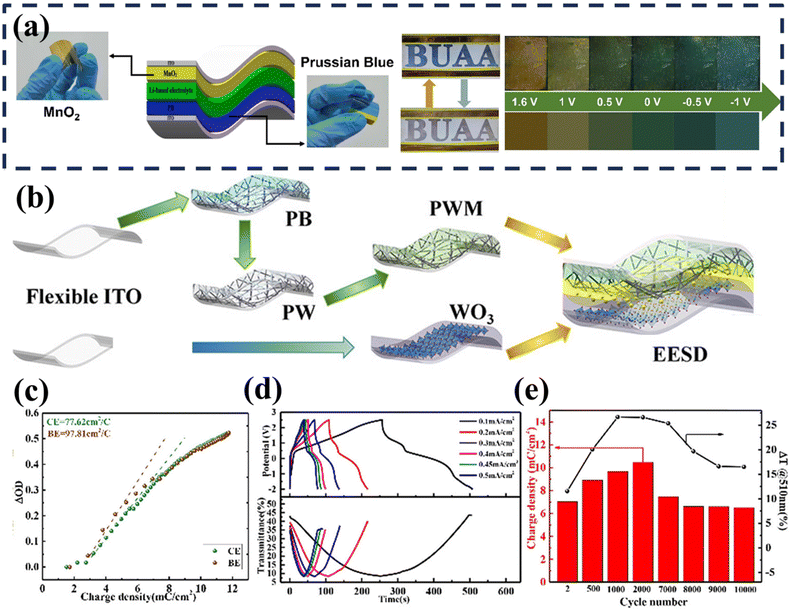 | ||
| Fig. 6 (a) Schematic diagram of the flexible M-ECD and real photos (up) and emulation images (down) at different voltages. Reproduced with permission.133 Copyright 2023 American Chemical Society. Schematic illustration (b) and relevant characterizations (c–e) of the present EESD. Reproduced with permission. Copyright 2022 American Chemical Society. | ||
In addition to constructing M-ECDs by a multilayered structure of different EC materials, introducing functional ionic gel electrolytes by coupling redox-active species (e.g., electrochemiluminescent (ECL) luminophores, EC dyes) instead of conventional electrolytes is also thought to be an effective way to achieve multicolor.147,148 Moon et al.147 fabricated a flexible M-ECD by a “cut-and-stick” strategy, designing an EC chromophore gel electrolyte by uniformly mixing poly(vinylidene fluoride-co-hexafluoro-propylene) (P(VDF-co-HFP)), ionic liquids (e.g., 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMI][TFSI]), 1-butyl-3-methylimidazolium tetrafluoroborate ([BMI][BF4])) and viologen together. The colors of the ECD can be easily tuned by the ionic liquid species due to the monomer–dimer equilibrium effects. Oh et al.148 demonstrated a flexible M-ECD by introducing monoheptyl viologen (MHV+) and diheptyl viologen (DHV2+) into the ionic gel electrolyte. Though the chemical structures of the two EC chromophores are similar, the EC behavior is significantly different when mixed due to the different color change potential. As a result, the fabricated flexible ECDs can show three colors of yellowish, blue, and magenta. In addition, the M-ECDs also have an excellent CE of 87.5 cm2 C−1 (magenta) and 91.3 cm2 C−2 (blue) with a low power consumption of 248 μW cm−2 (magenta) and ∼72 μW cm−2 (blue), showing that the voltage-tunable M-ECDs are very promising in low-power displays. To further improve the coloration and charge dissipation of M-ECDs, Liu et al.149 fabricated a flexible M-ECD by using a multicolor PANI electrode and 1-methyl-4,4′-bipyridyl iodide (MBI). The MBI can act as both electrolyte and cathodic EC layer, which can simplify the structure and enrich the color of the ECD. As a result, the device can display a wide color range (red, yellow, green, blue, and purple) with a wide band optical modulation (58.1% at 550 nm and 35% at 800 nm), fast switching time of 1 s/1.9 s (modulating 77%-colored/bleached), and high CE of 140.6 cm2 C−1.
 | ||
| Fig. 7 (a) Preparation and mechanism of the F–P cavity-type electrochromic supercapacitor electrodes; (b) full-device fabrication with fantastic patterns. Reproduced with permission.143 Copyright 2020 American Chemical Society. (c) Schematic structure of M-ECD; (d) corresponding optical images of devices with WO3 layers of different thicknesses. Reproduced with permission.142 Copyright 2023 Elsevier B.V. | ||
3.4 Flexible smart windows
The intensification of the energy crisis is a consequence of the increase in global energy demand. In response, there has been a continued exploration of renewable energy sources and energy-saving technology. As a manually operated device designed to control the level of light entering a building, EC-based smart windows have received much attention in academic and industry due to their potential to reduce the buildings’ energy consumption and improve the energy efficiency.160 Though several products have been successfully commercialized in the market, the conventional EC smart windows still suffer from issues of high cost, complexity, rigidity, long response time and inferior cycling stability. Compared with rigid smart windows, flexible EC-based smart windows are lightweight and can be glued to curved architectural structures due to their excellent mechanical stability, which is more convenient for practical applications.161 For instance, Koo et al.162 demonstrated a flexible EC-based smart window by using an amorphous-quantized WO3·H2O modified ITO/PEN as EC electrode, LiClO4/PMMA as gel electrolyte, and a H2PtCl6·6H2O modified ITO/PEN as counter-electrode. The amorphous-quantized WO3·H2O can provide abundant active sites and oxygen deficiencies, which can boost the charge transfer and buffer the stress during the coloration/bleaching process. As a result, the prepared flexible ECDs manifest an excellent flexibility with a bending radius of 1.3 cm and superior bendability maintaining 76.1% of the initial optical modulation after 300 bending cycles. To overcome the poor conductivity and high charge transport barrier, Nguyen et al.163 prepared a WO3/Ti3C2Tx MXene hybrid film by a spraying method and further fabricated a large-size flexible smart window. Owing to the introduction of highly conductive 2D MXene, the charge transport barrier of WO3-based ECD is greatly reduced, thus displaying a significantly improved EC behavior with an optical modulation of 57.6%, a CE of 126 cm2 C−1 and fast switching times of 1.5 s/2.7 s (bleaching/coloration). In addition, this strategy is very suitable for scale-up; as shown in Fig. 8a, a device with an area of 15 × 15 cm can be successfully constructed and demonstrated. | ||
| Fig. 8 (a) Photograph images of a flexible EC smart window. Reproduced with permission.150 Copyright 2021 Royal Society of Chemistry. (b) Schematic diagram and large-area applications (about 20 × 30 cm) of the flexible solid ECD. Reproduced with permission.151 Copyright 2021 Springer Nature. | ||
Recently, ITO/PET-free flexible ECDs have attracted tremendous interest due to the limitations of intrinsic brittleness and high cost of ITO, as well as poor adhesion of ITO on PET. For example, Wang et al.164 reported a flexible EC electrode by electrochemically depositing a homogeneous WO3 film on a flexible Ag nanofiber network-based transparent conductor. The sandwich structure of WO3/Ag/WO3 not only can improve the electrochemical stability in the oxidizing electrolyte, but also can homogenize the electrodeposition of WO3 in the gaps among the Ag networks. The obtained hybrid EC film exhibits a high optical modulation of 89.7% and excellent cycling stability without damage after 200 bending cycles. The fabricated flexible all-solid-state ECD can color uniformly under −1 V and be pasted on any curved surface, showing practical applicability in sunglasses and smart windows. To improve the electrochemical stability of Ag, Cai et al.165 coated the Ag grid with a PEDOT:PSS protection layer. The prepared transparent conductor displays excellent stability without obvious conductivity degradation after 2 months and superior bendability with only 7.5% optical modulation degradation after 1200 bending cycles. The fabricated flexible ECD exhibits a large optical modulation of 81.9% and high CE of 124.5 cm2 C−1, manifesting promising application in flexible electronics and optoelectronic devices.
Apart from the metal nanowires/grids-based flexible EC smart windows, MXene-based transparent conductors and flexible smart windows have also attracted tremendous attention recently. For example, Li et al.166 reported an all-MXene-based flexible EC smart window, where MXene (Ti3C2Tx)-derived 2D TiO2 and transparent MXene film were used as a flexible EC layer and flexible conductor, respectively. The assembled 2D TiO2/Ti3C2Tx heterostructures possess well-balanced porosity and good interconnection, which endowed the EC electrode with fast ion/electron transport, and excellent mechanical and electrochemical stability. As a result, the fabricated flexible solid ECD can deliver excellent cycling stability showing 92% transmittance modulation retention after 1000 EC cycles. In addition, the flexible solid device can be easily scaled up, and a large-area size device of 30 × 20 cm2 can be readily constructed, demonstrating practical large-area applications in next-generation flexible smart windows and wearable optoelectronics (Fig. 8b).
3.5 Other types of flexible multifunctional ECD
Apart from the above illustrated examples, other types of flexible multifunctional ECD have also been constructed to meet the different demands in recent years, which greatly broadens the practical applications of flexible multifunctional ECDs in electronics and optoelectronics. For example, conventional ECDs can only work in a limited temperature range due to the poor ionic conductivity of the electrolyte and macroscopic phase separation issues between electrodes/electrolytes at extreme temperatures (below 0 °C and above 60 °C). To overcome these issues, Poh et al.167 formulated polymerized ionogels by the one-step photopolymerization of vinyl-functionalized ionic liquids (BVIMTFSI), acrylate-terminated crosslinking agents (PEGDA700), and 1,3-ethylmethylimidazolium bis(trifluoromethylsulfonyl)imide (EMIMTFSI) ionic liquid. The designed ionogel electrolyte exhibits excellent transparency and stretchability, and possessed superior physicochemical stability (air, thermal and electrochemical stability), manifesting excellent thermally robust behavior in various conditions. The as-fabricated flexible ECD achieved a fast switching time (1.5/1.9 s at 572 nm), high color contrast (45.2% and 56.4%), excellent cycling stability (90% contrast retention after 3000 switching cycles), and wide temperature operation (−20 to 100 °C). Qiu et al.77 reported a transparent flexible ethylene glycol-assisted polyvinyl alcohol (PVA) based hydrogel electrolyte with Zn2+ and Al3+ as co-charge carrier. As shown in Fig. 9a–c, the obtained hydrogel electrolyte can deliver a high ionic conductivity of 2.9 mS cm−1 and 4.5 × 10−2 mS cm−1 with a transmittance of 94% and 94.3% at 60 °C and −40 °C, respectively. In addition, the hydrogel electrolyte has the merits of low cost, facile preparation, and good physicochemical stability. The assembled flexible Al/W10O49 ECD can work well at a low temperature of −40 °C with high optical modulation of 43% at 633 nm. | ||
| Fig. 9 (a–c) Various tests of the PVA-based hydrogel at various temperatures. Reproduced with permission.74 Copyright 2023 Elsevier Ltd. (d) Diagram of the actuation in microporous structure induced by the ionic adsorption/desorption and synchronous color change and actuation induced by the lattice contraction and expansion. Reproduced with permission.156 Copyright 2022 Wiley-VCH GmbH. (e) Schematic illustration of a skin-attached multi-sensor. Reproduced with permission.43 Copyright 2023 Elsevier B.V. | ||
Due to the potential application in biomimetic dual-stealth camouflage, robotics, and biomedicine, EC-actuators also have garnered significant attention and have been developed rapidly over the past few years. However, traditional ITO/glass transparent conductors are rigid, and can hardly demonstrate actuation. To address this issue, Li et al.168 designed an EC-actuator by employing PEDOT:PSS-protected Ag nanowires as a transparent conductor and W10O49 nanowires as an EC layer. Benefiting from the lattice contraction/recovery induced by the reversible Li+ intercalation/deintercalation into the W10O49 nanowires, the formed bifunctional film displays impressive EC and actuator behaviors with a fast-switching time of 4.1/2.9 s (coloration/bleaching), high CE of 119.2 cm2 C−1, and excellent actuation performance with a largest bending angle of 238° at 5 s. To further extend the dual-responsive color/shape change mode, Ling et al.169 reported a back-to-back structure multicolor EC-actuator by depositing V2O5 nanowires and single-walled CNTs on a porous polymer membrane, where the V2O5 and CNTs were used as multicolor EC materials and conductive current collectors. Owing to the lattice expansion/recovery of V2O5 nanowires induced by the reversible de-/intercalation of Li+, the composite film exhibits a synchronous color change (yellow, green and dark turquoise-blue) and actuation (Fig. 9d). The air-working EC-actuator can be easily fabricated by using two composite films stacked together. The produced device can also show a high actuation distance about ±9.7 mm with a reversible color change between yellow and green.
The traditional sensors are connected by signal processors and display devices together with an external electric circuit, which makes it difficult to realize portable wearable applications. In contrast, ECDs can visually display the strength of sensor signals through color changes, so the combination of EC technology and sensor technology can directly identify the sensor information by the naked eye.170,171 For example, Kim et al.45 reported an interactive display system by integrating EC devices and temperature and strain sensors together, which can visually display the skin temperature and wrist movement. The EC materials can change color between magenta, violet, and blue at −1.5 V, 0.5 V, and 1.5 V, respectively. With this EC sensor system, the wrist bending and temperature change of the skin can induce the color change in the ECD patterns. This work suggests the potential application of stretchable arrays of ECDs to effective interactive skin-attachable display devices for multi-sensor signals (Fig. 9e).
4 Conclusion and perspectives
In recent years, with the significant development of wearable and portable electronics, the interest in flexible multifunctional ECDs is growing rapidly. Compared with the traditional rigid glass-based ECDs, flexible multifunctional ECDs provide better functionality and can be easily integrated with curved surfaces. This review systematically discusses the recent advances in flexible multifunctional ECDs, including flexible self-powered ECDs, flexible ECESDs, flexible M-ECDs, and flexible smart windows. The configuration of flexible ECDs, design principles, integration mechanisms, and material optimization are also discussed. However, the flexible multifunctional ECDs are still in their infancy, and more effort should be devoted to address the issues of scalability, cost, device delamination, dissociation and degradation before the practical application. The first challenge is to reduce the cost of flexible multifunctional ECDs. Currently, the most popular flexible transparent conductor is ITO/PET due to its high optical transparency and electronic conductivity. However, the costly preparation process and rare indium as well as poor adhesion between ITO and PET limit its further use in flexible ECDs. As an alternative, ITO-free transparent conductors, including metal nanowires, conducting polymers, carbon materials, and MXene, have made significant progress in recent years. Unfortunately, the comprehensive performance, including transparency, electronic conductivity, and electrochemical stability, still cannot compete with ITO. The second challenge is to improve the cycling stability and deformability of flexible multifunctional ECDs. Unlike the conventional rigid ECDs, the flexible multifunctional ECDs need to work repeatedly under static, bending, twisting and stretching conditions, which easily induce electrochemical performance deterioration due to the delamination and electrolyte leakage. Therefore, more attention needs to be paid to improving the adhesion stability by introducing chemical bonds or electrostatic interactions between different layers, to satisfy the various working conditions. The third challenge is the mass production of EC materials with low cost, good EC performance, and a facile preparation process. Currently, the most popular synthesis methods are hydrothermal, electrodeposition, sol–gel, electrospinning, etc., which are costly and still limited to the laboratory scale. There is an urgent need for a breakthrough in large-scale preparation methods with acceptable cost, easy morphology control, and quantity regulation. In addition, current EC materials are at the nanoscale, and unpredictable and irreversible side reactions often occur during the coloration/bleaching process due to the large active surface area. Therefore, the in-depth understanding of the side reactions by using advanced in situ characterization methods, including in situ X-ray diffraction, and in situ Raman/infrared spectroscopy, needs to be further investigated. The fourth challenge is to design solid-state electrolytes with high ionic conductivity and thermal stability, excellent electrochemical inertness, and outstanding deformability. To avoid the leakage of liquid electrolyte, solid-state electrolytes are more practical for flexible multifunctional ECDs. However, currently reported solid-state electrolytes have poor ionic conductivity and are easily cracked under mechanical strain/stress. Therefore, more effort needs to be paid to the design of high ionic conductivity, stretchable, and self-healing solid-state electrolytes to meet the demands of various types of deformable ECD. Last but not least is that the scale-up method must be reliable according to industrial manufacturing standards. To date, the most widely reported methods, including electrodeposition, spray coating, spin coating, and rod coating, are still limited to the laboratory scale, and large-scale methods with controllable thickness and uniformity need to be further developed. Therefore, practical adaptations to industrial manufacturing standards need to be developed, which requires the collaboration between industry and academia. Overall, the further development of flexible multifunctional ECDs requires a systematic optimization of transparent conductors, EC materials, electrolytes, sealing and packaging materials, and device structure design. We believe that with the continuous performance improvement in flexible multifunctional ECDs by the optimization and integration of different EC materials, design of hybrid approaches, in-depth understanding of the mechanism, and development of fabrication technologies, the practical applications will proliferate in the near future.Author contributions
All authors conceptualized, curated data, wrote, reviewed and edited the manuscript before submission.Data availability
No new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China (no. 52102117), Key Science and Technology Program of Henan Province (no. 202102310212).References
- R. Zheng, Y. Wang, C. Jia, Z. Wan, J. Luo, H. A. Malik, X. Weng, J. Xie and L. Deng, ACS Appl. Mater. Interfaces, 2018, 10, 35533–35538 CrossRef CAS PubMed.
- A. K. Sasmal and T. Pal, J. Indian Chem. Soc., 2021, 98, 100073 Search PubMed.
- J. H. Day, Chem. Rev., 1963, 65–80 CAS.
- T. Yamase, Chem. Rev., 1998, 98, 307–326 CAS.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 Search PubMed.
- R. J. Mortimer, Chem. Soc. Rev., 1997, 26, 147 CAS.
- S. J. Lee, T.-G. Lee, S. Nahm, D. H. Kim, D. J. Yang and S. H. Han, J. Alloys Compd., 2020, 815, 152399 Search PubMed.
- Y. Zhang, B. Xu, F. Zhao, H. Li, J. Chen, H. Wang and W. W. Yu, FlexMat, 2024, 1, 23–45 Search PubMed.
- J. R. Platt, J. Chem. Phys., 1961, 34, 862–863 Search PubMed.
- S. K. Deb, Appl. Opt., 1969, 8(Suppl 1), 192–195 Search PubMed.
- J. Svensson and C. G. Granqvist, Thin Solid Films, 1985, 126, 31–36 Search PubMed.
- C.-J. Tang, J.-M. Ye, Y.-T. Yang and J.-L. He, Opt. Mater., 2016, 55, 83–89 Search PubMed.
- S. Huang, Y. Liu, M. Jafari, M. Siaj, H. Wang, S. Xiao and D. Ma, Adv. Funct. Mater., 2021, 31, 2010022 Search PubMed.
- H. Gong, A. Li, G. Fu, M. Zhang, Z. Zheng, Q. Zhang, K. Zhou, J. Liu and H. Wang, J. Mater. Chem. A, 2023, 11, 8939–8949 Search PubMed.
- J. Li, A. Levitt, N. Kurra, K. Juan, N. Noriega, X. Xiao, X. Wang, H. Wang, H. N. Alshareef and Y. Gogotsi, Energy Storage Mater., 2019, 20, 455–461 Search PubMed.
- J. Kim, M. Rémond, D. Kim, H. Jang and E. Kim, Adv. Mater. Technol., 2020, 5, 1900890 Search PubMed.
- L. Wang, M. Guo, J. Zhan, X. Jiao, D. Chen and T. Wang, J. Mater. Chem. A, 2020, 8, 17098–17105 CAS.
- X. Yu, P. Guo, J. Chen, S. Li and H. Li, Responsive Mater., 2024, 2, e20240013 CrossRef.
- J. Yan, S. Li, B. Lan, Y. Wu and P. S. Lee, Adv. Funct. Mater., 2019, 30, 1902564 CrossRef.
- Z. Wang, X. Wang, S. Cong, F. Geng and Z. Zhao, Mater. Sci. Eng., R, 2020, 140, 100524 CrossRef.
- L. Manjakkal, L. Pereira, E. Kumi Barimah, P. Grey, F. F. Franco, Z. Lin, G. Jose and R. A. Hogg, Prog. Mater. Sci., 2024, 142, 101244 CrossRef CAS.
- H. Wang, C.-J. Yao, H.-J. Nie, L. Yang, S. Mei and Q. Zhang, J. Mater. Chem. C, 2020, 8, 15507–15525 RSC.
- W. Wu, S. Guo, J. Bian, X. He, H. Li and J. Li, J. Energy Chem., 2024, 93, 453–470 CrossRef CAS.
- A. L. S. Eh, A. W. M. Tan, X. Cheng, S. Magdassi and P. S. Lee, Energy Technol., 2017, 6, 33–45 CrossRef.
- B. Wang, W. Zhang, F. Zhao, W. W. Yu, A. Y. Elezzabi, L. Liu and H. Li, Nano Mater. Sci., 2023, 5, 369–391 CrossRef.
- A. L.-S. Eh, J. Chen, X. Zhou, J.-H. Ciou and P. S. Lee, ACS Energy Lett., 2021, 6, 4328–4335 CAS.
- D. He, C. Su, C. Zhao, G. Yan, Z. Zhao and W. Mai, Chem. Eng. J., 2022, 438, 135469 CrossRef CAS.
- W. Li, T. Bai, G. Fu, Q. Zhang, J. Liu, H. Wang, Y. Sun and H. Yan, Sol. Energy Mater. Sol. Cells, 2022, 240, 111709 CAS.
- A. Viñuales, Y. Alesanco, G. Cabañero, J. Sobrado and R. Tena-Zaera, Sol. Energy Mater. Sol. Cells, 2017, 167, 22–27 Search PubMed.
- R. Brooke, E. Mitraka, S. Sardar, M. Sandberg, A. Sawatdee, M. Berggren, X. Crispin and M. P. Jonsson, J. Mater. Chem. C, 2017, 5, 5824–5830 RSC.
- P. Cossari, A. Cannavale, S. Gambino and G. Gigli, Sol. Energy Mater. Sol. Cells, 2016, 155, 411–420 CrossRef CAS.
- H. Lee, M. Kim, I. Kim and H. Lee, Adv. Mater., 2016, 28, 4541–4548 CrossRef CAS PubMed.
- W. Kang, C. Yan, C. Y. Foo and P. S. Lee, Adv. Funct. Mater., 2015, 25, 4203–4210 Search PubMed.
- W. Kang, M. F. Lin, J. Chen and P. S. Lee, Small, 2016, 12, 6370–6377 CrossRef CAS PubMed.
- G. Fu, H. Gong, T. Bai, Q. Zhang and H. Wang, J. Mater. Sci.: Mater. Electron., 2023, 34, 1316 CAS.
- C. Miao, H. Du, X. Zhang and H. V. Tippur, Cellulose, 2021, 29, 557–569 Search PubMed.
- A. W. Lang, A. M. Österholm and J. R. Reynolds, Adv. Funct. Mater., 2019, 29, 1903487 Search PubMed.
- Y. Djaoued, V. H. Phong, S. Badilescu, P. V. Ashrit, F. E. Girouard and V.-V. Truong, Thin Solid Films, 1997, 293, 108–112 CrossRef CAS.
- S. Macher, M. Rumpel, M. Schott, U. Posset, G. A. Giffin and P. Löbmann, ACS Appl. Mater. Interfaces, 2020, 12, 36695–36705 CrossRef CAS PubMed.
- W. Li, J. Sun, J. Zhang, O. A. Ganiyat and Y. Cui, Results Surf. Interfaces, 2021, 2, 100002 Search PubMed.
- G. Cai, J. Wang and P. S. Lee, Acc. Chem. Res., 2016, 49, 1469–1476 CAS.
- S. Huang, Y. Liu, F. Yang, Y. Wang, T. Yu and D. Ma, Environ. Chem. Lett., 2022, 20, 3005–3037 CrossRef CAS.
- Z. Ke, A. Abtahi, J. Hwang, K. Chen, J. Chaudhary, I. Song, K. Perera, L. You, K. N. Baustert, K. R. Graham and J. Mei, J. Am. Chem. Soc., 2023, 145, 3706–3715 CrossRef CAS.
- B. Wu, Y. Guo, C. Hou, Q. Zhang, Y. Li and H. Wang, Nano Energy, 2021, 89, 106487 CrossRef CAS.
- D. S. Kim, Y. H. Lee, J. W. Kim, H. Lee, G. Jung and J. S. Ha, Chem. Eng. J., 2022, 429, 132289 CrossRef CAS.
- C. Ma, H. Liu, C. Teng, L. Li, Y. Zhu, H. Yang and L. Jiang, ACS Appl. Mater. Interfaces, 2020, 12, 55372–55381 CrossRef CAS.
- H. Peng, M. Pan, H. Jiang, W. Huang, X. Wang, Q. Yang, S. Chen and B. Yan, ACS Appl. Mater. Interfaces, 2022, 14, 42402–42411 CrossRef CAS.
- S. Guo, R. Zhu, J. Chen, W. Liu, Y. Zhang, J. Li and H. Li, Microsyst. Nanoeng., 2024, 10, 89 CAS.
- J. Zhao, S. Zhang, S. Chang, C. Li, C. Fang, X. Xia, L. Shen, J. Yang Lee, C. Cao, X. Zhang and Y. Xuan, Chem. Eng. J., 2024, 480, 148010 CAS.
- D. A. Barus, K. Sebayang, J. Ginting and R. T. Ginting, J. Phys.: Conf. Ser., 2018, 1116, 032006 Search PubMed.
- H. Zhang, J. Feng, F. Sun, D. Zhou, G. Cao, Z. Wu, S. Wang, F. Su, Y. Tian and Y. Tian, Adv. Mater., 2023, 4, 995–1004 RSC.
- G. Cai, S. Park, X. Cheng, A. L.-S. Eh and P. S. Lee, Sci. Technol. Adv. Mater., 2018, 19, 759–770 CrossRef CAS.
- W. Zhao, J. Wang, B. Tam, H. Zhang, F. Li, A. Du and W. Cheng, Adv. Opt. Mater., 2023, 11, 2202774 CrossRef CAS.
- S. Weng, Z. Cao, K. Song, W. Chen, R. Jiang, A. A. Rogachev, M. A. Yarmolenko, J. Zhou and H. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 18164–18172 CrossRef CAS PubMed.
- C.-T. Lee, S. Han, Y.-X. Zhao, Y.-C. Hung, T.-H. Hsu, H.-Y. Hsieh and K.-W. Weng, Surf. Coat. Technol., 2019, 363, 426–429 CAS.
- Y. Liang, S. Cao, Q. Wei, R. Zeng, J. Zhao, H. Li, W. W. Yu and B. Zou, Nano-Micro Lett., 2021, 13, 196 CrossRef CAS PubMed.
- S. Huang, R. Zhang, P. Shao, Y. Zhang and R.-T. Wen, Adv. Opt. Mater., 2022, 10, 2200903 CrossRef CAS.
- R. Goel, R. Jha and C. Ravikant, Chem. Pap., 2023, 77, 2885–2903 CrossRef CAS.
- S. H. Sutar, B. M. Babar, K. B. Pisal, A. I. Inamdar and S. H. Mujawar, J. Energy Storage, 2023, 73, 109035 CrossRef.
- D. Ma, A. Lee-Sie Eh, S. Cao, P. S. Lee and J. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 1443–1451 Search PubMed.
- J. Wang, W. Zhao, B. Tam, H. Zhang, Y. Zhou, L. Yong and W. Cheng, Chem. Eng. J., 2023, 452, 139655 CAS.
- H. Sun, W. Wang, Y. Xiong, Z. Jian and W. Chen, Chin. Chem. Lett., 2024, 35, 109213 Search PubMed.
- D. Zhang, J. Wang, Z. Tong, H. Ji and H. Y. Qu, Adv. Funct. Mater., 2021, 31, 2106577 CAS.
- J. Wang, J. Liu, M. Hu, J. Zeng, Y. Mu, Y. Guo, J. Yu, X. Ma, Y. Qiu and Y. Huang, J. Mater. Chem. A, 2018, 6, 11113–11118 CAS.
- C. S. Pinto, V. H. R. Souza, A. Schmidt and A. J. G. Zarbin, Synth. Met., 2023, 293, 117259 CAS.
- Y. Luo, J.-P. Liu, L.-K. Li and S.-Q. Zang, Inorg. Chem., 2023, 62, 14385–14392 CrossRef CAS.
- Y. Sui, Y. Ma, Y. Gao, J. Song, Y. Ye, H. Niu, W. Ma, P. Zhang and C. Qin, New J. Chem., 2021, 45, 10654–10663 RSC.
- K.-C. Lee, C.-W. Chang-Jian, E.-C. Cho, J.-H. Huang, W.-T. Lin, B.-C. Ho, J.-A. Chou and Y.-S. Hsiao, Sol. Energy Mater. Sol. Cells, 2019, 195, 1–11 CrossRef CAS.
- E. Eren, M. F. Aydın and A. U. Oksuz, J. Solid State Electrochem., 2020, 24, 1057–1065 CrossRef CAS.
- Z. Wang, X. Wang, S. Cong, J. Chen, H. Sun, Z. Chen, G. Song, F. Geng, Q. Chen and Z. Zhao, Nat. Commun., 2020, 11, 302 CrossRef CAS PubMed.
- W. Zhang, H. Li, E. Hopmann and A. Y. Elezzabi, Nanophotonics, 2020, 10, 825–850 CrossRef.
- Q. Wang, S. Cao, Q. Meng, K. Wang, T. Yang, J. Zhao and B. Zou, Mater. Horiz., 2023, 10, 960–966 RSC.
- X. Liu, T. Cao, W. Yao, L. Shen, J. Xu, F. Jiang and Y. Du, J. Colloid Interface Sci., 2020, 570, 382–389 CrossRef CAS.
- W. Zhang, H. Li, W. W. Yu and A. Y. Elezzabi, Light: Sci. Appl., 2020, 9, 121 CrossRef CAS PubMed.
- V. Primiceri, M. Pugliese, C. T. Prontera, A. G. Monteduro, M. Esposito, A. Maggiore, A. Cannavale, R. Giannuzzi, G. Gigli and V. Maiorano, Sol. Energy Mater. Sol. Cells, 2022, 240, 111657 CrossRef CAS.
- P. Barbosa, L. Rodrigues, M. Silva, M. Smith, A. Gonçalves and E. Fortunato, J. Mater. Chem., 2010, 20, 723–730 RSC.
- B. Qiu, X. Xiao, G. Xu and G. Dong, Mater. Today Chem., 2023, 33, 101703 CrossRef CAS.
- B. Xu, J. Chen, Z. Ding, J. Hu, Y. Zhang, H. Li and H. Wang, Small Sci., 2023, 3, 2300025 CrossRef CAS.
- M. Han, C. H. Cho, H. Jang and E. Kim, J. Mater. Chem. A, 2021, 9, 16016–16027 RSC.
- C. Gu, A.-B. Jia, Y.-M. Zhang and S. X.-A. Zhang, Chem. Rev., 2022, 122, 14679–14721 CrossRef CAS PubMed.
- N. C. Davy, M. Sezen-Edmonds, J. Gao, X. Lin, A. Liu, N. Yao, A. Kahn and Y.-L. Loo, Nat. Energy, 2017, 2, 17104 CrossRef CAS.
- A. Cánovas-Saura, R. Ruiz, R. López-Vicente, J. Abad, A. Urbina and J. Padilla, Electron. Mater., 2021, 2, 174–185 CrossRef.
- H. Zhang, F. Sun, J. Feng, H. Ling, D. Zhou, G. Cao, S. Wang, F. Su, Y. Tian and Y. Tian, Cell Rep. Phys. Sci., 2022, 3, 101193 Search PubMed.
- Z. He, B. Gao, T. Li, J. Liao, B. Liu, X. Liu, C. Wang, Z. Feng and Z. Gu, ACS Sustainable Chem. Eng., 2018, 7, 1745–1752 Search PubMed.
- S. Bi, W. Jin, X. Han, X. Cao, Z. He, K. Asare-Yeboah and C. Jiang, Nano Energy, 2022, 102, 107629 CAS.
- J. Huang, Z. Ren, Y. Zhang, P. W.-K. Fong, H. T. Chandran, Q. Liang, K. Yao, H. Tang, H. Xia, H. Zhang, X. Yu, Z. Zheng and G. Li, Adv. Energy Mater., 2022, 12, 2201042 CAS.
- L. Zhou, D. Liu, L. Liu, L. He, X. Cao, J. Wang and Z. L. Wang, Research, 2021, 2021, 4673028 CAS.
- S. Zhao, X. Gao, L. Chen, W. Huang and Y. Liu, Appl. Mater. Today, 2022, 28, 101543 CrossRef.
- H. Gong, S. Wang, M. Xie and H. Wang, Sol. Energy Mater. Sol. Cells, 2022, 248, 112018 CrossRef CAS.
- J.-L. Wang, S.-Z. Sheng, Z. He, R. Wang, Z. Pan, H.-Y. Zhao, J.-W. Liu and S.-H. Yu, Nano Lett., 2021, 21, 9976–9982 CrossRef CAS.
- H. Li, W. Zhang and A. Y. Elezzabi, Adv. Mater., 2020, 32, 2003574 CrossRef CAS PubMed.
- Q. Ma, J. Chen, H. Zhang, Y. Su, Y. Jiang and S. Dong, ACS Energy Lett., 2023, 8, 306–313 CrossRef CAS.
- F. Zhao, C. Li, S. Li, B. Wang, B. Huang, K. Hu, L. Liu, W. W. Yu and H. Li, Adv. Mater., 2024, 2405035, DOI:10.1002/adma.202405035.
- J. Hu, Y. Zhang, B. Xu, Y. Ouyang, Y. Ma, H. Wang, J. Chen and H. Li, Chem. Commun., 2024, 60, 566–569 RSC.
- Y. An, Y. Tian, C. Liu, S. Xiong, J. Feng and Y. Qian, ACS Nano, 2021, 15, 15259–15273 CrossRef CAS.
- W. Nie, H. Cheng, Q. Sun, S. Liang, X. Lu, B. Lu and J. Zhou, Small Methods, 2024, 8, 2201572 CrossRef CAS.
- Y. Chen, Q. Zhao, Y. Wang, W. Liu, P. Qing and L. Chen, Electrochim. Acta, 2021, 399, 139334 CrossRef CAS.
- C. Xiong, T. Wang, Z. Zhao and Y. Ni, SmartMat, 2022, 4, e1158 CrossRef.
- P. Yang, P. Sun and W. Mai, Mater. Today, 2016, 19, 394–402 CrossRef CAS.
- D. S. Dalavi, R. S. Desai and P. S. Patil, J. Mater. Chem. A, 2022, 10, 1179–1226 RSC.
- A. Amiri, A. Bruno and A. A. Polycarpou, Carbon Energy, 2023, 5, e320 CrossRef CAS.
- Y. Chen, S. Li, J. Chen, L. Gao, P. Guo, C. Wei, J. Fu and Q. Xu, J. Colloid Interface Sci., 2024, 664, 360–370 Search PubMed.
- S. Li, Q. Tian, J. Chen, Y. Chen, P. Guo, C. Wei, P. Cui, J. Jiang, X. Li and Q. Xu, Chem. Eng. J., 2023, 457, 141265 CrossRef CAS.
- X. Jiao, G. Li, Z. Yuan and C. Zhang, ACS Appl. Energy Mater., 2021, 4, 14155–14168 CAS.
- Y. Shi, M. Sun, Y. Zhang, J. Cui, Y. Wang, X. Shu, Y. Qin, H. H. Tan, J. Liu and Y. Wu, Sol. Energy Mater. Sol. Cells, 2020, 212, 110579 CAS.
- L. Shen, L. Du, S. Tan, Z. Zang, C. Zhao and W. Mai, Chem. Commun., 2016, 52, 6296–6299 CAS.
- T. Hao, S. Wang, H. Xu, X. Zhang, J. Xue, S. Liu, Y. Song, Y. Li and J. Zhao, Chem. Eng. J., 2021, 426, 130840 CAS.
- P. Zhang, Q. Sui, Z. Liu, C. Hu, C. Li, X. Guo, J. Wang and G. Cai, Chem. Eng. J., 2024, 498, 155277 Search PubMed.
- G. Cai, X. Cheng, M. Layani, A. W. M. Tan, S. Li, A. L.-S. Eh, D. Gao, S. Magdassi and P. S. Lee, Nano Energy, 2018, 49, 147–154 Search PubMed.
- M. Hassan, P. Li, J. Lin, Z. Li, M. S. Javed, Z. Peng and K. Celebi, Small, 2024, 20, 2400278 CAS.
- H. Li, L. McRae, C. J. Firby, M. Al-Hussein and A. Y. Elezzabi, Nano Energy, 2018, 47, 130–139 CrossRef CAS.
- P. Zhang, F. Zhu, F. Wang, J. Wang, R. Dong, X. Zhuang, O. Schmidt and X. Feng, Adv. Mater., 2016, 29, 1604491 CrossRef.
- R. U. Amate, P. J. Morankar, G. T. Chavan, A. M. Teli, R. S. Desai, D. S. Dalavi and C.-W. Jeon, Electrochim. Acta, 2023, 459, 142522 CrossRef CAS.
- G. Cai, R. Zhu, S. Liu, J. Wang, C. Wei, K. J. Griffith, Y. Jia and P. S. Lee, Adv. Energy Mater., 2022, 12, 2103106 CrossRef CAS.
- K. Xu, L. Wang, G. Liu, C. Ge, L. Wang, W. Wang and M. Chen, Energy Environ. Mater., 2023, 6, e12362 CrossRef CAS.
- K. Zhou, H. Wang, J. Jiu, J. Liu, H. Yan and K. Suganuma, Chem. Eng. J., 2018, 345, 290–299 CrossRef CAS.
- W. Zhang, J. Cao, H. Li, C. Du, S. Chen, L. Cao, J. Xu, B. Lu and G. Zhang, J. Energy Storage, 2024, 98, 113154 CrossRef.
- L. Zhang, Y. Chen, Z. Jiang, J. Chen, C. Wei, W. Wu, S. Li and Q. Xu, Energy Environ. Mater., 2024, 7, e12507 CrossRef CAS.
- L. Gao, Y. Ma and M. Cao, Int. J. Hydrogen Energy, 2024, 49, 1–10 CrossRef CAS.
- L. Gao, H. Zhan, G. Feng, Y. Ma, C. Zhang, Y. Zhang and M. Cao, J. Energy Storage, 2024, 97, 112890 CrossRef.
- T. G. Yun, J. Lee, H. S. Kim, J. Y. Cheong, S. H. Kim, Y. Kim, S. Lee and I.-D. Kim, Adv. Mater., 2023, 35, 2301141 CrossRef CAS PubMed.
- H. Li, L. McRae, C. J. Firby and A. Y. Elezzabi, Adv. Mater., 2019, 31, 1807065 CrossRef PubMed.
- Z. Tong, R. Lian, R. Yang, T. Kang, J. Feng, D. Shen, Y. Wu, X. Cui, H. Wang, Y. Tang and C.-S. Lee, Energy Storage Mater., 2022, 44, 497–507 Search PubMed.
- H. Li, C. J. Firby and A. Y. Elezzabi, Joule, 2019, 3, 2268–2278 Search PubMed.
- Z. Tong, T. Kang, Y. Wan, R. Yang, Y. Wu, D. Shen, S. Liu, Y. Tang and C.-S. Lee, Adv. Funct. Mater., 2021, 31, 2104639 Search PubMed.
- Y. Ding, H. Sun, Z. Li, C. Jia, X. Ding, C. Li, J.-G. Wang and Z. Li, J. Mater. Chem. A, 2023, 11, 2868–2875 Search PubMed.
- T. G. Yun, J. Lee, H. S. Kim, J. Y. Cheong, S. H. Kim, Y. Kim, S. Lee and I. D. Kim, Adv. Mater., 2023, 35, 2301141 Search PubMed.
- S. Sun, C. Tang, Y. Jiang, D. Wang, X. Chang, Y. Lei, N. Wang and Y. Zhu, Sol. Energy Mater. Sol. Cells, 2020, 207, 110332 Search PubMed.
- S. B. Singh, D. T. Tran, K. U. Jeong, N. H. Kim and J. H. Lee, Small, 2021, 18, 2104462 Search PubMed.
- G. Cai, J. Chen, J. Xiong, A. Lee-Sie Eh, J. Wang, M. Higuchi and P. S. Lee, ACS Energy Lett., 2020, 5, 1159–1166 Search PubMed.
- Q. Huang, J. Hu, M. Yin, Y. Zhu and R.-T. Wen, Sol. Energy Mater. Sol. Cells, 2024, 267, 112706 Search PubMed.
- U. Linderhed, I. Petsagkourakis, P. A. Ersman, V. Beni and K. Tybrandt, Flexible Printed Electron., 2021, 6, 045014 Search PubMed.
- K. Tang, Y. Zhang, Y. Shi, J. Cui, X. Shu, Y. Wang, J. Liu, J. Wang, H. H. Tan and Y. Wu, J. Mater. Chem. C, 2018, 6, 12206–12216 RSC.
- H. Zhao, Y. Chen, L. Zhao, X. Liang and Z. Liu, Sol. Energy Mater. Sol. Cells, 2023, 257, 112374 CrossRef CAS.
- D. Shin, J. Kim, S. Choi, G. Song, A. Rougier and C. S. Lee, Sol. Energy Mater. Sol. Cells, 2023, 257, 112341 CrossRef CAS.
- J. Wu, D. Qiu, H. Zhang, H. Cao, W. Wang, Z. Liu, T. Tian, L. Liang, J. Gao and F. Zhuge, J. Electrochem. Soc., 2018, 165, D183–D189 CrossRef CAS.
- S. Sun, S. Cui, F. Wang, M. Gao, W. Wei, J. Dong, X. Xia, Q. Zhu and J. Zhang, ACS Appl. Electron. Mater., 2022, 4, 4724–4732 CrossRef CAS.
- Q. Liu, X. Ou, Y. Niu, L. Li, D. Xing, Y. Zhou and F. Yan, Angew. Chem., Int. Ed., 2024, 63, e202317944 CrossRef CAS PubMed.
- X. Huang, J. Chen, H. Xie, F. Zhao, S. Fan and Y. Zhang, Sci. China Mater., 2022, 65, 2217–2226 Search PubMed.
- H. Zhang, S. Ming, Y. Liang, L. Feng and T. Xu, Int. J. Electrochem., 2020, 15, 1044–1057 Search PubMed.
- H. Fan, K. Li, X. Liu, K. Xu, Y. Su, C. Hou, Q. Zhang, Y. Li and H. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 28451–28460 CAS.
- X. Gao, Y. Wang, M. Wu, C. Zhi, J. Meng and L. Zhang, Dyes Pigm., 2023, 219, 111642 CrossRef CAS.
- Y. Ding, M. Wang, Z. Mei and X. Diao, ACS Appl. Mater. Interfaces, 2023, 15, 15646–15656 Search PubMed.
- Y. Ding, M. Wang, Z. Mei and X. Diao, ACS Appl. Mater. Interfaces, 2022, 14, 48833–48843 CAS.
- W. Xue, Y. Zhang, F. Liu, Y. Dou, M. Yan and W. Wang, Research, 2023, 6, 0227 Search PubMed.
- W. Zhang, H. Li and A. Y. Elezzabi, Adv. Funct. Mater., 2021, 32, 2108341 Search PubMed.
- H. C. Moon, C.-H. Kim, T. P. Lodge and C. D. Frisbie, ACS Appl. Mater. Interfaces, 2016, 8, 6252–6260 Search PubMed.
- H. Oh, D. G. Seo, T. Y. Yun, C. Y. Kim and H. C. Moon, ACS Appl. Mater. Interfaces, 2017, 9, 7658–7665 CAS.
- G. Liu, Z. Wang, J. Wang, H. Liu and Z. Li, J. Colloid Interface Sci., 2024, 655, 493–507 CrossRef CAS PubMed.
- X. Ran, J. Ren, S. Zhang, Y. Wu and S. Wu, ACS Appl. Mater. Interfaces, 2023, 15, 41763–41771 Search PubMed.
- Q. Zhang, X. Li, M. Qin, F. Guan, Y. Gong, R. Wang, J. Xu and G. Chen, ACS Appl. Electron. Mater., 2021, 3, 4441–4447 Search PubMed.
- L. Li, Z. Yu, C. Ye and Y. Song, Adv. Funct. Mater., 2024, 34, 2311845 Search PubMed.
- F. J. Rodriguez, D. E. Aznakayeva, O. P. Marshall, V. G. Kravets and A. N. Grigorenko, Adv. Mater., 2017, 29, 1606372 Search PubMed.
- J. Zhao, M. Qiu, X. Yu, X. Yang, W. Jin, D. Lei and Y. Yu, Adv. Opt. Mater., 2019, 7, 1900646 CrossRef CAS.
- A. Rao, S. Zhang, J. Hu, L. Zheng, Q. Yao, K. Lin, C. Niu, L. Wang, M. Yang, Y. Lv and Q. Chen, J. Alloys Compd., 2023, 969, 172310 CrossRef CAS.
- J. Chen, Z. Wang, Z. Chen, S. Cong and Z. Zhao, Nano Lett., 2020, 20, 1915–1922 CrossRef CAS PubMed.
- J. Chen, Y. Li, T. Zhang, X. Zha, X. Tang, X. Mu, P. Sun, G. Song, S. Cong, Q. Chen and Z. Zhao, Laser Photonics Rev., 2022, 16, 2200303 CrossRef CAS.
- L. Zheng, S. Zhang, Q. Yao, K. Lin, A. Rao, C. Niu, M. Yang, L. Wang and Y. Lv, Ceram. Int., 2023, 49, 13355–13362 CrossRef CAS.
- X. Tang, Z. Hu, Z. Wang, J. Chen, X. Mu, G. Song, P. Sun, Z. Wen, J. Hao, S. Cong and Z. Zhao, eScience, 2022, 2, 632–638 CrossRef.
- Y. Zhang, B. Xu, B. Huang, T. He, F. Meng, W. Tian, Y. Zhu, J. Wu, H. Wang, H. Li and J. Chen, ACS Energy Lett., 2024, 9, 4162–4171 CrossRef.
- Y. Zhao, Q. Liu, Y. Wang, H. Liu, M. Lv, P. Cheng, Y. Fu, J. Li and D. He, Cell Rep. Phys. Sci., 2022, 3, 101100 CrossRef CAS.
- J. Koo, V. Amoli, S. Y. Kim, C. Lee, J. Kim, S.-M. Park, J. Kim, J. M. Ahn, K. J. Jung and D. H. Kim, Nano Energy, 2020, 78, 105199 CrossRef CAS.
- V.-T. Nguyen, B. K. Min, S. K. Kim, Y. Yi and C.-G. Choi, J. Mater. Chem. C, 2021, 9, 3183–3192 RSC.
- Y. Wang, Z. Meng, H. Chen, T. Li, D. Zheng, Q. Xu, H. Wang, X. Y. Liu and W. Guo, J. Mater. Chem. C, 2019, 7, 1966–1973 RSC.
- G. Cai, P. Darmawan, M. Cui, J. Wang, J. Chen, S. Magdassi and P. S. Lee, Adv. Energy Mater., 2016, 6, 1501882 CrossRef.
- R. Li, X. Ma, J. Li, J. Cao, H. Gao, T. Li, X. Zhang, L. Wang, Q. Zhang, G. Wang, C. Hou, Y. Li, T. Palacios, Y. Lin, H. Wang and X. Ling, Nat. Commun., 2021, 12, 1587 CrossRef CAS PubMed.
- W. C. Poh, A. L. S. Eh, W. Wu, X. Guo and P. S. Lee, Adv. Mater., 2022, 34, 2206952 CrossRef CAS.
- K. Li, Y. Shao, H. Yan, Z. Lu, K. J. Griffith, J. Yan, G. Wang, H. Fan, J. Lu, W. Huang, B. Bao, X. Liu, C. Hou, Q. Zhang, Y. Li, J. Yu and H. Wang, Nat. Commun., 2018, 9, 4798 CrossRef PubMed.
- Y. Ling, H. Fan, K. Wang, Z. Lu, L. Wang, C. Hou, Q. Zhang, Y. Li, K. Li and H. Wang, Small, 2022, 18, 2107778 CrossRef CAS PubMed.
- C. Li, M. Zhen, K. Wang, L. Liu, W. Zhang, Y. Wang, X. Fan, W. Hou and J. Xiong, ACS Appl. Mater. Interfaces, 2023, 15, 40772–40780 CrossRef CAS.
- Z. Yu, G. Cai, X. Liu and D. Tang, Anal. Chem., 2021, 93, 2916–2925 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |




