Graveyard effects of antimicrobial nanostructured titanium over prolonged exposure to drug resistant bacteria and fungi†
Louisa Z. Y.
Huang
a,
Rowan
Penman
a,
Rashad
Kariuki
a,
Pierre H. A.
Vaillant
a,
Soroosh
Gharehgozlo
a,
Z. L.
Shaw
b,
Vi Khanh
Truong
 c,
Jitraporn
Vongsvivut
c,
Jitraporn
Vongsvivut
 d,
Aaron
Elbourne
d,
Aaron
Elbourne
 *a and
Rachel A.
Caruso
*a and
Rachel A.
Caruso
 *a
*a
aSchool of Science, College of STEM, RMIT University, Melbourne, Victoria 3000, Australia. E-mail: rachel.caruso@rmit.edu.au; aaron.elbourne@rmit.edu.au
bSchool of Engineering, College of STEM, RMIT University, Melbourne, Victoria 3000, Australia
cHealthcare Engineering Innovation Group, Department of Biomedical Engineering & Biotechnology, College of Medicine and Health Science, Khalifa University, Abu Dhabi, United Arab Emirates
dInfrared Microspectroscopy (IRM) Beamline, ANSTO – Australian Synchrotron, Clayton, Victoria 3168, Australia
First published on 16th December 2024
Abstract
Innovations in nanostructured surfaces have found a practical place in the medical area with use in implant materials for post-operative infection prevention. These textured surfaces should be dual purpose: (1) bactericidal on contact and (2) resistant to biofilm formation over prolonged periods. Here, hydrothermally etched titanium surfaces were tested against two highly antimicrobial resistant microbial species, methicillin-resistant Staphylococcus aureus and Candida albicans. Two surface types – unmodified titanium and nanostructured titanium – were incubated in a suspension of each microbial strain for 1 day and 7 days. Surface topography and cross-sectional information of the microbial cells adhered to the surfaces, along with biomass volume and live/dead rate, showed that while nanostructured titanium was able to kill microbes after 1 day of exposure, after 7 days, the rate of death becomes negligible when compared to the unmodified titanium. This suggests that as biofilms mature on a nanostructured surface, the cells that have lysed conceal the nanostructures and prime the surface for planktonic cells to adhere, decreasing the possibility of structure-induced lysis. Synchrotron macro-attenuated total reflection Fourier transform infrared (macro ATR-FTIR) micro-spectroscopy was used to elucidate the biochemical changes occurring following exposure to differing surface texture and incubation duration, providing further understanding into the effects of surface morphology on the biochemical molecules (lipids, proteins and polysaccharides) in an evolving and growing microbial colony.
1. Introduction
Bacteria and fungi are single cell organisms that are able to adapt to and multiply in both biotic and abiotic environments.1–4 This is largely due to their ability to form a biofilm – a three-dimensional structure of aggregated microorganisms within a self-produced, extracellular matrix (ECM) comprising extracellular polymeric substances (EPS).5–7 It acts as a protective, ideal environment for the colony to replicate and spread, while providing advantages to the overall survivability of a colony, such as increased resistance to antimicrobials and host-immune responses.Staphylococcus aureus is a highly virulent bacterial strain that is difficult to treat due to its increasing resistance to many antibiotics giving rise to the development of methicillin-resistant (MRSA) and vancomycin-resistant strains.8–11 While MRSA is part of the regular microflora of the human skin and nose, it can cause infection if introduced into wounds or during surgical procedures. MRSA is able to spread via surfaces, such as implant materials, making this infection one of the leading causes of hospital acquired infections.12–15 Similarly, Candida albicans is also a common opportunistic hospital fungal strain that coexists with the microflora on human skin and gastro-intestinal tract.16,17 Only when the immune status of the host is disturbed does the overproduction of C. albicans occur. If introduced into the bloodstream, such as during medical procedures, C. albicans can be associated with an increased mortality rate in patients. Candida infections are also exhibiting drug resistant capabilities to existing antifungal treatments, contributing to the rise in antimicrobial resistance.17–19
Hospital acquired infections (HAIs) of antibiotic resistant microbes on implant surfaces is a serious problem and is becoming an increasing burden to the healthcare system.20–22 High rates of HAIs occurred in intensive care units and in surgical and orthopaedic settings.23 Once a biofilm forms at an implant–tissue interface, it is difficult to treat and remove. Commonly, antibiotics are administered to treat the infection and serious infections can often necessitate implant removal.24–26 While developing new technologies for infection treatment is important, prevention of the spread of highly infectious microbes on surfaces is crucial. Preventing the attachment and further development of biofilm on a surface can be achieved via coatings, nanoparticle deposition and surface topographical features.27–30
Recently, strategies in imparting surfaces with nanostructures as a method of contact killing against microbes have shown promising results. Taking inspiration from cicada and dragonfly wings, nanopillars on these surfaces are able to mitigate the attachment of infection causing microbes.31–35 While there is a lack of consensus in the literature on the mechanobactericidal mechanism of nanostructured surface,32,36,37 a proposed mechanism indicates that the nanostructures physically penetrate through the membrane, interrupting the physiological and biochemical process of the cell membrane and inducing cell lysis. This physical form of cell deactivation is particularly important as it is drug-free and microbes cannot build resistance against it. However, antimicrobial coatings and nanostructured surfaces have the potential to exhibit a major drawback whereby the debris from dead cellular remnants can cover the functionality of the interface.38–44 Buildup of dead bacteria not only decreases the overall efficacy and coverage of the biocidal mechanism but also can trigger an immune response.45 The phenomenon has been described as ‘graveyard effects’ and recent developments in antimicrobial coatings have shown various strategies to circumvent this disadvantage.45 While this issue has been discussed as the main drawback of antimicrobial coatings, rarely has this issue been raised for nanostructured surfaces which are likely to experience the same flaw.
Many studies have shown the effectiveness of nanostructures over relatively short periods of incubation time, typically ranging from 1–24 hours.46–48 While these studies offer initial surface microbiocidal effects, these short incubation times do not reflect real world situations of how the surface would cope with the large cellular debris coverage that occurs after days of incubation.
In the literature, there has been minimal discussion on the so called ‘graveyard effects’ that nanostructures have the potential to experience. When a cell undergoes cell lysis as a result of the physical nanostructure interaction, the internal matrix of the cell spills out and the membrane would leave a covering on the nanostructure, ultimately diminishing its mechanomicrobiocidal capability. While not thoroughly explored, recent studies have offered mitigation methods such as regenerating and degradable surfaces.41,49,50
Here, we aim to elucidate the viability of nanostructured titanium as an antimicrobial surface over prolonged periods of time (1 and 7 days). MRSA and C. albicans were used as model bacterial and fungal cells as they are virulent pathogens that exhibit antimicrobial resistance. Insights into the growth of biofilm over 1 and 7 days indicated a trend of growth of each biofilm atop the differing surface topographies. Synchrotron-based macro attenuated total reflection-Fourier Transform Infrared (macro ATR-FTIR) was used to obtain high-resolution spectral maps of each system, providing the biochemical changes of the lipids, proteins and polysaccharides in these microbial cells under different growth conditions.
2. Experimental section
2.1. Titanium disc surface preparation
Commercially pure titanium discs were cut from Grade 2 titanium rods using a Wire Electrical Discharge Machine to dimensions of 10 mm in diameter and a thickness of 3 mm. These discs were polished using various polishing pads with an average grain size ranging from 30–70 μm. The surfaces were then cleaned in Milli-Q water (18.2 MΩ cm) to remove residue, then sonicated in 1.0 M HNO3 for 30 minutes followed by further rinsing in Milli-Q water. Finally, the surfaces were rinsed in ethanol and dried under a stream of nitrogen gas. This surface is labelled as control.2.2. Hydrothermally etched titanium discs
Etched titanium surfaces were prepared by placing the control (unmodified) discs in 100 mL volume Teflon lined titanium hydrothermal vessels containing 30 mL of aqueous 2.0 M NaOH (Sigma Aldrich). The vessel was then placed within an oven at 150 °C for 3 h. This surface is labelled as nanostructured.2.3. Bacterial strains and growth conditions
Methicillin-Resistant Staphylococcus aureus ATCC® 700699 (MRSA) was obtained from the American Type Culture Collection. For each experiment, bacterial cultures were grown on Luria–Bertani (DIFCO, U.S.A.) agar overnight at 37 °C. Bacterial cells were collected from the culture via an inoculation loop and suspended in Luria–Bertani nutrient broth. The density of the bacterial suspensions was then adjusted to an optical density of 0.1 at a wavelength of 600 nm, where an OD600 of 0.1 is approximately equal to 1 × 106 cells per mL.2.4. Fungal strains and growth conditions
The fungi Candida albicans 18-24511395 clinical isolates were obtained from South Australia Pathology Laboratory. Fungal cultures were cultured on potato dextrose broth agar plates for 2 days at 25 °C. Fungal suspensions were made in potato dextrose broth liquid medium with an OD600 = 0.1.2.5. Prolonged incubation conditions
Samples were incubated in their respective microbial broths in a 6-well plate in a volume adequate to cover the surface of the samples. Replicates were taken from the same, single inoculate and incubated simultaneously to prevent major biological variation. At 25 °C, the samples were incubated in a shaking incubator at 80 rpm to ensure the incorporation of oxygen for the cells and prevent anaerobic microbial contamination. Fresh broth was aliquoted into each well at 24-hour intervals. After 1 or 7 days, the samples were removed and prepared dependent on the analysis technique required, as outlined below.2.6. Confocal scanning laser microscopy (CSLM)
CSLM was performed on a ZEISS LSM 880 Airyscan upright microscope. Bacterial and fungal cells were dyed using a LIVE/DEAD BacLight Bacterial Viability Kit (including SYTO 9 and propidium iodide, Invitrogen Molecular Probes) according to the manufacturer's protocol.51 The SYTO 9 dye binds to nucleic acids in viable and non-viable cells and fluoresces green when excited at 485 nm. Propidium iodide (PI) is only capable of entering cells that are no longer viable once they have undergone significant membrane damage. In addition, PI has a higher binding affinity towards nucleic acids present in bacterial cells than the SYTO 9 dye. PI fluoresces red when excited by a 535 nm wavelength laser, therein representing dead cells. At least three areas of interest were examined, obtaining 3 × 3 imaging grids of 377 μm × 377 μm confocal images for each sample investigated. Z-stack imaging at 1 μm intervals was utilised to generate reconstructed three-dimensional images of the biofilms using the Zen Black software (Zeiss, Oberkochen, Germany).2.7. Biomass volume script
A custom python script was used to compute the red and green pixel count in each confocal data set by iterating through height images and summing the counts of each colour across such images. These values were subsequently combined to provide the total red and green pixel count in each data set.2.8. Statistical analysis
The t-values of the data that underwent statistical analysis were calculated. The t-value obtained for the degrees of freedom of the data set was compared with the critical t-value at the alpha level of 0.05 to obtain the p-value.2.9. Scanning electron microscopy (SEM)
Images of the surfaces of the control and nanostructured titanium were acquired on a FEI Verios 460L SEM operated at 1–3 kV and 16–25 pA using Immersion Mode and Beam Deceleration Mode. For top-view cellular imaging, all samples were affixed using a formaldehyde/glutaraldehyde 3% solution in 0.1 M sodium cacodylate buffer (pH = 7.4) for 3 hours. After this, the cells atop the samples were washed in MilliQ water and dehydrated using a series of 30, 80, 90, and 100% ethanol solutions, air-dried, then coated in a thin film of iridium (4–7 nm in thickness).2.10. Focused ion beam-scanning electron microscope (FIB-SEM)
Cross-sectional images to show interaction of the cells with the titanium was performed using the FEI Scios Dualbeam FIB-SEM. Cells were prepared using the same method as section 2.9. The region of interest was first coated with 100 nm of platinum (Pt) using the electron beam (0.8 nA at 2 kV). Then, a secondary protective layer of Pt was deposited using the gallium ion gun at 0.1 nA at 30 kV for a thickness of 400–500 nm. Ion slicing was operated at 30 kV/0.1 nA. Imaging was then carried out at 3–5 kV and 13–25 pA.2.11. Atomic force microscopy (AFM)
AFM images were obtained using a JPK Nanowizard 4 (JPK BioAFM Business, Am Studio 2D, 12489 Berlin, Germany) operated in QI mode (intermittent force mode) to obtain the height profiles of all surfaces and roughness (Sq) values for 10 μm × 10 μm images. All discs were imaged in air using AC240 cantilevers (Oxford Instrument, Asylum Research, Santa Barbara, CA, USA, nominal spring constant kc = 2 N m−1). All cantilevers were tuned prior to use using the thermal spectrum method in combination with inverse level sensitivity as measured by force spectroscopy, with the diameter of the AFM tip being 8 nm at the apex. Processing of AFM data involved using a combination of the JPK software (https://www.jpk.com/) and the Gwyddion software package.52 Images were used to obtain the height values, and root mean squared (RMS) roughness values. The arithmetical mean height (Sa) was the value selected to represent each surface's RMS.2.12. Synchrotron macro attenuated total reflection-Fourier transform infrared (macro ATR-FTIR) microspectroscopy
Synchrotron macro ATR-FTIR microspectroscopic analysis of the microbial biofilms was performed on the Infrared Microspectroscopy (IRM) beamline at the ANSTO – Australian Synchrotron (Victoria, Australia), using a Bruker Hyperion 3000 FTIR microscope equipped with a liquid nitrogen-cooled narrow-band mercury cadmium telluride detector, which was coupled to a VERTEX V80v FTIR spectrometer (Bruker Optik GmbH, Ettlingen, Germany).The spatially resolved distribution of the chemical functional groups present in the MRSA and C. albicans biofilms was imaged in macro ATR-FTIR mapping mode.53–56 An in-house developed macro ATR-FTIR device equipped with a 250 μm diameter facet germanium (Ge) ATR crystal (nGe = 4.0) and a 20× IR objective (NA = 0.60; Bruker Optik GmbH, Ettlingen, Germany) was used. The unique combination of the high NA objective used in this device and the high refractive index of the Ge ATR crystal when coupled to the synchrotron-IR beam, allowed the surface characterisation of the microbial biofilm samples to be performed at a high spatial resolution down to ∼2 μm at the amide I band (∼1650 cm−1).53
The samples imaged are outlined in section 2.5. To make the substrate appropriate for analysis, when the time point lapsed the substrate was removed from the microbial suspension and left to completely air dry, allowing the macro ATR-FTIR experiment to be performed directly atop the growth environment. Further advantages of this technique include avoiding the use of chemical fixation and sonication and re-suspension of cells removed from the titanium substrate.
In brief, the microbial cells attached on the titanium substrate were placed onto the sample stage of the macro ATR-FTIR unit. The Ge ATR crystal was brought to the focal point of the synchrotron-IR beam, and a background spectrum was recorded in air using 4 cm−1 spectral resolution and 256 co-added scans. After that, the microbial biofilm samples were brought into contact with the sensing facet of the Ge ATR crystal, and a synchrotron macro ATR-FTIR chemical map was acquired. The penetration depth of this technique is <1 μm, providing spectral information that contains the key biochemical composition of the biofilm surfaces.
Every spectrum was collected with a beam defining aperture, providing a nominal measurement area of 3.13 μm diameter per pixel, at 0.5 μm step intervals to further enhance the spatial resolution of the technique. For each pixel, the synchrotron macro ATR-FTIR spectrum was recorded within a spectral range of 3900–750 cm−1 using 4 cm−1 spectral resolution and 8 co-added scans. Blackman-Harris 3-Term apodisation, power-spectrum phase correction, and a zero-filling factor of 2 were set as the default acquisition parameters using the OPUS 8.0 software suite (Bruker).
To generate a chemical map, the raw spectra were processed with 25-point smoothing, baseline corrected using a concave rubberband correction (10 iterations and 64 baseline points), and vector-normalized. These processed spectra were subsequently used to generate chemical maps by integrating the area under the relevant peaks using OPUS 8.0 software.
2.13. Hierarchical cluster analysis (HCA) and principal component analysis (PCA)
The multivariate data analysis including HCA and PCA was performed using CytoSpec v. 1.4.02 (Cytospec Inc., Boston, MA, USA) and The Unscrambler X 10.3 software package (CAMO Software AS, Oslo, Norway), respectively. In this study, the HCA served as a quality control measure, for selecting clusters for PCA analysis based on the following criteria: (i) high signal-to-noise ratio, (ii) strong amide I peak intensity in 1705–1600 cm−1 spectral range, and (iii) regions of the spectral map that maintained good contact with the Ge crystal. Two spectral ranges, including 3035–2780 cm−1 and 1755–1000 cm−1, were used for both HCA and PCA, as these regions contain the molecular information most relevant to the microbial biofilm samples, particularly protein, lipid, and polysaccharides, as well as nucleic acids (DNA and RNA).54 HCA analysis was carried out using Ward's algorithm and 5 clusters based on processed vector-normalised 2nd derivative spectra.For the subsequent PCA, 2nd derivative spectra were similarly generated using Savitzky–Golay algorithm with 9 smoothing points and a polynomial order of 3, in order to remove the broad baseline offset and curvature.57 In addition, the 2nd derivative spectra were processed by extended multiplicative scatter correction (EMSC), which removes light-scattering artefacts and normalises the spectra to account for pathlength differences.58 This data analysis approach has been used previously for the FTIR analysis of microbial biofilms.59 After EMSC, the PCA was performed using The Unscrambler X 10.3 software package.
3. Results and discussion
3.1. Nanostructured titanium characterisation
The use of nanostructured surfaces as drug-free, self-sanitising surfaces has become a highly investigated area.31,48,60–62 Multiple methods of synthesis include reactive ion beam etching, lithography, chemical oxidation and electrochemical anodisation. Here, titanium was hydrothermally etched to provide a uniform nanostructured morphology via a top down synthesis, as previously described.63–65 The control (unmodified titanium) surface (Fig. 1a) is relatively flat, with the only roughness visible as a result of the polishing protocol. Upon these polished surfaces, titanium nanostructures were synthesised via hydrothermal reaction as described in the Experimental section. The length and width of the structures are dependent on the concentration of etching solution, duration in the oven and the temperature.66 This facile synthesis route allows for uniform structures to form across the surface of the titanium. Fig. 1b shows the morphology of nanostructured titanium to possess a wire-like, mesh structure. Measurements of the surface features were undertaken via AFM and SEM and are summarised in Table S1.†3.2. Prolonged microbial incubation experimental setup
Biofilm growth on surfaces is a major issue in implant associated infections. Often, implants that are overcome with infection causing pathogens that do not respond to prescribed antibiotics, require removal and replacement. This exposes the patient to unnecessary surgeries, putting them at risk of complications. Mitigating infections on surfaces is a large area of interest. Recent research has only investigated the effects of antimicrobial surfaces over short incubation periods ranging from 5 minutes to 24 hours.31,67,68 While investigation of these surfaces over short periods of time are important in understanding the immediate interaction at the cell-nanostructure interface, prolonging the exposure of infectious microbes on these surfaces is important to investigate. Especially when the recovery time for implantation can be a minimum of 3 months and infections can accumulate immediately after insertion or months to years later.69–71 Therefore, in this study incubation times were chosen to understand the effects of continuous cell lysis on the nanostructured titanium surfaces after an extended period of time.To minimise biological and external (temperature, time etc.) variations in the experiment, the incubation for surface and time point replicates were carried out simultaneously. Inoculations for both replicates were taken from the same parent inoculate and the broth for each well was refreshed when 24 hours elapsed since the previous refresh for the 7 day incubation (Fig. 2). The chosen microbial suspension was then added to the well plate and the volume added was enough to cover the surface of the titanium. First attempts at experimental replicates caused large standard deviations in the CLSM results when the data was analysed (Fig. S1†). This may have been caused by experiments taken from different agar plates as bacteria and fungi can inherently exhibit large changes in growth and preferential surface attachment. Therefore, it was found appropriate to perform replicates concurrently to reduce experimental variation.
The experiments were compared in two different ways; first, both surface types were compared at the same time point (i.e., control titanium compared with nanostructured titanium after day 7 incubation) and second, the same surface type compared against two different time points (i.e., nanostructured titanium at day 1 and day 7). This provides not only the differences in evolution in biofilm over 7 days, but also the effect the surface morphology has on the growth of the microbial colony. Further, the biomass data from the randomised regions for each of the titanium replicates are displayed in Fig. S2† and shows the variations in the microbial growth in response to the surface.
3.3. Visualising microbial debris accumulation on nanostructured surfaces
To visualise the interactions of the microbes with the titanium surfaces, SEM was employed to obtain representative images of the cell–surface interaction after 1 and 7 days incubation (Fig. 3). MRSA on the control (unmodified titanium) was seen to have good morphology, with the integrity of the cells maintained, indicating the MRSA is in a healthy, viable condition after 1 day. At 7 days, MRSA shows signs of changes in morphology as the biofilm matures, with the elongation of the cell aiding in attachment to the titanium. Comparatively, the C. albicans response was similar to the MRSA with the integrity of the fungal cells maintained after 1 day, but at 7 days there are signs of dead cell remnants with the cells that are still viable having achieved irreversible attachment.72,73 On the nanostructured titanium, MRSA exhibited a large production of EPS on the surface, obscuring the nanostructures (Fig. 3 and S3†). Low- and high-resolution images of the nanostructured surface covered by EPS and instances of MRSA producing EPS can be observed in Fig. S3c and d.† The EPS coating appeared well developed at day 7, with MRSA cells grown atop the EPS-covered nanostructures. For C. albicans, the cell response after 1 day shows interaction with the nanostructured surfaces and a slight covering of the nanostructured surfaces was seen after 7 days.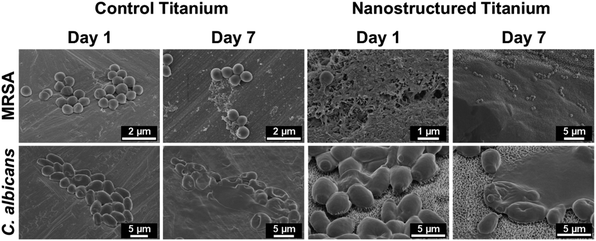 | ||
| Fig. 3 SEM images of MRSA (top) and C. albicans (bottom) grown on control (left) and nanostructured (right) titanium after 1 and 7 days. | ||
Further elucidation of the cell–surface interaction was investigated using FIB-SEM (Fig. 4). Cross-sectional images comparing the attachment of cells on unmodified titanium and nanostructured titanium revealed the differences in how the cells adhere. MRSA and C. albicans both appear to adhere in a continuous attachment to unmodified titanium. The cell's interaction with the nanostructured titanium surface shows the irregular contact points of the surface with the cell membrane. Once a cell has adhered to the nanostructure, the biofilm is not the component that interacts with the surface and therefore these images are only indicative of the cell–surface interaction that leads to biofilm accumulation.
 | ||
| Fig. 4 FIB-SEM cross-sectional images of MRSA (top) and C. albicans (bottom) on control (left) and nanostructured titanium (right). | ||
While these images appear to indicate that the biomass is less apparent than that of the CLSM images (Fig. 5 and 6), it is important to understand that the preparation of the samples for each characterisation technique involves different steps. Of the many preparation methods for SEM imaging of cellular samples,74–77 the protocol outlined in the Experimental section was chosen to cause the least disruption to the attached cells, while still making the sample viable for SEM viewing.
3.4. Detection of development of biomass on nanostructured titanium
Fluorescence imaging of stained microbial cells were collected using CLSM at day 1 and 7. Fig. 5 shows the response of MRSA after 1-day and 7-days incubation. The unmodified, control titanium surface shows an average of 3.3 ± 2.6% dead MRSA cells after 1 day incubation and a healthy coverage of cells over the titanium with the biomass volume at (3.37 ± 1.51) × 105 μm3. Side views of the Z-stack in Fig. 5 show both surface attached and planktonic cells. Custom python code provided the pixel number for each pixel that fluoresced in a CLSM scan, directly translating to the biofilm amount. Counting the total pixels that fluoresce in an image rather than using thresholding methods provides a more accurate way of comparing biofilm growth by taking into account the variations in cell–surface coverage and biofilm formation between time points and surface types. Nanostructured titanium exhibited similar biofilm growth as the control titanium surface. However, the death rate on nanostructured titanium was much greater and reached 56.5 ± 27.9% for MRSA and showed a biomass of (5.16 ± 1.56) × 105 μm3 after one-day.At the 7-day mark, the control titanium surface was overwhelmed with biovolume of MRSA cells and of the (3.88 ± 1.60) × 106 μm3 of biomass volume, only 4.9 ± 2.8% were dead cells. Comparing the biomass volume of the nanostructured surface after 7 days ((2.81 ± 0.81) × 106 μm3), the volume was similar to the control surface ((3.88 ± 1.60) × 106 μm3) when the error is taken into account. Of the biomass on the nanostructured surface, the percentage of dead cells was 12.1 ± 5.8% of MRSA after 7-days.
The growth of C. albicans had a similar trend to MRSA when comparing the control and nanostructured titanium surfaces. At the 1-day mark, both of the surface types exhibited a comparable biofilm growth after 1 day incubation (Fig. 6) with the surfaces having an average biomass of (6.58 ± 7.50) × 105 μm3 on the control and (1.02 ± 1.18) × 106 μm3 on the nanostructure. The large standard deviation in the measurement shows the natural variation in biological behaviour of C. albicans on replicates of the same surface type (incubated in separate well-plates) and the variations of the cell communities on the same surface (Fig. S2†). CLSM images were taken in randomised areas on a single surface (Fig. 2) and visually significant differences in the growth and attachment of the cell colonies were seen only a couple of hundred micrometres apart. Multiple scans were taken to include the varied cellular response (Fig. S2†). Minimal death rate was observed for the control and nanostructured surfaces after 1 day, 3.8 ± 4.6% and 5.4 ± 4.2%, respectively.
At the 7-day incubation time point, C. albicans growth on the nanostructured surface had a larger average biomass volume ((4.21 ± 2.64) × 106 μm3) when compared to the control titanium response ((2.44 ± 1.71) × 106 μm3). The difference in fungal death rate between both surface types was negligible when the error is taken into account with the control surface exhibiting 1.0 ± 1.4% death and the nanostructure surface exhibiting 2.1 ± 3.5% fungal death. Here, the large standard error shows the natural variation in the attachment behaviour of mature fungal biofilms. This is further evident in Fig. S2,† where CLSM imaging of at least 3 randomised areas on one surface greatly vary. It is important to note the overall differences between the antimicrobial efficiencies of the nanostructured surface between MRSA and C. albicans have previously been studied by our group.64 This difference in effectiveness can be attributed to the distinction in composition of the microbial membranes between bacterial and fungal strains. These factors contribute to how they respond to the nanostructure upon initial adhesion and how they undergo cell lysis on the surface.
Nevertheless, these experiments display the inability for nanostructured surfaces to: (1) greatly inhibit biofilm growth after 7-days incubation and (2) show a greater amount of mechanomicrobiocidal activity after 7-days when compared to the control surface with no antimicrobial activity.
3.5. Biomass volume change as dependent on exposure time
Observing the progression of biofilm production on the control and nanostructured surfaces is important in understanding the evolution of biofilm on surfaces over periods of time longer than the common, shorter incubation time periods typically used in other experimental studies. While shorter incubation times are suitable for understanding the initial attachment mechanisms of bacteria and fungi on a surface, as the microbes mature and produce biosensing molecules in response to the surface properties, there are notable changes in the biofilm growth surface.The biomass increase between 1 day and 7 days on the control surfaces was 11.5-fold for MRSA (Fig. 5i) and 3.7-fold for C. albicans (Fig. 6i) and on the nanostructured surfaces the increase was 5.4 fold for MRSA (Fig. 5i) and 4.1 fold for C. albicans (Fig. 6i). It is well known that bacteria are able to adapt to an environment, attach preferentially and ensure the survival and proliferation of the colony.78–80 Bacterial adhesion consists of four main stages: surface sensing, attachment, colonisation and biofilm maturation and migration. During the last stage, bacteria cluster together via adhesin molecules, extracellular DNA (eDNA) and cell wall proteins that aid in the formation of microcolonies.81 As the biofilm matures, the ECM made up of bacterial polymers and eDNA of dead bacterial or host cells is produced further to protect the colony and ensure an ideal environment for nutrients and intercellular communication.82,83 Since these nanostructured surfaces examined here do not exhibit antifouling properties, that is these surfaces do not prevent microbial attachment, it is unsurprising that the biofilm volume increases from day 1 to day 7 and that a higher biofilm volume is observed after 1 day on the nanostructured surface compared to the control (Fig. 5i and 6i). Nanotextured surfaces have a greater surface area and provide a better environment for cellular debris to accumulate when cells lyse and is discussed further in section 3.8. This is further evident in the decrease in dead cells on the nanostructured surface after 7 days to 12.1 ± 5.8% from 56.5 ± 27.9% after 1 day for MRSA (Fig. 5ii).
C. albicans followed the same trend for biofilm growth as MRSA, but to a lesser extent. The control surface exhibited an increase in biofilm growth of 73% from 1 day incubation to 7 days (Fig. 6i). The death rate for C. albicans was comparable between day 1 and day 7 when the standard deviation is taken into account. The nanostructured titanium surface saw an increase in cellular attachment and growth and a decrease in death after 7 days (Fig. 6ii).
From these findings, it is evident that nanostructures do not maintain antimicrobial activity after 7 days. The nanostructures become coated with the cellular debris of lysed cells, covering the contact points and forming a viable, ‘primed’ foundation for planktonic cells to become sessile in ideal conditions. SEM imaging of the nanostructured surfaces shows the mature biofilm does cause an increase in EPS production (Fig. 3).
3.6. Analysis of biofilm production using synchrotron macro ATR-FTIR microspectroscopy
High-resolution synchrotron macro-ATR-FTIR microspectroscopy was utilised to probe changes in the biochemical compositions of the MRSA and C. albicans cells at 1 and 7 days of growth atop control titanium and nanostructured titanium. The two main spectral ranges containing the key biological information of the microbes, and the biofilms are 2980–2800 cm−1 and 1755–1000 cm−1. These include ν(C–H) stretching modes of methyl/methylene groups (2980–2800 cm−1) indicative of lipids, amide I band (1695–1590 cm−1) representative of proteins, ν(PO2−) of the phosphodiester backbone of nucleic acids (DNA and RNA) at ∼1080 cm−1, as well as ν(C–O) and ν(C–C) of –CH2OH skeleton structure of polysaccharides (1175–1000 cm−1). Representative spectra for all samples are shown in Fig. S4.† Areas with high absorption intensity of the amide I and methyl/methylene groups indicate high concentrations of cells.54,84 Synchrotron macro ATR-FTIR spatio-chemical maps were produced, providing spatial distribution of proteins, lipids and polysaccharides in the MRSA and C. albicans cells grown on the surface of control and nanostructured titanium substrates (Fig. 7 and 8). | ||
| Fig. 7 Synchrotron macro ATR-FTIR chemical maps observed for MRSA at day 1 (a–f) and day 7 (g–l) on control titanium and nanostructured titanium, which were obtained by integrating areas under ν(C–H) peaks of CH2/CH3 groups for lipids (top row), amide I band for proteins (middle row), and ν(C–O)/ν(C–C) peaks of polysaccharides (bottom row). Colour scale from dark blue to white corresponds to minimum to maximum integrated peak values. Scale bar represents 20 μm. For chemicals maps with the absorbance intensity maximised per sample, see Fig. S5.† | ||
 | ||
| Fig. 8 Synchrotron macro ATR-FTIR chemical maps observed for C. albicans at day 1 (a–f) and day 7 (g–l) on control titanium and nanostructured titanium, which were obtained by integrating areas under ν(C–H) peaks of CH2/CH3 groups for lipids (top row), amide I band for proteins (middle row), and ν(C–O)/ν(C–C) peaks of polysaccharides (bottom row). Colour scale from dark blue to white corresponds to minimum to maximum integrated peak values. Scale bar represents 20 μm. For chemicals maps with the absorbance intensity maximised per sample, see Fig. S6.† | ||
After 1 day of exposure on the control titanium, MRSA expressed a greater average absorbance from the protein signals, which decreased when compared to the nanostructured titanium response (Table S2†). However, as the cells matured, the polysaccharide signals become less defined for MRSA after 7 days on nanostructured titanium. C. albicans on the control and nanostructured titanium exhibited an increase in average absorbance of polysaccharides after 7 days (Table S3†). This may suggest greater expression in the cellular structure responsible for adhesion in the C. albicans membrane, such as chitin and glucans that mainly consist of polysaccharides.85 Resolving the effects of the changes in biochemical signals of the microbes is complex and requires the use of multivariate data analysis.
3.7. Changes in biochemical signals influenced by nanostructured titanium and exposure time
As it is difficult to ascertain the differences and slight variations through spectral and spatiochemical comparison, multivariate data analysis including HCA and PCA was applied to increase the discriminatory accuracy among spectra. First, the second derivatisation was applied to each set of spectra to enhance the features of hidden and overlapping bands, prior to noise reduction and vector normalisation. Secondly, HCA was applied onto the pre-processed normalised 2nd derivative spectra as described previously in the Experimental section. High quality spectra were selected from the HCA to ensure low-quality spectra were filtered out for the subsequent PCA analysis (Fig. S4†). Lastly, the spectral clusters from the HCA analysis were pretreated via EMSC before PCA was performed to provide greater data interpretability by normalising the spectra. Using PCA reduces the dimensionality for analysis of large datasets, giving a greater distinction of the spectral changes in forms of score and loading plots for comparison purposes.54,86,87 In this study, the intensity of the loadings for each principal component (PC) larger than 0.05 (or smaller than −0.05, i.e., ±0.05) are designated as significant.Using the PCA approach described above, the spectra datasets of the microbial cells grown on the two titanium surfaces (i.e., control and nanostructured titanium), were compared for day 1 and day 7 of incubation using the first 2 PCs. The PC1 represents the direction of maximum variance in the data, capturing the most significant pattern or trend, whilst PC2 captures the second most significant variance that represents the next dominant trend after accounting for PC1. Fig. 9 displays the plotted PCA score values obtained for MRSA for each sample type. PC1 describes 98% with PC2 describing 1% of the spectral variation for MRSA at day 1 (Fig. 9a). On day 7, PC1 and PC2 account for 86% and 7%, respectively (Fig. 9d).
Firstly, the separation between the two datasets in the PCA score plot along PC1 axis strongly suggests distinct differences in biochemical signatures between cells grown on the two titanium surfaces (Fig. 9a and d). Specific biochemical components (i.e., functional groups) in the cellular constitutes that influence the differentiation between these two datasets (i.e., two types of titanium substrates) can be identified using the PC1 loading plots. After 1 day of incubation on the control and nanostructured titanium surfaces, the PC1 loadings indicate that the key differences involve protein, nucleic acid and polysaccharide components. In particular, the positive loadings of amide I/II bands at 1630 cm−1 and 1540 cm−1 were found to be stronger in the control group, suggesting a higher protein proportion in the cells attached to the control titanium surfaces. Similarly, the substantial positive loadings at ∼1200 cm−1 and 1160 cm−1, which represent ν(PO2−) stretches of the phosphodiester backbone of nucleic acids (DNA and RNA) and ν(CO–O–C) of glycogen, respectively, further indicates higher amounts of nucleic acid and polysaccharide components in the control dataset.86 As MRSA is a Gram-positive bacterium, it is encased in a peptidoglycan layer that is predominantly made up of polysaccharides.88 Therefore, this cellular matter is the primary component that interacts with (and attaches to) the surface. The loaded peaks in this spectral region are only significant in the first PC, suggesting that the majority of the spectra that were compared, exhibit differences in the polysaccharide composition or concentration. This biochemical change would primarily be influenced by the contrast in dead cell percentage (Fig. 5) between the control and nanostructured titanium surface after 1 day of incubation.
On the other hand, the PC1 loading plot obtained after 7 days of incubation reveals lipids and proteins as the most influential components leading to the separation of the two groups in the score plot. Specifically, the positive loaded peaks at 1621 cm−1 (amide I: antiparallel β-sheets), and 1727 cm−1 and 1388 cm−1 (i.e., ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of lipid esters and δ(CH3/CH2) of lipids, respectively) indicate slightly higher proportions of protein and lipids in the control dataset, while the strong negative loaded peak at 1672 cm−1 suggests a substantial increase of protein components in the cells attaching to nanostructured titanium surfaces and relating to protein in the β-turn conformation.86
O) of lipid esters and δ(CH3/CH2) of lipids, respectively) indicate slightly higher proportions of protein and lipids in the control dataset, while the strong negative loaded peak at 1672 cm−1 suggests a substantial increase of protein components in the cells attaching to nanostructured titanium surfaces and relating to protein in the β-turn conformation.86
Secondly, the clustering density and spreading distance observed on each cluster also reflects divergence in the spectra collected and is related to biochemical variation within the population, which mainly involves the variation of lipid and protein proportions within the same group as evidenced through the PC2 loading patterns. Variance in healthy cells can be attributed to the regular divergence of the microbial population in the scanned region. According to the score plot along the PC2 axis (Fig. 9a), the MRSA cells on the control titanium surface at day 1 exhibit a slightly larger chemical variation when compared to those grown on the nanostructured titanium surface, which agrees well with the growth patterns observed in Fig. 5. The results from both techniques indicate that nanostructured titanium substrates led to increased cell death for MRSA after 1 day on the surface. Furthermore, Fig. 9b shows the PCA score plot obtained at day 7 for MRSA cells, which reveals an increase in clustering distance along PC2 axis for the cluster corresponding to the nanostructured surfaces when compared to the same nanostructure surfaces on day 1. Furthermore, the clustering distance within the populations along the PC2 axis observed for the nanostructured titanium surface appears to be substantially larger in day 7 compared to day 1, while it remains relatively the same for those of the control group. This suggests that the biochemical variation in the control group remains the same in day 1 and day 7 according to the clustering distance along PC2 axis. The nanostructured surface, on the other hand, led to a larger variation in the cell population. Based on the PC2 loading plot observed for day 7, the key variation in biochemical components involves a loaded peak at 1706 cm−1 representative of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching mode of total lipids, as well as those at 1673 and 1629 cm−1 related to protein in β-turn and anti-parallel β-sheet secondary structures, respectively.
O) stretching mode of total lipids, as well as those at 1673 and 1629 cm−1 related to protein in β-turn and anti-parallel β-sheet secondary structures, respectively.
Lipids were more strongly detected in the MRSA grown atop control titanium after 1 and 7 days and can likely be attributed to the EPS expression in the cells to form a biofilm. Interestingly, the biofilm atop the nanostructured surfaces expressed a stronger presence of protein components over lipid presence. MRSA possess the strong capacity to irreversibly attach to surfaces and possess multiple mechanisms for adhesion. Being a Gram-negative bacterium, MRSA has a bilayer membrane, with the outer layer containing multiple proteins from the ‘microbial surface components recognising adhesive matrix molecules’ family as well as the glycocalyx (made up of glycoproteins and polysaccharides) which aid in adhesion to surfaces and other bacteria in the EPS.89–91 The glycocalyx contributes to biofilm maturation and forms electrostatic and hydrogen bonds between the EPS and the surface.92 Further, exoenzymes are produced to degrade the EPS to provide the colony with carbon and energy once the bacteria accumulate into multilayer cell clusters.91 Recently discovered phenol-soluble modulins (PSMs) are a family of short, amphipathic peptides found in pathogenic staphylococci that can be α-helical or β-fold and form the structure of biofilm and surface-attached agglomerations around the bacterial cell.93,94 The role of PSMs aid in the detachment of cells for proliferation and form biofilm channels to transport nutrients within the colony.95–97 Mechanical stress on staphylococcal surface proteins can change the conformation of the protein structure, increasing their adhesive ability.98,99 Differences in the protein expression atop nanostructured titanium in this study, suggests that nanostructures can induce changes in protein expression due to the mechanical stress on the membrane and further, enzyme production by MRSA can increase as the biofilm increases to support nutrient transport.
For interaction of C. albicans with the control and nanostructured titanium surfaces, the PCA score plots obtained on both day 1 and day 7 show separation between the two datasets from different titanium surfaces (Fig. 10a and d). This indicates that the biofilm colonies possess diverse biochemical compositions when growing on different titanium structures. By comparing the PC1 loading plots, the biochemical changes observed in C. albicans are different from those of MRSA. Here, the PC1 loadings for C. albicans on day 1 indicate a strong negative loading at ∼1160 cm−1 (i.e., ν(CO–O–C) of glycogen) with an observable negative loading at ∼1690 cm−1 (i.e., amide I), which together suggest higher proportions of polysaccharides and aggregated β-sheet secondary protein structures in the nanostructured titanium group (Fig. 10b). The cells on the control surfaces, on the other hand, possess slightly higher lipid compositions according to the positive loaded peaks at ∼2930 cm−1 and ∼1380 cm−1, which can be assigned to ν(C–H) stretching and δ(CH3/CH2) bending modes of lipids, respectively (Fig. 10b).
The PC1 loading plot obtained after 7 days of incubation shows that the key changes are now the nucleic acids at ∼1090 cm−1 (i.e., ν(PO2−) stretches of the phosphodiester backbone) and carbohydrates at ∼1050 cm−1 (i.e., ν(C–O) coupled with δ(C–O) of C–OH groups of carbohydrates) – both of which appeared to be stronger in the control group (Fig. 10e). On the contrary, the cells attached onto the nanostructured titanium surfaces present a distinctively strong loading at 1025 cm−1, suggesting an increase in polysaccharide components (i.e., ν(C–C) coupled with δ(CH2) of α-CH2 in –CH2OH groups of polysaccharides) (Fig. 10e).
Unlike the MRSA, the dispersion of the data points along PC2 for C. albicans showed a substantially higher biochemical variation in the control group after day 7, while the variation within the cells grown on the nanostructured titanium substrate remains relatively the same. According to the PC2 loading plot observed for day 7 (Fig. 10f), the key variation in biochemical components include the strong loaded peak at 1155 cm−1 attributable to νas(CO–O–C) of glycogen and nucleic acids (DNA and RNA), with some contribution from the loading at 2920 cm−1 from ν(C–H) from methylene (–CH2) groups of lipids and amide I of protein at ∼1650 cm−1. C. albicans is a yeast-type fungi with the cell wall being made up of 80–90% polysaccharides, 6–25% proteins and 1–7% lipids while the biofilm matrix comprises of 55% proteins and their glycosylated counterparts, 25% of carbohydrates, 15% of lipids, and 5% of noncoding DNA.100,101 Mostly, polysaccharides are cross-linked to proteins and surrounding adhesin molecules. Glycoproteins facilitate adhesion and cell–cell aggregation and linked with lipids such as glycosylphosphatidylinositol.102 This complex array of interlinked biomolecules means it is difficult to discern why one biochemical region is expressed more greatly than the other. Studies investigating the effect of micro- and nano-pillar surfaces suggest that elevated levels of protein in C. albicans occurs under external, mechanical stress, leading to the hypothesis that C. albicans is able to adapt to environmental stresses.103,104 Kollu et al. investigated the effect of nanostructured surface interactions with C. albicans and found that the concentration of the polysaccharide chitin increased in the first 4 hours of exposure and decreased to similar levels as the control surface after a further 4 hours.105 They suggested that the transient chitin increase occurred from the stress response of the nanostructured surfaces and the C. albicans is able to recover from the mechanical stress or the nanostructures stop being a challenge to the C. albicans cell. Consequently, the only strong protein peak from C. albicans was detected at day 1 incubation on the nanostructured titanium (Fig. 10c), correlating with the notion that protein expression increases under external stresses and at day 7, C. albicans is able to adapt to the nanostructure, reducing the protein concentration and regulate the production of other important biomolecules. This adaption to the nanostructure may correlate with the decrease in dead cells and increase in biofilm volume observed in Fig. 6.
3.8. Understanding the research climate of nanostructured titanium surfaces
Antimicrobial surfaces have been heavily researched as the next step in implant infection mitigation. Changes in surface chemistry, such as through coatings or nanostructures, can provide an alternative method to reduce adhesion or kill attaching bacteria and fungi upon contact. Many studies examine the effects of these engineered surfaces over a short amount of time (5–30 min,31,67 1–6 hours,37,106 18–24 hours68,107) and overlook extended exposure times of microbes on a nanostructure, especially in understanding the biochemistry of the biofilm that develops. Hayles et al. found nanostructured titanium increases the sensitivity of C. albicans towards antifungal drugs after 3 days of biofilm growth.108 Cao et al. incubated Staphylococcus epidermidis on nanostructured titanium for 6 days and determined that the anti-biofilm mechanism is dependent on the morphology of the nanostructures.46 In a study by Wandiyanto et al., osteoblast cells were able to attach and proliferate on nanostructured titanium pre-infected with Pseudomonas aeruginosa or S. aureus.109 Self-cleaning abilities have been investigated by Nguyen et al. on silicon nanopillar arrays that showed bacterial debris would begin to self-remove after 10 to 20 minutes of monitoring for both P. aeruginosa and S. aureus.107 However, the findings in this paper suggest that the situation is likely more complex. We find that MRSA and C. albicans biomass growth increases atop nanostructured surfaces and microbial death declines at 7 days. Investigation of the biomass using macro ATR-FTIR revealed that MRSA and C. albicans colonies atop nanostructured titanium adjusted the expression of protein at 7 days of exposure, suggesting an increase in virulence at a macromolecular level to overcome mechanical stresses and continue biofilm production.Further, we observe that the nanostructured surface loses its inherent, passive, physical antimicrobial properties due to the “graveyard effect”. This theory was proposed by Elbourne et al. where they described that the accumulation of bacterial debris (membrane and intracellular components) post cell lysis would hinder further mechano-microbiocidal interactions and therefore decrease antimicrobial efficiency.45 Several studies also have discussed this drawback41–44,46,110,111 and suggested that debris from dead cells can provide nutritional and structural support for further biofilm development,112–114 similar to the role of dead bacterial cells within the biofilm.115 Multiple mechano-microbiocidal mechanisms have been proposed in the literature including adhesion and penetration,116–120 stretching of the membrane,116,117,120,121 nanostructure bending,122–125 and surface-induced biological stress (reactive oxide species (ROS) production).37,126,127 This has been widely reported on nanostructures of differing morphologies ranging from ordered, uniform pillar arrays31,128 and multidirectional, heterogeneous structures48,61 of various dimensions. It is understood that this mechanism is mainly physical, as proven by the landmark study by Ivanova et al. and it is hypothesised that the nanopillars penetrate the membrane and induce cell lysis.31 Computational modelling of nanopillar arrays suggested that once a bacterium is adhered to nanopillars, the suspension of the cell between the pillars induces stress and stretches the membrane beyond the deformation limit where the cell then splits open.117 Other modelling suggests the dimensions of the nanopillars, such as radius, spacing and length, have an influence on the mechanical penetration of the structures into the membrane.129,130 It has also been proposed that the EPS layer produced by Gram-negative bacteria is involved in the strong adhesion on the nanopillars leading to shearing of the membrane.32 This was later refuted as EPS takes significantly longer to develop when the mechanobactericidal mechanism has been observed over shorter time frames.36 Recently, a few studies added an additional mechanism that suggested the mechanical stress from the nanostructures induces ROS production in the cells which triggers the bacteria to self-destruct.126,127 Due to these many conflicting proposed mechanisms, the true microbial mode of action is still up for debate. However, each circumstance relies on the ability of the microbe to directly interact with the interface. In this study, the colonised bacteria and subsequent dead microbial cells disrupt any direct contact that could be made to the interface, forming a biological scaffold that new bacterial and fungal cells (whether planktonic or in the process of replication) can colonise and thrive unimpeded by the now covered nanostructure.
To overcome this, technologies need to incorporate nanostructures alongside secondary and tertiary antimicrobial techniques. Previous functionalisation of antimicrobial surfaces has involved metal nanoparticles,63,131,132 hydrogel layers50,133,134 and antibiotics108,135,136 that can dissolve away from the surface and kill the microbes in solution or that have not made direct contact with the surface. These surfaces also have a drawback in that this secondary mechanism can be depleted over time. New studies are investigating self-refreshing materials with that main aim to ‘kill and release’ bacteria. These ‘smart’ surfaces can switch between microbial killing and microbial releasing and often require stimuli to trigger the release of the killed bacteria to maintain a sufficient level of antibacterial performance and reusability. The stimuli can be temperature,137,138 light,139,140 pH,141,142 electrical,143 magnetic144 or hydration145,146,146 responsive. Some materials have been developed to respond to the infection itself and initiate response to release the bacteria via the acidic microenvironment caused by bacterial metabolism.142 Physical, on-demand dynamic deformation of nanoscale structures was observed to remove adhered bacteria using MXene multilayers.147 These materials that respond to external or environmental changes to reduce the accumulation of killed bacteria would provide nanostructured, antimicrobial surfaces that rely on contact killing to be effective to regenerate and extend the life of medical devices and reduce the development of antimicrobial resistance.
4. Conclusion
Nanostructured titanium surfaces have been investigated to be a viable surface to naturally mitigate the proliferation of bacteria, fungi and other micro-organisms on materials more commonly used for medical devices. These studies are often conducted under minimum incubation periods, such as 1 to 24 hours, which is not applicable in the clinical application of these materials if implanted within the body. Therefore, prolonged incubations were conducted in this study to compare the response of MRSA and C. albicans on control titanium and nanostructured titanium at 1 and 7 days. The response for MRSA after 1 day on the nanostructured surface showed an increase in bacterial cell death and had a similar average biomass volume when compared to the untreated titanium. After 7 days, MRSA had greater biofilm growth than the C. albicans. C. albicans also exhibited a similar trend in biofilm growth and cellular death to the MRSA but as the errors are greater in the C. albicans data, a distinct trend was more difficult to discern. Taking the errors into consideration, C. albicans had a greater increase in biomass growth and decrease in dead cells on the nanostructured surface after 7 days compared to the control surface and 1 day incubation for both surfaces. The overall trend from these experiments indicates that nanostructured surfaces exhibit increased biomass volume and decreased antimicrobial efficacy after 7 days when compared to 1 day. Synchrotron macro ATR-FTIR spatio-chemical maps provided distribution of proteins, lipids and polysaccharides in the cells grown on the surface of control and nanostructured titanium substrates. Nanostructured titanium caused protein expression in MRSA to increase after 7 days, whereas C. albicans initially expressed greater protein concentrations at 1 day while protein absorbance was not highly detected at 7 days. These findings suggest the ability of MRSA and C. albicans to adapt at a macromolecular level and overcome the external, mechanical stresses of the mechano-microbiocidal surface. For nanostructured materials to become effective in medical devices and surgical settings, it is important to take into account the accumulation of killed microbial cells that prevent the main mechanomicrobiocidal mode of action to occur. Further developments in nanostructured titanium would provide long lasting antimicrobial performance and provide a method of mitigating infections of antimicrobial resistant pathogens.Data availability
All relevant data are within the manuscript and its additional files. Further clarification on data is available upon request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. Z. Y. H., R. P., S. G., and Z. L. S. acknowledges this research was supported by AINSE Ltd. Postgraduate Research Awards. A. E. is supported by an Australian Research Council (ARC) Discovery Early Career Research Award (DECRA) (DE220100511). R. A. C. acknowledges an ARC Discovery Project (DP180103815). The synchrotron macro ATR-FTIR experiment was performed on the IRM beamline at the Australian Synchrotron, part of ANSTO, through the merit access (Proposal ID. 19281 and 19590). The authors acknowledge the facilities, and the scientific and technical assistance of the RMIT Microscopy & Microanalysis Facility (RMMF), a linked laboratory of Microscopy Australia, enabled by NCRIS.References
- H. Chen, X. Zhou, B. Ren and L. Cheng, Virulence, 2020, 11, 337–348 CrossRef CAS.
- H. H. Tuson and D. B. Weibel, Soft Matter, 2013, 9, 4368–4380 RSC.
- L. Wang, D. Fan, W. Chen and E. M. Terentjev, Sci. Rep., 2015, 5, 15159 CrossRef CAS.
- C. A. Kumamoto and M. D. Vinces, Annu. Rev. Microbiol., 2005, 59, 113–133 CrossRef CAS.
- D. Lopez, H. Vlamakis and R. Kolter, Cold Spring Harbor Perspect. Biol., 2010, 2, a000398–a000398 Search PubMed.
- R. M. Donlan, Emerging Infect. Dis., 2002, 8, 881–890 CrossRef PubMed.
- J. W. Costerton, Z. Lewandowski, D. E. Caldwell, D. R. Korber and H. M. Lappin-Scott, Annu. Rev. Microbiol., 1995, 49, 711–745 CrossRef CAS PubMed.
- H. F. Chambers and F. R. DeLeo, Nat. Rev. Microbiol., 2009, 7, 629–641 CrossRef CAS PubMed.
- F. D. Lowy, J. Clin. Invest., 2003, 111, 1265–1273 CrossRef CAS PubMed.
- P. D. Stapleton and P. W. Taylor, Sci. Prog., 2002, 85, 57–72 CrossRef CAS PubMed.
- W. A. McGuinness, N. Malachowa and F. R. DeLeo, Yale J. Biol. Med., 2017, 90, 269 CAS.
- M. Ribeiro, F. J. Monteiro and M. P. Ferraz, Biomatter, 2012, 2, 176–194 CrossRef.
- D. Campoccia, L. Montanaro and C. R. Arciola, Biomaterials, 2006, 27, 2331–2339 CrossRef CAS.
- A. S. Lee, H. de Lencastre, J. Garau, J. Kluytmans, S. Malhotra-Kumar, A. Peschel and S. Harbarth, Nat. Rev. Dis. Primers, 2018, 4, 18033 CrossRef.
- M. Šiširak, A. Zvizdić and M. Hukić, Bosnian J. Basic Med. Sci., 2010, 10, 32–37 CrossRef PubMed.
- J. G. S. Souza, R. C. Costa, A. A. Sampaio, V. L. Abdo, B. E. Nagay, N. Castro, B. Retamal-Valdes, J. A. Shibli, M. Feres, V. A. R. Barão and M. Bertolini, iScience, 2022, 25, 103994 CrossRef CAS.
- S. Costa-de-Oliveira and A. G. Rodrigues, Microorganisms, 2020, 8, 154 CrossRef CAS PubMed.
- Y. Lee, E. Puumala, N. Robbins and L. E. Cowen, Chem. Rev., 2021, 121, 3390–3411 CrossRef CAS.
- S. G. Whaley, E. L. Berkow, J. M. Rybak, A. T. Nishimoto, K. S. Barker and P. D. Rogers, Front. Microbiol., 2017, 7, 2173 Search PubMed.
- H. W. Boucher, G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg and J. Bartlett, Clin. Infect. Dis, 2009, 48, 1–12 CrossRef PubMed.
- K. Bush, P. Courvalin, G. Dantas, J. Davies, B. Eisenstein, P. Huovinen, G. A. Jacoby, R. Kishony, B. N. Kreiswirth and E. Kutter, Nat. Rev. Microbiol., 2011, 9, 894–896 CrossRef CAS PubMed.
- C. J. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. Robles Aguilar, A. Gray, C. Han, C. Bisignano, P. Rao and E. Wool, Lancet, 2022, 399, 629–655 CrossRef CAS.
- M. K. Abban, E. A. Ayerakwa, L. Mosi and A. Isawumi, Heliyon, 2023, 9, e20561 CrossRef PubMed.
- D. El-Sayed and A. Nouvong, Clin. Podiatr. Med. Surg., 2019, 36, 627–649 CrossRef PubMed.
- J. M. Schierholz and J. Beuth, J. Hosp. Infect., 2001, 49, 87–93 CrossRef CAS.
- A. Kellish, A. Shahi, J. Rodriguez Jr., K. Usmani, M. Boniello, A. Oliashirazi, G. Keneth, D. Fuller, R. Mashru and H. Dolch, Arch. Bone Jt Surg., 2022, 514 Search PubMed.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Nano–Micro Lett., 2015, 7, 219–242 CrossRef CAS.
- C. M. Nobre, N. Pütz, B. König, S. Rupf and M. Hannig, Front. Bioeng. Biotechnol., 2020, 8, 598311 CrossRef PubMed.
- K. Pajor, Ł. Pajchel, A. Zgadzaj, U. Piotrowska and J. Kolmas, Int. J. Mol. Sci., 2020, 21, 5006 CrossRef CAS.
- C. Bergemann, S. Zaatreh, K. Wegner, K. Arndt, A. Podbielski, R. Bader, C. Prinz, U. Lembke and J. B. Nebe, World J. Transplant., 2017, 7, 193 CrossRef PubMed.
- E. P. Ivanova, J. Hasan, H. K. Webb, V. K. Truong, G. S. Watson, J. A. Watson, V. A. Baulin, S. Pogodin, J. Y. Wang, M. J. Tobin, C. Löbbe and R. J. Crawford, Small, 2012, 8, 2489–2494 CrossRef CAS PubMed.
- C. D. Bandara, S. Singh, I. O. Afara, A. Wolff, T. Tesfamichael, K. Ostrikov and A. Oloyede, ACS Appl. Mater. Interfaces, 2017, 9, 6746–6760 CrossRef CAS.
- D. P. Linklater, H. K. D. Nguyen, C. M. Bhadra, S. Juodkazis and E. P. Ivanova, Nanotechnology, 2017, 28, 245301 CrossRef PubMed.
- T. Diu, N. Faruqui, T. Sjöström, B. Lamarre, H. F. Jenkinson, B. Su and M. G. Ryadnov, Sci. Rep., 2014, 4, 7122 CrossRef CAS.
- S. M. Kelleher, O. Habimana, J. Lawler, B. O'reilly, S. Daniels, E. Casey and A. Cowley, ACS Appl. Mater. Interfaces, 2016, 8, 14966–14974 CrossRef CAS.
- D. P. Linklater, S. Juodkazis, S. Rubanov and E. P. Ivanova, ACS Appl. Mater. Interfaces, 2017, 9, 29387–29393 CrossRef CAS.
- S. Zhao, Z. Li, D. P. Linklater, L. Han, P. Jin, L. Wen, C. Chen, D. Xing, N. Ren, K. Sun, S. Juodkazis, E. P. Ivanova and L. Jiang, Nano Lett., 2022, 22, 1129–1137 CrossRef CAS PubMed.
- H. Koo, R. N. Allan, R. P. Howlin, P. Stoodley and L. Hall-Stoodley, Nat. Rev. Microbiol., 2017, 15, 740–755 CrossRef CAS.
- X. Wang, M. Shan, S. Zhang, X. Chen, W. Liu, J. Chen and X. Liu, Adv. Sci., 2022, 9, 2104843 CrossRef CAS.
- D. Huang, J. Wang, K. Ren and J. Ji, Biomater. Sci., 2020, 8, 4052–4066 RSC.
- T. Wei, Q. Yu and H. Chen, Adv. Healthcare Mater., 2019, 8, 1801381 CrossRef CAS PubMed.
- Q. Yu, Z. Wu and H. Chen, Acta Biomater., 2015, 16, 1–13 CrossRef CAS PubMed.
- L. Mi and S. Jiang, Angew. Chem., Int. Ed., 2014, 53, 1746–1754 CrossRef CAS.
- Y. He, X. Wan, K. Xiao, W. Lin, J. Li, Z. Li, F. Luo, H. Tan, J. Li and Q. Fu, Biomater. Sci., 2019, 7, 5369–5382 RSC.
- A. Elbourne, V. K. Truong, S. Cheeseman, P. Rajapaksha, S. Gangadoo, J. Chapman and R. J. Crawford, in Methods in Microbiology, ed. V. Gurtler, A. S. Ball and S. Soni, Academic Press, 2019, vol. 46, pp. 61–92 Search PubMed.
- Y. Cao, B. Su, S. Chinnaraj, S. Jana, L. Bowen, S. Charlton, P. Duan, N. S. Jakubovics and J. Chen, Sci. Rep., 2018, 8, 1071 CrossRef PubMed.
- P. Zhou, S. Long, F. Mao, H. Huang, H. Li, F. He, R. Zhang, L. Ren, J. Chen and S. Wei, Biomed. Mater., 2020, 15, 035002 CrossRef CAS.
- A. Elbourne, V. E. Coyle, V. K. Truong, Y. M. Sabri, A. E. Kandjani, S. K. Bhargava, E. P. Ivanova and R. J. Crawford, Nanoscale Adv., 2019, 1, 203–212 RSC.
- A. Nouri, A. Rohani Shirvan, Y. Li and C. Wen, Smart Mater. Manuf., 2023, 1, 100001 Search PubMed.
- J. Leng, Y. He, Z. Yuan, B. Tao, K. Li, C. Lin, K. Xu, M. Chen, L. Dai and X. Li, Bioact. Mater., 2021, 6, 4670–4685 CAS.
- L. Boulos, M. Prévost, B. Barbeau, J. Coallier and R. Desjardins, J. Microbiol. Methods, 1999, 37, 77–86 CrossRef.
- D. Nečas and P. Klapetek, Open Phys., 2012, 10, 181–188 CrossRef.
- J. Vongsvivut, D. Pérez-Guaita, B. R. Wood, P. Heraud, K. Khambatta, D. Hartnell, M. J. Hackett and M. J. Tobin, Analyst, 2019, 144, 3226–3238 RSC.
- S. Cheeseman, Z. L. Shaw, J. Vongsvivut, R. J. Crawford, M. F. Dupont, K. J. Boyce, S. Gangadoo, S. J. Bryant, G. Bryant, D. Cozzolino, J. Chapman, A. Elbourne and V. K. Truong, Molecules, 2021, 26, 3890 CrossRef CAS.
- Z. L. Shaw, S. Cheeseman, L. Z. Y. Huang, R. Penman, T. Ahmed, S. J. Bryant, G. Bryant, A. J. Christofferson, R. Orrell-Trigg, C. Dekiwadia, V. K. Truong, J. P. Vongsvivut, S. Walia and A. Elbourne, J. Mater. Chem. B, 2022, 10, 7527–7539 RSC.
- R. Penman, R. Kariuki, Z. L. Shaw, C. Dekiwadia, A. J. Christofferson, G. Bryant, J. Vongsvivut, S. J. Bryant and A. Elbourne, J. Colloid Interface Sci., 2024, 654, 390–404 CrossRef CAS PubMed.
- A. Savitzky and M. J. Golay, Anal. Chem., 1964, 36, 1627–1639 CrossRef CAS.
- A. Kohler, C. Kirschner, A. Oust and H. Martens, Appl. Spectrosc., 2005, 59, 707–716 CrossRef CAS PubMed.
- E. Correa, H. Sletta, D. I. Ellis, S. Hoel, H. Ertesvåg, T. E. Ellingsen, S. Valla and R. Goodacre, Anal. Bioanal. Chem., 2012, 403, 2591–2599 CrossRef CAS.
- T. S. Heckmann and J. D. Schiffman, ACS Appl. Nano Mater., 2020, 3, 977–984 CrossRef CAS.
- C. M. Bhadra, V. Khanh Truong, V. T. H. Pham, M. Al Kobaisi, G. Seniutinas, J. Y. Wang, S. Juodkazis, R. J. Crawford and E. P. Ivanova, Sci. Rep., 2015, 5, 1–12 Search PubMed.
- L. Zhao, H. Wang, K. Huo, L. Cui, W. Zhang, H. Ni, Y. Zhang, Z. Wu and P. K. Chu, Biomaterials, 2011, 32, 5706–5716 CrossRef CAS PubMed.
- L. Z. Y. Huang, A. Elbourne, Z. L. Shaw, S. Cheeseman, A. Goff, R. Orrell-Trigg, J. Chapman, B. J. Murdoch, R. J. Crawford, D. Friedmann, S. J. Bryant, V. K. Truong and R. A. Caruso, J. Colloid Interface Sci., 2022, 628, 1049–1060 CrossRef CAS PubMed.
- L. Z. Y. Huang, Z. L. Shaw, R. Penman, S. Cheeseman, V. K. Truong, M. J. Higgins, R. A. Caruso and A. Elbourne, ACS Appl. Bio Mater., 2024, 7, 344–361 CrossRef CAS.
- A. N. Banerjee, V. C. Anitha and S. W. Joo, Sci. Rep., 2017, 7, 1–20 CrossRef.
- A. Jaggessar, T. Tesfamicheal, H. Wang, C. Yan and P. K. Yarlagadda, Procedia Manuf., 2019, 30, 373–379 CrossRef.
- K. Nakade, K. Jindai, T. Sagawa, H. Kojima, T. Shimizu, S. Shingubara and T. Ito, ACS Appl. Nano Mater., 2018, 1, 5736–5741 CrossRef CAS.
- D. P. Linklater, P. H. Le, A. Aburto-Medina, R. J. Crawford, S. Maclaughlin, S. Juodkazis and E. P. Ivanova, Int. J. Mol. Sci., 2023, 24, 1298 CrossRef CAS.
- W. Zimmerli, J. Intern. Med., 2014, 276, 111–119 CrossRef CAS PubMed.
- F. Schömig and M. Putzier, J. Spine Surg., 2020, 6, 772–776 CrossRef.
- M. Zabaglo and T. Sharman.
- C. R. Armbruster and M. R. Parsek, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 4317–4319 CrossRef CAS PubMed.
- M. H. Muhammad, A. L. Idris, X. Fan, Y. Guo, Y. Yu, X. Jin, J. Qiu, X. Guan and T. Huang, Front. Microbiol., 2020, 11, 928 CrossRef PubMed.
- R. Bhattacharya, S. Saha, O. Kostina, L. Muravnik and A. Mitra, Appl. Microsc., 2020, 50, 1–6 Search PubMed.
- J. T. Y. Lee and K. L. Chow, Scanning, 2012, 34, 12–25 CrossRef CAS PubMed.
- S. Nikara, E. Ahmadi and A. A. Nia, J. Agric. Food Res., 2020, 2, 100036 Search PubMed.
- M. Schu, E. Terriac, M. Koch, S. Paschke, F. Lautenschläger and D. A. Flormann, PLoS One, 2021, 16, e0254165 CrossRef CAS PubMed.
- M. Katsikogianni and Y. Missirlis, eCM, 2004, 8, 37–57 CrossRef CAS PubMed.
- Y. H. An and R. J. Friedman, J. Biomed. Mater. Res., 1998, 43, 338–348 CrossRef CAS.
- C. Berne, C. K. Ellison, A. Ducret and Y. V. Brun, Nat. Rev. Microbiol., 2018, 16, 616–627 CrossRef CAS PubMed.
- T. Das, P. K. Sharma, B. P. Krom, H. C. Van Der Mei and H. J. Busscher, Langmuir, 2011, 27, 10113–10118 CrossRef CAS PubMed.
- A. Dell'Anno and R. Danovaro, Science, 2005, 309, 2179–2179 CrossRef.
- N. S. Jakubovics, R. C. Shields, N. Rajarajan and J. G. Burgess, Lett. Appl. Microbiol., 2013, 57, 467–475 CrossRef CAS PubMed.
- S. Kaminskyj, K. Jilkine, A. Szeghalmi and K. Gough, FEMS Microbiol. Lett., 2008, 284, 1–8 CrossRef CAS PubMed.
- R. Garcia-Rubio, H. C. de Oliveira, J. Rivera and N. Trevijano-Contador, Front. Microbiol., 2020, 10, 2993 CrossRef PubMed.
- J. Vongsvivut, P. Heraud, A. Gupta, M. Puri, D. McNaughton and C. J. Barrow, Analyst, 2013, 138, 6016 RSC.
- L. Ong, A. P. Pax, A. Ong, J. Vongsvivut, M. J. Tobin, S. E. Kentish and S. L. Gras, Food Chem., 2020, 332, 127327 CrossRef CAS PubMed.
- T. J. Silhavy, D. Kahne and S. Walker, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414–a000414 Search PubMed.
- T. J. Foster, Trends Microbiol., 2019, 27, 927–941 CrossRef CAS PubMed.
- C. Cucarella, C. Solano, J. Valle, B. Amorena, Í. Lasa and J. R. Penadés, J. Bacteriol., 2001, 183, 2888–2896 CrossRef CAS PubMed.
- N. Merino, A. Toledo-Arana, M. Vergara-Irigaray, J. Valle, C. Solano, E. Calvo, J. A. Lopez, T. J. Foster, J. R. Penadés and I. Lasa, J. Bacteriol., 2009, 191, 832–843 CrossRef CAS PubMed.
- R. V. Sionov and D. Steinberg, Microorganisms, 2022, 10, 1239 CrossRef CAS PubMed.
- C. Mehlin, C. M. Headley and S. J. Klebanoff, J. Exp. Med., 1999, 189, 907–918 CrossRef CAS.
- Q. Peng, X. Tang, W. Dong, N. Sun and W. Yuan, Antibiotics, 2022, 12, 12 CrossRef.
- S. Periasamy, H.-S. Joo, A. C. Duong, T.-H. L. Bach, V. Y. Tan, S. S. Chatterjee, G. Y. C. Cheung and M. Otto, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 1281–1286 CrossRef CAS PubMed.
- M. Otto, Int. J. Med. Microbiol., 2014, 304, 164–169 CrossRef CAS PubMed.
- K. M. Towle, C. T. Lohans, M. Miskolzie, J. Z. Acedo, M. J. van Belkum and J. C. Vederas, Biochemistry, 2016, 55, 4798–4806 CrossRef CAS.
- P. Vitry, C. Valotteau, C. Feuillie, S. Bernard, D. Alsteens, J. A. Geoghegan and Y. F. Dufrêne, mBio, 2017, 8, e01748–e01717 CrossRef CAS PubMed.
- L. F. Milles, K. Schulten, H. E. Gaub and R. C. Bernardi, Science, 2018, 359, 1527–1533 CrossRef CAS PubMed.
- W. L. Chaffin, J. L. López-Ribot, M. Casanova, D. Gozalbo and J. P. Martínez, Microbiol. Mol. Biol. Rev., 1998, 62, 130–180 CrossRef CAS.
- M. Cavalheiro and M. C. Teixeira, Front. Med., 2018, 5, 28 CrossRef.
- T. Kinoshita, J. Lipid Res., 2016, 57, 4–5 CrossRef CAS.
- L. G. Chivukula and D. LaJeunesse, ACS Biomater. Sci. Eng., 2023, 9, 6724–6733 CrossRef CAS.
- P. H. Le, D. P. Linklater, A. Aburto-Medina, S. Nie, N. A. Williamson, R. J. Crawford, S. Maclaughlin and E. P. Ivanova, Adv. Mater. Interfaces, 2023, 2300314 CrossRef CAS.
- N. V. Kollu and D. R. LaJeunesse, ACS Omega, 2021, 6, 1361–1369 CrossRef CAS PubMed.
- S. D. Puckett, E. Taylor, T. Raimondo and T. J. Webster, Biomaterials, 2010, 31, 706–713 CrossRef CAS.
- D. H. K. Nguyen, C. Loebbe, D. P. Linklater, X. Xu, N. Vrancken, T. Katkus, S. Juodkazis, S. Maclaughlin, V. Baulin, R. J. Crawford and E. P. Ivanova, Nanoscale, 2019, 11, 16455–16462 RSC.
- A. Hayles, R. Bright, J. Wood, D. Palms, P. Zilm, T. Brown, D. Barker and K. Vasilev, Adv. Mater. Interfaces, 2022, 9, 2102353 CrossRef CAS.
- J. V. Wandiyanto, V. K. Truong, M. Al Kobaisi, S. Juodkazis, H. Thissen, O. Bazaka, K. Bazaka, R. J. Crawford and E. P. Ivanova, Materials, 2019, 12, 1575 CrossRef CAS.
- X. Chu, F. Yang and H. Tang, Chin. J. Chem., 2022, 40, 2988–3000 CrossRef CAS.
- R. Bright, D. Fernandes, J. Wood, D. Palms, A. Burzava, N. Ninan, T. Brown, D. Barker and K. Vasilev, Mater. Today Bio, 2022, 13, 100176 CrossRef CAS PubMed.
- I. Guzmán-Soto, C. McTiernan, M. Gonzalez-Gomez, A. Ross, K. Gupta, E. J. Suuronen, T.-F. Mah, M. Griffith and E. I. Alarcon, iScience, 2021, 24, 102443 CrossRef.
- Q. M. Parks, R. L. Young, K. R. Poch, K. C. Malcolm, M. L. Vasil and J. A. Nick, J. Med. Microbiol., 2009, 58, 492–502 CrossRef CAS PubMed.
- T. S. Walker, K. L. Tomlin, G. S. Worthen, K. R. Poch, J. G. Lieber, M. T. Saavedra, M. B. Fessler, K. C. Malcolm, M. L. Vasil and J. A. Nick, Infect. Immun., 2005, 73, 3693–3701 CrossRef CAS PubMed.
- D. López, H. Vlamakis, R. Losick and R. Kolter, Mol. Microbiol., 2009, 74, 609–618 CrossRef.
- A. Velic, A. Jaggessar, T. Tesfamichael, Z. Li and P. K. D. V. Yarlagadda, Nanomaterials, 2021, 11, 2472 CrossRef CAS PubMed.
- S. Pogodin, J. Hasan, V. A. Baulin, H. K. Webb, V. K. Truong, T. H. Phong Nguyen, V. Boshkovikj, C. J. Fluke, G. S. Watson, J. A. Watson, R. J. Crawford and E. P. Ivanova, Biophys. J., 2013, 104, 835–840 CrossRef CAS PubMed.
- M. T. Alameda, M. R. Osorio, P. Pedraz and I. Rodríguez, Adv. Mater. Interfaces, 2022, 9, 2200608 CrossRef CAS.
- A. Valiei, N. Lin, J.-F. Bryche, G. McKay, M. Canva, P. G. Charette, D. Nguyen, C. Moraes and N. Tufenkji, Nano Lett., 2020, 20, 5720–5727 CrossRef CAS PubMed.
- A. Velic, J. Hasan, Z. Li and P. K. Yarlagadda, Biophys. J., 2021, 120, 217–231 CrossRef CAS PubMed.
- T. Liu, Q. Cui, Q. Wu, X. Li, K. Song, D. Ge and S. Guan, J. Phys. Chem. B, 2019, 123, 8686–8696 CrossRef CAS PubMed.
- E. P. Ivanova, D. P. Linklater, M. Werner, V. A. Baulin, X. Xu, N. Vrancken, S. Rubanov, E. Hanssen, J. Wandiyanto, V. K. Truong, A. Elbourne, S. Maclaughlin, S. Juodkazis and R. J. Crawford, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12598–12605 CrossRef CAS.
- D. P. Linklater, M. De Volder, V. A. Baulin, M. Werner, S. Jessl, M. Golozar, L. Maggini, S. Rubanov, E. Hanssen, S. Juodkazis and E. P. Ivanova, ACS Nano, 2018, 12, 6657–6667 CrossRef CAS.
- Y. Luan, S. Liu, M. Pihl, H. C. van der Mei, J. Liu, F. Hizal, C.-H. Choi, H. Chen, Y. Ren and H. J. Busscher, Curr. Opin. Colloid Interface Sci., 2018, 38, 170–189 CrossRef CAS.
- P. K. Sahoo, R. Janissen, M. P. Monteiro, A. Cavalli, D. M. Murillo, M. V. Merfa, C. L. Cesar, H. F. Carvalho, A. A. De Souza and E. P. Bakkers, Nano Lett., 2016, 16, 4656–4664 CrossRef CAS.
- X. Zhao and K. Drlica, Curr. Opin. Microbiol., 2014, 21, 1–6 CrossRef CAS PubMed.
- J. Jenkins, J. Mantell, C. Neal, A. Gholinia, P. Verkade, A. H. Nobbs and B. Su, Nat. Commun., 2020, 11, 1–14 CrossRef PubMed.
- E. P. Ivanova, J. Hasan, H. K. Webb, G. Gervinskas, S. Juodkazis, V. K. Truong, A. H. F. Wu, R. N. Lamb, V. A. Baulin, G. S. Watson, J. A. Watson, D. E. Mainwaring and R. J. Crawford, Nat. Commun., 2013, 4, 2838 CrossRef.
- G. S. Watson, D. W. Green, J. A. Watson, Z. Zhou, X. Li, G. S. P. Cheung and M. Gellender, Adv. Mater. Interfaces, 2019, 6, 1801646 CrossRef.
- X. Li, Phys. Chem. Chem. Phys., 2016, 18, 1311–1316 RSC.
- S. Maher, D. Linklater, H. Rastin, S. T.-Y. Liao, K. Martins De Sousa, L. Lima-Marques, P. Kingshott, H. Thissen, E. P. Ivanova and D. Losic, ACS Biomater. Sci. Eng., 2022, 8, 314–327 CrossRef CAS PubMed.
- E. Gutierrez, P. A. Burdiles, F. Quero, P. Palma, F. Olate-Moya and H. Palza, ACS Biomater. Sci. Eng., 2019, 5, 6290–6299 CrossRef CAS.
- G. Gao, Y.-W. Jiang, H.-R. Jia and F.-G. Wu, Biomaterials, 2019, 188, 83–95 CrossRef CAS.
- Y. He, J. Leng, K. Li, K. Xu, C. Lin, Z. Yuan, R. Zhang, D. Wang, B. Tao, T. J. Huang and K. Cai, Biomaterials, 2021, 278, 121164 CrossRef CAS PubMed.
- T. Suchý, L. Vištejnová, M. Šupová, P. Klein, M. Bartoš, Y. Kolinko, T. Blassová, Z. Tonar, M. Pokorný, Z. Sucharda, M. Žaloudková, F. Denk, R. Ballay, Š. Juhás, J. Juhásová, E. Klapková, L. Horný, R. Sedláček, T. Grus, Z. Čejka, Z. Čejka, K. Chudějová and J. Hrabák, Biomedicines, 2021, 9, 531 CrossRef.
- M. Stigter, J. Bezemer, K. De Groot and P. Layrolle, J. Controlled Release, 2004, 99, 127–137 CrossRef CAS PubMed.
- R. Jiang, Y. Yi, L. Hao, Y. Chen, L. Tian, H. Dou, J. Zhao, W. Ming and L. Ren, ACS Appl. Mater. Interfaces, 2021, 13, 60865–60877 CrossRef CAS.
- H. Fu, W. Bing and X. Liu, Prog. Org. Coat., 2024, 187, 108105 CrossRef CAS.
- Q. Liu and L. Liu, Langmuir, 2019, 35, 1450–1457 CrossRef CAS PubMed.
- T. Wei, W. Zhan, Q. Yu and H. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 25767–25774 CrossRef CAS PubMed.
- Y. Song, L. Zhang, L. Dong, H. Li, Z. Yu, Y. Liu, G. Lv and H. Ma, ACS Appl. Mater. Interfaces, 2021, 13, 46065–46075 CrossRef CAS.
- L. Zhang, Y. Yang, Y.-H. Xiong, Y.-Q. Zhao, Z. Xiu, H.-M. Ren, K. Zhang, S. Duan, Y. Chen and F.-J. Xu, Bioact. Mater., 2023, 25, 1–12 CAS.
- L. Děkanovský, R. Elashnikov, M. Kubiková, B. Vokatá, V. Švorčík and O. Lyutakov, Adv. Funct. Mater., 2019, 29, 1901880 CrossRef.
- J. Marqués-Marchán, M. M. Fernandes, S. Lanceros-Méndez and A. Asenjo, Appl. Mater. Today, 2023, 34, 101900 CrossRef.
- Q. Yu, J. Cho, P. Shivapooja, L. K. Ista and G. P. López, ACS Appl. Mater. Interfaces, 2013, 5, 9295–9304 CrossRef CAS PubMed.
- Y. Yi, R. Jiang, Z. Liu, H. Dou, L. Song, L. Tian, W. Ming, L. Ren and J. Zhao, J. Hazard. Mater., 2022, 432, 128685 CrossRef CAS.
- M. Asadi Tokmedash, N. Nagpal, P.-Y. Chen, J. S. VanEpps and J. Min, Adv. Mater. Interfaces, 2023, 10, 2202350 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03238a |
| This journal is © The Royal Society of Chemistry 2025 |






