Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in the chemistry and biology of plant oxylipin hormones
Yuho
Nishizato
a,
Taichi
Okumura
a,
Kotaro
Matsumoto
a and
Minoru
Ueda
 *ab
*ab
aDepartment of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan. E-mail: minoru.ueda.d2@tohoku.ac.jp
bDepartment of Molecular and Chemical Life Sciences, Graduate School of Life Sciences, Tohoku University, Sendai 980-8578, Japan
First published on 25th April 2025
Abstract
Jasmonates, including jasmonic acid (JA) and its derivatives, are lipid-based signaling molecules critical for plant growth, development, and defense. Among these, jasmonoyl-L-isoleucine (JA-Ile) has been identified as a bioactive plant hormone that mediates various physiological responses. JA-Ile functions in planta as a ‘molecular glue’ in protein–protein associations to induce the defense-related gene expression for plant–pathogen and plant–insect communications, and it affects many aspects of plant development and stress responses. This review explores the historical journey of jasmonate research, emphasizing the discovery of JA-Ile, its biosynthesis, function as a molecular glue, and the ligand–receptor co-evolutional aspect. The elucidation of the SCFCOI1-JAZ receptor complex and the crystallization of this co-receptor system marked significant advancements in understanding the chemical background of jasmonate biology. This review focuses on the advances in the chemistry and biology of jasmonate bioscience in the past two decades.
1. Introduction
Plant hormones orchestrate intricate networks, regulating growth, development, and stress responses.1 Plants are fascinating organisms because they rely on endogenous small molecules and plant hormones as key regulators for most of their physiological functions. This reliance means that a significant portion of plant physiology can, in principle, be controlled using organic small molecules, making plants attractive subjects of study for organic chemists.Jasmonates, which are relatively recent members of the plant hormone family, are oxylipins which are oxidized derivatives of poly-unsaturated fatty acids (PUFAs) (Fig. 1).2,3 Oxylipins have garnered significant attention in recent years for their roles as signaling molecules.4 Prostaglandins (PGE2 in Fig. 1), a well-known class of biologically active oxylipins, are found not only in mammals but also across a diverse range of organisms, including insects and marine species. Similarly, molecules known as phytoprostanes (16-B1-PhytoP in Fig. 1) have been identified in plants.5 Notably, plants primarily utilize oxylipins called jasmonates, including jasmonoyl-L-isoleucine (JA-Ile in Fig. 1), as plant hormones. Jasmonates, have attracted attention for their roles in both plant defense and development.6 In particular, JA-Ile induces defense responses against herbivorous insects and pathogenic infections and affect the growth and fertility of plants. The jasmonate family includes jasmonic acid (7-iso-JA in Fig. 1 and Table 1) and its conjugated derivatives like JA-Ile. Among these, JA-Ile has emerged as a biologically active molecule essential for jasmonate signaling in vascular plants.
Herbivorous insects and pathogens pose a significant threat to global food production, contributing to an estimated 20% loss in the yields of major crops worldwide.7 While chemical pesticides have been widely employed to combat these threats, their use raises several concerns, including environmental impact and the development of resistance. However, plants have developed sophisticated defense mechanisms against herbivorous insects and pathogens, with JA-Ile serving as a key player in these responses.6 Manipulating jasmonate signaling could enhance resistance to herbivorous insects and pathogens and improve stress tolerance. The potential of jasmonates for biotechnological applications is vast, ranging from genetic engineering of plant hormone biology to the development of jasmonate-based agrochemicals. Harnessing the natural defense mechanisms of plants for crop protection in agroecosystems offers a promising and sustainable approach to enhancing food production.
In this review, we focus on the chemistry and biochemistry of jasmonates. The biological aspects of jasmonates have been comprehensively covered in excellent review articles published in the past, which are recommended for further ref. 6 and 8–11.
2. Jasmonates as plant oxylipin hormones
2.1 How JA-Ile was identified as a plant hormone: a historical perspective
The following section describes how JA-Ile was discovered and recognized as a plant hormone. This long journey exemplifies how a combination of natural product chemistry, genetic research, and some serendipity, is essential for a natural product to achieve a status of biological significance.The first jasmonates were isolated in the early 1960s as volatile compounds (Fig. 2A). As the old saying goes, “no perfume without jasmine”. The determination of the pivotal fragrance component was of great value, with about 80% of the fragrance compositions on the market contained the precious jasmine essential oil component until the mid-20th century.12 Methyl jasmonate (MeJA, Fig. 2A) was isolated from Jasminum grandiflorum, highlighting its role as an odorant.13 JA was identified as a cyclopentanone derivative, derived from PUFA precursors, such as α-linolenic acid (Fig. 1), through the octadecanoid pathway.14 Subsequent studies demonstrated its growth-regulatory effects and senescence induction, paving the way for detailed biochemical investigations.15–17 This period also saw the isolation of JA from various plant species, underscoring its ubiquity in the plant kingdom.18
Around 1990, it became clear that the JA and other jasmonates accumulate in plants in response to pathogen infection and wounding, and that genes whose expression was induced by pathogenic infection or wounding were also up-regulated by JA treatment (Table 1).19 Additionally, JA is recognized as a signal in fertility, senescence, and environmental stress responses, such as heat tolerance or cold stress. All these aspects have been comprehensively described in the first historical overview in ref. 19. JA also plays a critical role in floral organ development and fertility. These findings attracted the attention of plant scientists and led to the identification of JA as wounding and pathogenic infection-induced potential defense “hormone” of plant. Another important step was the discovery of coronatine (COR) (Fig. 2A and Table 1), a bacterial phytotoxin, inducing highly potent jasmonate responses.20–22 Thus, COR was considered as “super jasmonate”,23 which might be caused by the fixed structure of the second ring of coronatine compared with JA-Ile.
Further progress came from genetic studies (Fig. 2B). The jar1 mutant of Arabidopsis thaliana, identified through screens for JA-insensitive phenotypes, exhibits defects in root inhibition and reduced accumulation of defense-related proteins (Fig. 2B).24,25 Jasmonic acid amido synthetase (JAR1) belongs to the Gretchen Hagen 3 (GH3) family enzyme which conjugates substrates with amino acids. GH3 acyl acid amido synthetases catalyze the conjugation of phytohormones with amino acids, playing key roles in maintaining plant hormone homeostasis of JA, auxins, and salicylic acid.26 Considering the structural similarity of JA and COR (Fig. 2A), plant scientists hypothesized that JA would be conjugated with isoleucine to form JA-Ile as a genuine bioactive form.19 Cloning of JAR1 (AtGH3.11) confirmed its role in JA-Ile biosynthesis, highlighting the functional specificity of this conjugate.27 This conjugation enhances the signaling capability of JA, transforming it into a biologically active JA-Ile hormone.28 The specificity of this reaction was confirmed through mutant analyses, particularly in jar1 mutants. This was followed by the discovery that JAR1 (AtGH3.11) and the partially redundant GH3.10 play similar roles in JA-Ile synthesis.29 These findings confirmed a pivotal link between JA and the bioactive form JA-Ile.
The identification of active plant hormone marked a significant breakthrough, as it enabled the search for a JA-Ile receptor. The JA-Ile receptor was identified in 2007. Following the pioneering achievement of the discovery of coi1 (Fig. 2B),30,31 a mutant insensitive to COR, it was revealed that JA-Ile binds to the COI1-JAZ co-receptor, which consists of the F-box protein COI1 and the transcriptional repressor JAZ, functioning as a molecular glue to link these two proteins (Fig. 2C).32,33 The COI1-JAZ co-receptor will be discussed in detail in a later section. This breakthrough made it possible to evaluate the structure–activity relationships of jasmonates, including JA-Ile, through in vitro assays based on their interaction with the receptor, marking a significant advancement in the field.
The remaining question concerns the relationship between stereochemistry and the bioactivity of JA-Ile (Fig. 2A). JA-Ile naturally exists as a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of (3R, 7R)-JA-Ile and (3R, 7S)-JA-Ile. However, most studies have utilized a mixture of JA-Ile synthesized from racemic JA, which comprises four isomers: (3S/R, 7R/S)-JA-Ile and (3R/S, 7S/R)-JA-Ile. The successful preparation of pure stereoisomers revealed that the (3R, 7S)-JA-Ile is the naturally occurring bioactive form of the plant hormone.34 It is also expected that JA-Ile can be inactivated through the epimerization of its active 7S form to the 7R form;34 however, no enzymes or mechanisms responsible for this process have been identified. Finally, the crystallization of COI1-JAZ complexed with JA-Ile/COR (PDB ID: 3OGL and 3OGM) provided definitive proof of JA-Ile as the ligand for this receptor (Fig. 2C).35 Structural studies revealed that enantiomeric specificity for (3R, 7S)-JA-Ile is critical for receptor binding and biological activity.
5 mixture of (3R, 7R)-JA-Ile and (3R, 7S)-JA-Ile. However, most studies have utilized a mixture of JA-Ile synthesized from racemic JA, which comprises four isomers: (3S/R, 7R/S)-JA-Ile and (3R/S, 7S/R)-JA-Ile. The successful preparation of pure stereoisomers revealed that the (3R, 7S)-JA-Ile is the naturally occurring bioactive form of the plant hormone.34 It is also expected that JA-Ile can be inactivated through the epimerization of its active 7S form to the 7R form;34 however, no enzymes or mechanisms responsible for this process have been identified. Finally, the crystallization of COI1-JAZ complexed with JA-Ile/COR (PDB ID: 3OGL and 3OGM) provided definitive proof of JA-Ile as the ligand for this receptor (Fig. 2C).35 Structural studies revealed that enantiomeric specificity for (3R, 7S)-JA-Ile is critical for receptor binding and biological activity.
Through this extensive history, which includes the isolation of JA, the discovery of its bioactivity in plants, the identification of JA/COR-insensitive mutants, the characterization of their coding proteins, and the identification of naturally occurring bioactive isomer, the crystal structure of COI1-JAZ complexed with JA-Ile, (3R, 7S)-JA-Ile (Fig. 2A) is confirmed as the genuine bioactive form of the plant hormone. Hereafter, (3R, 7S)-JA-Ile will simply be referred to as JA-Ile. The role of JA-Ile in plants extends to abiotic and biotic stress responses, enhancing resilience against environmental challenges.6 In addition, JA-Ile mediates the production of secondary metabolites in plants.36,37 JA-Ile is not only a key plant hormone in plant science research but also an exceptionally intriguing compound for natural product chemists due to its remarkable biological activities.
2.2 New family members of jasmonates
Beside JA, JA-Ile is a predominant oxylipin plant hormone; however, it has become increasingly evident that other oxylipins also function as plant hormones or endogenous bioactive compounds. Of particular interest is the inclusion of JA-Ile metabolites and biosynthetic precursors among these bioactive oxylipins. One such example is (+)-cis-12-oxo-phytodienoic acid (cis-OPDA, Fig. 3 and Table 1), a biosynthetic precursor of JA-Ile. Cis-OPDA is thought to play a dual role in plant biology as both a JA biosynthetic precursor and an independent signaling molecule.38–41 Emerging evidence highlights cis-OPDA-specific bioactivities independent of JA-Ile. Studies with mutants lacking JA biosynthesis reveal JA-Ile-independent unique roles of cis-OPDA in resilience against environmental stresses, including drought tolerance, thermotolerance, and stomatal closure.42–44 The electrophilic nature of cis-OPDA allows covalent linking with cysteine residues in target proteins, influencing redox homeostasis and gene activation. Notably, cis-OPDA directly binds to plastidic cyclophilin 20-3 (CYP20-3), stabilizing enzymes involved in cysteine synthesis and modulating thiol-based redox signaling pathways.45–47 These functions underscore the versatility of cis-OPDA as a signaling molecule. However, the detailed mode of action of cis-OPDA awaits the identification of its in vivo targets. Very recent results suggest that downstream metabolites of cis-OPDA, such as tetranor-cis-OPDA (tn-cis-OPDA) and 4,5-didehydro-JA (4,5-ddh-JA), also function as JA-Ile-independent signaling molecules and could be genuine bioactive forms of cis-OPDA (Fig. 3 and Table 1).48,49 Additionally, other biosynthetic precursors of JA-Ile, such as dinor-cis/iso-12-oxo-phytodienoic acid (dn-cis-OPDA and dn-iso-OPDA, Fig. 3 and Table 1), function as plant hormones in bryophytes, instead of JA-Ile.50,51 Bryophytes, particularly Marchantia polymorpha, are regarded as models for understanding the origins of land plants.52 A unique and fascinating example on the molecular co-evolution of plant hormone and their receptor was reported on M. polymorpha. In addition to dn-cis/iso-OPDA, Δ4-dn-iso-OPDA, which is biosynthesized from C20-PUFA, was found as a novel plant hormone of M. polymorpha (Fig. 3 and Table 1).53,54 The set of these diverse ‘ancestral’ jasmonates is required for the full activation of jasmonate response in M. polymorpha. As a JA-Ile metabolite, 12-hydroxy-jasmonoyl-L-isoleucine (12-OH-JA-Ile, Fig. 3 and Table 1) has recently been identified as an “attenuated plant hormone” that activates only a subset of JA-Ile-mediated biological activities.55–58 The details of 12-OH-JA-Ile and ‘ancestral’ jasmonate in M. polymorpha are summarized in the following sections. | ||
| Fig. 3 Biosynthetic precursors and metabolites of JA-Ile, functioning as endogenous bioactive signaling molecules. | ||
3. Receptor and signal transduction of plant oxylipin
3.1 Plant hormones JA-Ile and auxin as molecular glues
Although it is not widely known, the first small molecule discovered to function as a molecular glue was the plant hormone auxin.59,60 Auxin (indole-3-acetic acid) was discovered through research initiated by Charles Darwin and is the most important plant hormone controlling growth, phototropism, and gravitropism.61 Auxin binds to the F-box protein Transport Inhibitor Response 1 (TIR1), a subunit of the E3 ubiquitin ligase, and its homologs, but the substrate remained unclear. In 2005, it was revealed that auxin induces protein–protein interactions (PPI) between TIR1 and its substrate, the transcriptional repressor Aux/IAA,62,63 and the crystal structure of the TIR1-Aux/IAA co-receptor complex with the ligand auxin was reported. Remarkably, this small molecule, with a molecular weight of just 175, was shown to be sandwiched between TIR1 and IAA to act as a molecular glue. Between 2007 and 2010, it was discovered that JA-Ile (MW 323) functions as a molecular glue by inducing PPI between the F-box protein COI1 and the transcriptional repressor JAZ. TIR1 and COI1 are highly homologous F-box proteins that share an evolutionary ancestor64 and ubiquitinate their substrate transcriptional repressors, resulting in their degradation via the 26S proteasome mechanism to trigger the expression of genes involved in signal transduction pathways. The molecular glue was made famous through the historical achievement by Handa and co-workers in 2010 (ref. 65) that elucidated the teratogenic mechanism of thalidomide. Thalidomide functions as a molecular glue, inducing PPIs between CRBN, a subunit of the E3 ubiquitin ligase, and substrates such as SALL4, leading to the degradation of substrate proteins and causing teratogenic effects. However, reports describing auxin and JA-Ile acting as molecular glues were published earlier than this renowned study. In particular, a decisive statement can be found in a paper published in 2007: “Thus, we conclude that auxin promotes SCF-TIR1-substrate binding by acting as a ‘molecular glue’ rather than an allosteric switch.”63 Currently, small molecules recognized as plant hormones include auxin, gibberellin, ethylene, abscisic acid, cytokinin, JA-Ile, brassinosteroids, strigolactones, salicylic acid, and peptide hormones.1 Among these, the ones that clearly act as molecular glues are auxin and jasmonates, including JA-Ile.32,33,35,62,63,66 On the other hand, molecules like gibberellin and abscisic acid function as allosteric drugs that induce conformational changes in receptors, enabling PPIs with target proteins.67The whole picture of mode of action of JA-Ile is illustrated in Fig. 4.6 The SCFCOI1-JAZ complex mediates jasmonate perception. COI1, an F-box protein, forms part of the Skp1-Cullin-F-box (SCF) ubiquitin ligase complex. JAZ proteins act as transcriptional repressors, binding to MYC transcription factors and preventing their activation. JA-Ile promotes PPI between COI1 and JAZ, leading to JAZ ubiquitination and degradation. Upon JAZ degradation, MED25 binds to the vacated MYC2 binding site, leading to the reassembly of the transcriptional machinery (Fig. 4). This releases MYC2 and other transcription factors, initiating jasmonate-responsive gene expression (Fig. 4). These insights paralleled similar advances in understanding auxin and gibberellin receptor mechanisms, establishing a conserved paradigm in hormone perception.67,68
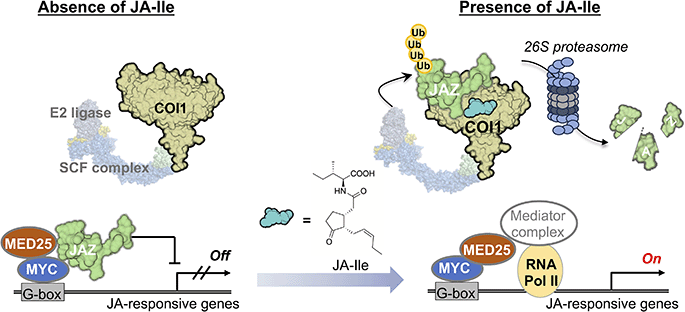 | ||
| Fig. 4 Mode of action of JA-Ile that involves promoting the protein–protein interaction between COI and JAZ, which triggers JAZ degradation via the 26S proteasome pathway. | ||
3.2 Crystal structure of COI1-JAZ complexed with JA-Ile
The crystal structures of the COI1/JA-Ile/JAZ1 complex and the COI1/COR/JAZ1 complex reported in 2010 represent a landmark achievement in the field of jasmonate bioscience.35 As of 2025, these remain the only reported crystal structures of the COI1-JAZ co-receptor complex. This groundbreaking discovery definitively confirms that JA-Ile and COR function as molecular “glues,” facilitating PPI between COI1 and JAZ. While COR has long been considered a biological mimic of JA-Ile, these crystal structures conclusively shown that it also acts as a structural mimic, forming an almost identical network of hydrogen bonds and hydrophobic interactions within the COI1-JAZ co-receptor complex (Fig. 5A and B). In this complex, JA-Ile and COR are anchored within the ligand-binding pocket of COI1, with their keto and carboxyl groups exposed on the surface of COI1, enabling the formation of hydrogen bonds with JAZ (Fig. 5C). In addition to interacting with COI1 in the same way as JA-Ile, COR is sandwiched between the side chains of two aromatic amino acid residues (Phe and Tyr) within the ligand-binding pocket of COI1, allowing it to bind to the COI1-JAZ co-receptor with higher affinity than JA-Ile.35 Along with JA-Ile and COR, the inositol pentakisphosphate (InsP5) molecule binds strongly to COI1, suggesting that it forms a hydrogen-bonding network with COI1-JAZ and JA-Ile/COR.35,69 Similarly, the auxin receptor TIR1 binds tightly to inositol hexakisphosphate (InsP6) at a comparable site,63 indicating a shared ligand-binding mechanism between TIR1 and COI1. | ||
| Fig. 5 (A and B) Binding poses of JA-Ile (PDB ID: 3OGL) and COR (PDB ID: 3OGM) within the COI1 ligand-binding pocket. (C) Interface between COI1 and JAZ. (D) Mechanisms by which the COI1-JAZ co-receptor perceives JA-Ile or COR. | ||
However, recent studies reveal that JA-Ile and COR form complexes with the COI1-JAZ co-receptor through distinct mechanisms (Fig. 5D).70,71 JA-Ile acts like a glue, directly inducing PPI between COI1 and JAZ in a one-step recognition mechanism. In contrast, COR employs a two-step recognition mechanism: first, it forms a stable complex with COI1 and then recruits JAZ. This difference in binding modes stems from variations in the ligands' affinities for COI1. COR binds more strongly to COI1 due to π–π stacking interactions between its fused 5,6-ring structure and Phe89 and Tyr444 within the COI1 ligand-binding pocket, allowing it to form a stable COI1-COR complex. These insights into the relationship between ligand structure, binding mode, and affinity could provide valuable for the future design of synthetic molecular glues.
3.3 Genetic redundancy of COI and JAZ leads to diverse JA-Ile responses
Genes encoding COI1 and JAZ often exhibit genetic redundancy, a result of gene duplication during evolution.72 This redundancy underscores the critical role of the COI1-JAZ co-receptor in plant survival because a genome of an organism encodes multiple copies of genes that are important for their survival. Most plants possess multiple COI1-JAZ co-receptor subtypes, enabling JA-Ile to trigger a wide array of biological responses, including herbivore defense, environmental stress responses, senescence, growth and reproduction, and secondary metabolite production. This functional versatility arises because each biological response is independently or redundantly regulated by specific receptor subtypes (Fig. 6A). On the other hand, JA-Ile can bind to all ten functional COI1-JAZ co-receptor pairs, simultaneously activating numerous JA-Ile-induced biological responses. However, this activation often comes at a cost, frequently described as a growth-defense trade-off, where defense responses inhibit plant growth, leading to dwarfing. Thus, plants face the constant dilemma of “to grow, or to defend” (prioritizing growth or defense). Resolving this dilemma is one of the most critical challenges in jasmonate bioscience. The number of COI and JAZ genes encoded in plant genomes varies among plant species. For example, Arabidopsis has a single COI1 gene, while rice (Oryza sativa) has three (OsCOI1a, OsCOI1b, and OsCOI2) (Fig. 6A).73 In rice, these genes function redundantly, although OsCOI2 plays a dominant role in JA-Ile-mediated responses.74,75As for JAZ genes, the Arabidopsis genome encodes 13 JAZ copies, including 10 functional JAZ copies, while rice has 15.72,76 These JAZ genes also exhibit function redundancy, which explains why knocking out a single JAZ gene often fails to produce noticeable phenotypic changes. Significant phenotypic changes are typically only observed in multiple knockout mutants, such as Arabidopsis jaz quintuple (jazQ) mutant, in which five of 13 JAZ genes are knocked out.77 JAZ proteins, classified as intrinsically disordered proteins,78 act as repressors of various transcription factors (TFs). Their most critical structural feature is the Jas motif near the C-terminus, which is essential for interactions with COI1 and MYC2 TFs, which are key TFs in jasmonate signaling (Fig. 6B).76 The short degron sequence ((V/L)P(Q/I)AR(R/K)) within the Jas motif is crucial for COI1 interaction in the presence of JA-Ile (Fig. 6B), facilitating JAZ degradation via the 26S proteasome pathway.35 The Jas motif can adopt different conformations depending on whether it interacts with MYC TF or COI1, enabling the switch between the repressive and active states of MYC TF (Fig. 6B).79 In the absence of JA-Ile, JAZ proteins bind to MYCs (MYC2/3/4/5), repressing their ability to activate the transcription of jasmonate-responsive genes. However, COI1 forms a ternary complex with JAZ and JA-Ile in the presence of JA-Ile, leading to JAZ polyubiquitination and subsequent degradation via the 26S proteasome.35 The degradation exposes the MYC interaction sites for coactivators such as MED25,80 linking the transcriptional machinery to jasmonate-responsive promoters (Fig. 4).81 Notably, three Arabidopsis JAZ proteins—JAZ7, JAZ8, and JAZ13—are classified as noncanonical JAZs due to their lack of the canonical degron sequence.82,83 As a result, noncanonical JAZs cannot interact with COI1 in the presence of JA-Ile, contributing to their stability even under jasmonate-stimulated conditions.
By interacting with diverse JA-Ile-responsive TFs, each JAZ subtype contributes to the regulation of various JA-Ile-triggered biological responses (Fig. 6B). For example, two major pathways are activated in defense responses: the MYC2 TF branch and the ERF/ORA TFs branch (Fig. 7A).84 The MYC2 branch activates defense against herbivores by inducing the expression of herbivory-related genes such as Vegetative Storage Protein 1/2 (VSP1/2).85 In contrast, the ERF/ORA branch is activated during necrotrophic pathogen attacks, inducing the expression of defense-related genes such as Plant Defensin 1.2 (PDF1.2) through APETALA2/Ethylene Response Factor (AP2/ERF) family TFs like ERF1 and ORA59.
 | ||
| Fig. 7 (A) An example of the JAZ-regulated signaling pathway in Arabidopsis. (B) JA-Ile playing a key role in regulating secondary metabolite production in plants. | ||
JA-Ile signaling also plays a critical role in regulating secondary metabolite production in plants. The involvement of jasmonates in secondary metabolite production was first demonstrated by the pioneering research led by Zenk.36 They showed that MeJA enhances secondary metabolite production in 36 types of plant cultured cells. Subsequent studies revealed that JA-Ile triggers the biosynthesis of various plant secondary metabolites, including glucosinolates and alkaloids, which contribute to feeding avoidance and defense against herbivore attack (Fig. 7B).86 Recent research has begun to uncover the molecular mechanisms underlying this process.37 In Arabidopsis, MYC TFs collaborate with MYB TFs to regulate glucosinolate biosynthesis.87 In tomato (Solanum lycopersicum), the production of α-tomatine, a steroidal alkaloid, is controlled by an AP2/ERF family TF JRE4, which acts in concert with MYC TFs.88,89
4. Synthetic molecules regulating plant oxylipin signaling
Recent advancements in the rational design of synthetic molecules have significantly contributed to our understanding of jasmonate biology, including antagonists, subtype-selective agonists, and novel compounds that modulate JA-Ile signaling.3 This section highlights several groundbreaking studies that highlight progress in this field.4.1 Coronalon: the first synthetic mimic of JA-Ile
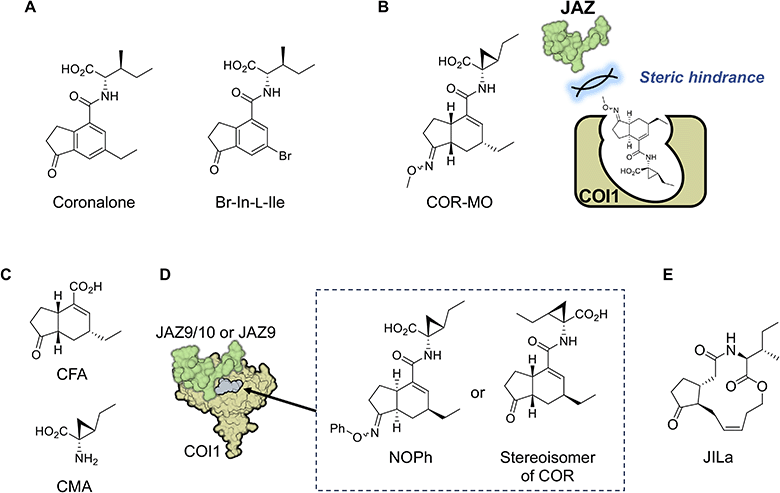
The first synthetic mimic of JA-Ile was reported in 2004.90 Based on the structure of COR, Boland and co-workers developed a simple synthetic mimic called coronalon, a 6-ethyl indanoyl-isoleucine conjugate (Fig. 8A), which was designed to replicate the activity of JA-Ile in plant defense signaling. Their research demonstrates that coronalon effectively induces the accumulation of defense-related secondary metabolites, modulates stress-related gene expression, and inhibits root growth at low micromolar concentrations. These findings suggest that coronalon could serve as a valuable tool for exploring various aspects of plant stress physiology as an effective and easily synthesized alternative to JA-Ile and COR. In a subsequent study, Boland and co-workers developed various bioactive 6-substituted-In-L-Ile analogs (Fig. 8A).91–93 This advancement paved the way for generating ligands with diverse biological activities that mimic JA-Ile. Collectively, these studies underscore the significance of synthetic JA-Ile/COR analogs in advancing jasmonate bioscience.4.2 Designed antagonist and agonist/antagonist from library screening
In 2010, the crystal structure of the JA-Ile/COR and COI1-JAZ co-receptor complex was reported,35 enabling the rational design of synthetic ligands based on their binding modes. This approach led to a major breakthrough in 2014, when the group led by Solano laid the groundwork for chemically modulating JA-Ile signaling by developing the first potent antagonist of the COI1-JAZ co-receptor.94 As discussed in the previous sections, COR binds to the ligand-binding pocket of COI1, exposing its ketone and carboxylate functionalities for hydrogen bonding with JAZ. Monte et al. modified the ketone moiety of COR into a methyl oxime to block JAZ recruitment.94 The designed antagonist, named COR-MO (Fig. 8B and Table 1), effectively blocks JA responses, demonstrating its potential as a tool for studying JA signaling. This work highlights the possibility of selectively disrupting JA-Ile signaling pathways to manipulate plant physiology without genetic modification.Watson and co-workers developed a scalable, gram-scale synthetic route for coronafacic acid (CFA) (Fig. 8C) and its analogs, enabling extensive structure–activity relationship studies.95 They synthesized a library of over 120 conjugates of CFA/analogs and amino acids. Biological assessments of these compounds against various weeds, combined with computational analyses, identified key structural features essential for potent activity. While conjugating alternative amino acids to the CFA core reduced biological activity, the critical structure was determined to be the CMA moiety (Fig. 8C), with modifications around the CFA core having minimal impact on biological activity.
Chini et al. introduced small-molecule antagonists that simultaneously target JA-Ile and auxin signaling pathways.96 These dual antagonists were identified through a chemical screening of over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds. Using biochemical assays and phenotypic analyses in vascular and nonvascular plants, they demonstrated that the antagonists effectively suppressed JA and auxin responses. This dual functionality offers a novel approach for studying the interplay between hormone pathways and manipulating plant development. The identification of Jarin-1, an inhibitor of JAR1, is another example of the discovery of synthetic molecules that regulate JA signaling through large-scale chemical screening.97 However, large-scale chemical screenings have failed to identify COI1-JAZ agonists, highlighting the challenges in developing such compounds and the inherent difficulty of screening for “molecular glue.”
000 compounds. Using biochemical assays and phenotypic analyses in vascular and nonvascular plants, they demonstrated that the antagonists effectively suppressed JA and auxin responses. This dual functionality offers a novel approach for studying the interplay between hormone pathways and manipulating plant development. The identification of Jarin-1, an inhibitor of JAR1, is another example of the discovery of synthetic molecules that regulate JA signaling through large-scale chemical screening.97 However, large-scale chemical screenings have failed to identify COI1-JAZ agonists, highlighting the challenges in developing such compounds and the inherent difficulty of screening for “molecular glue.”
4.3 Uncoupling of growth-defense tradeoff using synthetic molecules
Ueda and co-workers expanded the concept of synthetic modulation by designing the first JAZ subtype-selective COI1-JAZ agonists.98 This work represents a unique example of the rational design of synthetic “molecular glue” with receptor-subtype selectivity. Recognizing the diversity among JAZ proteins which regulate various aspects of plant responses, the study aimed to achieve precision in targeting specific JAZ subtypes. Through chemical synthesis and structural analysis, they developed a synthetic molecular glue capable of selectively triggering COI1-JAZ9/10 co-receptor formation. This unique agonistic molecular glue, named NOPh (Fig. 8D), modulated specific JA-Ile responses, triggering defense responses against pathogenic infection without causing growth inhibition. Unlike JA-Ile and COR which bind to all 10 COI1-JAZ co-receptors in plants and activate defense responses and growth inhibition,99 NOPh provided a way to escape the growth-defense tradeoff in JA-Ile signaling. This study demonstrated the potential for fine-tuned manipulation of plant signaling pathways to enhance specific traits, such as pathogen resistance. In a subsequent study, Ueda and co-workers constructed a chemical library composed of 16 stable stereoisomers of COR to identify a subtype-specific agonist of the COI1-JAZ co-receptor.100 One stereoisomer exhibited high specificity for JAZ9 (Fig. 8D), enabling precise modulation of JA responses. This discovery enhances our understanding of the structural basis of JA-Ile perception and provides a toolkit for selectively activating desired responses. Such innovations represent a significant step toward precision agriculture, enabling targeted interventions to optimize plant performance under varying environmental conditions. However, this requires chemical stability of the molecule in the field and easy supply through large-scale synthesis.In 2017, Boland's group also addressed the growth-defense tradeoff by the developing JA-Ile-macrolactones (JILa, Fig. 8E), a class of synthetic cyclized 12-OH-JA-Ile compounds.101,102 JILa was found to uncouple the typical antagonistic relationship between growth and defense responses in wild tobacco (Nicotiana attenuata).99 The application of JILa allowed plants to activate robust defense mechanisms against herbivores while maintaining growth rates. This effect was partly attributed to enhanced accumulation of the alkaloid nicotine in wild tobacco. However, the mechanism of action of JIla that confers this unique biological activity remains largely unknown.
These findings challenge the long-held paradigm that enhanced defense responses suppress growth, opening new avenues for developing crops capable of achieving high yields while remaining resilient to biotic stress.
The studies reviewed here collectively highlight the potential of synthetic chemistry and chemical design in advancing our understanding and application of plant hormone signaling pathways.103,104 The rational design of synthetic molecules to modulate jasmonate perception represents a transformative approach in plant biology. From antagonists that block JA signaling to subtype-selective agonists that fine-tune specific responses, these innovations offer powerful tools for basic research and agricultural applications. They open new avenues for the precise modulation of JA-Ile responses, allowing researchers to dissect the complex roles of JA-Ile in plant physiology and engineer crops with tailored traits.
5. Biosynthesis and metabolism of plant oxylipin hormone
5.1 Canonical biosynthetic pathway of plant oxylipin hormone
JA biosynthesis is triggered by various stimuli, including mechanical wounding, herbivory, pathogen attacks, and developmental processes such as flower and seed maturation.6 These signals activate the expression of genes encoding JA biosynthetic enzymes, leading to increased JA production. By the late 1990s, researchers had identified genes encoding key JA biosynthetic enzymes, elucidating the octadecanoid pathway.8,105 These discoveries enabled genetic manipulation studies, offering insights into the roles of individual enzymes and their contributions to the biosynthetic pathway. The identification of key enzymes and the elucidation of regulatory mechanisms have significantly advanced modern jasmonate research.JA biosynthesis begins with α-linolenic acid, which is released from chloroplast membranes by phospholipase A1 (Fig. 9). This substrate undergoes oxygenation by 13-lipoxygenase (13-LOX) to form 13-hydroperoxy-octadecatrienoic acid (13-HPOT) (Fig. 9). LOX enzymes specifically oxygenate fatty acids at the C-13 position, initiating downstream reactions. Subsequent steps involve allene oxide synthase (AOS)106 and allene oxide cyclase (AOC),107 producing the critical intermediate cis-OPDA (Fig. 9). The crystallization of AOS, a cytochrome P450 (CYP) enzyme, provided detailed insights into its catalytic mechanism. The stereochemical specificity of AOC in generating enantiomerically pure (+)-cis-OPDA underscores its essential role in maintaining JA bioactivity.8,105,108
The early stages of JA biosynthesis occur in chloroplasts, where LOX, AOS, and AOC are localized (Fig. 9). Subsequent steps take place in peroxisomes, ensuring efficient intermediate transport while maintaining cellular organization. Transporter proteins such as JASSY and COMATOSE facilitate the transfer of cis-OPDA to peroxisomes (Fig. 9),109,110 where OPDA reductase 3 (OPR3)111 reduces it to 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid (OPC-8:0) (Fig. 9). OPC-8:0 undergoes three rounds of β-oxidation to yield JA (Fig. 9). In the cytosol, JA is primarily conjugated with isoleucine by the enzyme JAR1,27,28,112 forming JA-Ile, the active signaling molecule recognized by the COI1-JAZ receptor complex in nucleus. Recently, AtGH3.10 was identified as another homolog of JA-conjugating GH3 enzyme contributing to JA-Ile biosynthesis (Fig. 9).29 JA can also conjugate with other amino acids, such as valine, leucine, and methionine etc., producing minor conjugates with varying biological activities across different plant species (Table 1).113
Comparisons with animal systems reveal striking parallels between plant oxylipins (including jasmonates) and eicosanoids, lipid-based signaling molecules in animals.114 Both pathways involve lipoxygenase enzymes and are activated by stress, highlighting convergent evolution in stress signaling mechanisms.
5.2 Non-canonical biosynthetic pathway of plant oxylipin hormone
In addition to the canonical octadecanoid pathway, two alternative biosynthetic pathways have been identified. One non-canonical pathway, thought to represent an ancient biosynthetic route, involves intermediates such as dinor-cis-OPDA (dn-cis-OPDA), tetranor-cis-OPDA (tn-cis-OPDA), and 4,5-didehydro-JA (4,5-ddh-JA) (Fig. 9), indicating evolutionary divergence in JA biosynthesis across plant lineages.115 Another alternative pathway originates from hexadecatrienoic acid (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 fatty acid) rather than α-linolenic acid, producing dinor-OPDA as an intermediate (Fig. 9).116,117 These alternative pathways contribute to the diversification of jasmonate signaling molecules.
3 fatty acid) rather than α-linolenic acid, producing dinor-OPDA as an intermediate (Fig. 9).116,117 These alternative pathways contribute to the diversification of jasmonate signaling molecules.
5.3 Catabolic turnover of plant oxylipin hormone attenuating jasmonate signaling in plants
Jasmonates undergo extensive catabolic conversions to maintain homeostasis and functional diversity. Constitutive activation of JA signaling can negatively impact plant survival, making mechanisms to attenuate JA signaling critical.77 The primary pathway for attenuating JA signaling involves CYP-mediated hydroxylation of JA-Ile at the C-12 position. CYP94B1/B3 catalyzes the production of 12-OH-JA-Ile (Fig. 10), while CYP94C1 facilitates its further oxidation to 12-COOH-JA-Ile (Fig. 10 and Table 1).118–121 12-OH-JA-Ile, a weakened JA-Ile derivative, binds to a subset of COI1-JAZ co-receptor pairs and exhibits reduced signaling activity.55–57 In contrast, 12-COOH-JA-Ile is fully inactive, with no COI1-JAZ affinity or bioactivity. Additionally, JA-Ile signaling can be attenuated by IAR3/ILL6 amide hydrolases, which hydrolyze JA-Ile to JA (Fig. 10).122,123 The 2-oxo-glutarate-dependent dioxygenases (2OGDs) JOX/JAOs also contribute by directly hydroxylating JA (Fig. 10), reducing JA-Ile accumulation.124,125 The resulting 12-OH-JA is further catabolized into 12-hydroxy jasmonoyl sulfate and 12-hydroxy jasmonoyl glucoside for complete inactivation (Table 1).8,126–128 11-hydroxy-JA (11-OH-JA) is another oxidative catabolite129 observed in many plant species and accumulates at higher levels than 12-OH-JA in A. thaliana.126 Thus, 11-OH-JA, alongside 12-OH-JA, are considered major shunt products in the JA catabolic pathway, although their biosynthesis and biological roles remain poorly understood. Additionally, JA can be methylated by jasmonic acid methyltransferase (JMT) to form MeJA (Fig. 10 and Table 1), a volatile compound involved in interplant signaling and herbivore defense.130Recently, a novel 2ODG named Jasmonate Induced Dioxigenase 1 (JID1) attenuated JA-Ile accumulation by catabolizing the JA biosynthetic intermediate cis-OPDA into modified OPDA (mo-OPDA).40,131 However, a subsequent study identified mo-OPDA as an artifact,132 leaving aspects of JID1's function elusive.
The jasmonate pathway exemplifies a sophisticated system of plant adaptation, integrating environmental and developmental signals. Its finely tuned biosynthesis and catabolism ensure dynamic responses, underscoring the complexity and adaptability of plant hormonal networks.
6. Molecular evolution of oxylipin plant hormone
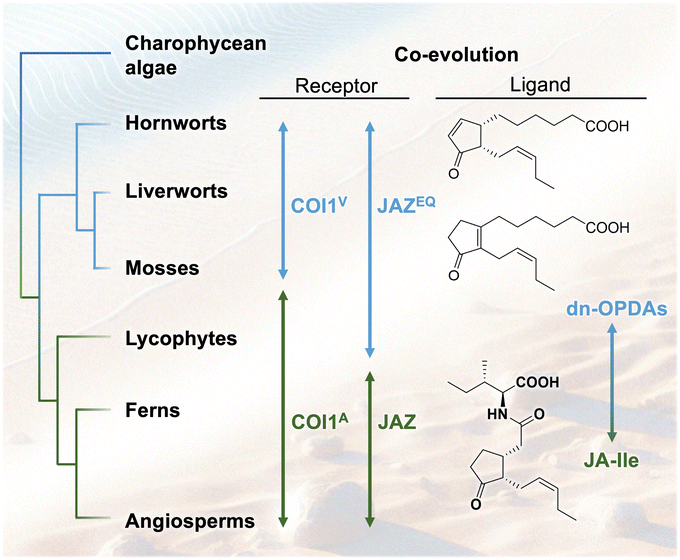
6.1 Plant evolution and oxylipin hormone
Plants first colonized land approximately 450 million years ago.52 Recent advancements in genomic science have enabled the evolutionary analysis of plant hormone signaling and biosynthetic mechanisms. The evolution of plant hormone signaling pathways is deeply intertwined with the adaptation of land plants to terrestrial environments, where they developed defense mechanisms against new biotic and abiotic stresses. Jasmonate hormones and their signaling pathways, which mediate defense responses to herbivores and plant pathogens, are thought to have evolved as advantageous mechanisms for plant survival on land. The perception of JA-Ile through its receptor, COI1, has evolved significantly across plant lineages, from algae to land plants (Fig. 11). This section focuses on the evolutionary aspects of JA perception and biosynthesis.6.2 Ligand–receptor co-evolution in plant oxylipin hormone
Jasmonate biosynthesis follows the octadecanoid pathway.41,133 The first evidence of jasmonate biosynthesis in terrestrial plants appears in charophytes, the closest relatives of land plants. Some charophytes possess partial JA biosynthesis genes, while more complex terrestrial algae, such as Klebsormidium nitens, contain a nearly complete pathway for producing cis/iso-OPDA, precursors of JA.51 This suggests that cis/iso-OPDA played an ancestral role in terrestrial adaptation before the evolution of JA-Ile as the primary signaling molecule in vascular plants. Liverworts and mosses lack OPR3 and JAR1, which are essential enzymes for JA-Ile synthesis. Almost no accumulation of JA-Ile occurs in M. polymorpha, a bryophyte considered to be a model of an “ancestral” land plant,52 supporting the hypothesis that different jasmonates evolved as functionally equivalent hormones in diverse plant lineages.COI1, an F-box protein, functions as a JA receptor in land plants. M. polymorpha represents the first lineage where a functional COI1-JAZ signaling module appears.52 In Marchantia, the MpCOI1-MpJAZ pathway regulates defense responses against herbivores and pathogens. Genetic mutants with impaired JA biosynthesis or MpCOI1 exhibit increased susceptibility to herbivores and pathogenic infection, aligning with findings in Arabidopsis. However, instead of binding JA-Ile, the bioactive ligand for MpCOI1 in M. polymorpha is dn-cis/iso-OPDA, intermediates of a non-canonical JA biosynthetic pathway.50 A mutation from valine (V) to alanine (A) in the ligand-binding pocket of MpCOI1 provides additional space, enabling changes in ligand specificity.50 Additionally, a mutation in the ligand-binding site of MpJAZ (from JAZEQ to JAZ) further alters the co-receptor's properties. MpJAZ in M. polymorpha has E200 and Q203 in the C-terminal loop region of the JAZ degron (JAZEQ). COI1V/JAZ could not perceive dn-OPDA or JA-Ile, whereas COI1A/JAZEQ could. This suggests that E200 and Q203 in JAZEQ are necessary for dn-OPDA recognition. However, additional residues outside the loop region may also be involved in ligand perception (Fig. 11).134 These mutations facilitate the co-evolution of hormone metabolites and receptor specificities, driving the transition of the hormone molecule from dn-OPDAs in bryophytes to JA-Ile in vascular plants. This represents a unique example of “ligand–receptor co-evolution,” in which an evolutionary shift in the hormone molecule and the COI1-JAZ co-receptor occur simultaneously, highlighting a critical step in the diversification of jasmonate signaling pathways (Fig. 11).
6.3 Shifts in the biosynthetic pathway causes evolution of the hormone structure
A recent study reveals that JA signaling remains activated in a Marchantia mutant (Mpfad5) with impaired dn-OPDA biosynthesis from C16/C18-PUFAs.116 This finding led to the discovery of an alternative hormone, Δ4-dn-OPDA (Δ4-dn-cis/iso-OPDA),53 biosynthesized from eicosapentaenoic acid (EPA), a C20-unsaturated fatty acid. These Δ4-dn-OPDAs also function as ligands for the MpCOI1-MpJAZ co-receptor. Notably, dn-iso-OPDA and Δ4-dn-iso-OPDA isomers possessing a tetra-substituted olefin are more abundant and exhibit stronger affinity for MpCOI1-MpJAZ than their cis-counterparts.53 Additionally, unique olefin isomerization occurs from Δ4-dn-cis-OPDA to Δ4-dn-iso-OPDA in Marchantia, suggesting that the cis-isomer serves as a biosynthetic precursor of the genuine bioactive hormone, the iso-isomer.53It is estimated that a similar correlation exists between dn-OPDAs. In bryophytes, including M. polymorpha, dn-iso-OPDA accumulates in greater amounts than dn-cis-OPDA and exhibits a higher affinity for MpCOI1-MpJAZ. These findings suggest that the isomerization between the two dn-OPDAs may generate the bioactive hormone dn-iso-OPDA in M. polymorpha. Notably, both dn-iso-OPDA in M. polymorpha and JA-Ile in A. thaliana are biosynthesized from a common precursor, cis-OPDA. The biosynthesis of dn-iso-OPDA requires olefin isomerization; however, this isomerization does not occur in vascular plants including A. thaliana. Based on these findings, we hypothesize that a “shift in the biosynthetic pathway” may have occurred during plant evolution, involving the loss of the isomerization enzyme and the emergence of reduction enzymes (OPR1/2/3) essential for JA-Ile biosynthesis. Consequently, this evolutionary shift in the biosynthetic pathway may have driven structural change of plant hormones (Fig. 12).
With the evolution of vascular plants, the JA-Ile signaling pathway became more prominent, supported by enzymes like OPR3 and JAR1. Selaginella moellendorffii is a lycophyte that produces dn-OPDA and JA-Ile and represents a transitional state in JA perception evolution.51 Ferns and seed plants have further diversified JA perception, leading to more specialized hormone–receptor interactions that regulate JA responses.51 These analyses suggest a unique trait of ligand–receptor co-evolution, specifically indicating a shift in ligand specificity during evolution.
6.4 Evolutional complexity in OPDA-amino acid conjugates
The emergence of cis-OPDA-amino acid conjugates such as cis-OPDA-Ile, cis-OPDA-Glu, cis-OPDA-Phe, etc. in Arabidopsis and other vascular plants provides additional layers of evolutional complexity.135,136 These conjugates lack affinity for the COI1-JAZ co-receptor and are biologically inactive. They are biosynthesized through the conjugation of cis-OPDA with amino acids, mediated by members of the AtGH3 enzyme family, and are subsequently hydrolyzed by amidohydrolases of the Indole-3-Acetyl-Leucine Resistant 1 (ILR1)-like family. This suggests that cis-OPDA-amino acid conjugates may serve as intermediates for cis-OPDA catabolism or as temporary storage forms during stress responses (Fig. 13A and Table 1).137 A similar conjugation mechanism between dn-OPDAs and amino acids was found in M. polymorpha.138 dn-iso-OPDA-amino acid conjugates (dn-iso-OPDA-AAs) were detected in wounded Marchantia. This conjugation reaction catalyzed by GRETCHEN-HAGEN 3A (MpGH3A) results in the inactivation of JA responses mediated by dn-iso-OPDA (Fig. 13B).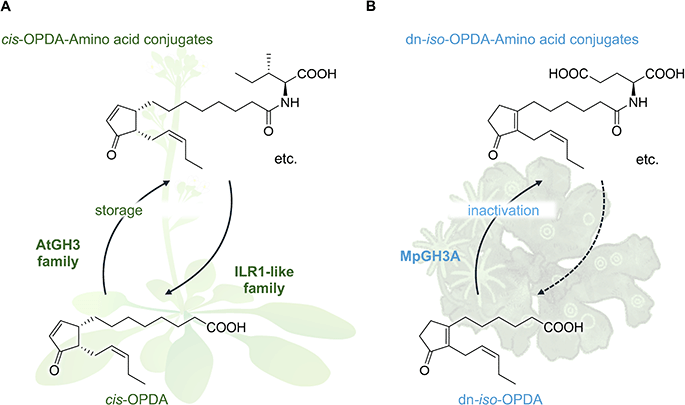 | ||
| Fig. 13 Conjugation of OPDAs with amino acids is a conserved mechanism for the deactivation of plant hormone in (A) Arabidopsis thaliana and (B) Marchantia polymorpha. | ||
The evolution of jasmonate perception, biosynthesis, and storage reflects a dynamic interplay between biochemical innovation and ecological adaptation. While many signaling components are conserved, key evolutionary shifts have shaped the specificity and function of jasmonate hormones. From ancestral dn-cis/iso-OPDA signaling in charophytes to the emergence of JA-Ile as the dominant ligand in vascular plants, modifications in COI1 and associated regulatory components have shaped the functional landscape of JA responses.
7. Principal conclusions
Jasmonates, once primarily recognized for their role in plant defense, have now emerged as multifaceted signaling molecules that orchestrate a wide range of plant responses-spanning herbivore and pathogen resistance to growth, development, stress tolerance, senescence, fertility, and secondary metabolism. The identification of JA-Ile as the principal plant oxylipin hormone represents a landmark in plant biology. JA-Ile functions as a “molecular glue” bridging the F-box receptor protein COI1 and diverse JAZ repressors. Through this single ligand–receptor interaction, plants can finely balance growth and defense, although this often comes at the cost of sacrificing one trait for the other.The discovery of JA-Ile and its receptor has also opened up opportunities to design synthetic molecules that activate or block particular branches of the jasmonate pathway, including antagonists and agonists. Notably, the development of JA-Ile receptor subtype-selective agonists has uncovered ways to uncouple the trade-off between growth and defense, hinting at the potential for more targeted agricultural interventions. Such “precision agriculture” approaches might enable growers to boost plant resilience against stress while preserving yield.
Beyond higher plants, evolutionary studies in basal land plants like M. polymorpha have shed light on how “ancestral jasmonates”, such as dn-OPDAs, served as active signals prior to the emergence of JA-Ile as the dominant ligand in vascular plants. These findings illustrate a co-evolution of plant hormones and their receptors, in which shifts in biosynthetic pathways and receptor-binding sites progressively shaped the specificity and complexity of jasmonate signaling. Ongoing research into jasmonate metabolism, especially the interplay of catabolic and storage pathways, will further clarify how plants turn jasmonate signals on and off to suit their ever-changing environment.
Despite remarkable progress, several questions remain on the precise mechanisms of JA-Ile transport, its turnover, and interactions with other signaling molecules, warranting further investigation. Emerging tools in synthetic biology and omics approaches promise to address these challenges, providing new insights into jasmonate biology. These insights promise new strategies to harness jasmonate biology, ranging from breeding and engineering efforts to the development of eco-friendly agrochemicals to sustainably enhance crop productivity and stress resilience. Continued exploration of mechanisms and applications will undoubtedly yield transformative insights into chemical manipulation of plant defense and enhance food production.
8. Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review. Crystallographic data used in this article (Fig. 2 and 5) has been deposited at the PBD under 3OGL and 3OGM and can be obtained from URL of data records, PDB DOI: https://doi.org/10.2210/pdb3OGL/pdb and https://doi.org/10.2210/pdb3OGM/pdb.9. Author contributions
All authors worked together on the manuscript.10. Conflicts of interest
There are no conflicts to declare.11. Acknowledgements
This work was financially supported by Grant-in-Aid for Scientific Research from JSPS (Japan) projects no. 23H00316, JPJSBP120239903, and a Grant-in-Aid for Transformative Research Areas (A) “Latent Chemical Space” [JP23H04880 and JP23H04883] from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to MU).12. References
- A. Santner, L. I. Calderon-Villalobos and M. Estelle, Nat. Chem. Biol., 2009, 5, 301–307 CrossRef CAS PubMed.
- M. H. Beale and J. L. Ward, Nat. Prod. Rep., 1998, 15, 533–548 RSC.
- M. Ueda, T. Kaji and W. Kozaki, Int. J. Mol. Sci., 2020, 21, 1124 CrossRef CAS PubMed.
- U. Jahn, J. M. Galano and T. Durand, Angew. Chem., Int. Ed. Engl., 2008, 47, 5894–5955 CrossRef CAS PubMed.
- J. M. Galano, Y. Y. Lee, C. Oger, C. Vigor, J. Vercauteren, T. Durand, M. Giera and J. C. Lee, Prog. Lipid Res., 2017, 68, 83–108 CrossRef CAS PubMed.
- G. A. Howe, I. T. Major and A. J. Koo, Annu. Rev. Plant Biol., 2018, 69, 387–415 CrossRef CAS PubMed.
- S. Savary, L. Willocquet, S. J. Pethybridge, P. Esker, N. McRoberts and A. Nelson, Nat. Ecol. Evol., 2019, 3, 430–439 CrossRef PubMed.
- C. Wasternack and I. Feussner, Annu. Rev. Plant Biol., 2018, 69, 363–386 CrossRef CAS PubMed.
- C. Wasternack and M. Strnad, Int. J. Mol. Sci., 2018, 19, 2539 CrossRef PubMed.
- S. Hu, K. Yu, J. Yan, X. Shan and D. Xie, Mol. Plant, 2023, 16, 23–42 CrossRef CAS PubMed.
- D. Gasperini and G. A. Howe, Plant Physiol., 2024, 195, 135–154 CrossRef CAS PubMed.
- C. Chapuis, Perfum. Flavor., 2011, 36, 36–48 CAS.
- E. Demole, E. Lederer and D. Mercier, Helv. Chim. Acta, 1962, 45, 675–685 CrossRef CAS.
- S. Blechert, W. Brodschelm, S. Holder, L. Kammerer, T. M. Kutchan, M. J. Mueller, Z. Q. Xia and M. H. Zenk, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 4099–4105 CrossRef CAS PubMed.
- W. Dathe, H. Ronsch, A. Preiss, W. Schade, G. Sembdner and K. Schreiber, Planta, 1981, 153, 530–535 CrossRef CAS PubMed.
- J. Ueda and J. Kato, Plant Physiol., 1980, 66, 246–249 CrossRef CAS PubMed.
- H. Yamane, H. Takagi, H. Abe, T. Yokota and N. Takahashi, Plant Cell Physiol., 1981, 22, 689–697 CAS.
- B. A. Vick and D. C. Zimmerman, Plant Physiol., 1984, 75, 458–461 CrossRef CAS PubMed.
- C. Wasternack, J. Plant Growth Regul., 2015, 34, 761–794 CrossRef CAS.
- A. Ichihara, K. Shiraishi, H. Sato, S. Sakamura, K. Nishiyama, R. Sakai, A. Furusaki and T. Matsumoto, J. Am. Chem. Soc., 1977, 99, 636–637 CrossRef CAS.
- A. Ichihara and S. Sakamura, Toxicon, 1983, 21, 187–190 CrossRef.
- Y. Koda, K. Takahashi, Y. Kikuta, F. Greulich, H. Toshima and A. Ichihara, Phytochemistry, 1996, 41, 93–96 CrossRef CAS.
- E. W. Weiler, T. M. Kutchan, T. Gorba, W. Brodschelm, U. Niesel and F. Bublitz, FEBS Lett., 1994, 345, 9–13 CrossRef CAS PubMed.
- P. E. Staswick, W. Su and S. H. Howell, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 6837–6840 CrossRef CAS PubMed.
- P. E. Staswick, I. Tiryaki and M. L. Rowe, Plant Cell, 2002, 14, 1405–1415 CrossRef CAS PubMed.
- J. M. Jez, Curr. Opin. Plant Biol., 2022, 66, 102194 CrossRef CAS PubMed.
- P. E. Staswick and I. Tiryaki, Plant Cell, 2004, 16, 2117–2127 CrossRef CAS PubMed.
- W. P. Suza and P. E. Staswick, Planta, 2008, 227, 1221–1232 CrossRef CAS PubMed.
- J. C. Delfin, Y. Kanno, M. Seo, N. Kitaoka, H. Matsuura, T. Tohge and T. Shimizu, Plant J., 2022, 110, 1082–1096 CrossRef CAS PubMed.
- D. X. Xie, B. F. Feys, S. James, M. Nieto-Rostro and J. G. Turner, Science, 1998, 280, 1091–1094 CrossRef CAS PubMed.
- B. Feys, C. E. Benedetti, C. N. Penfold and J. G. Turner, Plant Cell, 1994, 6, 751–759 CrossRef CAS.
- A. Chini, S. Fonseca, G. Fernandez, B. Adie, J. M. Chico, O. Lorenzo, G. Garcia-Casado, I. Lopez-Vidriero, F. M. Lozano, M. R. Ponce, J. L. Micol and R. Solano, Nature, 2007, 448, 666–671 CrossRef CAS PubMed.
- B. Thines, L. Katsir, M. Melotto, Y. Niu, A. Mandaokar, G. Liu, K. Nomura, S. Y. He, G. A. Howe and J. Browse, Nature, 2007, 448, 661–665 CrossRef CAS PubMed.
- S. Fonseca, A. Chini, M. Hamberg, B. Adie, A. Porzel, R. Kramell, O. Miersch, C. Wasternack and R. Solano, Nat. Chem. Biol., 2009, 5, 344–350 CrossRef CAS PubMed.
- L. B. Sheard, X. Tan, H. Mao, J. Withers, G. Ben-Nissan, T. R. Hinds, Y. Kobayashi, F. F. Hsu, M. Sharon, J. Browse, S. Y. He, J. Rizo, G. A. Howe and N. Zheng, Nature, 2010, 468, 400–405 CrossRef CAS PubMed.
- H. Gundlach, M. J. Muller, T. M. Kutchan and M. H. Zenk, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 2389–2393 CrossRef CAS PubMed.
- C. Wasternack and M. Strnad, Nat. Biotechnol., 2019, 48, 1–11 CAS.
- G. H. Jimenez Aleman, V. P. Thirumalaikumar, G. Jander, A. R. Fernie and A. Skirycz, Phytochemistry, 2022, 204, 113432 CrossRef CAS PubMed.
- N. Taki, Y. Sasaki-Sekimoto, T. Obayashi, A. Kikuta, K. Kobayashi, T. Ainai, K. Yagi, N. Sakurai, H. Suzuki, T. Masuda, K. Takamiya, D. Shibata, Y. Kobayashi and H. Ohta, Plant Physiol., 2005, 139, 1268–1283 CrossRef CAS PubMed.
- R. Yi, Y. Li and X. Shan, Plant Cell Rep., 2024, 43, 206 CrossRef CAS PubMed.
- C. Wasternack and B. Hause, Ann. Bot., 2013, 111, 1021–1058 CrossRef CAS PubMed.
- D. Maynard, H. Groger, T. Dierks and K. J. Dietz, J. Exp. Bot., 2018, 69, 5341–5354 CAS.
- I. Monte, S. Kneeshaw, J. M. Franco-Zorrilla, A. Chini, A. M. Zamarreno, J. M. Garcia-Mina and R. Solano, Curr. Biol., 2020, 30, 962–971 CrossRef CAS PubMed.
- Y. Chang, M. Shi, Y. Sun, H. Cheng, X. Ou, Y. Zhao, X. Zhang, B. Day, C. Miao and K. Jiang, Curr. Biol., 2023, 33, 1071–1081 CrossRef CAS PubMed.
- S. W. Park, W. Li, A. Viehhauser, B. He, S. Kim, A. K. Nilsson, M. X. Andersson, J. D. Kittle, M. M. Ambavaram, S. Luan, A. R. Esker, D. Tholl, D. Cimini, M. Ellerstrom, G. Coaker, T. K. Mitchell, A. Pereira, K. J. Dietz and C. B. Lawrence, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 9559–9564 CrossRef CAS PubMed.
- D. Maynard, A. Viehhauser, M. Knieper, A. Dreyer, G. Manea, W. Telman, F. Butter, K. Chibani, R. Scheibe and K. J. Dietz, Biomolecules, 2021, 11, 457 CrossRef CAS PubMed.
- S. M. Muller, S. Wang, W. Telman, M. Liebthal, H. Schnitzer, A. Viehhauser, C. Sticht, C. Delatorre, M. Wirtz, R. Hell and K. J. Dietz, Plant J., 2017, 91, 995–1014 CrossRef PubMed.
- M. Ueda, R. Saito, Y. Nishizato, T. Kitajima and N. Kato, Nat. Commun., 2025, 16 DOI:10.21203/rs.3.rs-5075946/v1.
- K. Mekkaoui, R. Baral, F. Smith, M. Klein, I. Feussner and B. Hause, bioRxiv, 2024, preprint, DOI:10.1101/2024.03.22.586262.
- I. Monte, S. Ishida, A. M. Zamarreno, M. Hamberg, J. M. Franco-Zorrilla, G. Garcia-Casado, C. Gouhier-Darimont, P. Reymond, K. Takahashi, J. M. Garcia-Mina, R. Nishihama, T. Kohchi and R. Solano, Nat. Chem. Biol., 2018, 14, 480–488 CrossRef CAS PubMed.
- A. Chini, I. Monte, A. M. Zamarreno, J. M. Garcia-Mina and R. Solano, New Phytol., 2023, 238, 2236–2246 CrossRef CAS PubMed.
- J. L. Bowman, T. Kohchi, K. T. Yamato, J. Jenkins, S. Shu, K. Ishizaki, S. Yamaoka, R. Nishihama, Y. Nakamura, F. Berger, C. Adam, S. S. Aki, F. Althoff, T. Araki, M. A. Arteaga-Vazquez, S. Balasubrmanian, K. Barry, D. Bauer, C. R. Boehm, L. Briginshaw, J. Caballero-Perez, B. Catarino, F. Chen, S. Chiyoda, M. Chovatia, K. M. Davies, M. Delmans, T. Demura, T. Dierschke, L. Dolan, A. E. Dorantes-Acosta, D. M. Eklund, S. N. Florent, E. Flores-Sandoval, A. Fujiyama, H. Fukuzawa, B. Galik, D. Grimanelli, J. Grimwood, U. Grossniklaus, T. Hamada, J. Haseloff, A. J. Hetherington, A. Higo, Y. Hirakawa, H. N. Hundley, Y. Ikeda, K. Inoue, S. I. Inoue, S. Ishida, Q. Jia, M. Kakita, T. Kanazawa, Y. Kawai, T. Kawashima, M. Kennedy, K. Kinose, T. Kinoshita, Y. Kohara, E. Koide, K. Komatsu, S. Kopischke, M. Kubo, J. Kyozuka, U. Lagercrantz, S. S. Lin, E. Lindquist, A. M. Lipzen, C. W. Lu, E. De Luna, R. A. Martienssen, N. Minamino, M. Mizutani, M. Mizutani, N. Mochizuki, I. Monte, R. Mosher, H. Nagasaki, H. Nakagami, S. Naramoto, K. Nishitani, M. Ohtani, T. Okamoto, M. Okumura, J. Phillips, B. Pollak, A. Reinders, M. Rovekamp, R. Sano, S. Sawa, M. W. Schmid, M. Shirakawa, R. Solano, A. Spunde, N. Suetsugu, S. Sugano, A. Sugiyama, R. Sun, Y. Suzuki, M. Takenaka, D. Takezawa, H. Tomogane, M. Tsuzuki, T. Ueda, M. Umeda, J. M. Ward, Y. Watanabe, K. Yazaki, R. Yokoyama, Y. Yoshitake, I. Yotsui, S. Zachgo and J. Schmutz, Cell, 2017, 171, 287–304 CrossRef CAS PubMed.
- S. Kneeshaw, G. Soriano, I. Monte, M. Hamberg, A. M. Zamarreno, J. M. Garcia-Mina, J. M. Franco-Zorrilla, N. Kato, M. Ueda, M. F. Rey-Stolle, C. Barbas, S. Michavila, S. Gimenez-Ibanez, G. H. Jimenez-Aleman and R. Solano, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2202930119 CrossRef CAS PubMed.
- T. Kaji, Y. Nishizato, H. Yoshimatsu, A. Yoda, W. Liang, A. Chini, G. Fernandez-Barbero, K. Nozawa, J. Kyozuka, R. Solano and M. Ueda, iScience, 2024, 27, 110191 CrossRef CAS PubMed.
- A. N. Poudel, R. E. Holtsclaw, A. Kimberlin, S. Sen, S. Zeng, T. Joshi, Z. Lei, L. W. Sumner, K. Singh, H. Matsuura and A. J. Koo, Plant Cell Physiol., 2019, 60, 2152–2166 CrossRef CAS PubMed.
- G. H. Jimenez-Aleman, M. Almeida-Trapp, G. Fernandez-Barbero, S. Gimenez-Ibanez, M. Reichelt, J. Vadassery, A. Mithöfer, J. Caballero, W. Boland and R. Solano, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2019, 1864, 158520 CrossRef CAS PubMed.
- R. Saito, T. Muto, H. Urano, T. Kitajima, N. Kato, E. Kwon and M. Ueda, Plant J., 2023, 115, 709–723 CrossRef CAS PubMed.
- A. N. Poudel, T. Zhang, M. Kwasniewski, R. Nakabayashi, K. Saito and A. J. Koo, Biochim. Biophys. Acta, 2016, 1861, 1396–1408 CrossRef CAS PubMed.
- S. L. Schreiber, Cell, 2021, 184, 3–9 CrossRef CAS PubMed.
- M. Konstantinidou and M. R. Arkin, Cell Chem. Biol., 2024, 31, 1064–1088 CrossRef CAS PubMed.
- C. Darwin and F. Darwin, The Power of Movement in Plants, third thousand, John Murray, London, 1882 Search PubMed.
- N. Dharmasiri, S. Dharmasiri and M. Estelle, Nature, 2005, 435, 441–445 CrossRef CAS PubMed.
- X. Tan, L. I. Calderon-Villalobos, M. Sharon, C. Zheng, C. V. Robinson, M. Estelle and N. Zheng, Nature, 2007, 446, 640–645 CrossRef CAS PubMed.
- C. Wang, Y. Liu, S.-S. Li and G.-Z. Han, Plant Physiol., 2015, 167, 872–886 CrossRef CAS PubMed.
- T. Ito, H. Ando, T. Suzuki, T. Ogura, K. Hotta, Y. Imamura, Y. Yamaguchi and H. Handa, Science, 2010, 327, 1345–1350 CrossRef CAS PubMed.
- J. Yan, C. Zhang, M. Gu, Z. Bai, W. Zhang, T. Qi, Z. Cheng, W. Peng, H. Luo, F. Nan, Z. Wang and D. Xie, Plant Cell, 2009, 21, 2220–2236 CrossRef CAS PubMed.
- A. Santner and M. Estelle, Nature, 2009, 459, 1071–1078 CrossRef CAS PubMed.
- N. Shabek and N. Zheng, Nat. Struct. Mol. Biol., 2014, 21, 293–296 CrossRef CAS PubMed.
- M. Cui, K. Zhang, R. Wu and J. Du, J. Comput.-Aided Mol. Des., 2022, 36, 141–155 CrossRef CAS PubMed.
- J. Yan, R. Yao, L. Chen, S. Li, M. Gu, F. Nan and D. Xie, Mol. Plant, 2018, 11, 1237–1247 CrossRef CAS PubMed.
- T. Kaji, K. Matsumoto, T. Okumura, M. Nakayama, S. Hoshino, Y. Takaoka, J. Wang and M. Ueda, iScience, 2024, 27, 108625 CrossRef CAS PubMed.
- A. Chini, S. Gimenez-Ibanez, A. Goossens and R. Solano, Curr. Opin. Plant Biol., 2016, 33, 147–156 CrossRef CAS PubMed.
- H. Y. Lee, J. S. Seo, J. H. Cho, H. Jung, J. K. Kim, J. S. Lee, S. Rhee and Y. Do Choi, PLoS One, 2013, 8, e52802 CrossRef CAS PubMed.
- H. Inagaki, K. Hayashi, Y. Takaoka, H. Ito, Y. Fukumoto, A. Yajima-Nakagawa, X. Chen, M. Shimosato-Nonaka, E. Hassett, K. Hatakeyama, Y. Hirakuri, M. Ishitsuka, E. Yumoto, T. Sakazawa, M. Asahina, K. Uchida, K. Okada, H. Yamane, M. Ueda and K. Miyamoto, Plant Cell Physiol., 2023, 64, 405–421 CrossRef CAS PubMed.
- X. Wang, Y. Chen, S. Liu, W. Fu, Y. Zhuang, J. Xu, Y. Lou, I. T. Baldwin and R. Li, New Phytol., 2023, 238, 2144–2158 CrossRef CAS PubMed.
- L. Pauwels and A. Goossens, Plant Cell, 2011, 23, 3089–3100 CrossRef CAS PubMed.
- Q. Guo, Y. Yoshida, I. T. Major, K. Wang, K. Sugimoto, G. Kapali, N. E. Havko, C. Benning and G. A. Howe, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E10768–E10777 CrossRef CAS PubMed.
- P. E. Wright and H. J. Dyson, Nat. Rev. Mol. Cell Biol., 2015, 16, 18–29 CrossRef CAS PubMed.
- F. Zhang, J. Yao, J. Ke, L. Zhang, V. Q. Lam, X. F. Xin, X. E. Zhou, J. Chen, J. Brunzelle, P. R. Griffin, M. Zhou, H. E. Xu, K. Melcher and S. Y. He, Nature, 2015, 525, 269–273 CrossRef CAS PubMed.
- C. An, L. Li, Q. Zhai, Y. You, L. Deng, F. Wu, R. Chen, H. Jiang, H. Wang, Q. Chen and C. Li, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E8930–E8939 CAS.
- Q. Zhai and C. Li, J. Exp. Bot., 2019, 70, 3415–3424 CrossRef CAS PubMed.
- C. Shyu, P. Figueroa, C. L. Depew, T. F. Cooke, L. B. Sheard, J. E. Moreno, L. Katsir, N. Zheng, J. Browse and G. A. Howe, Plant Cell, 2012, 24, 536–550 CrossRef CAS PubMed.
- C. Thireault, C. Shyu, Y. Yoshida, B. St Aubin, M. L. Campos and G. A. Howe, Plant J., 2015, 82, 669–679 CrossRef CAS PubMed.
- N. Aerts, M. Pereira Mendes and S. C. M. Van Wees, Plant J., 2021, 105, 489–504 CrossRef CAS PubMed.
- E. Breeze, Plant Cell, 2019, 31, 9–10 CrossRef CAS PubMed.
- A. Mithöfer and W. Boland, Annu. Rev. Plant Biol., 2012, 63, 431–450 CrossRef PubMed.
- F. Schweizer, P. Fernandez-Calvo, M. Zander, M. Diez-Diaz, S. Fonseca, G. Glauser, M. G. Lewsey, J. R. Ecker, R. Solano and P. Reymond, Plant Cell, 2013, 25, 3117–3132 CrossRef CAS PubMed.
- M. Nakayasu, N. Shioya, M. Shikata, C. Thagun, A. Abdelkareem, Y. Okabe, T. Ariizumi, G. I. Arimura, M. Mizutani, H. Ezura, T. Hashimoto and T. Shoji, Plant J., 2018, 94, 975–990 CrossRef CAS PubMed.
- S. Panda, A. Jozwiak, P. D. Sonawane, J. Szymanski, Y. Kazachkova, A. Vainer, H. Vasuki Kilambi, E. Almekias-Siegl, V. Dikaya, S. Bocobza, H. Shohat, S. Meir, G. Wizler, A. P. Giri, R. Schuurink, D. Weiss, H. Yasuor, A. Kamble and A. Aharoni, New Phytol., 2022, 233, 1220–1237 CrossRef CAS PubMed.
- G. Schuler, A. Mithöfer, I. T. Baldwin, S. Berger, J. Ebel, J. G. Santos, G. Herrmann, D. Holscher, R. Kramell, T. M. Kutchan, H. Maucher, B. Schneider, I. Stenzel, C. Wasternack and W. Boland, FEBS Lett., 2004, 563, 17–22 CrossRef CAS PubMed.
- Y. Nakamura, C. Paetz, W. Brandt, A. David, M. Rendon-Anaya, A. Herrera-Estrella, A. Mithöfer and W. Boland, J. Chem. Ecol., 2014, 40, 687–699 CrossRef CAS PubMed.
- R. Lauchli and W. Boland, Chem. Rec., 2003, 3, 12–21 CrossRef CAS PubMed.
- A. Walter, C. Mazars, M. Maitrejean, J. Hopke, R. Ranjeva, W. Boland and A. Mithöfer, Angew Chem Int Ed Engl, 2007, 46, 4783–4785 CrossRef CAS PubMed.
- I. Monte, M. Hamberg, A. Chini, S. Gimenez-Ibanez, G. Garcia-Casado, A. Porzel, F. Pazos, M. Boter and R. Solano, Nat. Chem. Biol., 2014, 10, 671–676 CrossRef CAS PubMed.
- M. M. Littleson, C. M. Baker, A. J. Dalencon, E. C. Frye, C. Jamieson, A. R. Kennedy, K. B. Ling, M. M. McLachlan, M. G. Montgomery, C. J. Russell and A. J. B. Watson, Nat. Commun., 2018, 9, 1105 CrossRef PubMed.
- A. Chini, I. Monte, G. Fernandez-Barbero, M. Boter, G. Hicks, N. Raikhel and R. Solano, Plant Physiol., 2021, 187, 1399–1413 CrossRef CAS PubMed.
- C. Meesters, T. Monig, J. Oeljeklaus, D. Krahn, C. S. Westfall, B. Hause, J. M. Jez, M. Kaiser and E. Kombrink, Nat. Chem. Biol., 2014, 10, 830–836 CrossRef CAS PubMed.
- Y. Takaoka, M. Iwahashi, A. Chini, H. Saito, Y. Ishimaru, S. Egoshi, N. Kato, M. Tanaka, K. Bashir, M. Seki, R. Solano and M. Ueda, Nat. Commun., 2018, 9, 3654 CrossRef PubMed.
- B. Huot, J. Yao, B. L. Montgomery and S. Y. He, Mol. Plant, 2014, 7, 1267–1287 CrossRef CAS PubMed.
- K. Hayashi, N. Kato, K. Bashir, H. Nomoto, M. Nakayama, A. Chini, S. Takahashi, H. Saito, R. Watanabe, Y. Takaoka, M. Tanaka, A. J. Nagano, M. Seki, R. Solano and M. Ueda, Commun. Biol., 2023, 6, 320 CrossRef CAS PubMed.
- G. H. Jimenez-Aleman, R. A. R. Machado, I. T. Baldwin and W. Boland, Org. Biomol. Chem., 2017, 15, 3391–3395 RSC.
- G. H. Jimenez-Aleman, R. A. Machado, H. Gorls, I. T. Baldwin and W. Boland, Org. Biomol. Chem., 2015, 13, 5885–5893 RSC.
- W. Dejonghe and E. Russinova, Plant Physiol., 2017, 174, 5–20 CrossRef CAS PubMed.
- V. Halder and E. Russinova, Nat. Chem. Biol., 2019, 15, 1025–1028 CrossRef CAS PubMed.
- M. Li, G. Yu, C. Cao and P. Liu, Plant Commun., 2021, 2, 100231 CrossRef CAS PubMed.
- D. Laudert, U. Pfannschmidt, F. Lottspeich, H. Hollander-Czytko and E. W. Weiler, Plant Mol. Biol., 1996, 31, 323–335 CrossRef CAS PubMed.
- I. Stenzel, B. Hause, O. Miersch, T. Kurz, H. Maucher, H. Weichert, J. Ziegler, I. Feussner and C. Wasternack, Plant Mol. Biol., 2003, 51, 895–911 CrossRef CAS PubMed.
- E. Hofmann, P. Zerbe and F. Schaller, Plant Cell, 2006, 18, 3201–3217 CrossRef CAS PubMed.
- S. Footitt, S. P. Slocombe, V. Larner, S. Kurup, Y. Wu, T. Larson, I. Graham, A. Baker and M. Holdsworth, EMBO J., 2002, 21, 2912–2922 CrossRef CAS PubMed.
- L. Guan, N. Denkert, A. Eisa, M. Lehmann, I. Sjuts, A. Weiberg, J. Soll, M. Meinecke and S. Schwenkert, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 10568–10575 CrossRef CAS PubMed.
- A. Stintzi and J. Browse, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 10625–10630 CrossRef CAS PubMed.
- A. Guranowski, O. Miersch, P. E. Staswick, W. Suza and C. Wasternack, FEBS Lett., 2007, 581, 815–820 CrossRef CAS PubMed.
- J. Yan, S. Li, M. Gu, R. Yao, Y. Li, J. Chen, M. Yang, J. Tong, L. Xiao, F. Nan and D. Xie, Plant Physiol., 2016, 172, 2154–2164 CrossRef CAS PubMed.
- T. Savchenko, J. W. Walley, E. W. Chehab, Y. Xiao, R. Kaspi, M. F. Pye, M. E. Mohamed, C. M. Lazarus, R. M. Bostock and K. Dehesh, Plant Cell, 2010, 22, 3193–3205 CrossRef CAS PubMed.
- A. Chini, I. Monte, A. M. Zamarreno, M. Hamberg, S. Lassueur, P. Reymond, S. Weiss, A. Stintzi, A. Schaller, A. Porzel, J. M. Garcia-Mina and R. Solano, Nat. Chem. Biol., 2018, 14, 171–178 CrossRef CAS PubMed.
- G. Soriano, S. Kneeshaw, G. Jimenez-Aleman, A. M. Zamarreno, J. M. Franco-Zorrilla, M. F. Rey-Stolle, C. Barbas, J. M. Garcia-Mina and R. Solano, New Phytol., 2022, 233, 1401–1413 CrossRef CAS PubMed.
- H. Weber, B. A. Vick and E. E. Farmer, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 10473–10478 CrossRef CAS PubMed.
- N. Kitaoka, T. Matsubara, M. Sato, K. Takahashi, S. Wakuta, H. Kawaide, H. Matsui, K. Nabeta and H. Matsuura, Plant Cell Physiol., 2011, 52, 1757–1765 CrossRef CAS PubMed.
- A. J. Koo, T. F. Cooke and G. A. Howe, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 9298–9303 CrossRef CAS PubMed.
- T. Heitz, E. Widemann, R. Lugan, L. Miesch, P. Ullmann, L. Desaubry, E. Holder, B. Grausem, S. Kandel, M. Miesch, D. Werck-Reichhart and F. Pinot, J. Biol. Chem., 2012, 287, 6296–6306 CrossRef CAS PubMed.
- A. J. Koo, C. Thireault, S. Zemelis, A. N. Poudel, T. Zhang, N. Kitaoka, F. Brandizzi, H. Matsuura and G. A. Howe, J. Biol. Chem., 2014, 289, 29728–29738 CrossRef CAS PubMed.
- M. G. Woldemariam, N. Onkokesung, I. T. Baldwin and I. Galis, Plant J., 2012, 72, 758–767 CrossRef CAS PubMed.
- E. Widemann, L. Miesch, R. Lugan, E. Holder, C. Heinrich, Y. Aubert, M. Miesch, F. Pinot and T. Heitz, J. Biol. Chem., 2013, 288, 31701–31714 CrossRef CAS PubMed.
- E. Smirnova, V. Marquis, L. Poirier, Y. Aubert, J. Zumsteg, R. Menard, L. Miesch and T. Heitz, Mol. Plant, 2017, 10, 1159–1173 CrossRef CAS PubMed.
- L. Caarls, J. Elberse, M. Awwanah, N. R. Ludwig, M. de Vries, T. Zeilmaker, S. C. M. Van Wees, R. C. Schuurink and G. Van den Ackerveken, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6388–6393 CrossRef CAS PubMed.
- O. Miersch, J. Neumerkel, M. Dippe, I. Stenzel and C. Wasternack, New Phytol., 2008, 177, 114–127 CrossRef CAS PubMed.
- S. K. Gidda, O. Miersch, A. Levitin, J. Schmidt, C. Wasternack and L. Varin, J. Biol. Chem., 2003, 278, 17895–17900 CrossRef CAS PubMed.
- S. Haroth, K. Feussner, A. A. Kelly, K. Zienkiewicz, A. Shaikhqasem, C. Herrfurth and I. Feussner, J. Biol. Chem., 2019, 294, 9858–9872 CrossRef CAS PubMed.
- H. Helder, O. Miersch, D. Vreugdenhil and G. Sembdner, Physiol. Plant., 1993, 88, 647–653 CrossRef CAS PubMed.
- H. S. Seo, J. T. Song, J. J. Cheong, Y. H. Lee, Y. W. Lee, I. Hwang, J. S. Lee and Y. D. Choi, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4788–4793 CrossRef CAS PubMed.
- R. Yi, R. Du, J. Wang, J. Yan, J. Chu, J. Yan, X. Shan and D. Xie, Cell Discov., 2023, 9, 39 CrossRef CAS PubMed.
- Y. Nishizato, Y. Muraoka, M. Morikawa, R. Saito, T. Kaji and M. Ueda, Biosci., Biotechnol., Biochem., 2024, 88, 885–891 CrossRef PubMed.
- C. Wasternack and S. Song, J. Exp. Bot., 2017, 68, 1303–1321 CAS.
- I. Monte, J. Caballero, A. M. Zamarreno, G. Fernandez-Barbero, J. M. Garcia-Mina and R. Solano, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2212155119 CrossRef CAS PubMed.
- V. Mik, T. Pospisil, F. Brunoni, J. Gruz, V. Nozkova, C. Wasternack, O. Miersch, M. Strnad, K. Flokova, O. Novak and J. Siroka, Phytochemistry, 2023, 215, 113855 CrossRef CAS PubMed.
- K. Flokova, K. Feussner, C. Herrfurth, O. Miersch, V. Mik, D. Tarkowska, M. Strnad, I. Feussner, C. Wasternack and O. Novak, Phytochemistry, 2016, 122, 230–237 CrossRef CAS PubMed.
- J. Siroka, A. Ament, V. Mik, T. Pospisil, M. Kralova, C. Zhang, M. Pernisova, M. Karady, V. Nozkova, Y. Nishizato, T. Kaji, R. Saito, M. Htitich, K. Flokova, C. Wasternack, M. Strnad, M. Ueda, O. Novak and F. Brunoni, Plant Physiol., 2024, 197, kiae636 CrossRef PubMed.
- W. Liang, A. M. Zamarreno, S. Torres-Montilla, A. de la Torre, J. C. Totozafy, T. Kaji, M. Ueda, M. Corso, J. M. Garcia-Mina, R. Solano and A. Chini, Plant Physiol., 2025, 197, kiae610 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |