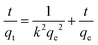Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Innovative CuBTC/g-C3N4 materials for tetracycline mitigation: adsorption, photocatalysis, and mechanistic perspectives†
Palkaran
Sethi
a,
Soumen
Basu
 *a and
Sanghamitra
Barman
*a and
Sanghamitra
Barman
 *b
*b
aDepartment of Chemistry and Biochemistry, Thapar Institute of Engineering and Technology, Patiala-147004, Punjab, India. E-mail: soumen.basu@thapar.edu
bDepartment of Chemical Engineering, Thapar Institute of Engineering and Technology, Patiala-147004, Punjab, India. E-mail: sbarman@thapar.edu
First published on 6th May 2025
Abstract
The widespread accumulation of antibiotic pollutants in water sources calls for advanced and efficient remediation strategies to curb environmental contamination. In this study, a CuBTC (copper benzene-1,3,5-tricarboxylate) with g-C3N4 heterojunction photocatalyst was synthesized via a hydrothermal approach in varying ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and comprehensively characterized using XRD, FESEM, EDS, HRTEM, EIS, UV-DRS, PL, TGA, FTIR, XPS, and BET measurements, confirming the composite's crystallinity, morphology, elemental composition, charge transport properties, optical behavior, stability, and porosity. Among the tested compositions, the 3
1) and comprehensively characterized using XRD, FESEM, EDS, HRTEM, EIS, UV-DRS, PL, TGA, FTIR, XPS, and BET measurements, confirming the composite's crystallinity, morphology, elemental composition, charge transport properties, optical behavior, stability, and porosity. Among the tested compositions, the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CuBTC/g-C3N4 composite exhibited the highest efficiency, achieving an impressive 97.4% degradation of 25 ppm tetracycline (TC) within just 60 minutes under UV illumination, with a remarkable rate constant of 0.02098 min−1. Stability assessments confirmed its excellent reusability over six consecutive cycles, with only a slight decline in performance to 82.7%. The adsorption behaviour of the composite was analyzed using six isotherm models—Langmuir, Freundlich, Halsey, Harkins–Jura, Temkin, and Dubinin–Radushkevich—along with five kinetic models, including pseudo-first-order, pseudo-second-order, intraparticle diffusion, Elovich, and liquid film models. Adsorption followed the Langmuir isotherm (R2 = 0.992) and pseudo-second-order kinetics (R2 = 0.968), while photocatalytic degradation aligned with pseudo-second-order kinetics (R2 = 0.993). Mechanistic studies identified superoxide radicals as the primary reactive species, supported by hydroxyl radicals, electrons, and holes in the degradation pathway. Mineralization studies revealed significant reductions in TOC (67.8%) and COD (68.6%), while LC-MS analysis provided a comprehensive degradation pathway, illustrating the breakdown of TC into intermediates through ring-opening and oxidative transformations. Thermodynamic assessments indicated that the degradation process was exothermic and spontaneous. ΔG, ΔH and ΔS values were found to be 92.7 J mol−1, −63.84 kJ mol−1, and −0.214 kJ mol−1 K−1 respectively.
1 CuBTC/g-C3N4 composite exhibited the highest efficiency, achieving an impressive 97.4% degradation of 25 ppm tetracycline (TC) within just 60 minutes under UV illumination, with a remarkable rate constant of 0.02098 min−1. Stability assessments confirmed its excellent reusability over six consecutive cycles, with only a slight decline in performance to 82.7%. The adsorption behaviour of the composite was analyzed using six isotherm models—Langmuir, Freundlich, Halsey, Harkins–Jura, Temkin, and Dubinin–Radushkevich—along with five kinetic models, including pseudo-first-order, pseudo-second-order, intraparticle diffusion, Elovich, and liquid film models. Adsorption followed the Langmuir isotherm (R2 = 0.992) and pseudo-second-order kinetics (R2 = 0.968), while photocatalytic degradation aligned with pseudo-second-order kinetics (R2 = 0.993). Mechanistic studies identified superoxide radicals as the primary reactive species, supported by hydroxyl radicals, electrons, and holes in the degradation pathway. Mineralization studies revealed significant reductions in TOC (67.8%) and COD (68.6%), while LC-MS analysis provided a comprehensive degradation pathway, illustrating the breakdown of TC into intermediates through ring-opening and oxidative transformations. Thermodynamic assessments indicated that the degradation process was exothermic and spontaneous. ΔG, ΔH and ΔS values were found to be 92.7 J mol−1, −63.84 kJ mol−1, and −0.214 kJ mol−1 K−1 respectively.
1. Introduction
For over half a century, antibiotics have served as a vital shield, protecting humans and animals from diverse pathogens. In recent times, however, their production and use have surged dramatically. Global antibiotic consumption was expected to soar to 128 billion defined daily doses by 2023, marking a staggering increase of over 200% since 2015.1 The persistence of antibiotics in water sources has become a global concern due to their detrimental effects on the environment and the potential risks they pose to aquatic life. Consequently, they are now classified as emerging pollutants.2–4 Key contributors to their presence in aquatic environments include pharmaceutical industries, municipal wastewater, hospital effluents, and sewage treatment plants.5,6 Tetracycline a broad-spectrum antibiotic is extensively used in human and veterinary medicine, including salmon farming. Their overuse can cause serious health issues like nephropathy, central nervous system disturbances, and increased antibiotic resistance.7 Residual antibiotics harm ecosystems and contribute to the emergence of bacterial strains with multi-drug resistance.8 Thus, practical technological solutions are necessary for their elimination. Various approaches, such as physical adsorption,9,10 chemical oxidation,11 and biological treatments,12 have been explored for removing tetracyclines (TCs). While each offers certain benefits, they often come with drawbacks, including complex procedures and risks of secondary pollution, limiting their practical applications.1,13 In contrast, photocatalysis presents advantages like simplicity, low energy consumption, high efficiency, and minimal secondary pollution.14,15 However, photocatalysis can be hindered by rapid recombination of photogenerated carriers and limited interaction with low-concentration antibiotics, reducing its effectiveness. To address these limitations, a promising strategy combines adsorption and photocatalysis.16–19 This approach enhances degradation by first rapidly capturing antibiotics through adsorption, followed by photocatalytic breakdown of the adsorbed pollutants and regeneration of the adsorbent.15 Various materials, such as mineral adsorbents, resins, activated carbons, silicon materials, polymers, metal oxides, charcoal, coordination polymers, and metal–organic frameworks, have been employed for antibiotic adsorption due to their high surface area and functional properties, which play a key role in enhancing adsorption efficiency.20 Coordination polymers (CPs) are highly crystalline and porous materials formed by transition metal ions and organic ligands.21 With adjustable pore sizes, vast surface areas, and diverse structures, they excel in adsorption, separation, and catalysis. Their unique combination of metal clusters and organic linkers allows for efficient light absorption and electron transfer, positioning them as exciting contenders in photocatalysis.8,22–26 CPs gain enhanced properties when combined with active materials. CP-carbon nanostructure composites have recently emerged as promising solutions for sustainable environmental applications. To enhance the photocatalytic activity of CPs, we integrate it with g-C3N4, transforming the composite into an efficient photocatalyst and combining the adsorption properties of CPs with the photocatalytic capabilities of g-C3N4. Graphite phase nitrogenized carbon (g-C3N4) is a non-metal semiconductor distinguished by its remarkable chemical stability, cost-effectiveness, and availability. Due to its remarkable photocatalytic efficiency, it is gaining heightened attention in the field of photocatalysis.27–29 Recently, research on CP/g-C3N4 composites has expanded significantly, primarily concentrating on applications such as photocatalysis, fuel cells, and the oxygen reduction reaction, among others. For instance, a Ce-based CP/g-C3N4 composite demonstrated superior dye removal efficiency compared to Ce-based CP or g-C3N4 alone for the degradation of methylene blue when exposed to UV-visible light.30 Cu-BTC, a well-known CP, has been widely explored for adsorption applications due to its high porosity and surface functionality. However, its direct application in antibiotic degradation has been limited due to its poor photo-reactivity as Cu-BTC itself does not effectively absorb any light.31 The incorporation of g-C3N4 enhances the composite's photocatalytic performance by facilitating UV light absorption. Compared to other reported composites, such as TiO2-based materials, Cu-BTC/g-C3N4 demonstrates superior adsorption capacity and photoreactivity, making it a promising candidate for antibiotic degradation offering a more effective and stable solution for environmental remediations.7 Also, a Bi-based CP/g-C3N4 composite demonstrated exceptional photocatalytic efficiency for both pollutant degradation (rhodamine B) and nitrogen fixation, offering a promising approach to converting wastewater into nutrient-rich irrigation water, addressing both environmental and agricultural sustainability.32 In another study, a range of MIL-88A composites doped with Ag/AgCl and supported on g-C3N4 were developed for the photocatalytic degradation of the herbicide Diuron.33 Another composite, AgFeO2/G@Cu2(BTC)3, exhibited superior photocatalytic efficiency under sunlight, achieving high removal rates for antibiotic pollutants and 87.1% COD reduction in real wastewater. The enhanced performance is due to efficient charge transfer via a Z-scheme mechanism facilitated by graphene.34 Also, Fe-based CP doped sesame stalk biochar exhibited higher degradation rates of 92.5% and 86.7% for norfloxacin and tetracycline respectively.35,36In comparison to CP/g-C3N4, MIL-88A/Ag/AgCl, and AgFeO2/G@Cu2(BTC)3 composites, the Cu-BTC/g-C3N4 composite exhibits superior performance in antibiotic degradation because of its distinct structural and functional characteristics. Its adsorption capacity is improved by its much larger surface area, which surpasses 1000 m2 g−1 and outperforms MIL-88A/Ag/AgCl and CP/g-C3N4. Achieving over 97% tetracycline degradation, the Z-scheme mechanism in Cu-BTC/g-C3N4 facilitates effective electron–hole separation, outperforming the plasmonic effect-based MIL-88A/Ag/AgCl and AgFeO2/G@Cu2(BTC)3 composites. Furthermore, compared to Ag/AgCl composites that are prone to degradation, Cu-BTC/g-C3N4 shows better stability and reusability, maintaining its catalytic efficiency for more cycles with less metal leaching. Its innovative nature is further enhanced by its economical design and environmentally friendly synthesis, which position Cu-BTC/g-C3N4 as an excellent material for environmentally friendly wastewater treatment. Most of the composites fail to achieve optimal charge separation or consistent performance. To address this, we developed a CuBTC/g-C3N4 (CgC) composite in different ratios as CgC11 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CgC13 (1
1), CgC13 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and CgC31 (3
3), and CgC31 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The catalysts were thoroughly analyzed employing techniques such as XRD, FESEM, EDS, HRTEM, EIS, UV-DRS, PL, TGA, FTIR, XPS, and BET. A sequence of experiments were meticulously crafted to elucidate the synergistic interaction between adsorption and photocatalysis of tetracycline. Thermodynamic parameters such as ΔH°, ΔG°, and ΔS° were diligently analyzed alongside adsorption isotherms, adsorption kinetics, and photocatalytic kinetic models. Additionally, potential mechanisms for the adsorption and photocatalytic degradation of tetracycline were proposed.
1). The catalysts were thoroughly analyzed employing techniques such as XRD, FESEM, EDS, HRTEM, EIS, UV-DRS, PL, TGA, FTIR, XPS, and BET. A sequence of experiments were meticulously crafted to elucidate the synergistic interaction between adsorption and photocatalysis of tetracycline. Thermodynamic parameters such as ΔH°, ΔG°, and ΔS° were diligently analyzed alongside adsorption isotherms, adsorption kinetics, and photocatalytic kinetic models. Additionally, potential mechanisms for the adsorption and photocatalytic degradation of tetracycline were proposed.
2. Experimental approach
2.1. Materials and methods
The materials were synthesized using high-purity reagents, including urea, cupric nitrate trihydrate, and trimesic acid all sourced from LOBA CHEMIE and GLR INNOVATIONS. Commercial tetracycline powder was obtained from Sigma Aldrich. Ultrapure double-distilled water was utilized for all solution preparations.2.2. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 EtOH–H2O solution, filtered through Büchner filtration, and air-dried for 24 hours.10,38,39
50 EtOH–H2O solution, filtered through Büchner filtration, and air-dried for 24 hours.10,38,39
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ethanol–water mixture was prepared by sonication for 1 hour in an ice bath. Then, benzene 1,3,5-tricarboxylic acid (0.421 g, 2 mmol) and Cu (NO3)2·3H2O (0.716 g, 3 mmol) were dissolved in a 12 mL ethanol–water mixture. This solution was combined with the g-C3N4 suspension and sonicated vigorously for 15 minutes. The mixture was then added to a Teflon-lined autoclave and further heated at 383 K for 24 hours. After cooling to room temperature, the resulting hybrid nanocomposites were collected and sonicated in an ethanol–water solution for 5–10 minutes. These were then filtered and air-dried for 24 hours. Three distinct CgC composites—CgC11, CgC13, and CgC31—were synthesized by varying the Cu-BTC to g-C3N4 ratio at 1
50 ethanol–water mixture was prepared by sonication for 1 hour in an ice bath. Then, benzene 1,3,5-tricarboxylic acid (0.421 g, 2 mmol) and Cu (NO3)2·3H2O (0.716 g, 3 mmol) were dissolved in a 12 mL ethanol–water mixture. This solution was combined with the g-C3N4 suspension and sonicated vigorously for 15 minutes. The mixture was then added to a Teflon-lined autoclave and further heated at 383 K for 24 hours. After cooling to room temperature, the resulting hybrid nanocomposites were collected and sonicated in an ethanol–water solution for 5–10 minutes. These were then filtered and air-dried for 24 hours. Three distinct CgC composites—CgC11, CgC13, and CgC31—were synthesized by varying the Cu-BTC to g-C3N4 ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (Scheme 1).
1, respectively (Scheme 1).
2.3. Characterization
Comprehensive characterization data, including analyses from FTIR, XRD, FESEM, BET, and other methods employed to verify the structural, surface, and morphological properties of the Cu-BTC/g-C3N4 composite, are available in the ESI.†2.4. Adsorption and photocatalytic degradation experiments
The adsorption and photocatalytic experiment details are provided in the ESI.† All statistical analyses were performed using OriginPro 2018 64-bit (OriginLab, USA). Nonlinear regression was applied to fit the adsorption isotherm and kinetic models, as these equations do not follow a linear trend. Standard deviations were calculated from triplicate experiments, and 95% confidence intervals were determined to assess data reliability.3. Results and discussion
3.1. Characterization of the CuBTC and g-C3N4 composites
CuBTC and g-C3N4 composites in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios, termed CgC11, CgC13, and CgC31, were synthesized using a hydrothermal technique. The octahedral CuBTC was formed in situ between gC3N4 sheets.
1 ratios, termed CgC11, CgC13, and CgC31, were synthesized using a hydrothermal technique. The octahedral CuBTC was formed in situ between gC3N4 sheets.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N within heterocycles. The triazine breathing mode was noted at 809 cm−1.43 For CB, distinctive peaks were observed at approximately 3300 cm−1 (O–H/–OH), 1644 and 1547 cm−1 (asymmetric –CO2–), 1442 and 1365 cm−1 (BTC carboxylate), and 1111 cm−1 (C–O–Cu), with benzene ring C–H bending vibrations at 760 and 728 cm−1.44 The observed shifts in key functional group vibrations, such as the stretching modes of Cu–O and C–N bonds, provided evidence of strong interactions between CB and gC in the composites. Specifically, the shift in the Cu–O stretching band indicated coordination changes due to the integration of g-C3N4, while the variations in the C–N stretching region suggested electronic interactions between the two components. The nanocomposites exhibited absorption peaks associated with these functional groups, with minor shifts in wavenumbers, indicating the successful formation of the hybrid nanocomposites.45
N within heterocycles. The triazine breathing mode was noted at 809 cm−1.43 For CB, distinctive peaks were observed at approximately 3300 cm−1 (O–H/–OH), 1644 and 1547 cm−1 (asymmetric –CO2–), 1442 and 1365 cm−1 (BTC carboxylate), and 1111 cm−1 (C–O–Cu), with benzene ring C–H bending vibrations at 760 and 728 cm−1.44 The observed shifts in key functional group vibrations, such as the stretching modes of Cu–O and C–N bonds, provided evidence of strong interactions between CB and gC in the composites. Specifically, the shift in the Cu–O stretching band indicated coordination changes due to the integration of g-C3N4, while the variations in the C–N stretching region suggested electronic interactions between the two components. The nanocomposites exhibited absorption peaks associated with these functional groups, with minor shifts in wavenumbers, indicating the successful formation of the hybrid nanocomposites.45
| Catalyst | Surface area (m2 g−1) | Average pore size (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| CB | 1223.9 | 1.66 | 0.5098 |
| gC | 97.5 | 11.23 | 0.2735 |
| CgC11 | 112.0 | 5.66 | 0.1585 |
| CgC13 | 71.8 | 17.29 | 0.3104 |
| CgC31 | 913.9 | 2.28 | 0.5202 |
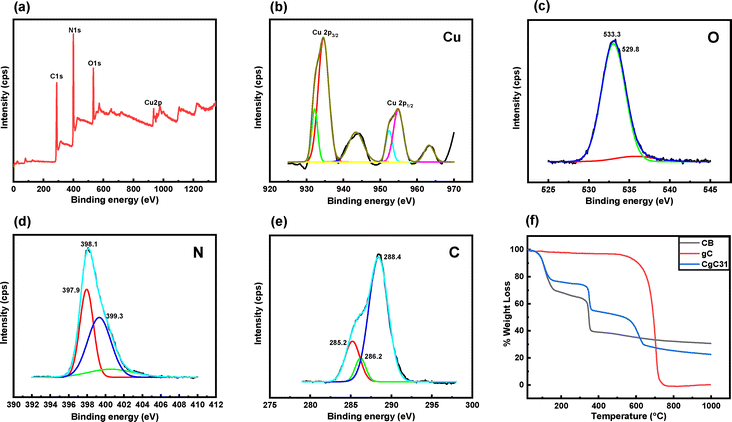 | ||
| Fig. 2 XPS spectra of CgC31, (a) survey spectra, (b) Cu 2p, (c) O 1s, (d) N 1s, and (e) C 1s, and (f) TGA curves of CB, gC, and CgC31. | ||
In Fig. 2(d), the nitrogen (N 1s) spectra displayed peaks corresponding to graphitic N, pyrrolic N, and pyridine N at 400.7 eV, 399.2 eV, and 398.7 eV, respectively. In the C 1s spectra in Fig. 2(e), a peak at 288.4 eV indicated the presence of BTC, while peaks at 286.2 eV and 285.2 eV corresponded to the C–H and C–C bonds. All results confirmed the successful synthesis of the CuBTC and g-C3N4 composite.47,48
 | (1) |
In this equation, the variables h, ν, α, and Eg represent Planck's constant, light frequency, absorption coefficient, and band gap energy, respectively. The band gap was determined where the linear portion of the plot intersects the x-axis.52 The calculated band gaps for CB, g-C3N4, CgC11, CgC13, and CgC31 photocatalysts were 1.85, 3.07, 3.05, 2.97, and 2.92 eV, respectively. Despite CB having the lowest band gap, it remained photo catalytically inactive, serving primarily as an adsorbent, while its composite with g-C3N4 especially CgC31 exhibited enhanced photocatalytic degradation under UV light due to the effective heterojunction formation and synergistic charge separation.
3.2. Impact of the catalyst dose
The effectiveness of photocatalysis heavily depends on using the optimal catalyst amount. Too much catalyst can lead to particle aggregation, reducing efficiency. Using the right dosage maximizes photon absorption and promotes efficient degradation. Tests with catalyst concentrations from 0.1 g L−1 to 0.6 g L−1 in Fig. 5(a) showed that degradation efficiency decreased beyond 0.1 g L−1 due to increased opacity, which scattered light and limited its impact on the solution. Additionally, the catalyst surface became saturated, offering no further improvement in degradation. Thus, 0.1 g L−1 was identified as the optimal concentration for future experiments.3.3. Impact of various lights
Comparative photocatalytic experiments under UV, visible, and natural sunlight were conducted to assess pollutant degradation. As shown in Fig. 5(b), decomposition reached 51.3% under sunlight and 45.8% when exposed to visible light, while the highest removal rate of 87.4% was achieved when exposed to UV light using the CgC31 heterojunction photocatalyst. These results highlight the superior effectiveness of UV light in driving pollutant degradation with the developed photocatalyst, making it more efficient.3.4. Impact of pH
pH plays a crucial role in controlling photocatalyst degradation efficiency, as it influences adsorption capacity, surface charge, and the ionization state of the catalyst.54 The photocatalyst's point of zero charge (PZC) was determined to be 4.57 in Fig. 5(d). Below pH 4.57, the surface is positively charged, and above it, negatively charged. Tetracycline (TC) exhibits different ionic forms depending on the pH due to its multiple pKa values (3.3, 7.7, 9.7, and 10.7). At pH < 3, TC is positively charged, while at pH > 7.7, it becomes anionic. TC is in a zwitterionic form in the pH range of 3.3–7.7.55 The degradation efficiency of TC by the CgC31 photocatalyst was lower in highly acidic or basic environments due to repulsive interactions between the ionic forms of TC and the charged catalyst surface. However, at pH 7, where TC is in its zwitterionic form, the electrostatic affinity between TC and the negatively charged photocatalyst surface enhanced degradation, achieving maximum removal efficiency (96.8%) under neutral to alkaline conditions in Fig. 5(c). This highlights the photocatalyst's optimal performance in real-world applications.3.5. Impact of temperature
The effect of temperature on tetracycline removal using CgC31 is shown in Fig. 5(f). Experiments conducted between 30 °C and 50 °C revealed a decrease in removal efficiency with increasing temperature, indicating an exothermic adsorption process. The highest removal was achieved at 30 °C, where TC molecules gained sufficient energy for adsorption. At higher temperatures, weakened electrostatic interactions caused a shift towards desorption. Therefore, 30 °C was chosen as the optimal temperature for the adsorption experiments. The temperature range (25–50 °C) was selected to evaluate the thermal effects on adsorption under conditions relevant to practical wastewater treatment. This range captures the influence of moderate thermal fluctuations on adsorption efficiency, as excessive temperatures may lead to material degradation or altered adsorption mechanisms.453.6. Adsorption isotherms
The fundamental equations and theoretical framework of adsorption isotherms are provided in the ESI.†The adsorption data were first analyzed using the Langmuir isotherm model, which assumes monolayer adsorption on a homogeneous CgC31 surface. Here, Qm represents the maximum adsorption capacity or the highest amount of tetracycline adsorbed per gram of CgC31 (mg g−1).56 The Langmuir constant, KL (L mg−1), reflects binding efficiency and was used to calculate the separation factor (RL). Fig. 6(a) inset shows that RL decreases as the initial concentration (C0) increases, with 0 < RL < 1 confirming favorable adsorption.
The Freundlich isotherm describes adsorption on heterogeneous surfaces in layers. An n value above 1 indicates effective sorption, and the Freundlich constant, approximately 3.546, suggests a suitable adsorption mechanism, while a 1/n value between 0 and 1 further supports favourable adsorption.57 The Freundlich plot is shown in Fig. 6(b). The Dubinin–Radushkevich (D–R) model, based on Polanyi's theory, indicates non-uniform adsorption, with an average adsorption energy (E) of 0.3429 kJ mol−1, suggesting physisorption in Fig. 6(c). The Temkin isotherm, seen in Fig. 6(e), proposes that the adsorption heat decreases as bonding increases between the adsorbate and the adsorbent. With β representing maximum bond energy, BT = RT/β was calculated at 10.22 kJ mol−1, indicating physical adsorption.58 The Harkins–Jura and Halsey in Fig. 5(e and f) isotherms illustrate heterogeneous pore distribution and multilayer adsorption. The Langmuir isotherm with the highest R2 value indicates monolayer adsorption on CgC31, while the D–R model confirms an uneven adsorbent surface.59 To further validate the model accuracy, statistical error values were calculated, where the Langmuir model exhibited the lowest χ2 (0.288) confirming its superior fit compared to the other models. These findings indicate monolayer adsorption as the dominant mechanism, consistent with previous studies (Table 2).60
3.7. Adsorption kinetics
The primary equations and theoretical framework for adsorption kinetics are detailed in the ESI.† The parameters and coefficients for the pseudo-first-order, pseudo-second-order, intraparticle diffusion, Elovich, and liquid film models are summarized in Table 3. A satisfactory correlation with the experimental data is observed across all models, with the pseudo-first-order kinetic model showing the highest fit (R2 = 0.96075) with 95% confidence intervals for the adsorption of tetracycline onto CgC31, suggesting that the rate of tetracycline adsorption onto CgC31 is primarily governed by the initial concentration of tetracycline in the solution, reflecting a surface-driven interaction. Additionally, strong correlations with intraparticle diffusion (R2 = 0.93594) and liquid film models (R2 = 0.96075) as compared to the other models as pseudo-second-order (R2 = 0.88119) and Elovich model (R2 = 0.89516) with similar confidence ranges suggest that both internal particle diffusion and boundary layer effects contribute significantly to the adsorption efficiency of tetracycline onto CgC31, highlighting a multi-step adsorption pathway (Fig. 7).613.8. Photocatalytic degradation kinetics
The photocatalytic degradation dynamics of the antibiotic were assessed by monitoring its concentration via UV-vis spectrophotometry, providing degradation rate and time data, with fundamental equations detailed in the ESI.† The kinetic analysis indicates that the degradation process aligns closely with pseudo-first-order kinetics, as evidenced by an R2 value near unity with a rate constant of k = 0.02098 min−1. This suggests that the reaction rate is primarily dependent on the concentration of the tetracycline pollutant, highlighting the efficiency of the composite in facilitating rapid degradation. The efficiency of various photocatalysts, including CB, gC, CgC11, CgC13, and TiO2-P25, was compared in a series of same experiments. The results revealed that CgC31 outperformed the others, exhibiting a rate constant of 0.02098, which was significantly higher than those of CB (1 × 10−16 min−1), gC (0.0038 min−1), TiO2-P25 (0.0062 min−1), CgC11 (0.003 min−1), and CgC13 (0.004 min−1). A synergy factor (R) was calculated using the below eqn (2) to assess the enhanced performance of the composite materials. | (2) |
This factor was derived using the photodegradation rate constants of the CB/gC composite and its components. The highest synergy factor of 5.53 was observed for CgC31 as compared to 0.789 for CgC11 and 1.053 for CgC13 which correlated with its superior photocatalytic degradation efficiency.62 The enhanced photocatalytic activity of the CgC31 composite can be attributed to the synergistic effects between Cu-BTC's high surface area and porosity and g-C3N4's electronic properties. Cu-BTC provides abundant active sites for adsorption, while gC facilitates charge transfer, reducing electron–hole recombination and improving photocatalytic efficiency. The intimate interfacial contact between the two materials enhances charge separation and strengthens adsorption interactions, leading to superior dye removal performance.63 This is further supported by literature findings on charge transfer dynamics in similar heterojunction systems.61
3.9. Thermodynamic studies
The thermodynamic analysis of tetracycline removal onto CgC31 indicates that the process is spontaneous at lower temperatures (303 K), as shown by the negative Gibbs free energy (ΔG° = −0.0927 kJ mol−1). However, at higher temperatures (308–323 K), ΔG° becomes positive suggesting reduced adsorption efficiency. A more negative ΔG with increasing temperature suggests enhanced feasibility of adsorption at higher temperatures. The negative enthalpy change (ΔH° = −64.8359 kJ mol−1) confirms that adsorption is exothermic, indicating strong interactions between tetracycline molecules and CgC31. Since adsorption releases heat, higher temperatures may reduce adsorption efficiency by shifting equilibrium toward desorption making the process more favorable at lower temperatures. The positive entropy change (ΔS° = 0.214286 J mol−1 K−1) suggests increased disorder at the solid–liquid interface, likely due to tetracycline molecules displacing water molecules on the adsorbent surface. However, as temperature increases, ΔS° slightly decreases, indicating a reduction in randomness. These findings highlight that tetracycline adsorption is thermodynamically favorable at moderate temperatures, but higher temperatures reduce its efficiency due to the increasing ΔG°.64 The theory, equations, and plot provided in Fig. S2 and Table S1 (ESI†) were used for the thermodynamic calculations.3.10. Possible adsorption and photocatalytic mechanism with the effect of scavengers
The adsorption mechanism of the CB/g-C3N4 composite for tetracycline hydrochloride can be explained through a combination of potential interactions including hydrogen bonding, pore filling, π–π interactions, van der Waals forces, and electrostatic interactions. The presence of hydroxyl and carboxyl groups on the composite surface suggests that hydrogen bonding with tetracycline's polar functional groups likely plays a significant role in adsorption.Additionally, the porous nature of CB/gC, observed through BET surface area analysis, points toward pore-filling as an effective means of trapping tetracycline molecules within the composite's structure. The π–π interactions between the aromatic rings of tetracycline and the composite could also contribute to the composite's affinity for the antibiotic, aligning with observed trends in adsorption studies of similarly structured pollutants.
Electrostatic forces may influence adsorption as well, given that the pH of the solution affects both the composite's charge and tetracycline's ionization state, which can favor attraction or repulsion based on the pH. These combined mechanisms suggest that the Cu-BTC/g-C3N4 composite leverages multiple types of interactions for effective adsorption of tetracycline, confirming its potential as a versatile adsorbent for environmental remediation.
The energy levels of the valence band (VB) and the conduction band (CBT) were determined using eqn (3) and (4) provided below.
| EVB = X − Ee + 0.5Eg | (3) |
| ECBT = EVB − Eg | (4) |
In these equations, ECBT and EVB denote the energies of the conduction band and the valence band, respectively. X signifies the compound's electronegativity, while Ee is the electron energy relative to hydrogen, which is set at 4.5 eV.
The ECBT values for CB and g-C3N4 were determined to be 0.52 eV and −0.99 eV, respectively, while their corresponding EVB values were determined to be 2.37 eV and 2.08 eV, respectively.65,66
The degradation process is primarily driven by electrons in the conduction band (CBT) and holes in the valence band (VB), along with reactive species such as superoxide and hydroxyl radicals, which are the key players.67 A scavenger study determined the predominant species involved in the degradation mechanism. Various scavengers were utilized, including dimethyl sulfoxide (DMSO), methanol, benzoquinone (BQ), and isopropyl alcohol (IPA), to selectively quench electrons (e−), holes (h+), superoxide radicals (O2˙−), and hydroxyl radicals (OH˙), respectively.52 In the absence of any trapping agents, the antibiotic underwent 87.2% degradation. However, the addition of scavengers to the reaction medium significantly suppressed the photocatalytic degradation rate. The comparable inhibition effects observed in Fig. 5(e) for all scavengers suggest that multiple reactive species, including holes, electrons, superoxide radicals, and hydroxyl radicals, were actively involved but superoxide radicals contributed the most to the degradation process.
So upon exposure to light, electrons within the valence bands of the semiconductors become excited and transition to their respective conduction bands. When the semiconductors are in close proximity, holes migrate from the more positively charged valence band of CB to the less positively charged valence band of gC, effectively inhibiting the recombination of photogenerated charge carriers. Concurrently, electrons transfer from the more negatively charged conduction band of gC to the less negatively charged conduction band of CB. Within CB's conduction band, these electrons participate in the reduction of oxygen to produce O2˙, which subsequently reacts with water to generate ˙OH radicals. These oxidative species effectively degrade TC into H2O, CO2, and other less toxic products. Additionally, the holes in the valence band of g-C3N4 react with water to produce OH˙ radicals, further oxidizing pollutants into simpler compounds. The effective separation of electron–hole pairs and the reduction of recombination greatly improve the photocatalytic performance of the CgC31 composite, as depicted in Scheme 3. The possible reaction steps are presented in the following equations.
| CB/gC + hv → e− + h+ | (5) |
| e−CBT + O2 → O2−˙ | (6) |
| O2−˙ + H2O → HOO˙ + OH˙ | (7) |
| h+VB + OH− → OH˙ | (8) |
| OH˙/h+ + pollutant → degraded products | (9) |
3.11. Mineralization studies
The intermediates formed during the photodegradation of organic dyes were identified by measuring total organic carbon (TOC) and chemical oxygen demand (COD) during irradiation. After 60 minutes of UV light exposure, TOC and COD reduction efficiencies for TC were observed to be 67.8% and 68.6%, respectively. As shown in Fig. 8(a), the solution was mineralized, with the remaining intermediates exhibiting low mineralization potential. The formation of intermediate organic compounds during the process, before complete conversion of TC into CO2 and simpler byproducts, led to a lower percentage of TOC and COD removal compared to the degradation rate. | ||
| Fig. 8 (a) TOC and COD values of tetracycline before and after degradation, (b) reusability studies and (c) XRD of CgC31 before and after degradation. | ||
3.12. Probable degradation pathway of tetracycline
The products and the intermediates generated during the photocatalytic degradation of tetracycline (TC) were analyzed using LC-MS. The corresponding mass spectra are presented in Fig. S3 (ESI†). The degradation process was found to proceed via three distinct pathways, as illustrated in Scheme 2. In pathway I, the first intermediate, product 1 (m/z = 462), undergoes further decomposition, yielding two secondary intermediates: product 2 (m/z = 434) and product 3 (m/z = 478). These intermediates lead to the formation of additional minor intermediates and ring-opened byproducts as the reaction progresses. Pathway II begins with the formation of product 8 (m/z = 434) through the loss of an N-methyl group, resulting from the cleavage of the N–C bond. This intermediate subsequently undergoes ring cleavage and dehydroxylation via oxidation by hydroxyl radicals, leading to the formation of product 9 (m/z = 304). In pathway III, product 11 (m/z = 475) is formed via a 1,3-dipolar cycloaddition at the double bond, followed by a rearrangement and oxidation by hydroxyl radicals. Product 11 is then converted to product 12 (m/z = 437) through N–C bond cleavage and hydroxyl substitution reactions. Further degradation transforms product 12 into product 13 (m/z = 210) via the loss of methyl and hydroxyl groups and the cleavage of the C–C bond, leading to the breakdown of rings. Subsequent oxidation of products 9 and 13 generates product 10, which is identified as the final intermediate before complete mineralization. The degradation process ultimately results in the production of simpler byproducts, primarily CO2 and H2O, completing the mineralization of TC (Scheme 3).3.13. Reusability studies
Evaluating the durability of a photocatalyst is essential for its practical application. To assess the repeatability and photostability of the CgC31 photocatalyst, recycling experiments were conducted under identical conditions to observe the photodegradation of TC over multiple cycles. Following each cycle, the catalyst was separated using centrifugation, cleaned, dried, and then reused in subsequent cycles. As illustrated in Fig. 8(b), after six cycles, the degradation efficiency remained around 80%, confirming the reusability of CgC31. The reduction in efficiency from 97.4% to 82.7% may be attributed to catalyst loss during recovery and the accumulation of untreated intermediates on the surface, which could block pores and active sites. Furthermore, the structural stability of the CgC31 catalyst was confirmed through XRD analysis after six cycles (Fig. 8(c)), indicating that the positions and intensities of the diffraction peaks remained consistent, with no additional peaks observed, confirming the preservation of the crystal structure. Hence, this promising adsorption and photocatalytic performance of our composite suggest its potential application in wastewater treatment plants for the removal of antibiotic contaminants. Its dual-functionality—adsorption and photocatalysis—allows for effective tetracycline degradation under UV light conditions, making it energy-efficient compared to conventional photocatalysts like TiO2. Additionally, its structural stability over multiple cycles indicates feasibility for long-term use in real-world treatment systems. Future studies could explore scaling up the composite for efficient removal of contaminants like heavy metals, antibiotics, dyes and other organic pollutants. The composite can be incorporated into advanced filtration systems in industrial wastewater plants.As shown in Table 4, our catalyst surpasses previously reported studies, demonstrating exceptional effectiveness even at lower dosages. The CB and gC combination stands out, achieving remarkable results in this work.
4. Conclusion
This study introduces an innovative CB/gC heterojunction photocatalyst synthesized via a sustainable hydrothermal approach, designed for effective antibiotic removal, particularly tetracycline, from wastewater. Characterization techniques including XRD, XPS, FE-SEM-EDS, TGA, BET, EIS, FTIR, HRTEM, UV-DRS, and PL validated the successful synthesis, structure, and stability of the composite. SEM imaging highlighted the seamless integration of gC onto CB, forming a heterojunction with enhanced light absorption and significant surface area. The CB/gC composite exhibited exceptional photocatalytic performance, achieving 97.4% degradation of 25 ppm tetracycline within 60 minutes under UV light. The degradation follows a pseudo-first-order reaction with a rate constant of 0.02098, which is 2.1 × 1014 times higher than that of pure CB and 5.5 times higher than that of pure gC3N4. This demonstrates strong synergy in the composite. Notably, the rate constant is 3.39 times greater than that of the commercial TiO2-P25 photocatalyst. Key parameters such as the catalyst dose, pollutant concentration, pH, light sources, and scavenger analysis were systematically optimized, revealing the dominant roles of ˙OH radicals, electrons, holes, and superoxide species in driving the degradation pathway. Notably, the catalyst demonstrated high reusability, maintaining over 82.7% degradation efficiency across six cycles. Post-use XRD analysis confirmed its structural resilience, while TOC and COD measurements indicated substantial reductions of 67.8% and 68.6%, respectively, reflecting effective pollutant mineralization. Degradation intermediates and products were mapped using LC-MS, offering insights into the degradation mechanism. This study establishes CB/gC as a powerful, sustainable solution for antibiotic remediation, with potential for broader applications in solar-driven environmental purification, positioning it as a robust tool for addressing water pollution challenges. However, one potential challenge in practical applications is that the presence of other contaminants in industrial wastewater may lead to pore blockage or surface fouling, potentially affecting the photocatalytic efficiency. However, this can be mitigated by optimizing pretreatment steps or periodic regeneration of the catalyst to maintain long-term performance. Also, the absence of ESR analysis limits the direct identification of reactive species.Author contributions
Palkaran Sethi: designed and performed the experiments, analyzed data, and co-wrote the paper. Soumen Basu: supervised the research, designed experiments and co-wrote the paper. Sanghamitra Barman: supervised the research, designed experiments, and co-wrote the paper.Data availability
The data supporting the findings of this study, including characterization results, adsorption and degradation kinetics, and isotherm studies, are available in the article as well as in the ESI† provided with this article.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to acknowledge Thapar Institute of Engineering and Technology for XRD, FESEM, and FTIR. The authors are also grateful to Dr Akansha Mehta, Fun Glass, Alexander Dubcek University, Slovakia for XPS analysis and Panjab University, Chandigarh, for HRTEM analysis.References
- N. Zhang, X. Ning, J. Chen, J. Xue, G. Lu and H. Qiu, Photocatalytic degradation of tetracycline based on the highly reactive interface between graphene nanopore and TiO2 nanoparticles, Microporous Mesoporous Mater., 2022, 338, 111958 CrossRef CAS
.
- Z. Yang, Q. Zhao, J. Gan, J. Zhang, M. Chen and Y. Zhu, Damage evolution characteristics of siliceous slate with varying initial water content during freeze-thaw cycles, Sci. Total Environ., 2024, 950, 175200 CrossRef CAS PubMed
.
- S. Chu, M. Lin, D. Li, R. Lin and S. Xiao, Adaptive reward shaping based reinforcement learning for docking control of autonomous underwater vehicles, Ocean Eng., 2025, 318, 120139 CrossRef
.
- Y. Yao, Y. Luo, Y. Yang, H. Sheng, X. Li and T. Li,
et al., Water free anaerobic co-digestion of vegetable processing waste with cattle slurry for methane production at high total solid content, Energy, 2014, 74, 309–313 CrossRef CAS
.
- S. Pal, Z. Ahamed and P. Pal, Removal of antibiotics and pharmaceutically active compounds from water Environment: Experiments towards industrial scale up, Sep. Purif. Technol., 2022, 295, 121249 CrossRef CAS
.
- S. Pal, Z. Ahamed and P. Pal, Removal of antibiotics and pharmaceutically active compounds from water Environment: Experiments towards industrial scale up, Sep. Purif. Technol., 2022, 295, 121249 CrossRef CAS
.
- A. H. Ragab, N. F. Gumaah, A. A. El Aziz Elfiky and M. F. Mubarak, Exploring the sustainable elimination of dye using cellulose nanofibrils- vinyl resin based nanofiltration membranes, BMC Chem., 2024, 18(1), 121 CrossRef CAS PubMed
.
- K. Li, S. Zou, G. Jin, J. Yang, M. Dou and L. Qin,
et al., Efficient removal of selenite in aqueous solution by MOF-801 and Fe3O4/MOF-801: Adsorptive behavior and mechanism study, Sep. Purif. Technol., 2022, 296, 121384 CrossRef CAS
.
- W. Xiong, G. Zeng, Z. Yang, Y. Zhou, C. Zhang and M. Cheng,
et al., Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent, Sci. Total Environ., 2018, 627, 235–244 CrossRef CAS PubMed
.
- P. Sethi, S. Barman and S. Basu, Strategic tuning of GO ratios in CuBTC-GO nanocomposites for next-generation tetracycline adsorption: A deep dive into isotherms, kinetics, and thermodynamics, Sep. Purif. Technol., 2025, 361, 131311 CrossRef CAS
.
- S. Shao and X. Wu, Microbial degradation of tetracycline in the aquatic environment: a review, Crit. Rev. Biotechnol., 2020, 40(7), 1010–1018 CrossRef CAS PubMed
.
- X. He, T. Kai and P. Ding, Heterojunction photocatalysts for degradation of the tetracycline antibiotic: a review, Environ. Chem. Lett., 2021, 19(6), 4563–4601 CrossRef CAS PubMed
.
- H. Jiang, Q. Wang, P. Chen, H. Zheng, J. Shi and H. Shu,
et al., Photocatalytic degradation of tetracycline by using a regenerable (Bi)BiOBr/rGO composite, J Cleaner Prod., 2022, 339, 130771 CrossRef CAS
.
- M. A. Abbasi, K. M. Amin, M. Ali, Z. Ali, M. Atif and W. Ensinger,
et al., Synergetic effect of adsorption-photocatalysis by GO−CeO2 nanocomposites for photodegradation of doxorubicin, J. Environ. Chem. Eng., 2022, 10(1), 107078 CrossRef CAS
.
- Z. Wu, Z. Chen, J. Chen, X. Ning, P. Chen and H. Jiang,
et al., Enhanced adsorption and synergistic photocatalytic degradation of tetracycline by MOF-801/GO composites via solvothermal synthesis, Environ. Sci.: Nano, 2022, 9(12), 4609–4618 RSC
.
- M. Pourshaban-Mazandarani, M. Ahmadian, A. Nasiri and A. Poormohammadi, CuCoFe2O4@AC magnetic nanocomposite as a novel heterogeneous Fenton-like nanocatalyst for Ciprofloxacin degradation from aqueous solutions, Appl. Water. Sci., 2023, 13(9), 179 CrossRef CAS
.
- A. Nasiri, M. R. Heidari, N. Javid and G. Yazdanpanah, New efficient and recyclable magnetic nanohybrid adsorbent for the metronidazole removal from simulated wastewater, J. Mater. Sci.: Mater. Electron., 2022, 33(33), 25103–25126 CrossRef CAS
.
- A. Nasiri, M. Malakootian and N. Javid, Desalination and Water Treatment Modelling and optimization of lead adsorption by CoFe2O4@CMC@HZSM-5 from aqueous solution using response surface methodology, Desalination Water Treat., 2022, 248, 134–148 CrossRef CAS
.
- G. Yazdanpanah, M. R. Heidari, N. Amirmahani and A. Nasiri, Heterogeneous Sono-Fenton like catalytic degradation of metronidazole by Fe3O4@HZSM-5 magnetite nanocomposite, Heliyon, 2023, 9(6), e16461 CrossRef CAS PubMed
.
- K. Althumayri, A. Guesmi, W. Abd El-Fattah, L. Khezami, T. Soltani, N. B. Hamadi and A. Shahat, Effective Adsorption and Removal of Doxorubicin from Aqueous Solutions Using Mesostructured Silica Nanospheres: Box–Behnken Design Optimization and Adsorption Performance Evaluation, ACS Omega, 2023, 8(15), 14144–14159 CrossRef CAS PubMed
.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341(6149), 1230444 CrossRef PubMed
.
- M. X. Wu and Y. W. Yang, Metal–Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy, Adv. Mater., 2017, 29(23), 1606134 CrossRef PubMed
.
- T. Xia, Y. Lin, W. Li and M. Ju, Photocatalytic degradation of organic pollutants by MOFs based materials: A review, Chin. Chem. Lett., 2021, 32(10), 2975–2984 CrossRef CAS
.
- T. Liu, Z. Li, X. Zhang, H. Tan, Z. Chen and J. Wu,
et al., Metal–Organic Framework-Intercalated Graphene Oxide Membranes for Selective Separation of Uranium, Anal. Chem., 2021, 93(48), 16175–16183 CrossRef CAS PubMed
.
- J. Yu, C. Mu, B. Yan, X. Qin, C. Shen and H. Xue,
et al., Nanoparticle/MOF composites: preparations and applications, Mater. Horiz., 2017, 4(4), 557–569 RSC
.
- R. M. Rego, G. Sriram, K. V. Ajeya, H. Y. Jung, M. D. Kurkuri and M. Kigga, Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification, J. Hazard. Mater., 2021, 416, 125941 CrossRef CAS PubMed
.
- Y. Deng, L. Tang, G. Zeng, Z. Zhu, M. Yan and Y. Zhou,
et al., Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism, Appl. Catal., B, 2017, 203, 343–354 CrossRef CAS
.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao and C. Zhou,
et al., Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution, Adv. Mater., 2017, 29(16), 1605148 CrossRef PubMed
.
- L. Zhang, X. Zhou, S. Liu, H. Liu, S. Zhu and Y. Mao,
et al., Two birds, one stone: Rational design of Bi-MOF/g-C3N4 photocatalyst for effective nitrogen fixation and pollutants degradation, J. Cleaner Prod., 2023, 425, 138912 CrossRef CAS
.
- Y. Cao, L. Wang, C. Wang, X. Hu, Y. Liu and G. Wang, Sensitive detection of glyphosate based on a Cu-BTC MOF/g-C3N4 nanosheet photoelectrochemical sensor, Electrochim. Acta, 2019, 317, 341–347 CrossRef CAS
.
- A. H. Ragab, N. F. Gumaah, A. A. El Aziz Elfiky and M. F. Mubarak, Exploring the sustainable elimination of dye using cellulose nanofibrils- vinyl resin based nanofiltration membranes, BMC Chem., 2024, 18(1), 121 CrossRef CAS PubMed
.
- Z. Durmus, R. Köferstein, T. Lindenberg, F. Lehmann, D. Hinderberger and A. W. Maijenburg, Preparation and characterization of Ce-MOF/g-C3N4 composites and evaluation of their photocatalytic performance, Ceram. Int., 2023, 49(14, Part B), 24428–24441 CrossRef CAS
.
- D. Akgün and M. Dükkancı, g-C3N4 supported Ag/AgCl@MIL-88A MOF based triple composites for highly efficient diuron photodegradation under visible LED light irradiation, J. Water Process Eng., 2023, 51, 103469 CrossRef
.
- E. M. El-Fawal, S. A. Younis and T. Zaki, Designing AgFeO2-graphene/Cu2(BTC)3 MOF heterojunction photocatalysts for enhanced treatment of pharmaceutical wastewater under sunlight, J. Photochem. Photobiol., A, 2020, 401, 112746 CrossRef CAS
.
- Y. Li, W. Qin, S. Chen, T. Gu, Y. Chen and F. Pei,
et al., Iron-based metal organic framework (Fe-MOF)-doped sesame stalk biochar enhances antibiotic degradation: Important role of free radicals and comparison of multiple degradation processes, Sep. Purif. Technol., 2025, 353, 128464 CrossRef CAS
.
- A. A. E. A. Elfiky, M. F. Mubarak, M. Keshawy, I. E. T. El Sayed and T. A. Moghny, Novel nanofiltration membrane modified by metal oxide nanocomposite for dyes removal from wastewater, Environ. Dev. Sustainability, 2024, 26(8), 19935–19957 CrossRef
.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3](n), Science, 1999, 283(5405), 1148–1150 CrossRef CAS PubMed
.
- S. Bordiga, L. Regli, F. Bonino, E. Groppo, C. Lamberti and B. Xiao,
et al., Adsorption properties of HKUST-1 toward hydrogen and other small molecules monitored by IR, Phys. Chem. Chem. Phys., 2007, 9(21), 2676–2685 RSC
.
- A. K. El-Sawaf, S. R. El-Dakkony, M. A. Zayed, A. M. Eldesoky, A. A. Nassar and A. El Shahawy,
et al., Green synthesis and characterization of magnetic gamma alumina nanoparticlesfor copper ions adsorption from synthetic wastewater, Results Eng., 2024, 22, 101971 CrossRef CAS
.
- D. Monga, D. Ilager, N. P. Shetti, S. Basu and T. M. Aminabhavi, 2D/2d heterojunction of MoS2/g-C3N4 nanoflowers for enhanced visible-light-driven photocatalytic and electrochemical degradation of organic pollutants, J. Environ. Manage., 2020, 274, 111208 CrossRef CAS PubMed
.
- H. J. Shin, K. K. Kim, A. Benayad, S. M. Yoon, H. K. Park and I. S. Jung,
et al., Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance, Adv. Funct. Mater., 2009, 19(12), 1987–1992 CrossRef CAS
.
- G. Zhang, C. Huang and X. Wang, Dispersing Molecular Cobalt in Graphitic Carbon Nitride Frameworks for Photocatalytic Water Oxidation, Small, 2015, 11(9–10), 1215–1221 CrossRef CAS PubMed
.
- S. Kumar, T. Surendar, A. Baruah and V. Shanker, Synthesis of a novel and stable g-C3N4–Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation, J. Mater. Chem. A, 2013, 1(17), 5333–5340 RSC
.
- V. Jabbari, J. M. Veleta, M. Zarei-Chaleshtori, J. Gardea-Torresdey and D. Villagrán, Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants, Chem. Eng. J., 2016, 304, 774–783 CrossRef CAS
.
- A. H. Ragab, B. S. Mettwally, M. F. Mubarak, A. Al-Ghamdi and M. Hemdan, Eco-friendly Electrospinning of Recycled Nylon 6,12 Waste for High-Performance Nonwoven Nanofibers in Sustainable Textile Applications, J. Inorg. Organomet. Polym. Mater., 2024, 34(4), 1491–1505 CrossRef CAS
.
- Y. Chen, B. Zhai, Y. Liang, Y. Li and J. Li, Preparation of CdS/g-C3N4/MOF composite with enhanced visible-light photocatalytic activity for dye degradation, J. Solid State Chem., 2019, 274, 32–39 CrossRef CAS
.
- Z. Huang, Q. Sun, K. Lv, Z. Zhang, M. Li and B. Li, Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs. (101) facets of TiO2, Appl. Catal., B, 2015, 164, 420–427 CrossRef CAS
.
- H. Niu, S. Liu, Y. Cai, F. Wu and X. Zhao, MOF derived porous carbon supported Cu/Cu2O composite as high performance non-noble catalyst, Microporous Mesoporous Mater., 2016, 219, 48–53 CrossRef CAS
.
- C. Zhao, Q. Yan, S. Wang, P. Dong and L. Zhang, Regenerable g-C3N4–chitosan beads with enhanced photocatalytic activity and stability, RSC Adv., 2018, 8(48), 27516–27524 RSC
.
- S. Singla, S. Basu and P. Devi, Solar light responsive 2D/2D BiVO4/SnS2 nanocomposite for photocatalytic elimination of recalcitrant antibiotics and photoelectrocatalytic water splitting with high performance, J. Ind. Eng. Chem., 2023, 118, 119–131 CrossRef CAS
.
- L. Zhang, Y. Meng, H. Shen, J. Li, C. Yang and B. Xie,
et al., Photocatalytic degradation of rhodamine B by Bi2O3@LDHs S-scheme heterojunction: Performance, kinetics and mechanism, Appl. Surf. Sci., 2021, 567, 150760 CrossRef CAS
.
- S. Singla, S. Sharma and S. Basu, MoS2/WO3 heterojunction with the intensified photocatalytic performance for decomposition of organic pollutants under the broad array of solar light, J. Cleaner Prod., 2021, 324, 129290 CrossRef CAS
.
- B. Garg, P. Hait and S. Basu, Unlocking solar energy's potential: Dual photocatalytic activities of g-C3N4/Sb2S3 for hydrogen evolution and tetracycline degradation in sunlight, J. Environ. Manage., 2024, 370, 122403 CrossRef CAS PubMed
.
- P. Wang and Q. Yuan, Photocatalytic degradation of tetracyclines in liquid digestate: Optimization, kinetics and correlation studies, Chem. Eng. J., 2021, 410, 128327 CrossRef CAS
.
- G. Alnaggar, A. Hezam, Q. A. Drmosh and S. Ananda, Sunlight-driven activation of peroxymonosulfate by microwave synthesized ternary MoO3/Bi2O3/g-C3N4 heterostructures for boosting tetracycline hydrochloride degradation, Chemosphere, 2021, 272, 129807 CrossRef CAS PubMed
.
- N. Mukwevho, R. Gusain, E. Fosso-Kankeu, N. Kumar, F. Waanders and S. S. Ray, Removal of naphthalene from simulated wastewater through adsorption-photodegradation by ZnO/Ag/GO nanocomposite, J. Ind. Eng. Chem., 2020, 81, 393–404 CrossRef CAS
.
- N. Ghasemi, M. Ghasemi, S. Moazeni, P. Ghasemi, N. S. Alharbi and V. K. Gupta,
et al., Zn(II) removal by amino-functionalized magnetic nanoparticles: Kinetics, isotherm, and thermodynamic aspects of adsorption, J. Ind. Eng. Chem., 2018, 62, 302–310 CrossRef CAS
.
- N. Wang, J. Chen, J. Wang, J. Feng and W. Yan, Removal of methylene blue by Polyaniline/TiO2 hydrate: Adsorption kinetic, isotherm and mechanism studies, Powder Technol., 2019, 347, 93–102 CrossRef CAS
.
- A. A. Inyinbor, F. A. Adekola and G. A. Olatunji, Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp, Water Resour. Ind., 2016, 15, 14–27 CrossRef
.
- M. F. Hussein, M. F. Mubarak, A. M. Al-Sirhani and R. Hosny, Examining the factors that impact the formation of barite scale in water injection operations: experimental study and quantification of scale formation, Discover Appl. Sci., 2024, 6(10), 519 CrossRef CAS
.
- A. H. Ragab, N. F. Gumaah, A. A. El Aziz Elfiky and M. F. Mubarak, Exploring the sustainable elimination of dye using cellulose nanofibrils- vinyl resin based nanofiltration membranes, BMC Chem., 2024, 18(1), 121 CrossRef CAS PubMed
.
- M. F. Mubarak, G. E. Khedr and H. M. El Sharkawy, Environmentally-friendly calcite scale mitigation: encapsulation of CDs@MS composite within membranes framework for nanofiltration, J. Alloys Compd., 2024, 999, 175061 CrossRef CAS
.
- A. K. El-Sawaf, A. A. Nassar, A. A. El Aziz Elfiky and M. F. Mubarak, Advanced microcrystalline nanocellulose-based nanofiltration membranes for the efficient treatment of wastewater contaminated with cationic dyes, Polym. Bull., 2024, 81(14), 12451–12476 CrossRef CAS
.
- N. Goyal, S. Barman and V. K. Bulasara, Efficient removal of bisphenol S from aqueous solution by synthesized nano-zeolite secony mobil-5, Microporous Mesoporous Mater., 2018, 259, 184–194 CrossRef CAS
.
- D. N. Mengesha, B. T. Shiferraw and H. Kim, Modification of the electronic structure of g-C3N4 using urea to enhance the visible light–assisted degradation of organic pollutants, Environ. Sci. Pollut. Res., 2023, 30(46), 102910–102926 CrossRef CAS PubMed
.
- J. Zhang, C. Su, X. Xie and P. Liu, Huq MdE. Enhanced visible light photocatalytic degradation of dyes in aqueous solution activated by HKUST-1: performance and mechanism, RSC Adv., 2020, 10(61), 37028–37034 RSC
.
- A. Kundu, S. Sharma and S. Basu, Modulated BiOCl nanoplates with porous g-C3N4 nanosheets for photocatalytic degradation of color/colorless pollutants in natural sunlight, J. Phys. Chem. Solids, 2021, 154, 110064 CrossRef CAS
.
- C. Maggu, S. Singla and S. Basu, Unleashing the power of sunlight: Bi2O3/Sb2S3 photocatalysis for sustainable wastewater remediation of Tetracycline and Rhodamine-B, J. Environ. Manage., 2024, 349, 119424 CrossRef CAS PubMed
.
- Y. Xiao, H. Wang, Y. Jiang, W. Zhang, J. Zhang and X. Wu,
et al., Hierarchical Sb2S3/ZnIn2S4 core–shell heterostructure for highly efficient photocatalytic hydrogen production and pollutant degradation, J. Colloid Interface Sci., 2022, 623, 109–123 CrossRef CAS PubMed
.
- S. Singla, P. Devi and S. Basu, Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite, Catalysts, 2023, 13(4), 731 CrossRef CAS
.
- H. Wang, M. Zhang, X. He, T. Du, Y. Wang and Y. Li,
et al., Facile prepared ball-like TiO2 at GO composites for oxytetracycline removal under solar and visible lights, Water Res., 2019, 160, 197–205 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nj00556f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |