A carbon quantum dot@mesoporous silicon composite-based fluorescent probe for chlortetracycline hydrochloride and gentamicin†
Lina
Gao
and
Jing
Wang
 *
*
School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, P. R. China. E-mail: wjwyj82@gxu.edu.cn
First published on 25th November 2024
Abstract
Antibiotics, such as chlortetracycline hydrochloride (CTC) and gentamicin (GTM), pose a significant threat to human health and ecosystems. Therefore, stable analytical tools to identify CTC and GTM are of great practical importance. For accuracy and sensitivity, mesoporous SBA-15 silica was chosen as the solid support material to enhance the photostability of the detection system. Carbon quantum dots (CDs) as fluorescence signal emitters were introduced into SBA-15, and a fluorescent nanoprobe, CDs@SBA-15, exhibiting remarkable selectivity and sensitivity was constructed. The composite-based nanoprobe showed remarkable selectivity toward GTM, enabling the sensitive detection of GTM. The fluorescence spectrum of CDs@SBA-15 was altered in a “turn-on” manner when GTM was present, and the detection limit was 29 nM. Compared with the non-fluorescence response of CDs toward GTM, the superior fluorescence response of CDs@SBA-15 toward GTM was due to the immobilization of the CDs and the spatially restricted domain effect of the pore channels of SBA-15. In addition, the nanoprobe showed excellent selectivity for CTC in a “turn-off” manner and could sensitively detect CTC in the ranges of 0–4.0 and 4.0–100.0 μM with a detection limit of 113 nM. Moreover, the feasibility of the fluorescent nanoprobe was verified using tap water samples.
1. Introduction
Since the discovery of penicillin by Alexander Fleming,1 a large number of antibiotics have been developed. Chlortetracycline hydrochloride (CTC) and gentamicin (GTM) are two drugs with therapeutic applications (Table S1, ESI†). Although they are beneficial for treating human and animal diseases due to their low price and high antimicrobial efficacy,2,3 when they are excreted into the environment through feces they can cause various health problems, such as nephrotoxicity, impaired immune function, and allergic reactions.4–6 Therefore, developing highly selective and sensitive analytical tools for detecting CTC and GTM in complicated matrices is significant.Researchers have invested much effort in developing analytical methods for CTC and GTM detection, such as chromatography,7,8 immunoassay,9,10 and electrochemical methods.11,12 Although these traditional methods can quantitatively detect CTC and GTM, most of them are expensive, cumbersome, and lack specificity. Nanomaterial-based fluorescence detection has unique advantages over the above analytical methods, including high sensitivity, a wide detection range, low cost, and simplicity of operation.13 Quantitative analysis of GTM typically relies on liquid chromatography or immunoassays; however, fluorescence-based detection of GTM in aminoglycoside antibiotics (AGs) remains rare (Table S1, ESI†). The fluorescent nanomaterials used for CTC detection include metal–organic frameworks, metal nanoclusters, and organic fluorescent molecules.13 Some of the above nanomaterials are made of precious metals with high cost, and others have limited applications due to their toxicity. Compared with these materials, carbon quantum dots (CDs) have specific advantages,14 such as a wide range of raw materials at low cost (e.g. piper betle leaf and kiwi fruit peel),15,16 good biocompatible, and low toxicity, allowing them to be widely used in the fields of optical sensing and bioimaging.17–19 Moreover, synthesizing CDs by using the hydrothermal method is widely adopted because of its simple operation, environmental friendliness and easy adjustment of the surface state.20 The use of CDs as a fluorescent material for CTC detection has been reported. However, due to the structural similarity among tetracycline antibiotics (TCs), CDs cannot achieve high selectivity for CTC in complex matrices. In addition, a high CDs concentration is prone to aggregation-caused quenching (ACQ), which limits their practical applications.21 The surface of CDs has many functional groups, so electron donors or electron acceptors in the surrounding medium can react with CDs for fluorescence quenching.22,23
The following two main strategies are employed to overcome the shortcomings of CDs: (i) self-quenching-resistant CDs prepared by surface passivation, self-assembly, and modulation of the carbon core structure, and (ii) matrix-assisted construction of CDs (using polymers, silicon substrate, and porous materials). Although much progress has been made in preparing self-quenching-resistant CDs, their optical properties are still unsatisfactory. By contrast, strategy (ii) remains one of the most successful methods against ACQ.21 Zhang et al.24 reported polymer wrapped CDs able to overcome ACQ; however, CDs aggregation may still occur if a mismatch occurs between the hydrophobic/hydrophilic properties of the CDs and the polymer.25 Silicon compounds can act as a bridge between inorganic and organic materials and serve as a hub for combining CDs with other promising organic/inorganic materials.26 Li et al.27 covalently linked CDs with silane coupling agents to enable uniform dispersion, resulting in intense fluorescence. Mesoporous silicon with the nanoconfinement effect may be a more promising material than common silane coupling agents.28,29 Among them, SBA-15 is one of the most ideal carriers due to its rich pore structure, large pore size, good chemical stability, and easy loading of other materials.30 Mu et al.31 prepared a series of CexWy/TS catalysts by encapsulating the active ingredient into SBA-15. The introduction of SBA-15 enhanced the interaction between analytes and active sites, and effectively avoided the agglomeration of active nanoparticles. However, only a few studies focused on the fluorescence sensing properties of composites of CDs and mesoporous silicon for antibiotic detection. Currently, synthesis strategies for CDs and SBA-15 composites often require a long time, complex procedures and demanding conditions.32,33 In comparison, incipient wetness impregnation and ultrasound-assisted methods are advantageous, e.g., accelerated reaction, short reaction time, simplicity, and low cost.34,35 Applying the above two methods would be a promising strategy to prepare composites of CDs and mesoporous silicon.
Based on the above findings, we envisage that composites of CDs and mesoporous silicon would improve the optical properties and detection performance of CDs for antibiotics through the nanoconfinement effect, thus killing two birds with one stone. Herein, CDs as fluorescent signals were introduced into SBA-15, and a fluorescent nanoprobe CDs@SBA-15 was constructed (Scheme 1a). The fluorescence detection performance of the nanoprobe for CTC and GTM was investigated, and the detection mechanism is discussed (Scheme 1b). Furthermore, the practical application of CDs@SBA-15 is evaluated for the fluorescence detection of CTC and GTM in tap water samples.
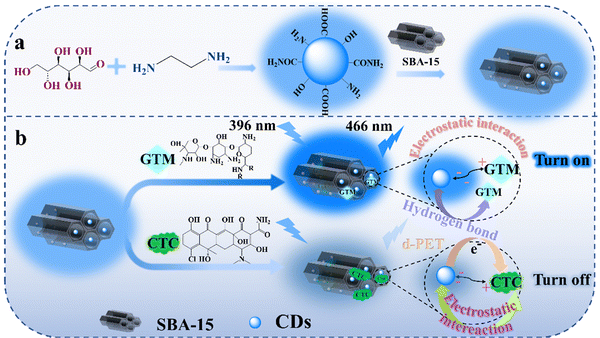 | ||
| Scheme 1 (a) Schematic diagram of the CDs@SBA-15 nanoprobe preparation and (b) its detection of GTM and CTC. | ||
2. Experimental section
2.1 Materials and instruments
SBA-15 was purchased from Nanjing XFNANO Materials Tech Co. Ltd (China). Glucose (Glu), ethylenediamine (EDA), chlortetracycline (CTC), tetracycline (TC), oxytetracycline (OTC), gentamycin (GTM), penicillin (PEG), erythromycin (ERM), clarithromycin (CAL), roxithromycin (ROX), sulfathiazole (STZ), sulfachloropyridazine (SCP), sulfamethoxypyridazine (SMP), sulfamethizole (SMT), sulfamethazine (SMR), sulfamethoxazole (SMZ), sulfathiazole (SIZ), NaCl, KCl, and alanine (Ala) were purchased from reputable suppliers. The instruments used in this work were a transmission electron microscope (TEM, Japan's JEOL JEM-F200), Fourier infrared spectroscope (FT-IR, Nicolet iS50), small-angle X-ray diffractometer (SXRD, Rigaku Miniflex 600, Japan), powder X-ray diffractometer (PXRD, Smartlab SE, Japan), nitrogen absorption–desorption isotherms (BET, Micro metrics ASAP 2460 in the USA), X-ray photoelectron spectrometer (XPS, Thermo Scientific K-Alpha, USA), Mettler Toledo S20K pH meter, 960 MC fluorescence spectrometer and UV 1901 UV-Vis spectrometer.2.2 Preparation of CDs and CDs@SBA-15
CDs were synthesized using hydrothermal methods according to the reported literature with slight adjustments.36 Briefly, Glu (0.1 g) and EDA (0.15 mL) in 50 mL of deionized water were transferred into a Teflon stainless steel autoclave and reacted at 200 °C for 4 hours. After cooling, the obtained solution was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 12 minutes and CDs was obtained by filtering through a 0.22 μm membrane filter.
000 rpm for 12 minutes and CDs was obtained by filtering through a 0.22 μm membrane filter.
CDs@SBA-15 was synthesized using incipient wetness impregnation and ultrasound-assisted methods according to reported literature with slight adjustments.37 A CDs solution (45 mL) was added to 0.3 g of SBA-15 dispersions in 30 mL of deionized water, the mixture was stirred for 30 minutes and then ultrasonicated for 30 minutes. After centrifuging, washing, and drying at 60 °C for 12 hours, CDs@SBA-15 was obtained as a brown solid powder (Scheme 1a).
2.3 Recognition experiments
0–100 μM GTM or CTC was added to 0.05 g L−1 of CDs@SBA-15 in HEPES buffer solution (20 mM). Then, the fluorescence spectra of CDs@SBA-15 in the presence and absence of GTM or CTC were obtained. The fluorescence intensity at 466 nm was recorded for the quantitative analysis of GTM and CTC. The fluorescence enhancement constants (or quenching constants) of CDs@SBA-15 against GTM (or CTC) were calculated according to the equation I/I0 − 1 = Kec [Q] (or I0/I − 1 = Ksv [Q]), where I0 is the fluorescence intensity of CDs@SBA-15 at 466 nm, I is the fluorescence intensity of CDs@SBA-15 at 466 nm after the addition of antibiotics, and [Q] is the concentration of antibiotics. The limits of detection (LOD) for GTM and CTC were calculated based on LOD = 3σ/s, where σ is the standard deviation of 10 blank solutions, and s is the slope of the standard curve.2.4 Selective, anti-interference and reproducible experiments
In the selectivity experiments, the fluorescence spectra of CDs@SBA-15 (0.05 g L−1) were measured in the presence of each of the analytes (100 μM), including metal cations, anions, amino acids and a variety of antibiotics. In the anti-interference experiments, the fluorescence spectra of CDs@SBA-15 in the presence of GTM or CTC (100 μM) and each of the interfering substances (100 μM) were plotted. In the reproducibility study, 0.05 g of CDs@SBA-15 was treated with GTM or CTC (100 μM) in 50 mL of deionized water, and after stirring at room temperature for 10 h, the mixture of CDs@SBA-15 and GTM (or CTC) was centrifuged, washed with 0.1 mM HCl solution, and dried at 60 °C for 12 h.2.5 Theoretical calculations
Theoretical calculations are based on the reported literature.38 The energies of the lowest unoccupied molecular orbitals (LUMOs) and the highest occupied molecular orbitals (HOMOs) were calculated using the Gaussian 09 software package. The geometric structures were optimized by the hybrid B3LYP, in combination with the 6-31G (d) basis set.3. Results and discussion
3.1 Characterization of CDs@SBA-15
PXRD displayed a characteristic CDs peak corresponding to the (002) lattice spacing of the crystalline graphite (Fig. 1a).37 SXRD displayed three characteristic diffraction peaks corresponding to the (100), (110), and (200) crystal facets, indicating the preservation of the hexagonal structure of SBA-15 after CD functionalization (the inset of Fig. 1a),39,40 which is consistent with the ordered parallel channels shown in the TEM image (Fig. 1b). As shown in the FT-IR spectra (Fig. 1c), SBA-15 and CDs@SBA-15 displayed the distortion vibration of Si–O at 964 cm−1 and the characteristic Si–O–Si peaks at 468, 809, and 1081 cm−1, further confirming the existence of the SBA-15 silicon framework.41 In the spectra of CDs, the peak at 1351 cm−1 was attributed to the characteristic C–O–H stretching vibration, and the peak at 1589 cm−1 was ascribed to the amide II band. In the spectra of CDs@SBA-15, the peak at 1650 cm−1 was attributed to the characteristic carbonyl stretching vibration of the amide I band, indicating the presence of CDs in the composite. The peak at 3615 cm−1 signaled the formation of hydrogen bonds, suggesting the existence of hydrogen bonds between CDs and SBA-15. The peaks in the range of 3075–3640 cm−1 were attributed to the Si–OH of SBA-15 and N–H of the CDs stretching vibrations. The absorption peaks of CDs@SBA-15 retained the absorption bands of CDs and SBA-15, and showed the characteristic peak of hydrogen bonding, indicating that CDs@SBA-15 was successfully prepared. The N2 adsorption–desorption isotherm revealed the type IV isotherms of the H1 hysteresis line (Fig. 1d), which is characteristic of mesoporous materials. Comparing the BET data of the composites with those of SBA-15,42 the specific surface area, total pore volume, and average pore diameter decreased to 434.8 m2 g−1, 0.95 cm3 g−1 and 87.1 Å (Table S2, ESI†), which indicated that CDs dispersed inside the pore channels of SBA-15 and the ordered mesoporous structure of SBA-15 was preserved after loading CDs.Fig. S1 (ESI†) shows the elemental composition of CDs@SBA-15, and four elements, Si, C, N, and O, were detected in the full-spectrum analysis. The presence of the following groups was confirmed in the high-resolution C 1s, N 1s, O 1s and Si 2p XPS spectra (Fig. 2a–d): C–C (284.8 eV), C–O/C–N (286.4 eV), O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.5 eV), C–N–C (398.47 eV), C–N–H (400.48 eV), N–C
O (288.5 eV), C–N–C (398.47 eV), C–N–H (400.48 eV), N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (401.58 eV), C
O (401.58 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (530.75 eV) Si–O–Si/C–O (532.88 eV) and Si–O (103.38 eV). The XPS analyses showed that the composite contains abundant oxygen/nitrogen-containing functional groups due to the introduction of CDs, which provided more active sites for CDs@SBA-15 to interact with antibiotics.
O (530.75 eV) Si–O–Si/C–O (532.88 eV) and Si–O (103.38 eV). The XPS analyses showed that the composite contains abundant oxygen/nitrogen-containing functional groups due to the introduction of CDs, which provided more active sites for CDs@SBA-15 to interact with antibiotics.
3.2 Optimization of fluorescence detection conditions
Detection conditions affect the optical properties and detection performance of the probe. Experimental conditions, including probe concentration, pH, and time, were optimized to achieve the best fluorescence sensing performance of CDs@SBA-15 for GTM and CTC. The normalized fluorescence excitation and emission spectra of CDs@SBA-15 showed maximum peaks at 396 and 466 nm, respectively, in an aqueous solution (Fig. S2, ESI†). As shown in Fig. S3a (ESI†), the fluorescence intensity at 466 nm gradually increased with the concentration of CDs@SBA-15 (0.025–3.0 g L−1), and the fluorescence intensity versus the concentration displayed an excellent linear relationship (Fig. S3b, F466 nm = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474.1216x – 1761.3238, R2 = 0.9909, ESI†). In subsequent experiments, the probe concentration was set at 0.05 g L−1.
474.1216x – 1761.3238, R2 = 0.9909, ESI†). In subsequent experiments, the probe concentration was set at 0.05 g L−1.
The effect of pH on the fluorescence spectra of CDs@SBA-15 in the presence and absence of GTM or CTC was investigated (Fig. S3c, ESI†). When 3.0 < pH < 8.0, the fluorescence intensity of CDs@SBA-15 at 466 nm gradually decreased with the increase in pH. When 8.0 < pH < 10.0, the fluorescence emission intensity of CDs@SBA-15 at 466 nm did not change significantly. This phenomenon is related to the protonation and deprotonation of functional groups on the CDs surface that could be attributed to photo-induced electron transfer (PET) (Fig. S4, ESI†).43 In the presence of GTM, the change value (ΔI) of fluorescence intensity at 466 nm increased firstly and then decreased with the increase in pH (as shown in the inset of Fig. S3c, ESI†). ΔI (Iprobe+GTM − Iprobe) was maximum at pH = 6.0, indicating that the optimal pH for detecting GTM is 6.0. ΔI (Iprobe − Iprobe+CTC) was maximum at pH = 5.0, therefore, CTC was subsequently detected at pH = 5.0.
As shown in Fig. S3d (ESI†), after GTM was added to the suspensions of the CDs@SBA-15 probe, the fluorescence intensity of the probe increased rapidly within 1 min and then remained unchanged after 5 min of reaction. Meanwhile, the fluorescence intensity was flat 1 min after the addition of an equal amount of CTC. Therefore, the incubation time for the assay was selected to be 5 min for GTM and 1 min for CTC. As shown in Fig. S5a (ESI†), the fluorescence intensity of CDs@SBA-15 was relatively stable in up to 100 mM NaCl, indicating that CDs@SBA-15 has good tolerance to high ionic strength. Moreover, the fluorescence behavior of CDs@SBA-15 was relatively stable within 1 month (Fig. S5b, ESI†), indicating its good photostability.
ACQ refers to the rapid weakening or even complete quenching of the fluorescence intensity of CDs with the increase in their concentration.44 When the concentration of CDs increased from 0.05 g L−1 to 3.0 g L−1, the fluorescence intensity first increased and then decreased until it was almost completely quenched (Fig. S6, ESI†). Moreover, the maximum emission peak shifted from 445 nm to 474 nm, with a red-shift of 29 nm (inset of Fig. S6, ESI†). The fluorescence of CDs is quenched as a function of their concentration, and ACQ limits their applications in detection and imaging. However, a linear relationship was observed between the fluorescence intensity of CDs@SBA-15 at 466 nm and its concentration (Fig. S3a and b, ESI†), indicating that the presence of SBA-15 facilitates the dispersion of CD particles, which in turn attenuates ACQ.23,45
3.3 Fluorescence detection of GTM and CTC
Under the optimal experimental conditions, the sensing performances of the CDs@SBA-15 probe for GTM and CTC were investigated. As shown in Fig. 3a, CDs@SBA-15 showed a “turn-on” fluorescence response toward GTM, and the fluorescence intensity at 466 nm was gradually enhanced with the GTM concentration. As shown in Fig. 3b, favorable linear relationships between the fluorescence intensity ratio (I/I0 − 1) of the probe and the GTM were observed in the concentration ranges of 0.2–2 μM (I/I0 − 1 = 0.1245 + 0.2014[QGTM]) and 2–100 μM (I/I0 − 1 = 0.5708 + 0.0027[QGTM]), and the fluorescence enhancement constant (Kec) values were 2.01 × 105 and 2.7 × 103, respectively. The small slope and Kec at high GTM concentrations may be due to saturation effects.46,47 The LOD was calculated to be 29 nM. Thus, the prepared probe had high sensitivity for GTM detection. | ||
| Fig. 3 (a) Fluorescence spectra of CDs@SBA-15 in the presence of different concentrations of GTM; (b) linearity between the fluorescence intensity ratio (I/I0 − 1) and the concentration of GTM. | ||
The fluorescence spectra of CDs before and after GTM addition (100 μM) are shown in Fig. S7 (ESI†). The fluorescence of the CDs did not change significantly in the presence of GTM. The different fluorescence response between CDs@SBA-15 and CDs toward GTM could be attributed to the spatial domain restriction effect of the SBA-15 pore size, which enhances the interaction between the CD particle immobilized in the pore and GTM. Therefore, compared to the CDs detection system, the CDs@SBA-15 composite probe enriches the detection pathway of GTM.28,29,48
To investigate the sensitivity of CDs@SBA-15 for the detection of CTC, CTC (0–100 μM) was sequentially added to CDs@SBA-15 suspensions. As shown in Fig. 4a, the fluorescence intensity at 466 nm gradually weakened with the increase in CTC concentration. The quenching of CDs@SBA-15 by CTC was discussed using the linear Stern–Volmer curve (Fig. 4b). Good linear relationships were found between the quenching degree (I0/I − 1) and CTC concentration (0–4.0 μM: I0/I − 1 = 0.0101 + 0.0516[QCTC], quenching constant (Ksv) = 5.16 × 104; 4.0–100.0 μM: I0/I − 1 = 0.1701 + 0.0093[QCTC], Ksv = 9.3 × 103), all with R2 values above 0.99. The LOD of the CDs@SBA-15 for CTC was calculated to be 113 nM. At high CTC concentrations, the linear relationship deviates downward and the slope shrunk, which may be caused by the co-existence of static and dynamic quenching49 and the saturation effects.46,47 CTC enhances the fluorescence of CDs at low concentrations and gradually quenches their fluorescence at high concentrations.36 Quantitatively analyzing CTC using the fluorescence spectra of CDs is difficult due to the relationship between the fluorescence intensity of CDs and the concentration of CTC; the same fluorescence intensity might correspond to multiple CTC concentrations. Hence, a continuous response pattern of CDs@SBA-15 to CTC is preferable. Compared with single CDs, the CDs@SBA-15 composite probe showed better linearity and lower LOD in the studied concentrations.
 | ||
| Fig. 4 (a) Fluorescence spectra of CDs@SBA-15 in the presence of different concentrations of CTC; (b) linearity between the fluorescence intensity ratio (I0/I − 1) and the concentration of CTC. | ||
3.4 Selectivity and anti-interference properties of the probe
The fluorescence response of CDs@SBA-15 to a variety of analytes, including five classes of antibiotics (structures are shown in Table S1, ESI†), metal cations, anions, and amino acids. Only AGs and TCs changed the fluorescence intensity ratio of the probe as shown in Fig. 5. By comparison, minimal or almost no changes were observed in the other investigated analytes. In particular, the probe responded to AGs (STR, KNM, and GTM) and TCs (TC, OTC, and CTC) in a “turn-on” manner and a “turn-off” manner, respectively, with fluorescence enhancement and reduction at 466 nm, respectively. The fluorescence response values of the probe to the three AGs in descending order were: I/I0 − 1 (GTM) ≫ I/I0 − 1 (KNM) > I/I0 − 1 (STR). Among TCs, the fluorescence quenching of the probe by CTC was the most severe, suggesting that the probe can achieve highly selective detection of CTC. Therefore, only the detection of GTM and CTC was discussed in this work.There may be a variety of analytes in actual water samples, and the effect of other analytes on GTM or CTC detection was further investigated from the perspective of practical application. As shown in Fig. S8 (ESI†), all the metal ions, anions, amino acids, and other antibiotics caused no significant changes in sensing GTM and CTC. The above results show that the probe has good anti-interference properties for the detection of GTM and CTC.
3.5 Possible sensing mechanisms
The zeta potentials of CDs@SBA-15 and GTM were measured in the pH range of 1.0–10.0 to explore the electrostatic interactions (Fig. S9, ESI†). The zeta potential of CDs@SBA-15 was approximately 5.0, and that of the GTM was approximately 7.1. When pH < 5.0 and pH > 7.1, CDs@SBA-15 and GTM carried the same type of charge and the fluorescence detection performance was reduced due to electrostatic repulsion. In the pH range of 5.0–7.1, electrostatic attraction, which is convenient for detection, dominated between CDs@SBA-15 and GTM. Fig. S10 (ESI†) is a plot of CTC distribution coefficients. CTC has three dissociation constants: pKa1 = 3.33, pKa2 = 7.44, and pKa3 = 9.33. The main form of CTC changed under different pH values, existing as CTCH3+ (pH < 3.33), CTCH2± (3.3< pH < 7.44), CTCH− (7.44 < pH < 9.33), and CTC2− (pH > 9.33).50 At pH < 3.33, the detection performance was poor due to electrostatic repulsion between the positively charged CDs@SBA-15 and CTC. In the pH range of 3.33–5.0, the surface charge of the CTC gradually changed from positive to neutral, so the detection performance of CDs@SBA-15 increased with the pH in this interval. Given that CTC has a large π-conjugated structure, a strong π–π stacking effect occurred between CTC and CDs, resulting in a slight red shift of the fluorescence spectra at high CTC concentrations (Fig. 4).51 When pH > 5.0 and a large amount of CTCH− and CTCH2− was present, the electrostatic repulsion between CDs@SBA-15 and CTC dominated again, and the detection ability of CDs@SBA-15 for CTC gradually decreased. The above results suggest that the electrostatic interaction between the probe and GTM (or CTC) contributes to the fluorescence sensing.52The inner filter effect (IFE) may be present when the absorption spectrum of the analyte overlaps with the excitation or emission spectrum of the probe. In this case, the fluorescence lifetime before and after adding the analyte remains unchanged.53 As shown in Fig. S11 and S12 (ESI†), the UV absorption spectrum of CTC significantly overlapped with the excitation spectrum of CDs@SBA-15. GTM has no UV absorption, so IFE is excluded in the detection mechanism for GTM. The fluorescence lifetime of CDs@SBA-15 was determined before and after GTM and CTC additions to further elucidate the mechanism (Fig. S13, ESI†). The fluorescence lifetime decay curve of CDs@SBA-15 can be described by a biexponential function, with 2.12 ns for the fast component T1 and 8.71 ns for the slow component T2. The average fluorescence lifetime of the probe was calculated to be 3.3 ns using the formula τaverage = (B1T1 + B2T2)/(B1 + B2), and the fluorescence lifetimes became 4.3 and 2.9 ns after GTM and CTC additions, respectively (Table S3, ESI†). Therefore, the quenching of CDs@SBA-15 induced by CTC was not due to the IFE. Another fluorescence detection mechanism is PET. According to the direction of electron transfer, PET is divided into a-PET and d-PET. In a-PET (Fig. S14a, ESI†), electrons are transferred from the acceptor to the fluorophore because the HOMO energy level of the fluorophore is lower than that of the acceptor. d-PET is opposite to a-PET (Fig. S14b, ESI†), where the fluorophore in the excited state transfers electrons on the LUMO energy level to the LUMO energy level of the acceptor, which in turn leads to fluorescence quenching.53 The LUMO and HOMO energy levels of GTM and CTC were calculated based on the DFT method (Table S4, ESI†). As shown in Fig. S15 (ESI†), the results showed that the HOMO energy level of GTM was higher than that of CTC. If a-PET is present in CTC detection, then the HOMO energy level of the CDs would be lower than that of CTC, and consequently of GTM. However, the probe showed a “turn-on” fluorescence response toward GTM, indicating that a-PET is not present. Meanwhile, the LUMO energy level of GTM was higher than that of CTC. On the basis of the completely opposite fluorescence response of the probe to GTM and CTC, the following electron transfer process was proposed: the electrons on the LUMO energy level of CDs are transferred to the LUMO energy level of CTC, leading to fluorescence quenching (d-PET), and cannot be transferred to the LUMO energy level of GTM. Therefore, d-PET contributes to the sensing of CTC, and the electron transfer process is not involved in GTM sensing.
Hydrophilic CDs with multiple active sites can interact with target detectors through hydrogen bonding and thus achieve a “turn-on” fluorescence response.54,55 GTM (Table S1, ESI†) and CDs (Fig. 1c) contain multiple hydrophilic groups (–NH2, –OH), suggesting the possibility of hydrogen bonding interactions in the detection mechanisms. The FT-IR spectra were investigated in the presence and absence of GTM. As shown in Fig. S16 (ESI†), the characteristic peaks of GTM were found in the range of 1400–1650 cm−1, where the peak at 1469 cm−1 was attributed to the characteristic C–N stretching vibration, and those at 1531 and 1633 cm−1 were ascribed to the N–H in-plane deformation vibration.56 After GTM was added to the probe, the C–N stretching vibration at 1469 cm−1 was observed and the in-plane deformation vibration of N–H at 1531 cm−1 was enhanced. The peak shapes of the probe located above 3000 cm−1 widened, the 3475 cm−1 stretching vibration shifted to a lower wave number by 166 cm−1 (3309 cm−1), and the vibration of the absorption peak located at 3613 cm−1 intensified. The above infrared data indicate the formation of strong hydrogen bonds between GTM and CDs.
As shown in Fig. S3c (ESI†), at high pH values, the probe, CTC, and GTM were negatively charged. As the electrostatic repulsion increased, the detection performance of the probe for CTC decreased dramatically. However, the detection performance of the probe for GTM remained excellent, possibly due to the effect of hydrogen bonding. The formed hydrogen bonds between CDs and GTM increased the rigidity of the CD structure, which in turn significantly enhanced the fluorescence.54 Based on the above analyses, the sensing mechanism of CDs@SBA-15 for GTM is based on electrostatic interaction and hydrogen bonding; the detection mechanism for CTC was based on electrostatic interaction and d-PET (Fig. 6).
3.6 Reproducibility study
The recycling property of probes is a crucial factor to be considered in fluorescence sensing. Therefore, the recycling performance of CDs@SBA-15 was evaluated based on the method described in Section 2.4. The infrared spectra revealed that CDs had numerous hydrophilic groups, making it challenging to separate them from the analyte after the reaction and impossible to recycle them, which is in contrast to the concept of green chemistry. Given that SBA-15 was insoluble in any solvent, CDs@SBA-15 could be recycled by centrifugal washing. The fluorescence could still reach 87% of the initial value after recycling three times (Fig. S17, ESI†), suggesting that the probe has a high practical value.3.7 Real sample detection
The sensing performance of CDs@SBA-15 as the probe for GTM and CTC was compared with reported detection methods (Table S4, ESI†). Electrochemical and chromatographic methods are highly sensitive, however, they suffer from the drawbacks of complex operation and high cost. Compared with these methods, CDs@SBA-15 offers advantages of simple operation and low cost. Compared with other related materials reported in previous literature reports based on the fluorescence method, CDs@SBA-15 can not only display differential responses to GTM and CTC, but also has lower detection limits, which could be attributed to the following reasons: (1) the type and nature of precursors used are critical to the sensing performance of CDs. CDs synthesized from precursors containing oxygen and nitrogen can facilitate changing the electron cloud density and energy band structure of CDs;57 (2) the introduction of SBA-15 attenuated the aggregation phenomenon of CDs and thus enhanced the photostability of CDs; in addition, due to nanoconfinement effect, the distance between the recognition group in the probe and the analyte is reduced, which in turn enhances sensitivity; (3) CDs@SBA-15 contains rich oxygen and nitrogen functional groups, which strengthens its binding ability with the analytes. It is well known that probes with a strong binding ability can more effectively capture analytes and enable more sufficient interaction between analytes and materials, thereby improving sensitivity.The fluorescence detection performance of the probe for GTM and CTC in tap water was examined to study its application to actual samples. As shown in Table 1, the experimental results indicated that the determined GTM and CTC concentrations were equivalent to their added concentrations, and the spiked recoveries ranged from 99.45 to 102.43%, with the standard deviations of the three determinations less than 2%. Therefore, CDs@SBA-15 can be applied to sensitively detect trace amounts of GTM and CTC in tap water.
| Sample | Spiked/μM | Found/μM | Recovery/% | RSD/% |
|---|---|---|---|---|
| Tap water (GTM) | 10.00 | 10.24 ± 0.19 | 102.43 ± 0.02 | 1.8 |
| 14.00 | 14.26 ± 0.22 | 101.89 ± 0.02 | 1.5 | |
| 20.00 | 20.38 ± 0.01 | 101.91 ± 0.01 | 0.96 | |
| Tap water (CTC) | 24.00 | 23.87 ± 0.27 | 99.45 ± 0.01 | 1.1 |
| 30.00 | 29.84 ± 0.52 | 99.46 ± 0.02 | 1.8 | |
| 34.00 | 34.24 ± 0.47 | 100.71 ± 0.01 | 1.4 |
4. Conclusion
A functionalized mesoporous silica composite CDs@SBA-15 was constructed and used as a sensitive fluorescent probe for the “turn-off” mode detection of CTC and the “turn-on” mode detection of GTM. In the composite-based probe, SBA-15 acted as a protective agent and insulating layer for CDs, attenuating the ACQ of CDs. Compared with CDs alone, the probe showed superior sensing properties for CTC, such as improved quantitative analysis abilities and reduced LOD. The excellent fluorescence response of the probe to GTM was also attributed to the spatial domain-limiting effect of the SBA-15 pore channels. Its excellent recycling performance makes the probe highly valuable for practical applications. Given the simple operation, easy preparation, and excellent detection capability of the proposed method, it can be used to design other highly sensitive fluorescent probes for detecting target pollutants by adjusting the composite nanomaterials with SBA-15 or the porous material composite with CDs.Author contributions
Lina Gao: software, validation, formal analysis, investigation, data curation, writing – original draft. Jing Wang: conceptualization, methodology, resources, visualization, project administration, funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Natural Science Foundation of China (NSFC Grant No. 22465007, 21966006 and 22167003) generously funded this research.References
- B. Bynum, Rediscovering penicillin, Lancet, 2018, 392, 1108–1109 Search PubMed.
- M. Glinka, W. Wojnowski and A. Wasik, Determination of aminoglycoside antibiotics: current status and future trends, TrAC, Trends Anal. Chem., 2020, 131, 116034 Search PubMed.
- X. Liu, D. Huang, C. Lai, G. Zeng, L. Qin, C. Zhang, H. Yi, B. Li, R. Deng, S. Liu and Y. Zhang, Recent advances in sensors for tetracycline antibiotics and their applications, TrAC, Trends Anal. Chem., 2018, 109, 260–274 Search PubMed.
- F. Yue, H. Li, Q. Kong, J. Liu, G. Wang, F. Li, Q. Yang, W. Chen, Y. Guo and X. Sun, Selection of broad-spectrum aptamer and its application in fabrication of aptasensor for detection of aminoglycoside antibiotics residues in milk, Sens. Actuators, B, 2022, 351, 130959 Search PubMed.
- Y. Gao, Z. Liu, Y. Li and D. Zou, Three-in-one multifunctional luminescent metal-organic gels/sodium alginate beads for high-performance adsorption and detection of chlortetracycline hydrochloride, and high-security anti-counterfeiting, Chem. Eng. J., 2023, 452, 139194 Search PubMed.
- C. Liu, B. Li, M. Liu and S. Mao, Demand, status, and prospect of antibiotics detection in the environment, Sens. Actuators, B, 2022, 369, 132383 Search PubMed.
- Q. Duan, H. Shi, L. Tan, Z. Liu, Q. Huang, W. Shen, L. Cao, H. K. Lee and S. Tang, Ultrahigh-performance supercritical fluid chromatography and detection of multiple biogenic amines in gentamicin sulfate: method development using computer-assisted modeling, Anal. Chem., 2022, 94, 7229–7237 CrossRef CAS PubMed.
- H. Ri, J. Piao, L. Cai, X. Jin, X. Piao, X. Jin, C. Jon, L. Liu, J. Zhao, H. Shang and D. Li, A reciprocating magnetic field assisted on-line solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry determination of trace tetracyclines in water, Anal. Chim. Acta, 2021, 1182, 338957 Search PubMed.
- P. Dai, Y. Zhang, Y. Hong, J. Xiong, H. Du, L. Duan, D. Wang, Y. Wang, W. Deng, B. D. Hammock and W. Yang, Production of high affinity monoclonal antibody and development of indirect competitive chemiluminescence enzyme immunoassay for gentamicin residue in animal tissues, Food Chem., 2023, 400, 134067 Search PubMed.
- A. Pollap and J. Kochana, Electrochemical immunosensors for antibiotic detection, Biosensors, 2019, 9, 61–88 Search PubMed.
- W. Dong, Z. Li, W. Wen, B. Liu and G. Wen, Novel CdS/MOF cathodic Photoelectrochemical (PEC) platform for the detection of doxorubicin hydrochloride and gentamicin sulfate, ACS Appl. Mater. Interfaces, 2021, 13, 57497 CrossRef CAS.
- L. Tu, Y. Rong, Z. Yu, S. Chen, J. Sun, Z. Li, J. Li and Y. Hou, Chlortetracycline degradation performance and mechanism in the self-biased bio-photoelectrochemical system constructed with an oxygen-defect-rich BiVO4/Ni9S8 photoanode, Chemosphere, 2022, 295, 133787 CrossRef CAS.
- Z. Zhang, H. Zhang, D. Tian, A. Phan, M. Seididamyeh, M. Alanazi, Z. Ping Xu, Y. Sultanbawa and R. Zhang, Luminescent sensors for residual antibiotics detection in food: recent advances and perspectives, Coord. Chem. Rev., 2024, 498, 215455 CrossRef CAS.
- W. Zhang, H. Zhong, P. Zhao, A. Shen, H. Li and X. Liu, Carbon quantum dot fluorescent probes for food safety detection: progress, opportunities and challenges, Food Control, 2022, 133, 108591 CrossRef.
- R. Atchudan, S. Chandra Kishore, P. Gangadaran, T. N. Jebakumar Immanuel Edison, S. Perumal, R. L. Rajendran, M. Alagan, S. Al Rashed, B. C. Ahn and Y. R. Lee, Tunable fluorescent carbon dots from biowaste as fluorescence ink and imaging human normal and cancer cells, Environ. Res., 2022, 204, 112365 CrossRef CAS.
- R. Atchudan, T. N. J. I. Edison, S. Perumal, R. Vinodh and Y. R. Lee, Betel-derived nitrogen-doped multicolor carbon dots for environmental and biological applications, J. Mol. Liq., 2019, 296, 111817 CrossRef CAS.
- R. Atchudan, T. N. Edison, S. Perumal, R. Vinodh, A. K. Sundramoorthy, R. S. Babu and Y. R. Lee, Leftover Kiwi Fruit Peel-Derived carbon dots as a highly selective fluorescent sensor for detection of ferric ion, Chemosensors, 2021, 9, 166 CrossRef CAS.
- R. Atchudan, T. N. J. I. Edison, K. R. Aseer, S. Perumal, N. Karthik and Y. R. Lee, Highly fluorescent nitrogen-doped carbon dots derived from phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink, Biosens. Bioelectron., 2018, 99, 303–311 CrossRef CAS PubMed.
- R. Atchudan, T. N. J. I. Edison, S. Perumal, N. Muthuchamy and Y. R. Lee, Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications, Fuel, 2020, 275, 117821 CrossRef CAS.
- N. Nazatul Akmal, Z. Mohammad Faiz and A. Che Azurahanim Che, Hydrothermal synthesis of carbon quantum dots: an updated review, J. Adv. Res. Fluid Mechan. Thermal Sci., 2023, 101(1), 192–206 Search PubMed.
- H. Wang, L. Ai, H. Song, Z. Song, X. Yong, S. Qu and S. Lu, Innovations in the solid-state fluorescence of carbon dots: strategies, optical manipulations, and applications, Adv. Funct. Mater., 2023, 33, 2303756 Search PubMed.
- L. Yang, J. Wen, K. Li, L. Liu and W. Wang, Carbon quantum dots: comprehensively understanding of the internal quenching mechanism and application for catechol detection, Sens. Actuators, B, 2021, 333, 129557 Search PubMed.
- C. Liu, L. Bao, B. Tang, J. Zhao, Z. Zhang, L. Xiong, J. Hu, L. Wu and D. Pang, Fluorescence-converging carbon nanodots-hybridized silica nanosphere, Small, 2016, 12, 4702–4706 CrossRef CAS.
- S. Zhang and J. He, In situ wrapping carbon dots towards robust, durable and transparent tri-layer films with precise spectral conversion and excellent self-cleaning properties, J. Mater. Chem. A, 2024, 12, 2070–2080 RSC.
- Z. Latif, K. Shahid, H. Anwer, R. Shahid, M. Ali, K. H. Lee and M. Alshareef, Carbon quantum dots (CQDs)-modified polymers: a review of non-optical applications, Nanoscale, 2024, 16, 2265–2288 RSC.
- H. Hu, Y. Wu and X. Gong, Organosilicon-based carbon dots and their versatile applications, Small, 2024, 20, 2305933 CrossRef CAS.
- J. Li, W. Li, H. Wang, X. Zhao and X. Gong, Solid-state fluorescent carbon dots-based composite optical waveguide film enables transparent and high-performance luminescent solar concentrators, Appl. Energy, 2024, 358, 122571 CrossRef CAS.
- Z. Zhou, F. Yu and J. Ma, Nanoconfinement engineering for enchanced adsorption of carbon materials, metal-organic frameworks, mesoporous silica, MXenes and porous organic polymers: a review, Environ. Chem. Lett., 2022, 20, 563–595 CrossRef CAS.
- L. Duan, C. Wang, W. Zhang, B. Ma, Y. Deng, W. Li and D. Zhao, Interfacial assembly and applications of functional mesoporous materials, Chem. Rev., 2021, 121, 14349 CrossRef CAS.
- J. A. S. Costa, R. A. De Jesus, D. O. Santos, J. B. Neris, R. T. Figueiredo and C. M. Paranhos, Synthesis, functionalization, and environmental application of silica-based mesoporous materials of the M41S and SBA-n families: a review. Journal of Environmental, Chem. Eng., 2021, 9, 105259 CAS.
- Y. Mu, X. Huang, Z. Tang and Q. Wang, Ordered mesoporous TiO2/SBA-15 confined CexWy catalysts for selective catalytic reduction of NO using NH3, New J. Chem., 2022, 46, 22030–22044 RSC.
- B. Lei, L. Wang, H. Zhang, Y. Liu, H. Dong, M. Zheng and X. Zhou, Luminescent carbon dots assembled SBA-15 and its oxygen sensing properties, Sens. Actuators, B, 2016, 230, 101–108 Search PubMed.
- X. Wang, X. Li, X. Li, Y. Wang, Q. Han and J. Li, Determination of 2,4,6-trinitrophenol by in-situ assembly of SBA-15 with multi-hydroxyl carbon dots, Anal. Chim. Acta, 2020, 1098, 170–180 Search PubMed.
- X. Zou, Z. Shen, X. Li, Y. Cao, Q. Xia, S. Zhang, Y. Liu, L. Jiang, L. Li, L. Cui and Y. Wang, Boosting CO2 methanation on ceria supported transition metal catalysts via chelation coupled wetness impregnation, J. Colloid Interface Sci., 2022, 620, 77–85 Search PubMed.
- Y. Ogura, K. Taniya, T. Horie, K. L. Tung, S. Nishiyama, Y. Komoda and N. Ohmura, Process intensification of synthesis of metal organic framework particles assisted by ultrasound irradiation, Ultrason. Sonochem., 2023, 96, 106443 CrossRef CAS.
- C. Wang, Q. Sun, M. Yang, E. Liu, W. Xue and J. Fan, Preparation of highly luminescent nitrogen-doped carbon quantum dots and their detection of tetracycline antibiotics, Colloids Surf., A, 2022, 653, 129982 Search PubMed.
- Q. Chang, S. Yang, C. Xue, N. Li, Y. Wang, Y. Li, H. Wang, J. Yang and S. Hu, Nitrogen-doped carbon dots encapsulated in the mesoporous channels of SBA-15 with solid-state fluorescence and excellent stability, Nanoscale, 2019, 11, 7247–7255 Search PubMed.
- Y. Liang, J. Wang, H. Zhang, P. Yin, T. Li, Q. Li, Q. Li, Y. Liu and H. Liu, A highly sensitive magnetic nano-fluorescent probe for singlet oxygen detection and screening of natural photosensitizers, Sens. Actuators, B, 2022, 369, 132346 Search PubMed.
- M. Shakeri, Z. Khatami Shal and P. Van Der Voort, An overview of the challenges and progress of synthesis,characterization and applications of plugged SBA-15 materials for heterogeneous catalysis, Materials, 2021, 14, 5082–5099 Search PubMed.
- J. Lee, Y. Park, P. Kim, H. Kim and J. Yi, Preparation of NaCl-incorporated plugged mesoporous silica using a cost-effective precursor and applications to the hydrodechlorination of chlorinated hydrocarbons, J. Mater. Chem., 2004, 14, 1050–1056 Search PubMed.
- H. Chaudhuri, S. Dash and A. Sarkar, SBA-15 functionalised with high loading of amino or carboxylate groups as selective adsorbent for enhanced removal of toxic dyes from aqueous solution, New J. Chem., 2016, 40, 3622–3634 Search PubMed.
- Y. Zhou, J. Wang, Q. Zhao, H. Cai and H. Zhang, Selective adsorption and removal of congo red based on ethylenediamine functionalized mesoporous silica, ChemistrySelect, 2022, 7, e202203280 Search PubMed.
- M. Lan, Y. Di, X. Zhu, T. Ng, J. Xia, W. Liu, X. Meng, P. Wang, C. Lee and W. Zhang, A carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism, Chem. Commun., 2015, 51, 15574 Search PubMed.
- H. Wu, L. Zhang, J. Yang, R. Bo, H. Du, K. Lin, D. Zhang, M. Ramachandran, Y. Shen, Y. Xu, X. Xue, Z. Ma, A. R. Lindstrom, R. Carney, T. Lin and Y. Li, Rotatable aggregation-induced-emission/aggregation-caused-quenching ratio strategy for real-time tracking nanoparticle dynamics, Adv. Funct. Mater., 2020, 30, 1910348 CrossRef CAS.
- L. Huang, H. Zhang, T. Zeng, J. Chen and S. Song, Synergistically enhanced heterogeneous activation of persulfate for aqueous carbamazepine degradation using Fe3O4@SBA-15, Sci. Total Environ., 2021, 760, 144027 Search PubMed.
- X. Zhou, X. Wu, H. He, H. Liang, X. Yang, J. Nie, W. Zhang, B. Du and X. Wang, Contrast-enhancing fluorescence detection of copper ions by functional fluorescent microgels, Sens. Actuators, B, 2020, 320, 128328 Search PubMed.
- T. Liu, G. Li, N. Zhang and Y. Chen, An inorganic–organic hybrid optical sensor for heavy metal ion detection based on immobilizing 4-(2-pyridylazo)-resorcinol on functionalized HMS, J. Hazard. Mater., 2012, 201–202, 155–161 Search PubMed.
- J. Wang, X. Zhang, H. Liu, D. Zhang, H. Nong, P. Wu, P. Chen and D. Li, Aggregation induced emission active fluorescent sensor for the sensitive detection of Hg2+ based on organic-inorganic hybrid mesoporous material, Spectrochim. Acta, Part A, 2020, 227, 117585 CrossRef CAS PubMed.
- H. Sohn, M. J. Sailor, D. Magde and W. C. Trogler, Detection of nitroaromatic explosives based on photoluminescent polymers containing metalloles, J. Am. Chem. Soc., 2003, 125, 3821–3830 CrossRef CAS PubMed.
- L. Chen, K. Zhu, Y. Liu, Y. Huang, L. Zhu, X. Li, Y. Chao and W. Zhu, Supported hybridization of MgCuFe-layered double hydroxides for efficient adsorption of chlortetracycline hydrochloride in sewage, J. Colloid Interface Sci., 2024, 653, 1052–1062 CAS.
- L. Li, L. Shi, J. Jia, O. Eltayeb, W. Lu, Y. Tang, C. Dong and S. Shuang, Red fluorescent carbon dots for tetracycline antibiotics and pH discrimination from aggregation-induced emission mechanism, Sens. Actuators, B, 2021, 332, 129513 CrossRef CAS.
- Q. Luo, T. Ren, Z. Lei, Y. Huang, Y. Huang, D. Xu, C. Wan, X. Guo and Y. Wu, Non-toxic chitosan-based hydrogel with strong adsorption and sensitive detection abilities for tetracycline, Chem. Eng. J., 2022, 427, 131738 CrossRef CAS.
- X. Li, S. Zhao, B. Li, K. Yang, M. Lan and L. Zeng, Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application, Coord. Chem. Rev., 2021, 431, 213686 Search PubMed.
- L. Li, L. Yang, D. Lin, S. Xu, C. Mei, S. Yu and C. Jiang, Hydrogen-bond induced enhanced emission ratiometric fluorescent handy needle for visualization assay of amoxicillin by smartphone sensing platform, J. Hazard. Mater., 2023, 444, 130403 Search PubMed.
- M. Yang, H. Li, J. Liu, W. Kong, S. Zhao, C. Li, H. Huang, Y. Liu and Z. Kang, Convenient and sensitive detection of norfloxacin with fluorescent carbon dots, J. Mater. Chem. B, 2014, 2, 7964–7970 RSC.
- T. Yamaguchi, S. Ko, J. Jung, H. Kim and J. Oh, Periodic charge matching driven immobilization of gentamicin in nanoclays for stable and long-term antibacterial coating, Dalton Trans., 2021, 50, 14216–14222 RSC.
- X. Zhang, X. Liao, Y. Hou, B. Jia, L. Fu, M. Jia, L. Zhou, J. Lu and W. Kong, Recent advances in synthesis and modification of carbon dots for optical sensing of pesticides, J. Hazard. Mater., 2022, 422, 126881 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj04517c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |




