Benzimidazole-based low-sensitivity and heat-resistant energetic materials: design and synthesis†
Ying
Liang
a,
Xian-Kun
Hu
a,
Zhang-Lei
Yang
a,
Miao-Miao
Liu
a,
Yao
Zhang
a,
Jin-Ting
Wu
 a,
Jian-Guo
Zhang
a,
Jian-Guo
Zhang
 b,
Ting-Xing
Zhao
*a,
Shan-Hu
Sun
b,
Ting-Xing
Zhao
*a,
Shan-Hu
Sun
 *a and
Shu-Min
Wang
*a
*a and
Shu-Min
Wang
*a
aSchool of Materials and Chemistry, Southwest University of Science and Technology, Mianyang 621010, China. E-mail: tingxingzhao@swust.edu.cn; shanhusun@126.com; shumin_kxwang@163.com
bState Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China
First published on 4th December 2024
Abstract
Heat-resistant and low-sensitivity energetic materials are urgently needed in demanding environments, such as deep oil wells, space blasting, and hypersonic weapons. Herein, through the processes of substitution, reduction, cyclization, nitration, and ammoniation, two nitro groups and two amino groups were successfully introduced into a benzimidazole framework to prepare a new heat-resistant energetic material, 4,6-diamino-5,7-dinitro-1H-benzo[d]imidazole (DADNBI). Single crystal X-ray diffraction was executed to verify the structure of the compound. Crystal DADNBI belongs to the C2/c space group and monoclinic crystal system. The thermal stability of DADNBI was analyzed through differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), and results showed that the decomposition temperature of DADNBI was 366 °C, which is higher than that of 2,4,6-trinitrotoluene (TNT) (Td: 295 °C), hexanitrostilbene (HNS) (Td: 318 °C), and 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole) (TKX-55) (Td: 335 °C) and comparable to that of 1,3,5-tritamino-2,4,6-trinitrobenzene (TATB) (Td: 360 °C). Non-isothermal thermal decomposition kinetics and Mayer bond pole calculations verified the excellent thermal stability of DADNBI from a theoretical perspective. The characteristic drop height (h50%) of DADNBI is 305 cm. All these parameters of DADNBI far exceed those of the reported 5,7-dinitro-1H-benzo[d]imidazole (DNBI). This work offers important guidelines from both theoretical and experimental perspectives for designing and synthesizing new insensitive heat-resistant energetic materials.
1. Introduction
Owing to their reliable explosive characteristics after long-term storage at high temperature, heat-resistant energetic materials are widely employed in special needs such as space exploration at high temperature and high vacuum and exploitation of deep well oil resources.1,2 In addition, when excavating at a depth of 7 km, the temperature inside the earth may reach 300 °C, or even up to 350 °C, increasing the demand for heat-resistant energetic materials.3 However, currently, the decomposition temperatures of most energetic materials are below 300 °C.4 Thus, the development of new low-sensitivity and heat-resistant energetic materials is essential. In the last decade, significant efforts have been undertaken to develop promising heat-resistant energetic materials. Most polynitrobenzenes have multiple modification sites, good heat resistance, flatness, and moisture resistance superiorities and are extensively exploited as heat-resistant energetic materials, such as 1,3,5-tritamino-2,4,6-trinitrobenzene (TATB), 2,4,6-trinitrotoluene (TNT), and 2,6-bis(picrylamino)-3,5-dinitropyridine (PYX) (Fig. 1a).5–8 In recent years, nitrogen-rich fused-ring framework energetic compounds have received significant attention.9–11 Fused heterocyclics are typically planar-conjugated molecules, and crystalline fillers driven by intermolecular and intramolecular hydrogen bonds as well as π–π interactions allow sliding and compression between molecular layers, thereby reducing mechanical sensitivity towards mechanical stimuli.12,13 Furthermore, the high nitrogen content of the N-heterocyclic skeleton guarantees a large number of C–N, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and N–N bonds in the molecular structure, providing a high positive heat of formation for the target energetic molecule (Fig. 1b).14 Therefore, the development of fused-ring energetic materials is considered a promising strategy to design and synthesize next-generation heat-resistant energetic materials.
N, and N–N bonds in the molecular structure, providing a high positive heat of formation for the target energetic molecule (Fig. 1b).14 Therefore, the development of fused-ring energetic materials is considered a promising strategy to design and synthesize next-generation heat-resistant energetic materials.
 | ||
| Fig. 1 Representative benzene (a) and fused heterocyclic (b) heat-resistant energetic materials, and this work (c). | ||
Benzimidazole is a typical fused-ring framework that has both the stable structure of the benzene ring and the energy and high nitrogen content of the azole ring, forming a two-ring-conjugated plane ring skeleton.15 The highly aromatic-conjugated system can give the molecule high thermal stability. Moreover, there are 5 modifiable sites in benzimidazole, which can introduce multiple energetic functional groups, such as nitro, nitramine, azide, dinitromethyl, and trinitromethyl. Among them, the nitro group is the most stable energetic substituent, and the more the nitro groups, the higher the energy.16 Nevertheless, in the presence of numerous nitro groups, intermolecular O–O interactions increase as sensitivity increases.17,18 Delightfully, the formation of intramolecular and intermolecular hydrogen bonding interactions between amino and nitro groups can improve the stability of molecules and regulate the way molecules stack, thus harmonizing the contradiction between energy and safety.19 For instance, in 1952, Carl Tabs Bahner first synthesized 5,7-dinitro-1H-benzo[d]imidazole (DNBI) by the cyclization of 1,2-diamino-4,6-dinitrobenzene.20 DNBI, as a bioactive substance, has been widely used in the medical field. However, as an energetic material, the detonation performance is insufficient, even if it has good flatness and two nitro groups.
In this paper, two nitro groups and two amino groups were successfully introduced into the DNBI to prepare a new heat-resistant energetic material, 4,6-diamino-5,7-dinitro-1H-benzo[d]imidazole (DADNBI) (Fig. 1c). Then, the crystal of DNDNBI was cultured and tested by single crystal X-ray diffraction for the first time. DADNBI crystalized in the monoclinic space group C2/c, the entire molecule was almost coplanar, and there were strong inter/intramolecular hydrogen bonding interactions. The thermal stability properties of DADNBI were analyzed by DSC and TGA and showed that the decomposition temperature of DADNBI was 366 °C. Then, the detonation properties of DADNBI were calculated, the theoretical detonation velocity was 7306 m s−1, the detonation pressure was 23.2 GPa, and the h50% was 305 cm. This work could offer important guidelines for further exploiting new heat-resistant and low-sensitivity energetic materials.
2. Results and discussion
2.1. Synthesis
The starting material o-nitroaniline was commercially available. As shown in Scheme 1, the chlorinated intermediate 2,4-dichloro-6-nitroaniline (2) was first prepared by an electrophilic substitution reaction between commercially available o-nitroaniline and N-chlorosuccinimide (NCS) at 60 °C (2, 78.5% yield). Subsequent reduction reaction of compound 2 with stannous chloride dihydrate yielded 5-dichloro-1,2-diaminobenzene (3, 85.4% yield). After that, compound 3 underwent an N-acylation reaction with formic acid under the catalysis of hydrochloric acid and then cycled by addition and dehydration to obtain 4,6-dichloro-1H-benzo[d]imidazole (4, 86.7% yield). Compound 4 was nitrated by a nitric–sulfuric mixed-acid system to produce nitro intermediate compound 4,6-dichloro-5,7-dinitro-1H-benzo[d]imidazole (5, 94.4% yield). Finally, the amination of compound 5 with aqueous ammonia in DMF obtained the target compound 4,6-diamine-5,7-dinitro-1H-benzo[d]imidazole (DADNBI, 86.5% yield). All the synthesized intermediates and the target compound DADNBI were characterized by nuclear magnetic resonance (NMR), mass spectra (MS), and infrared spectra (IR) (see the ESI†). The gradual evaporation of dimethyl sulfoxide (DMSO) at room temperature produced a single crystal of DADNBI·DMSO. The crystal structure of compound DADNBI was further confirmed by single-crystal X-ray diffraction.2.2. Crystal structure
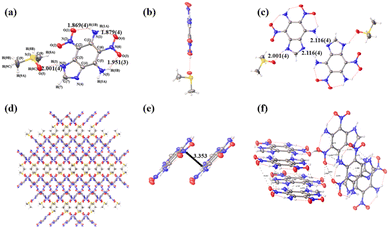
Single crystals of DADNBI·DMSO were obtained by slow evaporation from DMSO at room temperature. The CCDC number (2333741) and detailed crystallographic data are shown in Table S1 (ESI†). The molecular structure of compound DADNBI·DMSO is shown in Fig. 2a. DADNBI·DMSO crystalizes in the monoclinic space group, C2/c, with eight formula units per unit cell (Z = 8). At 150 K, the crystal density of DADNBI is 1.648 g cm−3 due to the presence of the solvent molecule DMSO, which reduces the accumulation coefficient of the crystals. For molecular conformation, the molecular structure of DADNBI in the single crystal data is consistent with NMR, MS, and IR. Detailedly, the fused ring, the two amino groups, and the two nitro groups are nearly coplanar, which could be verified by the torsion angles O(2)–N(2)–C(2)–C(3) = −0.59°, N(1)–C(1)–C(2)–C(3) = −179.88°, O(4)–N(6)–C(6)–C(5) = −174.51° and N(5)–C(5)–C(4)–N(4) = −2.03° (Fig. 2a and b). Similar to most cyclobenzenes, some strong intramolecular hydrogen bonds are distributed in the crystal structure. For example, three strong intramolecular interactions (1.869 Å, 134.042°; 1.879 Å, 129.889°; and 1.951 Å, 125.638°) were located between the two nitro groups and the two amino groups. These strong intramolecular hydrogen bonds can effectively improve the thermal stability of explosives.19 Besides, the intermolecular hydrogen bonding enhances the stability of the crystal structure. Separately, the nitrogen atom without hydrogen in the diazole ring forms intermolecular hydrogen bonds (2.116 Å, 151.919°) with the amino group in another molecule. The hydrogen atoms on nitrogen in the diazole ring form an intermolecular hydrogen bond (2.001 Å, 152.453°) with the oxygen atoms in the solvent molecule (Fig. 2c). Additionally, weak associations existing widely between molecules, such as N⋯N, N⋯O, and O⋯O interactions are also easily observed (Fig. 2f). These interactions result in the compound DADNBI·DMSO, presenting a cross-like stacking arrangement with layer spacing of 3.353 Å (Fig. 2d and e).The Hirshfeld surface and two-dimensional (2D) fingerprint of DADNBI were calculated to further investigate the weak interaction of DADNBI. As shown in Fig. 3, the entire ring is almost coplanar, with almost no bumps or deformations (Fig. 3a). The red and blue areas on Hirshfeld's surface indicate high and low close contacts, respectively. The red region is distributed on the side of the plate, which is a strong interaction, mainly N⋯H, O⋯H hydrogen bond interactions. These strong interactions occur around nitro, amino, and imino, accounting for 47% of the total intermolecular interactions (Fig. 3b). These intermolecular weak interactions can also be obtained from 2D fingerprints (Fig. 3c). DADNBI may have good molecular stability due to a large number of hydrogen bond interactions and the planar conjugated structure of the molecules.
 | ||
| Fig. 3 (a) DADNBI in different directions on the Hirshfeld surfaces. (b) Contact percentage contribution of DADNBI molecular interactions. (c) Two-dimensional fingerprint plots for DADNBI. | ||
2.3. Physicochemical and energetic properties
Thermal stability is an essential criterion for evaluating the safety of energetic materials in an extreme environment.21,22 The thermostability of DADNBI was investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) at a heating rate of 5 °C min−1 under a nitrogen atmosphere. The corresponding DSC and TG-DTG curves of DADNBI are displayed in Fig. 4a and b, respectively. For DADNBI, there is no melting process before its decomposition, which illustrates that DADNBI begins to decompose directly from its solid state and demonstrates the excellent thermal stability of DADNBI. Upon further heating, DADNBI has a sharp exothermic peak in its DSC curve with an onset decomposition temperature of 366 °C and a peak decomposition temperature of 374 °C, which is consistent with its TG-DTG curves, indicating that severe decomposition has occurred near this temperature. | ||
| Fig. 4 (a) DSC curves of DADNBI (5 °C min−1). (b) TG-DTG curves of DADNBI (5 °C min−1). (c) DSC curves of DADNBI at different heating rates. (d) Kissinger and Ozawa curves of DADNBI. | ||
To further study the thermal performance of DADNBI, the thermal stability of DADNBI was tested at different heating rates of 5, 10, 15, and 20 °C min−1. With an increase in heating rate, the peak decomposition temperature on the DSC curve moves towards higher temperatures to 374, 384, 389, and 389 °C, respectively (Fig. 4c). This means that as the rate of warming increases, DADNBI requires higher temperatures to respond.
In comparison, the thermostability of DADNBI is much better than those of common heat-resistant energetic materials of TNT (Td: 295 °C), HNS (Td: 318 °C), and 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole) (TKX-55) (Td: 335 °C) and comparable to those of TATB (Td: 360 °C).23 This high thermal stability of DADNBI may be attributed to the presence of an extensive π-electron conjugation system in the molecular framework, as well as alternative arrangements of nitro and amino groups in the molecular structure.8
In addition, the thermal decomposition kinetics of DADNBI under non-isothermal conditions were studied using the methods of Kissinger (1) and Ozawa (2).24–26 The equations are as follows:
 | (1) |
 | (2) |
According to the peak decomposition temperature at different heating rates, the fitting curves of Kissinger's and Ozawa's equations were obtained, and the Ea values obtained were 296.68 kJ mol−1 and 292.48 kJ mol−1, respectively, indicating that the calculated results of the two methods agree well, which implies the excellent thermal stability of DADNBI (Fig. 4d).
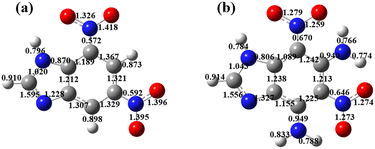
To explain the high thermal stability of DADNBI, the Mayer bond poles of compounds DNBI and DADNBI were calculated by the Gaussian09 and Multiwfnv3.8 (DFT, B3LYP/6-311G**). Generally, the weakest chemical bond (C–NO2) in a molecule determines its thermal stability.27 Thus, the higher Mayer bond poles usually tend to correspond to higher thermal stability. As shown in Fig. 5a and b, comparing the weakest Mayer bond poles of DNBI and DADNBI, the Mayer bond poles of DNBI were calculated to be 0.592 for C5–NO2 and 0.572 for C7–NO2. The Mayer bond poles of DADNBI were 0.646 for C5–NO2 and 0.670 for C7–NO2. Therefore, the Mayer bond order of the weakest bond in DNBI is lower than that of DADNBI, which is consistent with the high thermal stability of DADNBI.
Detonation performances are significant parameters to evaluate the damage ability of explosives.28–30 Using Gaussian 09 software (DFT, B3LYP/6-311G**), the enthalpy of formation of DADNBI was calculated to be 26.8 kJ mol−1 (ESI†). Meanwhile, the detonation velocity and detonation pressure were calculated using the Kamlet–Jacobs (K–J) equations. DADNBI has a detonation velocity of 7306 m s−1 and a detonation pressure of 23.2 GPa, which is superior to those of TNT and DNBI and comparable to HNS. All features of DADNBI are shown in Table 1. Mechanical sensitivity can evaluate the safety of compounds and is an important parameter for assessing the stability of energetic materials.31 Characteristic drop height (h50%) is one of the parameters used to measure the sensitivity of energetic compounds. The sensitivity of the compounds can be expressed by the value of h50%. The greater the value of h50%, the lower the sensitivity of energetic compounds.29 The h50% was obtained by theoretical calculation (DFT, B3LYP/6-31G). The h50% of DADNBI is 305 cm, which is significantly superior to the previous energetic materials TNT, HNS, and DNBI. With all of these features, DADNBI may be used as an insensitive heat-resistant energetic material.
| Compounds | DADNBI | TNTg,k | DNBIh | HNSi,k | TATBj,k |
|---|---|---|---|---|---|
| a Onset decomposition temperature. b Calculated density of DADNBI. c Calculated heat of formation. d Calculated detonation velocity. e Calculated detonation pressure. f h 50% is a characteristic drop height (2.5 kg). g Ref. 4. h Ref. 20. i Ref. 32. j Ref. 6. k Ref. 33. | |||||
| T d (°C) | 366 | 295 | 240 | 318 | 360 |
| ρ (g cm−3) | 1.73 | 1.65 | 1.65 | 1.74 | 1.93 |
| ΔHfc (kJ mol−1) | 26.8 | −67.0 | 105.4 | 78.2 | −139.7 |
| D v (m s−1) | 7306 | 6881 | 6602 | 7612 | 8179 |
| P (GPa) | 23.2 | 19.5 | 18.3 | 24.3 | 30.5 |
| h 50% (cm) | 305 | 98 | 140 | 54 | 490 |
3. Conclusion
In conclusion, a new heat-resistant fused ring compound DADNBI was prepared by substitution, reduction, cyclization, nitration, and ammoniation, and its structure was characterized by single crystal X-ray diffraction, NMR, MS, and IR spectroscopy, respectively. From the DSC and TGA tests, the decomposition temperature of DADNBI could reach 366 °C, which is higher than that of traditional heat-resistant energetic materials TNT (Td: 295 °C) and HNS (Td: 318 °C), and comparable to TATB (Td: 360 °C). The non-isothermal thermal decomposition kinetics calculations also evidenced the excellent thermal stability of DADNBI. The Mayer bond pole was subsequently used to explain the high thermal stability of DADNBI. Compared with DNBI, the weakest Mayer bond order in DADNBI is larger than that of DNBI, so DADNBI has a higher molecular stability. By theoretical calculation, DADNBI also has low sensitivity (h50%: 305 cm). All these parameters of DADNBI far exceed those of DNBI. These systematic investigations on the thermal stability of DADNBI may in turn offer important guidelines for designing and synthesizing new insensitive heat-resistant energetic materials.Author contributions
Ying Liang conducted the molecular design and synthesis, collected and analyzed data, performed theoretical calculations, and wrote the manuscript. Xian-Kun Hu conducted the molecular synthesis and data processing. Zhang-Lei Yang carried out data processing and theoretical calculation. Miao-Miao Liu and Yao Zhang performed the molecular synthesis. Jin-Ting Wu and Jian-Guo Zhang carried out theoretical calculations. Ting-Xing Zhao and Shan-Hu Sun revised the manuscript and directed the research. Shu-Min Wang designed the study, directed the research and provided the main funding.Data availability
All relevant data supporting this study are available in the ESI,† and in the paper.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China No. 22075260, the Doctoral Research Foundation of Southwest University of Science and Technology No. 22zx7129 and No. 22zx7134.Notes and references
- H. Li, L. Zhang, N. Petrutik, K. Wang, Q. Ma, D. Shem-Tov, F. Zhao and M. Gozin, Molecular and crystal features of thermostable energetic materials: Guidelines for architecture of “bridged” compounds, ACS Cent. Sci., 2019, 6, 54–75 CrossRef PubMed.
- J. Cai, C. Xie, J. Xiong, J. Zhang, P. Yin and S. Pang, High performance and heat-resistant pyrazole-1,2,4-triazole energetic materials: Tuning the thermal stability by asymmetric framework and azo-bistriazole bridge, Chem. Eng. J., 2022, 433, 134480 CrossRef CAS.
- J. Zhang, J. Zhang, G. H. Imler, D. A. Parrish and J. M. Shreeve, Sodium and potassium 3,5-dinitro-4-hydropyrazolate: three-dimensional metal-organic frameworks as promising super-heat-resistant explosives, ACS Appl. Energy Mater., 2019, 2, 7628–7634 CrossRef CAS.
- M. Deng, F. Chen, S. Song, S. Huang, Y. Wang and Q. Zhang, From the sensitive primary explosive ICM-103 to insensitive heat-resistant energetic materials through a local azide-to-amino structural modification strategy, Chem. Eng. J., 2022, 429, 132172 CrossRef CAS.
- S. Vangara, N. Kommu, V. Thaltiri, M. Balaraju and A. K. Sahoo, Polynitro-N-aryl-C-nitro-pyrazole/imidazole derivatives: Thermally stable-insensitive energetic materials, J. Org. Chem., 2022, 87, 7202–7212 CrossRef CAS PubMed.
- Q. Ma, G. Zhang, J. Li, Z. Zhang, H. Lu, L. Liao, G. Fan and F. Nie, Pyrazol-triazole energetic hybrid with high thermal stability and decreased sensitivity: Facile synthesis, characterization and promising performance, Chem. Eng. J., 2020, 379, 122331 CrossRef CAS.
- Z. Zhang, W. Geng, W. Yang, Q. Ma, W. Li, G. Fan and Y. Chen, Heat-resistant energetic materials deriving from benzopyridotetraazapentalene: Halogen bonding effects on the outcome of crystal structure, thermal stability and sensitivity, Propellants, Explos., Pyrotech., 2021, 46, 593–599 CrossRef CAS.
- R. Lv, L. Jiang, J. Wang, S. Huang, S. Song, L. Wei, Q. Zhang and K. Wang, A zwitterionic fused-ring framework as a new platform for heat-resistant energetic materials, J. Mater. Chem. A, 2024, 12, 10050–10058 RSC.
- Y. Liu, G. Zhao, Y. Tang, J. Zhang, L. Hu, G. H. Imler, D. A. Parrish and J. M. Shreeve, Multipurpose [1,2,4]triazolo[4,3-b][1,2,4,5] tetrazine-based energetic materials, J. Mater. Chem. A, 2019, 7, 7875–7884 RSC.
- L. Hu, P. Yin, G. H. Imler, D. A. Parrish, H. Gao and J. M. Shreeve, Fused rings with N-oxide and -NH2: Good combination for high density and low sensitivity energetic materials, Chem. Commun., 2019, 55, 8979–8982 RSC.
- S. Chen, W. Zhang, Y. Wang and Q. Zhang, [1,2,4]triazolo[4,3-b]pyridazine as a building block towards low-sensitivity high-energy materials, Chem. Eng. J., 2021, 421, 129635 CrossRef CAS.
- Y. Kang, Y. Dong, Y. Liu, H. Gao, Y. Wang and J. M. Shreeve, Halogen bonding (C-F⋯X) and its effect on creating ideal insensitive energetic materials, Chem. Eng. J., 2022, 440, 135969 CrossRef CAS.
- S. Chen, Y. Liu, Y. Feng, X. Yang and Q. Zhang, 5,6-fused bicyclic tetrazolo-pyridazine energetic materials, Chem. Commun., 2020, 56, 1493–1496 RSC.
- L. Zhang, Q. Lang, M. Zhu, X. Zhang, S. Jiang, M. Lu and Q. Lin, Enhancing conjugation effect to develop nitrogen-rich energetic materials with higher energy and stability, ACS Appl. Mater. Interfaces, 2024, 16, 10211–10217 CrossRef CAS PubMed.
- X. Zheng, Y. Xue, C. Dai, H. Yang and G. Cheng, The skeleton of 5,7-fused bicyclic imidazole-diazepine for heat-resistant energetic materials, Def. Technol., 2023, 27, 193–199 CrossRef.
- C. Li, T. Zhu, C. Lei, G. Cheng, C. Xiao and H. Yang, Construction of p-nitropyrazole-1,3,4-triazole framework energetic compounds: Towards a series of high-performance heat-resistant explosives, J. Mater. Chem. A, 2023, 11, 12043–12051 RSC.
- Q. Sun, N. Ding, C. Zhao, Q. Zhang, S. Zhang, S. Li and S. Pang, Full-nitro-nitroamino cooperative action: Climbing the energy peak of benzenes with enhanced chemical stability, Sci. Adv., 2022, 8, 3176 CrossRef PubMed.
- Q. Yu, F. Li, P. Yin, S. Pang, R. J. Staples and J. M. Shreeve, Bridged and fused triazolic energetic frameworks with an azo building block towards thermally stable and applicable propellant ingredients, J. Mater. Chem. A, 2021, 9, 24903–24908 RSC.
- Q. Sun, W. Chen, N. Ding, C. Zhao, Z. Jiang, S. Li and S. Pang, Unraveling the direct effect of hydrogen bonding on density and thermostability of energetic materials through isomerism, Chem. Eng. J., 2022, 444, 136539 CrossRef CAS.
- C. T. Bahner, H. A. Rutter Jr and L. M. Rives, Substituted benzimidazoles, J. Am. Chem. Soc., 1952, 74, 3689–3690 CrossRef CAS.
- X. Yu, J. Tang, C. Lei, H. Yang and G. Cheng, Intramolecular connections of tetrazoles with N,N′-alkylene and ether bridges: Toward energetic materials with high nitrogen and thermal stability, Cryst. Growth Des., 2024, 24, 455–460 CrossRef CAS.
- D. Su, J. Cai, P. Yin and S. Pang, Regioisomeric N-C functionalization of an asymmetric N-rich framework: A promising pathway to heat-resistant energetic materials, CrystEngComm, 2023, 25, 4902–4906 RSC.
- T. M. Klapötke and T. G. Witkowski, 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole) (TKX-55): thermally stable explosive with outstanding properties, ChemPlusChem, 2016, 81, 357–360 CrossRef PubMed.
- H. E. Kissinger, Reaction kinetics in differential thermal analysis, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- T. Ozawa, A new method of analyzing thermogravimetric data, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- Z. Cheng, Z. Zhang, Q. Ma, L. Yang, H. Yang, G. Cheng, G. Fan and W. Yang, A potential insensitive-highly-energetic material through conjugation-promoted N-oxidation strategy, Chem. Eng. J., 2022, 436, 131990 CrossRef CAS.
- W. J. Geng, Q. Ma, Y. Chen, W. Yang, Y. F. Jia, J. S. Li, Z. Q. Zhang, G. J. Fan and S. M. Wang, Structure-performance relationship in thermally stable energetic materials: Tunable physical properties of benzopyridotetraazapentalene by incorporating amino groups, hydrogen bonding, and π–π interactions, Cryst. Growth Des., 2020, 20, 2106–2114 CrossRef CAS.
- B. C. Tappan, P. R. Bowden, J. P. Lichthardt, M. M. Schmitt and L. G. Hill, Evaluation of the detonation performance of insensitive explosive formulations based on 3,3′ diamino-4,4′-azoxyfurazan (DAAF) and 3-nitro-1,2,4-triazol-5-one (NTO), J. Energ. Mater., 2017, 36, 169–178 CrossRef.
- M. H. Keshavarz, H. Motamedoshariati, R. Moghayadnia, H. R. Nazari and J. Azarniamehraban, A new computer code to evaluate detonation performance of high explosives and their thermochemical properties, part I, J. Hazard. Mater., 2009, 172, 1218–1228 CrossRef CAS PubMed.
- J. Ma, A. K. Chinnam, G. Cheng, H. Yang, J. Zhang and J. M. Shreeve, 1,3,4-oxadiazole bridges: A strategy to improve energetics at the molecular level, Angew. Chem., Int. Ed., 2021, 60, 5497–5504 CrossRef CAS PubMed.
- P. He, J. G. Zhang, L. Wu, J. T. Wu and T. L. Zhang, Computational design and screening of promising energetic materials: Novel azobis(tetrazoles) with ten catenated nitrogen atoms chain, J. Phys. Org. Chem., 2017, 30, 1–12 CrossRef.
- B. M. Rice and J. J. Hare, A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules, J. Phys. Chem. A, 2002, 106, 1770–1783 CrossRef CAS.
- Y. Zhao, W. Cao, F. Huang, Y. Han and X. Long, Evaluation of detonation performance and working capacity of explosives by optimized VLW EOS, Combust. Flame, 2022, 235, 111734 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental section, crystallographic data for target compound, and spectra. CCDC 2333741. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4nj04471a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |