Selective separation and complexation of trivalent actinides and lanthanides using an unsymmetric pyridine-derived triazinyl and amide extractant†
Chenchen
Zhu‡
a,
Yuxiao
Guo‡
c,
Xiao
Yang
a,
Xiaofan
Yang
 a,
Shihui
Wang
a,
Chao
Xu
a,
Shihui
Wang
a,
Chao
Xu
 c,
Chengliang
Xiao
c,
Chengliang
Xiao
 *a and
Lei
Xu
*a and
Lei
Xu
 *b
*b
aCollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310058, China. E-mail: xiaoc@zju.edu.cn
bKey Laboratory of Nuclear Agricultural Sciences of Ministry of Agriculture and Zhejiang Province, Institute of Nuclear Agricultural Sciences, Zhejiang University, Hangzhou, 310058, China. E-mail: hgxulei@zju.edu.cn
cInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
First published on 25th November 2024
Abstract
Unsymmetric N-heterocyclic extractants have been proven to have good application prospects in the separation of trivalent actinides over lanthanides from highly active liquid waste. In this article, a novel unsymmetric pyridine-based extractant functionalized with triazinyl and amide groups (N-ethyl-N-(p-tolyl)-6-(5,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanobenzo[e][1,2,4]triazin-3-yl)picolinamide (Et-Tol-CA-ATP)) was designed and used for the extraction and complexation towards trivalent lanthanide and actinide ions. The ligand Et-Tol-CA-ATP exhibited moderate extraction ability but high selectivity towards Am(III) over Eu(III) (SFAm(III)/Eu(III) = 16.9) under highly acidic HNO3 conditions. The complexation mechanism and extraction behaviors of Et-Tol-CA-ATP for Am(III) and typical lanthanides were thoroughly investigated by NMR spectroscopy, UV-vis spectrophotometry, time-resolved laser fluorescence spectroscopy (TRLFS), high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) analysis and DFT calculations. The stability constants for the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Nd(III) complexes with Et-Tol-CA-ATP in CH3CN were determined as 2.26 ± 0.02 and 4.38 ± 0.01, respectively. The geometric structures of the 1
2 Nd(III) complexes with Et-Tol-CA-ATP in CH3CN were determined as 2.26 ± 0.02 and 4.38 ± 0.01, respectively. The geometric structures of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes of Am(III) and Eu(III) coordinated with Et-Tol-CA-ATP and their differences in the bonding nature leading to the extraction selectivity were further illustrated using DFT calculations. This work describes the successful synthesis of a new unsymmetric pyridine-based triazinyl and amide extractant to selectively separate Am(III) over Eu(III) from highly acidic HNO3 solutions, providing an alternative strategy for developing some new unsymmetric extractants for the separation of trivalent minor actinides.
2 complexes of Am(III) and Eu(III) coordinated with Et-Tol-CA-ATP and their differences in the bonding nature leading to the extraction selectivity were further illustrated using DFT calculations. This work describes the successful synthesis of a new unsymmetric pyridine-based triazinyl and amide extractant to selectively separate Am(III) over Eu(III) from highly acidic HNO3 solutions, providing an alternative strategy for developing some new unsymmetric extractants for the separation of trivalent minor actinides.
Introduction
In the pursuit of addressing global climate change and attaining dual carbon goals, the demand for clean and sustainable energy increases greatly. Nuclear energy as a clean and high-density energy plays an important role in the global clean energy structure. Still, one of the biggest obstacles to the sustainable development of nuclear energy is the continuous generation of large amounts of spent nuclear fuel (SNF) from nuclear plants worldwide.1,2 The plutonium and uranium reduction extraction (PUREX) process is industrially employed to recover the majority of U and Pu from SNF.3,4 However, the raffinate of the PUREX process, also known as high active liquid waste (HLW), still contains minor actinides (MAs) characterized by long half-lives and intense radioactivity and considerable amounts of lanthanides (the abundance of trivalent lanthanides is around one to two magnitudes larger than MAs), posing serious threats to the environment and human beings.5 The partitioning and transmutation (P&T) strategy has been proposed to solve the problems of those long-lived radioactive nuclides, especially for MAs containing in HLW.6 The large neutron capture cross-section of lanthanides containing in HLW can significantly reduce the transmutation efficiency of trivalent MAs, necessitating the separation of trivalent actinides (An(III)) from lanthanides (Ln(III)) prior to transmutation.7,8 However, the selective separation of An(III) over Ln(III) from HLW is very challenging due to the chemical similarities of trivalent lanthanide ions and MAs (similar ionic radius, same coordination numbers and modes) in aqueous solutions, high acidity (3–4 M HNO3), strong radioactivity and complex composition of HLW.9,10 According to the hard–soft acid–base (HSAB) theory, the coordination bonds formed by ligands containing soft donor atoms (such as S and N atoms) and softer 5f actinide ions are slightly stronger than those formed with 4f lanthanide ions.11,12 Therefore, the selective separation of An(III) over Ln(III) can be achieved by adjusting the soft and hard degrees of those N or S donor ligands.13–152,6-Bis(1,2,4-triazin-3-yl)pyridine (BTP) (Fig. 1) is one of the earliest soft N-donor ligands capable of direct extraction for trivalent MAs over Ln(III) from highly acidic HNO3 (>1.0 M) solutions with high efficiency (SFAm/Eu ≤ 150).16 Kolarik et al.17 first developed and studied the extraction properties of alkyl-substituted BTPs for Am(III) over Eu(III) in nitric acid solutions and the results showed that iPr-BTPs had strong extraction ability and high selectivity for Am(III) over Eu(III) (SFAm(III)/Eu(III) ≤ 120) even without the addition of any synergistic agents. However, those alkylated BTPs underwent rapid hydrolytic degradation when in contact with high-concentration nitric acid, followed by the formation of alcohol or ketone, ultimately leading to the loss of extraction ability for MAs.18 To strengthen the hydrolytic stability of BTPs, Trumm et al.19 designed and synthesized a camphor ring substituted BTP type ligand CA-BTP (Fig. 1). The occupation of the pseudo-benzylic positions by tertiary and quaternary carbon atoms can effectively prevent the hydrolytic degradation of CA-BTP and the bridged-ring system of the camphor scaffold and meanwhile fastened the ligand's extraction kinetics (10 min) for f-block elements. The results of solvent extraction studies showed that CA-BTP holds satisfactory extraction performance for Am(III) over Eu(III) (SFAm(III)/Eu(III) ≤ 100) in 1 M HNO3 medium. However, most BTP-derived ligands generally have difficulties in back-extraction and tend to undergo acidolysis under high acidity conditions (>1 M HNO3), which limits the industrial applications of BTPs. In 1995, Hudson et al.20 first developed a diamide pyridine-based (DAP) ligand, N,N′-dibutyl-N,N′-dimethylpyridine-2,6-dicarboxamide, based on the monoamide type ligands, which showed certain selectivity for the separation of An(III) over Ln(III) (SFAm(III)/Eu(III) = 1.4) in TPH. Later, Babain et al.21,22 also studied a series of dialkyldiaryl diamide pyridine-based ligands for the separation of MAs, among which EtTDPA (Fig. 1) showed the best extraction selectivity for An(III) over Ln(III) (SFAm(III)/Eu(III) = 4.4) in nitrobenzotrifluoride (F-3) from 3 M HNO3 medium. Due to the presence of hard oxygen donor atoms, these diamide pyridine-based ligands exhibit good stability under strong acidic conditions but with relatively low separation efficiency towards An(III) over Ln(III).
 | ||
| Fig. 1 Molecular structures of some tridental pyridine-derived extractants for An(III)/Ln(III) separation mentioned in this study. | ||
In addition to those symmetrical ligands previously discussed, some advancements have also been made in the separation and complexation of trivalent f-block elements with unsymmetric ligands over the past two decades. Hudson et al.23 have developed hemi-BTPs (Fig. 1) type ligands by replacing the triazine ring on one side of BTP with a pyridine ring. The extraction results showed that diethyl-substituted hemi-BTPs can only extract An(III) from less-acidic HNO3 solutions when using 2-bromodecylic acid as a synergistic agent. To improve the extraction performance of hemi-BTPs, Girnt et al.24 replaced the triazine rings on both sides of the BTP framework with pyridine and pentapyrazole, respectively, to obtain the DMPBIPY ligand, which exhibited moderate selectivity for Am(III) over Eu(III) (SFAm(III)/Eu(III) ≤ 8), but it also suffers from severe protonation problems under high acidity conditions. Guillet et al.25 added an amide group to BTPs and predicted that the presence of the amide group would reduce the extraction selectivity towards An(III) and Ln(III) compared to BTP using theoretical calculations. Recently, Miao et al.26 have designed and synthesized two unsymmetric pyridine triazinyl-derived ligands BuPh-MTP and Ph2-MTP (Fig. 1), which combined the merits of phosphoryl and triazinyl groups. The results showed that these two ligands had moderate extraction ability and high selectivity for Am(III) over Eu(III) in highly concentrated nitric acid solutions (SFAm(III)/Eu(III) ≤ 26). It provided a brand-new concept for designing unsymmetric ligands by combining different functional groups into one extractant. More recently, Yang et al.27 systemically summarized and compared the existing unsymmetric ligands and pointed out the direction in ligand design for the separation of MAs over lanthanides.
Notably, the current research on unsymmetric ligands has just begun and only a few of the existing ligands have been reported for separation and complexation with f-block elements. Further investigation into the “structure–effect” relationship of unsymmetric ligands towards trivalent lanthanides and actinides is warranted. In this work, a novel unsymmetric pyridine framework ligand Et-Tol-CA-ATP (Fig. 1) was designed and synthesized. The Et-Tol-CA-ATP ligand combines both triazinyl and amide functional groups, along with the utilization of the camphor scaffold of CA-BTP and the N-ethyl-tolyl group of EtTDPA. The extraction performance of pyridine-based triazinyl and amide bifunctional ligands towards groups of Ln(III) has been reported.25 However, to the best of our knowledge, the complexation mechanism for trivalent f-block elements and separation behaviors towards An(III) and Ln(III) by pyridine-based triazinyl and amide bifunctional ligands has not been reported yet. Herein, solvent extraction, 1H-NMR titration, HR-ESI-MS analysis, UV-vis titration, time-resolved fluorescence spectrophotometry and DFT calculations were used to comprehensively study the extraction behaviors and the underlying complexation mechanisms of Et-Tol-CA-ATP towards Am(III) and typical lanthanide ions.
Experimental section
Chemicals
Chemical reagents (6-methylpicolinonitrile, hydrazine hydrate, camphor quinone, selenium dioxide, and N-ethyl-p-toluidine) and other reagents used in synthetic experiments were of analytical grade or higher and used as obtained without further purification. Tracers of 241Am and 152/154Eu were provided by the China Institute of Atomic Energy. The ligand Et-Tol-CA-ATP used in solvent extraction and complexation experiments was prepared in our lab using the following procedures.Synthesis
The synthetic route of Et-Tol-CA-ATP is shown in Fig. 2. A triazinyl ring was introduced into the pyridine framework on one side, followed by the oxidation of methyl on the other side of the pyridine ring and through the amidation to obtain the final target product.Compound 1. 1H NMR (500 MHz, DMSO, 25 °C): δ (ppm) 7.70 (m, 1H), 7.62 (dd, J = 9.6, 5.8 Hz, 1H), 7.16 (m, 1H), 5.71 (s, 2H), 5.30 (s, 2H), 2.48 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the target compound 2 as a white powder (0.91 g, 25.0%). The 1H NMR spectrum of the product is shown in Fig. S2 (ESI†).
1) to obtain the target compound 2 as a white powder (0.91 g, 25.0%). The 1H NMR spectrum of the product is shown in Fig. S2 (ESI†).
Compound 2. 1H NMR (500 MHz, CDCl3, 25 °C): δ (ppm) 8.37 (dd, J = 45.6, 7.8 Hz, 1H), 7.76 (m, 1H), 7.30 (d, J = 7.7 Hz, 1H), 3.24 (dd, J = 47.3, 4.4 Hz, 1H), 2.74 (d, J = 1.9 Hz, 3H), 2.32 (m, 1H), 2.06 (m, 1H), 1.46 (m, 1H), 1.44 (s, 3H), 1.42 (s, 1H), 1.11 (d, J = 4.1 Hz, 3H), 0.66 (d, J = 2.2 Hz, 3H).
Compound 4. 1H NMR (500 MHz, CDCl3, 25 °C): δ (ppm) 8.82 (d, J = 7.9 Hz, 1H), 8.39 (d, J = 7.7 Hz, 1H), 8.14 (t, J = 7.4 Hz, 1H), 3.24 (dd, J = 88.7, 4.0 Hz, 1H), 2.39 (d, J = 11.1 Hz, 1H), 2.14 (dd, J = 17.8, 10.1 Hz, 1H), 1.50 (d, J = 14.6 Hz, 1H), 1.42 (m, 3H), 1.31 (d, J = 27.6 Hz, 1H), 1.15 (s, 3H), 0.67 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain Et-Tol-CA-ATP as a yellow solid (1.01 g, 40.7%). The 1H NMR spectrum and HR-ESI-MS spectrum of Et-Tol-CA-ATP are shown in Fig. S4 and S5 (ESI†), respectively.
2) to obtain Et-Tol-CA-ATP as a yellow solid (1.01 g, 40.7%). The 1H NMR spectrum and HR-ESI-MS spectrum of Et-Tol-CA-ATP are shown in Fig. S4 and S5 (ESI†), respectively.
Et-Tol-CA-ATP. 1H NMR (500 MHz, MeOD, 25 °C): δ (ppm) 8.32 (d, J = 7.9 Hz, 1H), 7.85 (t, J = 7.7 Hz, 1H), 7.45 (dd, J = 13.6, 7.8 Hz, 1H), 7.10 (d, J = 8.1 Hz, 2H), 6.98 (d, J = 8.0 Hz, 2H), 4.05–3.94 (m, 2H), 3.24 (d, J = 4.1 Hz, 1H), 2.42 (d, J = 10.9 Hz, 1H), 2.19 (d, J = 11.2 Hz, 1H), 2.15 (s, 3H), 1.43 (d, J = 8.6 Hz, 3H), 1.39 (s, 1H), 1.37 (s, 1H), 1.25 (t, J = 7.3 Hz, 3H), 1.17 (s, 3H), 0.65 (d, J = 5.1 Hz, 3H).
Solvent extraction
Caution! The radioactive nuclides 152,154Eu and 241Am can cause serious health risks to humans, and thus all the radioactive experiments were carried out in special facilities to avoid ionizing radiation. Kinetic measurements were conducted using non-radioactive Ln(III) to determine the extraction equilibrium time. Equal volumes (0.5 mL each) of the organic and aqueous phases were mixed and vigorously shaken using a vortex oscillator set at 2000 rpm for varying durations, indicating that extraction equilibrium could be reached within 10 minutes (Fig. S8, ESI†). A slope analysis experiment was performed to uncover the stoichiometric ratio of extracts during solvent extraction.28 The organic phases were obtained by dissolving different amounts of Et-Tol-CA-ATP in F-3 with concentrations varying from 5 mM to 30 mM. The 3.0 M HNO3 solutions spiked with trace amounts of 241Am(III) and 152,154Eu(III) were used as the aqueous phases. Equal volumes (0.5 mL) of organic phase and aqueous phases were mixed and then shaken in centrifuge tubes for 180 min to reach the extraction equilibrium. After phase separation, the radioactivity counts of 241Am(III) and 152,154Eu(III) before and after extraction were measured using a Quantulus 1220 Ultra Model instrument to calculate the corresponding distribution ratio (D) and separation factor (SF) values according to eqn (1) and (2) (where X0 and X1 represent the radioactivity counts in the aqueous phase before and after extraction, respectively). | (1) |
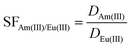 | (2) |
Besides, the effects of HNO3 concentration on the extraction ability and selectivity of Et-Tol-CA-ATP towards An(III) and Ln(III) experiments were also determined.29,30 The organic phase was prepared by dissolving Et-Tol-CA-ATP (10 mM) in F-3 and the aqueous phases consisted of HNO3 of different concentrations (0.5 M to 4 M), which were spiked with trace amounts of 241Am(III) and 152,154Eu(III). Using the same experimental procedures as the above extraction slope analysis, the resulting extraction data of D and SFAm(III)/Eu(III) values were obtained.
NMR titration
To reveal the complexation patterns between the ligand and f-block elements, the NMR titrations of lanthanides with Et-Tol-CA-ATP were performed by collecting the 1H NMR spectra of the ligand upon CD3OD addition with different equivalents of lanthanides.31,32 Diamagnetic La(III) and Lu(III) were selected as the titrants to reflect the greatest difference of Ln(III) series. La(NO3)3, Lu(NO3)3 and the ligand Et-Tol-CA-ATP were directly dissolved in CD3OD (25.0 mM), respectively, to prepare stock solutions for titration. Solutions of Et-Tol-CA-ATP were added to a series of NMR tubes, which were added with certain amounts of La(III) and Lu(III), respectively. The molar ratios of the lanthanide ion to the ligand (M/L ratio) were set in the range of 0 to 4.0. Then the 1H NMR spectra of each sample were collected using a Bruker Avance III 500M instrument. The titration was stopped until no changes were found in the chemical shifts of characteristic protons in the tested spectra.HR-ESI-MS
The HR-ESI-MS method facilitates the exploration of complexation patterns between metal ions and organic ligands in the gas phase.33,34 The stock solutions of lanthanide complexes for the HR-ESI-MS test were prepared by dissolving certain amounts of Eu(NO3)3 and Et-Tol-CA-ATP in HPLC-grade acetonitrile (with a concentration of 1.0 mM), respectively. The molar ratios of metal to ligand were set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by the addition of certain volumes of Eu(NO3)3 and Et-Tol-CA-ATP stock solutions, respectively. The resulting mixtures were diluted to 0.1 mM and added to 3.0 mL screw glass vials. After being shaken for 5 min to reach complexation equilibrium, the high-resolution mass spectra of the ligand Et-Tol-CA-ATP and its complexes with Eu(III) were collected using an Agilent 6545 Model Q-TOF mass spectrometer.
3 by the addition of certain volumes of Eu(NO3)3 and Et-Tol-CA-ATP stock solutions, respectively. The resulting mixtures were diluted to 0.1 mM and added to 3.0 mL screw glass vials. After being shaken for 5 min to reach complexation equilibrium, the high-resolution mass spectra of the ligand Et-Tol-CA-ATP and its complexes with Eu(III) were collected using an Agilent 6545 Model Q-TOF mass spectrometer.
UV-vis titration
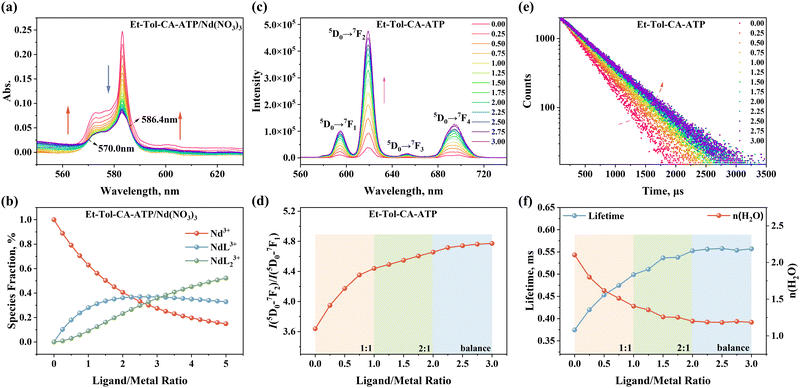
The UV-vis titration experiments were carried out using a Hitachi UH-5300 model spectrophotometer (Japan) in CH3CN at 298 ± 1 K. The stock solutions of 5.0 mM Nd(NO3)3 and 50.0 mM Et-Tol-CA-ATP in CH3CN were prepared respectively for titration. 50.0 mM Et4NNO3 was applied to maintain the ionic strength in all stock solutions. Each 50 μL of ligand solution was added to Nd(III) solution with an initial volume of 2.0 mL in a 1.0 cm quartz cell. The mixture was stirred thoroughly for 3 min to guarantee the equilibrium of the complexation reaction. The characteristic absorption spectra of Nd(III) in the wavelength within 550–630 nm were collected after each titration and the titration was stopped until no further significant changes were observed in spectra. The stability constants for the Nd(III) complexes species and the corresponding molar absorptivity as well as species fraction curves during titration were obtained by fitting the spectral data using the HypSpec program.35TRLFS titration
TRLFS titration was performed to investigate the changes in the coordination environments of Eu(III) along with an increase in the concentration of Et-Tol-CA-ATP. The TRLFS titration experiment was conducted using a FLSP920 model fluorometer and the excitation wavelength was set at 394 nm. The stock solutions of 1.0 mM Eu(NO3)3 in CH3OH and 10.0 mM Et-Tol-CA-ATP in CH3OH were prepared and each was added with 10.0 mM Et4NNO3 to control the ionic strength. Each 50 μL of ligand solution was gradually added into Eu(III) solution with an initial volume of 2.0 mL in a 1.0 cm quartz cell and the mixture was stirred for 2 min to guarantee complexation equilibrium. The fluorescence emission spectra within the wavelength range of 560–740 nm and the lifetime of Eu(III) complexes along with the change of ligand/metal molar ratios were recorded after each titration and the titration was stopped until no further significant changes were observed in titration spectra.Computational details
All the theoretical calculations were carried out with the density functional theory (DFT) method at the B3LYP level using the Gaussian 16 package.36–38 Geometry optimizations without symmetry restrictions were conducted independently in the gas phase. Quasi-relativistic effective core potentials (RECPs) were taken into consideration, and 60 and 28 core electrons substituted by RECPs were chosen for Am and Eu, respectively. In addition, the affiliated segmented contraction scheme (ECP60MWB-SEG for Am and ECP28MWB-SEG for Eu) basis sets were applied for valence electrons.39,40 For other light atoms (C, H, N, and O), the standard Pople basis set 6-311G(d) was used in optimization calculations. The spin–orbit coupling was neglected in this work. The natural atomic charges and Wiberg bond indices (WBIs) were calculated using natural bond orbital (NBO) analyses using the same method.41 Following this, the molecular electrostatic potential (ESP) map of the ligand was computed using the Multiwfn 3.4 software program and was visualized using Visual Molecular Dynamics (VMD) software.42Results and discussion
Solvent extraction studies
The effect of HNO3 concentrations on the extraction ability and selectivity of Et-Tol-CA-ATP towards Am(III) over Eu(III) was also determined at 298 K. Et-Tol-CA-ATP exhibited limited extraction capacity but notable selectivity for Am(III) over Eu(III). As shown in Fig. 3a, the DAm(III) values were higher than those of DEu(III) under all acidity conditions, suggesting that Et-Tol-CA-ATP holds stronger extraction ability for Am(III) than Eu(III). Both DAm(III) and DEu(III) values increased as the HNO3 concentration increased from 0.5 M to 3 M and the maximum DAm(III) value of 0.49 was found at 3 M HNO3 and the resulting largest SFAm(III)/Eu(III) value was up to 16.9 at 3 M HNO3, implying that Et-Tol-CA-ATP has excellent selectivity for Am(III) over Eu(III) under high acidity.
Fig. 3b displays the extraction results of Am(III) and Eu(III) by varying the concentrations of Et-Tol-CA-ATP in F-3 from 3.0 M HNO3 solution. As the ligand concentration increased, the DAm(III) and DEu(III) values increased gradually and the resulting SFAm(III)/Eu(III) values stabilized at around 16, indicating that the ligand concentration did not affect the extraction selectivity. The slopes of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAm(III) and log
DAm(III) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu(III)vs. log [Et-Tol-CA-ATP] were calculated to be 1.91 ± 0.14 and 1.88 ± 0.17, respectively, which implied that the 1
DEu(III)vs. log [Et-Tol-CA-ATP] were calculated to be 1.91 ± 0.14 and 1.88 ± 0.17, respectively, which implied that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal/ligand complex species was dominant during extraction. Based on the electric neutralization principle of the extracted species, the extraction reaction can be depicted as follows:
2 metal/ligand complex species was dominant during extraction. Based on the electric neutralization principle of the extracted species, the extraction reaction can be depicted as follows:
| Lnaq.3+ + 2Lorg. + 3NO3aq.− ↔ [Ln(L)2(NO3)3]org. |
A comparison of extraction performance between Et-Tol-CA-ATP and some other structurally similar pyridine-based ligands is listed in Table 1. EtTDPA22 and CA-BTP20 are symmetrical pyridine-based ligands that contain the same side group as Et-Tol-CA-ATP. BuPh-MTP26 and Ph2-MTP26 are unsymmetrical ligands that contain phosphoryl and triazinyl groups (Fig. 1). It can be found that the SFAm(III)/Eu(III) value of Et-Tol-CA-ATP was in between EtTDPA and CA-BTP, and similar to those of BuPh-MTP and Ph2-MTP. However, the DAm(III) value of Et-Tol-CA-ATP was smaller than those of the ligands.
Besides, the extraction kinetics results indicate that Et-Tol-CA-ATP requires around 10 minutes to reach extraction equilibrium (Fig. S8, ESI†), which is longer than the equilibrium times of EtTDPA (1 min)43 and similar to that of CA-BTP (10 min).20
NMR titration studies
NMR titration spectroscopy is an effective method to interpret complexation patterns between the organic ligand and f-block elements. The spectral changes in the 1H NMR spectra of Et-Tol-CA-ATP titrated with La(NO3)3 in CD3OD are shown in Fig. 4a, suggesting that only one complex species was formed during titration regardless of the change in M/L ratios. Initially, in the absence of La(III) in solution (M/L = 0.0), the 1H NMR peaks located at 8.32, 7.85, 7.45, 7.10 and 6.98 ppm corresponded to the protons on the aromatic ring of free Et-Tol-CA-ATP. With the addition of La(III), obvious chemical changes were observed for those peaks, indicating the formation of one new complex species in solution. Further increasing the La(III) molar ratios in solution, the peaks at 8.56 and 7.93 ppm gradually shifted downfield until the M/L ratio was larger than 1.0. At M/L ≥ 1.0, the 1H NMR pattern of those characteristic peaks remained almost unaltered and only a slight change in the chemical shift of those peaks was observed, which inferred that only one complex species was formed during titration. According to the previously discussed extraction slope analysis, the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species is most likely during titration.
2 complex species is most likely during titration.
In contrast to La(III) with Et-Tol-CA-ATP, two stable complex species were formed during the titration of Et-Tol-CA-ATP with Lu(NO3)3. Combined with the results of slope analysis, it was inferred that the stoichiometric ratios of these two stable complexes were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. As shown in Fig. 4b, with the addition of Lu(III) (M/L = 0.3), five characteristic peaks of the free ligand located at 8.32, 7.85, 7.45, 7.10 and 6.98 ppm disappeared, accompanied by the emergence of one new group of broad peaks located at 9.03–8.87, 8.14, 7.58–7.31 and 7.05–6.85 ppm. Considering the large excess of the ligand in this titration point, it was reasonable to presume that the 1
2, respectively. As shown in Fig. 4b, with the addition of Lu(III) (M/L = 0.3), five characteristic peaks of the free ligand located at 8.32, 7.85, 7.45, 7.10 and 6.98 ppm disappeared, accompanied by the emergence of one new group of broad peaks located at 9.03–8.87, 8.14, 7.58–7.31 and 7.05–6.85 ppm. Considering the large excess of the ligand in this titration point, it was reasonable to presume that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species was formed. At M/L = 0.5, one new narrow peak located at 8.79 ppm emerged, and meanwhile, the intensity of the board peak located at 9.03–8.87 decreased, and three other broad peaks located at 8.14, 7.58–7.31 and 7.05–6.85 ppm became sharper, suggesting the formation of another new complex species in solution. When the M/L ratio was greater than 1.0, the pattern and chemical shift of these three new groups of peaks located at 7.01, 7.41 and 7.30 remained almost constant, indicating that 1
2 complex species was formed. At M/L = 0.5, one new narrow peak located at 8.79 ppm emerged, and meanwhile, the intensity of the board peak located at 9.03–8.87 decreased, and three other broad peaks located at 8.14, 7.58–7.31 and 7.05–6.85 ppm became sharper, suggesting the formation of another new complex species in solution. When the M/L ratio was greater than 1.0, the pattern and chemical shift of these three new groups of peaks located at 7.01, 7.41 and 7.30 remained almost constant, indicating that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species had been completely replaced by the 1
2 complex species had been completely replaced by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex species and the 1
1 complex species and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 species was dominant in solution. It means that during the titration process, in the first titration stage, the 1
1 species was dominant in solution. It means that during the titration process, in the first titration stage, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species was formed, and upon further increasing the concentration of metal ions a 1
2 complex species was formed, and upon further increasing the concentration of metal ions a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal/ligand complex species was also formed and became dominant. The differences in NMR titration results of the ligand with La(III) and Lu(III) may stem from the variations in the metal radius.44
1 metal/ligand complex species was also formed and became dominant. The differences in NMR titration results of the ligand with La(III) and Lu(III) may stem from the variations in the metal radius.44
HR-ESI-MS analysis
HR-ESI-MS tests for Et-Tol-CA-ATP complexation with Eu(III) in acetonitrile were conducted on samples with equivalent amounts of metal and ligand (M/L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Fig. 4c) as well as on samples with excess ligand (M/L = 1
1, Fig. 4c) as well as on samples with excess ligand (M/L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Fig. 4d). The measured m/z values of the ligand and complex species were compared to the theoretical m/z values simulated by ChemDraw software as listed in Table S1 (ESI†). As shown in Fig. 4c, the peaks observed at m/z = 428.2516, 450.2286 and 887.4671 were ascribed to the ligand species of [L + H]+, [L + Na]+ and [2L + Na]+, respectively. The peak with m/z values of 1129.368 was assigned to the complex of [Eu(L)2(NO3)2]+, indicating that only 1
3, Fig. 4d). The measured m/z values of the ligand and complex species were compared to the theoretical m/z values simulated by ChemDraw software as listed in Table S1 (ESI†). As shown in Fig. 4c, the peaks observed at m/z = 428.2516, 450.2286 and 887.4671 were ascribed to the ligand species of [L + H]+, [L + Na]+ and [2L + Na]+, respectively. The peak with m/z values of 1129.368 was assigned to the complex of [Eu(L)2(NO3)2]+, indicating that only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal/ligand complex species was present in the sample of M/L = 1
2 metal/ligand complex species was present in the sample of M/L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As in the sample of M/L = 1
1. As in the sample of M/L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 4d), the results were almost identical to those obtained with equivalent amounts of metal and ligand, revealing only the existence of ligand species and the 1
3 (Fig. 4d), the results were almost identical to those obtained with equivalent amounts of metal and ligand, revealing only the existence of ligand species and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal/ligand complex species. The instability of the 1
2 metal/ligand complex species. The instability of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex may explain its absence in the mass spectrometry results, which is consistent with the findings from the extraction slope analysis that 1
1 complex may explain its absence in the mass spectrometry results, which is consistent with the findings from the extraction slope analysis that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species are dominant. Notably, the abundance of the complex species peaks was much lower than that of ligands, indicative of the relatively weak coordination ability of Et-Tol-CA-ATP for Eu(III).
2 complex species are dominant. Notably, the abundance of the complex species peaks was much lower than that of ligands, indicative of the relatively weak coordination ability of Et-Tol-CA-ATP for Eu(III).
UV-vis spectrophotometric titration studies
The stability constants of the Nd(III) complexes formed with Et-Tol-CA-ATP in CH3CN were determined by the UV-vis titration experiment. Nd(III) was selected as a representative of lanthanide ions because of its similar ionic radius to that of Am(III) and it has an obvious characteristic UV-vis absorption band in the wavelength range of 560–630, which is very sensitive to the coordination environments of Nd(III) and thus can be used to investigate its complexation with organic ligands.45 As shown in Fig. 5a, free Nd(III) had an absorption band centered at 583.2 nm, and with the addition of a ligand, the intensity of this absorption band decreased gradually and meanwhile the shape of the peak turned from sharp to flat. Notably, the intensity of the absorbance band in the wavelength range of 550.0–570.0 nm increased resulting in the formation of one isosbestic point at 570.0 nm. These changes observed in spectra verified the complexation reaction between Nd(III) and the ligand. Further analysis with the HypSpec program suggested that the spectral changes in the titration of Nd(III) with Et-Tol-CA-ATP could be best fitted by the successive formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species and the corresponding stability constants (log
2 complex species and the corresponding stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) were determined as 2.26 ± 0.02 and 4.38 ± 0.01, respectively. The comparison of the stability constants for complexes formed between Nd(III) and other unsymmetrical pyridine-based ligands is also provided in Table 2. Compared with other structurally similar ligands, the stability constants for the formation of 1
β) were determined as 2.26 ± 0.02 and 4.38 ± 0.01, respectively. The comparison of the stability constants for complexes formed between Nd(III) and other unsymmetrical pyridine-based ligands is also provided in Table 2. Compared with other structurally similar ligands, the stability constants for the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Nd(III) complexes with Et-Tol-CA-ATP are much smaller, indicative of the weaker coordination ability of Et-Tol-CA-ATP towards trivalent f-block elements, which also explains the relatively low distribution ratios of Am(III) and Eu(III) in subsequent solvent extraction experiments. As for the stoichiometric difference found in the complex species formed between these tridentate ligands and Nd(III), which probably resulted from various factors such as the binding strength, substituent effect and solvation effect and it remains a complex issue that necessitates further investigation.
2 Nd(III) complexes with Et-Tol-CA-ATP are much smaller, indicative of the weaker coordination ability of Et-Tol-CA-ATP towards trivalent f-block elements, which also explains the relatively low distribution ratios of Am(III) and Eu(III) in subsequent solvent extraction experiments. As for the stoichiometric difference found in the complex species formed between these tridentate ligands and Nd(III), which probably resulted from various factors such as the binding strength, substituent effect and solvation effect and it remains a complex issue that necessitates further investigation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) of Nd(III) complexes with Et-Tol-CA-ATP and other typical unsymmetric pyridine-derived ligands
β) of Nd(III) complexes with Et-Tol-CA-ATP and other typical unsymmetric pyridine-derived ligands
| Ligand | Reaction | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β β |
Ionic medium | Ref. |
|---|---|---|---|---|
| *N.A. denotes not available. *P.W. denotes present work. | ||||
| C5-hemi-BTP | L + Nd3+ ⇌ Nd(L)3+ | 3.01 ± 0.02 | 10 mM Et4NNO3 | 21 |
| Ph2-MTP | 3L + Nd3+ ⇌ Nd(L)33+ | 7.06 ± 0.02 | 50 mM Et4NNO3 | 24 |
| BuPh-MTP | 3L + Nd3+ ⇌ Nd(L)33+ | 6.67 ± 0.01 | 24 | |
| DMAPA | L− + Nd3+ ⇌ Nd(L)2+ | 4.44 ± 0.02 | N.A. | 46 |
| 2L− + Nd3+ ⇌ Nd(L)2+ | 7.90 ± 0.03 | |||
| 3L− + Nd3+ ⇌ Nd(L)3 | 10.28 ± 0.03 | |||
| Et-Tol-CA-ATP | L + Nd3+ ⇌ Nd(L)3+ | 2.26 ± 0.02 | 50 mM Et4NNO3 | P.W. |
| 2L + Nd3+ ⇌ Nd(L)23+ | 4.38 ± 0.01 | |||
As depicted in Fig. 5b, with the ligand/metal ratio (L/M) increasing from 0 to 5.0, the fraction of free Nd(III) gradually decreased. The fraction value of NdL3+ reached its maximum at L/M = 2.5 and subsequently declined and meanwhile the fraction of NdL23+ continued to increase. Otherwise, the ligand absorption in the wavelength of 550–630 nm was also considered to reduce the impact of backline uplift. The molar absorptivity of Nd(III) species and the absorption spectrum of Et-Tol-CA-ATP within the testing wavelength range are presented in Fig. S6 (ESI†).
TRLFS titration studies
TRLFS analysis provides a unique insight into the coordination environment of Eu(III) when complexed with Et-Tol-CA-ATP in solution.47,48 The fluorescence emission spectra of Eu(III) contains valuable information about the electron transition process from the excited state 5D0 to ground states 7F of 5D0 → 7F0, (λ = 579 nm), 5D0 → 7F1 (λ = 594 nm), 5D0 → 7F2 (λ = 619 nm), 5D0 → 7F3 (λ = 651 nm), and 5D0 → 7F4 (λ = 702 nm).49 The electron transition of 5D0 → 7F2 is of particular concern due to its “hypersensitive effect”,50,51 and thus the asymmetry factor AF is defined as the intensity ratio of 5D0 → 7F2 and 5D0 → 7F1, denoting the asymmetry degree of complex species in solution.52,53 Besides, as the vibration of hydroxyl groups bonded to Eu(III) in the first coordination sphere can significantly quench fluorescence, the variation in the fluorescence lifetime is able to reflect the replacement of water molecules by other ligands in the first coordination layer of Eu(III) during titration.49The resulting changes in fluorescence emission spectra and the calculated asymmetry factor AF during titration of Eu(III) with Et-Tol-CA-ATP are shown in Fig. 5c and d, respectively. With an increase in the molar ratio of the ligand/metal, the fluorescence intensity of Eu(III) increased significantly, suggesting that Et-Tol-CA-ATP had a strong “fluorescence enhancement effect” for Eu(III). Additionally, the asymmetry factor (AF) exhibited a discernible rise from 3.64 to 4.77 with a notable surge occurring at L/M = 0.25, which signifies a progressive escalation in species asymmetry within the system.
Fluorescence lifetime τ is related to the number of hydration water molecules n(H2O) in the first coordination sphere of Eu(III), which was deduced as eqn (3) based on the empirical law.49
 | (3) |
The lifetime τ and the number of n(H2O) in the first coordination sphere of Eu(III) as a function of L/M molar ratios are shown in Fig. 5e and f, respectively. It was found that with an increase of the L/M ratio, the lifetime τ of Eu(III) complexes gradually increased from 0.35 ms to 0.55 ms until the L/M ratio reached the maximum of 2.0 and then almost kept constant. Accordingly, the number of n(H2O) decreased from 2.1 to 1.2, laterally confirming the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Eu(III) species by the replacement of H2O molecules with Et-Tol-CA-ATP molecules in the first coordination layer of Eu(III) during titration. Notably, both the curves of AF (Fig. 5d) and τ (Fig. 5f) undergo a dramatic change during this phase as the L/M ratio increases from 0.0 to 1.0. Subsequently, as the L/M ratio continues to increase from 1.0 to 2.0, the rate of curve transformation slows down. If the L/M ratio is beyond 2.0, the curve remains nearly unchanged. These trends provide further confirmation of the formation of 1
2 Eu(III) species by the replacement of H2O molecules with Et-Tol-CA-ATP molecules in the first coordination layer of Eu(III) during titration. Notably, both the curves of AF (Fig. 5d) and τ (Fig. 5f) undergo a dramatic change during this phase as the L/M ratio increases from 0.0 to 1.0. Subsequently, as the L/M ratio continues to increase from 1.0 to 2.0, the rate of curve transformation slows down. If the L/M ratio is beyond 2.0, the curve remains nearly unchanged. These trends provide further confirmation of the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species in the system.
2 complex species in the system.
DFT calculation studies
The electronic structures and bonding nature of Et-Tol-CA-ATP complexes with Am(III) and Eu(III) were investigated using the DFT calculation method. The optimized structure and the corresponding electrostatic potential (ESP) map of the Et-Tol-CA-ATP molecule are depicted in Fig. 6 (where the N atom in the pyridine ring was denoted as N1 and in the triazine ring was denoted as N2). The ESP map showed that the N1, N2 donor atoms and O donor atoms in the amide group had more negative electrostatic potential values than those of other atoms, which means that the rotation of the amide group is needed before binding with actinide and lanthanide cations to form the final stable complexes.Despite concerted efforts, crystalline structures of lanthanide complexes with the ligand proved elusive. Fortunately, quantum chemical calculations provide a valuable avenue to understand the structures of these complexes. Considering the TRLFS titration results (Fig. 5d), the metal is most likely coordinated to one water molecule in both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species as confirmed in 1H NMR and HR-ESI-MS analysis studies. Therefore, the optimization of the molecular structures of Am(III) and Eu(III) complexes with stoichiometric ratios of M(L)(NO3)3(H2O) and [ML2(NO3)(H2O)]2+ (M = Am or Eu) in the gas phase was undertaken.
2 complex species as confirmed in 1H NMR and HR-ESI-MS analysis studies. Therefore, the optimization of the molecular structures of Am(III) and Eu(III) complexes with stoichiometric ratios of M(L)(NO3)3(H2O) and [ML2(NO3)(H2O)]2+ (M = Am or Eu) in the gas phase was undertaken.
As shown in Fig. 6, the coordination modes of M(L)(NO3)3(H2O) and [ML2(NO3)(H2O)]2+ in deca-coordinate and ennea-coordinate, respectively, were proposed based on literature reports54,55 and further validated through computational analysis. As listed in Table 3, the length of Am-N bonds appears marginally longer than those of the corresponding Eu–N bonds. However, upon accounting for the ionic radii of Am(III) and Eu(III) (rAm(III) = 0.98 Å and rEu(III) = 0.95 Å),56 the revised Am–N distances are marginally shorter than the corresponding Eu–N distances, which implies that Et-Tol-CA-ATP manifests a more robust interaction with Am(III) than Eu(III), agreeing well with the solvent extraction results. Besides, the shorter bond distance of Eu–O than that of Am–O indicates the stronger affinity of oxygen atoms with Eu(III) than Am(III). Thus, the selectivity of Et-Tol-CA-ATP towards Am(III) over Eu(III) in the solvent extraction system is attributed to the nitrogen atoms in pyridine and triazine rings rather than the oxygen atoms in amide groups.
| Complex | M–N1 | M–N2 | M–O | M–N1WBI | M–N2WBI | M–OWBI | Q M | Q N1 | Q N2 | Q O |
|---|---|---|---|---|---|---|---|---|---|---|
| Am(L)(NO3)3(H2O) | 2.711 | 2.686 | 2.563 | 0.208 | 0.223 | 0.262 | 1.375 | −0.431 | −0.294 | −0.613 |
| Eu(L)(NO3)3(H2O) | 2.712 | 2.679 | 2.528 | 0.202 | 0.220 | 0.273 | 1.295 | −0.417 | −0.281 | −0.605 |
| [Am(L)2(NO3)(H2O)]2+ | 2.674 | 2.641 | 2.443 | 0.233 | 0.253 | 0.344 | 1.437 | −0.456 | −0.313 | −0.640 |
| [Eu(L)2(NO3)(H2O)]2+ | 2.671 | 2.648 | 2.382 | 0.229 | 0.251 | 0.353 | 1.366 | −0.442 | −0.298 | −0.637 |
To elucidate the bonding nature of the M–N bonds, NBO analyses were conducted for all 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 species. The Wiberg bond index (WBI) in NBO analysis is widely accepted to measure the degree of covalency.36 As delineated in Table 3, the WBIs of M–N bonds fall within the range of 0.202 to 0.223 and these diminutive values signify a predominance of ionic interactions with electrostatic forces exerting a dominant influence on the M–N bonds. Notably, the WBI values of Am–N bonds surpass those of Eu–N, indicative of the heightened covalent attributes within the Am–N bonds. Moreover, the natural charges of Am (QAm) exceed those of Eu, underscoring that the ligand has more pronounced affinity towards Am(III) over Eu(III). The natural charges on the N atoms of the pyridine ring (QN1), triazine ring (QN2) and the O atom (QO) are all in the range of −0.456 to −0.417, −0.313 to −0.281, and −0.640 to −0.605, respectively. The more negative QO value indicates that O has stronger electronegativity and is more likely to form ionic bonds with metal ions.
2 species. The Wiberg bond index (WBI) in NBO analysis is widely accepted to measure the degree of covalency.36 As delineated in Table 3, the WBIs of M–N bonds fall within the range of 0.202 to 0.223 and these diminutive values signify a predominance of ionic interactions with electrostatic forces exerting a dominant influence on the M–N bonds. Notably, the WBI values of Am–N bonds surpass those of Eu–N, indicative of the heightened covalent attributes within the Am–N bonds. Moreover, the natural charges of Am (QAm) exceed those of Eu, underscoring that the ligand has more pronounced affinity towards Am(III) over Eu(III). The natural charges on the N atoms of the pyridine ring (QN1), triazine ring (QN2) and the O atom (QO) are all in the range of −0.456 to −0.417, −0.313 to −0.281, and −0.640 to −0.605, respectively. The more negative QO value indicates that O has stronger electronegativity and is more likely to form ionic bonds with metal ions.
Conclusions
A new bifunctional unsymmetric pyridine-based ligand Et-Tol-CA-ATP combining the merits of triazinyl and amide groups was designed and synthesized. Solvent extraction studies proved the selective extraction capability of Et-Tol-CA-ATP towards Am(III) over Eu(III) from highly acidic HNO3 solutions. The separation factors of Am(III) over Eu(III) by Et-Tol-CA-ATP ranged from 2.6 to 16.9, with the concentration of HNO3 varying from 0.5 to 3.0 M. The formation of the main 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal/ligand complex species during the extraction process was demonstrated by slope analysis. The results of NMR titration studies indicated that only 1
2 metal/ligand complex species during the extraction process was demonstrated by slope analysis. The results of NMR titration studies indicated that only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species were formed between La(III) and Et-Tol-CA-ATP, while for Lu(III), both 1
2 complex species were formed between La(III) and Et-Tol-CA-ATP, while for Lu(III), both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species were formed. Besides, the formation of 1
2 complex species were formed. Besides, the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes between Eu(III) and Et-Tol-CA-ATP was confirmed by HR-ESI-MS analysis. UV-vis titration studies revealed the formation of similar 1
2 complexes between Eu(III) and Et-Tol-CA-ATP was confirmed by HR-ESI-MS analysis. UV-vis titration studies revealed the formation of similar 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex species between Et-Tol-CA-ATP and Nd(III). The corresponding stability constants were determined as 2.26 ± 0.02 and 4.38 ± 0.01, respectively. Further TRLFS studies unveiled a “fluorescence enhancement effect” during the complexation process of Et-Tol-CA-ATP with Eu(III), elucidating the displacement of water molecules by Et-Tol-CA-ATP in the first coordination sphere of Eu(III) during titration. Quantum chemical calculations revealed the molecular structures of 1
2 complex species between Et-Tol-CA-ATP and Nd(III). The corresponding stability constants were determined as 2.26 ± 0.02 and 4.38 ± 0.01, respectively. Further TRLFS studies unveiled a “fluorescence enhancement effect” during the complexation process of Et-Tol-CA-ATP with Eu(III), elucidating the displacement of water molecules by Et-Tol-CA-ATP in the first coordination sphere of Eu(III) during titration. Quantum chemical calculations revealed the molecular structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Am(III) and Eu(III) complexes as confirmed in NMR and solvent extraction studies. Further NBO analysis implies that the higher prevalence of covalency in An–N bonds than that of Eu–N bonds potentially leads to the selectivity of Et-Tol-CA-ATP towards Am(III) over Eu(III). This work delves into the complexation mechanism between one novel unsymmetric ligand and trivalent f-block elements, providing an effective method for the design of more efficient unsymmetric ligands for the separation of trivalent actinides in the future.
2 Am(III) and Eu(III) complexes as confirmed in NMR and solvent extraction studies. Further NBO analysis implies that the higher prevalence of covalency in An–N bonds than that of Eu–N bonds potentially leads to the selectivity of Et-Tol-CA-ATP towards Am(III) over Eu(III). This work delves into the complexation mechanism between one novel unsymmetric ligand and trivalent f-block elements, providing an effective method for the design of more efficient unsymmetric ligands for the separation of trivalent actinides in the future.
Author contributions
Chenchen Zhu: conceptualization, investigation, data curation, methodology, formal analysis, and writing – original draft; Yuxiao Guo: methodology, formal analysis, and writing – original draft; Xiao Yang: methodology and formal analysis; Xiaofan Yang: visualization; Shihui Wang: validation; Chao Xu: resources; Chengliang Xiao: supervision, funding acquisition, and project administration; Lei Xu: funding acquisition and review & editing.Data availability
There is no database used in this manuscript. All the experimental data in this manuscript can be obtained in the main text or the ESI.† If some additional data are needed, please contact the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the financial support from the National Natural Science Foundation of China (No. 2206168 and U20672132).References
- G. H. Marcus, Prog. Nucl. Energy, 2005, 47, 27–31 CrossRef CAS
.
- R. C. Ewing, Nat. Mater., 2015, 14, 252–257 CrossRef CAS PubMed
.
- J. E. Birkett, M. J. Carrott, O. D. Fox, C. J. Jones, C. J. Maher, C. V. Roube, R. J. Taylor and D. A. Woodhead, Chimia, 2005, 59, 898 CrossRef CAS
.
- J. M. Mckibben, Radiochim. Acta, 1984, 36, 3–16 Search PubMed
.
- D. Serrano-Purroy, P. Baron, B. Christiansen, R. Malmbeck, C. Sorel and J.-P. Glatz, Radiochim. Acta, 2005, 93, 351–355 CrossRef CAS
.
- M. Salvatores and G. Palmiotti, Prog. Part. Nucl. Phys., 2011, 66, 144–166 CrossRef CAS
.
- J. Magill, V. Berthou, D. Haas, J. Galy, R. Schenkel, H.-W. Wiese, G. Heusener, J. Tommasi and G. Youinou, Nucl. Energy, 2003, 42, 263–277 CAS
.
- M. Salvatores, Nucl. Eng. Des., 2005, 235, 805–816 CrossRef CAS
.
- A. Bhattacharyya, T. Gadly, P. K. Mohapatra, S. K. Ghosh, D. Manna, T. K. Ghanty and V. K. Manchanda, RSC Adv., 2012, 2, 7066–7073 RSC
.
- K. L. Nash, Solvent Extr. Ion Exch., 1993, 11, 729–768 CrossRef CAS
.
- C. Adam, P. Kaden, B. B. Beele, U. Müllich, S. Trumm, A. Geist, P. J. Panak and M. A. Denecke, Dalton Trans., 2013, 42, 14068–14074 RSC
.
- D. E. Smiles, E. R. Batista, C. H. Booth, D. L. Clark, J. M. Keith, S. A. Kozimor, R. L. Martin, S. G. Minasian, D. K. Shuh and S. C. E. Stieber, Chem. Sci., 2020, 11, 2796–2809 RSC
.
- C. Xiao, C. Wang, L. Yuan, B. Li, H. He, S. Wang, Y. Zhao, Z. Chai and W. Shi, Inorg. Chem., 2014, 53, 1712–1720 CrossRef CAS PubMed
.
- M. Iqbal, R. G. Struijk, J. Huskens, M. Sypula, A. Wilden, G. Modolo and W. Verboom, New J. Chem., 2012, 36, 2048 RSC
.
- M. Heitzmann, C. Gateau, L. Chareyre, M. Miguirditchian, M.-C. Charbonnel and P. Delangle, New J. Chem., 2010, 34, 108–116 RSC
.
- F. Lewis, M. Hudson and L. Harwood, Synlett, 2011, 2609–2632 Search PubMed
.
- Z. Kolarik, U. Mullich and F. Gassner, Solvent Extr. Ion Exch., 1999, 17, 1155–1170 Search PubMed
.
- C. Hill, D. Guillaneux, L. Berthon and C. Madic, J. Nucl. Sci. Technol., 2002, 39, 309–312 Search PubMed
.
- S. Trumm, A. Geist, P. J. Panak and T. Fanghänel, Solvent Extr. Ion Exch., 2011, 29, 213–229 Search PubMed
.
- L. Nigond, N. Condamines, P. Y. Cordier, J. Livet, C. Madic, C. Cuillerdier, C. Musikas and M. J. Hudson, Sep. Sci. Technol., 1995, 30, 2075–2099 CrossRef CAS
.
- M. Y. Alyapyshev, V. A. Babain, I. V. Smirnov and A. Y. Shadrin, Czech. J. Phys., 2006, 56, D469–D475 CrossRef CAS
.
- V. A. Babain, M. Yu Alyapyshev and R. N. Kiseleva, Radiochim. Acta, 2007, 95, 217–223 CrossRef CAS
.
- M. J. Hudson, M. G. Drew, M. R. S. Foreman, C. Hill, N. Huet, C. Madic and T. G. Youngs, Dalton Trans., 2003, 1675–1685 RSC
.
- D. Girnt, P. W. Roesky, A. Geist, C. M. Ruff, P. J. Panak and M. A. Denecke, Inorg. Chem., 2010, 49, 9627–9635 CrossRef CAS
.
- G. L. Guillet, I. D. Hyatt, P. C. Hillesheim, K. A. Abboud and M. J. Scott, New J. Chem., 2013, 37, 119–131 RSC
.
- Y. Miao, L. Xu, X. Yang, S. Wang, J. Zhang, C. Xu and C. Xiao, Inorg. Chem., 2022, 61, 17911–17923 CrossRef CAS PubMed
.
- X. Yang, L. Xu, D. Fang, A. Zhang and C. Xiao, Chem. Commun., 2024, 60, 11415–11433 RSC
.
- X. Yang, S. Wang, L. Xu, C. Liu, C. Jin, A. Zhang and C. Xiao, Sep. Purif. Technol., 2023, 320, 124124 CrossRef CAS
.
- L. Xu, N. Pu, Y. Li, P. Wei, T. Sun, C. Xiao, J. Chen and C. Xu, Inorg. Chem., 2019, 58, 4420–4430 CrossRef CAS
.
- X. Yang, L. Xu, Y. Hao, R. Meng, X. Zhang, L. Lei and C. Xiao, Inorg. Chem., 2020, 59, 17453–17463 CrossRef CAS
.
- L. Xu, A. Zhang, Y. Lu, H. Yang and Z. Liu, RSC Adv., 2016, 6, 99859–99866 RSC
.
- Y. Fang, X. Yuan, L. Wu, Z. Peng, W. Feng, N. Liu, D. Xu, S. Li, A. Sengupta and P. K. Mohapatra, Chem. Commun., 2015, 51, 4263–4266 RSC
.
- C. Xiao, C. Wang, L. Mei, X. Zhang, N. Wall, Y. Zhao, Z. Chai and W. Shi, Dalton Trans., 2015, 44, 14376–14387 RSC
.
- X. Kong, Q. Wu, X. Zhang, C. Wang, K. Hu, Z. Chai, C. Nie and W. Shi, J. Mol. Liq., 2020, 300, 112287 CrossRef CAS
.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739–1753 CrossRef CAS
.
- C. Wang, W. Shi, J. Lan, Y. Zhao, Y. Wei and Z. Chai, Inorg. Chem., 2013, 52, 10904–10911 Search PubMed
.
- M. Kaneko, M. Watanabe and T. Matsumura, Dalton Trans., 2016, 45, 17530–17537 RSC
.
- P. Huang, C. Wang, Q. Wu, J. Lan, Z. Chai and W. Shi, Radiochim. Acta, 2020, 108, 517–526 CrossRef CAS
.
- M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1989, 90, 1730–1734 CrossRef CAS
.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, J. Chem.
Phys., 1994, 100, 7535–7542 CrossRef
.
- J. K. Badenhoop and F. Weinhold, J. Chem. Phys., 1997, 107, 5406–5421 CrossRef CAS
.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graph. Model., 1996, 14, 33–38 CrossRef CAS
.
- A. Paulenova, M. Yu Alyapyshev, V. A. Babain, R. S. Herbst and J. D. Law, Sep. Sci. Technol., 2008, 43, 2606–2618 Search PubMed
.
- G. Ferru, B. Reinhart, M. K. Bera, M. Olvera de la Cruz, B. Qiao and R. J. Ellis, Chem. – Eur. J., 2016, 22, 6899–6904 CrossRef CAS
.
- G. Tian, S. J. Teat and L. Rao, Inorg. Chem., 2014, 53, 9477–9485 Search PubMed
.
- H. Cao, P. Wei, N. Pu, Y. Zhang, Y. Yang, Z. Wang, T. Sun, J. Chen and C. Xu, Inorg. Chem., 2022, 61, 6063–6072 Search PubMed
.
- T. Rabung, M. C. Pierret, A. Bauer, H. Geckeis, M. H. Bradbury and B. Baeyens, Geochim. Cosmochim. Acta, 2005, 69, 5393–5402 Search PubMed
.
- F. Kou, S. Yang, H. Qian, L. Zhang, C. M. Beavers, S. J. Teat and G. Tian, Dalton Trans., 2016, 45, 18484–18493 Search PubMed
.
- X. Tan, M. Fang and X. Wang, Molecules, 2010, 15, 8431–8468 Search PubMed
.
- T. Rabung, T. Stumpf, H. Geckeis, R. Klenze and J. I. Kim, Radiochim. Acta, 2000, 88, 711–716 CAS
.
- T. Stumpf, A. Bauer, F. Coppin, T. Fanghänel and J.-I. Kim, Radiochim. Acta, 2002, 90, 345–349 Search PubMed
.
- Y. Yang, J. Liu, L. Yang, K. Li, H. Zhang, S. Luo and L. Rao, Dalton Trans., 2015, 44, 8959–8970 Search PubMed
.
- P. J. Panak and A. Geist, Chem. Rev., 2013, 113, 1199–1236 CAS
.
- M. G. Drew, D. Guillaneux, M. J. Hudson, P. B. Iveson and C. Madic, Inorg. Chem. Commun., 2001, 4, 462–466 CAS
.
- C. Boucher, M. G. Drew, P. Giddings, L. M. Harwood, M. J. Hudson, P. B. Iveson and C. Madic, Inorg. Chem. Commun., 2002, 5, 596–599 Search PubMed
.
- A. Klamt and G. Schüürmann, J. Chem. Soc. Perkin Trans., 1993, 799–805 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H NMR spectra of Et-Tol-CA-ATP and intermediates synthesized in this work. Supplementary data for HR-ESI-MS analysis, UV-vis titration and solvent extraction studies. Supplementary data for the coordinates of optimized structures of the ligand and its Eu(III) complexes calculated using the DFT method. See DOI: https://doi.org/10.1039/d4nj04483e |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |