Open Access Article
Open Access ArticleWO3/Nb2CTx MXene 2D–2D heterojunction as a high performance photoanode for photoelectrochemical water splitting†
Maida
Murtaza
a,
Waqas Ali
Shah
 ab and
Amir
Waseem
ab and
Amir
Waseem
 *a
*a
aDepartment of Chemistry, Quaid-i-Azam University, Islamabad-45320, Pakistan. E-mail: amir@qau.edu.pk
bSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 10th June 2025
Abstract
Photoelectrochemical (PEC) water splitting plays a key role in the production of green hydrogen, which is a sustainable energy source and non-exploitative to the environment. Therefore, the development of efficient photocatalysts is essential for enabling green hydrogen generation. In this work, the synergistic effect between tungsten oxide (WO3) and Nb2CTx (MXene) was explored for PEC water oxidation, and a composite catalyst was prepared using a hydrothermal and sonication approach to obtain a 2D/2D WO3/Nb2CTx heterojunction. WO3 is a promising photocatalyst owing to its optimal band gap, stability, and cost-effectiveness, but its efficiency is hindered by poor charge transfer, rapid recombination, and weak visible light absorption. Integrating Nb2CTx, a highly conductive MXene, enhances charge separation, reduces electron–hole recombination, and strengthens photocatalytic activity. This synergy increases the number of catalytic sites, improves visible light absorption, and stabilizes WO3, leading to a more efficient and durable material for solar energy conversion and water splitting. The Tauc plot of the composite shows a slightly lower bandgap (2.56 eV) than that of pristine WO3 (2.74 eV), while the charge separation efficiency of the composite is confirmed by its lower photoluminescence intensity than that of pristine WO3. Among the synthesized catalysts, WO3@Nb2C3 showed improved PEC-OER activity by attaining a photocurrent density of 4.71 mA cm−2 at 1.23 V vs. RHE compared to the pristine WO3 that attained a photocurrent density of 2.15 mA cm−2 at the same potential. This strategy appears promising for designing catalysts for PEC water oxidation in a solar-driven hydrogen-powered future.
1. Introduction
The environment has been severely degraded because of the widespread reliance on non-renewable energy sources, such as coal, oil, and natural gas. Burning fossil fuels as a continuous energy source has become hazardous to the environment, as it is the main cause of global warming, air contamination, extreme weather patterns, and ecosystem deterioration, making life less sustainable on the planet. Therefore, there is an urgent global need to transition to cleaner and renewable energy sources such as wind, hydropower, and solar energy.1,2 Photoelectrochemical (PEC) water splitting is the process of generating green hydrogen via the electrolysis of water using solar energy. PEC water splitting is a great substitute for nonrenewable energy sources because it produces green hydrogen without dependence on fossil fuels; hence, it is a great technique for producing clean energy, as it assures a more environmentally friendly, sustainable, and resilient hydrogen-powered future.3,4 Therefore, stable, abundant, and cost-effective photocatalysts are required to conduct photoelectrochemical water splitting efficiently. Some important parameters to consider when choosing an efficient photocatalyst include light harvesting efficiency, band gap energy, easy transfer of electrons from the valence band to the conduction band and less electron–hole recombination.2 Various studies have been conducted on different photocatalysts such as BiVO4,5 CdS,6 Bi2WO6,7 WO3,8 and g-C3N4.9 These efficient catalysts, however, suffer from limited visible light absorption, faster charge recombination and poor stability. To overcome these problems, the formation of heterostructures has been reported, for example, with BiVO4,10,11 TiO2,12–15 Fe2O3,16,17 ZnO,18 silicon-based n–i–p heterojunction19 and surface engineering for defect passivation/reduction.20 WO3 has attracted significant interest among them because it has many aspects that make it an ideal candidate for use as a photoanode material. Bulk WO3 is an n-type semiconductor material with an indirect band gap of 2.4–2.8 eV, where the filled O 2p orbital forms the valence band, while the conduction band is formed by the empty W 5d orbital.21,22 With visible light absorption coverage of 12% of the energy range of the solar spectrum, it offers chemical stability, straightforward synthesis, strong photocatalytic oxidizing ability, cost effectiveness, abundance, and non-toxicity.23 However, WO3 is susceptible to photo corrosion, and bare tungsten oxide has quite a low photocatalytic activity because of faster electron–hole recombination; there is a need to develop a strategy for overcoming these problems and enhancing the photocatalytic activity of WO3.22 In the literature, many strategies have been employed to enhance the photocatalytic performance of WO3, such as morphological engineering, modification through doping with various elements, oxygen vacancy studies,23,24 and crystal phase control.25 One of the strategies is to form composites with suitable semiconductor materials to create heterostructures that can result in an increased number of active sites, increased surface area and enhanced photocatalytic efficiency of tungsten oxide by an increase in the rate of charge separation and decrease in the rate of electron–hole recombination.26–28 A class of 2D materials called MXenes are transition metal carbides or nitrides with the general formula Mn+1XnTx, where n is a number from 1 to 3, M is a transition metal, X is the carbide or nitride, and Tx is the surface functional group, which is usually –F, –OH, or –O. MXenes are obtained from the MAX phase by the process of etching through the removal of the “A” layer (Al, Ge, or Si).29 MXene can prove to be a great choice for creating heterostructures with WO3 because of its high conductivity, structural support and stability, photostability, hydrophilicity, and corrosion-resistant nature.30,31 MXenes have multiple exposed metal sites, many surface groups and layered structures; all these properties agree with the formation of a heterostructure of MXene and WO3 to improve the overall photocatalytic activity of WO3 for PEC water oxidation. A few studies have reported composite formation using MXene with WO3; for example, ZnO/WO3/Ti3C2 has been tested for PEC water splitting.32 Another study reported the use of Ti3C2 MXene/WO3 for photodegradation studies of tetracycline antibiotics, and the composite was synthesized using in situ growth of WO3 nanosheets on the MXene surface in a one-pot solvothermal synthesis strategy.33 In another similar study, hydrothermal and ultrasonic treatments were used to develop a 2D/2D WO3/Ti3C2 heterojunction and were used for the photocatalytic degradation of methylene blue dye under visible light irradiation.34 Similarly, a WO3/MXene (Ti3C2Tx) composite was reported earlier using electrostatic attraction between WO3 nanorods and MXene (Ti3C2Tx) for supercapacitor applications.35 Few studies have been conducted on different kinds of MXene, such as niobium carbide, vanadium carbide, or molybdenum carbide, and there is still room for investigating them as PEC catalysts. In previous studies, niobium carbide MXene has been used as a photoactive material and for charge storage in the literature.36 In this study, WO3 nanorods and niobium carbide (Nb2CTx) MXene composites are prepared by applying the hydrothermal method, followed by sonication. The synergistic effect of WO3 and Nb2CTx is explored, where both the components of the heterostructure complement the photocatalytic properties and surface characteristics of each other, resulting in enhanced photoelectrochemical OER activity. Nb2CTx facilitates charge extraction, while WO3 provides an electronic structure that drives the water oxidation reaction efficiently. WO3, which is susceptible to photo corrosion, can undergo photo corrosion during the water oxidation reaction, but it is protected by Nb2CTx because it stabilizes the surface of WO3 and has a noncorrosive nature and tungsten oxide is not degraded over time and therefore has enhanced stability. Hence, the interaction of tungsten oxide with niobium carbide seems promising, as it can result in increased light absorption efficiency, enhanced charge separation, decreased electron–hole recombination, increased number of catalytic sites, prolonged stability, and enhanced interface contact surface area. This study is expected to serve as a pathway for designing highly efficient photocatalysts driven by visible light. The as-prepared samples were characterized using powder XRD, XPS, FESEM/EDX, HRTEM, and PL techniques.2. Experimental
2.1. Materials and chemicals
Ammonium metatungstate, hydrochloric acid (HCl), sodium sulfate (Na2SO4), and hydrofluoric acid (HF) were purchased from Sigma-Aldrich. The MAX phase (Nb2AlC) was purchased from Chemazone, Inc. Nanochemazone. Fluorine-doped tin oxide (FTO) plates were obtained from Sigma-Aldrich. Deionized water was used to prepare the electrolytes and reaction mixtures.2.2. Synthesis of WO3
WO3 nanorods were synthesized by applying the hydrothermal method, in which 0.77 g of ammonium meta tungstate was dissolved in 12.5 ml distilled water and 0.53 ml hydrochloric acid was added to it. The homogenized mixture was then transferred into a Teflon-lined autoclave and kept at 180 °C for 4 hours. After centrifugation and washing at 5000 rpm with distilled water three times, the material was kept at 70 °C for 6 hours and then kept for annealing at 500 °C for half an hour, and yellowish powder was formed.372.3 Synthesis of Nb2CTx
Two grams of Nb2AlC were slowly added into 40 ml of HF, and the solution was magnetically stirred at room temperature for 90 hours.38 After this period, the material was washed through centrifugation at 5000 rpm multiple times with DI water. The washed material was then kept for drying overnight at 60 °C.2.4 Synthesis of WO3/Nb2CTx heterojunction
WO3/Nb2CTx heterojunction was prepared by applying the sonication approach. 100 mg of hydrothermally prepared WO3 was dissolved in 10 ml of DI water, and Nb2CTx sheets were added to it (5, 10, and 15 weight%). The solution was kept at sonication for 12 hours; then, the resulting suspension was dried overnight at 100 °C.34 The resulting materials were named WO3@Nb2C1, WO3@Nb2C2, and WO3@Nb2C3 for 5, 10, and 15 weight% of Nb2CTx, respectively. The ultrasonication approach is beneficial for synthesizing such heterojunctions because high shear forces generated by intense sound waves can be helpful in the uniform distribution of particles and can break the agglomerates of particles to ensure uniform dispersibility. As a result of ultrasonic treatment, bubbles are formed, and these bubbles rupture swiftly and hence induce local high temperature and pressure within the material, which ultimately can induce surface modification in both WO3 and Nb2CTx. A schematic of the synthesis is depicted in Fig. 1. | ||
| Fig. 1 Schematic of the synthesis showing pure WO3, Nb2CTx MXene and composite WO3@Nb2CTx formation. | ||
2.5 Photoelectrochemical characterization and preparation of the working electrode
Catalyst ink was prepared by applying the spin coating method, and FTO was used. For cleaning, the FTO was immersed in acetone, ethanol, and DI water for 15 minutes in each solvent. 100 mg of the prepared photocatalyst was added into 5 ml of ethanol (20 mg ml−1) to prepare a slurry. This dispersion (1000 microliters) was spin coated at 1000 rpm on FTO substrates to procure WO3 and WO3@Nb2C thin films, which were dried at 60 °C for 5 min. A thin uniform film was then obtained by repeating the coating and drying for 5 cycles. All PEC measurements were performed using an electrochemical workstation (Biologic VSP-300 Potentiostat) with three-electrode cell configurations: platinum wire as the counter electrode, Ag/AgCl as the reference electrode, and catalyst-coated FTO as the working electrode. The experiment was performed in a quartz cell. As the light source solar simulator, Oriel LCS-100 with a 100 W Xe lamp and 1.0 sun AM 1.5 G filter was used. The electrolyte used was a 0.5 M Na2SO4 solution. The Nernst equation was used for the conversion of potential to vs. RHE. Linear sweep voltammetry (LSV) and cyclic voltammetry (CV) measurements were performed in the potential range of 0.6–1.6 V vs. RHE under illumination and in the dark as well. Chopped light LSVs were also performed in the potential range of 0.6–1.6 V vs. RHE at a scan rate of 5 mV s−1 using a mechanical chopper with 5 seconds light ON and 5 seconds light OFF intervals. Electrochemical impedance spectroscopy (EIS) was performed under illumination in the frequency range of 100–100 mHz at a potential of 0.8 V vs. RHE. Electrochemical surface area (ECSA) and double layer capacitance (Cdl) were calculated by taking CV cycles in the dark in the non-faradaic potential range at various scan rates. The Mott–Schottky analysis was performed to determine the flat band potential and donor density.3 Results and discussion
3.1 Characterizations
The synthesis of all the materials was studied by powder X-ray diffraction (XRD), and the XRD was recorded in the range of 2θ 5–70°. The etching of the commercially available niobium aluminum carbide (Nb2AlC) MAX phase to Nb2CTx MXene was confirmed by XRD patterns, as illustrated in Fig. S1.† The MAX phase shows a prominent diffraction pattern at the 2θ values of 12.7° (002), 33.3° (100), 38.7° (103), 39.1° (006), and 52.3° (106) of crystalline Nb2AlC, which matches well with the standard pattern (JCPDS 30-0033) (Fig. S1†).38,39 After treatment with HF to etching the Al layer, a characteristic broad signal was observed at a 2θ value of 8.9°, which corresponds to the (002) crystal plane of Nb2CTx MXene, corresponding to the d-spacing of 0.98 nm, similar to the previously reported study.40 The appearance of this new signal as well as the substantial decrease in the intensity of the diffraction signal at 39° (006); others confirm the formation of Nb2CTx MXene after successful etching.38,40 The synthesis of WO3 is confirmed by analyzing the XRD pattern, showing the prominent signals at 2θ values of 23.1°, 23.5°, 24.4°, 33.3°, 33.6°, 34.2°, and 49.9° and it matches well with the standard pdf # 431035 (Fig. 2a) and shows the monoclinic phase of the WO3.22 The synthesis of WO3@Nb2CTx heterojunction with different ratios of Nb2CTx (5–15%) was also confirmed by the XRD pattern shown in Fig. 2(c–e), and the broad signal at 8.9 shows that the Nb2CTx is incorporated successfully to produce WO3@Nb2CTx composite with varying ratios.41 The XRD of the composites shows no significant shift in WO3 peaks, suggesting physical interfacial interaction between WO3 and Nb2CTx MXene, similar to previously reported studies where composite synthesis was carried out through electrostatic attraction between WO3 nanorods and Ti3C2Tx MXene for supercapacitor applications.35 | ||
| Fig. 2 XRD patterns of the WO3 reference card (a), WO3 (b), WO3@Nb2C1 (c), WO3@Nb2C2 (d), and WO3@Nb2C3 (e). The insets show the magnified patterns of (c)–(e). | ||
The syntheses of WO3 and WO3@Nb2C3 composites were also confirmed by analyzing the XPS survey scan. Fig. 3 shows that all the desired elements (W, O, Nb, and C) are present on their respective binding energies, which shows the successful incorporation of Nb2CTx MXene with WO3. The absence of Al 2p and 2s signals at ≈74 and ≈118 eV in both survey scans also confirms successful etching.42 The high-resolution spectra of W 4f core levels show a well-defined doublet at binding energies at 35.6 and 37.7 eV and can be assigned to W 4f7/2 and W 4f5/2 for W6+ (Fig. 3b) and a weak doublet at 34.3 eV and 36.4 eV for W5+.33,43 The high resolution XPS core level Nb 3d region shows spin orbital splitting at binding energies of 203.3 and 206.1 eV, which corresponds to 3d5/2 and 3d3/2 of Nb–C bonds, respectively, for both Nb2CTx and WO3@Nb2C3 composite (Fig. 3c). Another week's broad doublet at 204.9 eV and 207.4 eV shows the presence of Nb–O bonds, as reported previously for Nb2CTx MXene.44,45 The core level XPS of the O 1s region of WO3 (Fig. 3d) shows the major peak fitting at 530.5 eV and 531.4, which can be assigned to lattice oxygen and oxygen absorbed on the surface, respectively.33 In the case of WO3@Nb2C3 composite, O 1s spectra show other peaks at 531.6 and 533 eV, which can be assigned to C–Nb–Ox and interlayer adsorbed water due to the presence of Nb2CTx MXene.30,45
Fig. S2† shows the FESEM images of the etched Nb2CTx MXene. It can be observed that the layered morphology of the Nb2CTx is present, and gaps in aluminum after etching are visible, which is a typical shape of 2D MXenes, as reported previously.30,46 Fig. S3† shows the small plate-like structures of pristine WO3 in the nanometer range (a) and flower-like arrangements when aggregated (b). During the composite formation, WO3 was smoothly distributed over the surface of the delaminated layers of Nb2CTx to produce the WO3@Nb2C3 composite. The sharp edges of the nanoplate become smooth in the composite owing to sonication, as shown in Fig. 4(a) and (b). Fig. 4c shows the EDX spectrum of the WO3@Nb2C3 composite with all the required component elements.
 | ||
| Fig. 4 FESEM images of the WO3@Nb2C3 composite at different resolutions (a and b) and EDX spectrum of the composite (c). | ||
Fig. 5 shows TEM images of the composite. Both components of the composite WO3 and Nb2CTx MXene are visible (Fig. 5a), showing successful composite formation; the fringes on Nb2CTx MXene are clearly visible and are related to interlayer spacing.40 The HRTEM (Fig. 5a) of WO3@Nb2C3 composite shows the fringes corresponding to plane (002) of Nb2CTx MXene with a d-spacing of 0.98 nm, while the WO3 shows the fringes corresponding to plane (200) of monoclinic phase with d-spacing of 0.36 nm at the composite interface.47
The investigation of the optical absorption properties of WO3 and WO3@Nb2C3 composite was carried out by UV-vis spectroscopy. The Tauc equation is used for the relation between the bandgap energy and absorption spectra, which is written as follows: (αhν) = k(hν − Eg)n.
Extrapolating the linear portion of the plot (hν) vs. energy (αhν)2 provides the band gap (Eg) within a wavelength range of 200–800 nm. Various parameters can be employed to induce alteration in Eg values, including doping, grain size, annealing treatment, and the nature of transition (direct or indirect).48 The bandgap energies of WO3 and WO3@Nb2C3 composite were observed as 2.74 and 2.56 eV, respectively (Fig. 6a), which agrees with previously reported studies.33,47 By the addition of Nb2CTx sheets to the catalyst, the band gap energy for WO3@Nb2C3 is lower than that of WO3, and it can be attributed to the overlapping of electronic band structures.47 As the band gap energy is reduced, it is beneficial for the generation of electrons and holes in visible light, and Nb2CTx serves to prevent the recombination of electrons and holes, which ultimately results in the enhancement of the photoelectrocatalytic efficiency of WO3@Nb2C3. Steady-state photoluminescence (PL) studies were conducted to interpret the behavior of photo-induced charge separation and recombination. Fig. 6b shows a broad emission peak under an excitation wavelength of 375 nm for pristine WO3 at a signal centered at about 465 nm, which agrees with faster electron–hole pair recombination as a result of the de-excitation emission from W 4f to O 2p. Two other peaks of low intensity at 500 nm as a shoulder and 545 nm could arise owing to the band transition of the trapped electrons on defect sites to the valence band, showing the role of W5+ species in WO3, which is evident in the XPS results.33 It is evident that the PL intensity significantly decreased upon the composite formation of WO3@Nb2C3 compared to pure WO3. This disparity in photoluminescence can be attributed to the boosted charge separation within the composite.23 The quenching of photoluminescence intensity reveals that the assimilation of Nb2CTx MXene on WO3 possibly accelerates spatial charge separation and overturns carrier recombination through the establishment of a supplementary nonradiative decay pathway across the WO3/Nb2CTx MXene interface.33 The increased charge separation and reduced recombination rate make the material highly suitable for various photocatalytic applications.21
 | ||
| Fig. 6 Spectroscopic characterization of Nb2CTx MXene, WO3 and WO3@Nb2C3 composites: (a) Tauc plots and (b) photoluminescence. | ||
3.2 Photoelectrochemical studies
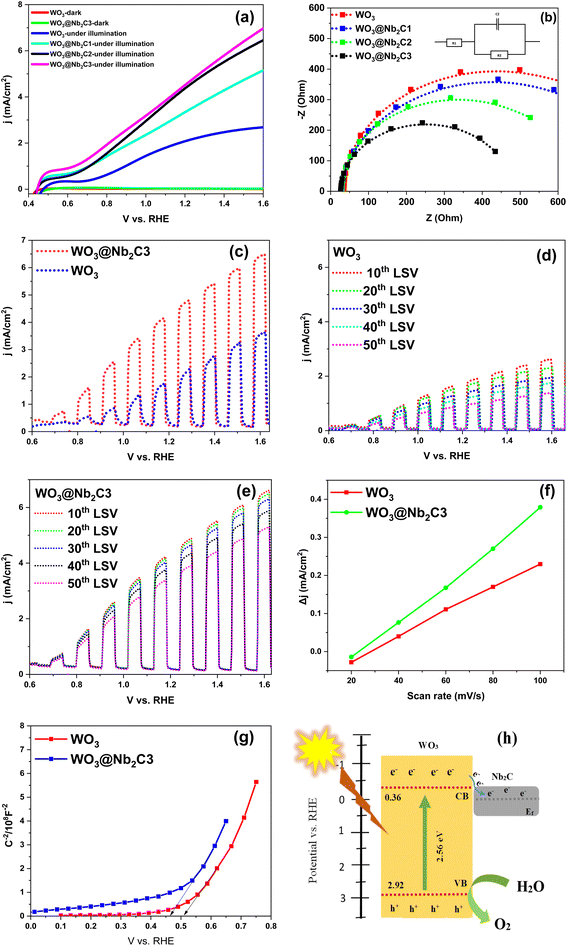
Photoelectrochemical catalytic activity was examined using an electrochemical workstation (Biologic VSP-300 potentiostat) with a 1.0 sun AM 1.5 G solar simulator as the light source in a 0.5 M Na2SO4 electrolyte. This work preferred a hole scavenger-free electrolyte, as the presence of a sacrificial agent can interrupt and mask some important information about the photoelectrochemical experiment representations. LSV measurements were performed for WO3, WO3@Nb2C1, WO3@Nb2C2, and WO3@Nb2C3 under illumination and in the dark as well. Fig. 7a shows that WO3 photocatalytic activity is enhanced by forming a heterojunction with Nb2CTx. Among the three composites with varying ratios of Nb2CTx, WO3@Nb2C3 outperformed the other catalysts by attaining a photocurrent density of 4.71 mA cm−2vs. 1.23 V RHE, while WO3, WO3@Nb2C1, and WO3@Nb2C2 attained photocurrent densities of 2.15 mA cm−2, 3.48 mA cm−2, and 4.56 mA cm−2 at 1.23 V RHE, respectively. The higher photocurrents demonstrate that more charge carriers pass through the circuit for reaction with water to evolve oxygen.EIS was performed under illumination in frequencies ranging from 100 kHz to 100 mHz at a potential of 0.8 V vs. RHE, investigating the charge transfer resistance. Fig. 7b shows the Nyquist plots for WO3, WO3@Nb2C1, WO3@Nb2C2, and WO3@Nb2C3. WO3@Nb2C3 has the smallest semicircle among them; the diameter of the semicircle is directly proportional to the Rct value. The smaller the semicircle, the smaller the resistance, the faster the charge transfer at the electrode–electrolyte interface, and hence the better the catalytic activity. Solution resistance (Rs) for WO3, WO3@Nb2C1, WO3@Nb2C2, and WO3@Nb2C3 were calculated to be 29.63 Ω, 24.89 Ω, 22.99 Ω, and 21.05 Ω, respectively. Rct values for WO3, WO3@Nb2C1, WO3@Nb2C2, and WO3@Nb2C3 were determined to be 665 Ω, 589 Ω, 416 Ω, and 309 Ω, respectively. The interaction of Nb2CTx with WO3 decreases charge transfer resistance by facilitating electron transfer and reducing electron–hole recombination, thereby providing better PEC-OER activity.
Chopped light LSV was performed with a mechanical chopper at an interval of 5 seconds, alternating light ON and light OFF. In PEC water oxidation, chopped light LSV can help optimize photoelectrodes because it provides information about light harvesting efficiency, generation of photocurrents, and the dynamics of charge carriers of the catalyst. WO3@Nb2C3 outperformed the bare WO3 in chopped light LSV (Fig. 7c). These results agree with the LSV as well. WO3@Nb2C3 attained a higher photocurrent. This shows that the addition of Nb2CTx MXene to WO3 helps in better charge separation, and it has reduced the electron–hole recombination as well; therefore, a higher photocurrent can be observed for the WO3@Nb2C3 heterojunction. The difference in the dark current when the light is OFF and the photocurrent when the light is ON is of crucial importance in evaluating the efficiency of the catalyst for the evolution of oxygen through the oxidation of water. As depicted in Fig. 6c, we can observe that the current drops to zero when there is no light and rises swiftly when the light is ON. This indicates that the reaction is mainly driven by the photo-induced charge carriers and not through the applied voltage. Chopped light LSV can also provide insights to determine the photostability of the catalyst when the catalyst is consecutively exposed to light for up to many cycles. We performed 50 consecutive chopped light LSVs to evaluate the photostability of our catalysts, and the comparison of the 10th, 20th, 30th, 40th, and 50th LSV is shown in Fig. 7d and e. WO3@Nb2C3 outperformed WO3. This indicates that the WO3@Nb2C3 heterojunction has prevented degradation and photo corrosion. These results are considerable for analyzing the long-term stability of catalysts under real operating parameters.
Double layer capacitance (Cdl) was determined for WO3 and WO3@Nb2C3 by taking multiple CV cycles in non-faradaic potential range at various scan rates of 20, 40, 60, 80, and 100 mV s−1 under dark conditions to study the intrinsic electrochemical behavior of the prepared catalysts without considering the activity induced by light (Fig. S4†). Δj was plotted against the scan rate, and the slope of this plot gave the Cdl value. WO3@Nb2C3 exhibited a higher Cdl value (2.01 mF) than WO3 (1.21) mF, ECSA is directly proportional to Cdl value (Fig. 7f). The higher the Cdl value, the higher the ECSA. ECSA is determined using the following formula: Cdl/Cs, where Cs is the specific capacitance value of the electrode material. WO3@Nb2C3 possessed a higher ECSA value (40.2 cm2) than pristine WO3 (24.2 cm2). A higher value of ECSA for WO3@Nb2C3 indicates that there are more catalytic sites available on it compared to WO3 and larger surface area availability for charge transfer and charge accumulation.
Mott–Schottky (MS) analysis was conducted for WO3 and WO3@Nb2C3 heterojunction in the dark to determine the flat band potential value Vfb and donor density Nd. The Mott–Schottky equation is given below:49,50
Conclusion
In this work, composites of WO3 with Nb2CTx MXene were successfully synthesized by applying a hydrothermal and ultrasonic approach to develop a 2D/2D WO3@Nb2CTx heterojunction. The prepared catalysts were characterized by XRD, XPS, FESEM, EDX, TEM, and UV-visible spectroscopy and photoelectrochemical measurements. The photocatalytic activity of WO3 was aimed to be enhanced by developing a heterojunction with Nb2CTx MXene. Nb2CTx is a conductive material and acts as a cocatalyst. Therefore, the composite WO3@Nb2C3 results in improved charge transfer and lower electron–hole recombination, which is the most common limitation of any photocatalyst. WO3@Nb2C3 showed a photocurrent density of 4.71 mA cm−2 at 1.23 V vs. RHE. The heterojunction WO3@Nb2C3 showed enhanced photostability, lower charge transfer resistance, lower band gap (2.56 eV), lower Vfb and higher Cdl value (2.1 mF). Nb2CTx serves as a conductive bridge, which facilitates electron transport from WO3 to the external circuit, thereby minimizing energy loss and increasing the availability of electrons for water oxidation. Its integration enhances the photocatalytic behavior of WO3 by improving stability, durability, and visible light absorption while offering protection against corrosion during the oxidation process. Additionally, Nb2CTx optimizes charge transfer, increases catalytic sites, and expands surface area, demonstrating the synergistic effect of the heterojunction in advancing photoelectrochemical water oxidation. This study highlights a promising pathway for developing stable and sustainable photocatalysts for solar-driven green energy production.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Pakistan Science Foundation for financial assistance under project no. PSF-NSFC-IV/Chem/C-QAU (27), and M. M. would like to thank the Higher Education Commission of Pakistan for indigenous PhD fellowship.References
- T. H. Wondimu, A. W. Bayeh, D. M. Kabtamu, Q. Xu, P. Leung and A. A. Shah, Recent progress on tungsten oxide-based materials for the hydrogen and oxygen evolution reactions, Int. J. Hydrogen Energy, 2022, 47, 20378–20397 CrossRef CAS
.
- G. Zheng, J. Wang, H. Liu, V. Murugadoss, G. Zu, H. Che, C. Lai, H. Li, T. Ding and Q. Gao, Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting, Nanoscale, 2019, 11, 18968–18994 RSC
.
- H. Zhang, Q. Ding, D. He, H. Liu, W. Liu, Z. Li, B. Yang, X. Zhang, L. Lei and S. Jin, A p-Si/NiCoSe x core/shell nanopillar array photocathode for enhanced photoelectrochemical hydrogen production, Energy Environ. Sci., 2016, 9, 3113–3119 RSC
.
- S. Zhou, K. Chen, J. Huang, L. Wang, M. Zhang, B. Bai, H. Liu and Q. Wang, Preparation of heterometallic CoNi-MOFs-modified BiVO4: a steady photoanode for improved performance in photoelectrochemical water splitting, Appl. Catal., B, 2020, 266, 118513 CrossRef CAS
.
- S. Tokunaga, H. Kato and A. Kudo, Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties, Chem. Mater., 2001, 13, 4624–4628 CrossRef CAS
.
- N. Bao, L. Shen, T. Takata and K. Domen, Self-templated synthesis of nanoporous CdS nanostructures for highly efficient photocatalytic hydrogen production under visible light, Chem. Mater., 2008, 20, 110–117 CrossRef CAS
.
- M. Shang, W. Wang, S. Sun, L. Zhou and L. Zhang, Bi2WO6 nanocrystals with high photocatalytic activities under visible light, J. Phys. Chem. C., 2008, 112, 10407–10411 CrossRef CAS
.
- P. Dong, G. Hou, X. Xi, R. Shao and F. Dong, WO 3-based photocatalysts: morphology control, activity enhancement and multifunctional applications, Environ. Sci.: Nano, 2017, 4, 539–557 RSC
.
- J. Wen, J. Xie, X. Chen and X. Li, A review on g-C3N4-based photocatalysts, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS
.
- M. Tayebi, Z. Masoumi, B. Seo, C.-S. Lim, H.-G. Kim and B.-K. Lee, Efficient and Stable MoOX@Mo-BiVO4 Photoanodes for Photoelectrochemical Water Oxidation: Optimization and Understanding, ACS Appl. Energy Mater., 2022, 5, 11568–11580 CrossRef CAS
.
- E. Omid Najafabadi, F. Razi Astaraei, M. Tayebi, Z. Masoumi, O. Moradlou, M. M. Momeni, H.-G. Kim, F. Karimian Bahnamiri, M. Khalili and B.-K. Lee, Embedding cobalt polyoxometalate in polypyrrole shell for improved photoelectrochemical performance of BiVO4 core, Mater. Chem. Phys., 2023, 309, 128430 CrossRef CAS
.
- D. Pal, D. Mondal, D. Maity, D. De, M. K. Ganesha, A. K. Singh and G. G. Khan, Single-atomic ruthenium dispersion promoting photoelectrochemical water oxidation activity of CeOx catalysts on doped TiO2 nanorod photoanodes, J. Mater. Chem. A, 2024, 12, 3034–3045 RSC
.
- D. Pal, D. Maity, D. De, M. K. Ganesha, A. K. Singh, S. Bhaladhare and G. G. Khan, Citrate modulation of CoAl(OH)x Catalyst/Sb–TiO2 nanorods interface boosting photocarrier separation and injection for enhanced water oxidation, Int. J. Hydrogen Energy, 2024, 51, 52–65 CrossRef CAS
.
- A. Sarkar, K. Karmakar, A. K. Singh, K. Mandal and G. G. Khan, Surface functionalized H2Ti3O7 nanowires engineered for visible-light photoswitching, electrochemical water splitting, and photocatalysis, Phys. Chem. Chem. Phys., 2016, 18, 26900–26912 RSC
.
- A. Sarkar, A. K. Singh, D. Sarkar, G. G. Khan and K. Mandal, Three-Dimensional Nanoarchitecture of BiFeO3 Anchored TiO2 Nanotube Arrays for Electrochemical Energy Storage and Solar Energy Conversion, ACS Sustainable Chem. Eng., 2015, 3, 2254–2263 CrossRef CAS
.
- A. K. Singh and D. Sarkar, Enhanced Light Absorption and Charge Carrier Management in Core-Shell Fe2O3@Nickel Nanocone Photoanodes for Photoelectrochemical Water Splitting, ChemCatChem, 2019, 11, 6355–6363 CrossRef CAS
.
- A. K. Singh and D. Sarkar, A facile approach for preparing densely-packed individual p-NiO/n-Fe2O3 heterojunction nanowires for photoelectrochemical water splitting, Nanoscale, 2018, 10, 13130–13139 RSC
.
- M. Tayebi, A. Tayyebi, Z. Masoumi and B.-K. Lee, Photocorrosion suppression and photoelectrochemical (PEC) enhancement of ZnO via hybridization with graphene nanosheets, Appl. Surf. Sci., 2020, 502, 144189 CrossRef CAS
.
- K. Shubham, M. K. Ganesha, H. Hakkeem, A. M. Chandran, A. S. Mary, A. Anand, D. De, D. Sarkar, G. G. Khan and A. K. Singh, High-performance silicon-based n–i–p heterojunction photoanode for efficient photoelectrochemical water splitting: fabrication, optimization, and large-scale application, J. Mater. Chem. A, 2025, 13, 10844–10854 RSC
.
- A. Anand, A. Raj, D. Mondal, D. Maity, M. K. Ganesha, A. K. Singh, D. De and G. G. Khan, Effective Surface Engineering for Defect Passivation and Reduction of Water Oxidation Overpotential in Benchmark 2D, Adv. Funct. Mater., 2025, 35, 2417398 CrossRef CAS
.
- Z. Ni, Q. Wang, Y. Guo, H. Liu and Q. Zhang, Research Progress of Tungsten Oxide-Based Catalysts in Photocatalytic Reactions, Catalysts, 2023, 13, 579 CrossRef CAS
.
- H. Quan, Y. Gao and W. Wang, Tungsten oxide-based visible light-driven photocatalysts: crystal and electronic structures and strategies for photocatalytic efficiency enhancement, Inorg. Chem. Front., 2020, 7, 817–838 RSC
.
- H. Yang, S. Li, S. Yu, X. Yu, H. Zhao, C. Wang, D. Ping and J. Y. Zheng, Strategies for enhancing the stability of WO3 photoanodes for water splitting: A review, Chem. Eng. Sci., 2025, 302, 120894 CrossRef CAS
.
- S. S. Kalanur, I.-H. Yoo, I.-S. Cho and H. Seo, Effect of oxygen vacancies on the band edge properties of WO3 producing enhanced photocurrents, Electrochim. Acta, 2019, 296, 517–527 CrossRef CAS
.
- Y. Li, Z. Tang, J. Zhang and Z. Zhang, Fabrication of vertical orthorhombic/hexagonal tungsten oxide phase junction with high photocatalytic performance, Appl. Catal., B, 2017, 207, 207–217 CrossRef CAS
.
- M. Tayebi and B.-K. Lee, The effects of W/Mo-co-doped BiVO4 photoanodes for improving photoelectrochemical water splitting performance, Catal. Today, 2021, 361, 183–190 CrossRef CAS
.
- M. Tayebi, Z. Masoumi and B.-K. Lee, Ultrasonically prepared photocatalyst of W/WO3 nanoplates with WS2 nanosheets as 2D material for improving photoelectrochemical water splitting, Ultrason. Sonochem., 2021, 70, 105339 CrossRef CAS PubMed
.
- M. Tayebi, Z. Masoumi, M. Kolaei, A. Tayyebi, M. Tayebi, B. Seo, C.-S. Lim, H.-G. Kim and B.-K. Lee, Highly efficient and stable WO3/MoS2-MoOX photoanode for photoelectrochemical hydrogen production; a collaborative approach of facet engineering and P-N junction, Chem. Eng. J., 2022, 446, 136830 CrossRef CAS
.
- C. Zhou, X. Zhao, Y. Xiong, Y. Tang, X. Ma, Q. Tao, C. Sun and W. Xu, A review of etching methods of MXene and applications of MXene conductive hydrogels, Eur. Polym. J., 2022, 167, 111063 CrossRef CAS
.
- M. Murtaza, L. Saleem, W. A. Shah, I. Ahmad, H. Alawadhi and A. Waseem, Delaminated Vanadium Carbide MXene Supported Two-Dimensional (2D) CoNH2BDC MOF Hybrid for Enhanced Water Splitting, Ind. Eng. Chem. Res., 2024, 63, 20915–20924 CrossRef CAS
.
- P. Chen, J. Ye, H. Wang, L. Ouyang and M. Zhu, Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting, J. Alloys Compd., 2021, 883, 160833 CrossRef CAS
.
- A. Sreedhar, Q. T. H. Ta and J.-S. Noh, 2D Ti3C2 MXene interfaced ZnO/WO3 thin film nanostructures towards improved photoelectrochemical water splitting, J. Electroanal. Chem., 2023, 940, 117509 CrossRef CAS
.
- X. Pang, S. Xue, T. Zhou, M. Qiao, H. Li, X. Liu, Q. Xu, G. Liu and W. Lei, Noble Metal-Free Heterojunction of Ultrathin Ti3C2 MXene/WO3 for Boosted Visible-Light-Driven Photoreactivity, Adv. Sustainable Syst., 2023, 7, 2100507 CrossRef CAS
.
- D. Jin Lee, G. Mohan Kumar, S. Sekar, H. Chang Jeon, D. Young Kim and P. Ilanchezhiyan, Ultrasonic processing of WO3 nanosheets integrated Ti3C2 MXene 2D-2D based heterojunctions with synergistic effects for enhanced water splitting and environmental remediation, Ultrason. Sonochem., 2023, 101, 106681 CrossRef CAS PubMed
.
- C. Peng, Z. Kuai, T. Zeng, Y. Yu, Z. Li, J. Zuo, S. Chen, S. Pan and L. Li, WO3 Nanorods/MXene composite as high performance electrode for supercapacitors, J. Alloys Compd., 2019, 810, 151928 CrossRef CAS
.
- J. Azadmanjiri, J. Regner, J. Sturala and Z. k. Sofer, Decoding Niobium Carbide MXene Dual-Functional Photoactive Cathode in Photoenhanced Hybrid Zinc-Ion Capacitor, ACS Mater. Lett., 2024, 6, 1338–1346 CrossRef CAS PubMed
.
- I. Székely, G. Kovács, L. Baia, V. Danciu and Z. Pap, Synthesis of Shape-Tailored WO3 Micro-/Nanocrystals and the Photocatalytic Activity of WO3/TiO2 Composites, Materials, 2016, 9, 258 CrossRef PubMed
.
- M. Naguib, J. Halim, J. Lu, K. M. Cook, L. Hultman, Y. Gogotsi and M. W. Barsoum, New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries, J. Am. Chem. Soc., 2013, 135, 15966–15969 CrossRef CAS PubMed
.
- G. Guan and F. Guo, A Review of Nb2CTx MXene: Synthesis, Properties and Applications, Batteries, 2023, 9, 235 CrossRef CAS
.
- M. Liu, D. Zhang, B. Liu, C. Tian, B. Zhao, Y. Wang, Y. Wang, Y. Hu, L. Kong, D. Luo and Z. Chen, Boosting the alkali metal ions storage performance of layered Nb2C with a molecular welding strategy, Nano Energy, 2022, 103, 107795 CrossRef CAS
.
- P. Wang, S. Guo, Y. Zhao, Z. Hu, Y. Tang, L. Zhou, T. Li, H.-Y. Li and H. Liu, WO3 nanoparticles supported by Nb2CTx MXene for superior acetone detection under high humidity, Sens. Actuators, B, 2024, 398, 134710 CrossRef CAS
.
- M. Murtaza, K. Farooq, W. A. Shah and A. Waseem, CoBDC MOF derived CoP/C couple with 2D Nb2CTx MXene as an efficient bifunctional catalyst for water splitting, Fuel, 2025, 394, 135095 CrossRef CAS
.
- T. Paik, M. Cargnello, T. R. Gordon, S. Zhang, H. Yun, J. D. Lee, H. Y. Woo, S. J. Oh, C. R. Kagan, P. Fornasiero and C. B. Murray, Photocatalytic Hydrogen Evolution from Substoichiometric Colloidal WO3–x Nanowires, ACS Energy Lett., 2018, 3, 1904–1910 CrossRef CAS
.
- Y. Tan, Z. Zhu, X. Zhang, J. Zhang, Y. Zhou, H. Li, H. Qin, Y. Bo and Z. Pan, Nb4C3Tx (MXene) as a new stable catalyst for the hydrogen evolution reaction, Int. J. Hydrogen Energy, 2021, 46, 1955–1966 CrossRef CAS
.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes), Appl. Surf. Sci., 2016, 362, 406–417 CrossRef CAS
.
- K. Farooq, M. Murtaza, Z. Yang, A. Waseem, Y. Zhu and Y. Xia, MXene boosted MOF-derived cobalt sulfide/carbon nanocomposites as efficient bifunctional electrocatalysts for OER and HER, Nanoscale Adv., 2024, 6, 3169–3180 RSC
.
- G. Bharath, S. Palanisamy, T. Sivaranjani, A. Karthigeyan, S. Rajakarthihan and F. Banat, Advancing nanoarchitectures of 2D WO3/MXene photoanode for enhanced photoelectrocatalytic oxidation of phenol and arsenic in synthetic wastewater, Environ. Res., 2024, 260, 119676 CrossRef PubMed
.
- S. Noureen, J. Sultana, M. Rani, I. Ahmad, W. A. Shah, M. M. Khan and A. Waseem, CeNiO3/rGO composite as an efficient photocatalyst for the removal of environmental pollutants, Fullerenes, Nanotubes Carbon Nanostruct., 2025, 33, 209–223 CrossRef CAS
.
- K. Sivula, Mott–Schottky Analysis of Photoelectrodes: Sanity Checks Are Needed, ACS Energy Lett., 2021, 6, 2549–2551 CrossRef CAS
.
- E. Usman, M. Barzgar Vishlaghi, A. Kahraman, N. Solati and S. Kaya, Modifying the Electron-Trapping Process at the BiVO4 Surface States via the TiO2 Overlayer for Enhanced Water Oxidation, ACS Appl. Mater. Interfaces, 2021, 13, 60602–60611 CrossRef CAS PubMed
.
- S. S. Kalanur, R. Singh and H. Seo, Enhanced solar water splitting of an ideally doped and work function tuned {002} oriented one-dimensional WO3 with nanoscale surface charge mapping insights, Appl. Catal., B, 2021, 295, 120269 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5na00345h |
| This journal is © The Royal Society of Chemistry 2025 |