Open Access Article
Open Access ArticlePreparation and characterization of nano guar gum/BF3/Fe3O4 as a novel bio-based Lewis acid catalyst for the one-pot green synthesis of pyrimido benzothiazoles under solvent-free conditions
Motahare
Hajihasani Bafghi
a,
Abdolhamid
Bamoniri
 *a and
Bi Bi
Fatemeh Mirjalili
*a and
Bi Bi
Fatemeh Mirjalili
 b
b
aDepartment of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, I. R. Iran. E-mail: bamoniri/kashanu.ac.ir
bDepartment of Chemistry, College of Science, Yazd University, Yazd, I. R. Iran. E-mail: fmirjalili/yazd.ac.ir
First published on 27th October 2025
Abstract
Nano guar gum/BF3/Fe3O4 is a bio-based nanocatalyst that was prepared, characterized and applied in one-pot three-component reactions of various aldehydes, 2-aminobenzothiazole and ethyl acetoacetate for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives at 80 °C under solvent-free conditions. The structure and properties of the heterogeneous bifunctional Lewis acid–base catalyst were studied via FT-IR, FESEM, TGA, EDS-MAP, XRD, VSM, and BET. Some unique characteristics of the magnetic nanocatalysts enabled the development of the catalytic activity using a simple, efficient, green and eco-friendly protocol. The catalyst was reused several times without a loss of activity.
Introduction
Guar gum is a novel agrochemical processed from the endosperm of the cluster bean (Cyamopsis tetragonoloba). It is largely used in the form of guar gum powder as an additive in food, pharmaceuticals, paper, textiles, explosives, oil well drilling, and the cosmetics industry.1,2 Industrial applications of guar gum are possible because of its ability to form hydrogen bonds with water molecules. Thus, it is chiefly used as a thickener and stabilizer.3,4Increasing awareness of green chemistry and other biological processes has inspired the development of an eco-friendly approach for the synthesis of nanoparticles because of their advantages, such as facile synthesis, cost-effectiveness, and compatibility with biomedical applications as well as large-scale commercial production.5 In recent years, many interesting methods have been applied for the green preparation of nano-sized magnetic particles.6
The modern focus on reducing pollution in organic reactions has led to the rise of heterogeneous catalytic systems as effective methods for waste reduction.7,8 Catalysts are now being immobilized on various supports, with iron oxide magnetic nanoparticles (Fe3O4 MNPs) playing a crucial role owing to their low toxicity and superparamagnetism.9,10 The combination of organic and inorganic materials at the nanoscale is increasingly used for its catalytic potential. Immobilizing catalysts on Fe3O4 MNPs enables easy separation without cumbersome methods such as centrifugation or filtration. These magnetic nanoparticles have been widely used in various organic transformations for their high activity levels.11 The 4H-pyrimido[2,1-b]benzothiazole derivatives are compounds used in the preparation of drugs.12,13 Recent studies have shown that these compounds can function as anti-tumor, anti-inflammatory, anti-bacterial and anti-fungal materials.14–17 Therefore, owing to the significant biological and therapeutic relevance of 4H-pyrimido[2,1-b]benzothiazole, various synthetic strategies, including one-step and multi-step methods, have been reported.18 One of the most attractive methods for the synthesis of 4H-pyrimido[2,1-b]benzothiazole is based on multi-component reactions (MCRs).19 The synthesis of 4H-pyrimido[2,1-b]benzothiazole is a three-component condensation reaction between aldehyde, β-ketoester and 2-aminobenzothiazole.20,21 Some of the catalysts that have previously been used for the synthesis of these products are Fe3O4@nano-cellulose-TiCl,22 nano-kaolin/Ti4+/Fe3O4,23 nano-Fe3O4@SiO2-TiCl3,24 Fe3O4@nano-cellulose/Cu(II),25 FNAOSiPPEA/Cu(II),26 and Fe3O4@nano-dextrin-OPO3H2.27
Despite the remarkable achievements for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives, there are limitations for some of these catalysts, such as inefficient separation of the catalyst from reaction mixtures, non-recyclability, and environmental limitations. Furthermore, this reaction was performed without a catalyst under harsh reaction conditions.28
Herein, we demonstrate a simple, environmentally friendly, and cost-effective method to prepare highly stable dispersions of a magnetic nanoparticle catalyst. The catalyst is based on a Lewis acid derived from a natural biopolymer and is applied for the synthesis of pyrimido[2,1-b]benzothiazoles.
Results and discussion
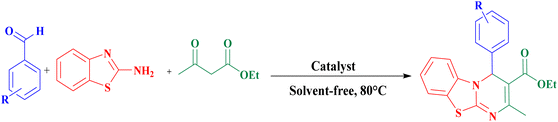
We report the synthesis and characterization of nano guar gum/BF3/Fe3O4 for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles via the condensation reaction of ethyl acetoacetate, aromatic aldehydes, and 2-amino benzothiazole (Scheme 1). | ||
| Scheme 1 Preparation of nano guar gum/BF3/Fe3O4/for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles. | ||
The structure of nano guar gum/BF3/Fe3O4 as a bio-based Lewis acid catalyst, was confirmed by Fourier transform infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), thermogravimetric analysis (TGA), energy-dispersive X-ray spectroscopy (EDS-map), Brunauer–Emmett–Teller (BET) surface area measurement, and vibrating sample magnetometry (VSM).
FT-IR spectroscopy of nano guar gum/BF3/Fe3O4
The FT-IR spectra of guar gum, nano guar gum/BF3, and nano guar gum/BF3/Fe3O4 are shown in Fig. 1. The FT-IR spectrum of guar gum (Fig. 1a) shows distinct peaks at 3389 cm−1, 2942 cm−1, and 1154 cm−1, which are related to O–H, C–H, and C–O vibrational stretching, respectively. In the nano guar gum/BF3/Fe3O4 spectrum (Fig. 1c), a distinct peak at 568 cm−1 is attributed to the Fe–O stretching vibration. In addition, the broad peak at 3400 cm−1 is attributed to the stretching vibration of O–H group.FESEM and particle size distribution histogram of guar gum and nano guar gum/BF3/Fe3O4
The particle size of guar gum (Fig. 2a) and nano guar gum/BF3/Fe3O4 (Fig. 2b) was studied using field emission scanning electron microscopy (FESEM) and particle size distribution histogram and was found to be less than 100 nm (Fig. 2 and 3).TGA of nano guar gum/BF3/Fe3O4
Fig. 4 shows the TGA and DTA curves of guar gum, and nano guar gum/BF3/Fe3O4.The thermal gravimetric analysis pattern of guar gum was detected by heating from 50 °C to 450 °C (Fig. 4a). Weight loss (10%) occurred at temperatures between 50 and 100 °C, which is related to the removal of guar gum moisture. The main weight loss (55%) was observed in the range of 225–310 °C, due to the burning of guar gum. The next stage of weight loss (33.5%) occurred in the temperature range of 310–450 °C. The yield of guar gum char at 450 °C is 1.5% of the original weight. The nanocatalyst showed a small initial weight loss at a temperature lower than 100 °C due to the removal of absorbed water and other organic solvents. At temperatures higher than 100 °C (180–300 °C), the highest weight loss is observed in the TGA curve, which was probably due to the decomposition of guar gum and BF3 from the catalyst.
XRD of nano guar gum/BF3/Fe3O4
The XRD patterns of guar gum (Fig. 5a), Fe3O4 NPs (Fig. 5b), and nano guar gum/BF3/Fe3O4 (Fig. 5c) are shown in Fig. 4. The signals in 2θ equal to 22° and 16° are related to the guar gum and, several peaks appeared at 2θ = 31°, 35°, 43°, 53°, 56°, and 61°, indicating that the original Fe3O4 crystalline structure was not destroyed.VSM of nano guar gum/BF3/Fe3O4
The magnetic properties of the Fe3O4 nanoparticles (Fig. 6a) and nano guar gum/BF3/Fe3O4 (Fig. 6b) were measured using a vibrating sample magnetometer (VSM) at a temperature of 300 K. The obtained results are shown in the form of magnetization curves in Fig. 6. According to these curves, the value of the specific saturation magnetization (Ms) of the Fe3O4 nanoparticles is about 47 emu g−1, which is reduced to 40 emu g−1 during the process of coating with guar gum and then connecting BF3 on the surface of the guar gum. Despite the use of Lewis acid BF3, the magnetism of the catalyst remains very high, and it can be easily separated from the reaction medium using an external magnet (Fig. 6). In addition, the samples exhibit zero coercivity and magnetic hysteresis, indicating their superparamagnetic behaviour at room temperature.EDX and EDS-map of nano guar gum/BF3/Fe3O4
The elemental composition of the nano guar gum/BF3/Fe3O4 catalyst was determined by EDX. As shown in Fig. 7, the presence of Fe, O, C, B, and F signals prove the catalyst structure. The percentage composition of Fe, C, O, F, and B elements is 42.35, 26.4, 18.52, 9.52, and 3.21% respectively. According to the results of the EDS-mapping analysis in Fig. 8, these elements are homogeneously distributed on the surface of the nanocatalyst.Inductively coupled plasma (ICP) spectroscopy was used to determine the iron content in the nano-guar gum/BF3/Fe3O4 catalyst, which was 53% before and 44% after the reaction.
BET measurements of nano guar gum/BF3/Fe3O4
The BET (Brunauer–Emmett–Teller) surface area of the prepared nanocatalyst was obtained by nitrogen adsorption and desorption measurements (Fig. 9). The N2 isotherms related to the type IV isotherm in the IUPAC classification have shown H3 type rings, which can indicate the existence of mesopores and also have non-hard pores. As shown in Table 1, according to BJH (Barrett–Joyner–Halenda) model analysis, the pore diameters were 30.466 m2 g−1, 0.1217 cm3 g−1, and 15.511 nm, respectively. | ||
| Fig. 9 N2 adsorption (blue line)-desorption (red line) isotherm and corresponding diagrams showing the pore size distributions (BJH, BET, Langmuir, t-plot). | ||
| BET plot | ||
|---|---|---|
| V m | 6.9997 | [cm3 (STP) g−1] |
| a s,BET | 30.466 | [m2 g−1] |
| C | 67.264 | |
| Total pore volume (p/p0 = 0.990) | 0.1181 | [cm3 g−1] |
| Mean pore diameter | 15.511 | [nm] |
| Langmuir plot | ||
|---|---|---|
| V m | 7.5497 | [cm3 (STP) g−1] |
| a s,Lang | 32.86 | [m2 g−1] |
| B | 0.875 | |
| t plot | ||
|---|---|---|
| Plot data | Adsorption branch | |
| a 1 | 27.755 | [m2 g−1] |
| V 1 | 0 | [cm3 g−1] |
| BJH plot | ||
|---|---|---|
| Plot data | Adsorption branch | |
| V p | 0.1217 | [cm3 g−1] |
| r p,peak (area) | 4.61 | [nm] |
| a p | 42.196 | [m2 g−1] |
Catalyst activity of nano guar gum/BF3/Fe3O4
The optimization study (Table 2) clearly demonstrates that the amount of catalyst, reaction temperature, and solvent play a crucial role in determining the yield and reaction time. Increasing the catalyst loading from 0.005 g to 0.02 g resulted in a significant increase in product yield and a remarkable reduction in reaction time, highlighting the importance of a higher number of accessible Lewis acidic sites in promoting the reaction. Likewise, increasing the temperature from 35 to 80 °C markedly enhanced the reaction efficiency, and under optimized conditions (0.02 g catalyst at 80 °C under solvent-free conditions), the highest yield of 96% was obtained within only 1.4 h. Examination of the solvent effects revealed that the use of ethanol or water provided inferior results compared to the solvent-free system. For instance, only a 42% yield was obtained in ethanol at room temperature, and even at elevated temperatures, the yield remained lower than that under solvent-free conditions. These findings emphasize that solvent-free conditions offer superior efficiency due to the increased effective collisions between reactant molecules and the absence of dilution of the catalytically active sites.| Entry | Conditions | Time (h) | Yieldb (%) |
|---|---|---|---|
| Solvent, temp. (°C), catalyst (g) | |||
| a Conditions: benzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), and 2-amino benzothiazole (1 mmol), solvent free. b Isolated yield. | |||
| 1 | —, r.t., nano guar gum/BF3/Fe3O4 (0.005) | 5 | 53 |
| 2 | —, r.t., nano guar gum/BF3/Fe3O4 (0.01) | 5 | 68 |
| 3 | —, r.t., nano guar gum/BF3/Fe3O4 (0.015) | 5.30 | 68 |
| 4 | —, r.t., nano guar gum/BF3/Fe3O4 (0.02) | 3.30 | 86 |
| 5 | —, 35, nano guar gum/BF3/Fe3O4 (0.02) | 3.30 | 85 |
| 6 | —, 55, nano guar gum/BF3/Fe3O4 (0.02) | 3 | 87 |
| 7 | —, 65, nano guar gum/BF3/Fe3O4 (0.02) | 2.30 | 87 |
| 8 | —, 80, nano guar gum/BF3/Fe3O4 (0.005) | 3 | 86 |
| 9 | —, 80, nano guar gum/BF3/Fe3O4 (0.01) | 2.30 | 87 |
| 10 | —, 80, nano guar gum/BF 3 /Fe 3 O 4 (0.02) | 1.40 | 96 |
| 11 | —, 80, nano guar gum/BF3/Fe3O4 (0.03) | 2 | 88 |
| 12 | —, 80, — | 4.30 | 51 |
| 13 | EtOH, r.t., nano guar gum/BF3/Fe3O4 (0.02) | 4 | 79 |
| 14 | EtOH, 80, nano guar gum/BF3/Fe3O4 (0.02) | 3.30 | 83 |
| 15 | H2O, 80, nano guar gum/BF3/Fe3O4 (0.02) | 5 | 41 |
| 16 | —, 80, nano guar gum/BF3 (0.02) | 2.30 | 80 |
| 17 | —, 80, nano guar gum (0.02) | 4.5 | 57 |
| 18 | —, 80, BF3 (0.02) | 1.5 | 70 |
| 19 | —, 80, Fe3O4 (0.02) | 3.50 | 36 |
To evaluate the contribution of each component of the catalyst, control experiments were conducted using guar gum or BF3 alone, which afforded only 57% and 51% yield, respectively. This comparison clearly indicates a synergistic effect among the three constituents, namely guar gum, BF3, and Fe3O4. In this system, guar gum acts as a natural support and stabilizing matrix for the nanoparticles, BF3 provides the necessary Lewis acid centers for carbonyl activation, and Fe3O4 increases the active surface area and enables facile magnetic separation of the catalyst. Altogether, the combination of these three components significantly enhanced the catalytic performance and led to increased yields in the target reaction.
The catalytic performance of nano guar gum/BF3/Fe3O4 was further examined in the synthesis of 4H-pyrimido[2,1-b]benzothiazoles using various substituted aromatic aldehydes, and the results are summarized in Table 3.
| Entry | R | Nano guar gum | Nano guar gum/BF3 | Nano guar gum/BF3/Fe3O4 | mp. (ref.) | |||
|---|---|---|---|---|---|---|---|---|
| Time (min) | Yieldb (%) | Time (min) | Yieldb (%) | Time (min) | Yieldb (%) | |||
| a Conditions: aldehyde (1 mmol), ethyl acetoacetate (1 mmol), 2-amino benzothiazole (1 mmol), 80 °C, Solvent free, catalyst (0.02 g). b Isolated yield. | ||||||||
| 1 | H | 270 | 57 | 150 | 80 | 100 | 96 | 177–180 (ref. 22) |
| 2 | 4-Cl | 195 | 80 | 80 | 85 | 65 | 90 | 89–91 (ref. 29) |
| 3 | 2,4-(Cl)2 | 240 | 71 | 75 | 83 | 70 | 89 | 132–134 (ref. 30) |
| 4 | 4-Br | 120 | 84 | 100 | 90 | 65 | 95 | 112–115 (ref. 31) |
| 5 | 2-NO2 | 135 | 69 | 60 | 79 | 55 | 85 | 121–123 (ref. 32) |
| 6 | 3-NO2 | 120 | 86 | 60 | 81 | 45 | 93 | 223–225 (ref. 33) |
| 7 | 4-NO2 | 100 | 86 | 60 | 78 | 60 | 96 | 172–174 (ref. 34) |
| 8 | 3-OH | 260 | 58 | 120 | 75 | 90 | 86 | 260–262 (ref. 27) |
| 9 | 4-OH | 200 | 61 | 120 | 80 | 100 | 88 | 209–212 (ref. 24) |
| 10 | 2-OEt | 230 | 66 | 105 | 71 | 90 | 85 | 173–176 (ref. 25) |
| 11 | 2,4-(OMe)2 | 250 | 75 | 90 | 78 | 75 | 87 | 165–167 (ref. 32) |
| 12 | 2-Hydroxynaphthalen | 180 | 68 | 120 | 83 | 105 | 89 | 220–222 |
The electronic nature of the substituents on the aldehydes strongly influenced the reaction outcome. Aromatic aldehydes bearing electron-withdrawing groups, such as nitro substituents at the ortho, meta, or para positions, afforded higher yields in shorter times. Notably, 2-nitrobenzaldehyde delivered the product in 92% yield within only 60 min, which was attributed to the increased electrophilicity of the carbonyl carbon, facilitating the initial Knoevenagel condensation step. Conversely, aldehydes containing electron-donating groups, such as –OCH3 or –OH, exhibited lower reactivity and produced lower yields (75% and 77%, respectively). This reduction in efficiency may be ascribed to the decreased electrophilicity of the carbonyl group as well as possible steric or hydrogen-bonding interactions that hinder the approach of 2-aminobenzothiazole to the aldehyde carbonyl.
In addition to electronic effects, steric factors also played an important role. Substrates with bulky or sterically hindered substituents, such as 2,4-dichlorobenzaldehyde and 2-hydroxynaphthaldehyde, required longer reaction times and produced relatively lower yields, indicating that steric hindrance restricts the access of the reactants to the activated carbonyl center. Overall, the data clearly demonstrate that the designed nanocatalyst accelerates the synthesis of 4H-pyrimido[2,1-b]benzothiazoles and maintains high efficiency across a broad range of substrates, with yields that are strongly correlated with the electronic and steric properties of the substituents.
To compare the efficiency of this magnetite bio-based nano-catalyst with other catalysts for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives, a summary of the results was collected in Table 4. The reaction efficiency of this catalyst is greater than that of other catalysts, and the reaction time is shorter than that of others.
| Entry | Conditions | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|
| Catalyst, solvent, temp. (°C) | ||||
| 1 | Nano-kaolin/Ti4+/Fe3O4, —, 100 °C | 1.50 | 95 | 23 |
| 2 | Nano-Fe3O4@SiO2-TiCl3, —, 100 °C | 0.50 | 90 | 24 |
| 3 | FNAOSiPPEA/Cu(II) (0.04 g), —, 100 °C | 1 | 97 | 26 |
| 4 | Fe3O4@NCs/Sb(V) (0.03), —, 90 °C | 0.50 | 98 | 34 |
| 5 | Nano guar gum/BF3/Fe3O4 (0.02), —, 80 °C | 1.40 | 96 | This work |
Reusability of nano guar gum/BF3/Fe3O4
To confirm the recyclability of the nano catalyst, after the completion of the reaction, the catalyst can be separated from the reaction mixture with a magnet, and after washing with chloroform (CHCl3) and drying at ambient temperature, it can be reused for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles. Therefore, the reusability of the catalyst for the model reaction was evaluated for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles (Fig. 10). | ||
| Fig. 10 Reusability of nano guar gum/BF3/Fe3O4 for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles. | ||
Proposed mechanism for synthesis of 4H-pyrimido[2,1-b]benzothiazoles
The synthesis of 4H-pyrimido[2,1-b]benzothiazoles proceeds via a multicomponent reaction involving aromatic aldehydes, ethyl acetoacetate, and 2-aminobenzothiazole in the presence of a boron-based catalyst. Initially, a Knoevenagel condensation between the aldehyde and ethyl acetoacetate affords an α,β-unsaturated intermediate, facilitated by activation of the carbonyl group through hydrogen bonding interactions with the boron catalyst. This activated olefin undergoes a Michael-type addition with the nucleophilic amino group of 2-aminobenzothiazole, followed by intramolecular cyclization and proton transfer. Subsequent dehydration results in the formation of the fused heterocyclic 4H-pyrimido[2,1-b]benzothiazole framework. The boron-based catalyst plays a key role in promoting enolate formation, stabilizing transition states, and enhancing the rate and selectivity of the reaction (Scheme 2).Experimental section
Materials and methods
Chemicals were purchased from Merck, Fluka, and Aldrich Chemical Companies. 1H NMR and 13C NMR spectra were recorded at 400 and 100 MHz, respectively. Fourier transform infrared (FT-IR) spectroscopy measurements (in KBr pellets or ATR) were recorded on a Bruker spectrometer. Melting points were determined on a Büchi B-540 apparatus. The X-ray diffraction (XRD) pattern was obtained by a Philips Xpert MPD diffractometer equipped with a Cu Kα anode (k = 1.54 Å) in the 2θ range from 10 to 80°.A Mira 3-XMU was used for the field emission scanning electron microscopy (FESEM). Vibrating sample magnetometry (VSM) measurements were obtained using a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co. Kashan Kavir, Iran). Energy-dispersive X-ray spectroscopy (EDS) of the nanocatalyst was measured by an EDS instrument and Phenom pro X. The EDX-MAP micrographs were obtained on the MIRA II detector SAMX (France). Thermal gravimetric analysis (TGA) was conducted using the “STA 504” instrument. A BELSORP MINI II nitrogen adsorption apparatus (Japan) was used for recording Brunauer–Emmett–Teller (BET) surface area measurements of the nanocatalyst at 77 K.
Purification of the nano guar gum
White guar gum powder is prepared in different processes.35 According to the results of FESEM, the size of guar gum particles is less than 50 nm. Therefore, to remove possible contamination and impurities, 5 g of nano guar gum was treated with 20 mL of EtOH at 60 °C for 4 h under reflux conditions. To remove possible impurities present in the purchased guar gum, it was washed several times with ethanol before use. Subsequently, the nano guar gum was filtered and washed with distilled water, and the yield was 99%.Preparation of Fe3O4
In a 250 mL flask, 100 mL of 0.05 M acetic acid (CH3COOH) was added. After that, FeCl3·6H2O (3.51 g, 13 mmol) and FeCl2·4H2O (1.29 g, 6.5 mmol) were added and the solution was stirred for 6 h at 80 °C. Then, 8 mL of NH4OH (25%), was added dropwise, and the solution was stirred for 45 min. The precipitated brown products were isolated from the solution by a magnet, washed 3 times with distilled water, and dried in an oven at 80 °C for 4 h. Fe3O4 was prepared using the co-precipitation method.Synthesis of nano guar gum/BF3/Fe3O4
In a 100 mL flask, 1 g of nano guar gum/BF3 catalyst is added to the Fe3O4 (0.3 g) solution in 20 mL of dichloromethane, which is dispersed by ultrasonic waves, and the final mixture is vigorously stirred by a mechanical stirrer for 6 h at 60 °C. Then the prepared catalyst nano guar gum/BF3/Fe3O4 is separated from the reaction mixture by an external magnet, washed with dichloromethane, and dried.General synthesis of 4H-pyrimido[2,1-b]benzothiazoles
For the synthesis of 4H-pyrimido[2,1-b]benzothiazoles, in a 50 mL round-bottom flask, 2-aminobenzothiazole (1 mmol), aldehyde (1 mmol), ethyl acetoacetate (1 mmol), and nano-Fe3O4/guar gum/BF3 (0.02 g) were mixed under solvent-free conditions. The reaction mixture was stirred at 80 °C for the durations specified in Table 2. After the end of the reaction (TLC), ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane 6
n-hexane 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 the mixture was dissolved in hot ethanol (5 mL), and the catalyst was separated by an external magnet. Then, by the dropwise addition of water (1 mL) to the reaction mixture, the precipitates of the product appeared as a pure solid in high yield.
3 the mixture was dissolved in hot ethanol (5 mL), and the catalyst was separated by an external magnet. Then, by the dropwise addition of water (1 mL) to the reaction mixture, the precipitates of the product appeared as a pure solid in high yield.
Conclusion
A magnetite guar gum-based nanocatalyst was prepared, characterized, and used for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles. The prepared nano guar gum/BF3/Fe3O4 shows high catalytic activity and good reusability. This method is non-toxic and biodegradable, and it may be used to prepare other biopolymer-based nanocatalysts for more interesting reactions.Conflicts of interest
There are no conflicts to declare.Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5na00201j.Acknowledgements
The Research Council of University of Kashan and Yazd University is gratefully acknowledged for the financial support for this work.References
- J. Anandan and S. Sivasankar, Ultrason. Sonochem., 2018, 40, 1–10 Search PubMed.
- S. Pandey and S. B. Mishra, Carbohydr. Polym., 2014, 113, 525 CrossRef CAS PubMed.
- T. Baran, N. Y. Baran and A. Mentes, Int. J. Biol. Macromol., 2019, 132, 1147–1154 CrossRef CAS PubMed.
- E. Abdel-Halim, M. El-Rafie and S. S. Al-Deyab, Carbohydr. Polym., 2011, 85, 692–697 CrossRef CAS.
- M.-N. Chen, L.-P. Mo, Z.-S. Cui and Z.-H. Zhang, Curr. Opin. Green Sustainable Chem., 2019, 15, 27–37 CrossRef.
- S. B. Singh and P. K. Tandon, J. Energy Chem. Eng., 2014, 2, 106–115 Search PubMed.
- J. Safari and L. Javadian, Iran. J. Catal., 2016, 6, 58–64 Search PubMed.
- F. Kiani, D. Elhamifar and S. Kargar, Nanoscale Adv., 2025, 7, 1552–1560 RSC.
- S. M. R. Mousavi and K. Rad-Moghadam, Nanoscale Adv., 2025, 7, 1901–1913 RSC.
- A. O. Moghanlou, M. Pourshahi, S. Atabak and N. M. Tarighi, Iran. J. Catal., 2023, 13, 187–192 CAS.
- F. Dadvar and D. Elhamifar, Nanoscale Adv., 2024, 6, 5398 RSC.
- Y. Yu, W.-F. Lu, Z.-J. Yang, N. Wang and X.-Q. Yu, Bioorg. Chem., 2021, 107, 104534 CrossRef PubMed.
- Y. Yu, W. Zhang, Q.-T. Gong, Y.-H. Liu, Z.-J. Yang, W.-X. He, N. Wang and X.-Q. Yu, J. Biotechnol., 2020, 324, 91–98 CrossRef PubMed.
- A. B. Atar, Y. S. Jeong and Y. T. Jeong, Tetrahedron, 2014, 70, 5207–5213 CrossRef.
- P. K. Sharma, A. Amin and M. Kumar, Open Med. Chem. J., 2020, 1, 14 Search PubMed.
- M. N. Bhoi, M. A. Borad, D. J. Jethava, P. T. Acharya, E. A. Pithawala, C. N. Patel, H. A. Pandya and H. D. Patel, Eur. J. Med. Chem., 2019, 177, 12–31 CrossRef PubMed.
- M. R. Anizadeh, M. A. Zolfigol, M. Yarie, M. Torabi and S. Azizian, Res. Chem. Intermed., 2020, 46, 3945–3960 CrossRef.
- B. Laha, A. R. Tiwari, E. Gravel, E. Doris and I. N. Namboothiri, Org. Biomol. Chem., 2024, 22, 1346 RSC.
- R. Talaei and A. Olyaei, Iran. J. Catal., 2016, 6, 339–343 Search PubMed.
- M. N. Bhoi, M. A. Borad, A. P. Solanki and H. D. Patel, Phosphorus, Sulfur Silicon Relat. Elem., 2023, 198, 822–835 CrossRef.
- P. K. Sahu, P. K. Sahu, Y. Sharma and D. D. Agarwal, J. Heterocycl. Chem., 2014, 51, 1193–1198 CrossRef.
- S. Azad and B. B. F. Mirjalili, RSC Adv., 2016, 6, 96928–96934 RSC.
- B. B. F. Mirjalili and R. Soltani, RSC Adv., 2019, 9, 18720–18727 RSC.
- S. A. Fazeli-Attar and B. B. F. Mirjalili, Res. Chem. Intermed., 2018, 44, 6419–6430 CrossRef.
- N. Safajoo, B. B. F. Mirjalili and A. Bamoniri, RSC Adv., 2019, 9, 1278–1283 RSC.
- D. Mallah and B. B. F. Mirjalili, BMC Chem., 2022, 16, 45 CrossRef PubMed.
- A. Dehghani Tafti, B. B. F. Mirjalili, N. Salehi and A. Bamoniri, J. Iran. Chem. Soc., 2022, 19, 4377–4388 CrossRef.
- Z. Moghadasi, A. N. Fajer, A. M. Abudken and H. A. Al-Bahrani, Nanomater. Chem., 2023, 1(1), 24–31 Search PubMed.
- S. S. Gong, R. Kong, C. Zheng, C. Fan, C. Wang, D. Z. Yang, Z. Z. Chen, S. Duo, S. Pu and Q. Sun, J. Mater. Chem., 2021, 9, 10029–10036 Search PubMed.
- K. Khazenipour, F. Moeinpour and F. S. Mohseni-Shahri, J. Chin. Chem. Soc., 2021, 68, 121–130 CrossRef.
- A. R. Moosavi-Zare, H. Goudarziafshar and P. Fashi, Res. Chem. Intermed., 2020, 46, 5567 CrossRef.
- B. B. F. Mirjalili and F. Aref, Res. Chem. Intermed., 2018, 44, 4519–4531 CrossRef.
- S. Singh and J. Lal, SN. Appl. Sci., 2020, 2, 1–9 Search PubMed.
- S. S. Hosseinikhah and B. B. F. Mirjalili, Polycyclic Aromat. Compd., 2022, 42, 1013–1022 CrossRef.
- G. Sharma, S. Sharma, A. Kumar, H. Ala'a, M. Naushad, A. A. Ghfar, G. T. Mola and F. J. Stadler, Carbohydr. Polym., 2018, 199, 534–545 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |