Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical synthesis of NdxCo1−xFe2O4 hybrid nanoparticles for permanent magnet applications: structural, magnetic and electrical properties
Saleh M.
Matar
a,
Galal H.
Ramzy
b,
Muhammad
Arif
c,
Ibrahim M.
Maafa
a,
Ayman
Yousef
a,
Nasser
Zouli
a,
Ahmed F. F.
Abouatiaa
a,
Abdel Samed M.
Adam
a,
Isam Y.
Qudsieh
a,
Ahmed I.
Ali
 *de,
Elbadawy A.
Kamoun
*de,
Elbadawy A.
Kamoun
 *fg and
Amr
Ali
h
*fg and
Amr
Ali
h
aDepartment of Chemical Engineering, College of Engineering and Computer Sciences, Jazan University, Jazan 45142, Saudi Arabia
bPhysics Department, Faculty of Science, Cairo University, Giza, 12613, Egypt
cDepartment of Applied Physics, Integrated Education Institute for Frontier Science and Technology and Institute of Natural Sciences, Kyung Hee University, Yongin 17104, Republic of Korea
dBasic Science Department, Faculty of Technology and Education, Helwan University, Saray–El Qoupa, El Sawah Street, 11281 Cairo, Egypt. E-mail: Ahmed_Ali_2010@techedu.helwan.edu.eg
eDepartment of Mechanical Engineering (Integrated Engineering Program), Kyung Hee University, 1732 Deogyeong-Daero, Yongin, Gyeonggi 17104, Republic of Korea
fDepartment of Chemistry, College of Science, King Faisal University, Al-Ahsa 31982, Saudi Arabia. E-mail: ekamoun@kfu.edu.sa; badawykamoun@yahoo.com
gPolymeric Materials Research Dep., Advanced Technology and New Materials Research Institute (ATNMRI), City of Scientific Research and Technological Applications (SRTA-City), New Borg Al-Arab City 21934, Alexandria, Egypt
hProduction Technology Department, Faculty of Technology & Education, Sohag University, 82524 Sohag, Egypt
First published on 18th March 2025
Abstract
Nd-doped CoFe2O4 spinel ferrites were synthesized via the sol–gel method, confirming a cubic spinel structure. Increasing Nd concentration expanded the lattice parameter (8.3900–8.4231 Å) and unit cell volume while reducing grain size. FT-IR analysis validated the spinel phase. Nd doping enhanced the dielectric constant by affecting space charge polarization and charge hopping, with conductivity following a Debye-type relaxation mechanism. Cole–Cole plots indicated grain boundary effects and polaron hopping conduction. Magnetic properties improved with Nd3+ content, with Ms and Hc reaching 5.621 emu g−1 and 143.43 Oe at 4% doping. A transition from an antiferromagnetic to a ferromagnetic state was observed, with a high Curie temperature (Tm) of 292 °C, confirming a stable ferromagnetic phase. These findings highlight Nd-doped CoFe2O4 as a promising candidate for permanent magnet applications.
1. Introduction
Recently, ferrites with a spinel structure have attracted significant attention due to their wide range of potential applications, including magnetic fluids, high-density magnetic recording, data storage devices, spintronics, solar cells, sensors, and catalysis.1,2 In the spinel cubic structure, oxygen ions are dispersed among 64 tetrahedral and 32 octahedral sites. The metal cations can occupy 8 A-sites and 16 B-sites, with migrating cations potentially filling up the vacant positions. Several factors including growth conditions such as the types of elements in the composite, doping, temperature, pressure, sintering time, fabrication method, and cation distribution between A and B-sites can all have an impact on the physical properties of the spinel structure.3 The most well-known member of the iron oxide family is cubic spinel structured cobalt ferrite (CoFe3O4) and is a promising candidate for a variety of magnetic recording applications because of its exceptional chemical and physical durability.4–6 Because of its exceptional electrical and magnetic properties, strong magneto-crystalline anisotropy, and chemical, mechanical, and thermal stability, CoFe2O4 is one of the most significant compounds that are being thoroughly researched.4,7 In the CoFe2O4 spinel ferrite structure, both Fe3+ and Co2+ ions occupy tetrahedral and octahedral interstitial sites.8–10 Additionally, the structure features a face-centered cubic (FCC) with a close-packed configuration of oxygen ions. CoFe2O4 offers several advantages because of the ferromagnetic state that arises from the super-exchange interactions between Fe3+ and Co2+ ions via O2− ions.11 However, an exceptionally high crossover rate is still a significant obstacle, which might be overcome by doping metal ions. In particular, rare earth ions, known for their high resistivity, are useful dopants for spinel ferrites.12–14 According to previously published reports, neodymium (Nd) doping into TiO2 increases carrier longevity, enabling charge transfer and serving as direct trapping spots.15 Owing to their wide range of applications, several methods including co-precipitation, combustion, sol–gel, solid–state reaction, and precipitation have been employed.16–18 Among these, the sol–gel technique offers a more simple and effective way to produce CoFe2O4 nanoparticles.19 Ni-doped ferrite magnetic nanoparticles (NixCo1−xFe2O4) have been synthesized, and their magnetic properties have been investigated at room temperature. Magnetic hysteresis loop measurements revealed that both the saturation magnetization (Ms) and coercivity (Hc) decreased linearly with increasing Nd3+ content.7 Likewise, magnetic nanoparticle Co0.5Nd0.5Fe2O4 spinel ferrites have been synthesized by means of the co-precipitation technique and their room temperature magnetic characteristics were investigated, and it was observed that with greater Nd+ doping levels, Ms reduced but Hc increased.20,21 In addition, Yadav et al.3 synthesized nano-crystalline ferrite particles (CoFe2−xNdxO4 at lower doping levels) and observed a simultaneous increase in both Ms and Hc with the addition of Nd3+ ions. Furthermore, the doping effect of elements like La, Er, and Ce on the ferrites has been investigated.22,23 It has been noted that adding these ions in place of Fe3+ leads to strain and structural alterations, which in turn weakens the particle's physical characteristics. Consequently, it is extremely important to monitor how Nd3+ doping in the B-site affects the structural, electrical, and magnetic characteristics of CoFe2O4 ferrite particles.24Nd3+ doped CoFe2O4 has significantly influenced its structural, magnetic, and electrical properties, due to its large ionic radius and trivalent nature. The incorporation of Nd3+ into the spinel lattice primarily affects cation distribution, resulting in lattice distortion and potential phase transformation. Nd3+ preferentially occupies octahedral sites, causing partial inversion of the spinel structure and altering Co2+–Fe3+ super-exchange interactions. This redistribution of cations modulates the material's magnetic behavior, potentially reducing saturation magnetization due to the non-magnetic nature of Nd3+ and modifying coercivity based on strain-induced anisotropy changes.25,26
The electrical properties of CoFe2O4 are also impacted by Nd doping, in which charge compensation mechanisms introduce oxygen vacancies, influencing conductivity and dielectric properties. The presence of these vacancies enhances leakage current characteristics, improving energy storage performance. Furthermore, dielectric constant variations suggest improved charge carrier mobility, which can be beneficial for high-frequency applications.27
Structurally, Nd doping induces strain within the CoFe2O4 lattice, potentially leading to phase segregation at higher doping concentrations. The formation of secondary phases, such as NdFeO3 or NdCoO3, is observed when the solubility limit is exceeded, affecting the thermal and mechanical stability of the material. This phase evolution highlights the complex interplay between ionic size effects, charge balance, and structural integrity in Nd-doped CoFe2O4.28,29
Neodymium (Nd), a lanthanide element, is widely used in the production of permanent magnets,30 which have diverse applications across various industries.31 Nd-based magnets are essential for hard disk drives, mobile phones, televisions, magnetic separators, and medical devices like magnetic resonance imaging (MRI) machines.32–34 However, developing cost-effective and environmentally sustainable methods for recovering neodymium from aquatic environments remains a critical challenge. The sol–gel method is ideal for synthesizing Nd-doped CoFe2O4, due to its high chemical homogeneity, precise stoichiometry, and controlled structural properties. It enables uniform ion mixing, minimizing phase segregation and ensuring material quality. Using metal nitrates or acetates as precursors allows accurate dopant control, preserving the spinel structure. Its low processing temperature reduces energy consumption and limits grain growth, resulting in fine nanoparticles with enhanced magnetic and electrical properties. The method also offers excellent phase purity, crystallinity, and tunable particle size, making it suitable for high-frequency applications and scalable for large-scale production.35 Based on this, we believe that Nd-doping in A-sites could enhance the performance of CoFe2O4, making it promising for advanced applications. This doping strategy significantly modifies structural, electrical, and magnetic properties, benefiting nanoelectronics. This study explores the impact of Nd doping on the structural, magnetic, and electrical properties of CoFe2O4, revealing key transformations that enhance functionality. Structurally, Nd incorporation expands the lattice, induces strain, and influences phase stability. It suppresses grain growth and promotes secondary phase formation at high doping levels. Magnetically, Nd alters super-exchange interactions, leading to spin canting, reduced saturation magnetization, and variable coercivity. Increased magnetic anisotropy highlights its tunability for applications. Electrically, Nd suppresses Fe2+/Fe3+ hopping, reducing conductivity while improving leakage current and dielectric properties, optimizing energy storage density and efficiency.
In this study, we investigate Nd-doping in the A-site of cobalt ferrite (NdxCo1−xFe2O4) nanoparticles synthesized via the sol–gel combustion method. Various characterization techniques were employed to analyze their magnetic and dielectric properties. The novelty of this work lies in Nd-doping, which has been less explored in cobalt ferrite. Nd3+ incorporation in the A-site enhances dielectric properties by reducing losses, improving high-frequency performance and optimizing magnetic behavior by influencing cation distribution within the spinel structure. This synergistic effect enhances electrical conductivity and overall material performance, making Nd-doped cobalt ferrite promising for applications in sensors, catalysis, high-frequency devices, and nanoelectronics. This research provides a foundation for the practical use of such nanoparticles.
2. Materials and methods
2.1. Materials
High purity cobalt nitrate (Co(NO3)2·6H2O (3N)) and iron nitrate (Fe(NO3)3·9H2O (3N)) were purchased from Al-Gomhouria Chemical Company in Cairo, Egypt. Neodymium nitrate (Nd(NO3)3·6H2O (3N)) was purchased from Sigma-Aldrich, Germany.2.2. Preparation of samples
NdxCo1−xFe2O4 (x = 0–0.5) powders, as listed in Table 1, were prepared using the sol–gel method. The detailed procedure, shown in Fig. 1, is as follows: first, stoichiometric amounts of high-purity cobalt nitrate (Co(NO3)2·6H2O, 3N), iron nitrate (Fe(NO3)3·9H2O, 3N), and neodymium nitrate (Nd(NO3)3·6H2O, 3N) were measured using a digital balance and mixed thoroughly with a mortar and pestle for 30 minutes to prepare the precursor. In the next step, the raw materials were mixed with ethyl alcohol as a solvent and sonicated for 20 minutes. The solution was then heated on a hot plate at 85 °C for 2 hours under magnetic stirring. After preparing the precursor solution, precipitation was induced by adjusting pH to 7 with an ammonium hydroxide solution (NaOH, 6 mol L−1) under constant stirring. The precipitates were allowed to settle and were subsequently collected by vacuum filtration. To ensure purity, the precipitates were washed multiple times with deionized water and ethanol to remove any residual ions or unreacted precursors. The collected precipitates were then subjected to a drying process before calcination. Drying of the mixture was carried out in an alumina crucible in an oven at 80 °C for 12 h to remove excess moisture and volatile components. This controlled drying step ensured uniform precursor decomposition and minimized agglomeration during calcination. The dried precursor was then ground into fine powder before being calcined at the optimized temperature for phase formation and crystallization. Finally, the powder was calcined at 600 °C for 2 h to remove impurities and unreacted components. The calcined powders were then sintered at 1150 °C for 3 h and ground for 30 minutes. They were then pressed using a homemade hydraulic press at 35 bar cm−2. The pellets were circular disks (∼10 mm in diameter and 1–2 mm thick) prepared using polyvinyl alcohol (PVA) as a binder. In the final stage, the compacted powder was re-sintered in air at 1300 °C for 3 hours.| NdxCo1−xFe2O4 | ||
|---|---|---|
| X = 0 | CoFe2O4 | S1 |
| X = 0.1 | Nd0.1Co0.9Fe2O4 | S2 |
| X = 0.2 | Nd0.2Co0.8Fe2O4 | S3 |
| X = 0.3 | Nd0.3Co0.7Fe2O4 | S4 |
| X = 0.4 | Nd0.4Co0.6Fe2O4 | S5 |
| X = 0.5 | Nd0.5Co0.5Fe2O4 | S6 |
 | ||
| Fig. 1 Schematic representation of Nd3+ doped-CoFe2O4 solid solution prepared by the sol–gel technique. | ||
2.3. Instrumental analyses
The crystal structure was examined using a Bruker D8 Advance X-ray diffractometer with CuKα radiation at room temperature. Surface morphology was analyzed via Scanning Microscopy (SEM) using a JSM-6360 microscope (JEOL, Japan). To identify the functional groups and vibrational modes of chemical bonds in the prepared ceramics, Fourier-transform infrared spectroscopy (FT-IR) measurement was conducted at room temperature using a Thermo-FT-IR 200 spectrometer (Thermo Scientific, USA).To measure the dielectric properties, silver paste contacts were made on the sample surface, followed by their annealing at 600 °C for 10 minutes. The frequency-dependent complex dielectric constants (ε′, ε′′, and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), AC conductivity (σAC), and complex impedance (Z′ and Z′′) were all investigated in the frequency range of 1 kHz to 100 kHz, with temperatures ranging from 25 °C to 200 °C, using an LCR meter (TH2826, 20 Hz–5 MHz). Additionally, the room-temperature magnetic properties, including M–H loops, were studied using a vibrating sample magnetometer (VSM7407, Lake Shore).
δ), AC conductivity (σAC), and complex impedance (Z′ and Z′′) were all investigated in the frequency range of 1 kHz to 100 kHz, with temperatures ranging from 25 °C to 200 °C, using an LCR meter (TH2826, 20 Hz–5 MHz). Additionally, the room-temperature magnetic properties, including M–H loops, were studied using a vibrating sample magnetometer (VSM7407, Lake Shore).
3. Results and discussion
3.1. X-ray diffraction
The X-ray diffraction pattern of NdxCo1−xFe2O4 (0.0 ≤ x ≤ 0.5) samples is shown in Fig. 2(a). In the XRD spectrum for all the samples, all the observed characteristic peaks such as (111), (220), (311), (400), (040), (242), (200), (422), (400), (533), and (211) are indexed to the cubic spinel structure of CoFe2O4 (JCPDS 22-1086).14 Additionally, a few small unindexed peaks, which may correspond to a secondary orthorhombic phase of NdFe2O4, are also observed. The XRD patterns reveal small additional peaks, indicating that the synthesized NdxCo1−xFe2O4 samples are in a primary spinel CoFe2O4 phase, with a secondary phase (NdFe2O4) also identified. These phases form due to the limited solubility of Nd3+ in the spinel lattice, leading to phase segregation at higher doping concentrations. Their presence affects both structural and functional properties. Structurally, they induce lattice strain, altering the crystallite size and microstructure. Functionally, they disrupt Fe3+(A)–O–Fe3+ (B-site) super-exchange interactions, reducing saturation magnetization (Ms). Additionally, NdFe2O4 affects electrical resistivity and dielectric properties, influencing energy storage and electronic applications. Controlling these secondary phases is crucial for optimizing material performance in specific technologies. Notably, with increasing Nd3+ ion doping, the lattice parameter expanded from 8.3900 to 8.4231 Å, leading to an increase in unit cell volume. Meanwhile, the grain size decreased due to the larger ionic radius of the Nd ion. This is expected because of the large ionic radius of Nd3+ in comparison to Fe3+, meaning that only a small portion of Fe3+ ions may be replaced by Nd3+. As a result, Nd3+ ions tend to collect near grain boundaries, promoting the development of the NdFe2O4 phase, as reported in ref. 36. | ||
| Fig. 2 The structural analysis of Nd(x)Co(1−x)Fe2O4: (a) X-ray diffraction and (b) FT-IR transmittance. | ||
The phase transformation in Nd-doped CoFe2O4 can be rationalized by several factors including ionic size effects, crystal field stabilization, and defect chemistry. The ionic radius of Nd3+ (0.983 Å) is larger than those of Co2+ (0.745 Å) and Fe3+ (0.645 Å), which causes lattice distortion upon substitution. Further, the larger Nd3+ ions preferentially replace Co2+ in A-sites, leading to strain in the spinel structure, which can drive phase transformation into secondary or non-spinel phases. The introduction of Nd3+ into CoFe2O4 affects charge neutrality, possibly requiring the formation of oxygen vacancies to maintain charge balance. Moreover, the presence of oxygen vacancies can destabilize the normal spinel structure and promote the formation of secondary phases such as orthoferrites (NdFeO3) or mixed phases (NdCoO3). Nd3+ has a strong preference for octahedral coordination, whereas Co2+ and Fe3+ are more flexible in their site occupancy within the spinel lattice. The substitution of Nd3+ disrupts the cation distribution in CoFe2O4, potentially leading to partial inversion of the spinel structure or the emergence of a perovskite-like phase.
At high Nd concentrations, the solubility limit of Nd in the CoFe2O4 lattice may be exceeded, leading to phase segregation. The Nd–O bond energy is different from those of Co–O and Fe–O, which affects the Gibbs free energy of the system and can drive phase transformation. In addition, the observed phase transformation in Nd-doped CoFe2O4 likely arises from a combination of lattice distortion, charge compensation mechanisms, and site occupancy changes. The trend aligns with reports on other rare-earth dopants, reinforcing the idea that large trivalent cations in the CoFe2O4 lattice tend to induce structural transitions due to ionic size mismatch and altered thermodynamic stability. Several studies have reported similar phase transformations in rare-earth-doped CoFe2O4 systems such as La-doped CoFe2O4. The La3+ substitution also induces lattice distortion and can result in secondary phases such as LaFeO3 at high doping levels. In Sm-doped CoFe2O4, Sm3+ doping has been found to reduce the crystallinity of CFO and promote structural disorder, sometimes leading to the appearance of a perovskite phase. In Gd-doped CoFe2O4, Gd3+ doping can induce a transition from a spinel to a mixed spinel-orthoferrite phase, due to strain effects and cation redistribution.37–39
The average crystallite size of CoFe2O4 and Nd-doped CoFe2O4 ferrite particles was determined using the classical Scherrer's formula.40
Dhkl = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (1) |
| Samples | Lattice parameter, a (Å) | Angles (o) | System | Symmetry | Volume (106 pm)3 | Grain size (nm) | Calculated density (gm cm−3) |
|---|---|---|---|---|---|---|---|
| CoFe2O4 | 8.3900 | 90 | Cubic |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
590.590 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 811.85 811.85 |
5.300 |
| Nd0.1Co0.9Fe2O4 | 8.4010 | 90 | Cubic |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
592.916 | 6870.35 | 5.296 |
| Nd0.2Co0.8Fe2O4 | 8.4140 | 90 | Cubic |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
595.672 | 4309.55 | 5.289 |
| Nd0.3Co0.7Fe2O4 | 8.4170 | 90 | Cubic |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
596.310 | 4206.43 | 5.284 |
| Nd0.4Co0.6Fe2O4 | 8.4211 | 90 | Cubic |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
597.182 | 4057.33 | 5.280 |
| Nd0.5Co0.5Fe2O4 | 8.4231 | 90 | Cubic |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
597.607 | 3476.13 | 5.782 |
3.2. FT-IR analysis
The FT-IR spectra of NdxCo1−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) samples with wavenumbers ranging from 400 to 3500 cm−1 are depicted in Fig. 2(b). All the samples have shown a fundamental absorption peak around 450 cm−1, confirming that the NdxCo1−xFe2O4 compounds exhibit a cubic spinel ferrite structure. This could be attributed to the stretching vibrations of metal–oxygen bonds.16,17,24 Since the atomic mass (A.M.) of Co is greater than that of Fe and the A.M. of O2− remains constant, the overall atomic mass of the metal increases due to the substitution of Fe by Co, as previously reported.41 However, the absorption band observed at approximately 688.4 cm−1 is attributed to the vibrations of iron–oxygen bonds in the A-site position.20 A broad absorption band observed in the range of 2942–3430 cm−1 is associated with phase A, which corresponds to the broadening of O–H stretching modes from hydroxyl groups.18 In addition, four other absorption bands are also observed that correspond to both (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and C–O vibrations. Both absorption bands observed at 1640 cm−1 and 1113 cm−1 represent the stretching mode of C
O) and C–O vibrations. Both absorption bands observed at 1640 cm−1 and 1113 cm−1 represent the stretching mode of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O vibrations, which in turn arise from esterification reactions.42,43
O and C–O vibrations, which in turn arise from esterification reactions.42,43
3.3. SEM investigation
To investigate and understand grain size distribution and topology of the grains, FE-SEM micrographs, as shown in Fig. 3(a–c), were analyzed. The FE-SEM micrographs reveal that with the gradual increase of Nd3+, the average grain size decreases, making precise statistical calculations challenging. Furthermore, it is evident that the lattice properties have changed when Nd+ is substituted in CoFe2O4 spinel ferrites, which results in an internal stress. Despite this, the Co-ferrite particles have different agglomeration diameters and a hexagonal shape. Here, on the other hand, a more homogeneous distribution of both small and large particles resulted in Nd-doped-ferrite particles exhibiting a roughly cubic structure. Furthermore, because Nd-ferrite grains are larger in ionic size and have a rounder shape than Co-ferrite grains, their surfaces are rougher. | ||
| Fig. 3 FE-SEM images graph displaying the prepared ferrites' elemental composition and surface morphology: (a) = S2, (b) = S3 and (c) = S4. | ||
The structural analysis confirmed a cubic spinel ferrite structure in NdxCo1−xFe2O4 (0.0 ≤ x ≤ 0.5), while at higher Nd concentrations, additional small peaks corresponding to the orthorhombic NdFe2O4 phase were observed. These peaks are consistent with previous studies on rare-earth doping in spinel ferrites, where excessive Nd substitution leads to the formation of secondary phases due to size and valence mismatch between Nd3+ and Fe3+ ions.26 Nd3+ ions have a larger ionic radius (0.983 Å) compared to Fe3+ (0.645 Å), which disrupts the spinel lattice and promotes the segregation of an orthorhombic phase at higher doping levels.44,45 Similar observations have been reported in Nd-doped cobalt ferrites (NdxCoFe2−xO4), where an increase in Nd content beyond a solubility threshold results in the emergence of a secondary rare-earth iron oxide phase.46 Additionally, the presence of the orthorhombic NdFe2O4 phase may be attributed to the stronger Nd–O bond energy, which affects the overall crystallographic stability, as observed in Nd-substituted NiFe2O4 and ZnFe2O4 systems.
Moreover, FT-IR spectra further confirm the cubic spinel structure, with characteristic metal–oxygen stretching vibrations. The absorption peaks around ν 450 cm−1 are attributed to tetrahedral Fe–O stretching, consistent with prior studies on spinel ferrites.47 The broad band at 688.4 cm−1, corresponding to Fe–O vibrations in the A-site, aligns with previously reported FT-IR studies of Ni-doped ferrites.48 The broad O–H stretching modes observed in the 2942–3430 cm−1 range indicate the presence of hydroxyl groups, suggesting partial surface hydration or adsorbed moisture, a common phenomenon in ferrite systems.49,50 These findings are in agreement with earlier reports on Nd doping in ferrites, where low Nd concentrations stabilize the cubic spinel phase, while higher Nd incorporation induces the segregation of an orthorhombic NdFe2O4 phase due to ionic radius mismatch and solubility limits.51 FT-IR analysis supports the structural interpretation, further validating the formation of the spinel lattice and secondary phases at higher Nd content.
3.4. Composition analysis
Fig. 4 depicts the FE-SEM images and EDS analysis of S1 and S5 samples. To evaluate the elemental composition of the prepared spinel ferrites, EDX analysis was performed on the area shown in FE-SEM images (Fig. 4). The detected elements are all presented in the EDX spectra, from which the proportion of each element was calculated, as shown in the EDX table. The EDX composition table indicates that the weight percentages of each element are nearly within the expected stoichiometric ratios, aligning well with the NdxCo1−xFe2O4 (0.0 ≤ x ≤ 0.5) composition. All compositions exhibit similar intensity peaks at the same positions, confirming the presence of Nd ions and being consistent with XRD analysis, as shown in Fig. 2. In addition, the EDX spectra show that extremely undesirable precursor materials, like nitrate ions, were successfully removed during the chemical process, and this results in the creation of the required oxide compounds. Here, Nd3+ has a larger ionic radius (1.109 Å) compared to Fe3+ (0.645 Å) and Co2+ (0.745 Å). The incorporation of Nd3+ into the CoFe2O4 spinel structure not only modifies the lattice parameters by inducing expansion due to its larger ionic radius but also influences the overall structural integrity and magnetic characteristics of the material. The preferential occupancy of Nd3+ in octahedral sites alters the cation distribution and disrupts the continuity of the crystal lattice. This substitution results in weaker Nd–O bonds compared to the stronger Fe–O interactions, inducing local distortions that can significantly affect crystallite size and increase defect density. Consequently, the presence of Nd3+ may impede grain growth, leading to finer microstructures, which in turn can enhance certain functional properties of the spinel ferrites, making them suitable for various applications in magnetic materials and electronics.3.5. Dielectric properties
To study the temperature dependence of dielectric and electrical properties, AC electrical measurements were carried out at 500 kHz within the temperature range of 20 °C to 200 °C. The permittivity (ε′) of the samples can be estimated using | (2) |
| D = tan(δ) = 1/(ωRpCp). | (3) |
To calculate AC conductivity (σac), the following relationship is employed:
σac = ωεoεr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tan(δ). tan(δ). | (4) |
| ω = 2πf | (5) |
The frequency dependence of the dielectric constant (permittivity) of NdxCo1−xFe2O4 (x = 0–0.5) in the frequency range (100 Hz–1 MHz) with varying temperatures (25 °C to 200 °C) is shown in Fig. 5. Interestingly, all samples showed a substantially high dielectric constant in the low-frequency band, with the dielectric constant dropping exponentially with increasing frequency. This is caused by the frequency-varying effects of ionic, dipolar, electronic, and space charge polarizations. However, eventually, the dielectric constant stabilizes at high frequencies, signifying that the material's polarization has reached saturation. Thereafter, there is no discernible change in the dielectric constant with increasing frequency, indicating the dominance of two different polarization mechanisms.52 This frequency-independent behavior is commonly observed in most ferrimagnetic materials and ascribed to the transfer of Fe3+ ions into Fe2+ ions during the sintering process. A dipolar ferrite material is usually produced when there are more Fe3+ ions than Fe2+ ions. Polarization relaxation occurs in ferrites when the coupling of Fe3+ and Fe2+ ions aligns with an alternating field.53 The decrease in the dielectric constant can be ascribed to space charge carriers,54 as it requires a certain time interval for orientation in response to the applied field. As the frequency increases, these space charge carriers lag behind the changing field, resulting in a reduced dielectric constant.55 Conversely, the dielectric constant, which measures permittivity, increases with temperature for all samples, reaching a maximum during the transition from ferroelectric to paraelectric. This increase may be due to space charge polarization, as higher temperature enhances the interfacial polarization, which in turn increases permittivity. However, with the application of an electric field on the dielectric materials, the polarization implies the harvesting charges, and the effects of temperature on ionic and electronic polarization are a minimal.35 Additionally, because of the different ionic radii of Nd3+ (1.109 Å) and Co2+ (0.745 Å), it seems the Nd3+ ions preferred the A-site position, causing a threefold increase in the dielectric constant with increasing Nd doping.56
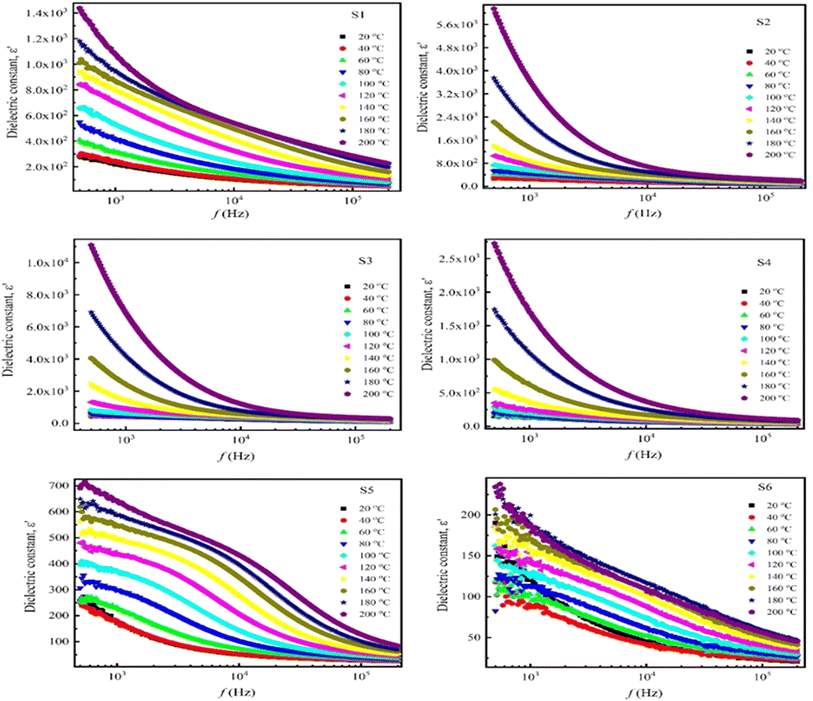 | ||
| Fig. 5 Dielectric constant (ε′) as a function of frequency in the temperature range (25–200 °C) of NdxCo1−xFe2O4 (x = 0–0.5). | ||
Fig. 6 shows the dielectric loss tangent (tan(δ)) as a function of frequency for all NdxCo1−xFe2O4 (x = 0–0.5) samples at different temperatures (30 to 200 °C). The dielectric loss tangent for all samples exhibits a similar trend to that of ε′. At low frequency, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is higher and decreases exponentially as frequency increases. This occurs because, at low frequencies, the applied field frequency is lower than the electron hopping frequency between Fe2+ and Fe3+ ions at the B-site, thus leading to greater dielectric loss. Conversely, at high frequencies, the dielectric loss becomes constant under the applied electric field. As the frequency improves, both space charge polarization and dielectric loss decrease because the electron hopping between Fe2+ and Fe3+ ions can no longer keep up with the applied field.57 Most of the energy loss at low frequencies was recognized due to huge resistant grain boundaries. Therefore, energy loss is higher at low frequencies and lower at high frequencies. This is because, at low frequencies, more energy is essential for electron hopping between ferrous (Fe2+) and ferric (Fe3+) ions, compared to the energy required at higher frequencies.58–60 Additionally, the temperature dependence of dielectric loss reveals that tan
δ is higher and decreases exponentially as frequency increases. This occurs because, at low frequencies, the applied field frequency is lower than the electron hopping frequency between Fe2+ and Fe3+ ions at the B-site, thus leading to greater dielectric loss. Conversely, at high frequencies, the dielectric loss becomes constant under the applied electric field. As the frequency improves, both space charge polarization and dielectric loss decrease because the electron hopping between Fe2+ and Fe3+ ions can no longer keep up with the applied field.57 Most of the energy loss at low frequencies was recognized due to huge resistant grain boundaries. Therefore, energy loss is higher at low frequencies and lower at high frequencies. This is because, at low frequencies, more energy is essential for electron hopping between ferrous (Fe2+) and ferric (Fe3+) ions, compared to the energy required at higher frequencies.58–60 Additionally, the temperature dependence of dielectric loss reveals that tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ rises as the temperature increases. This behavior is most likely because of exchange between Fe ions in octahedral positions, which is influenced by the movement of charge carriers.61 Moreover, the dielectric loss tangent is minimized for doped samples x ≈ 0.1 and x ≈ 0.5.
δ rises as the temperature increases. This behavior is most likely because of exchange between Fe ions in octahedral positions, which is influenced by the movement of charge carriers.61 Moreover, the dielectric loss tangent is minimized for doped samples x ≈ 0.1 and x ≈ 0.5.
 | ||
Fig. 6 Frequency-dependent dielectric loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at altered temperatures ranging from 25 °C to 200 °C for NdxCo1−xFe2O4 (x = 0–0.5). δ) at altered temperatures ranging from 25 °C to 200 °C for NdxCo1−xFe2O4 (x = 0–0.5). | ||
3.6. AC conductivity
The AC technique distinguishes between various mechanisms that contribute to the material's overall conductivity response, like electrode response and the electrical conduction through grains and their boundaries. However, the DC technique reflects the total conductivity response of the material. Fig. 7 presents the ln(σac) versus ln(f) plots with temperatures. The analysis reveals that all samples exhibit a nearly identical behavior with two distinct slopes, implying a rise in conductivity with rising temperature. The slopes at higher frequencies are steeper than those at lower frequencies, as high-frequency radiation exerts a greater driving force on charge carriers, resulting in higher AC conductivity at elevated frequencies.62 According to Koops model, ferrite samples behave like multilayer capacitors which are combined grain with boundary,63 resulting in the increase in the conductivity at higher frequency and elevated temperature. At lower frequencies, a nearly continuous plateau is noted, where electronic charge carriers are hindered from hopping between the more resistive grain boundaries. However, with increasing frequencies, the conductive grains were active, enabling charge carriers hopping between neighboring ions.64 In DC conductivity, charge carriers follow the shortest path between ions, which includes jumps across regions with higher resistance, such as the spaces between cations, a factor that is not required in AC conduction. As a result, AC conduction may involve lower activation energy.65–67 | ||
| Fig. 7 AC conductivity through variation of ln(σac) dependence ln(f) plots with temperatures (20–200 °C) of all samples. | ||
3.7. Dielectric-temperature dependence
Fig. 8(a) illustrates the dielectric constant (ε′) of NdxCo1−xFe2O4 as a function of temperature for all samples at an applied frequency of 500 kHz. It is observed that ε′ increases monotonically with rising temperature. However, at higher temperatures, the dielectric constant remains nearly constant. This is because the dipoles acquire sufficient thermal energy to align with the applied field, resulting in higher dielectric constant values. In contrast, at lower temperatures, there is insufficient energy to fully polarize the dipoles.68 | ||
| Fig. 8 Temperature-dependent ac conductivity of Nd3+ doped cobalt-ferrite at different temperatures. | ||
Furthermore, with the increased doping concentration of Nd3+ ions, the dielectric constant decreases as well.69 This is ascribed to the large ionic radius of Nd3+, which preferentially occupies octahedral B-sites, replacing Fe3+ ions. Consequently, Nd3+ doping in Co-ferrites forces Fe3+ to move its position from B to A sites, and in turns it can reduce the Fe3+ number in the B-site, leading to decreased interstitial polarization.70,71 As a result, the dielectric constantly reduces.
Fig. 8(b) shows the σacversus frequency plot for all NdxCo1−xFe2O4 (x = 0.0 to 0.5) composites with varying temperature (25–200 °C) at a constant frequency of 500 kHz. The temperature-dependent AC conductivity measurements show that σac gradually increases with the increase of temperature for all samples. Specifically, in the low-temperature region (T < 100 °C), σac increases slightly. However, it becomes more significant when the temperature increases (T > 100 °C). This is due to the increased applied field, which enhances the electron mobility and hence leads to higher conductivity values.
The behavior of AC conductivity with varying frequency was described using a large/small polaron model. This concept states that when an electric field is applied, electrons begin to migrate and polarize the surrounding lattice by creating polarons. Massive polarons are produced when the distortion increases the lattice constant; tiny polarons appear when the distortion is equal to the lattice constant.72,73
3.8. Impedance spectroscopy
The real (Z′) and imaginary (Z′′) parts of the impedance were calculated for NdxCo1−xFe2O4 using the following equation:74Z′ = Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (6) |
Z′′ = Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (7) |
 | ||
| Fig. 9 Real impedance (Z′) as a function of frequency for S1, S2, S3, S4, S5 and S6 from room temperature to 200 °C. | ||
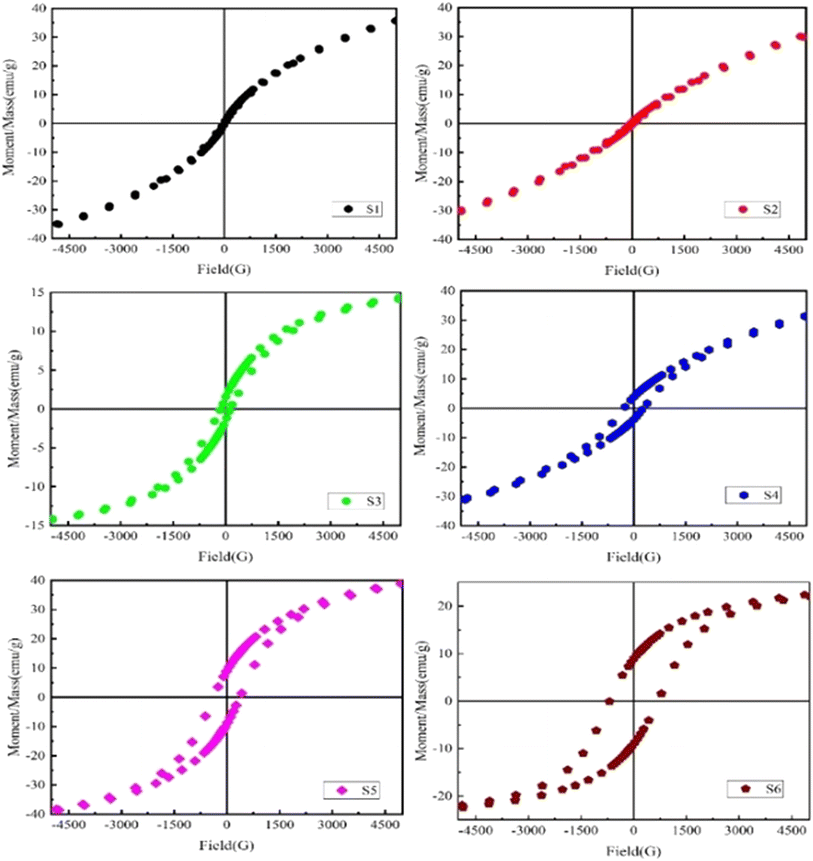
3.9. Magnetic properties (M–H hysteresis loops)
The magnetic hysteresis loops (M–H) of the manufactured NdxCo1−xFe2O4 solid solutions (x = 0–0.5) were measured using VSM under an applied field of ±40 kOe, as shown in Fig. 12. The M–H loops reveal the effect of the Nd ions on the magnetic properties including coercivity (Hc), remnant magnetization (Mr), and saturation magnetization (Ms). All the samples showed a narrow, “S”-shaped M–H curve, being the characteristic of the ferromagnetic state. The squareness ratio (Mr/Ms) increases with increasing Nd doping. Typically, in cobalt ferrite (CoFe2O4), Co2+ ions occupy the octahedral (B-site) positions, while Fe3+ ions reside in both tetrahedral (A) and octahedral (B) sites.80,81 However, with the doping of Nd3+ ions, it prefers the tetragonal structure at B-sites, causing migration of Co2+ and Fe3+ ions from B-sites to A-sites, thereby leading to an increased squareness (Mr/Ms) ratio.The saturation magnetization (Ms) and retentivity (Mr) both increase with rising Nd3+ concentrations up to x = 0.1 and 0.3, after which they begin to decrease, as depicted in Fig. 12. For the sample with 2% Nd doping, Ms is approximately 3.08 emu g−1 and Hc is around 64.26 Oe. For the 4% doped sample, these values rose to 5.621 emu g−1 and 143.43 Oe, correspondingly. Owing to the larger magnetic moment of Nd3+ (3.65 μB) in the position of a lower magnetic moment of Co2+ (3 μB) at B-sites, the saturation magnetization increases. However, the increased coercivity can be related to the existence of the NdFe2O4 phase of the parent compound with the primary phase, causing significant lattice distortion.82 Notably, our findings show higher magnetization as compared to previous reports about Nd-doped BFO synthesized via the sol–gel method.83,84 This strong saturation magnetization at room temperature (300 K) is linked to the variations in chemical composition and cation distribution, as suggested by the Néel sub-lattices model.85 These findings offer promising potential for applications in memory devices and spintronics. The physical traits of Nd-doped CoFe2O4, including particle size, morphology, density, porosity, and cation distribution, significantly influence its magnetic properties. Larger particles enhance magnetization (Ms) through stronger exchange interactions, while smaller particles increase coercivity (Hc) due to single-domain behavior but may induce superparamagnetic at extreme sizes. Morphology affects anisotropy, with elongated structures increasing Hc. A higher density improves exchange coupling, increasing Ms and decreasing Hc, whereas porosity weakens interactions, reducing Ms while increasing Hcvia domain wall pinning. Nd3+ doping alters cation distribution, reducing Ms and modifying Hc, with excessive doping leading to secondary phase formation. For high-frequency applications, controlled particle size and porosity minimize eddy current losses, while Nd-induced anisotropy enhances ferromagnetic resonance, making Nd-doped CoFe2O4 suitable for transformers, sensors, and spintronic devices.25
4. Conclusions
Spinel ferrites doped with the rare-earth element Nd3+, forming the solid solution NdxCo1−xFe2O4 (x; 0.0–0.5), were effectively synthesized using the sol–gel technique. XRD analysis confirmed the cubic phase spinel structure of CoFe2O4. The Nd doping significantly influenced the dielectric properties of the prepared materials, which was ascribed to the reduction of Co2+ ions and the incorporation of Nd3+ ions. FT-IR analysis confirms the cubic spinel ferrite structure of NdxCo1−xFe2O4, with characteristic metal–oxygen stretching vibrations. AC conductivity as a function of frequency was explained using the large/small polaron model. The impedance and electrical modulus show a Debye-type relaxation phenomenon. Cole–Cole plots exhibited semicircular arcs at 30, 40, and 60 °C, which indicates that the conduction mechanism in Nd-doped CoFe2O4 is governed by grain boundary effects and space charge polarization, supporting the polaron hopping conduction mechanism. Further, Nd-doped CoFe2O4 exhibits enhanced magnetic properties with increasing Nd3+ concentration, reaching 5.621 emu g−1 (Ms) and 143.43 Oe (Hc) for the 0.4 doped sample. Strong saturation magnetization at 300 K suggests a key role of chemical composition and cation distribution, as explained by the Néel sub-lattice model. Compared to Nd-doped BFO, our samples show superior magnetic performance. A transition from an antiferromagnetic to a ferromagnetic state was observed, with a high Curie temperature (Tm) of 292 °C, confirming a stable ferromagnetic phase, making Nd-doped CoFe2O4 promising for permanent magnet applications.Ethical approval
No ethical approval was granted to conduct the experiments involved within the manuscript.Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.Author contributions
Amr Ali and Ahmed I. Ali: formal analyses, experiments and data analysis; Ahmed I. Ali: project administration, resources, and writing – the original draft; Galal H. Ramzy: data analysis and software; Muhammad Arif, Nasser Zouli, Ahmed F. F. Abouatiaa, and Abdel Samed M. Adam: wrote – the original draft of the manuscript; and Saleh M. Matar, Ahmed I. Ali and Elbadawy A. Kamoun: supervision, software, and wrote – reviewed and editing the final draft. All authors approved the current version of the draft for submission.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors gratefully acknowledge the funding of the Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, through project no. RG24-S0134.References
- M. Houshiar, et al., Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties, J. Magn. Magn. Mater., 2014, 371, 43–48 CAS.
- Z. Zi, et al., Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles, J. Magn. Magn. Mater., 2009, 321(9), 1251–1255 CrossRef CAS.
- R. S. Yadav, et al., Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties, J. Magn. Magn Mater., 2016, 399, 109–117 CrossRef CAS.
- A. Giri, et al., Photomagnetism and structure in cobalt ferrite nanoparticles, Appl. Phys. Lett., 2002, 80(13), 2341–2343 CrossRef CAS.
- R. Arulmurugan, et al., Mn–Zn ferrite nanoparticles for ferrofluid preparation: Study on thermal–magnetic properties, J. Magn. Magn Mater., 2006, 298(2), 83–94 CrossRef CAS.
- S. Jauhar, et al., Tuning the properties of cobalt ferrite: a road towards diverse applications, RSC Adv., 2016, 6(100), 97694–97719 RSC.
- K. V. Chandekar, M. Shkir and S. AlFaify, A structural, elastic, mechanical, spectroscopic, thermodynamic, and magnetic properties of polymer coated CoFe2O4 nanostructures for various applications, J. Mol. Struct., 2020, 1205, 127681 CrossRef CAS.
- R. Melo, P. Banerjee and A. Franco, Hydrothermal synthesis of nickel doped cobalt ferrite nanoparticles: optical and magnetic properties, J. Mater. Sci.: Mater. Electron., 2018, 29, 14657–14667 CrossRef CAS.
- S. Ammar and F. Fiévet, Polyol synthesis: A versatile wet-chemistry route for the design and production of functional inorganic nanoparticles, Nanomaterials, 2020, 10(6), 1217 CrossRef CAS PubMed.
- A. Pinheiro, et al., Exchange bias and superspin glass behavior in nanostructured CoFe2O4-Ag composites, J. Magn. Magn. Mater., 2020, 497, 165940 CrossRef CAS.
- M. Rajendran, et al., Magnetic properties of nanocrystalline CoFe2O4 powders prepared at room temperature: variation with crystallite size, J. Magn. Magn. Mater., 2001, 232(1–2), 71–83 CrossRef CAS.
- I. Edelman, et al., Effect of gadolinium on magnetic circular dichroism and electron magnetic resonance of ε-Fe2O3 nanoparticles formed in borate glasses, J. Non-Cryst. Solids, 2019, 506, 68–79 CrossRef CAS.
- M. Kurian, et al., Structural, magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods, J. Adv. Ceram., 2015, 4, 199–205 CrossRef CAS.
- L. Fkhar, et al., Magnetic and structural properties of novel neodymium-tin spinel ferrite nanoparticles, J. Supercond. Novel Magn., 2019, 32(2), 381–384 CrossRef CAS.
- R. Pandiyan, Growth by Radio Frequency Sputtering and Characterisation of Rare Earth Doped Wide Bandgap Oxides, University of Trento, 2013 Search PubMed.
- K. Jalaiah, et al., Co-dopant affect on the structural, electrical and magnetic properties of zirconium and copper co-substituted Ni0. 75Zn0. 25Fe2O4 spinel ferrites synthesized by sol-gel method, Chin. J. Phys., 2018, 56(5), 2039–2051 CrossRef CAS.
- İ. Şabikoğlu, et al., The effect of neodymium substitution on the structural and magnetic properties of nickel ferrite, Prog. Nat. Sci.: Mater. Int., 2015, 25(3), 215–221 CrossRef.
- G. Ahmadpour, et al., Microstructure, composition and magnetic properties of Nd-(Fe1-xCox)-B oxide magnetic particles synthesized by Pechini-type chemical method, Adv. Powder Technol., 2021, 32(11), 3964–3979 CrossRef CAS.
- A. Hunyek, C. Sirisathitkul and P. Harding, Synthesis and characterization of CoFe2O4 particle by PVA sol-gel method, Adv. Mater. Res., 2010, 93, 659–663 Search PubMed.
- Z. Mahhouti, et al., Chemical synthesis and magnetic properties of monodisperse cobalt ferrite nanoparticles, J. Mater. Sci.: Mater. Electron., 2019, 30, 14913–14922 CAS.
- N. H. Kumar, et al., Structural, optical, thermoelectric, and magnetic properties of terbium doping on zinc cobalt nanoparticles and applications, Indian J. Phys., 2023 DOI:10.1007/s12648-023-02906-6.
- M. Ahmed, N. Okasha and M. Gabal, Transport and magnetic properties of Co–Zn–La ferrite, Mater. Chem. Phys., 2004, 83(1), 107–113 CrossRef CAS.
- M. Ahmed, N. Okasha and A. Ebrahem, Correlation of the physico chemical properties of Zn-substituted Li–La ferrite, Ceram. Int., 2005, 31(3), 361–369 CrossRef CAS.
- P. Kharazi, R. Rahimi and M. Rabbani, Copper ferrite-polyaniline nanocomposite: structural, thermal, magnetic and dye adsorption properties, Solid State Sci., 2019, 93, 95–100 CrossRef CAS.
- F. Azam, H. Ali and M. Anis-ur-Rehman, Investigation of Structural, Electrical, and Resistive Switching Effect of Co-Gd Ferrites by Substituting Nd+3 for Rram Applications, SSRN: https://ssrn.com/abstract=5129776 Search PubMed.
- M. Almessiere, et al., Exploring dielectric and electrical characteristics in Sr0. 5Ba0. 5SnxFe12-xO19/CoFe2O4 nanocomposites, Ceram. Int., 2024, 50(24), 52330–52343 CrossRef CAS.
- F. R. Ghadi and G. A. Hodtani, Copula-based analysis of physical layer security performances over correlated Rayleigh fading channels, IEEE Trans. Inf. Forensics Secur., 2020, 16, 431–440 Search PubMed.
- P. Anil, et al., Structural and dielectric characteristics of CoFe2O4/nitrogen-enriched reduced graphene oxide composites via a one-pot solvothermal method, J. Mater. Sci., 2025, 1–25 Search PubMed.
- K. Srinivasamurthy, et al., Electrochemical performance of Sr-doped cobalt nickel ferrite ceramics for supercapacitor applications, J. Energy Storage, 2025, 114, 115735 CrossRef.
- X. Du and T. E. Graedel, Global rare earth in-use stocks in NdFeB permanent magnets, J. Ind. Ecol., 2011, 15(6), 836–843 CrossRef CAS.
- Y. Zhang, et al., Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: A review, Metals, 2020, 10(6), 841 CrossRef CAS.
- S. S. Kalyankar, et al., Composite Materials For Adsorption of Rare Earth Metal Ions, Water, Air, Soil Pollut., 2024, 235(10), 661 CrossRef CAS.
- M. Firdaus, et al., Review of high-temperature recovery of rare earth (Nd/Dy) from magnet waste, J. Sustain. Metall., 2016, 2, 276–295 CrossRef.
- H. Wang, T. N. Lamichhane and M. P. Paranthaman, Review of additive manufacturing of permanent magnets for electrical machines: A prospective on wind turbine, Mater. Today Phys., 2022, 24, 100675 CrossRef CAS.
- K. Vani, et al., Impact of rare earth Tb3+ substitution in cobalt ferrites: Tuning structural, dielectric, magnetic properties and photocatalytic activity, Ceram. Int., 2025, 51(1), 240–251 CrossRef CAS.
- P. G. Bercoff, C. Herme and S. E. Jacobo, The influence of Nd–Co substitution on the magnetic properties of non-stoichiometric strontium hexaferrite nanoparticles, J. Magn. Magn. Mater., 2009, 321(14), 2245–2250 CrossRef CAS.
- R. S. Yadav, et al., Structural and Magnetic Properties of CoFe2− xGdxO4 (0.0≤ x ≥ 0.1) Spinel Ferrite Nanoparticles Synthesized by Starch-Assisted Sol–Gel Auto-combustion Method, J. Supercond. Novel Magn., 2015, 28, 1797–1806 CrossRef CAS.
- W. Song, et al., Enhancing the performance of spinel La-doped CoFe2O4 oxygen carriers for chemical looping hydrogen generation, Green Carbon, 2024, 2(4), 393–400 CrossRef.
- I. A. A. Amar, New materials for electrochemical synthesis of ammonia, Doctoral Thesis, Department of Pure and Applied Chemistry, University of Strathclyde, 2014.
- A. Patterson, The Scherrer formula for X-ray particle size determination, Phys. Rev., 1939, 56(10), 978 CrossRef CAS.
- V. Evangelou, Pyrite Oxidation and its Control, CRC press, 2018 Search PubMed.
- S. Vivekanandhan, M. Venkateswarlu and N. Satyanarayana, Effect of different ethylene glycol precursors on the Pechini process for the synthesis of nano-crystalline LiNi {sub 0.5} Co {sub 0.5} VO {sub 4} powders, Mater. Chem. Phys., 2005, 91(1), 54–59 CrossRef CAS.
- S. Vivekanandhan, M. Venkateswarlu and N. Satyanarayana, Effect of different ethylene glycol precursors on the Pechini process for the synthesis of nano-crystalline LiNi0. 5Co0. 5VO4 powders, Mater. Chem. Phys., 2005, 91(1), 54–59 CrossRef CAS.
- X.-L. Song, et al., NdFe2O4 Nanoparticles: Synthesis, Characterization, and Magnetic Properties, Sci. Adv. Mater., 2020, 12(6), 810–814 CrossRef CAS.
- X. Song, et al., Study on the controllable preparation of Nd3+ doped in Fe3O4 nanoparticles for magnetic protective fabrics, Molecules, 2023, 28(7), 3175 CrossRef CAS PubMed.
- A. Moreno-Góngora, Sonochemical Synthesis of Ferrite Nanoparticles, 2023 Search PubMed.
- H. Dawoud, L. Ouda and S. Shaat, FT-IR studies of nickel substituted polycrystalline zinc spinel ferrites for structural and vibrational investigations, Chem. Sci. Trans., 2017, 8, 179–188 Search PubMed.
- R. S. Yadav, et al., Structural, magnetic, dielectric, and electrical properties of NiFe2O4 spinel ferrite nanoparticles prepared by honey-mediated sol-gel combustion, J. Phys. Chem. Solids, 2017, 107, 150–161 CrossRef CAS.
- I. Petrila and F. Tudorache, Annealing temperature effects on humidity sensor properties for Mg0. 5W0. 5Fe2O4 spinel ferrite, Sensors, 2022, 22(23), 9182 CrossRef CAS PubMed.
- M. C. Mathpal, et al., State of art of spinel ferrites enabled humidity sensors, Spinel Nanoferrites: Synthesis, Properties and Applications, 2021, pp. 437–475 Search PubMed.
- M. Saha, et al., Structural, optical, dielectric, and magnetic properties of spinel MFe2O4 (M= Co and Zn) nanoparticles synthesized by CTAB-assisted hydrothermal method, Ceram. Int., 2022, 48(23), 35719–35732 CrossRef CAS.
- S. A. Abdelwahab, et al., Influence of TiO2/GO weight ratio on the structure, mechanical, and electrical properties of SiO2–Al2O3 glass–ceramics, J. Mater. Sci.: Mater. Electron., 2021, 32, 11092–11106 CrossRef CAS.
- E. Murad and J. Cashion, Mössbauer Spectroscopy of Environmental Materials and Their Industrial Utilization, Springer Science & Business Media, 2011 Search PubMed.
- S. Torabi, et al., Strategy for enhancing the dielectric constant of organic semiconductors without sacrificing charge carrier mobility and solubility, Adv. Funct. Mater., 2015, 25(1), 150–157 CrossRef CAS.
- M. Sajedi Alvar, P. W. Blom and G.-J. A. Wetzelaer, Space-charge-limited electron and hole currents in hybrid organic-inorganic perovskites, Nat. Commun., 2020, 11(1), 4023 CrossRef CAS PubMed.
- M. Zannen, et al., Structural, optical, and electrical properties of Nd-doped Na0. 5Bi0. 5TiO3, Mater. Chem. Phys., 2012, 134(2–3), 829–833 CrossRef CAS.
- R. Ranga, et al., Effect of La3+ ion doping on Structural, Magnetic and Dielectric behaviour of Mg0. 5Co0. 5Fe2O4 (0≤ x≤ 0.1), Phys. Chem. Chem. Phys., 2025,(10) 10.1039/D4CP04836A.
- Y. Alanazi, et al., Improved structural and dielectric traits of Cd-Zn spinel ferrites by co-substitution of La 3+ and Er 3+ cations, J. Ovonic Res., 2024, 20(6), 803–811 CrossRef.
- A. I. Ali, et al., Dielectric and dynamic antibacterial investigations of organic–inorganic conductive membranes based on oxidized cellulose with BNKT nanoceramics, Cellulose, 2023, 30(14), 9027–9046 CrossRef CAS.
- R. N. Chikhale, V. S. Shinde and P. G. Bhatia, Investigate structural, morphological, electrical, dielectric and magnetic properties of dysprosium doped Cobalt-Nickel ferrites and their response to gamma irradiation, Nucl. Instrum. Methods Phys. Res., B, 2024, 550, 165320 CrossRef CAS.
- L. Banaj and S. Agrawal, Dielectric behavior of Zr4+ doped MgFe2O4 spinel ferrite synthesized by solid-state reaction method, Curr. Appl. Phys., 2023, 56, 47–65 CrossRef.
- A. Hakeem, et al., Magnetic, dielectric and structural properties of spinel ferrites synthesized by sol-gel method, J. Mater. Res. Technol., 2021, 11, 158–169 CrossRef CAS.
- J.-S. An, et al., Unveiling of interstice-occupying dopant segregation at grain boundaries in perovskite oxide dielectrics for a new class of ceramic capacitors, Energy Environ. Sci., 2023, 16(5), 1992–2002 RSC.
- R. Pawar, et al., Inter-atomic bonding and dielectric polarization in Gd3+ incorporated Co-Zn ferrite nanoparticles, Phys. B, 2017, 510, 74–79 CrossRef CAS.
- D. Almond, G. Duncan and A. West, The determination of hopping rates and carrier concentrations in ionic conductors by a new analysis of ac conductivity, Solid State Ionics, 1983, 8(2), 159–164 CrossRef CAS.
- A. S. Fawzi, A. Sheikh and V. Mathe, Structural, dielectric properties and AC conductivity of Ni (1−x) ZnxFe2O4 spinel ferrites, J. Alloys Compd., 2010, 502(1), 231–237 CrossRef CAS.
- E. Oumezzine, et al., Frequency and temperature dependence of conductance, impedance and electrical modulus studies of Ni0. 6Cu0. 4Fe2O4 spinel ferrite, J. Alloys Compd., 2017, 726, 187–194 CrossRef CAS.
- P. P. Mohapatra, S. Pittala and P. Dobbidi, Temperature dependent broadband dielectric, magnetic and electrical studies on Li1-xMg2xFe5-xO8 for microwave devices, J. Mater. Res. Technol., 2020, 9(3), 2992–3004 CrossRef CAS.
- A. Rout and S. Agrawal, Structural, morphological and electrical properties of new type Dy doped Ca6-xNa2Y2 (SiO4) 6 (OH) 2 hydroxyapatite compound synthesized by Co–precipitation method, J. Electroceram., 2022, 48(2), 74–94 CrossRef CAS.
- M. Kamran and M. Anis-ur-Rehman, Resistive switching effect in RE-Doped cobalt ferrite nanoparticles, Ceram. Int., 2022, 48(12), 16912–16922 CrossRef CAS.
- M. Kamran and M. Anis-ur-Rehman, Enhanced transport properties in Ce doped cobalt ferrites nanoparticles for resistive RAM applications, J. Alloys Compd., 2020, 822, 153583 CrossRef CAS.
- M. Jebli, et al., Structural and morphological studies, and temperature/frequency dependence of electrical conductivity of Ba 0.97 La 0.02 Ti 1− x Nb 4x/5 O3 perovskite ceramics, RSC Adv., 2021, 11(38), 23664–23678 RSC.
- S. Dhanya, J. Satapathy and N. P. Kumar, Electrical properties of (Nd, Cr) co-doped Bismuth Ferrites synthesized via solid state method, Mater. Sci. Eng. B, 2023, 293, 116466 CrossRef CAS.
- A. I. Ali, et al., Dielectric and dynamic antibacterial investigations of organic–inorganic conductive membranes based on oxidized cellulose with BNKT nanoceramics, Cellulose, 2023, 30(14), 9027–9046 CrossRef CAS.
- M. Khosrozadeh, et al., Complex impedance spectroscopy, dielectric response, and magnetic properties of the La0. 7 Sr0. 3BO3 (B= Mn, Fe, Co, or Ni) perovskite oxides, Ceram. Int., 2024, 50(1), 315–328 CrossRef CAS.
- J. Mahapatro and S. Agrawal, Effect of Eu3+ ions on electrical and dielectric properties of barium hexaferrites prepared by solution combustion method, Ceram. Int., 2021, 47(14), 20529–20543 CrossRef CAS.
- K. Y. Butt, et al., The study of structural, magnetic and dielectric properties of spinel ferrites for microwave absorption applications, Appl. Phys. A, 2021, 127(9), 714 CrossRef CAS.
- M. A. Almessiere, et al., Electrical and dielectric properties of rare earth substituted hard-soft ferrite (Co0. 5Ni0. 5Ga0. 01Gd0. 01Fe1. 98O4) x/(ZnFe2O4) y nanocomposites, J. Mater. Res. Technol., 2021, 15, 969–983 CrossRef CAS.
- N. Acharya and R. Sagar, Structure and electrical properties characterization of NiMn2O4 NTC ceramics, Inorg. Chem. Commun., 2021, 132, 108856 CrossRef CAS.
- C. Stein, et al., Structural and magnetic properties of cobalt ferrite nanoparticles synthesized by co-precipitation at increasing temperatures, AIP Adv., 2018, 8, 056303 CrossRef.
- G. Allaedini, S. M. Tasirin and P. Aminayi, Magnetic properties of cobalt ferrite synthesized by hydrothermal method, Int. Nano Lett., 2015, 5, 183–186 CrossRef CAS.
- G. Yuan, et al., Structural transformation and ferroelectromagnetic behavior in single-phase Bi1− xNdxFeO3 multiferroic ceramics, Appl. Phys. Lett., 2006, 89, 052905 Search PubMed.
- D. Wang, et al., Sol–gel synthesis of Nd-doped BiFeO3 multiferroic and its characterization, Ceram. Int., 2015, 41(7), 8768–8772 CrossRef CAS.
- U. Venkat and P. Seenuvasakumaran, Synthesis, structural, micro structural, optical, magnetic and dielectric properties of Nd doped multiferroic bismuth iron oxide, Results Mater., 2023, 17, 100359 CrossRef CAS.
- S. S. Abbas, et al., Ce-substituted Co 0.5 Ni 0.5 Fe2O4: Structural, morphological, electrical, and dielectric properties, Electron. Mater. Lett., 2015, 11, 100–108 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |