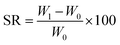Open Access Article
Open Access ArticleA mussel-like, biodegradable natural hydrogel can effectively prevent adhesion after cardiac surgery†
Jie
Jiang
a,
Liang
Chen
b,
Yan
Zhang
b and
Yan
Liu
 *b
*b
aDepartment of Cardiac Surgery, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, China
bDepartment of Cardiology, Nanjing First Hospital, Nanjing Medical University, Changle Road No. 68, Nanjing, Jiangsu 210006, China. E-mail: njyyly2024@163.com
First published on 9th June 2025
Abstract
Postoperative cardiac tissue damage can lead to adhesions between the heart and surrounding tissues. These adhesions may result in restricted cardiac function, reduced quality of heart surgeries, and an increased risk of severe bleeding during reoperations. Therefore, it is essential to develop an effective anti-adhesion therapy to address postoperative cardiac adhesions. In this study, we propose a simple and easy-to-prepare adhesive hydrogel designed to prevent adhesions following cardiac surgery while maintaining normal cardiac pumping function. Dopamine was modified onto GelMA chains via a straightforward amidation reaction and mixed with silk fibroin to form a hydrogel (GelMA-DA/SF) under ultraviolet irradiation. In vitro experiments demonstrated that the GelMA-DA/SF hydrogel exhibited strong wet adhesion and could effectively degrade within 14 days. In vivo experiments showed that the GelMA-DA/SF hydrogel effectively prevented postoperative adhesions by reducing inflammation and friction. Therefore, the GelMA-DA/SF hydrogel is a reliable material for preventing postoperative adhesions and offers new insights and directions for subsequent therapeutic approaches.
1. Introduction
Pericardial adhesion refers to the adhesion formed between the heart, major blood vessels, and surrounding tissues.1,2 It is also a major postoperative complication of cardiac surgery.3,4 Tissue adhesion is essentially a self-defense response of the body, often causing symptoms such as pain, stiffness, and loss of function.5,6 In cardiovascular surgery, particularly in cases of congenital heart disease, many patients require a second or even a third cardiac surgery.7 After the first heart surgery, varying degrees of adhesion develop between the heart, major blood vessels, and surrounding tissues over time, significantly increasing the risk of fatal complications during subsequent surgeries.8 Adhesions primarily occur due to inflammation and coagulation processes triggered by surgery, injury, or irritation. Initially, these adhesions manifest as inflammatory responses, which damage the monolayer of cells on the basement membrane in tissues.9 A large amount of inflammatory cells and fibrin-rich serous material exudes, further leading to fibroblast attachment and the formation of the vascular system. These issues, along with a decrease in fibrinolytic activity, can result in the deposition of the extracellular matrix (ECM) and the formation of adhesions.10 Currently, the primary approach to resolving postoperative adhesions is surgical adhesion lysis, but this method can lead to more severe recurrent adhesions.11Recently, there has been a growing number of reports on anti-adhesion biomaterials.12 These materials can effectively prevent postoperative adhesions by forming a physical barrier that isolates fibrin deposits from inflamed tissue surfaces.13 However, several issues remain with their practical application. On one hand, anti-adhesion materials are difficult to completely degrade in vivo, potentially leading to postoperative infections and secondary injuries. On the other hand, common anti-adhesion materials have difficulty remaining at the wound site for extended periods, resulting in limited postoperative protection. Liu et al. designed a novel Janus hydrogel adhesive, where one side firmly adheres to gastric tissue, while the other side effectively prevents postoperative adhesions. Although this material exhibits good adhesive properties, the article did not report the duration of its adhesive effectiveness.14 Guo et al. reported an underwater adhesive with anti-swelling properties that lasts for 7 days. However, the hydrogel contains various organic polymers, which not only increase the material's biotoxicity but also make it difficult to degrade, potentially causing secondary inflammation.15 Therefore, we propose a biomaterial with wet adhesive properties that can effectively secure the heart and pericardium, reducing damage caused by postoperative friction.16,17
Injectable hydrogels are considered ideal materials for preventing postoperative adhesions.18 On one hand, due to the injectability of hydrogel precursors, the liquid can be injected into the required site and form hydrogels under specific conditions, thereby increasing the contact area between the material and the tissue.19 On the other hand, hydrogels can serve as a barrier in the body to reduce friction between tissues. A variety of hydrogels derived from natural materials or synthetic polymers have been used to prevent postoperative adhesions.20 Among various injectable hydrogels, UV-polymerizable hydrogels have received increasing attention for preventing postoperative adhesions due to their excellent operability. However, these hydrogels still face several challenges in biomedical applications, such as non-biodegradability, low mechanical strength, and rapid erosion at the injection site. Particularly, when used as anti-adhesive agents to prevent postoperative adhesions in vivo, common hydrogels tend to exhibit a short retention time. Therefore, developing new hydrogels with excellent biodegradability and biocompatibility for anti-adhesion applications remains a pressing need.21
Gelatin methacrylate (GelMA) has been widely used in biomedical engineering due to its excellent biocompatibility, controllable degradability, and photo-crosslinking properties.22 On one hand, GelMA gels contain Arg-Gly-Asp (RGD) motifs and matrix metalloproteinases, which can provide sites for cell attachment. On the other hand, many studies have shown that catechol modification, inspired by mussels, can improve the poor adhesive properties of GelMA.23,24 For example, it has been reported that catechol-modified GelMA adhesive nanosheets can effectively adhere to uneven tissue surfaces under wet conditions for extended periods.25 Furthermore, various polymers, such as gelatin and silk fibroin, can form photo-crosslinked network structures by modifying photoactive groups and can be prepared as multifunctional wound dressings together with other hydrogels.26 To date, developing photo-crosslinked hemostatic wound dressings with good adhesiveness, antibacterial properties, and hemostatic properties remains a major challenge.
As shown in Fig. 1, in this study, we developed a degradable, stable, adhesive, photopolymerizable hydrogel, GelMA-DA/SF. By characterizing the mechanical properties and adhesive strength of hydrogels with different compositions, we identified the most suitable hydrogel formulation, GelMA-DA/SF-2. Through in vitro and in vivo experiments, we demonstrated that the hydrogel we designed exhibits excellent biocompatibility, anti-inflammatory effects, and other functions. More importantly, the GelMA-DA/SF hydrogel reduces friction between the heart and pericardium, preventing the formation and worsening of cardiac adhesions following cardiac surgery.
 | ||
| Fig. 1 Schematic illustration of the GelMA-DA/SF hydrogel preventing postoperative cardiac adhesions in rats. | ||
2. Materials and methods
2.1 Reagents and cells
Gelatin methacrylate (GelMA), 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), dopamine hydrochloride (DA), 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylacetophenone (2959), and silk fibroin (avg. mol wt 100 kDa, SF) were purchased from Sigma. DMEM medium, DCFH-DA detection kit, live/dead staining (AM/PI) kit, and CCK-8 kit were obtained from Nanjing KeyGen Biotech Co., Ltd. Mouse fibroblast L929 cells were purchased from the Cell Research Institute of the Chinese Academy of Sciences.2.2 Preparation of GelMA-DA/SF
| GelMA-DA (w/w) | SF (w/w) | 2959 (w/w) | |
|---|---|---|---|
| GelMA-DA/SF-1 | 5 | 10% | 1% |
| GelMA-DA/SF-2 | 5 | 20% | 1% |
| GelMA-DA/SF-3 | 5 | 30% | 1% |
2.3 Morphological characterization
The prepared hydrogel substrate was cut into 1.5 cm × 1.5 cm pieces and frozen at −20 °C. The pieces were then freeze-dried and treated with liquid nitrogen. The samples were broken in half, with the cross-section facing upwards, fixed onto a scanning electron microscope (SEM, FEI Quanta S 4800) stage using conductive double-sided tape, and then sputter-coated with gold for observation.2.4 Infrared spectroscopy
The prepared hydrogel substrate (200 mg) was frozen at −20 °C and then freeze-dried. The dried hydrogel was ground into a powder and pressed into a pellet under a 150 W infrared heat lamp, followed by analysis using an infrared spectrometer (PerkinElmer Spectrum 100 FTIR Spectrometer).2.5 Swelling analysis
Swelling tests were conducted by immersing the dried hydrogel in deionized water. The hydrogel was weighed at regular intervals after wiping off surface water at room temperature. The swelling ratio (SR) was calculated using the formula:where W0 is the sample weight before and W1 is the weight after swelling, respectively.
2.6 Mechanical and adhesion performance testing
Rheological measurements were performed to assess the viscoelastic properties of the hydrogel using a rotational rheometer (MCR302, Anton Paar GmbH, Austria). The hydrogel sample (approximately 1 mL) was placed between the plates and the precursor solution exposed to UV light and subjected to oscillatory shear stress. The storage modulus (G′) and loss modulus (G′′) were recorded as a function of temperature or time. The gelation process was monitored by measuring the dynamic moduli under continuous oscillation at a frequency of 1 Hz. The temperature sweep was conducted over a range of 20 °C to 37 °C, and the gelation point was determined based on the crossover of G′ and G′′.Then, take 10 μL of hydrogel precursor solution and apply it to the front end of a gelatin-coated glass slide. Then, overlap it with another gelatin-coated glass slide, ensuring the contact area between the two glass slides is the region coated with the hydrogel precursor solution. Place the overlapped glass slides under a UV lamp (50 mW and λ of 345–385 nm) for 5 minutes and immediately remove them afterward. Test the samples using a tensile testing machine (FLR-303, Flora Automation Technology). Fix the overlapped glass slides in the machine's clamps and set the tensile rate parameter to 1 mm min−1. Measure the maximum load that the two glass slides can withstand before separating and calculate the adhesion strength of the hydrogel. Each sample group is tested in triplicate.
2.7 Cytotoxicity testing
Seed 5000 L929 cells in each well of a 96-well plate. Use complete DMEM (100 μL) for the control group, and culture the experimental group with the extract solution of hydrogel samples. After culturing the cells in the extract solution for 1, 3, and 5 days, wash the wells with PBS. Then, prepare a 10% CCK-8 working solution using the complete DMEM medium and CCK-8 reagent. Add 100 μL of the CCK-8 working solution to each well of the plate and incubate it at 37 °C in the dark for 2 hours. After incubation, place the plate on a shaker to mix thoroughly, and then use a Multiskan FC Microplate Reader (US Thermo) to measure the absorbance of each well at 450 nm.Co-culture the hydrogel extract samples with 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L929 cells in a 24-well plate. Use complete DMEM (100 μL) for the control group, while the experimental group is cultured with the extract solution of the hydrogel samples. After 24 hours of culture, wash the wells thoroughly with PBS to remove the medium. Then, treat the wells with a live/dead staining reagent by adding 200 μL of staining solution to each well and incubate for 30 minutes in a cell culture incubator. Finally, capture images and analyze them using a fluorescence microscope (Olympus BX53, Japan).
000 L929 cells in a 24-well plate. Use complete DMEM (100 μL) for the control group, while the experimental group is cultured with the extract solution of the hydrogel samples. After 24 hours of culture, wash the wells thoroughly with PBS to remove the medium. Then, treat the wells with a live/dead staining reagent by adding 200 μL of staining solution to each well and incubate for 30 minutes in a cell culture incubator. Finally, capture images and analyze them using a fluorescence microscope (Olympus BX53, Japan).
2.8 Real-time polymerase chain reaction (RT-PCR)
QRT-PCR was used to measure the gene expression levels. Total RNA was extracted from cells using TRIzol reagent (Invitrogen). RNA purity was checked using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RNA was reverse transcribed using the PrimeScript™ RT kit (Takara, Tokyo, Japan) and then the obtained cDNAs were quantified using RT-qPCR assay labelled with an SYBR Green PCR Mix Kit (Takara) according to the instructions. The RT-qPCR results were quantified with the 2-ΔΔCT method.2.9 In vivo anti-adhesion testing
A cardiac adhesion model was used to test the in vivo anti-adhesion performance of the hydrogels.27 Male SD rats (10–12 weeks) were obtained from Beijing Sibeifu Biotechnology Co., Ltd. SD rats were anesthetized with tribromoethanol, intubated, and mechanically ventilated. A thoracotomy was performed to expose the heart, and the pericardium was torn to expose it further. The exposed heart surface was punctured 100 times using a 30 mL needle and air-dried for 10 minutes. The experimental group injected 200 μL of GelMA-DA/SF hydrogel droplets onto the surface of the injured heart and irradiated it with 50 mW at a wavelength of 345–385 nm for 5 minutes to form the hydrogel, then closed the thoracic cavity and sutured the wound. In the control group, no treatment was applied before wound closure. After two weeks, the rats were dissected to observe the adhesion status. Tissue samples from the adhesion area, heart, lungs, spleen, liver, and kidneys were collected, paraffin-embedded, sectioned, and stained with H&E and Masson's trichrome. Images were obtained using a microscope (Olympus BX53, Japan) and Olympus imaging software.2.10 Statistical analysis
Statistical significance in all graphs was indicated by P values (***, P < 0.001). Statistical differences were considered significant, and NS indicated no significant difference.3. Results and discussion
3.1 Synthesis and characterization of GelMA-DA
GelMA-DA was synthesized using the method shown in Fig. 2A. Briefly, under the catalysis of EDC/NHS, the carboxyl groups on the GelMA chain were activated, allowing an amidation reaction with the amine groups on DA. The reaction product was characterized by infrared spectroscopy (Fig. 2B), where the characteristic peaks at 1550 cm−1 and 1679 cm−1 corresponded to the amide I and amide II bands, respectively. Additionally, ultraviolet (UV) spectroscopy was performed on the samples before and after DA grafting. As shown in Fig. 2C, DA exhibited a significant UV absorption peak at 280 nm, and the intensity of absorption increased with the DA concentration in the solution, due to the catechol groups on DA. These data confirm the successful synthesis of GelMA-DA. Subsequently, GelMA-DA was mixed with SF and polymerized under UV light to form a GelMA-DA/SF hydrogel (Fig. 2D). The hydrogel precursor displayed excellent injectability and rapid gelation, laying the foundation for subsequent in vivo experiments. SEM images showed that the GelMA-DA/SF hydrogel formed a three-dimensional network (Fig. 2E). When comparing the GelMA-DA/SF hydrogel with existing cardiac repair adhesives, our approach does not require complex synthesis steps, and the raw materials are all natural components with excellent biosafety. However, to improve the underwater adhesion strength of the material, we introduced catechol groups through chemical modification, which still somewhat limits the clinical application of the GelMA-DA/SF hydrogel. In future experiments, we hope to prepare porous or fibrous structures through electrospinning or freeze-drying to enhance underwater adhesion via mechanical interlocking. This still requires further validation. In summary, we grafted dopamine onto GelMA using EDC/NHS catalysis to form GelMA-DA. After confirming its structure through spectral characterization, we copolymerized it with SF to obtain a GelMA-DA/SF hydrogel with injectability, rapid gelation, and a three-dimensional network structure.3.2 Mechanical properties and adhesion performance of GelMA-DA/SF hydrogels
To ensure that the GelMA-DA/SF hydrogel we designed can effectively prevent postoperative adhesions, it is necessary for the hydrogel to have sufficient wet adhesion strength to stabilize the heart's adhesion to the pericardium, reducing friction between the heart and surrounding tissues. Additionally, the GelMA-DA/SF hydrogel should be biodegradable post-surgery to minimize inflammation and wound complications caused by foreign materials. Therefore, we adjusted the SF content and labeled the hydrogels as GelMA-DA/SF-1, GelMA-DA/SF-2, and GelMA-DA/SF-3, and characterized their mechanical properties and adhesion strength.As shown in Fig. 3A, the swelling ratio of the hydrogels increased over time, reaching equilibrium at around 8 hours. The swelling ratio also increased with higher SF concentrations. Furthermore, we analyzed the in vitro degradation performance of the hydrogels. As shown in Fig. 3B, the hydrogels gradually degraded over time, reaching equilibrium after approximately 3–4 days. The degradation rate of GelMA-DA/SF increased with higher SF concentrations, which is related to the degree of crosslinking within the hydrogel. Furthermore, we observed that after 14 days, more than 90% of the GelMA-DA/SF-1 group had degraded. This excellent degradability prevents secondary infections caused by foreign implants. Currently reported in vivo adhesives are often chemically synthesized gels, which, although they offer stable adhesion strength, still require surgical removal of the implant after treatment. In contrast, our polysaccharide-based hydrogel can degrade stably within 14 days, offering significant application value.
Rheological analysis further evaluated the mechanical properties of the hydrogels. As shown in Fig. 3C, within the range of 0.01–100 rad s−1, the storage modulus (G′) of the hydrogels was consistently greater than the loss modulus (G′′), indicating the successful formation of GelMA-DA/SF hydrogels. The storage modulus of GelMA-DA/SF increased with higher SF concentrations, consistent with the degradation results. Under 1–100% strain, the hydrogels exhibited a crossover point between G′ and G′′. These data indicate that the designed hydrogels have good degradability, making them suitable for preventing in vivo adhesions.
The GelMA-DA/SF hydrogels also demonstrated excellent adhesion properties. As shown in Fig. 4A, the hydrogels adhered to various substrates and could attach wet rat viscera to glass slides. According to the procedure in Fig. 4B, we tested the adhesion strength of the hydrogels. As shown in Fig. 4C, the adhesion strength of pure GelMA-DA was only 1.4 kPa, which is related to the poor mechanical properties of GelMA hydrogels. After adding SF, the adhesion strength of GelMA-DA/SF significantly increased. As the SF concentration increased, the adhesion strength of GelMA-DA/SF hydrogels first increased and then decreased. This can be attributed to two factors. First, when the SF concentration is low, it may act as a reinforcing material, filling the structural gaps in GelMA to form a more compact network, thereby improving mechanical properties and adhesion strength. However, an excessive amount of SF may hinder the crosslinking of GelMA itself, forming an overly rigid structure that makes the hydrogel brittle and unable to effectively adhere to the surface, thus reducing adhesion strength. Additionally, SF can affect the swelling rate of the hydrogel. Appropriate swelling can promote interface wetting (such as filling surface micropores), but excessive swelling can lead to the accumulation of volumetric expansion stress, weakening the interfacial bonding. This results in our material exhibiting a trend of first increasing and then decreasing adhesion strength as the SF concentration increases. We also tested the stability of the adhesion strength of GelMA-DA/SF hydrogels. As shown in Fig. 4D, the adhesion strength of all hydrogel groups decreased over time, and the rate of decrease slowed with higher SF concentrations. On the 14th day, the adhesion strength of the GelMA-DA/SF-2 group was only 0.16 kPa. On one hand, the GelMA-DA/SF hydrogel provided adhesion prevention post-surgery, and on the other hand, it ensured post-surgical biodegradability. The GelMA-DA/SF-3 hydrogel maintained a high adhesion strength even after 14 days, which could hinder post-surgical heart recovery. Conversely, the GelMA-DA/SF-1 material exhibited poor mechanical properties. Therefore, we selected the GelMA-DA/SF-2 hydrogel for subsequent in vivo experiments.
3.3 Biocompatibility and anti-inflammatory properties
Good biocompatibility is essential for the success of in vivo experiments.28 The cytotoxicity of GelMA-DA/SF-2 hydrogels was evaluated using live/dead staining and the CCK-8 assay. As shown in Fig. 5A, cells remained in good condition after 5 days of culture. The CCK-8 assay results also indicated that cell viability remained above 95% (Fig. 5B), demonstrating the excellent biocompatibility of our materials. Furthermore, our material also demonstrates excellent hemocompatibility (Fig. S1†). To test the in vivo biocompatibility of the PMPC polymer, we directly injected the GelMA-DA/SF-2 precursor solution into the peritoneal cavity and irradiated it with UV light for 10 minutes to form the hydrogel. After 14 days, we collected blood from the rats and performed hematological and clinical biochemical tests, as well as pathological evaluations of the organs. The hematological and clinical biochemical results were similar and showed no systemic toxicity (Tables 3 and 4). Moreover, we found no significant differences between the two groups in the HE staining (Fig. S3†), indicating that GelMA-DA/SF exhibits excellent in vivo biosafety.Additionally, we tested the anti-inflammatory properties of GelMA-DA/SF-2 hydrogels. An inflammation model was established by co-culturing cells with LPS for 24 hours. Subsequently, GelMA-DA/SF hydrogel extracts were added, and ROS levels were measured after 24 hours. As shown in Fig. 5C and S2,† compared to the control group, the ROS levels in the GelMA-DA/SF group were significantly reduced. Furthermore, qt-PCR was used to detect the expression of inflammatory cytokines after GelMA-DA/SF-2 treatment. As shown in Fig. 5D, the expression of TNF-α, IL-6, and IL-8 was significantly reduced in the treatment group. These results indicate that GelMA-DA/SF-2 hydrogels not only have excellent biocompatibility but also possess strong anti-inflammatory effects in vitro, providing a basis for post-surgical recovery.
3.4 The construction of the rat cardiac adhesion model
Based on previous studies, a rat cardiac adhesion model was selected to evaluate the anti-adhesion performance of GelMA-DA/SF-2 hydrogels. As shown in Fig. 6A, a schematic diagram illustrates the model. The procedure began with a midline neck incision to separate the thyroid. A left anterior chest incision was then made to separate the pectoralis major and serratus anterior muscles. The thoracic cavity was opened between the 4th and 5th ribs. To induce trauma, the heart surface was pricked 100 times and air-dried for 30 minutes. A drainage tube was placed before closing the thoracic cavity. After removing the tracheal tube, the tracheal and thoracic incisions were sutured.As illustrated in Fig. 6B, the thoracic cavities were reopened on days 3, 7, and 14 to assess adhesion formation. No adhesion was observed on day 3, partial adhesion between the chest wall and heart was observed on day 7, and significant adhesion was seen by day 14. Thus, day 14 was selected as the sampling point for subsequent experiments. Injection of the GelMA-DA/SF-2 hydrogel into the pericardial space reduced friction between the heart and pericardium, thereby decreasing the likelihood of postoperative adhesions.
3.5 In vivo anti-adhesion performance
As shown in Fig. 7A, after 14 days of treatment, there was no adhesion tissue between the heart and chest walls of the GelMA-DA/SF-2 hydrogel group. However, the heart and chest walls of the model group were tightly adhered. Further histological analysis, including H&E and Masson staining, was conducted to evaluate tissue recovery in the treatment group. As illustrated in Fig. 7B, the results from the H&E and Masson staining corroborated the previous findings. In the staining images of the model group (as indicated by arrows), a significant amount of adhesion tissue was observed between the myocardium and ribs. The GelMA-DA/SF-2 hydrogel demonstrated a reduction in collagen deposition, which is likely a critical factor in preventing the formation of adhesions.The adhesion scores of the two groups were evaluated using a scoring system that ranged from no adhesion to very dense vascularized adhesion.29 Over 50% of the GelMA-DA/SF-2 hydrogel group scored 0, whereas the majority of the model group scored 4 or 5 (Fig. 7C and Table 2). Additionally, a pathological analysis of cardiac adhesion tissue was performed. As depicted in Fig. 7D and E, the treatment group showed a marked reduction in the expression of inflammation-related cytokines.
| Score | The condition of abdominal-blind cecum adhesion |
|---|---|
| 0 | No adhesions |
| 1 | Very little adhesion |
| 2 | Small to moderate amounts of adhesion |
| 3 | Moderate to substantial adhesion |
| 4 | Severe adhesion |
| Parameters | GelMA-DA/SF-2 | Control |
|---|---|---|
| a White blood cell (WBC); red blood cell (RBC); hemoglobin (HgB); platelet (PLT); neutrophil proportion (NEUT); lymphocyte proportion (LYMPH); monocyte proportion (MONO); eosinophil proportion (EO); basophil proportion (BASO). | ||
| WBC [109 L−1] | 8.62 ± 1.35 | 8.62 ± 1.53 |
| RBC [1012 L−1] | 7.34 ± 1.35 | 7.22 ± 0.31 |
| HgB [g L−1] | 146.67 ± 2.36 | 165.28 ± 4.73 |
| PLT [109 L−1] | 658.25 ± 46.36 | 612.67 ± 42.34 |
| NEUT [%] | 18.17 ± 2.13 | 20.36 ± 3.42 |
| LYMPH [%] | 81.25 ± 4.22 | 75.44 ± 3.68 |
| MONO [%] | 4.74 ± 0.59 | 4.25 ± 0.64 |
| EO [%] | 0.37 ± 0.29 | 0.37 ± 0.06 |
| BASO [%] | 0.12 ± 0.02 | 0.11 ± 0.04 |
| WBC [109/L] | 9.25 ± 1.48 | 9.45 ± 1.23 |
| Parameters | GelMA-DA/SF-2 | Control |
|---|---|---|
| a Alanine amino transferase (ALT); aspartate amino transferase (AST); alkaline phosphatase (ALP); blood urea nitrogen (BUN); total protein (TP); glucose (GLU); cholesterol (CHOL); total bilirubin (TBIL); creatine kinase isoenzyme (CK-MB); creatinine (CR). | ||
| ALT [U L−1] | 59.34 ± 4.65 | 62.33 ± 3.68 |
| AST [U L−1] | 126.17 ± 8.52 | 131.58 ± 7.69 |
| ALP [U L−1] | 94.68 ± 7.68 | 98.57 ± 7.35 |
| BUN [mmol L−1] | 5.77 ± 0.44 | 6.74 ± 0.78 |
| TP [g L−1] | 85.37 ± 4.17 | 93.03 ± 8.46 |
| GLU [mmol L−1] | 0.59 ± 0.29 | 0.54 ± 0.06 |
| CHOL [mmol L−1] | 2.36 ± 0.44 | 2.52 ± 0.24 |
| TBIL [mmol L−1] | 1.20 ± 0.14 | 0.90 ± 0.16 |
| CK-MB [U L−1] | 424.29 ± 36.58 | 384.29 ± 21.34 |
| CR [μmol L−1] | 30.67 ± 2.52 | 30.42 ± 1.53 |
To further assess the impact of GelMA-DA/SF-2 hydrogels on rat organs, a pathological examination was carried out on the hearts, livers, spleens, lungs, and kidneys of both the SD rat model group and the GelMA-DA/SF-2 hydrogel group. As shown in Fig. 8A, in comparison to the treatment group, the model group exhibited more void-like structures between myocardial fibers in the left ventricular tissues, likely due to adhesion-induced cardiac dysfunction. Rats in the model group also demonstrated extensive inflammatory cell infiltration and significant pulmonary congestion in the lungs. Furthermore, varying levels of hepatic, splenic, and renal congestion were noted in all rats, indicating right ventricular dysfunction, which was more pronounced in the model group. In contrast, the GelMA-DA/SF-2 hydrogel group showed only mild cardiac dysfunction, characterized by splenic and renal congestion, which is likely attributed to direct cardiac injury during model establishment rather than cardiac adhesion. Overall, the GelMA-DA/SF-2 hydrogel effectively prevented cardiac adhesions and helped maintain the normal function of other organs in the rats.
4. Conclusion
In this study, we developed a simple and easily prepared adhesive hydrogel for the prevention of post-cardiac surgery adhesions. The ratio of components in the GelMA-DA/SF hydrogel was optimized through rheological analysis and adhesion performance testing, and the selected GelMA-DA/SF-2 hydrogel was used for subsequent experiments. The in vitro results showed that the GelMA-DA/SF hydrogel exhibited excellent wet adhesion properties, could adhere to various substrates, and degraded by 80% within 14 days. Additionally, the GelMA-DA/SF hydrogel demonstrated good biocompatibility and anti-inflammatory effects. In vivo experiments indicated that the GelMA-DA/SF hydrogel could prevent cardiac adhesions by reducing inflammation and minimizing friction between the heart and pericardium. Therefore, the GelMA-DA/SF hydrogel we designed is a safe and effective approach, providing new insights and methods for preventing postoperative adhesions in cardiac surgery patients.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Nanjing Medical University and experiments were approved by the Animal Ethics Committee of Nanjing First Hospital.Data availability
The data are available in the main manuscript, ESI† files, and from the corresponding authors upon reasonable request.Author contributions
J. J. contributed to the experimental design and data analysis, L. C. and Y. Z. were responsible for the synthesis and characterization of the biodegradable natural hydrogel, and Y. L. supervised the project and provided guidance throughout the research process. All the authors reviewed the manuscript.Conflicts of interest
The authors declare that they have no competing interests.References
- S. J. Wu, J. Wu, S. J. Kaser, H. Roh, R. D. Shiferaw and H. Yuk, et al., A 3D printable tissue adhesive, Nat. Commun., 2024, 15(1), 1215 CrossRef CAS.
- P. Chansoria, A. Chaudhari, E. L. Etter, E. E. Bonacquisti, M. K. Heavey and J. Le, et al., Instantly adhesive and ultra-elastic patches for dynamic organ and wound repair, Nat. Commun., 2024, 15(1), 4720 Search PubMed.
- Y. Wang, W. Zhai, H. Zhang, S. Cheng and J. Li, Injectable Polyzwitterionic Lubricant for Complete Prevention of Cardiac Adhesion, Macromol. Biosci., 2023, 23(4), e2200554 CrossRef.
- L. M. Stapleton, A. N. Steele, H. Wang, H. Lopez Hernandez, A. C. Yu and M. J. Paulsen, et al., Use of a supramolecular polymeric hydrogel as an effective post-operative pericardial adhesion barrier, Nat. Biomed. Eng., 2019, 3(8), 611–620 Search PubMed.
- J. Stehlik, J. Kobashigawa, S. A. Hunt, H. Reichenspurner and J. K. Kirklin, Honoring 50 Years of Clinical Heart Transplantation in Circulation, Circulation, 2018, 137(1), 71–87 Search PubMed.
- H. E. Emam and T. I. Shaheen, Design of a dual pH and temperature responsive hydrogel based on esterified cellulose nanocrystals for potential drug release, Carbohydr. Polym., 2022, 278, 118925 CrossRef CAS.
- S. Li, W. Ahmed, S. Wang, X. Hu, B. Wang and Z. Wang, et al., Bi-layered polyurethane nanofiber patches with asymmetrical surface prevent postoperative adhesion and enhance cardiac repair, Composites, Part B, 2024, 283, 111668 CrossRef CAS.
- E. Zhang, J. Li, Y. Zhou, P. Che, B. Ren and Z. Qin, et al., Biodegradable and injectable thermoreversible xyloglucan based hydrogel for prevention of postoperative adhesion, Acta Biomater., 2017, 55, 420–433 CrossRef CAS.
- H. Fan, J. Wang and J. P. Gong, Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion, Adv. Funct. Mater., 2020, 31(11), 2009334 Search PubMed.
- Y. Lv, F. Cai, X. Zhao, X. Zhu, F. Wei and Y. Zheng, et al., Bioinspired Microstructured Janus Bioadhesive for the Prevention of Abdominal and Intrauterine Adhesions, Adv. Funct. Mater., 2024, 34(21), 2314402 CrossRef CAS.
- T. Wu and W. Liu, Functional hydrogels for the treatment of myocardial infarction, NPG Asia Mater., 2022, 14(9), 1–15 CAS.
- D. Zhu, Z. Li, K. Huang, T. G. Caranasos, J. S. Rossi and K. Cheng, Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair, Nat. Commun., 2021, 12(1), 1412 Search PubMed.
- M. Fujita, G. M. Policastro, A. Burdick, H. T. Lam, J. L. Ungerleider and R. L. Braden, et al., Preventing post-surgical cardiac adhesions with a catechol-functionalized oxime hydrogel, Nat. Commun., 2021, 12(1), 3764 Search PubMed.
- C. Cui, T. Wu, X. Chen, Y. Liu, Y. Li and Z. Xu, et al., A Janus Hydrogel Wet Adhesive for Internal Tissue Repair and Anti-Postoperative Adhesion, Adv. Funct. Mater., 2020, 30(49), 2005689 Search PubMed.
- Y. Liang, H. Xu, Q. Han, M. Xu, J. Zhang and J. Wang, et al., A Janus hydrogel sealant with instant wet adhesion and anti-swelling behavior for gastric perforation repair, Nano Today, 2024, 54, 102105 CrossRef CAS.
- G. M. Taboada, K. Yang, M. J. N. Pereira, S. S. Liu, Y. Hu and J. M. Karp, et al., Overcoming the translational barriers of tissue adhesives, Nat. Rev. Mater., 2020, 5(4), 310–329 Search PubMed.
- S. Huang, D. Lei, Q. Yang, Y. Yang, C. Jiang and H. Shi, et al., A perfusable, multifunctional epicardial device improves cardiac function and tissue repair, Nat. Med., 2021, 27(3), 480–490 CrossRef CAS PubMed.
- Y. Liu, Q. Wang, X. Liu, P. Nakielski, F. Pierini and X. Li, et al., Highly Adhesive, Stretchable and Breathable Gelatin Methacryloyl-based Nanofibrous Hydrogels for Wound Dressings, ACS Appl. Bio Mater., 2022, 5(3), 1047–1056 CrossRef CAS PubMed.
- M. Montgomery, S. Ahadian, L. Davenport Huyer, M. Lo Rito, R. A. Civitarese and R. D. Vanderlaan, et al., Flexible shape-memory scaffold for minimally invasive delivery of functional tissues, Nat. Mater., 2017, 16(10), 1038–1046 CrossRef CAS PubMed.
- L. Song, L. Li, T. He, N. Wang, S. Yang and X. Yang, et al., Peritoneal adhesion prevention with a biodegradable and injectable N,O-carboxymethyl chitosan-aldehyde hyaluronic acid hydrogel in a rat repeated-injury model, Sci. Rep., 2016, 6(1), 37600 CrossRef CAS.
- C. K. Roy, H. L. Guo, T. L. Sun, A. B. Ihsan, T. Kurokawa and M. Takahata, et al., Self-Adjustable Adhesion of Polyampholyte Hydrogels, Adv. Mater., 2015, 27(45), 7344–7348 CrossRef CAS.
- P. Rao, T. L. Sun, L. Chen, R. Takahashi, G. Shinohara and H. Guo, et al., Tough Hydrogels with Fast, Strong, and Reversible Underwater Adhesion Based on a Multiscale Design, Adv. Mater., 2018, 30(32), e1801884 CrossRef PubMed.
- A. Hasan, A. Khattab, M. A. Islam, K. A. Hweij, J. Zeitouny and R. Waters, et al., Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction, Adv. Sci., 2015, 2(11), 1500122 CrossRef.
- Z. Zhang, J. Ni, L. Chen, L. Yu, J. Xu and J. Ding, Encapsulation of cell-adhesive RGD peptides into a polymeric physical hydrogel to prevent postoperative tissue adhesion, J. Biomed. Mater. Res., Part B, 2012, 100(6), 1599–1609 CrossRef PubMed.
- J. Yu, K. Wang, C. Fan, X. Zhao, J. Gao and W. Jing, et al., An Ultrasoft Self-Fused Supramolecular Polymer Hydrogel for Completely Preventing Postoperative Tissue Adhesion, Adv. Mater., 2021, 33(16), e2008395 CrossRef.
- Y. He, Q. Li, P. Chen, Q. Duan, J. Zhan and X. Cai, et al., A smart adhesive Janus hydrogel for non-invasive cardiac repair and tissue adhesion prevention, Nat. Commun., 2022, 13(1), 7666 CrossRef CAS.
- M. Cencer, Y. Liu, A. Winter, M. Murley, H. Meng and B. P. Lee, Effect of pH on the Rate of Curing and Bioadhesive Properties of Dopamine Functionalized Poly(ethylene glycol) Hydrogels, Biomacromolecules, 2014, 15(8), 2861–2869 Search PubMed.
- L. Yuan, H. Wei, Z. Pan, X. Deng, L. Yang and Y. Wang, et al., A bioinspired injectable antioxidant hydrogel for prevention of postoperative adhesion, J. Mater. Chem. B, 2024, 12(28), 6968–6980 RSC.
- X. Wang, L. Xiang and Y. Peng, et al., Gelatin/Polycaprolactone Electrospun Nanofibrous Membranes: The Effect of Composition and Physicochemical Properties on Postoperative Cardiac Adhesion, Front. Bioeng. Biotechnol., 2022, 10, 862276 ( Front. Bioeng. Biotechnol. , 2021 , 9 , 792893 ) CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5na00005j |
| This journal is © The Royal Society of Chemistry 2025 |