 Open Access Article
Open Access ArticleUnique advantages and applications of polysaccharide microneedles as drug delivery materials and in treatment of skin diseases
Chao
Liu†
 abc,
Meng
Liu†
ab,
Xin
Li†
ab,
Yimei
Hu
ab,
Lingling
Zhang
a,
Feng-Ming
You
*ab,
Gang
Fan
abc,
Meng
Liu†
ab,
Xin
Li†
ab,
Yimei
Hu
ab,
Lingling
Zhang
a,
Feng-Ming
You
*ab,
Gang
Fan
 *b and
Yiman
Ge
*ab
*b and
Yiman
Ge
*ab
aHospital of Chengdu University of Traditional Chinese Medicine, Chengdu, 610072, China. E-mail: fmdoc@163.com
bChengdu University of Traditional Chinese Medicine, Chengdu, 611137, China. E-mail: fangang1111@163.com; geyiman@cdutcm.edu.cn
cChongqing Key Laboratory of Sichuan-Chongqing Co-construction for Diagnosis and Treatment of Infectious Diseases Integrated Traditional Chinese and Western Medicine, China
First published on 12th April 2025
Abstract
Owing to its non-invasive nature, painless drug delivery, and controlled drug loading capacity, the microneedle (MN) technology has recently garnered significant attention in clinical practice. For instance, it has been pervasively employed as an innovative transdermal delivery method in skin disease therapy. However, traditional MN techniques have been associated with challenges regarding biocompatibility, biodegradability, and drug release precision, limiting their clinical efficacy and increasing the risk of side effects resulting from uneven drug distribution. To address these issues, polysaccharide materials have been proposed as viable alternatives to be used in MN technologies. In addition to their excellent biocompatibility and biodegradability, polysaccharide materials such as alginate, chitosan, and Hyaluronic Acid (HA), among other Traditional Chinese Medicine (TCM)-extracted polysaccharides (such as Bletilla and notoginseng), could also exert anti-inflammatory and antibacterial effects, promoting tissue regeneration. These attributes enable polysaccharide-based MNs to improve the local drug concentration, reduce systemic side effects, minimize patient discomfort, and lower treatment risks, making them particularly suitable for treating skin conditions such as eczema, psoriasis, and acne. This article systematically reviews the properties of various polysaccharide materials, as well as the preparation methods of polysaccharide-based MNs and their therapeutic effects as reported in animal models and clinical trials. Our findings could lay a solid theoretical foundation for developing polysaccharide-based MN technologies and fostering their widespread clinical application.
| Chao Liu Chao Liu is currently working at the Hospital of Chengdu University of Traditional Chinese Medicine and the College of Medical Technology, Chengdu University of Traditional Chinese Medicine. They have been engaged in research and clinical work related to traditional Chinese medicine and medical technology in this hospital and college. Research interests: they are currently focused on research on polysaccharide-based microneedles and their applications in the treatment of skin diseases, as well as exploration of the combination of traditional Chinese medicine and modern medical technology. |
| Yimei Hu Yimei Hu is currently working at the Hospital of Chengdu University of Traditional Chinese Medicine and Chengdu University of Traditional Chinese Medicine. They are engaged in teaching, research and clinical work related to traditional Chinese medicine in the hospital and university. They are interested in research on traditional Chinese medicine polysaccharides and their pharmacological activities in skin diseases. |
| Lingling Zhang Lingling Zhang is currently working at the Hospital of Chengdu University of Traditional Chinese Medicine. |
| Feng-Ming You Feng-Ming You is currently working at the Hospital of Chengdu University of Traditional Chinese Medicine and the TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine. |
| Gang Fan Gang Fan is currently working at the School of Ethnic Medicine, Chengdu University of Traditional Chinese Medicine. They are engaged in teaching and research in the fields of ethnic medicine and related biomedical fields. They are interested in the exploration of the integration of traditional ethnic medicine and modern medical technology, especially in the application of polysaccharide materials. |
| Yiman Ge Yiman Ge is currently working at the Department of Clinical Laboratory, Hospital of Chengdu University of Traditional Chinese Medicine and Chengdu University of Traditional Chinese Medicine. They participated in the work of the clinical laboratory and related research in the hospital and university. They focus on research on biomarkers and diagnostic methods related to skin diseases, as well as the application of polysaccharide-based materials in diagnosis and treatment. |
1 Introduction
Presently, skin disease therapy presents numerous challenges, including pharmacotherapeutic inefficacies in penetrating the skin barrier, poor patient compliance during treatment, and the risk of side effects. Due to skin barrier-imposed limitations on osmotic quantity and duration of Active Pharmaceutical Ingredients (APIs), traditional treatment approaches often fail to achieve optimal results and meet patient demands.1The drug delivery efficiency of topical creams is relatively low, with research data indicating that only 10–20% of active ingredients can penetrate the skin barrier to complete transdermal absorption.2 Although transdermal patches can overcome the physical barrier of the stratum corneum, their drug bioavailability remains significantly limited. While the addition of chemical penetration enhancers can improve drug permeability to some extent, the improvement is very limited.3 Traditional subcutaneous injections can achieve a drug delivery efficiency of 90–100%, but because they require puncturing into the dermis layer rich in nociceptors, they are often accompanied by significant pain responses, seriously affecting patients' treatment compliance. In contrast, microneedle (MN) delivery systems physically cross the stratum corneum barrier, precisely delivering drugs to the superficial skin layer without pain nerve distribution, achieving painless drug delivery while ensuring 100% delivery efficiency. (Table 1 presents a comparative discussion of various transdermal drug delivery systems.)
| Dosage form | Mechanism | Pain | Efficiency | Bioavailability | Compliance |
|---|---|---|---|---|---|
| Cream | Passive transdermal permeation | None | Low | Low | Moderate |
| Patch | Transcorneal diffusion | None | Relatively low | Relatively low | High |
| Injection needle | Dermal injection | Present | Relatively high | Relatively high | Low |
| Microneedle | Stratum corneum penetration | None | High | High | High |
Microneedle (MN) technology, an innovative transdermal drug delivery approach, could penetrate the skin barrier with micrometer-scale precision. As a result, it can deliver drugs directly into the skin to enhance local drug concentration and bioavailability while reducing systemic side effects and the dosing frequency.4 This technique has been established to significantly improve the drug absorption efficiency and patient compliance. Furthermore, the length of MNs often ranges in several hundred microns, which is sufficient for penetrating the epidermal layer without reaching deeper nociceptors, ensuring significantly reduced pain sensation during drug administration (Fig. 1). Due to these attributes, the MN technology effectively overcomes limitations associated with traditional transdermal drug delivery systems.5,6
Based on material composition, the MN technology could be classified into metal, polymer, protein, and macromolecular polysaccharide types.7–10 Although metal MNs offer high intensity, biocompatibility issues have been reported to limit their practical application. Furthermore, polymer MNs have shown instability in degradability and drug release control. Protein MNs, on the other hand, may easily decompose or degenerate due to their complex preparation processes. Conversely, polysaccharides have demonstrated good biocompatibility, low immunogenicity, easy biodegradation, and a unique medicinal value.11 Polysaccharide materials are also readily available from various sources, making them easy to process to form good gels.12,13
In addition to offering a means of drug delivery, polysaccharide MN materials also possess inherent medicinal values. For instance, due to its natural antimicrobial properties, chitosan exhibits effectiveness against bacterial skin infections.14 Additionally, Hyaluronic Acid (HA) can retain moisture and promote skin repair, making it suitable for addressing dry and inflammatory skin diseases.15 On the other hand, due to its superior gel-forming capabilities and flexibility in chemical modifications, alginate has been recognized as an excellent hemostatic biomaterial.16 It has also been established that polysaccharides generally possess unique hemostatic and antibacterial properties which could expedite the healing of superficial skin lesions and mitigate the risk of infections.17 Moreover, Panax notoginseng polysaccharides have been increasingly incorporated into MN technologies due to their notable antioxidant, antitumor, and antidiabetic properties.18 These attributes collectively suggest the dual advantage of polysaccharide MNs in the treatment of dermatological diseases, highlighting their potential clinical significance.
Although the applications of polysaccharide hydrogels in obesity management and polysaccharide MNs in treating ophthalmic diseases have been systematically reviewed in the existing literature,19–22 the use of polysaccharide MNs in treating dermatological conditions is yet to be comprehensively reviewed. This article systematically reviews the preparation processes of polysaccharide MNs and analyzes their clinical value in drug delivery, enhancing patient compliance, and promoting treatment innovations. We also summarize their applications and transdermal delivery mechanisms across various skin diseases.
2 Classification and delivery mechanisms of MNs
It is worth noting that MNs can be further classified into solid, dissolving, coating, hydrogel, and hollow types (Fig. 2 and Table 2). Solid MNs physically penetrate the skin's cutin layer, creating a microchannel for the direct delivery of drug molecules to the dermis.23 On the other hand, hollow MNs utilize the tip cavity and passive diffusion mechanisms such as pressure or electromigration to deliver drugs to the target position.24 Hydrogel MNs absorb body fluids after insertion into the skin, then swell and promote drug diffusion and release.25 Dissolving MNs leverage fluid hydration post-insertion, rapidly releasing drug payloads directly below the skin.26 Finally, coating MNs surface coat with drug solutions or dispersions to achieve drug release.27| Types | Materials | Advantages | Disadvantages |
|---|---|---|---|
| Solid MNs | Metal and ceramics, among others | Possess mechanical strength and not easily broken | There is residue, causing strong discomfort |
| Coated MNs | PVP and PEG, among others | Flexible drug release is strong, no residue | The coating process is complex, and the coat easily falls off |
| Hollow MNs | Silicon and metal, among others | Controllable (active drug release AND release rate modulation) and less skin damage | Small diameter, easy to block, and easy to break |
| Hydrogel MNs | PVG and PVP, among others | Dissolves completely without residue | Low mechanical strength |
| Dissolving MNs | Polysaccharides and PVP, among others | Degradable, safe, and rapid dissolution | Weak penetration and delayed dissolution |
Polysaccharide MNs primarily comprise the dissolving and hydrogel types. Dissolving MNs are often made of soluble materials such as HA, chitosan, or alginate, which have good biocompatibility and degradability. Moreover, polysaccharides are often the preferred materials for preparing dissolving MNs. Notably, hydrogel polysaccharide molecules could also be modified chemically for superior compatibility and versatility. Under biological catalysis, the amplification of the degradation of polysaccharide-based water gel MNs could be accelerated, allowing for the accurate control of drug release kinetics. Furthermore, when used to coat needle surfaces, polysaccharide components such as trehalose or glucan could function as stabilizers, thickeners, and emulsifiers. In addition to improving coating uniformity, these polysaccharides could also maintain drug activity, as well as ensure drug stability during storage and precise release.28
3 Types of polysaccharide materials
Polysaccharide materials play crucial roles in a wide range of applications, and their nature and function depend largely on their source and chemical structure (Fig. 3 and Table 3).| Name of polysaccharide | Sources | Advantages |
|---|---|---|
| Hyaluronic acid | Vertebrates, microbes, and shark skin | Moisturizing effect and promotes healing |
| Alginate | Macroalgae and wakame | Easy to shape and forms a stable structure |
| Chitosan | Crustaceans and arthropods | Anticancer, antibacterial, and anti-inflammatory |
| Panax notoginseng polysaccharides | Notoginseng | Immunomodulatory and antitumor |
| Pectin | Peels | Gelling agent, healthfulness, and sustainability |
| Xanthan gum | Xanthomonas species | Thickening agent, provides stability, and resistant to processing |
| Glucan | Specific bacteria | Immunogenic and activates immunity |
| Carboxymethyl cellulose | Cellulose is chemically modified | Good biocompatibility and soluble in water |
| Chondroitin sulfate | Shark cartilage, cows, pigs, etc. | Good solubility and high bioavailability |
| Bletilla striata polysaccharides | Bletilla striata | Anti-inflammatory and promotes healing |
3.1 Marine polysaccharides
Studies have also reported HA's good solubility both in vivo and in vitro, further highlighting its potential for application in MNs. Liu et al. developed HA MNs and assessed their drug delivery capabilities using high molecular weight dextran (FD4) as the model drug. According to the results, HA-FD4 demonstrated a significantly higher transdermal permeability than the traditional FD4 solution, leading to a substantial increase in FD4 accumulation in the skin. Furthermore, in addition to successfully penetrating rat skin without bending or cracking, the array MNs also dissolved within 5 min, demonstrating their efficient drug delivery capabilities and good mechanical properties.31 Moreover, studies have reported that HAMNs could create an acidic and anaerobic environment, preserving the chemical structure and biological activity of the target drug. Zhu et al. prepared 5-aminolevulinic acid (5-ALA)-loaded HA MNs and assessed their efficacy in superficial tumor photodynamic therapy. These MNs effectively penetrated the stratum corneum, delivering 5-ALA to the skin tissue while also providing an acidic and oxygen-free milieu. Notably, this environment significantly reduced the formation of inactive dimers via Schiff base reactions, thereby maintaining the chemical structure and biological activity of 5-ALA, a feature that further enhanced the therapeutic effect of 5-ALA in photodynamic treatment.32
It is also noteworthy that MNs have been shown to interact with specific cell receptors, thus promoting drug internalization. Shen et al. developed an HA-coated liposome MN. The modification of the liposome surface with HA enabled it to bind with the CD44 protein. This binding promoted methotrexate (MTX) internalization and activated immune cells in the skin. Furthermore, the enhanced interaction between HA–CD44–MTX–Lipo and HaCaT cells promoted the apoptosis of the latter. These findings suggest that HA–MTX–Lipo MNs could significantly inhibit psoriasis development, thus reducing skin erythema, desquamation, and thickening. Moreover, these findings underscore the potential of HA MNs in promoting drug internalization and enhancing healing processes.33
It is also noteworthy that alginate could be combined with other polymers via chemical or physical cross-linking, further enhancing the performance of MNs. Zhao et al. developed an expandable MN comprising polyvinyl alcohol and sodium alginate hydrogel. According to their findings, adding sodium alginate via strong hydrogen bonding promoted crosslinking within the two matrices, significantly improving the hydrogel's overall performance.38 Alginate has also shown great promise in the field of drug delivery. Zhou et al. used a Ca2+-optimized alginate-expandable MN in the transdermal delivery of 3-O-ethyl ascorbic acid (EAA). This MN exhibited excellent mechanical performance and biocompatibility and significantly increased EAA transdermal permeability. Furthermore, the sodium alginate MN remained intact even after 16 h of transdermal drug delivery, demonstrating its good stability and applicability in acidic drug delivery scenarios. These findings highlight the versatility of alginate in enhancing the performance of MNs and broadening their potential applications in the realm of drug delivery.39
3.2 Animal polysaccharides
3.3 Plant polysaccharides
3.4 TCM polysaccharides
3.5 Microbial polysaccharides
4 Molecular immunology of polysaccharides
Different polysaccharides exert diverse immunoregulatory effects in immunomodulation through specific receptors and signalling pathways. Low-molecular-weight hyaluronic acid (HA, <500 kDa) and its fragments (<10 kDa) can activate receptors such as TLR2/4 and CD44–RHAMM complexes, trigger the MyD88/NF-κB signalling pathway, induce the release of pro-inflammatory factors and exacerbate inflammation;67–70 their immunological effects have molecular weight and modification specificity: unmodified HA has no significant effect on monocyte activation, while N-acetylated or butylated HA enhances the pro-inflammatory response through TLR4 activation, and sulfated HA exhibits immunoregulatory properties.71–75 In addition, the interaction between HA and CD44 also mediates macrophage migration, polarization (M1/M2 phenotypic conversion), and the maturation and antigen presentation of dendritic cells (DCs).76,77After chitosan is taken up by immune cells through phagocytosis, it activates the cGAS-STING pathway: mitochondrial ROS induces dsDNA release, which triggers I-type IFN production through the STING-TBK1/IKK signalling axis and promotes Th1-type immune responses;78–80 compared with alum, it not only induces Th17-related IL-23 secretion but also synergistically activates TLR9 with CpG, significantly promoting IL-12 secretion to enhance Th1 polarization, demonstrating its advantage as a vaccine adjuvant.81
Chondroitin Sulfate (CS), as a negatively charged glycosaminoglycan, regulates immune function through differential modifications at sulfated sites and molecular conformation: it inhibits NF-κB nuclear translocation by binding to TLR4, blocks the LPS/CD44 signalling pathway, and reduces the release of pro-inflammatory factors such as TNF-α, IL-1β, and IL-6, while N-deacetylated derivatives (CSA) activate NF-κB to promote inflammation;82–88 in dendritic cells (DCs), CS inhibits Th1/Th17 differentiation through a TLR4-dependent mechanism, and the CSA subtype affects T cell polarization by regulating IL-12 and IFN-γ secretion. CS also has molecular weight specificity (only CSE is effective) in inhibiting the excessive activation of DCs through the RPTPσ receptor, thereby maintaining immune homeostasis.89
The immunomodulatory function of Bacillus subtilis polysaccharide (BSP) is related to its molecular weight and glycosidic bond structure. It induces the proliferation of splenocytes through the TLR2/MyD88 signalling axis and restores the thymus index in the cyclophosphamide-induced immunosuppression model, demonstrating multi-target biological activity.90,91
5 Preparation technology of polysaccharide microneedles
Polysaccharide-based microneedle (MN) technology has shown significant potential in drug delivery systems for the treatment of skin diseases, owing to its versatility and customizability. Importantly, mastering the manufacturing processes of this advanced technology is critical for optimizing its performance and expanding its clinical applications. In this section, we review key MN fabrication methods, focusing on their unique characteristics and their impact on MN functionality.Specifically, we will discuss various MN preparation techniques, including micromolding, atomized spray droplet blowing, and 3D printing (Fig. 4). Each of these methods offers distinct advantages and limitations in terms of mechanical strength, drug-loading capacity, and scalability. By analyzing these fabrication techniques, this review aims to provide valuable insights into their practical applications and clinical relevance, thereby laying a foundation for future research and broader implementation of MN technologies in the treatment of skin diseases. (Table 4 provides an overview of microneedle fabrication techniques and their resulting types.)
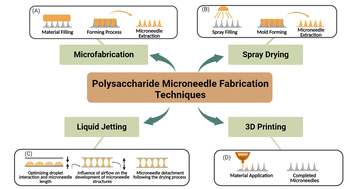 | ||
| Fig. 4 Microneedle preparation technologies. (A) Microfabrication (B) spray drying (C) liquid jetting (D) 3D printing. | ||
5.1 Microfabrication
Microfabrication, which involves MN structure design, template preparation, material injection, template removal, and post-processing steps, is the primary manufacturing strategy for polysaccharide-based microneedles (MNs) (Fig. 4A). The process begins with the creation of a master mold using solid materials such as metal or silicon, typically through micro-milling or lithography. This master mold serves as the basis for casting polysaccharide templates. During the casting process, liquid prepolymers are injected into the mold cavities, with a vacuum or centrifugal force applied to ensure complete filling and uniform distribution. Subsequently, an MN array is formed by curing the injected material through either a drying process or a crosslinking reaction. Finally, the cured polysaccharide MNs are released from the mold during the demolding step.106Polydimethylsiloxane (PDMS) is frequently used as a template material in micromolding due to its low adhesion to cured materials, excellent thermal stability, and flexibility. These properties make PDMS particularly suitable for high-viscosity polysaccharides, ensuring precise replication of the MN structure. However, a notable limitation of this method is the requirement for vacuum circulation during several steps, which is necessary to remove air bubbles and ensure proper micro-injection molding. This additional complexity may increase the overall manufacturing time and cost.
Micromolding remains a widely used technique due to its high precision and adaptability to various polysaccharide materials. However, further optimization of the process, particularly in reducing the reliance on vacuum systems, could enhance its scalability and cost-effectiveness for large-scale production.
5.2 Spray drying
Spray drying is a manufacturing process that involves atomizing liquid polymers into microscale droplets. This process begins with the application of force to the liquid polymer, typically through ultrasonic atomization, which generates fine droplets. These droplets are then directed through an air nozzle to form microscale molds (Fig. 4B). The droplets subsequently undergo multiple drying stages, leading to polymer solidification and the formation of a microneedle (MN) array.107One of the key advantages of atomization spraying is its ability to eliminate the need for centrifugal or vacuum steps, which are commonly required in traditional micromolding processes. By doing so, this method addresses several limitations of micromolding, such as the challenges associated with high-viscosity materials, and enables scalable mass production. Additionally, atomization spraying ensures complete mold filling, regardless of changes in material viscosity, making it particularly suitable for temperature-sensitive, high-viscosity, or high-concentration polysaccharide materials.
Despite these advantages, atomization spraying is not without its drawbacks. Sterilization remain a significant concern, as the process may introduce risks of contamination or cross-contamination during the handling and reuse of therapeutic materials. Furthermore, ensuring the safety and consistency of the final product requires careful optimization of the manufacturing process.
5.3 Liquid jetting
Liquid jetting is an innovative approach for the fabrication of polysaccharide-based microneedles (MNs). This method offers significant advantages, including moderate temperature conditions (4–25 °C) and rapid manufacturing times (≤10 min), which minimize drug loss and preserve the integrity of heat-sensitive materials.108The fabrication process begins with the precise deposition of polysaccharide solution droplets onto a flat substrate. A second substrate is then placed on top of the droplet array, and the height of the MNs can be precisely controlled by adjusting the upward movement of the second substrate. Directional airflow is subsequently applied to thin and solidify the droplets into the desired MN structures. Finally, the two substrates are separated, yielding MN arrays on both sides (Fig. 4C).
This method is particularly advantageous for its compatibility with high-viscosity polysaccharide solutions and its suitability for materials sensitive to heat. The ability to precisely control MN height allows for the customization of MN properties, such as the penetration depth and drug-loading capacity, making it a versatile and efficient fabrication technique. Overall, droplet blowing provides a mild and scalable approach for the rapid production of polysaccharide MNs, with potential applications in drug delivery systems for heat-sensitive and high-viscosity formulations.
5.4 3D printing
In 3D printing (Fig. 4D), objects are constructed by sequentially adding materials layer by layer to a Computer-Aided Design (CAD) model. This advanced technique enables the creation of complex structures with exceptional precision and design flexibility. Moreover, 3D printing exhibits excellent load-bearing capacity, making it particularly suitable for the customization of pharmaceuticals and medical devices. By addressing several limitations of traditional dosage forms, 3D printing provides a versatile platform for innovative drug delivery systems and medical applications.Various 3D printing technologies have been developed, including Fused Deposition Modeling (FDM), Two-Photon Polymerization (2PP), Stereolithography (SLA), Digital Light Processing (DLP), and Continuous Liquid Interface Production (CLIP). Each of these techniques offers unique advantages: for example, FDM is widely used for its cost-effectiveness and material versatility, while 2PP provides nanoscale resolution suitable for intricate biomedical structures. SLA and DLP are known for their high precision and smooth surface finishes, whereas CLIP enables rapid, continuous production of complex geometries. Despite these advancements, the range of materials currently compatible with 3D printing remains relatively limited, particularly for polysaccharide-based systems. This limitation poses challenges for the broader application of 3D printing in polysaccharide microneedle (MN) fabrication.
Nevertheless, ongoing research and technological advancements are expected to expand the range of polysaccharide materials suitable for 3D printing. Such developments will likely enhance the adaptability of 3D printing for MN-based drug delivery systems, enabling the production of more sophisticated and functional biomedical devices with tailored properties.109
The successful implementation of the aforementioned preparation methods for polysaccharide-based microneedles largely depends on the type and properties of the hydrogel. As the primary material of microneedles, hydrogels not only determine the mechanical strength and drug-loading capacity of the microneedles but also influence their molding precision and biocompatibility. In particular, the application of liquid hydrogels in micromolding, spray-coating, and blow-spinning methods can significantly simplify the fabrication process and improve the production efficiency. Therefore, an in-depth investigation into hydrogel preparation methods, especially chemical crosslinking and physical crosslinking techniques, is of great significance for optimizing microneedle performance and advancing their clinical applications. The following sections will provide a detailed overview of the preparation methods for polysaccharide-based hydrogels.
Chemical crosslinking involves the formation of covalent bonds between polysaccharide molecules through the introduction of crosslinking agents or chemical reactions, thereby producing hydrogels with a three-dimensional network structure. Commonly used crosslinking agents include glutaraldehyde, epoxy compounds, and multifunctional carboxylic acids. For instance, chitosan and gelatin–sodium alginate can form stable hydrogels under the action of crosslinking agents.110,111 The advantage of this method lies in its ability to precisely control the mechanical properties and degradation rate of the hydrogel by adjusting the concentration of the crosslinking agent and reaction conditions. However, chemical crosslinking may introduce residual crosslinking agents or by-products, necessitating strict optimization of reaction conditions to ensure biological safety.
Physical crosslinking is a strategy for constructing hydrogel networks through non-covalent interactions, which has garnered significant attention in the biomedical field due to its mild preparation conditions and dynamic reversibility. For instance, natural polysaccharides such as chitosan and Bletilla striata polysaccharide, which are rich in functional groups, can form hydrogels through various physical crosslinking mechanisms, including hydrogen bonding and metal coordination.112,113 Unlike chemical crosslinking, physical crosslinking does not require the use of chemical crosslinking agents, thereby avoiding potential toxicity issues while endowing hydrogels with excellent biocompatibility and dynamic self-healing capabilities.
6 Factors affecting the performance of polysaccharide MNs
The essence of microneedle (MN) technology lies in its microscale needle structures, which enable the penetration of the skin's stratum corneum while avoiding contact with nociceptive nerve endings. This unique feature facilitates painless drug delivery, making MNs an attractive alternative to conventional transdermal and injectable drug delivery systems. The stratum corneum, as the outermost layer of the skin, serves as a primary barrier to drug penetration, and the ability of MNs to bypass this barrier without causing pain is a key advantage of this technology.The design of polysaccharide MNs is critically influenced by several factors, including needle size, shape, density, and arrangement. These parameters directly affect the penetration efficiency, drug loading capacity, and overall patient comfort. For instance, needle length and tip sharpness determine the depth of penetration, while needle density and arrangement influence the drug distribution and release profiles. Additionally, the mechanical strength of the MNs must be sufficient to ensure successful skin penetration without breakage, particularly for polysaccharide-based systems.
This section will comprehensively review these design factors, focusing on their impact on MN performance and patient experience. By understanding the interplay between these parameters, we aim to provide insights into optimizing polysaccharide MNs for enhanced drug delivery efficiency and clinical applicability.
6.1 Size
The length of microneedles (MNs) typically ranges from tens to hundreds of microns. To achieve effective skin penetration, the length must be sufficient to bypass the stratum corneum, the outermost barrier of the skin, without reaching the nociceptive nerve endings in the dermis. Based on current studies, an optimal MN length is approximately 1000 microns, as this ensures adequate penetration while minimizing the risk of pain or nerve damage.114In addition to length, the diameter of MNs plays a critical role in determining their mechanical strength and drug-loading capacity. Smaller-diameter MNs are advantageous for easier skin penetration due to reduced resistance; however, they exhibit lower structural strength and are more prone to breakage during insertion. Conversely, larger-diameter MNs offer greater mechanical stability and higher drug-loading capacity but may face challenges in penetrating the skin effectively. Therefore, the diameter of MNs should be carefully designed within a range of tens to hundreds of micrometers to balance penetration efficiency, mechanical integrity, and drug delivery performance.
6.2 Shape
The geometry of microneedles (MNs) plays a critical role in determining their penetration efficiency, drug-loading capacity, and mechanical stability. Due to its concentrated tip, a tapered MN design facilitates easier skin penetration by reducing skin resistance. In contrast, square or prism-like MN shapes provide a larger surface area, which enhances the drug-loading capacity and allows for more efficient drug delivery. These differences highlight the importance of tailoring MN geometry to specific applications.Additionally, MNs with hook or barb-like designs offer unique advantages by anchoring the needles more securely in the skin, thereby preventing slippage during drug delivery. This feature is particularly beneficial for applications requiring prolonged skin contact or sustained drug release.
In vitro evaluations of the penetration properties of various MN array types, conducted using CT imaging, have provided further insights into the relationship between the MN geometry and mechanical performance. When MNs of the same diameter and length were tested, it was observed that increasing the number of vertices in the needle design improved their mechanical properties. This finding suggests that more complex geometries can enhance the structural integrity of MNs, making them more resistant to deformation or breakage during insertion.115
6.3 Density
The density of microneedles (MNs), defined as the number of needles per square centimeter, is a critical factor influencing their performance and applicability. A high-density MN array offers the advantage of delivering larger quantities of drugs within a confined area, thereby improving the drug delivery efficiency. However, this design may also exert greater pressure on the skin, potentially leading to increased irritation or discomfort for the patient. This trade-off highlights the need for careful optimization of MN density to balance drug delivery efficiency and patient comfort.The choice of polysaccharide materials for MN fabrication is particularly important, as these materials directly influence the mechanical properties, thickness, and density of the needles. Polysaccharides with higher mechanical strength and flexibility enable the production of thinner and denser MN arrays, which can enhance the drug delivery efficiency while maintaining structural integrity. Therefore, material selection plays a pivotal role in optimizing MN design for specific therapeutic applications.116
6.4 Rules
Microneedles (MNs) are often arranged in a matrix pattern, which facilitates even pressure distribution and ensures effective skin penetration by each needle. This arrangement is particularly advantageous for achieving uniform drug delivery. However, in some designs, MNs are arranged randomly to mimic the disordered structures found in natural biological systems. Such random arrangements may reduce the skin's adaptive responses, such as inflammation or barrier reinforcement, thereby enhancing drug delivery efficiency.117,118The size, shape, density, and arrangement of MNs are interdependent parameters that require careful optimization to achieve optimal performance (Fig. 5). For instance, larger MN arrays with a lower needle density can minimize skin damage, reduce patient discomfort, and promote more uniform drug delivery. Proper spacing between larger MNs is also critical to prevent stress concentration, which could otherwise compromise the structural integrity of the skin and the MN array.
The morphology and density of MN arrays are equally important, as they directly influence the stability of the needles and their drug delivery capabilities. Different MN shapes within specific arrangements can significantly affect the drug penetration depth and release profiles. For example, tapered MNs may enhance the penetration efficiency, while barbed designs can improve retention within the skin. High-density MN arrays, on the other hand, require specialized configurations to ensure force equilibrium during insertion and to achieve uniform drug release.
Therefore, to regulate the overall performance and efficacy of MNs, the size, shape, density, and arrangement must be carefully evaluated and tailored to the intended application. Additionally, the choice of materials used for MN fabrication plays a crucial role in determining these parameters, further emphasizing the need for a holistic approach to MN design.
7 Application of polysaccharide MNs in skin disease treatment
Hyaluronic acid (HA), a key component of the extracellular matrix (ECM), is highly abundant in both the epidermis and dermis, accounting for approximately 50% of the total HA in the body.119 In the dermis, HA plays a critical role in regulating water balance, osmotic pressure, and ionic currents, thereby reinforcing the structural integrity of the extracellular domain. Furthermore, HA contributes to skin stability through electrostatic interactions, which help maintain the cohesion of dermal components and enhance the mechanical properties of the skin.120,121In the epidermis, HA's remarkable water-binding capacity and swelling properties help preserve the skin's organizational structure and mitigate wrinkle formation, contributing to improved skin elasticity and hydration.122,123 These physiological properties not only support the maintenance of healthy skin but also enhance the therapeutic efficacy of treatments targeting skin disorders.
Beyond its role in maintaining skin physiology, HA's suitability as a polysaccharide material for microneedle (MN) fabrication lies in its excellent drug delivery properties. The biocompatibility, hydrophilicity, and ability to encapsulate and release therapeutic agents make HA-based polysaccharide MNs particularly effective for treating a range of skin conditions. For instance, polysaccharide MNs have demonstrated potential for delivering drugs to manage psoriasis, atopic dermatitis, and hyperplastic scars, among other skin diseases (Fig. 6). These advantages highlight the versatility of polysaccharide-based MNs in both maintaining skin health and addressing complex dermatological conditions.
7.1 Atopic dermatitis
Atopic dermatitis (AD) is a common chronic inflammatory skin disorder characterized by intense itching and significant discomfort. Its pathogenesis is complex and multifactorial, involving hereditary factors, immune system dysfunction, environmental triggers, and skin infections, among other contributors.124 AD often manifests as red, inflamed, and scaly skin, accompanied by severe itching, which can significantly impact patients' quality of life.Hyaluronic acid (HA), a naturally occurring polysaccharide, rapidly dissolves and exhibits excellent moisturizing properties. It strengthens the skin barrier, reduces water loss, and alleviates dryness, making it a promising therapeutic agent for AD. Song et al. developed HA-based double microneedle (MN) patches using a two-step manufacturing process, enabling the incorporation of vitamin D3 (VD3) for AD treatment. The HA in these patches not only provided sufficient mechanical strength for minimally invasive penetration into AD-affected skin but also delivered potent moisturizing effects upon rapid dissolution.
To evaluate the therapeutic efficacy of the integrated drug delivery microneedle (IDMN) patch, BALB/c mice were randomly divided into four groups: a control group receiving saline, a VD3 treatment group receiving VD3, an MN group using empty IDMN, and an IDMN group receiving VD3 through IDMN. Treatments were administered every three days for a total of four sessions. In a previous study, control mice exhibited severe erythema (redness), desquamation (skin peeling), and lichenification (thickened, leathery skin), which are hallmark symptoms of AD. The VD3 group showed limited local drug efficacy in alleviating these symptoms. In contrast, the MN group demonstrated significant improvement, with the mouse skin appearing nearly normal after 12 days, showing minimal erythema, desquamation, and lichenification.125 These therapeutic effects were attributed to HA's moisturizing properties, which enhanced skin hydration and repair. Furthermore, the HA in the drug delivery system provided protection and facilitated the rapid release of therapeutic agents, contributing to the effective treatment of AD.
Chen et al. further advanced this approach by developing a double-layer microneedle patch composed of “poly(lactic-co-glycolic acid) (PLGA)/sodium hyaluronate (HA)” for AD treatment. This innovative design featured curcumin (CUR)-loaded PLGA tips, while the base layer incorporated gallic acid (GA) stabilized with L-ascorbic acid (AA) in an HA matrix. The HA in the base layer protected GA from degradation and dissolved rapidly in interstitial fluid upon skin insertion, triggering the release of GA within 5 minutes.
To investigate the therapeutic effects of HA microneedles on AD skin lesions, Nc/Nga mice were divided into four groups: a healthy group (without DNCB exposure), an AD group (DNCB untreated, repeat application), a CUR MN group (CUR-loaded MN treatment), and a CUR/GA MN group (CUR/GA-loaded MN treatment). DNCB was repeatedly applied locally to induce significant lesions and stabilize AD symptoms on the mice's back skin. Images of dorsal skin lesions were analyzed to evaluate the therapeutic effects of the MN patches, and dermatitis scores were calculated. After two weeks of DNCB induction on day 0, the average dermatitis scores in all DNCB-exposed mice increased to approximately 4.5, indicating severe AD symptoms. Treatment with CUR/GA MN patches demonstrated significant therapeutic advantages, particularly in rapidly controlling AD severity. Within two days, mice treated with CUR/GA MN patches showed lower dermatitis scores compared to those treated with CUR MN patches alone, suggesting that CUR/GA MN patches can effectively and quickly alleviate AD symptoms.126
7.2 Wound healing
Wound healing is a complex process modulated by multiple factors that contribute to the formation of scars. Achieving global wound healing remains challenging, imposing significant economic pressure on healthcare systems. Chitosan (CS), a natural alkaline polysaccharide derived from arthropod chitin, has been extensively investigated due to its excellent biocompatibility and antimicrobial properties. CS functions as a positively charged polyelectrolyte in aqueous solutions, with high adsorption capacity. It can penetrate negatively charged bacterial cell walls owing to its positive charge, leading to intracellular liquid leakage and bacterial death. Consequently, CS has broad applications in the production of microneedles and other advanced composite materials for skin wound healing.127Hasnain et al. developed a chitosan/polyvinyl alcohol (CS/PVA) hydrogel microneedle patch loaded with curcumin (CUR) and L-arginine (ARG) (CUR-ARG MNs).128 CUR, a natural polyphenolic compound with strong antioxidant, anti-inflammatory, and antimicrobial properties, has been recognized as an innovative agent for wound healing. Studies have shown that CUR alleviates wound inflammation by scavenging reactive oxygen species (ROS) and reducing oxidative stress through both enzymatic and non-enzymatic mechanisms. Additionally, CUR promotes the transition of wound healing from the inflammatory phase to the proliferative phase, characterized by accelerated angiogenesis, enhanced collagen deposition, faster re-epithelialization, and earlier tissue formation. Topical application of CUR is particularly effective for skin wound repair, as it allows the drug to directly target the injured site. However, the instability of CUR in the aqueous microenvironment of the skin and its limited cellular uptake pose challenges for its localized delivery. To address these issues, advanced drug delivery systems have been developed to improve the stability, solubility, and sustained release of CUR at wound sites. For instance, CUR-loaded nanocomposite hydrogels based on chitosan (CS) and oxidized alginate have been shown to significantly enhance its wound-healing efficacy.
In Hasnain et al.'s study, CUR and ARG were successfully incorporated into CS/PVA hydrogel microneedle patches to enhance their therapeutic potential. ARG, an amino acid with wound-healing and antimicrobial properties, complements the effects of CUR. The study found that when CS was used alone, its insufficient mechanical strength caused structural and morphological changes during the healing process, limiting its antibacterial activity and restricting its clinical applications. However, the hydroxyl and amino groups on the surface of CS enable convenient chemical modifications. Both theoretical and experimental studies have demonstrated that introducing amino acid moieties, such as L-arginine, L-asparagine, or L-lysine, into CS significantly broadens its range of applications. Experimental findings further revealed that incorporating CUR into microneedle patches promotes tissue regeneration and collagen deposition, thereby substantially accelerating wound healing rates.
Chitosan (CS), derived from arthropod chitin, functions as a positively charged polyelectrolyte in aqueous solutions under acidic conditions, specifically at pH levels below its pKa (typically around 6.5). In such environments, chitosan exhibits strong adsorption properties. Its positive charge enables it to penetrate the negatively charged bacterial cell walls, leading to intracellular fluid leakage, ultimately causing bacterial cell death. Due to these properties, chitosan has been widely utilized in the fabrication of microneedles. Yu et al. developed a microneedle array patch using electrostatic and hydrogen bond interactions between Kangfuxin (KFX), CS, and a mixture of fucoidan glue (FD) to accelerate full-thickness wound healing.129 It was observed that the KFX-MN patch exhibited good biocompatibility and antibacterial properties while also promoting epithelial reshaping, which enhanced full-thickness wound healing in rats.
7.3 Hyperplastic scar
Scar formation is initiated by abnormal chronic inflammation in the wound area, primarily driven by excessive fibroblast proliferation and abnormal collagen deposition. This process involves numerous signaling molecules, including growth factors, cytokines, and chemokines, which collectively contribute to the dysregulated wound healing response. Additionally, scar formation is influenced by various intrinsic and extrinsic factors, such as race, gender, age, wound tension, location, and mode of injury. These factors interact in complex ways, contributing to the heterogeneity and variability observed in scar formation.130Polysaccharides derived from Bletilla Striata (BSP) have garnered significant attention due to their excellent biocompatibility, cost-effectiveness, and multifunctional therapeutic properties, including wound healing, hemostasis, anti-inflammatory, antioxidant, and antibacterial effects. Notably, BSP has been shown to reduce hydroxyproline (HYP) production, a key marker of collagen deposition. Zhang et al. developed a microneedle patch combining carboxymethyl chitosan (CMCH) with BSP for the synergistic treatment of hypertrophic scars. CMCH exhibits superior water solubility, biodegradability, and non-immunogenicity, making it an ideal material for biomedical applications. Furthermore, CMCH has been demonstrated to inhibit the expression of transforming growth factor-β1 (TGF-β1) in hypertrophic scar fibroblasts (HSFs), a critical pathway in scar formation.
The BSP-CMCH microneedle patch has shown promising potential for wound healing and scar management. Studies have revealed that the patch consists of uniformly structured needles with sufficient mechanical strength for effective skin penetration, rapid dissolution, and minimal cytotoxicity. Importantly, this microneedle system has demonstrated significant therapeutic effects, including reducing hypertrophic scar thickness, lowering HYP and TGF-β1 expression levels in the scar tissue, enhancing collagen fiber alignment, and minimizing dermal hyperemia and hyperplasia. These findings underscore the potential of BSP and CMCH as effective materials for the treatment of hypertrophic scars, offering a novel and efficient approach to scar management.131
7.4 Skin tumors
Superficial skin tumors (SSTs) are among the most common human tumors and are primarily categorized into hemangioma, actinic keratosis, and squamous cell carcinoma. These tumors are caused by excessive skin cell proliferation and genetic mutations, with their development influenced by both environmental and genetic factors.132,133 Effective treatment strategies for SSTs often require localized and sustained drug delivery systems to minimize systemic toxicity and enhance therapeutic efficacy.Dextran (Dex), a natural polysaccharide, has been widely utilized in the biomedical field due to its excellent biocompatibility and safety profile. Recent research on modified glucans, such as oxidized glucan, carboxymethyl dextran, acetal-modified glucan, and methacrylated dextran (DexMA), has demonstrated their potential in fabricating biocompatible and mechanically stable light-crosslinked hydrogels. Huang et al. synthesized a novel DexMA with a 5% higher degree of substitution (DS) compared to previously reported formulations. This modification enabled the formation of a denser crosslinked network, significantly enhancing the hydrogel's mechanical strength and stability. Characterization of the 30% DexMA hydrogel revealed its low viscosity, optimal mechanical properties, and excellent biosafety, making it a suitable material for biomedical applications.
Using this hydrogel, insoluble microneedles were successfully fabricated. The higher DS of DexMA improved the microneedles' mechanical strength, penetration efficiency, and drug release performance. These microneedles demonstrated sufficient mechanical durability to achieve sustained release of small-molecule drugs at a delivery depth exceeding 600 microns. Furthermore, microneedles loaded with doxorubicin (DOX) and trastuzumab (Tra) exhibited significant synergistic effects in tumor treatment. The combination of DOX and Tra likely enhances therapeutic efficacy by targeting distinct pathways involved in tumor progression, thereby improving the overall treatment outcomes. This research highlights the potential of DexMA-based microneedles as a safe and effective local drug delivery strategy for the treatment of SSTs. The findings underscore the importance of material modifications, such as increasing DS, in optimizing microneedle performance and advancing localized cancer therapies.134
7.5 Psoriasis
Psoriasis is a chronic inflammatory disease characterized by the formation of erythematous plaques on the skin, often accompanied by scaling and inflammation. This condition is primarily caused by excessive proliferation and disordered growth of keratinocytes, lymphocytic infiltration into the dermal layer, and the expansion of dermal blood vessels. In addition to skin manifestations, psoriasis can induce pathological changes in nails (e.g., pitting and onycholysis) and joints (e.g., psoriatic arthritis), significantly affecting patients' quality of life.135,136Chitosan (CS), a natural polysaccharide, has garnered attention as an ideal drug carrier due to its excellent biocompatibility and biodegradability, which minimize skin irritation and improve drug delivery efficiency. However, its low solubility under physiological conditions and the complexity of preparing chitosan microneedles (CS MNs) have limited its widespread application. To address these limitations, chitosan was modified with methacrylic anhydride (MA), resulting in a methyl propylene acyl chitosan hydrogel (CSMA), which exhibits improved solubility and enhanced potential for psoriasis treatment.
Dai and colleagues successfully developed microneedle patches using CSMA hydrogels (CSMA hMNs) to deliver therapeutic agents, including methotrexate (MTX) and niacinamide (NIC), for the treatment of psoriasis. These CSMA hMN patches demonstrated excellent morphology and mechanical strength, withstanding a force of 0.7 N, ensuring effective skin penetration. In vitro studies revealed that the microneedles exhibited slow-release properties, which reduced the MTX toxicity and dosing frequency. In vivo experiments further demonstrated that the CSMA hMN patch effectively inhibited psoriasis-induced skin thickening and spleen enlargement in mice while maintaining good biological safety at therapeutic doses.
The development of CSMA hMNs highlights the potential of modified chitosan and other biocompatible materials in advancing hydrogel-based microneedle systems for psoriasis management. These systems offer significant advantages, including improved drug delivery efficiency, reduced systemic toxicity, and enhanced patient compliance. This research provides valuable insights into the design of innovative therapeutic strategies for chronic inflammatory skin diseases.137
7.6 Other types of skin diseases
Acne vulgaris is a prevalent chronic skin disease, particularly among adolescents, characterized by papules, pustules, and nodules, which can cause permanent scarring in severe cases.138,139 The combination of photothermal therapy with dissolving microneedles and transdermal administration of antibacterial agents exhibits a synergistic treatment effect on acne vulgaris. Hyaluronic acid (HA) has been widely employed as a substrate for microneedles due to its excellent biocompatibility, degradability, and ability to promote the healing of acne lesions. Wang et al. developed a microneedle composed of HA specifically aimed at treating acne vulgaris. HA, a vital component of the dermal matrix, plays a key role in creating micro-pores in the skin, which not only helps maintain elasticity and luminosity but also enhances the healing process of acne lesions. However, high molecular weight HA (>600 kDa) has limited permeability in the skin, while low molecular weight HA (approximately 5–50 kDa) is susceptible to brittle fracture despite its favorable mechanical properties. In contrast, HA with medium molecular weight (∼100–300 kDa) has sufficient strength, making it ideal for microneedle preparation. In this study, a combination of low and medium molecular weight HA was used to prepare soluble HA microneedles. This method effectively avoided the shortcomings of single-material innovation by implementing a synergistic “1 + 1 > 2” effect.The combination of hyaluronic acid (HA) with microneedles enhances their antibacterial and anti-inflammatory properties, thereby improving their therapeutic potential. The in vivo anti-acne efficacy of various microneedle formulations was evaluated using a mouse acne model. The successful establishment of the model was confirmed by the thickening and redness of the ears in the model mice. Starting on day 8, mice in the experimental groups were treated with different microneedle formulations, and the healing progress, redness, and changes in ear thickness were monitored daily until day 12. The results showed that mice in the model group exhibited pronounced redness, swelling, and inflammation in their right ear, accompanied by increased auricle thickness. In contrast, the group treated with Acropass, a commercially available microneedle patch, showed only minimal improvement. Notably, the groups treated with HA E@P-HA MNs + NIR, EO-HA MNs, and E@P-EO-HA MNs + NIR microneedles demonstrated significant reductions in ear swelling. While the HA E@P-HA MNs + NIR and EO-HA MN groups still exhibited some residual redness, their therapeutic outcomes were comparable. By contrast, the E@P-EO-HA MNs + NIR group achieved nearly complete clearance of redness, along with a significant reduction in auricle thickness, resulting in a near-normal appearance. These findings underscore the strong therapeutic potential of HA-based microneedles in the treatment of acne vulgaris.140
Leishmaniasis is a serious tropical disease caused by various species of the genus Leishmania, transmitted through the bite of female sandflies. The typical symptom is the formation of an ulcer at the site of the sandfly bite.141 Carboxymethyl cellulose (CMC) offers significant benefits in microneedle (MN) development, particularly due to its low degradation rate, which allows for controlled dissolution and drug release while improving the mechanical strength. Building on these properties, Reza Zare and colleagues developed a soluble MN patch using a blend of biodegradable polymers, polyvinylpyrrolidone (PVP) and CMC, for the transdermal delivery of amphotericin B (AmB). PVP provides robust mechanical strength, ensuring effective skin penetration, while CMC contributes to the overall stability and performance. Studies show that these microneedles can successfully puncture the stratum corneum, enabling AmB to penetrate the skin effectively. After implantation, the microneedles rapidly dissolve, and the formed micropores fully recover within 30 minutes. Flow cytometry analysis demonstrated significant anti-leishmaniasis activity for AmB-loaded microneedle patches, highlighting their potential as a novel treatment strategy for this disease.142
8 Clinical application progress
At present, various-scale human clinical trials have been conducted on microneedle therapy in multiple skin diseases, medical aesthetics, and special medical scenarios, and its efficacy and safety have been verified in multiple fields:In the field of skin disease treatment, Lim et al. (2024) confirmed through a single-blind self-controlled trial that when treating post-surgical scars with siRNA microneedle patches, the scar volume at 30 days and 60 days was reduced by 10.7% (P < 0.05) and 29.4% (P = 0.005), respectively, compared to the silicone patch control group, the core mechanism being the inhibition of excessive extracellular matrix deposition;143 for melasma, Cassiano et al. (2022) conducted a factorial design trial, showing that microneedles combined with triple cream or tranexamic acid could reduce the number of melanocytes by 41% (P < 0.01) and improve the basement membrane structure.144 Hofny et al. (2023) further confirmed that the efficacy of microneedles combined with trichloroacetic acid was significantly better than that of a single dermabrasion at 1–3 months (P < 0.05);145 in nail psoriasis, Yew et al. (2022) found that when treating nail psoriasis with microneedles loaded with steroid hormones for 4 months, the improvement in the Nail Psoriasis Severity Index (NAPSI) was continuously better than that achieved with traditional ointments (P = 0.028);146 in acne treatment, Tai et al. (2022) showed in a 28 day intervention that the anti-acne microneedle patch could reduce the volume of skin lesions (with a shrinkage rate of over 22% in 28 days) and improve pigmentation deposition, and there were no adverse events;147 in vitiligo patients, Ebrahim et al. (2021) conducted a randomized controlled trial, showing that microneedle combined with tacrolimus ointment treatment for 6 months achieved ≥ 75% pigmentation improvement in 50% of patients (vs. 29.2% in the control group, P = 0.003), and the improvement was more significant in the limbs;148 in atopic dermatitis studies, Song et al. (2023) confirmed through a side-by-side control that the effect of hyaluronic acid microneedle patches in repairing the epidermal barrier was significantly better than that of non-puncture patches (P < 0.05);149 for rosacea (redness and telangiectasia type of rosacea), Bageorgou et al. (2019) conducted a randomized controlled trial involving 20 female patients, with group A using 500 mg/5 mL tranexamic acid solution for cold wet compress on the right nasolabial fold and group B using tranexamic acid solution on microneedles followed by wet compress (once every 15 days for a total of 4 times), and the results showed that the average improvement score of the Investigator Global Assessment Scale (IGA-RSS) in group B was 3 levels higher than that of group A (2 levels, P < 0.05), and the improvement effect lasted for more than 4 months, and all patients were well tolerated, confirming the potential application of microneedles combined with drugs in vascular abnormal expansion-type skin diseases.150
In the field of medical aesthetics, Zasada et al. (2019) compared and found that the effect of microneedles combined with vitamin C in improving skin firmness, elasticity, and hydration was better than that of non-puncture aesthetic therapy;151 Choi et al. (2017) confirmed that after 8 weeks of treatment with transparent microneedle patches, the improvement of crow's feet wrinkles were better than essence liquid and without irritation;152 for skin rejuvenation, Pruettijarai et al. (2022) conducted a prospective trial using a split design (right side – nasolabial fold microneedle patch + 1.8% hyaluronic acid solution; left side – only microneedle patch) for 8 weeks and found that the Merz aesthetic scale scores of both groups in the nasolabial fold were significantly improved, with no inter-group differences, confirming that both microneedles alone or combined with hyaluronic acid solution can effectively improve facial wrinkles and have good safety.153
In special medical scenarios, such as in the treatment of skin diseases, medical aesthetics, and special medical scenarios, microneedle therapy has been applied in various scales of human clinical trials, and its efficacy and safety have been verified in multiple fields. In special medical scenarios, in the treatment of alopecia areata, the study by Qiao et al. (2025) included 80 patients and showed that the efficacy of microneedle transdermal delivery of hormones was comparable to that of traditional injection, but the pain score was significantly lower (VAS: 4.00 ± 1.17 vs. 5.28 ± 2.10, P = 0.0047);154 in the treatment of plantar warts, Khan et al. (2023) found that the complete remission rate of the mitomycin C microneedle group was 76.7%, significantly higher than that of the cryotherapy group (56.7%);155 in the field of blood glucose control, for type I diabetes, Rini et al. (2015) found that microneedle delivery of insulin analogues in the subcutaneous area could optimize the pharmacokinetic characteristics and achieve more precise meal-time blood glucose regulation. 3 days of continuous monitoring showed stable efficacy and no serious adverse events. The subjects' feedback on their usage experience and willingness reached the clinically acceptable standard, providing a new direction for breaking through the limitations of traditional insulin injection therapy.156
9 Challenges and prospects of polysaccharide microneedles
9.1 Manufacturing complexity and scalability limitations
As an emerging drug delivery system, polysaccharide microneedles have demonstrated clinical application potential in ophthalmology, dermatology, and wound treatment for diabetes. However, their actual promotion still faces some application bottlenecks. Firstly, the complexity of manufacturing processes and the difficulty of large-scale production pose challenges. This technology requires precise processing methods such as microforming, 3D printing, or electrospinning to strictly control the size accuracy, geometric shape, and uniformity of drug loading of microneedles. However, the viscoelasticity and degradation characteristics of polysaccharide materials may lead to a decrease in the stability of microneedle structures, often requiring the combination of synthetic high-molecular materials to optimize performance. Moreover, polysaccharides derived from natural sources usually have poor mechanical properties. To ensure they are strong enough to penetrate the skin, they must be blended with other polymers, nanostructures, or cross-linked.159 Although this can improve product characteristics, it significantly increases the complexity of the process. Some advanced technologies, such as light-curing 3D printing, can achieve high-precision processing, but the equipment cost and energy consumption issues limit the economic feasibility of large-scale production.Secondly, although the adhesion properties of polysaccharide materials can prolong the drug release time, if the material parameters are not precisely controlled, problems such as premature burst release or insufficient retention of drugs in the later stage may occur, thereby affecting the stability of treatment effects.
Finally, the clinical safety and long-term efficacy verification system are incomplete. Most current research results are limited to animal experiments and in vitro model validations, lacking systematic large-scale human clinical trial data support. At the same time, applying microneedles may cause local tissue irritation or secondary infection risks, especially in immunosuppressed patient groups, and their safety needs to be carefully evaluated. How to establish a standardized biosafety evaluation system and clarify the degradation and drug metabolism patterns of materials during long-term use remain key issues that need to be addressed urgently.
9.2 Long-term stability and industrial production challenges
In practical clinical applications, polysaccharide microneedles encounter several key limitations. Firstly, the issue of long-term storage stability is the main obstacle. Polysaccharide molecules are susceptible to environmental conditions, such as temperature, humidity, and light, which may cause structural changes. Seasons and environments also influence the molecular weights and structures of polysaccharides. For instance, polysaccharides harvested under varying climatic conditions may exhibit structural heterogeneity, further complicating standardization.157 Some polysaccharides will degrade at temperatures above 70 °C or under continuous light exposure, resulting in a significant reduction in antioxidant activity; at the same time, improper storage conditions may cause the hardness of the microneedle body to decrease, directly affecting the efficiency of skin puncture. Light-proof packaging, low-temperature (4 °C) storage, and controlled dry environments should be adopted, and chelating agents should be added to inhibit metal ion catalytic degradation.Secondly, the variability in industrial production constrains large-scale applications. The differences in molecular weight, purity, and source of natural polysaccharide materials (such as differences in extraction from animals and plants) directly affect microneedles' mechanical properties and drug-loading efficiency. Critically, the natural polysaccharide components exhibit significant batch-to-batch variations. The fluctuations in their molecular weight distribution and structural characteristics may lead to deviations in the reproducibility and consistency of the drug release curves.158 Therefore, quality control standards such as gel electrophoresis and mass spectrometry analysis must be established. The complexity of the preparation process further intensifies the challenge: the micro-moulding method requires precise control of temperature and pressure, 3D printing has strict requirements for layer thickness accuracy, and the uniformity of soluble microneedle coatings is easily affected by fluctuations in solution viscosity. Improvement directions include developing standardized processes such as automated spraying technology.
Some polysaccharides (such as β-glucan) have specific molecular structures that potentially induce immunogenicity through pattern recognition receptors. Thus, they must be systematically evaluated for their biocompatibility through in vitro cytotoxicity tests and in vivo immune index detection. Finally, the risk of immunogenicity needs to be strictly controlled. Some polysaccharides (such as bacterial lipopolysaccharides) may trigger immune responses, while the immunomodulatory effect of chitosan is affected by differences in molecular weight and degree of deacetylation. Countermeasures include selecting low-immunogenic polysaccharides from plant sources or reducing the risk by acetylation modification. Residual cross-linking agents (such as glutaraldehyde) and impurities such as backing materials (latex) in the preparation process may cause sensitization, and compliance with GMP standards and strict regulations for the detection of heavy metals, endotoxins, etc. in residues are required.
Moreover, the dynamic interactions between polysaccharide carriers and the biological microenvironment (such as enzymatic degradation in the intestine or pH gradient changes in tumour tissues) may destroy the drug delivery structure or lead to loss of control over the release kinetics, directly affecting the controllability of drug delivery. Additionally, microneedle puncture may damage the skin barrier, increasing the risk of pathogen invasion. Diabetic patients, due to impaired skin barrier function, are more prone to infection. Specific assessment of individual differences and local inflammatory responses is necessary.
9.3 Clinical validation and regulatory standardization
Given the regulatory challenges of natural source polysaccharide microneedles, a systematic solution must be constructed to promote clinical application. Standardization of quality control is the primary step. A raw material traceability system should be established to identify geographical indications of plant or animal origin (such as the white peony polysaccharide from a specific origin), and components can be identified through Fourier Transform Infrared (FTIR) spectroscopy, Nuclear Magnetic Resonance (NMR) spectroscopy, and High-Performance Liquid Chromatography (HPLC). At the same time, the repeatability of the production process should be guaranteed. The extraction and purification of natural polysaccharides and the microneedle forming process need to be optimized through experimental design and process analytical technology (PAT), such as near-infrared spectroscopy, which can be applied for online monitoring to control the concentration and homogeneity of the polysaccharide solution in real-time and ensure process stability.Clinical validation and risk control should be implemented in stages. In the Phase I clinical trial, the local safety of a single administration is evaluated, and skin irritation and immune indicators such as IgE levels are monitored. In the Phase II trial, the efficacy and dose effect of drug-loaded microneedles are verified through clinical disease models in rats. In the Phase III trial, multi-centre randomized controlled trials covering different ethnic groups and skin types should be conducted. After the drug is marketed, a drug safety surveillance system should be established for at least 5 years to track patients' infection and allergy occurrence rates with chronic wounds.
9.4 Methodological variability and research reproducibility
Methodological variability significantly affects the comparability of research results on polysaccharide microneedles, with the root cause being systematic errors caused by differences in experimental design, operational procedures, and analytical tools. The specific impacts are manifested in three aspects: first, differences in measurement tools may lead to deviations in key parameters, such as variations in 3D printer models or scanning electron microscopy resolutions used by different laboratories, which directly affect the measurement results of microneedle diameters and needle body strength; second, when experimental conditions are not standardized, fluctuations in environmental temperature and humidity may change the dissolution rate or drug release curve of polysaccharide microneedles, resulting in a decline in the comparability of cross-study data; third, inconsistent data analysis methods (such as differences in linear regression and machine learning algorithms) may cause deviations in result interpretation, typically manifested as misjudgments of immunogenicity risks.To reduce the interference of methodological variability, a systematic standardization strategy needs to be implemented: firstly, establish a unified experimental design framework, formulate standardized operating procedures (SOPs), clearly define microneedle preparation parameters (centrifugation speed and drying time) and characterization methods (atomic force microscopy for detecting needle body roughness), set a reproducibility threshold according to the ICH Q2(R1) guidelines, and establish a list of control variables to record environmental temperature and humidity, raw material batches, and operator qualifications to ensure data traceability. Secondly, standardize data preprocessing methods using Z-score normalization or range scaling to eliminate dimensional differences (e.g., normalizing drug release rate to the 0–1 interval). Introduce prediction intervals through statistical control methods to assess method repeatability and conduct deviation investigations for abnormal data exceeding historical intervals.
Finally, promote cross-platform validation collaboration, conduct joint research by multiple laboratories to verify key parameters (such as puncture force measurement), establish industry-recognized reference standards, and enhance the reproducibility of conclusions by sharing experimental data and analysis codes through open source.
Data availability
No primary research results, software or code has been included, and no new data were generated or analysed as part of this review.Author contributions
Conceptualization: Chao Liu, Meng Liu and Xin Li; methodology: Chao Liu, Meng Liu, Xin Li and Yimei Hu; investigation: Chao Liu, Meng Liu, Xin Li, Yimei Hu, Lingling Zhang, Feng-Ming You, Gang Fan and Yiman Ge; resources: Feng-Ming You and Gang Fan; data curation: Chao Liu, Meng Liu and Xin Li; writing – original draft preparation: Chao Liu; writing – review & editing: Meng Liu, Xin Li, Yimei Hu, Lingling Zhang, Feng-Ming You, Gang Fan and Yiman Ge; visualization: Yiman Ge; supervision: Feng-Ming You and Gang Fan; project administration: Feng-Ming You; funding acquisition: Feng-Ming You.Conflicts of interest
The authors declare no conflict of interest. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 82104878 and 82174262) and the Province Natural Science Foundation of Sichuan (Grant No. 2024NSFSC1854), which has been instrumental in facilitating our research and enabling the successful completion of this study.References
- T. Waghule, G. Singhvi, S. K. Dubey, M. M. Pandey, G. Gupta, M. Singh and K. Dua, Microneedles: A smart approach and increasing potential for transdermal drug delivery system, Biomed. Pharmacother., 2019, 109, 1249–1258, DOI:10.1016/j.biopha.2018.10.078.
- L. K. Vora, A. H. Sabri, Y. Naser, A. Himawan, A. R. J. Hutton, Q. K. Anjani, F. Volpe-Zanutto, D. Mishra, M. Li, A. M. Rodgers, A. J. Paredes, E. Larrañeta, R. R. S. Thakur and R. F. Donnelly, Long-acting microneedle formulations, Adv. Drug Deliv. Rev., 2023, 201, 115055, DOI:10.1016/j.addr.2023.115055.
- M. Gupta, U. Agrawal and S. P. Vyas, Nanocarrier-based topical drug delivery for the treatment of skin diseases, Expet Opin. Drug Deliv., 2012, 9(7), 783–804, DOI:10.1517/17425247.2012.686490.
- J. Li, R. Ge, K. Lin, J. Wang, Y. He, H. Lu and H. Dong, Advances in the Application of Microneedles in the Treatment of Local Organ Diseases, Small, 2024, 20(6), e2306222, DOI:10.1002/smll.202306222.
- J. Gupta, H. S. Gill, S. N. Andrews and M. R. Prausnitz, Kinetics of skin resealing after insertion of microneedles in human subjects, J. Control. Release., 2011, 154(2), 148–155, DOI:10.1016/j.jconrel.2011.05.021.
- M. I. Haq, E. Smith, D. N. John, M. Kalavala, C. Edwards, A. Anstey, A. Morrissey and J. C. Birchall, Clinical administration of microneedles: skin puncture, pain and sensation, Biomed. Microdevices, 2009, 11(1), 35–47, DOI:10.1007/s10544-008-9208-1.
- J. Li, B. Liu, Y. Zhou, Z. Chen, L. Jiang, W. Yuan and L. Liang, Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery, PLoS One, 2017, 12(2), e0172043, DOI:10.1371/journal.pone.0172043.
- W. Duan, K. Xu, S. Huang, Y. Gao, Y. Guo, Q. Shen, Q. Wei, W. Zheng, Q. Hu and J. W. Shen, Nanomaterials-incorporated polymeric microneedles for wound healing applications, Int. J. Pharm., 2024, 659, 124247, DOI:10.1016/j.ijpharm.2024.124247.
- H. Lu, J. Wang, J. Li, B. Gao and B. He, Advanced Silk Fibroin Biomaterials-Based Microneedles for Healthcare, Macromol. Biosci., 2023, 23(11), e2300141, DOI:10.1002/mabi.202300141.
- M. Kenchegowda, U. Hani, A. Al Fatease, N. Haider, K. V. R. N. S. Ramesh, S. Talath, H. V. Gangadharappa, G. Kiran Raj, S. H. Padmanabha and R. A. M. Osmani, Tiny titans- unravelling the potential of polysaccharides and proteins based dissolving microneedles in drug delivery and theranostics: A comprehensive review, Int. J. Biol. Macromol., 2023, 253(Pt 5), 127172, DOI:10.1016/j.ijbiomac.2023.127172.
- J. Pushpamalar, A. K. Veeramachineni, C. Owh and X. J. Loh, Biodegradable Polysaccharides for Controlled Drug Delivery, ChemPlusChem, 2016, 81(6), 504–514, DOI:10.1002/cplu.201600112.
- I. A. Duceac, M. C. Stanciu, M. Nechifor, F. Tanasă and C. A. Teacă, Insights on Some Polysaccharide Gel Type Materials and Their Structural Peculiarities, Gels, 2022, 8(12), 771, DOI:10.3390/gels8120771.
- K. Valachová, M. E. Hassan and L. Šoltés, Hyaluronan: Sources, Structure, Features and Applications, Molecules, 2024, 29(3), 739, DOI:10.3390/molecules29030739.
- G. Ye, R. Jimo, Y. Lu, Z. Kong, Y. Axi, S. Huang, Y. Xiong, L. Zhang, G. Chen, Y. Xiao, P. Li, K. Gou and R. Zeng, Multifunctional natural microneedles based methacrylated Bletilla striata polysaccharide for repairing chronic wounds with bacterial infections, Int. J. Biol. Macromol., 2024, 254(Pt 2), 127914, DOI:10.1016/j.ijbiomac.2023.127914.
- K. N. How, W. H. Yap, C. L. H. Lim, B. H. Goh and Z. W. Lai, Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review, Front. Pharmacol., 2020, 11, 1105, DOI:10.3389/fphar.2020.01105.
- Y. Xie, P. Gao, F. He and C. Zhang, Application of Alginate-Based Hydrogels in Hemostasis, Gels, 2022, 8(2), 109, DOI:10.3390/gels8020109.
- Z. He, Y. He, X. Meng, Z. Ge and H. Sun, Structural characteristics and wound-healing effects of Bletilla striata fresh tuber polysaccharide, Int. J. Biol. Macromol., 2024, 278(Pt 3), 134679, DOI:10.1016/j.ijbiomac.2024.134679.
- S. Zhang, C. Ding, X. Liu, Y. Zhao, Q. Ding, S. Sun, J. Zhang, J. Yang, W. Liu and W. Li, Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus, Molecules, 2023, 28(9), 3733, DOI:10.3390/molecules28093733.
- S. Ouyang, F. Wang, Y. Liu, Z. Hu, M. Li, Y. Wu, Z. Li, J. Qian, L. Wang and S. Ma, Current status of research on polysaccharide-based functional gradient gel materials: A review, Carbohydr. Polym., 2024, 344, 122520, DOI:10.1016/j.carbpol.2024.122520.
- G. Gopan, J. Jose, K. B. Khot and A. Bandiwadekar, The use of cellulose, chitosan and hyaluronic acid in transdermal therapeutic management of obesity: A review, Int. J. Biol. Macromol., 2023, 244, 125374, DOI:10.1016/j.ijbiomac.2023.125374.
- Q. Bao, X. Zhang, Z. Hao, Q. Li, F. Wu, K. Wang, Y. Li, W. Li and H. Gao, Advances in Polysaccharide-Based Microneedle Systems for the Treatment of Ocular Diseases, Nano–Micro Lett., 2024, 16(1), 268, DOI:10.1007/s40820-024-01477-3.
- L. K. Vora, K. Moffatt, I. A. Tekko, A. J. Paredes, F. Volpe-Zanutto, D. Mishra, K. Peng, R. Raj Singh Thakur and R. F. Donnelly, Microneedle array systems for long-acting drug delivery, Eur. J. Pharm. Biopharm., 2021, 159, 44–76, DOI:10.1016/j.ejpb.2020.12.006.
- P. W. R. Ananda, D. Elim, H. S. Zaman, W. Muslimin, M. G. R. Tunggeng and A. D. Permana, Combination of transdermal patches and solid microneedles for improved transdermal delivery of primaquine, Int. J. Pharm., 2021, 609, 121204, DOI:10.1016/j.ijpharm.2021.121204.
- S. Gade, K. Glover, D. Mishra, S. Sharma, O. Guy, R. F. Donnelly, L. K. Vora and R. R. S. Thakur, Hollow microneedles for ocular drug delivery, J. Control. Release., 2024, 371, 43–66, DOI:10.1016/j.jconrel.2024.05.013.
- X. Zhang, Y. Liang, D. Luo, P. Li, Y. Chen, X. Fu, Y. Yue, R. Hou, J. Liu and X. Wang, Advantages and disadvantages of various hydrogel scaffold types: A research to improve the clinical conversion rate of loaded MSCs-Exos hydrogel scaffolds, Biomed. Pharmacother., 2024, 179, 117386, DOI:10.1016/j.biopha.2024.117386.
- M. Starlin Chellathurai, S. Mahmood, Z. Mohamed Sofian, C. Wan Hee, R. Sundarapandian, H. N. Ahamed, C. S. Kandasamy, A. R. Hilles, N. M. Hashim and A. K. Janakiraman, Biodegradable polymeric insulin microneedles-a design and materials perspective review, Drug Deliv., 2024, 31(1), 2296350, DOI:10.1080/10717544.2023.2296350.
- J. Jiang, H. S. Gill, D. Ghate, B. E. McCarey, S. R. Patel, H. F. Edelhauser and M. R. Prausnitz, Coated microneedles for drug delivery to the eye, Investig. Ophthalmol. Vis. Sci., 2007, 48(9), 4038–4043, DOI:10.1167/iovs.07-0066.
- Y. Shin, J. Kim, J. H. Seok, H. Park, H. R. Cha, S. H. Ko, J. M. Lee, M. S. Park and J. H. Park, Development of the H3N2 influenza microneedle vaccine for cross-protection against antigenic variants, Sci. Rep., 2022, 12(1), 12189, DOI:10.1038/s41598-022-16365-2.
- K. T. Dicker, L. A. Gurski, S. Pradhan-Bhatt, R. L. Witt, M. C. Farach-Carson and X. Jia, Hyaluronan: a simple polysaccharide with diverse biological functions, Acta Biomater., 2014, 10(4), 1558–1570, DOI:10.1016/j.actbio.2013.12.019.
- S. Liu, M. N. Jin, Y. S. Quan, F. Kamiyama, H. Katsumi, T. Sakane and A. Yamamoto, The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin, J. Control. Release., 2012, 161(3), 933–941, DOI:10.1016/j.jconrel.2012.05.030.
- S. Liu, M. N. Jin, Y. S. Quan, F. Kamiyama, K. Kusamori, H. Katsumi, T. Sakane and A. Yamamoto, Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin, Eur. J. Pharm. Biopharm., 2014, 86(2), 267–276, DOI:10.1016/j.ejpb.2013.10.001.
- J. Zhu, L. Dong, H. Du, J. Mao, Y. Xie, H. Wang, J. Lan, Y. Lou, Y. Fu, J. Wen, B. Jiang, Y. Li, J. Zhu and J. Tao, 5-Aminolevulinic Acid-Loaded Hyaluronic Acid Dissolving Microneedles for Effective Photodynamic Therapy of Superficial Tumors with Enhanced Long-Term Stability, Adv. Healthcare Mater., 2019, 8(22), e1900896, DOI:10.1002/adhm.201900896.
- S. Shen, X. Zheng, X. Dong, M. Fang, A. Wan, T. Zhu, Q. Yang, J. Xie and Q. Yan, Methotrexate-loaded hyaluronan-modified liposomes integrated into dissolving microneedles for the treatment of psoriasis, Eur. J. Pharmaceut. Sci., 2024, 195, 106711, DOI:10.1016/j.ejps.2024.106711.
- M. Torlopov, O. Shevchenko, N. Drozd and E. Udoratina, Ethylenediamine-modified alginate - A hemocompatible platform for polymer-drug conjugates, Int. J. Biol. Macromol., 2025, 287, 138326, DOI:10.1016/j.ijbiomac.2024.138326.
- W. Jiao, W. Chen, Y. Mei, Y. Yun, B. Wang, Q. Zhong, H. Chen and W. Chen, Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate, Molecules, 2019, 24(23), 4374, DOI:10.3390/molecules24234374.
- H. Geng, M. Chen, C. Guo, W. Wang and D. Chen, Marine polysaccharides: Biological activities and applications in drug delivery systems, Carbohydr. Res., 2024, 538, 109071, DOI:10.1016/j.carres.2024.109071.
- H. Dawud, N. Edelstein-Pardo, K. Mulamukkil, R. J. Amir and A. Abu Ammar, Hydrogel Microneedles with Programmed Mesophase Transitions for Controlled Drug Delivery, ACS Appl. Bio Mater., 2024, 7(3), 1682–1693, DOI:10.1021/acsabm.3c01133.
- J. Zhao, J. Lv, G. Ling and P. Zhang, A swellable hydrogel microneedle based on cerium-metal organic frame composite nanozyme for detection of biomarkers, Int. J. Biol. Macromol., 2024, 254(Pt 1), 127745, DOI:10.1016/j.ijbiomac.2023.127745.
- Z. Zhou, M. Xing, S. Zhang, G. Yang and Y. Gao, Process optimization of Ca2+ cross-linked alginate-based swellable microneedles for enhanced transdermal permeability: More applicable to acidic drugs, Int. J. Pharm., 2022, 618, 121669, DOI:10.1016/j.ijpharm.2022.121669.
- V. V. Perelygin, M. V. Zharikov, I. V. Zmitrovich and T. A. Nekrasova, Chitin and Its Derivative Chitosan: Distribution in Nature, Applications, and Technology Research (A Review), Int. J. Med. Mushrooms, 2024, 26(10), 69–81, DOI:10.1615/IntJMedMushrooms.2024055012.
- R. C. Cheung, T. B. Ng, J. H. Wong and W. Y. Chan, Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications, Mar. Drugs, 2015, 13(8), 5156–5186, DOI:10.3390/md13085156.
- J. J. Wang, Z. W. Zeng, R. Z. Xiao, T. Xie, G. L. Zhou, X. R. Zhan and S. L. Wang, Recent advances of chitosan nanoparticles as drug carriers, Int. J. Nanomed., 2011, 6, 765–774, DOI:10.2147/IJN.S17296.
- P. Wen, Y. Wang, C. Zhang, P. He, Z. Lin, Z. Hu and W. Lu, Liposome-loaded dissolvable microneedle patches for more efficient intradermal antigen delivery of Hepatitis B vaccine, Int. J. Pharm., 2025, 669, 125023, DOI:10.1016/j.ijpharm.2024.125023.
- M. C. Chen, K. Y. Lai, M. H. Ling and C. W. Lin, Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design, Acta Biomater., 2018, 65, 66–75, DOI:10.1016/j.actbio.2017.11.004.
- Y. H. Chen, K. Y. Lai, Y. H. Chiu, Y. W. Wu, A. L. Shiau and M. C. Chen, Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination, Acta Biomater., 2019, 97, 230–238, DOI:10.1016/j.actbio.2019.07.048.
- R. Sharma, K. Kuche, P. Thakor, V. Bhavana, S. Srivastava, N. K. Mehra and S. Jain, Chondroitin Sulfate: Emerging biomaterial for biopharmaceutical purpose and tissue engineering, Carbohydr. Polym., 2022, 286, 119305, DOI:10.1016/j.carbpol.2022.119305.
- M. M. Abdallah, N. Fernández, A. A. Matias and M. D. R. Bronze, Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods, Carbohydr. Polym., 2020, 243, 116441, DOI:10.1016/j.carbpol.2020.116441.
- Z. Shu, Y. Cao, Y. Tao, X. Liang, F. Wang, Z. Li, Z. Li and S. Gui, Polyvinylpyrrolidone microneedles for localized delivery of sinomenine hydrochloride: preparation, release behavior of in vitro & in vivo, and penetration mechanism, Drug Deliv., 2020, 27(1), 642–651, DOI:10.1080/10717544.2020.1754524.
- K. Fukushima, A. Ise, H. Morita, R. Hasegawa, Y. Ito, N. Sugioka and K. Takada, Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats, Pharm. Res., 2011, 28(1), 7–21, DOI:10.1007/s11095-010-0097-7.
- X. Liang, S. Guo, X. Kuang, X. Wan, L. Liu, F. Zhang, G. Jiang, H. Cong, H. He and S. C. Tan, Recent advancements and perspectives on processable natural biopolymers: Cellulose, chitosan, eggshell membrane, and silk fibroin, Sci. Bull., 2024, 69(21), 3444–3466, DOI:10.1016/j.scib.2024.08.023.
- N. Qiang, Z. Liu, M. Lu, Y. Yang, F. Liao, Y. Feng, G. Liu and S. Qiu, Preparation and Properties of Polyvinylpyrrolidone/Sodium Carboxymethyl Cellulose Soluble Microneedles, Materials, 2023, 16(9), 3417, DOI:10.3390/ma16093417.
- M. S. Rahman, M. S. Hasan, A. S. Nitai, S. Nam, A. K. Karmakar, M. S. Ahsan, M. J. A. Shiddiky and M. B. Ahmed, Recent Developments of Carboxymethyl Cellulose, Polymers, 2021, 13(8), 1345, DOI:10.3390/polym13081345.
- L. Chen, H. Zhou, L. Hao, H. Chen and X. Zhou, Soy protein isolate-carboxymethyl cellulose conjugates with pH sensitivity for sustained avermectin release, R. Soc. Open Sci., 2019, 6(7), 190685, DOI:10.1098/rsos.190685.
- S. Yun, S. J. Lee, S. S. Giri, H. J. Kim, S. G. Kim, S. W. Kim, S. J. Han, J. Kwon, W. T. Oh and S. Chang Park, Vaccination of fish against Aeromonas hydrophila infections using the novel approach of transcutaneous immunization with dissolving microneedle patches in aquaculture, Fish Shellfish Immunol., 2020, 97, 34–40, DOI:10.1016/j.fsi.2019.12.026.
- L. An, G. Chang, L. Zhang, P. Wang, W. Gao and X. Li, Pectin: Health-promoting properties as a natural galectin-3 inhibitor, Glycoconj. J., 2024, 41(2), 93–118, DOI:10.1007/s10719-024-10152-z.
- Y. K. Demir and O. Kerimoglu, Novel use of pectin as a microneedle base, Chem. Pharmaceut. Bull., 2015, 63(4), 300–304, DOI:10.1248/cpb.c14-00759.
- Y. K. Demir, A. Ü. Metin, B. Şatıroğlu, M. E. Solmaz, V. Kayser and K. Mäder, Poly (methyl vinyl ether-co-maleic acid)-Pectin based hydrogel-forming systems: Gel, film, and microneedles, Eur. J. Pharm. Biopharm., 2017, 117, 182–194, DOI:10.1016/j.ejpb.2017.04.018.
- L. Bai, T. Wang, Q. Deng, W. Zheng, X. Li, H. Yang, R. Tong, D. Yu and J. Shi, Dual properties of pharmacological activities and preparation excipient: Bletilla striata polysaccharides, Int. J. Biol. Macromol., 2024, 254(Pt 1), 127643, DOI:10.1016/j.ijbiomac.2023.127643.
- X. Qu, X. Guo, T. Zhu, Z. Zhang, W. Wang and Y. Hao, Microneedle patches containing mesoporous polydopamine nanoparticles loaded with triamcinolone acetonide for the treatment of oral mucositis, Front. Bioeng. Biotechnol., 2023, 11, 1203709, DOI:10.3389/fbioe.2023.1203709.
- P. Zhou, S. Zhao, C. Huang, Y. Qu and C. Zhang, Bletilla striata polysaccharide microneedle for effective transdermal administration of model protein antigen, Int. J. Biol. Macromol., 2022, 205, 511–519, DOI:10.1016/j.ijbiomac.2022.02.116.
- C. Wang, L. Zheng, S. Liu, X. Guo, Y. Qu, M. Gao, X. Cui and Y. Yang, A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice, Int. J. Biol. Macromol., 2020, 149, 1084–1097, DOI:10.1016/j.ijbiomac.2020.02.034.
- C. Wang, S. Liu, J. Xu, M. Gao, Y. Qu, Y. Liu, Y. Yang and X. Cui, Dissolvable microneedles based on Panax notoginseng polysaccharide for transdermal drug delivery and skin dendritic cell activation, Carbohydr. Polym., 2021, 268, 118211, DOI:10.1016/j.carbpol.2021.118211.
- N. Dabholkar, S. Gorantla, T. Waghule, V. K. Rapalli, A. Kothuru, S. Goel and G. Singhvi, Biodegradable microneedles fabricated with carbohydrates and proteins: Revolutionary approach for transdermal drug delivery, Int. J. Biol. Macromol., 2021, 170, 602–621, DOI:10.1016/j.ijbiomac.2020.12.177.
- H. J. Choi, J. M. Song, B. J. Bondy, R. W. Compans, S. M. Kang and M. R. Prausnitz, Effect of Osmotic Pressure on the Stability of Whole Inactivated Influenza Vaccine for Coating on Microneedles, PLoS One, 2015, 10(7), e0134431, DOI:10.1371/journal.pone.0134431.
- Y. A. Mahmoud, M. E. El-Naggar, A. Abdel-Megeed and M. El-Newehy, Recent Advancements in Microbial Polysaccharides: Synthesis and Applications, Polymers, 2021, 13(23), 4136, DOI:10.3390/polym13234136.
- J. Liang, Y. Yu, C. Li, Q. Li, P. Chen, W. Li, W. Liu, Z. Li, Y. Liu, S. Zhang and X. Zhang, Tofacitinib combined with melanocyte protector α-MSH to treat vitiligo through dextran based hydrogel microneedles, Carbohydr. Polym., 2023, 305, 120549, DOI:10.1016/j.carbpol.2023.120549.
- A. C. Petrey and C. A. de la Motte, Hyaluronan, a crucial regulator of inflammation, Front. Immunol., 2014, 5, 101, DOI:10.3389/fimmu.2014.00101.
- L. A. Johnson and D. G. Jackson, Hyaluronan and Its Receptors: Key Mediators of Immune Cell Entry and Trafficking in the Lymphatic System, Cells, 2021, 10(8), 2061, DOI:10.3390/cells10082061.
- N. Nagy, H. F. Kuipers, P. L. Marshall, E. Wang, G. Kaber and P. L. Bollyky, Hyaluronan in immune dysregulation and autoimmune diseases, Matrix biology, Matrix Biol., 2019, 78, 292–313, DOI:10.1016/j.matbio.2018.03.022.
- A. M. Carvalho, R. L. Reis and I. Pashkuleva, Hyaluronan Receptors as Mediators and Modulators of the Tumor Microenvironment, Adv. Healthcare Mater., 2023, 12(5), e2202118, DOI:10.1002/adhm.202202118.
- Y. Dong, A. Arif, M. Olsson, V. Cali, B. Hardman, M. Dosanjh, M. Lauer, R. J. Midura, V. C. Hascall, K. L. Brown and P. Johnson, Endotoxin free hyaluronan and hyaluronan fragments do not stimulate TNF-α, interleukin-12 or upregulate co-stimulatory molecules in dendritic cells or macrophages, Sci. Rep., 2016, 6, 36928, DOI:10.1038/srep36928.
- F. M. Spinelli, D. L. Vitale, A. Icardi, I. Caon, A. Brandone, P. Giannoni, V. Saturno, A. Passi, M. García, I. Sevic and L. Alaniz, Hyaluronan preconditioning of monocytes/macrophages affects their angiogenic behavior and regulation of TSG-6 expression in a tumor type-specific manner, FEBS J., 2019, 286(17), 3433–3449, DOI:10.1111/febs.14871.
- O. Babasola, K. J. Rees-Milton, S. Bebe, J. Wang and T. P. Anastassiades, Chemically modified N-acylated hyaluronan fragments modulate proinflammatory cytokine production by stimulated human macrophages, J. biol. chem., 2014, 289(36), 24779–24791, DOI:10.1074/jbc.M113.515783.
- F. M. Spinelli, P. Rosales, S. Pluda, D. L. Vitale, A. Icardi, C. Guarise, A. Reszegi, I. Kovalszky, M. García, I. Sevic, D. Galesso and L. Alaniz, The effects of sulfated hyaluronan in breast, lung and colorectal carcinoma and monocytes/macrophages cells: Its role in angiogenesis and tumor progression, IUBMB Life, 2022, 74(10), 927–942, DOI:10.1002/iub.2604.
- J. Kajahn, S. Franz, E. Rueckert, I. Forstreuter, V. Hintze, S. Moeller and J. C. Simon, Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation, Biomatter, 2012, 2(4), 226–236, DOI:10.4161/biom.22855.
- A. M. Carvalho, R. L. Reis and I. Pashkuleva, Hyaluronan Receptors as Mediators and Modulators of the Tumor Microenvironment, Adv. Healthcare Mater., 2023, 12(5), e2202118, DOI:10.1002/adhm.202202118.
- L. Alaniz, M. M. Rizzo and G. Mazzolini, Pulsing dendritic cells with whole tumor cell lysates, Methods Mol. Biol., 2014, 1139, 27–31, DOI:10.1007/978-1-4939-0345-0_3.
- X. Cai, Y. H. Chiu and Z. J. Chen, The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling, Mol. Cell, 2014, 54(2), 289–296, DOI:10.1016/j.molcel.2014.03.040.
- G. N. Barber, STING: infection, inflammation and cancer, Nat. Rev. Immunol., 2015, 15(12), 760–770, DOI:10.1038/nri3921.
- E. C. Carroll, L. Jin, A. Mori, N. Muñoz-Wolf, E. Oleszycka, H. B. T. Moran, S. Mansouri, C. P. McEntee, E. Lambe, E. M. Agger, P. Andersen, C. Cunningham, P. Hertzog, K. A. Fitzgerald, A. G. Bowie and E. C. Lavelle, The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons, Immunity, 2016, 44(3), 597–608, DOI:10.1016/j.immuni.2016.02.004.
- A. Mori, E. Oleszycka, F. A. Sharp, M. Coleman, Y. Ozasa, M. Singh, D. T. O'Hagan, L. Tajber, O. I. Corrigan, E. A. McNeela and E. C. Lavelle, The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses, Eur. J. Immunol., 2012, 42(10), 2709–2719, DOI:10.1002/eji.201242372.
- C. X. Xu, H. Jin, Y. S. Chung, J. Y. Shin, M. A. Woo, K. H. Lee, G. N. Palmos, B. D. Choi and M. H. Cho, Chondroitin sulfate extracted from the Styela clava tunic suppresses TNF-alpha-induced expression of inflammatory factors, VCAM-1 and iNOS by blocking Akt/NF-kappaB signal in JB6 cells, Cancer Lett., 2008, 264(1), 93–100, DOI:10.1016/j.canlet.2008.01.022.
- C. Jomphe, M. Gabriac, T. M. Hale, L. Héroux, L. E. Trudeau, D. Deblois, E. Montell, J. Vergés and P. du Souich, Chondroitin sulfate inhibits the nuclear translocation of nuclear factor-kappaB in interleukin-1beta-stimulated chondrocytes, Basic Clin. Pharmacol. Toxicol., 2008, 102(1), 59–65, DOI:10.1111/j.1742-7843.2007.00158.x.
- A. L. da Cunha, J. A. K. Aguiar, F. S. Correa da Silva and Y. M. Michelacci, Do chondroitin sulfates with different structures have different activities on chondrocytes and macrophages?, Int. J. Biol. Macromol., 2017, 103, 1019–1031, DOI:10.1016/j.ijbiomac.2017.05.123.
- J. N. Gouze, E. Gouze, M. P. Popp, M. L. Bush, E. A. Dacanay, J. D. Kay, P. P. Levings, K. R. Patel, J. P. Saran, R. S. Watson and S. C. Ghivizzani, Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1beta, Arthritis Res. Ther., 2006, 8(6), R173, DOI:10.1186/ar2082.
- F. Taraballi, B. Corradetti, S. Minardi, S. Powel, F. Cabrera, J. L. Van Eps, B. K. Weiner and E. Tasciotti, Biomimetic collagenous scaffold to tune inflammation by targeting macrophages, J. Tissue Eng., 2016, 7, 2041731415624667, DOI:10.1177/2041731415624667.
- W. Zhang, F. Sun, H. Niu, Q. Wang and J. Duan, Mechanistic insights into cellular immunity of chondroitin sulfate A and its zwitterionic N-deacetylated derivatives, Carbohydr. Polym., 2015, 123, 331–338, DOI:10.1016/j.carbpol.2015.01.059.
- G. K. Tan and Y. Tabata, Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-κB nuclear translocation, Acta Biomater., 2014, 10(6), 2684–2692, DOI:10.1016/j.actbio.2014.02.025.
- S. Hatano and H. Watanabe, Regulation of Macrophage and Dendritic Cell Function by Chondroitin Sulfate in Innate to Antigen-Specific Adaptive Immunity, Front. Immunol., 2020, 11, 232, DOI:10.3389/fimmu.2020.00232.
- Y. Wang, S. Han, R. Li, B. Cui, X. Ma, X. Qi, Q. Hou, M. Lin, J. Bai and S. Li, Structural characterization and immunological activity of polysaccharides from the tuber of Bletilla striata, Int. J. Biol. Macromol., 2019, 122, 628–635, DOI:10.1016/j.ijbiomac.2018.10.201.
- Q. Peng, M. Li, F. Xue and H. Liu, Structure and immunobiological activity of a new polysaccharide from Bletilla striata, Carbohydr. Polym., 2014, 107, 119–123, DOI:10.1016/j.carbpol.2014.02.042.
- Z. Sartawi, C. Blackshields and W. Faisal, Dissolving microneedles: Applications and growing therapeutic potential, J. Control. Release., 2022, 348, 186–205, DOI:10.1016/j.jconrel.2022.05.045.
- J. J. Norman, S. O. Choi, N. T. Tong, A. R. Aiyar, S. R. Patel, M. R. Prausnitz and M. G. Allen, Hollow microneedles for intradermal injection fabricated by sacrificial micromolding and selective electrodeposition, Biomed. Microdevices, 2013, 15(2), 203–210, DOI:10.1007/s10544-012-9717-9.
- J. G. Turner, L. R. White, P. Estrela and H. S. Leese, Hydrogel-Forming Microneedles: Current Advancements and Future Trends, Macromol. Biosci., 2021, 21(2), e2000307, DOI:10.1002/mabi.202000307.
- G. Anbazhagan, S. B. Suseela and R. Sankararajan, Design, analysis and fabrication of solid polymer microneedle patch using CO2 laser and polymer molding, Drug Delivery Transl. Res., 2023, 13(6), 1813–1827, DOI:10.1007/s13346-023-01296-w.
- B. Zhou, Z. Guo, P. Zhao, H. Wang, S. Dong, B. Cheng, J. Yang, B. Li and X. Wang, Fabrication and characterization of coated microneedle patches based on PEGDA for transdermal administration of metformin, Drug Delivery Transl. Res., 2024, 14(1), 131–142, DOI:10.1007/s13346-023-01387-8.
- J. D. Kim, M. Kim, H. Yang, K. Lee and H. Jung, Droplet-born air blowing: novel dissolving microneedle fabrication, J. Control. Release., 2013, 170(3), 430–436, DOI:10.1016/j.jconrel.2013.05.026.
- P. Papadimitriou, E. G. Andriotis, D. Fatouros and D. Tzetzis, Design and Prototype Fabrication of a Cost-Effective Microneedle Drug Delivery Apparatus Using Fused Filament Fabrication, Liquid Crystal Display and Semi-Solid Extrusion 3D Printing Technologies, Micromachines, 2022, 13(8), 1319, DOI:10.3390/mi13081319.
- T. N. Tarbox, A. B. Watts, Z. Cui and R. O. Williams 3rd, An update on coating/manufacturing techniques of microneedles, Drug Delivery Transl. Res., 2018, 8(6), 1828–1843, DOI:10.1007/s13346-017-0466-4.
- M. G. McGrath, S. Vucen, A. Vrdoljak, A. Kelly, C. O'Mahony, A. M. Crean and A. Moore, Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration, Eur. J. Pharm. Biopharm., 2014, 86(2), 200–211, DOI:10.1016/j.ejpb.2013.04.023.
- S. Ross, N. Scoutaris, D. Lamprou, D. Mallinson and D. Douroumis, Inkjet printing of insulin microneedles for transdermal delivery, Drug Delivery Transl. Res., 2015, 5(4), 451–461, DOI:10.1007/s13346-015-0251-1.
- D. Baykara, T. Bedir, E. Ilhan, M. E. Mutlu, O. Gunduz, R. Narayan and C. B. Ustundag, Fabrication and optimization of 3D printed gelatin methacryloyl microneedle arrays based on vat photopolymerization, Front. Bioeng. Biotechnol., 2023, 11, 1157541, DOI:10.3389/fbioe.2023.1157541.
- L. Zhang, R. Guo, S. Wang, X. Yang, G. Ling and P. Zhang, Fabrication, evaluation and applications of dissolving microneedles, Int. J. Pharm., 2021, 604, 120749, DOI:10.1016/j.ijpharm.2021.120749.
- C. Yeung, S. Chen, B. King, H. Lin, K. King, F. Akhtar, G. Diaz, B. Wang, J. Zhu, W. Sun, A. Khademhosseini and S. Emaminejad, A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery, Biomicrofluidics, 2019, 13(6), 064125, DOI:10.1063/1.5129684.
- C. P. P. Pere, S. N. Economidou, G. Lall, C. Ziraud, J. S. Boateng, B. D. Alexander, D. A. Lamprou and D. Douroumis, 3D printed microneedles for insulin skin delivery, Int. J. Pharm., 2018, 544(2), 425–432, DOI:10.1016/j.ijpharm.2018.03.031.
- A. Tucak, M. Sirbubalo, L. Hindija, O. Rahić, J. Hadžiabdić, K. Muhamedagić, A. Čekić and E. Vranić, Microneedles: Characteristics, Materials, Production Methods and Commercial Development, Micromachines, 2020, 11(11), 961, DOI:10.3390/mi11110961.
- M. G. McGrath, S. Vucen, A. Vrdoljak, A. Kelly, C. O'Mahony, A. M. Crean and A. Moore, Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration, Eur. J. Pharm. Biopharm., 2014, 86(2), 200–211, DOI:10.1016/j.ejpb.2013.04.023.
- J. D. Kim, M. Kim, H. Yang, K. Lee and H. Jung, Droplet-born air blowing: novel dissolving microneedle fabrication, J. Control. Release., 2013, 170(3), 430–436, DOI:10.1016/j.jconrel.2013.05.026.
- A. Prabhu, V. Baliga, R. Shenoy, A. D. Dessai and U. Y. Nayak, 3D printed microneedles: revamping transdermal drug delivery systems, Drug Delivery Transl. Res., 2025, 15(2), 436–454, DOI:10.1007/s13346-024-01679-7.
- J. Yang, G. Liang, T. Xiang and W. Situ, Effect of crosslinking processing on the chemical structure and biocompatibility of a chitosan-based hydrogel, Food Chem., 2021, 354, 129476, DOI:10.1016/j.foodchem.2021.129476.
- M. Zussman and M. Zilberman, Injectable metronidazole-eluting gelatin-alginate hydrogels for local treatment of periodontitis, J. Biomater. Appl., 2022, 37(1), 166–179, DOI:10.1177/08853282221079458.
- X. Yue, S. Zhao, M. Qiu, J. Zhang, G. Zhong, C. Huang, X. Li, C. Zhang and Y. Qu, Physical dual-network photothermal antibacterial multifunctional hydrogel adhesive for wound healing of drug-resistant bacterial infections synthesized from natural polysaccharides, Carbohydr. Polym., 2023, 312, 120831, DOI:10.1016/j.carbpol.2023.120831.
- P. Li, Z. Feng, Z. Yu, Y. Chen, P. Li, Z. Yang, S. Li and S. Jin, Preparation of chitosan-Cu2+/NH3 physical hydrogel and its properties, Int. J. Biol. Macromol., 2019, 133, 67–75, DOI:10.1016/j.ijbiomac.2019.03.011.
- Z. Wang, M. Xiao, Z. Li, X. Wang, F. Li, H. Yang, Y. Chen and Z. Zhu, Microneedle Patches-Integrated Transdermal Bioelectronics for Minimally Invasive Disease Theranostics, Adv. Healthcare Mater., 2024, 13(17), e2303921, DOI:10.1002/adhm.202303921.
- P. Makvandi, M. Kirkby, A. R. J. Hutton, M. Shabani, C. K. Y. Yiu, Z. Baghbantaraghdari, R. Jamaledin, M. Carlotti, M. Mazzolai, V. Mattoli and R. F. Donnelly, Engineering Microneedle Patches for Improved Penetration: Analysis, Skin Models and Factors Affecting Needle Insertion, Nano–Micro Lett., 2021, 13(1), 93, DOI:10.1007/s40820-021-00611-9.
- M. Starlin Chellathurai, S. Mahmood, Z. Mohamed Sofian, C. Wan Hee, R. Sundarapandian, H. N. Ahamed, C. S. Kandasamy, A. R. Hilles, N. M. Hashim and A. K. Janakiraman, Biodegradable polymeric insulin microneedles-a design and materials perspective review, Drug Deliv., 2024, 31(1), 2296350, DOI:10.1080/10717544.2023.2296350.
- H. Teymourian, F. Tehrani, K. Mahato and J. Wang, Lab under the Skin: Microneedle Based Wearable Devices, Adv. Healthcare Mater., 2021, 10(17), e2002255, DOI:10.1002/adhm.202002255.
- Q. Yan, S. Shen, Y. Wang, J. Weng, A. Wan, G. Yang and L. Feng, The Finite Element Analysis Research on Microneedle Design Strategy and Transdermal Drug Delivery System, Pharmaceutics, 2022, 14(8), 1625, DOI:10.3390/pharmaceutics14081625.
- R. K. Reed, K. Lilja and T. C. Laurent, Hyaluronan in the rat with special reference to the skin, Acta Physiol. Scand., 1988, 134(3), 405–411, DOI:10.1111/j.1748-1716.1988.tb08508.x.
- N. G. Kotla, S. R. Bonam, S. Rasala, J. Wankar, R. A. Bohara, J. Bayry, Y. Rochev and A. Pandit, Recent advances and prospects of hyaluronan as a multifunctional therapeutic system, J. Control. Release., 2021, 336, 598–620, DOI:10.1016/j.jconrel.2021.07.002.
- E. Papakonstantinou, M. Roth and G. Karakiulakis, Hyaluronic acid: A key molecule in skin aging, Derm. Endocrinol., 2012, 4(3), 253–258, DOI:10.4161/derm.21923.
- K. M. Kapoor, D. I. Saputra, C. E. Porter, L. Colucci, C. Stone, E. E. A. Brenninkmeijer, J. Sloane, K. Sayed, K. K. Winaya and D. Bertossi, Treating Aging Changes of Facial Anatomical Layers with Hyaluronic Acid Fillers, Clin. Cosmet. Invest. Dermatol., 2021, 14, 1105–1118, DOI:10.2147/CCID.S294812.
- Y. Cui, F. Wang, J. J. Voorhees and G. J. Fisher, Rejuvenation of Aged Human Skin by Injection of Cross-linked Hyaluronic Acid, Plast. Reconstr. Surg., 2021, 147(1S-2), 43S–49S, DOI:10.1097/PRS.0000000000007620.
- K. He, J. Zang, T. Ren, S. Feng, M. Liu, X. Zhang, W. Sun, J. Chu, D. Xu and F. Liu, Therapeutic Potential and Mechanisms of Mesenchymal Stem Cell and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Atopic Dermatitis, J. Inflamm. Res., 2024, 17, 5783–5800, DOI:10.2147/JIR.S479444.
- L. Song, J. Chi, Z. Li, Y. Tao, Y. Sun, Q. Zhou, S. Lu, Q. Huang, S. Huang, X. Lu, M. Wu, Y. Yang, L. Chen, X. Li, K. Shi and J. Xiao, An inflammation-responsive double-layer microneedle patch for recurrent atopic dermatitis therapy, Int. J. Pharm., 2023, 643, 123215, DOI:10.1016/j.ijpharm.2023.123215.
- Y. L. Chen, C. C. Chang, Y. C. Lin and M. C. Chen, Double-layered PLGA/HA microneedle systems as a long-acting formulation of polyphenols for effective and long-term management of atopic dermatitis, Biomater. Sci., 2023, 11(14), 4995–5011, 10.1039/d3bm00182b.
- S. Mantry, A. Behera, S. Pradhan, L. Mohanty, R. Kumari, A. Singh and M. K. Yadav, Polysaccharide-based chondroitin sulfate macromolecule loaded hydrogel/scaffolds in wound healing- A comprehensive review on possibilities, research gaps, and safety assessment, Int. J. Biol. Macromol., 2024, 279(Pt 3), 135410, DOI:10.1016/j.ijbiomac.2024.135410.
- M. Hasnain, T. Kanwal, K. Rehman, S. R. U. Rehman, S. Aslam, T. Roome, S. Perveen, M. B. Zaidi, S. Saifullah, S. Yasmeen, A. Hasan and M. R. Shah, Microarray needles comprised of arginine-modified chitosan/PVA hydrogel for enhanced antibacterial and wound healing potential of curcumin, Int. J. Biol. Macromol., 2023, 253(Pt 1), 126697, DOI:10.1016/j.ijbiomac.2023.126697.
- X. Yu, C. Wang, Y. Wang, L. Li, X. Gao, T. Zhu, P. An, Z. Meng, W. Wang, T. Wu and Y. Hao, Microneedle Array Patch Made of Kangfuxin/Chitosan/Fucoidan Complex Enables Full-Thickness Wound Healing, Front. Chem., 2022, 10, 838920, DOI:10.3389/fchem.2022.838920.
- Y. Liu, S. Wang, F. Yang, X. Wang, J. Zhang, X. Han, X. Zhang and Z. Wang, Application and progress of new technologies and new materials in the treatment of pathological scar, Front. Chem., 2024, 12, 1389399, DOI:10.3389/fchem.2024.1389399.
- N. Zhang, L. Xue, A. Younas, F. Liu, J. Sun, Z. Dong and Y. Zhao, Co-delivery of triamcinolone acetonide and verapamil for synergistic treatment of hypertrophic scars via carboxymethyl chitosan and Bletilla striata polysaccharide-based microneedles, Carbohydr. Polym., 2022, 284, 119219, DOI:10.1016/j.carbpol.2022.119219.
- A. Lomas, J. Leonardi-Bee and F. Bath-Hextall, A systematic review of worldwide incidence of nonmelanoma skin cancer, Br. J. Dermatol., 2012, 166(5), 1069–1080, DOI:10.1111/j.1365-2133.2012.10830.x.
- US Preventive Services Task Force, D. C. Grossman, S. J. Curry, D. K. Owens, M. J. Barry, A. B. Caughey, K. W. Davidson, C. A. Doubeni, J. W. Epling Jr, A. R. Kemper, A. H. Krist, M. Kubik, S. Landefeld, C. M. Mangione, M. Silverstein, M. A. Simon and C. W. Tseng, Behavioral Counseling to Prevent Skin Cancer: US Preventive Services Task Force Recommendation Statement, JAMA, 2018, 319(11), 1134–1142, DOI:10.1001/jama.2018.1623.
- S. Huang, H. Liu, S. Huang, T. Fu, W. Xue and R. Guo, Dextran Methacrylate Hydrogel Microneedles Loaded with Doxorubicin and Trametinib for Continuous Transdermal Administration of Melanoma, Carbohydr. Polym., 2020, 246, 116650, DOI:10.1016/j.carbpol.2020.116650.
- F. Grän, A. Kerstan, E. Serfling, M. Goebeler and K. Muhammad, Current Developments in the Immunology of Psoriasis, Yale J. Biol. Med., 2020, 93(1), 97–110 Search PubMed.
- M. Tokuyama and T. Mabuchi, New Treatment Addressing the Pathogenesis of Psoriasis, Int. J. Mol. Sci., 2020, 21(20), 7488, DOI:10.3390/ijms21207488.
- P. Dai, X. Ge, C. Sun, H. Jiang, W. Zuo, P. Wu, C. Liu, S. Deng, J. Yang, J. Dai and Y. Ju, A Novel Methacryloyl Chitosan Hydrogel Microneedles Patch with Sustainable Drug Release Property for Effective Treatment of Psoriasis, Macromol. Biosci., 2023, 23(12), e2300194, DOI:10.1002/mabi.202300194.
- H. Chen, T. C. Zhang, X. L. Yin, J. Y. Man, X. R. Yang and M. Lu, Magnitude and Temporal Trend of Acne Vulgaris Burden in 204 Countries and Territories from 1990 to 2019: An Analysis from the Global Burden of Disease Study 2019, Br. J. Dermatol., 2022, 186(4), 673–683, DOI:10.1111/bjd.20882.
- D. A. Satish, S. Aurangabadkar, S. T. Tahiliani, R. Damisetty, A. Tiwari, K. D. S, N. Madnani, A. Saraswat, A. Das, D. Sen and S. Jadhwar, Role of Trifarotene in the Management of Acne in Indian Patients: Insights From an Indian Dermatology Experts' Meeting, Cureus, 2024, 16(7), e65800, DOI:10.7759/cureus.65800.
- Q. Wang, Z. Gan, X. Wang, X. Li, L. Zhao, D. Li, Z. Xu, C. Mu, L. Ge and D. Li, Dissolving Hyaluronic Acid-Based Microneedles to Transdermally Deliver Eugenol Combined with Photothermal Therapy for Acne Vulgaris Treatment, ACS Appl. Mater. Interfaces, 2024, 16(17), 21595–21609, DOI:10.1021/acsami.4c01790.
- J. K. de Oliveira, T. Ueda-Nakamura, A. G. Corrêa, R. Petrilli, R. F. V. Lopez, C. V. Nakamura and R. Auzely-Velty, Liposome-Based Nanocarrier Loaded with a New Quinoxaline Derivative for the Treatment of Cutaneous Leishmaniasis, Mater. Sci. Eng. C, 2020, 110, 110720, DOI:10.1016/j.msec.2020.110720.
- M. R. Zare, M. Khorram, S. Barzegar, B. Sarkari, Q. Asgari, S. Ahadian and K. Zomorodian, Dissolvable Carboxymethyl Cellulose/Polyvinylpyrrolidone Microneedle Arrays for Transdermal Delivery of Amphotericin B to Treat Cutaneous Leishmaniasis, Int. J. Biol. Macromol., 2021, 182, 1310–1321, DOI:10.1016/j.ijbiomac.2021.05.075.
- D. Z. J. Lim, Y. Y. Chun, F. N. S. Y. Tan, A. Y. Monteiro, H. M. Cheng, J. Y. Lee, Y. Tan, T. T. Y. Tan and H. L. Tey, Small Interfering RNA Microneedle Patches Versus Silicone Sheets in Reducing Postoperative Scars: A Randomized Single-Blinded Intraindividually Controlled Clinical Trial, Br. J. Dermatol., 2024, 192(1), 19–26, DOI:10.1093/bjd/ljae347.
- D. P. Cassiano, A. C. C. Espósito, K. M. Hassun, M. M. D. A. Lima, E. V. A. Lima, L. D. B. Miot, H. A. Miot and E. Bagatin, Histological Changes in Facial Melasma After Treatment with Triple Combination Cream with or Without Oral Tranexamic Acid and/or Microneedling: A Randomised Clinical Trial, Indian J. Dermatol. Venereol. Leprol., 2022, 88(6), 761–770 CrossRef PubMed.
- E. R. Hofny, A. A. Abdel-Motaleb, S. A. Hamed and H. O. Twisy, Trichloroacetic Acid With Microneedling Versus Trichloroacetic Acid Alone for Treating Melasma, Dermatol. Surg., 2023, 49(1), 66–71, DOI:10.1097/DSS.0000000000003641.
- Y. W. Yew, C. Z. Y. Phuan, X. Zhao, E. S. T. Tan, W. S. Chong and H. L. Tey, Novel Transdermal Device for Delivery of Triamcinolone for Nail Psoriasis Treatment, Ann. Acad. Med. Singap., 2022, 51(1), 16–23 Search PubMed.
- M. Tai, C. Zhang, Y. Ma, J. Yang, Z. Mai, C. Li and G. Leng, Acne and Its Post-Inflammatory Hyperpigmentation Treatment by Applying Anti-Acne Dissolving Microneedle Patches, J. Cosmet. Dermatol., 2022, 21(12), 6913–6919 CrossRef PubMed.
- H. M. Ebrahim, R. Elkot and W. Albalate, Combined Microneedling with Tacrolimus vs. Tacrolimus Monotherapy for Vitiligo Treatment, J. Dermatol. Treat., 2021, 32(8), 999–1004, DOI:10.1080/09546634.2020.1716930.
- J. H. Song, E. J. An, C. Y. Sung, D. H. Jeong, G. Lee and S. Y. Park, A Comparative Study on a Biodegradable Hyaluronic Acid Microneedle Patch with a Needleless Patch for Dry Skin in Atopic Dermatitis: A Single-Blinded, Split-Body, Randomized Controlled Trial, Arch. Dermatol. Res., 2023, 315(3), 569–581, DOI:10.1007/s00403-022-02400-9.
- F. Bageorgou, V. Vasalou, V. Tzanetakou and G. Kontochristopoulos, The New Therapeutic Choice of Tranexamic Acid Solution in Treatment of Erythematotelangiectatic Rosacea, J. Cosmet. Dermatol., 2019, 18(2), 563–567, DOI:10.1111/jocd.12724.
- M. Zasada, A. Markiewicz, Z. Drożdż, P. Mosińska, A. Erkiert-Polguj and E. Budzisz, Preliminary Randomized Controlled Trial of Antiaging Effects of L-Ascorbic Acid Applied in Combination with No-Needle and Microneedle Mesotherapy, J. Cosmet. Dermatol., 2019, 18(3), 843–849, DOI:10.1111/jocd.12727.
- S. Choi, H. J. Kwon, G. R. Ahn, E. J. Ko, K. H. Yoo, B. J. Kim, C. Lee and D. Kim, Hyaluronic Acid Microneedle Patch for the Improvement of Crow's Feet Wrinkles, Dermatol. Ther., 2017, 30(6), e12546, DOI:10.1111/dth.12546.
- U. Pruettijarai, J. Meephansan, O. Prapapan, P. Pureesrisak, P. Sirithanabadeekul, K. Tantisantisom, S. Thongma, Y. Rayanasukha, P. Adulyaritthikul and P. Khanchaitit, Efficacy of a Novel Microneedle Patch for Rejuvenation of the Nasolabial Fold, Skin Res, Technol., 2022, 28(6), 786–791, DOI:10.1111/srt.13199.
- R. Qiao, J. Zhu, J. Fang, H. Shi, Z. Zhang, J. Nie, Y. Ge, T. Lin and Y. Jiang, Microneedle Transdermal Delivery of Compound Betamethasone in Alopecia Areata-A Randomized Controlled Trial, J. Am. Acad. Dermatol., 2025, 92(2), 269–275, DOI:10.1016/j.jaad.2024.09.059.
- F. A. Khan, M. Hussain, B. M. Khan, S. Afsar, M. Shafique, S. U. Haq, N. Akbar and A. Siddique, To Compare Efficacy Between Cryotherapy and Mitomycin Microneedling for the Treatment of Plantar Warts, J. Ayub Med. Coll. Abbottabad, 2023, 35(1), 133–136, DOI:10.55519/JAMC-01-10932.
- C. J. Rini, E. McVey, D. Sutter, S. Keith, H. J. Kurth, L. Nosek, C. Kapitza, K. Rebrin, L. Hirsch and R. J. Pettis, Intradermal Insulin Infusion Achieves Faster Insulin Action than Subcutaneous Infusion for 3-Day Wear, Drug Deliv. Transl. Res., 2015, 5(4), 332–345, DOI:10.1007/s13346-015-0239-x.
- Y. Sun, X. Jing, X. Ma, Y. Feng and H. Hu, Versatile Types of Polysaccharide-Based Drug Delivery Systems: From Strategic Design to Cancer Therapy, Int. J. Mol. Sci., 2020, 21(23), 9159, DOI:10.3390/ijms21239159.
- D. Gaikwad, R. Sutar and D. Patil, Polysaccharide Mediated Nanodrug Delivery: A Review, Int. J. Biol. Macromol., 2024, 261(Pt 1), 129547, DOI:10.1016/j.ijbiomac.2024.129547.
- D. F. S. Fonseca, C. Vilela, A. J. D. Silvestre and C. S. R. Freire, A Compendium of Current Developments on Polysaccharide and Protein-Based Microneedles, Int. J. Biol. Macromol., 2019, 136, 704–728, DOI:10.1016/j.ijbiomac.2019.04.163.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |





