Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanoflower-like ZnO–carbon quantum dot heterostructures for solar-driven degradation of methylene blue: a high-performance and recyclable photocatalyst for sustainable wastewater treatment
Hitesh
Bansal†
,
Palkaran
Sethi†
and
Soumen
Basu
 *
*
Department of Chemistry and Biochemistry, Thapar Institute of Engineering & Technology, Patiala-147004, India. E-mail: soumen.basu@thapar.edu
First published on 29th September 2025
Abstract
Developing robust, high-performance photocatalysts for environmental remediation remains a critical scientific pursuit. This work successfully synthesized novel ZnO–carbon quantum dot (CQD) heterostructured nanocomposites with distinct nanoflower-like morphology by incorporating 5%, 10%, and 15% CQDs onto ZnO surfaces. The strategic integration of CQDs not only enhanced solar light harvesting and facilitated superior charge carrier separation, but also improved the recyclability and stability of the composites, surpassing the limitations of conventional photocatalytic systems. Comprehensive characterization using XRD, FTIR, XPS, BET, PL, UV-Vis-DRS, FE-SEM, EDS, and HR-TEM analyses confirmed the synthesized composites' high crystallinity, enlarged surface area, morphology, and light response. Photocatalytic studies conducted under natural sunlight irradiation demonstrated an impressive 97.7% degradation of methylene blue (MB) in merely 60 minutes, following pseudo-first-order kinetics and achieving a rate constant of about 0.047 min−1, which is 5.7 times greater than that of the benchmark TiO2–P25 catalyst. Systematic investigations of solution pH, photocatalyst dosage, light sources, and radical scavenging further validated the robustness and versatility of the composite. Impressively, the ZnO/CQD nanocomposite retained 85% of its photocatalytic efficiency after six consecutive cycles, demonstrating exceptional reusability and operational stability. The photocatalytic degradation pathway was determined using LC-MS analysis, showing notable decreases of 65% in TOC (total organic carbon) and 58% in COD (chemical oxygen demand), which confirmed effective mineralization. Given the persistence, toxicity, and carcinogenic potential of MB in aquatic ecosystems, this study introduces a sustainable, scalable, and highly effective solar-driven photocatalyst. These outstanding results position the ZnO/CQD nanocomposite as a leading candidate for next-generation wastewater remediation technologies and offer a compelling advancement warranting publication in high-impact scientific forums.
1. Introduction
The rapid growth of global urban populations has significantly escalated the demand for clean water, resulting in critical shortages and emphasizing the urgent need to address water scarcity challenges.1 This crisis is further intensified by the increasing contamination of freshwater resources with industrial pollutants, including synthetic dyes, heavy metals, and persistent organic compounds.2 Among these, synthetic dyes pose a serious environmental threat due to their structural complexity, resistance to degradation, and toxicity.3 Extensively used in textiles, printing, leather, cosmetics, pharmaceuticals, and food industries,4 these dyes can severely impact aquatic ecosystems and human health even at low concentrations.Methylene blue (MB), a widely used cationic dye in medical and industrial applications, is known for its carcinogenicity, mutagenicity, and persistence in water bodies.5–7 Its removal from wastewater is imperative, yet conventional treatment methods—including biological processes,8 chemical oxidation,9 adsorption,10 photocatalysis,11 and membrane-based physical separation12—often fall short due to high cost, incomplete degradation, and secondary pollution. Photocatalysis, as a form of the advanced oxidation process (AOP), has emerged as a promising alternative due to its ability to completely mineralize organic contaminants under mild conditions.13 This technique involves the generation of reactive oxygen species (ROS) through light-activated semiconductors, which oxidize and break down complex organic molecules.
Several semiconductor materials—such as TiO2, Fe2O3, CdS, ZnS, GaP, and P2Mo18—have been extensively investigated for photocatalytic applications.14–16 Among them, ZnO has garnered significant attention due to its high electron mobility, strong oxidizing potential, low cost, chemical stability, and environmental compatibility.17–19 Despite these advantages, ZnO suffers from a wide band gap (∼3.2 eV), which restricts its activity to the UV region, and rapid recombination of photoinduced charge carriers, both of which limit its overall photocatalytic efficiency.20
To overcome these limitations, various strategies have been employed, including element doping, surface modification, and formation of heterojunctions. As a new class of carbon nanomaterials, carbon quantum dots (CQDs), which exhibit fluorescence, have also gained attention as co-catalysts in photocatalysis due to their broad visible light absorption, high photostability, upconversion photoluminescence and excellent electron transfer properties.21 When composited with ZnO, CQDs can significantly enhance visible light responsiveness, improve charge separation, and promote photocatalytic activity. The synergistic interaction between ZnO and CQDs results in efficient suppression of electron–hole recombination and facilitates prolonged charge carrier lifetimes.22
Several CQD-modified ZnO systems have been studied for organic pollutant degradation. For example, lanthanum-doped ZnO nanotubes showed effective MB degradation under sunlight at pH 9 within 90 min.23 Also, sulfur-doped ZnO films degraded rhodamine B in 150 min under visible light with H2O2 assistance.24 In another study, GO/ZnO composites doped with Ag or Au exhibited improved photocatalytic degradation of MB, although performance varied with metal dopants.25 Nitrogen-doped CQDs from biomass demonstrated effective Malachite Green degradation under sunlight,26 and ZnO/N,S-CQDs were used for ciprofloxacin removal under simulated light but showed reduced activity in real water samples.27 However, most of these systems still face issues such as slow kinetics, limited reusability, narrow pH ranges, or incomplete mineralization—factors that restrict their practical applications.
To address these challenges, we developed a novel ZnO/CQD heterostructured nanocomposite with a flower-like ZnO morphology and varying CQD loadings (5%, 10%, and 15% by weight). The nanoflower morphology of ZnO provides a significant surface area and numerous active sites, whereas the CQDs improve visible light absorption and facilitate interfacial charge transfer. Its structural and optical properties were extensively characterized using XRD, XPS, FTIR, BET, PL, UV-vis DRS, FE-SEM, EDS, and HR-TEM. The photocatalytic performance was evaluated for MB degradation under natural sunlight, with investigations into catalyst dosage, pH, light source, degradation kinetics, and scavenger effects. LC-MS analysis was used to elucidate degradation intermediates, while TOC and COD measurements confirmed mineralization. The optimized ZnO/CQD composite showed rapid degradation, high reusability, and excellent solar-light-driven performance, addressing the critical limitations of prior systems and offering a scalable, sustainable solution for wastewater treatment.
2. Materials and reagents
2.1. Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) (98% AR), L-ascorbic acid (99% extra pure), sodium hydroxide (NaOH) (97% extra pure), and methylene blue powder were obtained from Loba Chemie. The solutions were prepared using ultrapure double-distilled water. Pure reagents were used without any further purification.2.2. Synthesis of zinc oxide nanoflowers
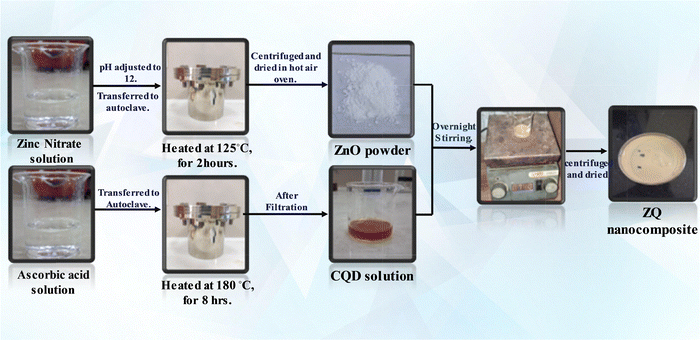
Zinc oxide (ZnO) nanoflowers were synthesized via a hydrothermal approach using Zn(NO3)2·6H2O as the precursor. Briefly, 4.46 g of Zn(NO3)2·6H2O was dissolved in 30 mL of deionized water under continuous magnetic agitation for 30 minutes to ensure complete dissolution. The solution's pH was adjusted to 12 by the dropwise addition of 5 M sodium hydroxide (NaOH) solution under constant stirring. The obtained homogeneous mixture was placed in a 100 mL Teflon-lined stainless-steel autoclave and subjected to heating at 125 °C for 2 hours in a muffle furnace, adhering to a previously documented procedure.28 Following natural cooling to room temperature, the precipitate was collected through centrifugation at 6000 rpm for 4 minutes. It was then thoroughly washed with deionized water and ethanol multiple times to eliminate unreacted species, and finally dried at ambient temperature. The obtained white powder was designated as ZnO (Z) for further use.2.3. Synthesis of CQDs
Carbon quantum dots (CQDs) were synthesized through a hydrothermal method using L-ascorbic acid as a carbon source. In this procedure, 2.0 g of L-ascorbic acid was carefully dissolved in 20 mL of deionized water while being subjected to magnetic stirring. The resulting clear solution was placed in a 50 mL Teflon-lined stainless-steel autoclave and subjected to heating at 180 °C for 8 hours in a muffle furnace, following the previously published procedure.29 A light-yellow suspension was obtained after the completion of the reaction. The suspension was filtered through standard filter paper, and the resulting light brown filtrate—containing the CQDs—was collected. The solid residue remaining on the filter paper was discarded. The obtained CQD solution was labeled as Q for subsequent composite synthesis.2.4. Synthesis of ZnO/CQD composites
To synthesize the ZnO/CQD nanocomposites, 950 mg of ZnO was carefully dispersed in a beaker containing 10 mL of the CQD solution. The solution was agitated for an extended period under ambient conditions to promote consistent interaction between ZnO and CQDs. After stirring, the suspension underwent centrifugation, and the resulting precipitate was meticulously washed with deionized water and ethanol to eliminate any residual impurities. The washed samples were subsequently dried overnight in a hot air oven. The obtained composite was designated as ZQ5, reflecting its CQD content. Similarly, other nanocomposites, namely ZQ10 and ZQ15, were prepared by varying the volume of CQD solution while keeping the ZnO weight constant, corresponding to 10% and 15% CQD loadings, respectively (Scheme 1).3. Results and discussion
3.1. Characterization methods
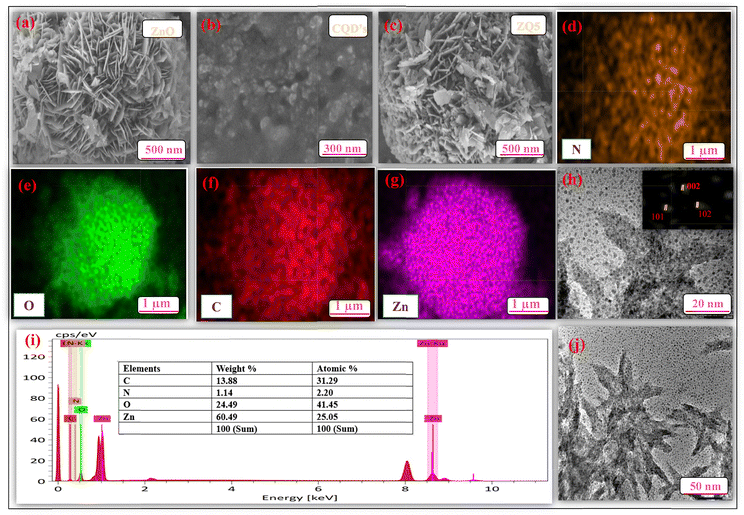
A comprehensive array of analytical methods was utilized to characterize the structural, morphological, optical, and compositional attributes of the synthesized nanocomposites. The UV-visible absorption spectra were obtained using a Shimadzu UV-2600 spectrophotometer, and the photoluminescence (PL) spectra were measured using a Shimadzu RF-6000 spectrofluorometer. The surface morphology and particle dimensions were analyzed using field emission scanning electron microscopy (FE-SEM, JEOL), while the internal structure at the nanoscale was further explored through high-resolution transmission electron microscopy (HR-TEM, JEOL JEM-2100 Plus). X-ray diffraction (XRD) was employed for the identification of crystallinity and the structural analysis. The materials' functional groups were characterized by Fourier-transform infrared (FTIR) spectroscopy using a Shimadzu IRTracer-100. Diffuse reflectance UV-vis spectroscopy (UV-DRS) was conducted to evaluate the optical band gap characteristics. The specific surface area and pore size distribution were determined from nitrogen adsorption–desorption isotherms employing the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods with a Belsorp Mini II analyzer. The elemental composition and chemical bonding states were examined through X-ray photoelectron spectroscopy (XPS) utilizing a Versa Probe III system. Liquid chromatography-mass spectrometry (LC-MS) was conducted using a Waters Micromass Q-Tof micro instrument to identify degradation intermediates and clarify the mechanism of photocatalytic degradation. Analyses of total organic carbon (TOC) and chemical oxygen demand (COD) were conducted to determine the extent of mineralization of the dye and evaluate the effectiveness of the photocatalytic process.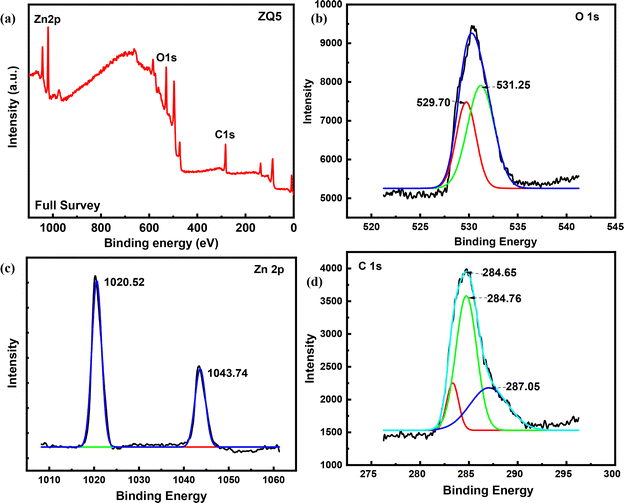 | ||
| Fig. 1 (a) XPS full spectrum of ZQ5, (b) spectrum of O 1s, (c) spectrum of Zn 2p, and (d) spectrum of C 1s. | ||
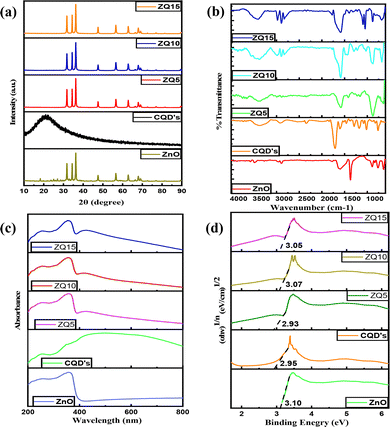
The high-resolution O 1s spectrum of the composite displays two distinct peaks at 530.34 eV and 531.81 eV, which are associated with Zn–O and C–O bonds, respectively. These changes, in combination with FTIR results, suggest that the carboxyl groups from CQDs react with the surface hydroxyl groups on ZnO. This interaction probably results in a decrease or the elimination of hydroxyl signals and the creation of new C–O bonds, as illustrated in Fig. 1(b).
Additionally, the C 1s spectrum (Fig. 1(d)) of the CQD/ZnO composite features a peak at 284.21 eV, linked to C–C bonds in CQDs. Peaks at 285.78 eV and 288.55 eV are associated with C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively. These spectral features confirm that CQDs have been successfully incorporated into the ZnO structure, forming a well-integrated composite.30
C bonds, respectively. These spectral features confirm that CQDs have been successfully incorporated into the ZnO structure, forming a well-integrated composite.30
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching associated with carbonyl groups. The bending vibrations of interstitial water molecules were evident at 752 cm−1, alongside a deformation band near 644 cm−1. Additionally, vibrational signatures associated with carbonate ions (CO32−) were identified at 1507, 1382, and 845 cm−1. A peak at 1560 cm−1 further confirmed the presence of symmetric C
O stretching associated with carbonyl groups. The bending vibrations of interstitial water molecules were evident at 752 cm−1, alongside a deformation band near 644 cm−1. Additionally, vibrational signatures associated with carbonate ions (CO32−) were identified at 1507, 1382, and 845 cm−1. A peak at 1560 cm−1 further confirmed the presence of symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations.28 CQDs exhibited a broad absorption region between 3135 and 3576 cm−1, characteristic of O–H and carboxyl groups. An absorption band at 1588 cm−1 was assigned to N–H bending, supported by a minor peak at 2351 cm−1, which further suggested the presence of amine-related functionalities. The C–O stretching vibration was observed at 1167 cm−1, confirming the oxygenated surface nature of CQDs.29 The FTIR spectra of the nanocomposites (ZQ5, ZQ10, and ZQ15) retained the key functional group signatures of both ZnO and CQDs, though slight shifts in peak positions were observed. These spectral changes imply successful surface interactions between ZnO and CQDs, confirming the formation of a hybrid composite without disrupting the core structure of either component.
O stretching vibrations.28 CQDs exhibited a broad absorption region between 3135 and 3576 cm−1, characteristic of O–H and carboxyl groups. An absorption band at 1588 cm−1 was assigned to N–H bending, supported by a minor peak at 2351 cm−1, which further suggested the presence of amine-related functionalities. The C–O stretching vibration was observed at 1167 cm−1, confirming the oxygenated surface nature of CQDs.29 The FTIR spectra of the nanocomposites (ZQ5, ZQ10, and ZQ15) retained the key functional group signatures of both ZnO and CQDs, though slight shifts in peak positions were observed. These spectral changes imply successful surface interactions between ZnO and CQDs, confirming the formation of a hybrid composite without disrupting the core structure of either component.
Upon compositing, ZQ5, ZQ10, and ZQ15 hybrids demonstrated a noticeable enhancement in visible light absorption compared to bare ZnO. This red-shift in absorption is attributed to the sensitizing role of CQDs, which improve the photon capture capability of the system and extend its optical response beyond the UV region. Additionally, a visible change in material color—from white for pure ZnO to a progressively darker shade in the composites—further supports the successful integration of CQDs and the corresponding increase in visible-light harvesting capacity.
To determine the optical band gap, Tauc's relation was used as follows:
| (αhν)1/n = A(hν − Eg) | (1) |
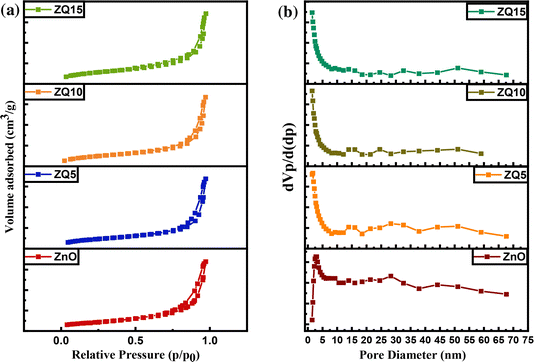
| Catalyst | Surface area (m2 g−1) | Average pore size (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| ZnO | 12.469 | 14.303 | 0.044 |
| ZQ5 | 11.353 | 12.689 | 0.036 |
| ZQ10 | 8.9343 | 12.031 | 0.0269 |
| ZQ15 | 14.582 | 10.761 | 0.0369 |
3.2. Photocatalytic activity
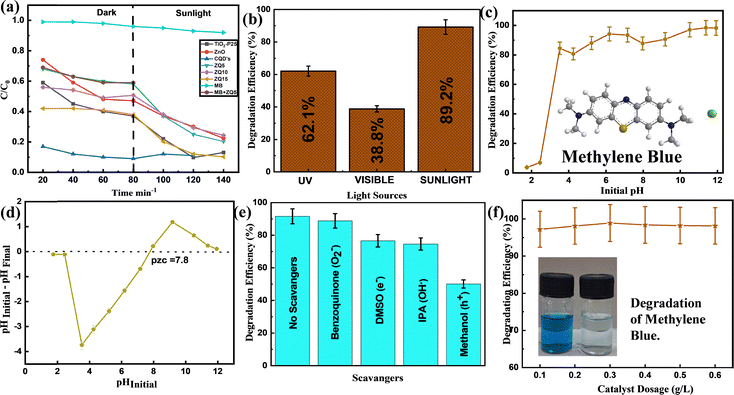
By tracking the methylene blue (MB) degradation under natural sunlight in Patiala, India, which has a subtropical climate, the ZQ5 nanocomposite's photocatalytic effectiveness was assessed (Thapar Institute of Engineering & Technology). The average sun irradiation during the experiments was measured using a LICOR pyranometer to be 850 W m−2. For comparative light source studies, visible-light irradiation from a 45 W compact fluorescent lamp (Philips) was used and UV light exposure was achieved using a 100 W mercury lamp.Photocatalytic experiments were conducted using a 10 mL aqueous solution of MB (10 ppm), where the catalyst dosage was maintained at 0.1 g L−1. To achieve adsorption–desorption equilibrium, the suspensions were stirred for 60 minutes in the dark before irradiation. The degradation process was then initiated under natural sunlight and continued for an additional 60 minutes. Absorbance measurements were taken at regular intervals using a UV-vis spectrophotometer, with the MB λmax identified at 660 nm. All degradation studies were conducted in triplicate, and the results were illustrated with error bars indicating a 5% margin to ensure reproducibility.
Photocatalytic degradation efficiency was calculated using the equation:
 | (2) |
Among all tested photocatalysts, ZQ5 showed the highest degradation efficiency of 97.2% within 60 minutes of solar exposure (Fig. 5(a)). The kinetic behavior of the photocatalytic reaction was further analyzed using a pseudo-first-order kinetic model:
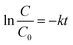 | (3) |
The synergy factor (R) was determined using the following equation:
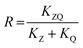 | (4) |
| Material | Rate constant (min−1) | Synergy factor (R) |
|---|---|---|
| TiO2–P25 | 0.0082 | — |
| ZnO | 0.015 | — |
| CQDs | 0.0092 | — |
| ZQ5 | 0.047 | 2.02 |
| ZQ10 | 0.019 | 0.81 |
| ZQ15 | 0.017 | 0.73 |
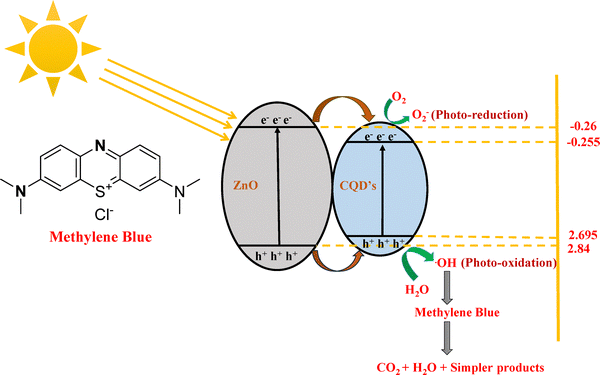
| ZnO/CQD + hν → e− + h+ | (5) |
| e−CB + O2 → O2˙− | (6) |
| O2˙− + H2O → OH˙ + HOO˙ | (7) |
| h+VB + OH− → OH˙ | (8) |
| OH˙/h+ + pollutant → degraded products | (9) |
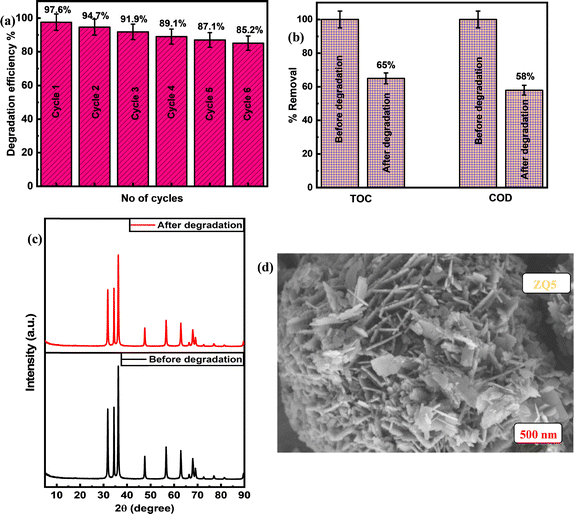
The findings underscore the impressive stability and regenerative capability of the ZQ5 nanocomposite, positioning it as a highly promising photocatalyst for a range of photocatalytic applications moving forward.
 | (10) |
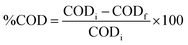 | (11) |
| Catalyst | Concentration of MB in ppm | Catalyst concentration in g L−1 | Reaction time in min | Light source | Degradation efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| ZnS/CdS | 10 | 0.1 | 360 | Visible light | 73 | 38 |
| WO3/TiO2 | 10 | 0.5 | 120 | Visible light | 27 | 39 |
| BiVO4/EDTA | 5 | 1 | 300 | Sunlight | 90.88 | 40 |
| (Yb,N)-TiO2 | 10 | 3 | 300 | Visible light | 93.55 | 41 |
| TiO2/polyaniline | 10 | 2.5 | 200 | UV light | 73 | 42 |
| ZnO/CQD | 10 | 0.1 | 60 | Sunlight | 97.7 | Present work |
4. Conclusion
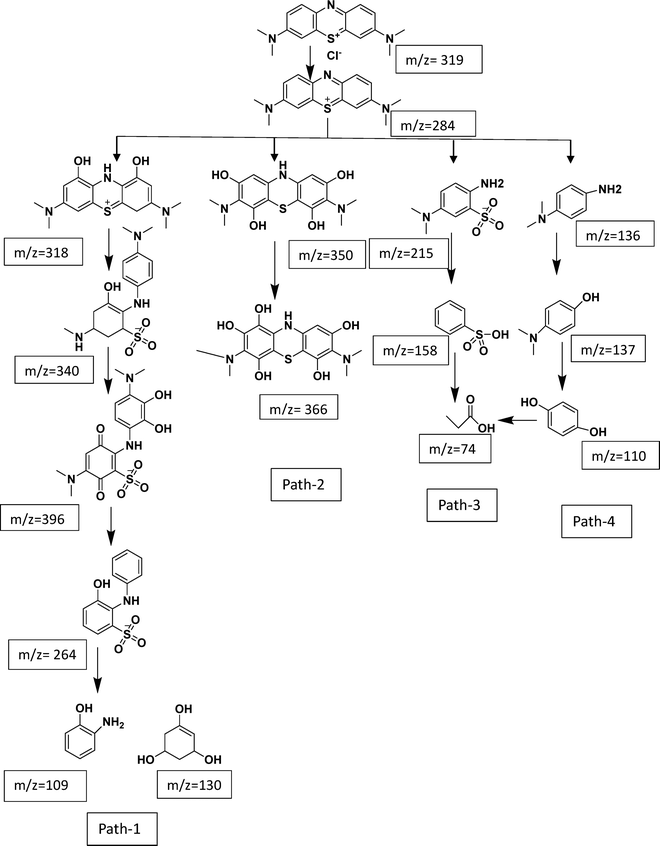
Using a hydrothermal method, a number of ZnO–CQD heterojunction photocatalysts with different molar ratios were effectively synthesized. Comprehensive characterization through XRD, FE-SEM, EDS, XPS, UV-DRS, BET, and HR-TEM confirmed the successful formation of a well-integrated heterostructure, where smaller CQDs were effectively deposited on larger ZnO nanoflowers. The photocatalytic activities of the synthesized samples were evaluated under natural sunlight using MB as a model pollutant. Among the composites, ZQ5 exhibited the most outstanding photocatalytic activity, with an apparent first-order rate constant (pseudo-first-order rate constant) of 0.047 min−1, which was 5.7 times higher than that of TiO2–P25 (0.0082 min−1), 3.1 times higher than that of ZnO (0.015 min−1), 5.1 times higher than that of CQDs (0.0092 min−1), 2.5 times higher than that of ZQ10 (0.019 min−1), and 2.8 times higher than that of ZQ15 (0.017 min−1). This remarkable enhancement is primarily attributed to the composite's improved charge separation, reduced recombination rate, and suitable band gap, as evidenced by PL, BET, and UV-DRS studies. Additional investigations—including catalyst dosage, pH influence, light source variation, scavenger analysis, and reusability—demonstrated the composite's robustness under varied conditions. Trapping experiments further confirmed that superoxide radicals (O2−) and electrons (e−) were the main active species responsible for MB degradation. The ZQ5 catalyst also exhibited excellent reusability, maintaining high photocatalytic efficiency over six consecutive cycles. Post-degradation XRD and FE-SEM analyses confirmed structural stability, with no significant changes in crystallinity or morphology. The degradation mechanism was further elucidated by LC-MS analysis, which identified intermediate products, validating successful mineralization of the dye. In addition, the ZQ5 photocatalyst demonstrated superior removal efficiencies of COD (58%) and TOC (65%) when compared to conventional physicochemical methods, emphasizing its practical potential in wastewater treatment. In summary, the ZQ5 nanocomposite stands out as a highly effective, reusable, and eco-friendly photocatalyst for solar-driven degradation of organic pollutants.Author contributions
Hitesh Bansal designed and performed the experiments, analyzed the data, and co-wrote the paper; Palkaran Sethi supervised the research, designed the experiments, and co-wrote the paper; Soumen Basu supervised the research, designed the experiments, and co-wrote the paper.Conflicts of interest
The authors declare no conflicts of interest regarding the conduct of this study and the publication of its results.Data availability
The data supporting the findings of this study, including characterization results, degradation kinetics, and LC-MS data, are available within the article as well as the SI file provided with this article. Supplementary information: characterization of the synthesized materials, kinetics plots, and LC-MS data. See DOI: https://doi.org/10.1039/d5ma00804b.Acknowledgements
We are grateful to DCBC and DPMS, TIET for providing us with characterization facilities. We are also thankful to the Department of Chemical Engineering for providing access to their UV-visible spectrophotometer facilities. We are also grateful to the Department of Science and Technology (DST), Government of India, for the financial support under the FIST program, which facilitated the FTIR analysis in this study. We are also grateful to the SAIF and CIL, Punjab University for providing us with LC/MS and HR-TEM characterization facilities and to IIC, IIT Roorkee, for XPS characterization.References
- S. Dong, J. Feng, M. Fan, Y. Pi, L. Hu, M. Liu, J. Sun and C. Du, Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review, RSC Adv., 2015, 5, 14610–14630, 10.1039/C4RA13734E.
- H. Deng, J. Yin, J. Ma, J. Zhou, L. Zhang, L. Gao and T. Jiao, Exploring the enhanced catalytic performance on nitro dyes via a novel template of flake-network Ni–Ti LDH/GO in-situ deposited with Ag3PO4 NPs, Appl. Surf. Sci., 2020, 543, 148821, DOI:10.1016/j.apsusc.2020.148821.
- J. Qu and M. Fan, The Current State of Water Quality and Technology Development for Water Pollution Control in China, Crit. Rev. Environ. Sci. Technol., 2010, 40, 519–560, DOI:10.1080/10643380802451953.
- I. Khan, I. Khan, M. Usman, M. Imran and K. Saeed, Nanoclay-mediated photocatalytic activity enhancement of copper oxide nanoparticles for enhanced methyl orange photodegradation, J. Mater. Sci.: Mater. Electron., 2020, 31, 8971–8985, DOI:10.1007/s10854-020-03431-6.
- A. P. Bhat and P. R. Gogate, Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: a review on aliphatic and aromatic amines, dyes, and pesticides, J. Hazard. Mater., 2021, 403, 123657, DOI:10.1016/j.jhazmat.2020.123657.
- R. Javaid and U. Qazi, Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview, Int. J. Environ. Res. Public Health, 2019, 16, 2066, DOI:10.3390/ijerph16112066.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Photocatalytic degradation pathway of methylene blue in water, Appl. Catal., B, 2001, 31, 145–157, DOI:10.1016/S0926-3373(00)00276-9.
- P. Zhang, M. Xiang, H. Liu, C. Yang and S. Deng, Novel Two-Dimensional Magnetic Titanium Carbide for Methylene Blue Removal over a Wide pH Range: Insight into Removal Performance and Mechanism, ACS Appl. Mater. Interfaces, 2019, 11, 24027–24036, DOI:10.1021/acsami.9b04222.
- C. Hou, B. Hu and J. Zhu, Photocatalytic degradation of methylene blue over TiO2 pretreated with varying concentrations of NaOH, Catalysts, 2018, 8, 575 CrossRef.
- P. Sethi, S. Barman and S. Basu, Strategic tuning of GO ratios in CuBTC-GO nanocomposites for next-generation tetracycline adsorption: a deep dive into isotherms, kinetics, and thermodynamics, Sep. Purif. Technol., 2025, 361, 131311, DOI:10.1016/j.seppur.2024.131311.
- P. Sethi, S. Basu and S. Barman, Innovative CuBTC/g-C3N4 materials for tetracycline mitigation: adsorption, photocatalysis, and mechanistic perspectives, New J. Chem., 2025, 49, 8454–8471, 10.1039/D5NJ00556F.
- M. Basibuyuk and C. F. Forster, An examination of the adsorption characteristics of a basic dye (Maxilon Red BL-N) on to live activated sludge system, Process Biochem., 2003, 38, 1311–1316, DOI:10.1016/S0032-9592(02)00327-8.
- G. Zhu, H. Fang, Y. Xiao and A. Hursthouse, The Application of Fluorescence Spectroscopy for the Investigation of Dye Degradation by Chemical Oxidation, J. Fluoresc., 2020, 30, 1271–1279, DOI:10.1007/s10895-020-02591-2.
- E. Alventosa-deLara, S. Barredo-Damas, M. I. Alcaina-Miranda and M. I. Iborra-Clar, Ultrafiltration technology with a ceramic membrane for reactive dye removal: optimization of membrane performance, J. Hazard. Mater., 2012, 209–210, 492–500, DOI:10.1016/j.jhazmat.2012.01.065.
- M. M. Russell, D. M. Kempisty, S. R. Kanel, S. Kurwadkar, S. W. Brittle, I. Sizemore and L. Yaal, Destruction of Aqueous Phase Organic Pollutants Using Ultraviolet Light-Emitting Diodes and Photocatalysis, Water, Air, Soil Pollut., 2018, 229, 139, DOI:10.1007/s11270-018-3785-2.
- Y. Zhang, M. Zhao, J. Huang, N. Zhao and H. Yu, Controllable Synthesis, Photocatalytic Property, and Mechanism of a Novel POM-Based Direct Z-Scheme Nano-Heterojunction α-Fe2O3/P2Mo18, Molecules, 2023, 28, 6671, DOI:10.3390/molecules28186671.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Recent developments in photocatalytic water treatment technology: a review, Water Res., 2010, 44, 2997–3027, DOI:10.1016/j.watres.2010.02.039.
- L. Kong, Y. Liu, L. Dong, L. Zhang, L. Qiao, W. Wang and H. You, Enhanced red luminescence in CaAl12O19: Mn4+ via doping Ga3+ for plant growth lighting, Dalton Trans., 2020, 49, 1947–1954, 10.1039/C9DT04086B.
- C. Y. Teh, T. Y. Wu and J. C. Juan, An application of ultrasound technology in synthesis of titania-based photocatalyst for degrading pollutant, Chem. Eng. J., 2017, 317, 586–612, DOI:10.1016/j.cej.2017.01.001.
- T. Hisatomi, J. Kubota and K. Domen, ChemInform Abstract: Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting, Chem. Soc. Rev., 2014, 43, 7520–7535, 10.1039/c3cs60378d.
- N. Roy and A. Roy, Zinc vacancy mediated structural phase transition and photoluminescence in nanocrystalline ZnS thin films, J. Mater. Sci.: Mater. Electron., 2014, 25, 1275–1279, DOI:10.1007/s10854-014-1721-9.
- J.-J. Xu, Y.-N. Lu, F.-F. Tao, P.-F. Liang and P.-A. Zhang, ZnO Nanoparticles Modified by Carbon Quantum Dots for the Photocatalytic Removal of Synthetic Pigment Pollutants, ACS Omega, 2023, 8, 7845–7857, DOI:10.1021/acsomega.2c07591.
- N. Roy, A. Chowdhury and A. Roy, Observation of negative differential resistance and electrical bi-stability in chemically synthesized ZnO nanorods, J. Appl. Phys., 2014, 115, 223502, DOI:10.1063/1.4882017.
- N. Roy and A. Roy, Growth and temperature dependent photoluminescence characteristics of ZnO tetrapods, Ceram. Int., 2015, 41, 4154–4160, DOI:10.1016/j.ceramint.2014.11.113.
- N. Roy, A. Chowdhury, T. Paul and A. Roy, Morphological, optical, and Raman characteristics of ZnO nanoflowers on ZnO-seeded Si substrates synthesized by chemical method, J. Nanosci. Nanotechnol., 2016, 16, 9738–9745 CrossRef CAS.
- L. K. Adams, D. Y. Lyon and P. J. J. Alvarez, Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions, Water Res., 2006, 40, 3527–3532, DOI:10.1016/j.watres.2006.08.004.
- S. Qu, X. Wang, L. Qipeng, X. Liu and L. Wang, A Biocompatible Fluorescent Ink Based on Water-Soluble Luminescent Carbon Nanodots, Angew. Chem., Int. Ed., 2012, 51, 12215, DOI:10.1002/anie.201206791.
- S. Mohan, M. Vellakkat, A. Aravind and R. U, Hydrothermal synthesis and characterization of Zinc Oxide nanoparticles of various shapes under different reaction conditions, Nano Express, 2020, 1, 030028, DOI:10.1088/2632-959X/abc813.
- H. Shabbir, T. Tokarski, D. Ungor and M. Wojnicki, Eco Friendly Synthesis of Carbon Dot by Hydrothermal Method for Metal Ions Salt Identification, Materials, 2021, 14, 7604, DOI:10.3390/ma14247604.
- X.-Y. Jin, W.-Y. Ying, R.-J. Che, P. Xiao, Y.-Q. Zhou, Y. Liu, M.-Y. Liu and S.-P. Chen, CQDs/ZnO composites based on waste rice noodles: preparation and photocatalytic capability, RSC Adv., 2022, 12, 23692–23703, 10.1039/D2RA03709B.
- H. Bozetine, Q. Wang, A. Barras, M. Li, T. Hadjersi, S. Szunerits and R. Boukherroub, Green chemistry approach for the synthesis of ZnO–carbon dots nanocomposites with good photocatalytic properties under visible light, J. Colloid Interface Sci., 2016, 465, 286–294, DOI:10.1016/j.jcis.2015.12.001.
- A. Mehta, A. Mishra, S. Kainth and S. Basu, Carbon quantum dots/TiO2 nanocomposite for sensing of toxic metals and photodetoxification of dyes with kill waste by waste concept, Mater. Des., 2018, 155, 485–493, DOI:10.1016/j.matdes.2018.06.015.
- A. Kumar, R. Kumar and G. Pandey, Synthesis, Characterization of Titania/Polyaniline/GO Nanocomposites, and Its Photocatalytic Activity Under UV-Visible Light, Macromol. Symp., 2018, 379, 1600192, DOI:10.1002/masy.201600192.
- A. Kundu, S. Sharma and S. Basu, Modulated BiOCl nanoplates with porous g-C3N4 nanosheets for photocatalytic degradation of color/colorless pollutants in natural sunlight, J. Phys. Chem. Solids, 2021, 154, 110064, DOI:10.1016/j.jpcs.2021.110064.
- S. Singla, S. Sharma and S. Basu, MoS2/WO3 heterojunction with the intensified photocatalytic performance for decomposition of organic pollutants under the broad array of solar light, J. Cleaner Prod., 2021, 324, 129290, DOI:10.1016/j.jclepro.2021.129290.
- H. Alamgholiloo, E. Asgari, S. Nazari, A. Sheikhmohammadi, N. Noroozi Pesyan and B. Hashemzadeh, Architecture of bimetallic-MOF/silicate derived Co/NC@mSiO2 as peroxymonosulfate activator for highly efficient ciprofloxacin degradation, Sep. Purif. Technol., 2022, 300, 121911, DOI:10.1016/j.seppur.2022.121911.
- S. Singla, P. Devi and S. Basu, Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite, Catalysts, 2023, 13, 731, DOI:10.3390/catal13040731.
- N. Soltani, E. Saion, M. Hussein, M. Erfani, A. Abedini, G. Bahmanrokh, M. Navasery and P. Vaziri, Visible Light-Induced Degradation of Methylene Blue in the Presence of Photocatalytic ZnS and CdS Nanoparticles, Int. J. Mol. Sci., 2012, 13, 12242–12258, DOI:10.3390/ijms131012242.
- L. Yang, Z. Si, D. Weng and Y. Yao, Synthesis, characterization and photocatalytic activity of porous WO3/TiO2 hollow microspheres, Appl. Surf. Sci., 2014, 313, 470–478, DOI:10.1016/j.apsusc.2014.05.230.
- W. Ma, Z. Li and W. Liu, Hydrothermal preparation of BiVO4 photocatalyst with perforated hollow morphology and its performance on methylene blue degradation, Ceram. Int., 2015, 41, 4340–4347, DOI:10.1016/j.ceramint.2014.11.123.
- J. Zhang, L. J. Xu, Z. Q. Zhu and Q. J. Liu, Synthesis and properties of (Yb, N)-TiO2 photocatalyst for degradation of methylene blue (MB) under visible light irradiation, Mater. Res. Bull., 2015, 70, 358–364, DOI:10.1016/j.materresbull.2015.04.060.
- K. R. Reddy, K. V. Karthik, S. B. B. Prasad, S. K. Soni, H. M. Jeong and A. V. Raghu, Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts, Polyhedron, 2016, 120, 169–174, DOI:10.1016/j.poly.2016.08.029.
- O. Intharaksa, S. Nanan, N. Patdhanagul, T. Panphojan, T. Srikakul, N. Tantisuwichwong, N. Tantisuwichwong and R. Dulyasucharit, Preparation of magnetic CuO/Fe3O4/ZnO photocatalyst for complete degradation of methylene blue under natural sunlight irradiation, J. Phys. Chem. Solids, 2023, 182, 111577, DOI:10.1016/j.jpcs.2023.111577.
- Y. Cao, G. Wu, Y. Huang and C. Huang, Synergistic Degradation of Methylene Blue by Hydrodynamic Cavitation Combined with Hydrogen Peroxide/Vitamin C System, ACS Omega, 2024, 9, 39997–40009, DOI:10.1021/acsomega.4c05815.
- J. Lin, Z. Luo, J. Liu and P. Li, Photocatalytic degradation of methylene blue in aqueous solution by using ZnO–SnO2 nanocomposites, Mater. Sci. Semicond. Process., 2018, 87, 24–31, DOI:10.1016/j.mssp.2018.07.003.
Footnote |
| † Both authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |